Vibralactone derivatives isolated from co-cultures of the basidiomycetes Stereum hirsutum and Boreostereum vibrans
Abstract
The basidiomycetes Stereum hirsutum and Boreostereum vibrans are two fungi of the same genus. In this study, chemical investigation on the co-cultures of the two congeneric fungi led to the isolation of eleven new vibralactone derivatives, hirsutavibrins A–K (1–11). The structures of 1–11 were elucidated by extensive NMR and HRESIMS spectroscopic analysis, and computational methods. Hirsutavibrins A (1) and B (2) showed weak cytotoxicity against the human lung cancer cell line A549. Hirsutavibrin D (4) showed moderate anti-nitric oxide activity in murine monocytic RAW 264.7 macrophages. This work not only expands the members of vibralactone derivatives with variable configurations but also opens a new avenue for fungal co-culturing study between congeneric fungi.Graphical Abstract
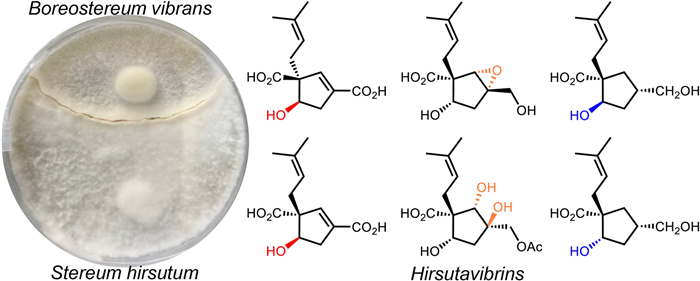
Keywords
Stereum hirsutum Boreostereum vibrans Basidiomycetes Co-culture Vibralactone derivatives1 Introduction
Natural products are a vital reservoir of compounds with intriguing structures and diverse bioactivity [1-6]. Microbes are particularly advantageous for natural product discovery due to their easy accessibility and amenability to genetic manipulation [7]. Secondary metabolites of fungal origin represent a crucial subgroup of natural products, exhibiting structural and biological diversity distinct from those found in plants and animals [8]. Several effective strategies are commonly employed to fully explore and expand the chemodiversity of microbial natural products. These include culturing in the presence of epigenetic modifiers [9] or in large-scale [10], co-culturing fungi with other fungi or bacteria [11], overexpressing key transcription factors within gene clusters of interest [12], and heterologous expression of biosynthetic gene clusters [13].
Our research group has long been dedicated to the chemical and biological study of specialized metabolites from higher fungi [14-18]. In previous work, vibralactone, a bicyclic β-lactone-containing meroterpene isolated from Boreostereum vibrans, a basidiomycetous fungus [19], was found to exhibit significant pancreatic lipase inhibitory activity (IC50 0.4 μg/mL). Subsequent studies on the cultures of B. vibrans resulted in the isolation of numerous vibralactone derivatives [20-23]. Both the total synthesis and biosynthesis of vibralactone have been successfully achieved [24-28]. Beyond our work with B. vibrans, we also focus on the chemodiversity of Stereum hirsutum HFG27, the type strain of the genus Stereum. Stereum hirsutum is a prolific producer of meroterpenoids [29], steroids [30], and sesquiterpenoids [31], and has also been found to produce trace amount of vibralactone (about 100 μg/L). Genetic analysis of these two fungi revealed that they both harbor the key biosynthetic genes for vibralactone and extensive homologous genes [26], but they also display distinct biosynthetic potential for other types of natural products.
Co-culturing microorganisms often involves combining two phylogenetically distinct fungi or a fungus with a bacterium to significantly stimulate the production of secondary metabolites during microbial interactions [32]. While only a few studies have focused on the co-culture of two pairs of basidiomycetous fungi from different families, such as co-culture Trametes versicolor with Ganoderma applanatum, Pleurotus ostreatus with Trametes robiniophila. As a result, eleven compounds, including phenolic glycosides and terpenoids, have been reported in total [33-35]. Boreostereum vibrans and Stereum hirsutum, two closely related fungal species with great potential of producing secondary metabolites, were co-cultured. Mixed cultures of these two fungi may increase the probability of producing a diverse range of compounds, potentially influenced by homologous biosynthetic enzymes. In this study, these two fungi were co-cultured to investigate their chemical profiles. This work details the isolation, structural characterization, and biological assessment of the resulting compounds.
