Marine natural products as a source of novel anticancer drugs: an updated review (2019–2023)
Abstract
Marine natural products have long been recognized as a vast and diverse source of bioactive compounds with potential therapeutic applications, particularly in oncology. This review provides an updated overview of the significant advances made in the discovery and development of marine-derived anticancer drugs between 2019 and 2023. With a focus on recent research findings, the review explores the rich biodiversity of marine organisms, including sponges, corals, algae, and microorganisms, which have yielded numerous compounds exhibiting promising anticancer properties. Emphasizing the multifaceted mechanisms of action, the review discusses the molecular targets and pathways targeted by these compounds, such as cell cycle regulation, apoptosis induction, angiogenesis inhibition, and modulation of signaling pathways. Additionally, the review highlights the innovative strategies employed in the isolation, structural elucidation, and chemical modification of marine natural products to enhance their potency, selectivity, and pharmacological properties. Furthermore, it addresses the challenges and opportunities associated with the development of marine-derived anticancer drugs, including issues related to supply, sustainability, synthesis, and clinical translation. Finally, the review underscores the immense potential of marine natural products as a valuable reservoir of novel anticancer agents and advocates for continued exploration and exploitation of the marine environment to address the unmet medical needs in cancer therapyGraphical Abstract
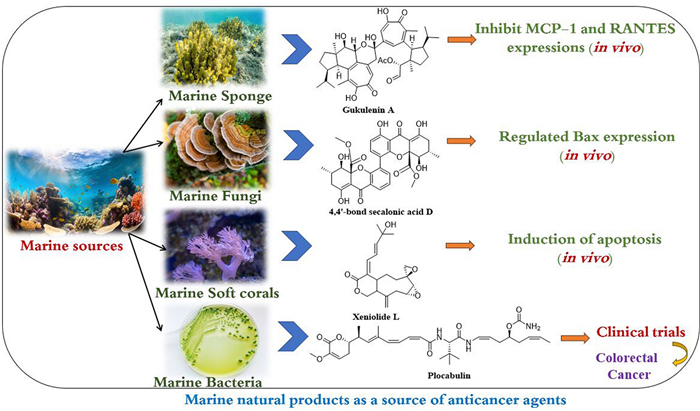
Keywords
Marine natural products Microorganism Anticancer Clinical trials Drugs1 Introduction
In the contemporary era, cancer represents a serious communal health issue and one of the main reasons for mortality worldwide, second to cardiovascular ailments [1]. Notably, tumors are not a modern ailment as previously mentioned, but it is an old one since it was mentioned in Egyptian papyrus [2]. Generally, cancer is a devastating disease that affects millions of individuals. It happens once cells in the body grow out of control, resulting in abnormal tissue growth [3]. The World Health Organization (WHO) reports that approximately one in six deaths worldwide is due to cancer, making it a significant global health issue [4]. According to recent statistics (GLOBOCAN 2020), almost 19.3 million new cancer cases with an estimated ten million cancer deaths were globally documented in 2020 [5].
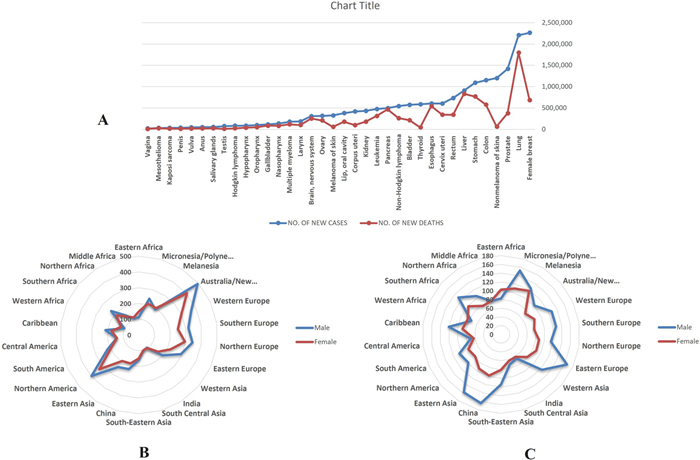
Furthermore, in 2040, the worldwide cancer encumbrance is predictable to be 28.4 million cases, a 47% growth from 2020 [5]. For instance, in 2023, 1,958,310 new cases of cancer and 609,820 cancer deaths are anticipated in the United States [6]. Figure 1A–C depicts the new cancer cases and deaths for 36 kinds of cancer in various world areas based on GLOBOCAN 2020. It is conspicuous that the lung, liver, and stomach cancers are the primary reasons for cancer mortality, where the most incidence occurs in Australia/New Zealand for both female and male sex, while the highest mortality cases belong to China and Eastern Europe countries [5].
A New Cases and Deaths for 36 Cancers and All Cancers Combined in 2020 based on GLOBOCAN 2020; B Incidence Rates (Age-Standardized Rate) for 22 World Areas and Sex for All Cancers Combined in 2020 based on GLOBOCAN 2020; C Mortality Rates (Age-Standardized Rate) for 22 World Areas and Sex for All Cancers Combined in 2020 based on GLOBOCAN 2020
Epidemiological studies have discussed the role of smoking, air pollution, alcohol consumption, genetic mutation, occupational exposure, viral infection, UV radiation, high obesity, junk food, and immunosuppression among others to contribute to cancer incidence [7, 8]. Despite the success of chemotherapy, radiation therapy, targeted therapy, surgery, hormone therapy, immunotherapy, and endocrine therapy in managing several types of cancer, still a plethora of patients die due to drug resistance, side effects of chemotherapy, and low immunity [9]. Thus, new approaches should be developed to combat cancer development or reduce the unwanted consequences with or without treatment [10, 11].
Marine creatures are considered one of the essential foundations of novel drugs. In this context, Carroll et al. claims that 1490 and 1425 new compounds have been discovered from marine sources in 2019 and 2021, respectively [12, 13]. Marine organisms, including algae (blue, red, green, red, and brown), microorganisms (bacteria, fungi), sponges, phytoplankton (dinoflagellates), mollusks (sea cucumbers and hares), coelenterates (sea anemones, gorgonians, and soft corals), and bryozoans, have long been known to produce a wide variety of secondary metabolites with diverse and complex chemical structures [14, 15]. Numerous of these compounds have been found to exhibit remarkable biological potential, including anticancer, antimicrobial, and anti-inflammatory properties [13]. Importantly, marine-derived compounds have shown promise as potent inhibitors of cancer cell growth and have demonstrated activity toward a wide spectrum of cancer types, among them some that are resistant to conventional chemotherapy [14]. These compounds offer a unique and largely untapped source of chemical diversity that can be exploited in the search for new and effective cancer treatments [16]. This has led to a growing body of research focused on the detection and improvement of marine-derived anticancer drugs, with many compounds undergoing preclinical and clinical evaluation.
The potential for discovery of new anticancer medicines from marine natural ingredients has attracted increasing attention in recent years. Thus, the main goal of the present manuscript is to screen the updated and latest investigation on marine natural products with anticancer activity as a continuation of our previously published review in 2019 [17]. The review will cover their action mechanism, pharmacological properties, and potential therapeutic applications. As well as the review highlights the challenges and opportunities associated with the detection and improvement of marine-derived anticancer drugs, and finally it discusses the prospects for future research in this exciting and rapidly evolving field.
2 Methodology
An extensive survey of the chemical compounds and anticancer activities of marine natural products was conducted in scientific databases, including Google Scholar and SciFinder. In the present review, search terms “marine algae’’, “marine soft corals’’, “microalgae’’, “marine bacteria’’, “marine fungi’’ were used either to search “in vivo’’ or “in vitro studies against cancer’’. Also, “clinical trials of marine against cancer’’ and “marketed marine drugs against cancer’’ were used for data collection. In total, 88 publications were included from the year 2019 to December 2023. From those, 61 studies were used to analyze recent updates and information related to marine anticancer compounds in the context of the topics mentioned above.
3 Different marine sources against cancer
3.1 Secondary metabolites of marine sponges against cancer
3.1.1 In vitro studies of bioactive compounds from marine sponge
The marine sources are rich in different classes of secondary metabolites with various pharmacological targets as shown in Fig. 2. The sedentary lifestyle of marine sponges allows them to produce various bioactive compounds to protect themselves from predators. These bioactive compounds have a variety of medical applications, i.e., cancer treatment [18-20]. Cytarabine, fludarabine phosphate, nelarabine, and eribulin mesylate are the four molecules produced by marine sponges (or by symbiotic cyanobacteria) that have received Food and Drug Administration (FDA) approval as anti-tumor medications. FDA and European Medicines Agency (EMA) later authorized fludarabine phosphate and nelarabine as anticancer drugs for leukemia and lymphoma [21, 22]. Herein we survey the different isolated bioactive compounds derived from marine sponge and involved in preclinical and clinical anticancer studies between 2019 and 2023 as illustrated in Tables 1, 2, and Figs. 3 and 4. It is significant to notice that we detailed the highly bioactive compounds in more depth.
Flowchart of the screened classes from different sources with the anticancer impact
In vitro studies of bioactive compounds isolated from marine sponge between 2019 and 2023
In-vivo studies of bioactive compounds isolated from marine sponge between 2019 and 2023
Structures of bioactive compounds isolated from marine sponge between 2019 and 2023 with in vitro studies
Structures of bioactive compounds isolated from marine sponge between 2019 and 2023 with in vivo studies
A natural quinazoline derivative named 2-Chloro-6-phenyl-8H-quinazolino[4,3-b]quinazolin-8-one (1), was obtained from marine sponge Hyrtios erectus. It showed a potentially anticancer impact against human breast cancer as confirmed by MTT assay using MCF-7 as in vitro model. Compound 1 exhibited Half-maximal inhibitory concentration (IC50) values of 13.04 ± 1.03 µg/mL and 22.67 ± 1.53 µg/mL for 48 and 24 h, compared with the positive control (cyclophosphamide: IC50 values of 8.11 ± 0.84 µg/mL and 15.11 ± 1.16 µg/mL for 48 and 24 h); respectively. The mechanism of action was explained by inducing breast carcinoma cells apoptosis via production of ROS and either extrinsic or intrinsic pathways of apoptosis [23]. In another study, the Indonesian marine sponge of Spongia sp. yielded three new bioactive compounds: ceylonamides G–I (2–4). This study assessed the inhibition of human prostate cancer DU145 cells growth in vitro using 2D monolayer cultures and spheroid of 3D cell culture. Bioactive compound 2 showed a significant effect with IC50 6.9 μM for 2D culture, and Medical Executive Committee (MEC) 10 μM for 3D spheroid cell culture in comparison with taxol with IC50 2.6 nM and MEC 10 nM, respectively. On the other hand, compounds 3 and 4didn't exhibit activity up to 100 μM. The mode of action for these compounds wasn’t clearly described [24]. Sakai and his team isolated a novel protein from the marine sponge Spongosorites sp. named by soritesidine (5). Investigation of the cytotoxicity was conducted in vitro through using the cancer cell line HeLa cells and L1210 murine leukemia cells. Soritesidine (5) showed a potential IC50 value of 0.062 and 12.11 ng/mL, respectively. The mechanism of action for this protein is not investigated yet [25]. Another new cycloheptapeptide named phakefustatin A (7), was obtained from Phakellia fusca a marine sponge by Wu and co-authors (2020). The cytotoxicity was evaluated against six cancer cell lines of human (HeLa, MCF-7, PC9, NCI-H460, SW480, and HepG2), and (H9c2 and HEK293T) nonmalignant cell lines in vitro. Compound 7 shows a highly significant effect only for HeLa, MCF-7, and NCI-H460 with IC50 values of 6.2 ± 0.3, 3.4 ± 1.2, and 7.1 ± 0.6 μM, respectively compared with PC cisplatin has IC50 values of 4.4 ± 0.3, 4.8 ± 0.8, and 3.2 ± 1.1 μM, respectively. The mode of action took place through apoptosis and cell growth inhibition via the pathway of Retinoid X receptor alpha (RXRα)-mediated phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt) signaling [26]. Three bioactive compounds, siphonellamides A and B (8 and 9), two new polyacetylene amides, and siphonellamide E (10), a new fatty amide, were obtained from the Red Sea marine sponge Siphonochalina siphonella. These substances were evaluated for cytotoxicity in vitro using MCF-7, HeLa, and A549 cancer cell lines.
. Compounds 8 and 9 demonstrated a strong cytotoxic effect on cancer cell lines. HeLa had IC50 values between 9.4 and 17.4 μM, whereas PC 5-Fluorouracil showed IC50 values of 28.4 μM.
. According to this study, the acetylene functionality may be responsible for their cytotoxic properties [27]. Surti and co-authors [28] obtained the bioactive compound Ilimaquinone (17) from marine sponge Hippospongia metachromia. This study evaluated the molecular mechanism of 17 on the anticancer through using colorectal cancer cell line HCT-116.
The results confirmed that compound 17 had a growth-inhibiting effect, with an IC50 of 17.89 μM.
Ilimaquinone triggers mitochondria-mediated apoptosis through the decrease in mitochondrial membrane potential and activation caspase-9/-3, DNA damage, and a reduction in Bcl-2 proportion [28]. Two new diterpenoids, kalihioxepane A and B (18 and 19), were separated from the marine sponge Acanthella cavernosa. The cytotoxic activity was evaluated using five cancer cell lines: K562, ASPC-1, H69AR, H69, and MDA-MB-231. The findings exhibited that exclusively compound 18 displayed a potential effect against K562, ASPC-1, and H69 with IC50 values of 6.57, 16.17, and 3.60 μmol/L, compared to positive control doxorubicin with IC50 0.252, 0.023, and 0.980 μmol/L, respectively. While compound 19 showed a potent effect against only one cell line, K562, with IC50 8.73 μmol/L compared with positive control (PC) doxorubicin with IC50 0.252 μmol/L. Both compounds showed significant cytotoxicity, indicating that the isocyano substituent was important [29]. Another study reported two secondary metabolites: pelorol and 5-epi-ilimaquinone (28 and 29), purified from Dactylospongia elegans. Two compounds were evaluated using 501Mel melanoma cells. The findings of the cell viability assay showed that compounds 28 and 29 have a highly significant effect with an IC50 value of 3.02 ± 1.06 and 1.71 ± 1.10 μM after 72 h, respectively. In a dependent manner in a concentration and time, the two compounds induced cell growth regression of 501Mel melanoma cells [30]. In combination, 11 bioactive compounds displayed significant effects against different cancer cell lines with IC50s below 20 µM. To obtain potential lead compounds as anticancer treatments, in vivo research is also strongly advised. In this regard, the most potent bioactive compounds require more thorough examination.
3.1.2 In vivo studies of bioactive compounds from marine sponge
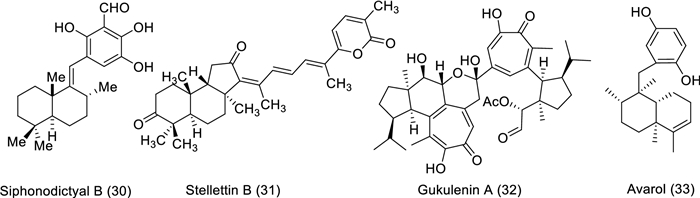
Siphonodictyal B (30), the biogenetic precursor of liphagal, was purified from the marine sponge Aka coralliphaga. In vivo study of colon cancer was conducted on siphonodictyal B (30) using the xenograft mouse model (Balb/c nude mice). The intraperitoneal administration (20 mg/kg) of compound 30 every third day showed a potent effect on tumor volume and weight (both significantly smaller) than in the control group. The mode of action was exhibited by the activation of the p38 MAPK pathway and the increase of p38 phosphorylation in tumor tissue [35]. Stellettin B (31) is a triterpenoid separated from Jaspis stellifera marine sponge. In vivo study of brain cancer was conducted to evaluate stellettin B effect on the inhibition of angiogenesis using a transgenic zebrafish embryo model. The findings showed that the embryos death rate was 0%, 0%, 6%, and 10% in correspondence to stellettin B concentrations of 10, 50 nM, 100, and 250 nM, respectively. While the percentage values of intersegmental vessels (ISVs) were 100% ± 0.5%, 66% ± 9.7%, 68% ± 11.1%, and 25% ± 10.5% of ISVs when 31 was administered at concentrations of 0, 50, 100, and 250 nM, respectively. Stellettin B decreased VEGF expression and caused a decline in VEGF expression as well as angiogenesis inhibition [36, 37]. Anh and his co-authors separated a bioactive compound named gukulenin A (32) from marine sponge Phorbas gukhulensis and investigated the anticancer activity in vivo using an ovarian cancer xenograft mouse model. Two doses (1 and 3 mg/kg) were applied only once each third day for 15 days and caused tumor growth suppression with 69.30% and 92.43% (inhibition of tumor weight), respectively. The mechanism of action for the compound 32 as an anticancer was explained by suppressing ovarian tumor growth through inhibition of monocyte chemoattractant protein-1 (MCP-1), regulated upon activation, normal T cell expressed and secreted (RANTES), and vascular endothelial growth factor (VEGF) expressions [38]. Avarol (33) is a sesquiterpene hydroquinone purified from the marine sponge Dysidea avara. In vivo, the study was conducted to investigate avarol effect on cancer using solid Ehrlich carcinoma (EC) and cervical cancer (CC-5) as a model. After three intraperitoneal administrations of (50 mg/kg) avarol exhibited an inhibition rate of 29% and 36% on EC and CC-5 tumor growth, respectively. Similarly, the compound 33 displayed potential antitumor activity via the inhibition of tumor growth [39].