2 Results and discussion
2.1 Structural elucidation of the previously undescribed compounds
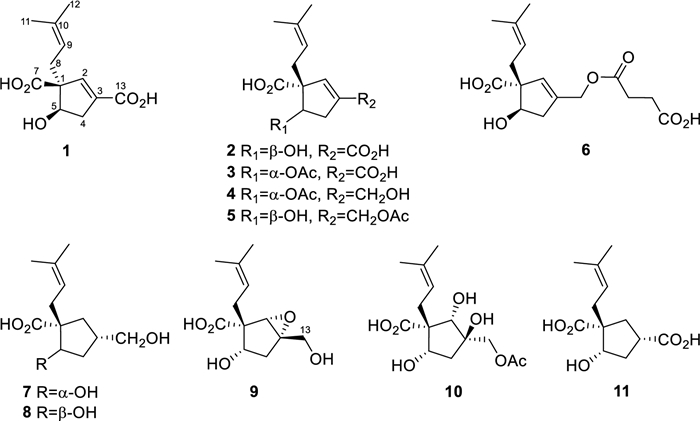
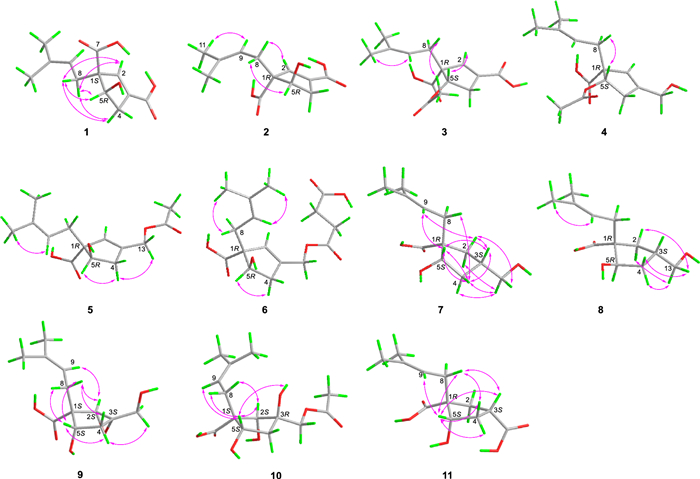
Hirsutavibrin A (1, Fig. 1) was isolated as a colorless oil. High-resolution positive electrospray ionization mass spectrometric [( +)-HRESIMS] analysis of 1 returned a protonated ion peak at m/z 241.10703 [M + H]+, corresponding to the molecular formula of C12H16O5 (mass error 0.08201 ppm) with five degrees of unsaturation. The 1H NMR spectrum of 1 (Table 1) revealed two olefinic protons at δH 6.72 (1H, s) and 5.12 (1H, t, J = 7.6 Hz), in addition to two methyl groups at δH 1.70 (3H, s) and 1.61 (3H, s). The 13C NMR (Table 2) and Distortionless Enhancement by Polarization Transfer (DEPT) spectroscopic data of 1 presented 12 carbon signals, including two CH3 at δC 18.2 (C-11) and 26.3 (C-12), two CH2 at δC 36.0 (C-8) and 40.8 (C-4), three methine carbons at δC 78.4 (C-5), 145.7 (C-2), and 120.6 (C-9), three proton-deficiency carbons at δC 67.4 (C-1), 135.9 (C-10), and 136.6 (C-3), and two carbonyl groups at δC 177.3 (C-7) and 169.1 (C-13). The 1H-1H COSY correlations of H-4/H-5, along with the HMBC correlations from H-2 to C-1, C-3, C-4, C-5, C-13 (Fig. 2) revealed the existence of a five-membered ring in 1, which, together with the two carbonyl groups and two double bonds mentioned above, satisfied the unsaturation degree of compound 1. The 1H–1H COSY correlation of H2-8/H-9, and the HMBC correlations from H3-12 to C-9, C-10, C-11, and from H-8 to C-1 indicated a prenyl group attach to C-1. In addition, the HMBC correlations from H-2 to C-13, from H-8 and H-5 to C-7 enabled the assignment of two carboxylic groups at C-1 and C-3, respectively. Therefore, the planar structure of 1 was established (Fig. 1), which highly resembled vibralactone E [21], except that the CH2OH group (C-13) was replaced by a COOH group in 1.