3.2 Secondary metabolites of marine algae against cancer
In accordance with our literature survey, no papers were found to discuss the role of bioactive compounds isolated from marine algae between 2019 and 2023 in treating cancer.
3.3 Secondary metabolites of marine bacteria against cancer
3.3.1 In vitro studies of bioactive compounds from marine bacteria
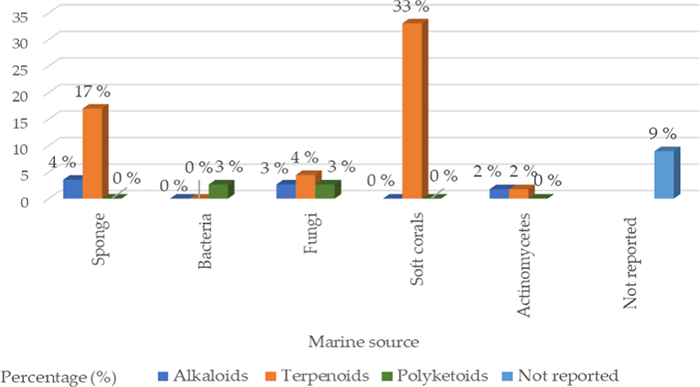
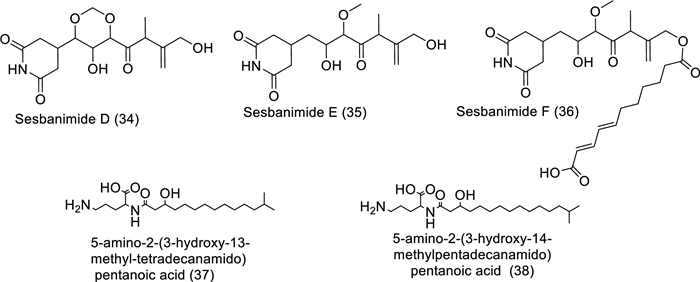
Antagonism is nature's own defense mechanism for surviving and existing. In order to protect themselves from other germs, bacteria develop various secondary metabolites, which are sources of bioactive substances that can be used in human therapeutic procedures. Potential sources of bioactive compounds such as alkaloids, polyketides, polycyclic aromatic hydrocarbons, and nonribosomal peptides (counting for about 70% of those newly found) can be found in the secondary metabolites of marine bacteria, as shown in Table 3 and Fig. 5 [40]. Sesbanimides D-F (34–36), as well as the known sesbanimides A and C, were separated from two different marine alphaproteobacterial species, namely Labrenzia aggregata PHM038 and Stappia indica PHM037. The above-mentioned substances significantly reduced the growth of breast, lung, as well as colorectal cancer cell lines [41]. From the Lacinutrix species strain, two isobranched lyso-ornithine lipids were found. A 3-hydroxy fatty acid is connected to an ornithine amino acid alpha amino group by an amide bond to form lyso-ornithine lipids, where the fatty acid sequences used were iso-C15:0 named as 5-amino-2-(3-hydroxy-13-methyltetradecanamido) pentanoic acid (37) and iso-C16:0 named as 5-amino-2-(3-hydroxy-14-methylpentadecanamido) pentanoic acid (38). A2058 human melanoma cells demonstrated cytotoxic activity in response to Lyso-Ornithine lipid 38 [42].
In vitro studies of bioactive compounds isolated from marine bacteria between 2019 and 2023
Structures of bioactive compounds isolated from marine bacteria between 2019 and 2023 with in vitro studies
3.4 Secondary metabolites of marine fungi against cancer
3.4.1 In vitro studies of bioactive compounds from marine fungi
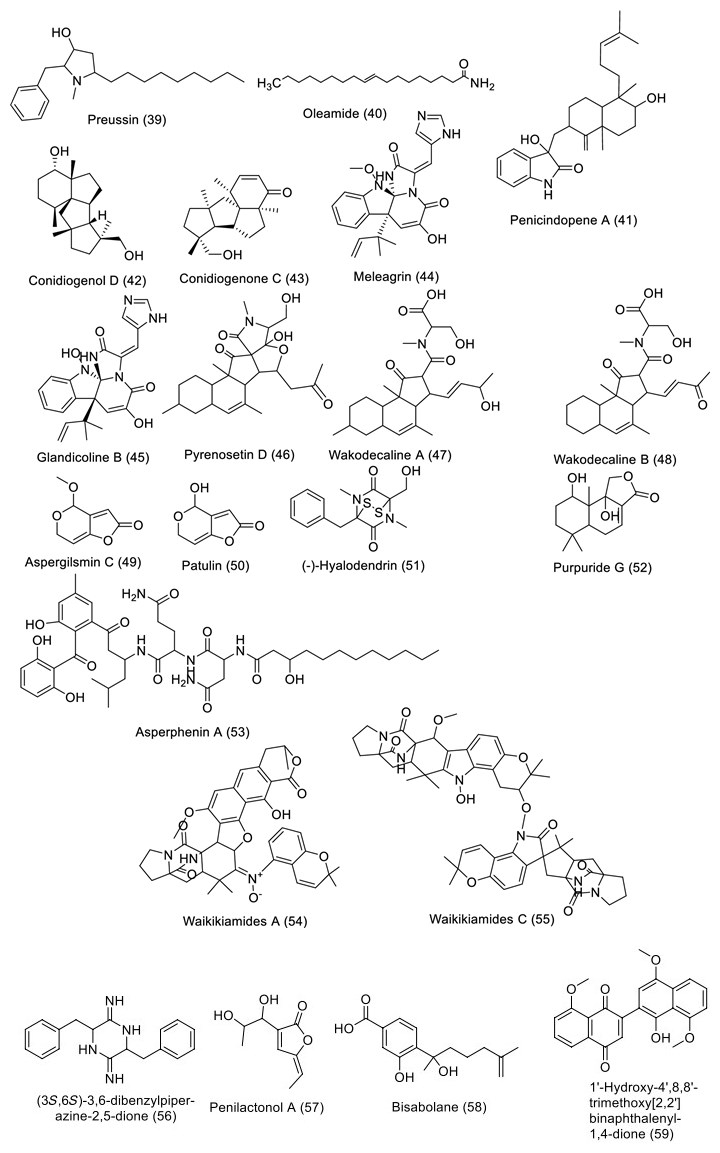
One of the primary marine environmental decomposers, marine fungi, has been found to produce distinctive biomolecules and possible enzymes. Preussin (39) is a hydroxyl pyrrolidine derivative (Table 4 and Fig. 6) that was found to have anticancer properties in MCF7 and other cancer cell lines after being separated from the fungus Aspergillus candidus that is connected to marine sponges (KUFA 0062). The antiproliferative and cytotoxic properties of preussin were examined on breast cancer cell lines (MCF7, SKBR3, and MDA-MB-221) as well as a non-tumor cell line (MCF12A). All examined cell lines were influenced by preussin's effects, as shown by the decline of cell survival and production in 2D and 3D cultures. Within MCF7, MCF12A, and SKBR3, preussin at 25 µM was sufficient to stop cell growth, but not in MDA-MB-231, in which the inhibition only happened at 50 µM [43]. Penicillium sp. ArCSPf, a marine sediment-resultant fungus, was separated from the Arabian Sea's eastern continental slope, and its active component of ethyl acetate extract displayed strong anticancer interest (IC50 = 22.79 g/mL) regarding MCF-7 breast cancer cells. LC–MS/MS analysis was used to identify the secondary metabolite (Z)-Octadec-9-enamide (oleamide (40)) of this fungus' active fraction [44]. Penicillium sp. YPCMAC1, a deep-sea fungus, yielded an indole diterpene known as penicindopene A (41). According to [45], penicindopene A had reasonable cytotoxicity against A549 and HeLa cell lines, with IC50 values of 15.2 and 20.5 µM, respectively. Conidiogenol D (42), conidiogenone C (43), meleagrin (44), and glandicoline B (45) were isolated through chemical analysis of an ethyl acetate extract of a deep-sea-derived Penicillium sp. All compounds showed a mild inhibitory impact on five esophageal cancer cell lines in the bioassay research, with IC50 values varying from 25 to 55 μM [46]. The endophytic bacterium Pyrenochaetopsis FVE-087, which emerged from the Baltic Fucus vesiculosus, was shown to include the pentacyclic decalinoylspirotetramic acid derivative pyrenosetin D (46), as well as the decalin precursors wakodecalines A (47) and B (48). These compounds were tested against the non-cancerous keratinocyte (HaCaT) and the human malignant melanoma (A-375) cell lines for their anticancer and toxic possibilities. With IC50 values of 77.5 and 39.3 μM, respectively, pyrenosetin D demonstrated toxicity against A-375 and HaCaT cells, whereas wakodecalines A and wakodecalines B were inert [47]. Highly oxygenated polyketides, viz., aspergilsmins A–G, as well as deoxytryptoquivaline, patulin, quinadoline, and tryptoquivaline, were isolated from Aspergillus giganteus NTU967 extracted from Ulva lactuca. Amid these, aspergilsmin C (49) and patulin (50) exhibited promising anticancer properties against prostate cancer PC-3 cells and human hepatocellular carcinoma SK-Hep-1 cells with IC50 values ranging from 2.7 to 7.3 μM [48]. The brown alga Pelvetia caniculata served as the source for the marine fungus Paradendryphiella salina PC 362H strain, which led to the separation of (-)-hyalodendrin (51) as the secondary metabolite responsible for the crude extract's cytotoxic properties. The anticancer effect of (-)-hyalodendrin was evident in cancer cells with spreading characteristics, such as colorectal cancer cells resistive to chemotherapy, and was not just restricted to the MCF7 cell lines. Further research revealed that (-)-hyalodendrin treatment of MCF7-Sh-WISP2 cells changed the phosphorylation level of p53 and changed the expression of HSP60, HSP70, and PRAS40 proteins [49]. The endophytic fungus Penicillium chrysogenum, extracted from the marine algae Chaetomorpha antennina, possesses anticancer properties that hinder the growth of HeLa cells and alter the apoptotic cell death cycle [50]. With IC50 values of 4.5 and 10.9 μM, respectively, purpuride G (52) was identified from the marine-sourced fungus Penicillium minioluteum ZZ1657, and it significantly suppressed human glioma U257 and U87MG cell lines [51]. A lipopeptidyl benzophenone metabolite called asperphenin A (53) was discovered during the marine-derived Aspergillus sp. fungus growth process. The substance showed powerful antiproliferative effects on many cancer cells. Asperphenin A stopped the G2/M cell cycle and then caused apoptosis in colon cancer cells, preventing them from proliferating. Asperphenin A causes reactive oxygen species in addition to having an impact on the cell cycle. The research also showed that the aryl ketone is crucial in the molecular structure of asperphenin A, which is responsible for its biological activity [52]. The structurally complicated diketopiperazine derivatives waikikiamides A (54) and waikikiamides C (55) were found in Aspergillus sp. FM242. According to [53], the two compounds had antiproliferative activity with IC50 values varying from 0.5 to 1.8 μM. The compound (3S, 6S)-3,6-dibenzylpiperazine-2,5-dione (56) was identified from a culture extract of Paecilomyces formous 17D47-2, which is derived from the sea. PANC-1 cells acclimated to conditions of low glucose with an IC50 value of 28 µM; however, in normal culture conditions, no effect was seen against PANC-1 cells up to 1000 µM [54]. A pentaketide derivative, penilactonol A (57), and sesquiterpenoids of the bisabolane type (58), were obtained from the marine alga-related fungus Penicillium chrysogenum LD-201810. Human cancer cell lines (BT-549, A549, HeLa, MCF-7, HepG2, and THP-1) were tested for cytotoxicity. Compound 57 had an IC50 value of 22.0 μM and was cytotoxic to the HepG2 cell line. With IC50 values of 21.2 and 18.2 μM, respectively, 11-dehydrosydonic acid also demonstrated noteworthy activity against A549 and THP-1 cell lines [55]. The marine fungus Hypoxylon rubiginosum FS521, which was obtained from a deep-sea deposit sample, was used to make 1′-hydroxy-4′,8,8′-trimethoxy[2,2']binaphthalenyl-1,4-dione (59). The compound showed substantial cytotoxic activity with IC50 values of 3.2, 1.8, 5.1, and 2.5 μM, respectively, when it was tested for its in vitro cytotoxic activity against the MCF-7, SF-268, A549, and HepG-2 tumor cell lines [56].
In vitro studies of bioactive compounds isolated from marine fungi between 2019 and 2023
Structures of bioactive compounds isolated from marine fungi between 2019 and 2023 with in vitro studies
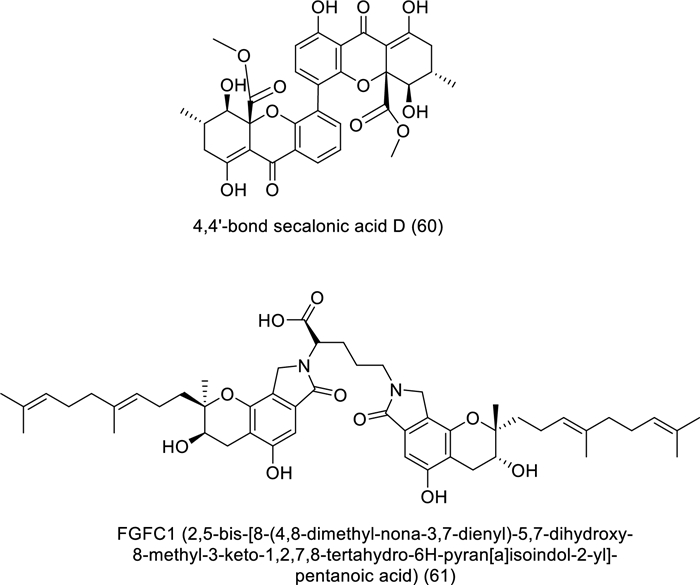
3.4.2 In vivo studies of bioactive compounds from marine fungi
The effect of FGFC1 (61) isolated from marine fungi Stachybotrys longispora FG216 on PC9 tumor transplant growth in BALB/c athymic nude mice was discussed as shown in Table 5 and Fig. 7 [57]. The study demonstrated that FGFC1 could inhibit PC9 cell development via controlling NF-κB signaling pathways, with no detectable effects on the mice's overall body weight.
In vivo studies of bioactive compounds isolated from marine fungi between 2019 and 2023
Structures of bioactive compounds isolated from marine fungi between 2019 and 2023 with in vivo studies
3.5 Secondary metabolites of marine soft corals against cancer
Soft corals are considered one of the richest sources of bioactive products, especially diterpenes, triterpenes, and steroids. Soft coral-derived metabolites have effective anticancer bioactivities against several cancer types [17]. Bioactive molecules extracted from marine soft corals and their impact against diverse cancer cell lines in in vitro studies are illustrated in Table 6 and Fig. 8.