Chemical structures of compounds 1–11
1H NMR spectroscopic data of compounds 1–6
13C NMR spectroscopic data of compounds 1–11
Key 1H-1H COSY and HMBC correlations of compounds 1–11
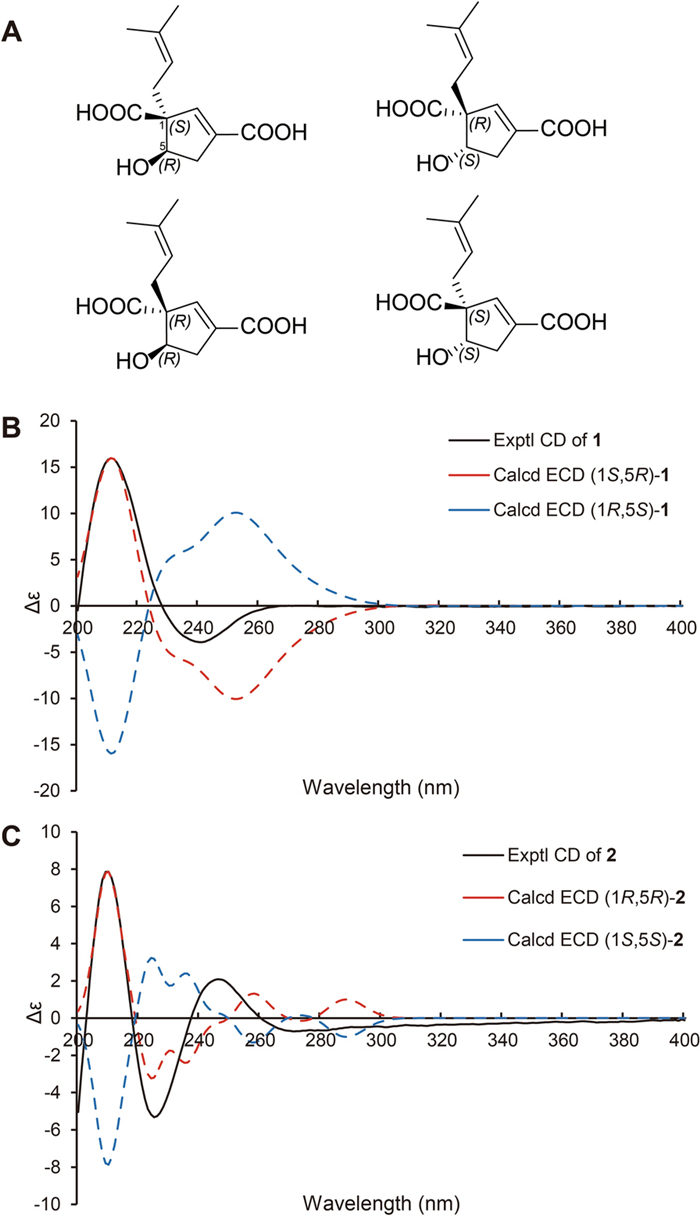
The conclusion of the relative configuration of 1 arrived by analyzing the ROESY spectrum (Fig. 3). The key correlations of H-5 (δH 4.22)/H-8a (δH 2.30), H-5/H-4α (δH 2.87), and H-8a/H-4α helped to locate H-5 and the prenyl group orienting the same side of the five-membered ring. Besides, almost all the prenyl group at C-1 from the previously reported vibralactone and its derivatives are β configuration, thus the relative configuration of 1 was determined to be 1R*, 5S*. The absolute configuration of 1 was determined by ECD calculations (Supplementary Material, Fig. 4). As a result, the calculated ECD of the stereoisomer 1S, 5R was most consistent with the experimental CD data of 1, while the calculated ECD data of 1R, 5S stereoisomer showed mirror Cotton effects at specific wavelengths (Fig. 4B). Therefore, the absolute configuration of 1 was assigned as depicted in Fig. 1, which was different from the previously reported vibralactone derivatives.
Key ROESY correlations of compounds 1–11
A The four possible stereoisomers of 1 and 2. B Comparisons of the experimental CD and calculated ECD of 1. C Comparisons of the experimental CD and calculated ECD of 2
Hirsutavibrin B (2, Fig. 1) was obtained as a colorless oil. Its molecular formula was determined to be C12H16O5 based on (+)-HRESIMS, which presented an [M + H]+ ion peak at m/z 241.10707 (calcd for C12H17O5, 241.10705). The 13C NMR and DEPT spectroscopic data of 2 (Table 2) showed two CH3 at δC 18.0 and 26.1, two CH2 at δC 30.9 and 39.9, three CH at δC 76.9, 145.1, and 120.7, three non-protonated carbons at δC 64.6, 135.5, and 135.4, and two carbonyl carbons at δC 178.0 and 168.5. The 1D NMR data showed high similarity with those of 1. By interpretating of the 2D NMR spectra, including HSQC, 1H–1H COSY, and HMBC spectra (Fig. 2), 2 was determined to have a planar structure identical to 1. The weak correlation signal of H-5 (δH 4.59)/H-8a (δH 2.60) in the ROESY spectrum indicating that H-5 and the prenyl group resided the opposite sides of the five-membered ring, which helped to establish the relative configuration of 2 as 1R*, 5R*. An ECD calculation workflow was applied to determine the absolute configuration of 2. As shown in Fig. 4C, the calculated ECD of the 1R, 5R stereoisomer matched well with the experimental CD data. Therefore, the evidence allowed the complete assignment of 2D structure and absolute configuration of 2 (Fig. 1).