In vitro studies of bioactive compounds isolated from marine soft corals between 2019 and2023
Structures of bioactive compounds isolated from marine soft corals between 2019 and 2023 with in vitro studies
Sarcophyton sp. afforded different bioactive metabolites with great biological activities. Major bioactive metabolites of soft corals are terpenes, molecules made up of isoprene building blocks, which undergo modification by re-arrangement or oxidation to form terpenoids. Because of their vast diversity, terpenoids are used for the treatment of many diseases, including cancer. In vitro studies revealed that marine-extracted secondary metabolites exerted the anticancer properties via suppression of protein synthesis and cell cycle inhibition, in addition to induction of programmed cell death [59]. Soft coral Klyxum flaccidum extracted cembrane diterpenoids such as flaccidenol A (62) and 7-epi-pavidolide D (63), flaccidodioxide (64), and flaccidodiol (65) were examined for cytotoxicity to human colorectal adenocarcinoma (DLD-1), lung adenocarcinoma (A549), and mouse lymphocytic leukemia (P388D1). Flaccidenol A as well as 7-epi-pavidolide D exhibited anti-proliferative activities against the screened cancer cells. The cytotoxic effects could be related to structure –function dependence; in other words, the presence of hydroperoxyl (as in flaccidenol A) enhanced the cytotoxic potency of the diterpenoid molecules [60]. Sarcoehrenbergilides D–F (66–68), cembrene–derived diterpenoids, extracted from Sarcophyton ehrenbergi were tested against human cancer cell lines like liver (HepG2), colon (Caco-2), and lung (A549). Bioassays revealed that A549 cell viability was inhibited by Sarcoehrenbergilides D–F, whereas HepG2 growth was slightly affected by Sarcoehrenbergilides E–F [61]. Cytotoxicity of Sarcophyton digitatum–isolated sardigitolides A–D (69–72) were evaluated against MCF-7, MDA-MB-231, HepG2, and HeLa cells. Sardigitolide B displayed anti-proliferative effects on breast cancer cell lines [62]. Xeniolides L (73) and M (74) were isolated from Xenia umbellate and evaluated for viability suppression of HepG2, PC‑3, and HT‑29, exhibiting potent anti-proliferative effects. Features of apoptosis were observed in both HepG2 and PC‑3 after treatment with xeniolide L, whereas exposure to xeniolide M produced apoptotic effects in HepG2 cells [63]. Litophyton nigrum isolated–linardosinenes A–C (75–77) and lineolemnenes A–D (78–81) were tested for their anti-proliferation against human lung epithelial carcinoma, THP-1, hepatocellular carcinoma, SNU-398, colon carcinoma, HT-29, pancreatic cancer, Capan-1 and lung cancer, A549, tumor cells. The results indicated that linardosinene B inhibited proliferation of THP-1, while linardosinene C and lineolemnene B were cytotoxic to SNU-398, as well as linardosinene C was cytotoxic toward HT-29 cell lines [64]. New cembranoids, sarcotenusenes A–C (82–84), extracted from Sarcophyton tenuispiculatum, were evaluated against MCF-7, MDA-MB-231, HepG2, and HeLa. The results demonstrated sarcotenusene A was a cytotoxic breast cancer cell line [65]. Newly discovered diterpenoids, asterolaurins O–R (85–88), were isolated from Asterospicularia laurae and examined for anti-proliferative potentials in MCF-7 (breast), Ca9-22 (oral), and SK-OV-3 (ovarian) cancer cells. Asterolaurins O–P suppressed proliferation of MCF-7 cell and strong activities were observed by asterolaurin O [66]. Sarcacutumolid A (89) was tested for anti-proliferative activity against human HepG-2 (liver), HeLa (cervix), and MCF-7 (breast) cell lines. Sarcacutumolid A exhibited anti-proliferative impact against colorectal cancer (Colo-205) [67]. Tuaimenals B–H (93–99), derived from Duva florida showed growth arrest to cervical cancer CaSki and C33A cell lines, Tuaimenals B, F, and G displayed forceful toxicity against the C33A cells [68]. Dendronestadione (100) extracted from soft coral Dendronephthya sp. showed significant cellular toxicity to a collection of human cancer cells made up of HepG2 (hepatocellular carcinoma), HT-29 (colorectal carcinoma), as well as PC (prostate carcinoma). Dendronestadione revealed a high effect on cancer cell lines [69]. Lobophytum catalai–isolated Lobocatalens A–G (101–107) cytotoxicity was evaluated to the human leukemia (K562), pancreatic (ASPC-1), and breast (MDA-MB-231) cancer cell lines. Cell viability assay showed lobocatalens G to be cytotoxic toward K562 human cancer cell line [70].
3.6 Secondary metabolites of marine actinomycetes against cancer
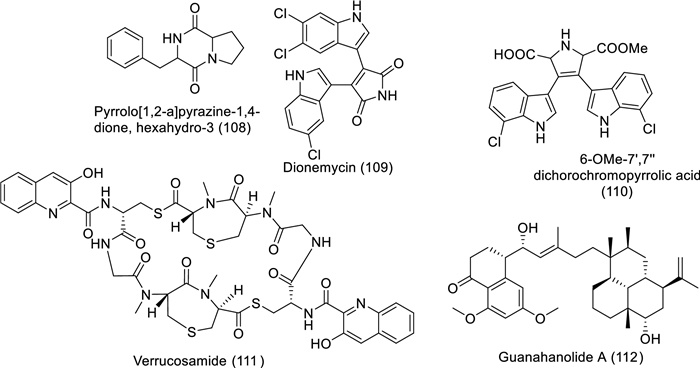
Actinomycetes, Gram-positive filamentous bacteria, are capable of producing various bioactive secondary metabolites, including anti-proliferative, cytotoxic, or antitumor molecules [72]. The secondary metabolites of marine actinomycetes that were identified, isolated, or classified as anticancer on in vitro models between 2019 and 2023 are displayed in Table 7 and Fig. 9.
In vitro studies of bioactive compounds isolated from marine actinomycin between 2019 and 2023
Structures of bioactive compounds isolated from marine actinomycetes between 2019 and 2023 with in vitro studies
The bulk of actinomycetes are hosted by marine sponges, specially Streptomyces being the most abundant genus [73] which has the potential to produce various novel bioactive compounds [73, 74]. Streptomyces have several verified antitumor molecules like bleomycin, dactinomycin, mitomycin, and doxorubicin [75, 76]. The anticancer activity of marine actinomycetes is attributed mainly to cytotoxic alkaloids [77]. The bioactive anticancer molecules act selectively toward malignant cells, leaving normal cells with minimal cytotoxic impact [73]. Streptomyces sp. VITSDK1 and LCJ85 showed potent anti-proliferative properties, and they effectively inhibit angiogenesis. Another study revealed that pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3 (108) extracted from marine actinomycetes Streptomyces akiyoshiensis GRG 6 (KY457710) was a cancer inhibitor to breast cancer cells MCF-7 (Nadar Rajivgandhi et al., 2020). Moreover, Streptomyces sp. SCSIO 11,791–isolated secondary metabolites have potent anticancer effects. Dionemycin (109) and 6-OMe-7′,7′′-dichorochromopyrrolic acid (110), chlorine and indole-containing alkaloids, were toxic to HepG2, MD1-MB-435, MCF10A, and HCT-116 cell lines. Furthermore, the bioactivity of dionemycin manifested cytotoxic criteria against MDA-MB-231 and NCI-H460 cell lines [78]. Nair and his colleges (2020) isolated a new cytotoxic thiodepsipeptide, verrucosamide (111), extracted from marine Verrucosispora sp. (CNX-026) and evaluated its cytotoxic effects on the NCI 60 cell line. Colon adenocarcinoma (COLO 205) and breast carcinoma (MDA-MB-468) cells showed significant sensitivity to the doses among the other cancer cell lines in the panel. The cytotoxicity was assigned to verrucosamide and related metabolite thiocoraline, which is also isolated from various strains of marine Verrucosispora sp. [79]. Recently, researchers extracted a meroterpenoid, guanahanolide A (112), from Streptomyces sp. RKBHB7 and elucidated its toxicity against NCI-60, breast cancer (MCF-7), human colon cancer (HCT-116), and HTB-26 in addition to Vero cell lines [80]. The meroterpenoid guanahanolide A consists of a sesterterpene skeleton, which is thought to result from cyclization of geranyl farnesyl diphosphate (GFPP, 2) by a terpene cyclase via a process similar to that described for labdane diterpene biosynthesis [80].
4 Clinical trials of marine natural products against cancer
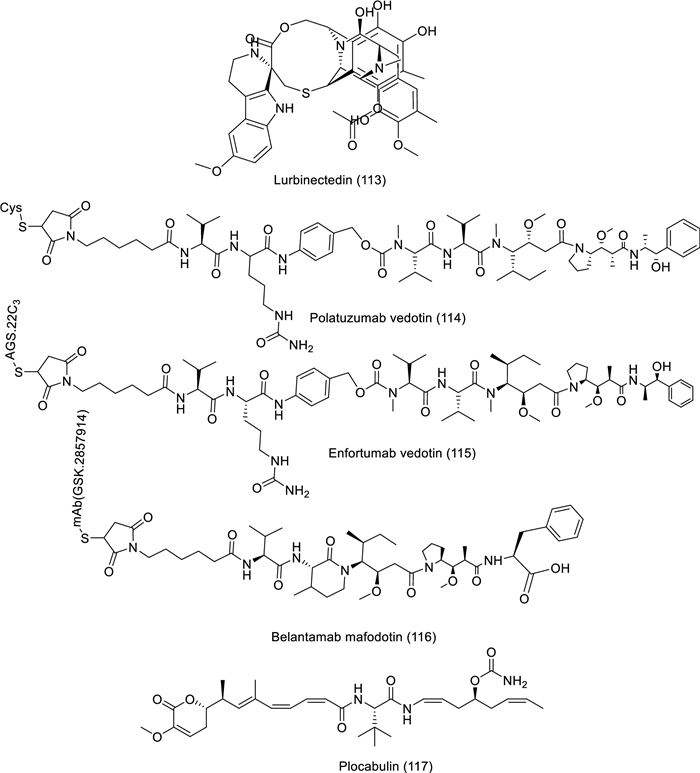
To date, several marine organisms' secondary metabolites have been reported with potential uses in the prevention of various cancers. Currently, nine of the 14 marine-derived medications now available on the market are used to treat cancer [16, 81, 82]. Herein we survey the clinical trials of derived bioactive compounds from different marine sources, which were registered or updated from one phase to another one between 2019 to now as shown in Table 8 and Fig. 10.