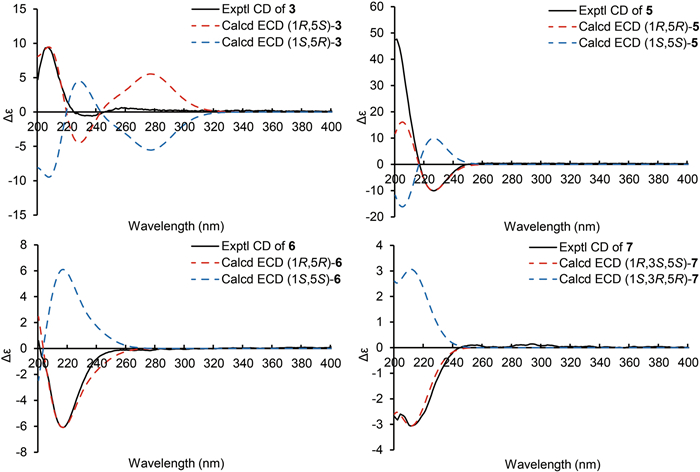
Hirsutavibrin C (3, Fig. 1) was obtained as a colorless oil. The molecular formula of 3 was determined as C14H18O6 according to the HRESIMS data (m/z 283.11765 [M + H]+, calcd for C14H19O6, 283.11761), indicating six degrees of unsaturation. The 1H and 13C NMR data of 3 (Tables 1 and 2) were closely similar to the data of 2, indicating the structure of 3 closely resembled that of 2, with the primary difference being associated with an extra acetoxyl group in 3. The methyl singlet at δH 1.98, the carbonyl carbon at δC 172.1, together with the key HMBC correlation from H-5 (δH 5.27) to δC 172.1 (Fig. 2) of 3 enabled the assignment of the acetoxyl group attaching to C-5. Although in the 13C NMR spectrum, the signal for C-13 was not detected, which is common for carboxylic groups [36], the HRESIMS result and the chemical shifts of C-2, C-3 and C-4 confirmed that a carboxylic acid group attached to C-3. The crucial and significant correlation of H-5/H-8 (δH 2.60, 2.35) in the ROESY spectrum (Fig. 3) assigned the H-5 and the prenyl group locating at the same side of the five-membered ring (Fig. 2). In addition, the calculated ECD spectrum of (1R, 5S)-3 showed similar adsorption trend with the experimental CD spectrum of 3. Therefore, the absolute configuration of 3 was established as 1R, 5S (Figs. 1 and 5).
ECD calculations of 3, 5–7
The colorless oil hirsutavibrin D (4, Fig. 1) had a molecular formula of C14H20O5 which returned from the (+)-HRESIMS analysis (m/z 269.13834 [M + H]+), revealing five degrees of unsaturation. In the 13C NMR spectrum of 4, 14 carbon signals were displayed (Table 2), which showed similarity to 3, except that an oxygenated methylene (C-13, δC 61.6) in 4 replaced the carbonyl group signal of 3. The key HMBC correlations from H-2 (δH 5.72) and H-13 (δH 4.11) to C-3 (δC 144.4), and from H-2 to C-13 (Fig. 2) supported the conclusion that the C-13 of 4 was a hydroxymethyl group instead of being a carboxylic acid group as in 3. The key ROESY correlation between H-5 and H-8 (Fig. 3) suggested that the prenyl group and H-5 were in the same plane of the five-membered ring. Therefore, compound 4 was identified as a C-13 reduced product of 3.
The molecular formula of hirsutavibrin E (5, Fig. 1) was determined as C14H20O5 according to the (+)-HRESIMS data (m/z 269.13834 [M + H]+, calcd. for C14H21O5). The 1D NMR spectroscopic data suggested that 5 (Table 2) was a congener of 4, except that the acetoxyl group was changed to locate at C-13 according to the 3J-HMBC correlations from the proton at δH 4.59 (H-13) to δC 170.9, and the 4J-HMBC correlations from methyl at δH 2.08 (13-OCOCH3) to C-13 (δC 62.8) (Fig. 2). The key correlation between H-5 and H-8 (Fig. 3) was absent in the ROESY spectrum, while between H-5 and H-4α was seen. Therefore, the relative configuration of 5 was speculated to be 1R*, 5R*. The calculated ECD curve of (1R, 5R)-5 showed a Cotton effect similar to the experimental one (Fig. 5), thus establishing the absolute configuration of 5 to be 1R, 5R.
Compound 6 (Fig. 1) was isolated as a colorless oil. The molecular formula was assigned as C16H22O7 based on the positive HRESIMS ion peak at m/z 327.14359 [M + H]+ (calcd for C16H23O7, 327.14383). Compared to the NMR data of 5 (Tables 1 and 2), the signals for acetyl group were absent in compound 6. In addition, four additional carbon resonances which were ascribable to two carbonyl groups (δC 174.1, 176.7) and two methylenes (δC 30.2, 30.3) (Table 2) were presented in 6. By interpreting the 2D NMR spectra, the four carbons were assigned to be a succinic acid moiety, which was determined by the correlations from H-2' (δH 2.58) and H-3' (δH 2.62) to C-1' (δC 174.1) and C-4' (δC 176.7), from H-13 (δH 4.61) to C-1' and C-3 (δC 138.3) (Fig. 2) in the HMBC spectrum. The correlation between H-5 and H-8 was not seen in the ROESY spectrum, suggesting a 1R*, 5R* configuration of 6. With the help of ECD calculations, an absolute configuration of 1R, 5R was assigned to 6 based on the highly similar Cotton effect between the experimental CD diagram and the calculated ECD data of (1R, 5R)-6 (Fig. 5). Therefore, compound 6 was trivially named hirsutavibrin F.
The 1H and 13C NMR data of 7 (Tables 2 and 3) displayed twelve carbon resonances which were classified into two CH3, four CH2 (one oxygenated), two sp3 CH (one oxygenated), one sp2 CH, one sp3 qC, and two sp2 qC (one carbonyl and one olefinic carbon). The NMR data of 7 displayed similarity to those of vibralactone E, except that the C-2–C-3 double bond was reduced to a single bond, which was corroborated by the key 1H-1H COSY correlations between H-2 (δH 2.31) and H-3 (δH 2.21) (Fig. 2). These assignments reached the conclusion that the molecular formula of 7 was C12H20O4, which was consistent with the HRESIMS analysis results (m/z 229.14349 [M + H]+, calcd. for C12H21O4, 229.14344). The key correlations of H-2β/H-3/H-4β/H-5 (δH 4.27)/H-8a (δH 2.51), H-2α/H-13/H-4α, and H-5/H-9 in the ROESY spectrum (Fig. 3) indicated the relative configurations of 7 to be 1R*, 3S*, 5S*. The ECD calculation steps were applied to deduce the absolute stereochemistry of 7. Comparative analysis of the measured and theoretically calculated ECD curves (Fig. 5) revealed a 1R, 3S, 5S configuration of 7. Compound 7 was named hirsutavibrin G.
1H NMR spectroscopic data of compounds 7–11
The molecular formula of compound 8 (Fig. 1) was deduced to be C12H20O4, which was identical with 7. Detailed interpretation of the NMR of 8 revealed the same planar structure as 7 (Tables 2 and 3). The lack of key ROESY signals between H-5 and H-8 indicated a 1R*, 3S*, 5R* configuration of 8 (Fig. 3). Considering the same biosynthetic pathways of 7 and 8, the absolute configurations of 8 were determined to be 1R, 3S, 5R. Compound 8 was trivially named hirsutavibrin H.
Hirsutavibrin I (9, Fig. 1) was isolated as a colorless oil. The (+)-HRESIMS sodium adduct ion peak at m/z 265.10452 [M + Na]+ (calcd for C12H18O5Na, 265.10519) revealed a chemical formula of C12H18O5 of 9. The 13C NMR data of 9 (Tables 2 and 3) displayed two methyls, three methylenes (one oxygenated), two oxygenated sp3 methines (δC 63.9, 75.0), two sp3 quaternary carbons (δC 59.7, 66.8), etc. These data are different from those of compounds 1–6, while are similar to those of compound 7, and are highly resemble to those of vibralactone B [20, 37]. Compound 9 was 18 Da larger than vibralactone B, suggesting that 9 was the lactone ring-opening product of vibralactone B. The crucial ROESY correlations of H-2 (δH 3.38)/H-8 (δH 2.31, 2.01)/H-5 (δH 3.76), H-2/H-9 (δH 5.08) indicated the 1S, 2S, 3S, 5S configuration of 9. Hence compound 9 was elucidated as depicted in Fig. 1.
The chemical formula of 10, obtained as a colorless oil, was determined to be C14H22O7 (m/z 303.14383 [M + H]+, Δ 0.00048 ppm) by (+)-HRESIMS. The 1D NMR data between 10 (Tables 2 and 3) and 5 were quite similar, except that the C-2/C-3 double bond between in 5 was replaced by a vicinal diol in 10, according to the key HMBC correlations from H-2 (δH 3.45) to C-1 (δC 54.3), C-3 (δC 76.9), C-5 (δC 70.6), from H-8 (δH 2.29) to C-2 (δC 83.7), and from H-13 (δH 3.97) to C-2, C-3, C-4 (δC 45.0), and from 3-OH (δH 4.60) to C-2, C-3, C-4, and C-13 (Fig. 2). The crucial ROESY correlations of H-2 (δH 3.45)/H-8/H-5 (δH 3.57)/3-OH (Fig. 3) indicated the 1S, 2S, 3R, 5S stereochemistry of 10. Therefore, the structure of compound 10 was established and named hirsutavibrin J.