Updated list of bioactive compounds derived from marine sources in clinical trials from 2019 to now
Structures of bioactive compounds derived from marine sources in clinical trials from 2019 to now
Lurbinectedin, an alkaloid analogue of trabectedin, was isolated from tunicate and assessed as a drug (phase 2) for fighting pancreatic cancer with the last update (August 10, 2022) and breast cancer (September 25, 2020). The antitumor activity of lurbinectedin is attributed to the inclusion of a tetrahydroβ‐carboline in their skeleton. The dose of (3.2 mg/m2 at day 1 of every cycle) was administered orally for the pancreatic cancer patients, but for breast cancer, the dose is (1 mg/vial and 4 mg/vial at day 1 every three weeks). This clinical study involved 19 participants with ages of 18 and older for pancreatic cancer and 111 participants with ages 18 to 75. The path of action of lurbinectedin was explained by the unalterable stalling of elongating RNA polymerase II on the DNA template and its specific breakdown by the ubiquitin/proteasome workings (https://clinicaltrials.gov, NCT05229588, NCT01525589, [83, 84]). Another example, Polatuzumab vedotin, is an antibody–drug conjugate isolated from mollusk/cyanobacterium involving a monoclonal anti-CD79b coupled to monomethyl auristatin E, which is an anti-mitotic cytotoxic agent. The polatuzumab vedotin was described as an interventional drug in phases 1 and 2 for treating diffuse large B cell lymphoma with last inform (November 3, 2021). It can be administered intravenously with a dosage (of 1.8 mg/kg on day 2 of cycle 1 and day 1 of succeeding cycles). The ages of the 20 participants in the study ranged from 12 to 70 years. Polatuzumab vedotin's mechanism of action was mainly by targeting the human B-cell surface antigen CD79b and monomethyl auristatin E, which blocks cell division and encourages apoptosis by attaching to tubulin and disabling the microtubule network (https://clinicaltrials.gov, NCT04491370, [85, 86]). Belantamab mafodotin is an antibody–drug conjugate isolated from mollusk/cyanobacterium that targets Nectin-4 conjugated to MMAE. The belantamab mafodotin was passed to phase 2 and examined for treating urothelial cancer and metastatic castration-resistant prostate cancer with the last update (June 15, 2022) and (July 3, 2023), respectively. According to urothelial cancer, the participants received a dosage of 1.2 mg/kg on days 1, 8, and 15 of each 28-day cycle intravenously (IV), while metastatic castration-resistant prostate cancer received a dosage of 1.2 mg/kg up to 125 mg intravenously (IV) on days 1, 8, and 15 as part of a 28-day cycle. Belantamab mafodotin's mechanism of action for urothelial cancer was explained by the cell-cycle arrest and apoptosis of Nectin-4-expressing cells (https://clinicaltrials.gov, NCT03219333, NCT04754191, [87]).
5 Marketed marine drugs
The anticancer sea-derived medications currently available on the pharmaceutical markets in the EU and/or the USA [22] are shown in Table 9.
Recent marine anticancer drugs allowed by the EMA and/or the FDA
6 Conclusions and future perspectives
The current review is an updated and expanded version of our prior review, which was published in this journal in 2019. For the current approved anticancer therapeutic agents isolated and identified from marine sources, the time period has been prolonged to include the last five years. According to WHO recent statistics, the cancer burden continues to rise globally. In this contemporary epoch, discovering novel therapeutic structures without side effects to combat lethal diseases, including cancer, is a crucial issue for scientific scholars and governments. Occasionally, preventing, treating, and rehabilitating strategies in cancer cases require a budget that is as high as the one spent in a war. Thus, seeking potential safe anticancer drugs has become an urgent demand. The oceans and seas cover almost 75% of the earth and thus are rich in secondary metabolites with various pharmacological targets. According to our literature survey we have found that, soft corals, sponges then fungi are the primary sources of anticancer drugs identified from marine sources. Terpenoids and alkaloids are the principal chemical classes of these drugs. It was found that these marine structures demonstrate potent preclinical anticancer and cytotoxic activities toward wide ranges of cell lines among them MCF-7, HeLa, PC, L1210 murine leukemia cells, NCI-H460, SW480, HepG2, K562, ASPC-1, H69AR, H69, MDA-MB-231, CRC, brain cancer, ovarian cancer, Ehrlich carcinoma and cervical cancer nonmalignant cell lines (H9c2 and HEK293T), esophageal cancer cell lines (EC109, EC9706, KYSE30, KYSE70, and KYSE450), and human hepatocellular carcinoma SK-Hep-1 compared to common positive control. Taxol, cyclophosphamide, cisplatin, and doxorubicin are some of the examples combating cancers via different mechanisms, including inducing cell growth inhibition and apoptosis via ROS production and the RXRα-mediated PI3K/Akt signaling pathway, suppressing the MAPK/ERK pathway and modulating the extrinsic pathway, activation of the p38 MAPK pathway and p38 phosphorylation in tumor tissue, inhibiting VEGF, MCP-1, RANTES expressions and angiogenesis, induced changes in the phosphorylation status and altered expression of HSP60, HSP70 and PRAS40 proteins, regulation of Bax expression, which is a biomarker of tumor growth and regulating NF-κB signaling pathways. Lately, six marine structures have been clinically approved as anticancer medications, among them 4 compounds, namely, Lurbinectedin, Polatuzumab vedotin, Enfortumab vedotin, and Belantamab mafodotin have been authorized by the FDA and/or EMA as anticancer drugs against ovarian cancer, breast cancer, urothelial cancer, and multiple myeloma, respectively. Taken together, it is nowadays evident that marine products are crucial for supplying a platform for several approved anticancer drugs.
Notes
Author contributions
Conceptualization; H.R.E., Investigation, M.S.R., N.E., M.F.E. and N.Y.; Methodology and data collection, M.S.R, M.F.E, F.M.K.A., I.D. M.D., and H.E.T., Writing—original draft, M.S.R., N.E., M.F.E. and N.Y; Writing—review & editing, M.D., H.Z., H.E., Z.G., and S.A.M.K. All authors have read and agreed to the published version of the manuscript.
Data availability
No data was used for the research described in the article.
Declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
-
1.Hulvat MC. Cancer incidence and trends. Surg Clin 2020;100: 469-81. PubMed Google Scholar
-
2.Falzone L, Salomone S, Libra M. Evolution of cancer pharmacological treatments at the turn of the third millennium. Front Pharmacol 2018;9: 1300. CrossRef PubMed Google Scholar
-
3.Cruz-Reyes N, Radisky DC. Inflammation, infiltration, and evasion—tumor promotion in the aging breast. Cancers 2023;15: 1836. CrossRef PubMed Google Scholar
-
4.WHO. Cancer. 2022. PubMed Google Scholar
-
5.Sung H, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71: 209-49. CrossRef PubMed Google Scholar
-
6.Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin 2023;73: 17-48. CrossRef PubMed Google Scholar
-
7.Tenore G, et al. Tobacco, alcohol and family history of cancer as risk factors of oral squamous cell carcinoma: case-control retrospective study. Appl Sci 2020;10: 3896. CrossRef PubMed Google Scholar
-
8.An S-Y, et al. Obesity is positively related and tobacco smoking and alcohol consumption are negatively related to an increased risk of thyroid cancer. Sci Rep 2020;10: 19279. CrossRef PubMed Google Scholar
-
9.Wang X, Zhang H, Chen X. Drug resistance and combating drug resistance in cancer. Drug Resist Combat Drug Resist Cancer 2019;2: 141-60. PubMed Google Scholar
-
10.Yosri N, et al. Marine organisms: pioneer natural sources of polysaccharides/proteins for green synthesis of nanoparticles and their potential applications. Int J Biol Macromol 2021;193: 1767-98. CrossRef PubMed Google Scholar
-
11.El-Seedi HR, et al. Exploring natural products-based cancer therapeutics derived from Egyptian flora. J Ethnopharmacol 2021;269: 113626. CrossRef PubMed Google Scholar
-
12.Carroll AR, Copp BR, Davis RA, Keyzers RA, Prinsep MR. Marine natural products. Nat Prod Rep 2021;38: 362-413. CrossRef PubMed Google Scholar
-
13.Carroll AR, Copp BR, Davis RA, Keyzers RA, Prinsep MR. Marine natural products. Nat Prod Rep 2023;40: 275-325. CrossRef PubMed Google Scholar
-
14.Lu W-Y, Li H-J, Li Q-Y, Wu Y-C. Application of marine natural products in drug research. Bioorg Med Chem 2021;35: 116058. CrossRef PubMed Google Scholar
-
15.El-Seedi HR, et al. Review of marine cyanobacteria and the aspects related to their roles: chemical, biological properties, nitrogen fixation and climate change. Mar Drugs 2023;21: 439. CrossRef PubMed Google Scholar
-
16.Montuori E, Hyde CAC, Crea F, Golding J, Lauritano C. Marine natural products with activities against prostate cancer: recent discoveries. Int J Mol Sci 2023;24: 1435. CrossRef PubMed Google Scholar
-
17.Khalifa SAM, et al. Marine natural products: a source of novel anticancer drugs. Mar Drugs 2019;17: 491. CrossRef PubMed Google Scholar
-
18.Hentschel U, Piel J, Degnan SM, Taylor MW. Genomic insights into the marine sponge microbiome. Nat Rev Microbiol 2012;10: 641-54. CrossRef PubMed Google Scholar
-
19.Perdicaris S, Vlachogianni T, Valavanidis A. Bioactive natural substances from marine sponges: new developments and prospects for future pharmaceuticals. Nat Prod Chem Res 2013;1: 2329-6836. CrossRef PubMed Google Scholar
-
20.Radjasa OK, et al. Highlights of marine invertebrate-derived biosynthetic products: their biomedical potential and possible production by microbial associants. Bioorg Med Chem 2011;19: 6658-74. CrossRef PubMed Google Scholar
-
21.Altmann K-H. Drugs from the oceans: marine natural products as leads for drug discovery. Chimia 2017;71: 646. CrossRef PubMed Google Scholar
-
22.Cappello E, Nieri P. From life in the sea to the clinic: the marine drugs approved and under clinical trial. Life 2021;11: 1390. CrossRef PubMed Google Scholar
-
23.De AK, et al. A natural quinazoline derivative from marine sponge Hyrtios erectus induces apoptosis of breast cancer cells via ROS production and intrinsic or extrinsic apoptosis pathways. Mar Drugs 2019;17: 658. CrossRef PubMed Google Scholar
-
24.Jomori T, Setiawan A, Sasaoka M, Arai M. Cytotoxicity of new diterpene alkaloids, ceylonamides G–I, isolated from Indonesian marine sponge of Spongia sp. Nat Prod Commun 2019;14: 1934578X19857294. PubMed Google Scholar
-
25.Sakai R, et al. Soritesidine, a novel proteinous toxin from the okinawan marine sponge Spongosorites sp. Mar Drugs 2019;17: 216. CrossRef PubMed Google Scholar
-
26.Wu Y, et al. Phakefustatins A–C: kynurenine-bearing cycloheptapeptides as RXRα modulators from the marine sponge Phakellia fusca. Org Lett 2020;22: 6703-8. CrossRef PubMed Google Scholar
-
27.Ki D-W, et al. New cytotoxic polyacetylene amides from the Egyptian marine sponge Siphonochalina siphonella. Fitoterapia 2020;142: 104511. CrossRef PubMed Google Scholar
-
28.Surti M, et al. Ilimaquinone (marine sponge metabolite) induces apoptosis in HCT-116 human colorectal carcinoma cells via mitochondrial-mediated apoptosis pathway. Mar Drugs 2022;20: 582. CrossRef PubMed Google Scholar
-
29.Wang Z, et al. Kalihioxepanes A—G: seven kalihinene diterpenoids from marine sponge Acanthella cavernosa collected off the south China Sea. Chin J Chem 2022;40: 1785-92. CrossRef PubMed Google Scholar
-
30.Carpi S, et al. Pro-apoptotic activity of the marine sponge Dactylospongia elegans metabolites pelorol and 5-epi-ilimaquinone on human 501Mel melanoma cells. Mar Drugs 2022;20: 427. CrossRef PubMed Google Scholar
-
31.Zhou M, et al. 12-deacetyl-12-epi-scalaradial, a scalarane sesterterpenoid from a marine sponge Hippospongia sp., induces HeLa cells apoptosis via MAPK/ERK Pathway and modulates nuclear receptor Nur77. Mar Drugs 2020;18: 643. CrossRef PubMed Google Scholar
-
32.Ki D-W, et al. New cytotoxic polyacetylene alcohols from the Egyptian marine sponge Siphonochalina siphonella. J Nat Med 2020;74: 409-14. CrossRef PubMed Google Scholar
-
33.Yu H-B, et al. Cytotoxic meroterpenoids from the marine sponge Dactylospongia elegans. Nat Prod Res 2021;35: 1620-6. CrossRef PubMed Google Scholar
-
34.Tran HN, Kim MJ, Lee Y-J. Scalarane sesterterpenoids isolated from the marine sponge Hyrtios erectus and their cytotoxicity. Mar Drugs 2022;20: 604. CrossRef PubMed Google Scholar
-
35.Chikamatsu S, et al. In vitro and in vivo antitumor activity and the mechanism of siphonodictyal B in human colon cancer cells. Cancer Med 2019;8: 5662-72. CrossRef PubMed Google Scholar
-
36.Cheng S-Y, et al. Anti-invasion and antiangiogenic effects of stellettin B through inhibition of the Akt/Girdin signaling pathway and VEGF in glioblastoma cells. Cancers 2019;11: 220. CrossRef PubMed Google Scholar
-
37.Feng C-W, et al. In vitro and in vivo neuroprotective effects of stellettin B through anti-apoptosis and the Nrf2/HO-1 pathway. Mar Drugs 2019;17: 315. CrossRef PubMed Google Scholar
-
38.Ahn J-H, Woo J-H, Rho J-R, Choi J-H. Anticancer activity of Gukulenin A isolated from the marine sponge Phorbas gukhulensis in vitro and in vivo. Mar Drugs 2019;17: 126. CrossRef PubMed Google Scholar
-
39.Stanojkovic TP, et al. Evaluation of in vitro cytotoxic potential of avarol towards human cancer cell lines and in vivo antitumor activity in solid tumor models. Molecules 2022;27: 9048. CrossRef PubMed Google Scholar
-
40.Mohamadkhani A. Gene cluster analysis of marine bacteria seeking for natural anticancer products. Jundishapur J Nat Pharm Prod 2021;16: e104665. CrossRef PubMed Google Scholar
-
41.Kačar D, et al. Identification of trans-AT polyketide clusters in two marine bacteria reveals cryptic similarities between distinct symbiosis factors. Environ Microbiol 2021;23: 2509-21. CrossRef PubMed Google Scholar
-
42.Kristoffersen V, et al. Two novel lyso-ornithine lipids isolated from an arctic marine Lacinutrix sp. bacterium. Molecules 2021;26: 5295. CrossRef PubMed Google Scholar
-
43.Malhão F, et al. Cytotoxic and antiproliferative effects of preussin, a hydroxypyrrolidine derivative from the marine sponge-associated fungus Aspergillus candidus KUFA 0062, in a panel of breast cancer cell lines and using 2D and 3D cultures. Mar Drugs 2019;17: 448. CrossRef PubMed Google Scholar
-
44.Farha AK, Hatha AM. Bioprospecting potential and secondary metabolite profile of a novel sediment-derived fungus Penicillium sp. ArCSPf from continental slope of Eastern Arabian Sea. Mycology 2019;10: 109-17. CrossRef PubMed Google Scholar
-
45.Liu L, et al. Penicindopene A, a new indole diterpene from the deep-sea fungus Penicillium sp. YPCMAC1. Nat Prod Res 2019;33: 2988-94. CrossRef PubMed Google Scholar
-
46.Cheng Z, et al. Three new cyclopiane-type diterpenes from a deep-sea derived fungus Penicillium sp. YPGA11 and their effects against human esophageal carcinoma cells. Bioorg Chem 2019;91: 103129. CrossRef PubMed Google Scholar
-
47.Fan B, et al. Pyrenosetin D, a new pentacyclic decalinoyltetramic acid derivative from the algicolous fungus Pyrenochaetopsis sp. FVE-087. Mar Drugs 2020;18: 281. CrossRef PubMed Google Scholar
-
48.Chen J-J, et al. Highly oxygenated constituents from a marine alga-derived fungus Aspergillus giganteus NTU967. Mar Drugs 2020;18: 303. CrossRef PubMed Google Scholar
-
49.Dezaire A, et al. Secondary metabolites from the culture of the marine-derived fungus Paradendryphiella salina PC 362H and evaluation of the anticancer activity of its metabolite hyalodendrin. Mar Drugs 2020;18: 191. CrossRef PubMed Google Scholar