( +)-HRESIMS spectrometric analysis established the molecular formula of 11 (Fig. 1) as C12H18O5Na (m/z 265.10461 [M + Na]+, mass error 0.11536 ppm). The 1D NMR spectroscopic data of 11 was similar to those of compound 7, except with the existence of a new carbonyl carbon (δC 181.0) in 11 while absence of the hydroxymethyl group in 7. This evidence indicated that compound 11 differs from 7 by the oxygenated status of C-13. The carbonyl group at δC 181.0 in compound 11 was assigned to a carboxylic group (C-13). The correlation from H-3 (δH 2.86) to C-13 in the HMBC spectrum (Fig. 2) as well as the molecular formula corroborated the above assignments. The vital ROESY correlations of H-8/H-5/H-3 suggested a 1R, 3S, 5S configuration of compound 11 (Fig. 3). Therefore, the structure of compound 11 was established and named hirsutavibrin K.
2.2 Biological activity evaluation of 1–11
The isolated compounds with abundant yield (1, 2, 4, 6) were subjected to a panel of biological activity screening, including the cytotoxicity against A549, a human lung cancer cell line, and anti-NO (nitric oxide) activity in murine monocytic RAW 264.7 macrophages. As a result, compounds 1 and 2 showed weak cytotoxicity activity toward A549 with the IC50 39.7 and 34.3 μM, respectively (positive control cisplatin, IC50 5.09 μM). Compound 4 displayed anti-NO activity with IC50 of 26.6, which were more significant than the positive control L-NG-monomethyl arginine (IC50 51.2 μM).
3 Conclusion
In conclusion, eleven new vibralactone derivatives (1 − 11) were obtained from the co-culture broth of the two basidiomycetous fungi Stereum hirsutum and Boreostereum vibrans. Comprehensive analyses of 1D & 2D NMR spectroscopic data and computational calculations allowed the establishment of the structures of all the isolates. Most of the isolates are structurally similar to vibralactone but display distinct configurations.
Co-culturing microbes typically involves ascomycetes or ascomycetes with bacteria, with few studies focusing on secondary metabolite discovery from co-cultures of two basidiomycetes. Our research demonstrates that co-culturing phylogenetically related fungal species is an effective approach for discovering natural products. For instance, previous studies on Boreostereum vibrans cultures primarily yielded vibralactone derivatives with the same absolute configuration as vibralactone. However, when Boreostereum vibrans was co-cultured with Stereum hirsutum, vibralactone derivatives with distinct configurations and additional modifications were discovered. Notably, none of the isolated compounds have been found from the individual cultures of each fungus. While further evidence is needed to confirm the involvement of new biosynthetic pathways in the modification of vibralactone, this study underscores that co-culturing two basidiomycetes is a promising strategy for natural product discovery. This work not only expands the members of vibralactone derivatives with different configurations but also opens a new avenue for fungal co-culturing study between congeneric fungi.
Notes
Acknowledgement
This work was financially supported by the National Natural Science Foundation of China (Grant number 82473810) and the Fundamental Research Funds for the Central Universities, South-Central Minzu University (Grant No. CPT22033). The authors thank the Analytical & amp; Measuring Center, School of Pharmaceutical Sciences, South-Central Minzu University for the spectra test, and the Bioactivity Screening Center of Natural Products, Kunming Institute of Botany, CAS for biological activity screening.
Author contributions
He-Ping Chen and Ji-Kai Liu: Conceptualization and writing—reviewing and editing, funding acquisition, methodology project administration; Jinjuan Wei, Zhe-Xi Li, and Gao-Ke Peng, investigation, formal analysis, writing-original draft preparation; Xinyang Li: Writing—reviewing and editing. All authors approved the final version for publication.