-
50.Parthasarathy R, et al. Molecular profiling of marine endophytic fungi from green algae: assessment of antibacterial and anticancer activities. Process Biochem 2020;96: 11-20. CrossRef PubMed Google Scholar
-
51.Ma M, Ge H, Yi W, Wu B, Zhang Z. Bioactive drimane sesquiterpenoids and isocoumarins from the marine-derived fungus Penicillium minioluteum ZZ1657. Tetrahedron Lett 2020;61: 151504. CrossRef PubMed Google Scholar
-
52.Bae SY, et al. Antitumor activity of asperphenin A, a lipopeptidyl benzophenone from marine-derived Aspergillus sp. fungus, by inhibiting tubulin polymerization in colon cancer cells. Mar Drugs 2020;18: 110. CrossRef PubMed Google Scholar
-
53.Wang F, et al. Waikikiamides A–C: complex diketopiperazine dimer and diketopiperazine-polyketide hybrids from a hawaiian marine fungal strain Aspergillus sp. FM242. Org Lett 2020;22: 4408-12. CrossRef PubMed Google Scholar
-
54.Tang R, Zhou D, Kimishima A, Setiawan A, Arai M. Selective cytotoxicity of marine-derived fungal metabolite (3S,6S)-3,6-dibenzylpiperazine-2,5-dione against cancer cells adapted to nutrient starvation. J Antibiot 2020;73: 873-5. CrossRef PubMed Google Scholar
-
55.Jiang L-L, et al. Cytotoxic secondary metabolites isolated from the marine alga-associated fungus Penicillium chrysogenum LD-201810. Mar Drugs 2020;18: 276. CrossRef PubMed Google Scholar
-
56.Zhang J, et al. Cytotoxic secondary metabolites from a sea-derived fungal strain of Hypoxylon rubiginosum FS521. Chin J Org Chem 2020;40: 1367. CrossRef PubMed Google Scholar
-
57.Feng J, et al. FGFC1 exhibits anti-cancer activity via inhibiting NF-κB signaling pathway in EGFR-mutant NSCLC cells. Mar Drugs 2022;20: 76. CrossRef PubMed Google Scholar
-
58.Chen L, et al. Isolation of 4,4′-bond secalonic acid D from the marine-derived fungus Penicillium oxalicum with inhibitory property against hepatocellular carcinoma. J Antibiot 2019;72: 34-44. CrossRef PubMed Google Scholar
-
59.Jayawardena TU, et al. Antiproliferative and apoptosis-inducing potential of 3$β$-hydroxy-$Δ$5-steroidal congeners purified from the soft coral Dendronephthya putteri. J Oceanol Limnol 2019;37: 1382-92. CrossRef PubMed Google Scholar
-
60.Tseng WR, et al. Bioactive capnosanes and cembranes from the soft coral Klyxum flaccidum. Mar Drugs 2019;17: 9-11. CrossRef PubMed Google Scholar
-
61.Hegazy MEF, et al. Sarcoehrenbergilides D-F: cytotoxic cembrene diterpenoids from the soft coral: Sarcophyton ehrenbergi. RSC Adv 2019;9: 27183-9. CrossRef PubMed Google Scholar
-
62.Huang C, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395: 497-506. CrossRef PubMed Google Scholar
-
63.Alarif WM, et al. Two new xeniolide diterpenes from the soft coral Xenia umbellata; displayed anti proliferative effects. Pharmacogn Mag 2020;16: 774-9. CrossRef PubMed Google Scholar
-
64.Yang F, et al. Uncommon nornardosinane, seconeolemnane and related sesquiterpenoids from Xisha soft coral Litophyton nigrum. Bioorg Chem 2020;96: 103636. CrossRef PubMed Google Scholar
-
65.Huang TY, et al. New hydroquinone monoterpenoid and cembranoid-related metabolites from the soft coral Sarcophyton tenuispiculatum. Mar Drugs 2021;19: 8. CrossRef PubMed Google Scholar
-
66.Lin YC, et al. Targeted isolation of xenicane diterpenoids from Taiwanese soft coral Asterospicularia laurae. Mar Drugs 2021;19: 123. CrossRef PubMed Google Scholar
-
67.Mohamed TA, et al. A new cembranoid from the Red Sea soft coral Sarcophyton acutum. Nat Prod Res 2024;38: 512. CrossRef PubMed Google Scholar
-
68.Welsch JT, et al. Tuaimenals B-H, merosesquiterpenes from the Irish deep-sea soft coral Duva florida with bioactivity against cervical cancer cell lines. J Nat Prod 2023;86: 182. CrossRef PubMed Google Scholar
-
69.Ghandourah MA. Cytotoxic ketosteroids from the Red Sea soft coral Dendronephthya sp. Open Chem 2023;21: 4-9. CrossRef PubMed Google Scholar
-
70.Zhang J, et al. Seven new lobane diterpenoids from the soft coral Lobophytum catalai. Mar Drugs 2023;21: 451. CrossRef PubMed Google Scholar
-
71.Chao CH, et al. Cembranolides and related constituents from the soft coral Sarcophyton cinereum. Molecules 2022;27: 1760. CrossRef PubMed Google Scholar
-
72.Nayaka S, et al. A potential bioactive secondary metabolites and antimicrobial efficacy of Streptomyces thermocarboxydus strain KSA-2, isolated from Kali River, Karwar. Curr Res Microbiol Infect 2020;1: 5-13. CrossRef PubMed Google Scholar
-
73.Fahmy NM, Abdel-Tawab AM. Isolation and characterization of marine sponge–associated Streptomyces sp. NMF6 strain producing secondary metabolite(s) possessing antimicrobial, antioxidant, anticancer, and antiviral activities. J Genet Eng Biotechnol 2021;19: 102. CrossRef PubMed Google Scholar
-
74.Nadar Rajivgandhi G, et al. Molecular identification and structural detection of anti-cancer compound from marine Streptomyces akiyoshiensis GRG 6 (KY457710) against MCF-7 breast cancer cells. J King Saud Univ Sci 2020;32: 3463-9. CrossRef PubMed Google Scholar
-
75.Bhattacharya B, Mukherjee S. Cancer therapy using antibiotics. J Cancer Ther 2015;06: 849-58. CrossRef PubMed Google Scholar
-
76.Demain AL, Vaishnav P. Natural products for cancer chemotherapy. Microb Biotechnol 2011;4: 687-99. CrossRef PubMed Google Scholar
-
77.Al-Ansari M, Kalaiyarasi M, Almalki MA, Vijayaraghavan P. Optimization of medium components for the production of antimicrobial and anticancer secondary metabolites from Streptomyces sp. AS11 isolated from the marine environment. J King Saud Univ Sci 2020;32: 1993-8. CrossRef PubMed Google Scholar
-
78.Song Y, et al. Chlorinated bis-indole alkaloids from deep-sea derived Streptomyces sp. SCSIO 11791 with antibacterial and cytotoxic activities. J Antibiot 2020;73: 542-7. CrossRef PubMed Google Scholar
-
79.Nair V, et al. Verrucosamide, a cytotoxic 1,4-thiazepane-containing thiodepsipeptide from a marine-derived actinomycete. Mar Drugs 2020;18: 549. CrossRef PubMed Google Scholar
-
80.Marchbank DH, Ptycia-Lamky VC, Decken A, Haltli BA, Kerr RG. Guanahanolide A, a meroterpenoid with a sesterterpene skeleton from coral-derived Streptomyces sp. Org Lett 2020;22: 6399-403. CrossRef PubMed Google Scholar
-
81.Riccio G, et al. Jellyfish as an alternative source of bioactive antiproliferative compounds. Mar Drugs 2022;20: 350. CrossRef PubMed Google Scholar
-
82.Riccio G, et al. Bioactivity screening of Antarctic sponges reveals anticancer activity and potential cell death via ferroptosis by mycalols. Mar Drugs 2021;19: 459. CrossRef PubMed Google Scholar
-
83.Cruz C, et al. Multicenter phase II study of lurbinectedin in BRCA-mutated and unselected metastatic advanced breast cancer and biomarker assessment substudy. J Clin Oncol Off J Am Soc Clin Oncol 2018;36: 3134-43. CrossRef PubMed Google Scholar
-
84.Jimenez PC, et al. Enriching cancer pharmacology with drugs of marine origin. Br J Pharmacol 2020;177: 3-27. CrossRef PubMed Google Scholar
-
85.Deeks ED. Polatuzumab vedotin: first global approval. Drugs 2019;79: 1467-75. CrossRef PubMed Google Scholar
-
86.Sehn LH, et al. Polatuzumab vedotin in relapsed or refractory diffuse large b-cell lymphoma. J Clin Oncol Off J Am Soc Clin Oncol 2020;38: 155-65. CrossRef PubMed Google Scholar
-
87.Rosenberg JE, et al. Pivotal trial of enfortumab vedotin in urothelial carcinoma after platinum and anti-programmed death 1/programmed death ligand 1 therapy. J Clin Oncol Off J Am Soc Clin Oncol 2019;37: 2592-600. CrossRef PubMed Google Scholar
-
88.Lonial S, et al. Belantamab mafodotin for relapsed or refractory multiple myeloma (DREAMM-2): a two-arm, randomised, open-label, phase 2 study. Lancet Oncol 2020;21: 207-21. CrossRef PubMed Google Scholar
Copyright information
© The Author(s) 2025
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.