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Ji-Kai Liu is the Editor-in-Chief of this journal but was not involved in the peer review or decision-making process in this article. The other authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
-
1.Feng L, Shang R-R, Wang XJ, Li L, Li X, Gong Y-X, Shi L-Y, Wang JW, Qian ZY, Tan NH, Wang Z. The natural alkaloid (−)-N-Hydroxyapiosporamide suppresses colorectal tumor progression as an NF-κB pathway inhibitor by targeting the TAK1–TRAF6 Complex. J Nat Prod 2023;86: 1449-62. CrossRef PubMed Google Scholar
-
2.Chen Y, Li Q, Li Y, Zhang W, Liang Y, Fu A, Wei M, Sun W, Chen C, Zhang Y, Zhu H. Quadriliterpenoids A − I, nine new 4, 4-dimethylergostane and oleanane triterpenoids from Aspergillus quadrilineatus with immunosuppressive inhibitory activity. Nat Prod Bioprospect 2024;14: 59. CrossRef PubMed Google Scholar
-
3.Ma R, Feng XY, Tang JJ, Ha W, Shi YP. 5α-Epoxyalantolactone from Inula macrophylla attenuates cognitive deficits in scopolamine-induced Alzheimer's disease mice model. Nat Prod Bioprospect 2024;14: 39. CrossRef PubMed Google Scholar
-
4.Peng W, Huang Q, Ke X, Wang W, Chen Y, Sang Z, Chen C, Qin S, Zheng Y, Tan H, Zou Z. Koningipyridines A and B, two nitrogen-containing polyketides from the fungus Trichoderma koningiopsis SC-5. Nat Prod Bioprospect 2024;14: 8. CrossRef PubMed Google Scholar
-
5.Sresuksai K, Sawadsitang S, Jantaharn P, Noppawan P, Churat A, Suwannasai N, Mongkolthanaruk W, Senawong T, Tontapha S, Moontragoon P, Amornkitbamrung V, McCloskey S. Antiproliferative polyketides from fungus Xylaria cf. longipes SWUF08–81 in different culture media. Nat Prod Bioprospect 2024;14: 6. PubMed Google Scholar
-
6.Zhou L, Akbar S, Wang MX, Chen HP, Liu JK. Tetra-, penta-, and hexa-nor-lanostane triterpenes from the medicinal fungus Ganoderma australe. Nat Prod Bioprospect 2022;12: 32. CrossRef PubMed Google Scholar
-
7.Zhang JJ, Tang X, Moore BS. Genetic platforms for heterologous expression of microbial natural products. Nat Prod Rep 2019;36: 1313-32. CrossRef PubMed Google Scholar
-
8.Schueffler A, Anke T. Fungal natural products in research and development. Nat Prod Rep 2014;31: 1425-48. CrossRef PubMed Google Scholar
-
9.Liu SL, Zhou L, Chen HP, Liu JK. Sesquiterpenes with diverse skeletons from histone deacetylase inhibitor modified cultures of the basidiomycete Cyathus stercoreus (Schwein) De Toni HFG134. Phytochemistry 2022;195: 113048. CrossRef PubMed Google Scholar
-
10.Hu Z, Ye Y, Zhang Y. Large-scale culture as a complementary and practical method for discovering natural products with novel skeletons. Nat Prod Rep 2021;38: 1775-93. CrossRef PubMed Google Scholar
-
11.Starnovskaya SS, Nesterenko LE, Popov RS, Kirichuk NN, Chausova VE, Chingizova EA, Chingizov AR, Isaeva MP, Yurchenko EA, Yurchenko AN. Metabolite profiles of Paragliomastix luzulae (formerly named as Acremonium striatisporum) KMM 4401 and its co-cultures with Penicillium hispanicum KMM 4689. Nat Prod Bioprospect 2024;14: 38. CrossRef PubMed Google Scholar
-
12.Lyu HN, Liu HW, Keller NP, Yin WB. Harnessing diverse transcriptional regulators for natural product discovery in fungi. Nat Prod Rep 2020;37: 6-16. CrossRef PubMed Google Scholar
-
13.Xu Y, Du X, Yu X, Jiang Q, Zheng K, Xu J, Wang P. Recent advances in the heterologous expression of biosynthetic gene clusters for marine natural products. Mar Drugs 2022;20: 341. CrossRef PubMed Google Scholar
-
14.Wang QY, Chen HP, Wu KY, Li X, Liu JK. Antibacterial and β-amyloid precursor protein-cleaving enzyme 1 inhibitory polyketides from the fungus Aspergillus chevalieri. Front Microbiol 2022;13: 1051281. CrossRef PubMed Google Scholar
-
15.Tang Y, Zhao ZZ, Yao JN, Feng T, Li ZH, Chen HP, Liu JK. Irpeksins A-E, 1, 10-seco-eburicane-type triterpenoids from the medicinal fungus Irpex lacteus and Their Anti-NO Activity. J Nat Prod 2018;81: 2163-8. CrossRef PubMed Google Scholar
-
16.Tang Y, Zhao ZZ, Hu K, Feng T, Li ZH, Chen HP, Liu JK. Irpexolidal represents a class of triterpenoid from the fruiting bodies of the medicinal fungus Irpex lacteus. J Org Chem 2019;84: 1845-52. CrossRef PubMed Google Scholar
-
17.Li X, Chen HP, Zhou L, Fan J, Awakawa T, Mori T, Ushimaru R, Abe I, Liu JK. Cordycicadins A-D, antifeedant polyketides from the entomopathogenic fungus Cordyceps cicadae JXCH1. Org Lett 2022;24: 8627-32. CrossRef PubMed Google Scholar
-
18.Wang QY, Chen HP, Tao H, Li X, Zhao Q, Liu JK. Penidaleodiolides A and B, cage-like polyketides with neurotransmission-regulating activity from the soil fungus Penicillium daleae L3SO. Org Lett 2024;26: 7632-7. CrossRef PubMed Google Scholar
-
19.Liu DZ, Wang F, Liao TG, Tang JG, Steglich W, Zhu HJ, Liu JK. Vibralactone: a lipase inhibitor with an unusual fused β-lactone produced by cultures of the basidiomycete Boreostereum vibrans. Org Lett 2006;8: 5749-52. CrossRef PubMed Google Scholar
-
20.Jiang MY, Wang F, Yang XL, Fang LZ, Dong ZJ, Zhu HJ, Liu JK. Derivatives of vibralactone from cultures of the basidiomycete Boreostereum vibrans. Chem Pharm Bull 2008;56: 1286-8. CrossRef PubMed Google Scholar
-
21.Jiang MY, Zhang L, Dong ZJ, Yang ZL, Leng Y, Liu JK. Vibralactones D-F from cultures of the basidiomycete Boreostereum vibrans. Chem Pharm Bull 2010;58: 113-6. CrossRef PubMed Google Scholar
-
22.Wang GQ, Wei K, Feng T, Li ZH, Zhang L, Wang QA, Liu JK. Vibralactones G-J from cultures of the basidiomycete Boreostereum vibrans. J Asian Nat Prod Res 2012;14: 115-20. CrossRef PubMed Google Scholar
-
23.Chen HP, Zhao ZZ, Li ZH, Dong ZJ, Wei K, Bai X, Zhang L, Wen CN, Feng T, Liu JK. Novel Natural oximes and oxime esters with a vibralactone backbone from the basidiomycete Boreostereum vibrans. Chemistryopen 2016;5: 142-9. CrossRef PubMed Google Scholar
-
24.Zhao PJ, Yang YL, Du LC, Liu JK, Zeng Y. Elucidating the biosynthetic pathway for vibralactone: a pancreatic lipase inhibitor with a fused bicyclic β-lactone. Angew Chem Int Ed 2013;52: 2298-302. CrossRef PubMed Google Scholar
-
25.Yang YL, Zhou H, Du G, Feng KN, Feng T, Fu XL, Liu JK, Zeng Y. A monooxygenase from Boreostereum vibrans catalyzes oxidative decarboxylation in a divergent vibralactone biosynthesis pathway. Angew Chem Int Ed 2016;55: 5463-6. CrossRef PubMed Google Scholar
-
26.Feng KN, Yang YL, Xu YX, Zhang Y, Feng T, Huang SX, Liu JK, Zeng Y. A hydrolase-catalyzed cyclization forms the fused bicyclic β-lactone in vibralactone. Angew Chem Int Ed 2020;59: 7209-13. CrossRef PubMed Google Scholar
-
27.Feng KN, Zhang Y, Zhang MF, Yang YL, Liu JK, Pan LF, Zeng Y. A flavin-monooxygenase catalyzing oxepinone formation and the complete biosynthesis of vibralactone. Nat Commun 2023;14: 3436. CrossRef PubMed Google Scholar
-
28.Nistanaki SK, Boralsky LA, Pan RD, Nelson HM. A concise total synthesis of (±)-vibralactone. Angew Chem Int Ed 2019;58: 1724-6. CrossRef PubMed Google Scholar
-
29.Zhao ZZ, Zhang F, He HJ, Wang Y, Du JH, Wang ZZ, Chen H, Liu JK, Stereuins AF. Isopentenyl benzene congeners with antibacterial and neurotrophic activities from Stereum hirsutum HFG27. Phytochemistry 2024;228: 114253. CrossRef PubMed Google Scholar
-
30.Zhao ZZ, Han KY, Li Z-H, Feng T, Chen HP, Liu JK. Cytotoxic ergosteroids from the fungus Stereum hirsutum. Phytochemistry Lett 2019;30: 143-9. CrossRef PubMed Google Scholar
-
31.Zhao ZZ, Zhao X, Si YY, Wang ZZ, Sun YJ, Chen HP, Feng WS, Liu JK. Structure elucidation of linear triquinane sesquiterpenoids, hirsutuminoids A-Q, from the fungus Stereum hirsutum and their activities. Phytochemistry 2022;200: 113227. CrossRef PubMed Google Scholar
-
32.Wang G, Ran H, Fan J, Keller NP, Liu Z, Wu F, Yin W-B. Fungal-fungal cocultivation leads to widespread secondary metabolite alteration requiring the partial loss-of-function VeA1 protein. Sci Adv 2022;8: eabo6094. CrossRef PubMed Google Scholar
-
33.Yao L, Zhu LP, Xu XY, Tan LL, Sadilek M, Fan H, Hu B, Shen XT, Yang J, Qiao B, Yang S. Discovery of novel xylosides in co-culture of basidiomycetes Trametes versicolor and Ganoderma applanatum by integrated metabolomics and bioinformatics. Sci Rep 2016;6: 33237. CrossRef PubMed Google Scholar
-
34.Yu G, Ge X, Wang Y, Mo X, Yu H, Tan L, Yang S. Discovery of novel terpenoids from the basidiomycete Pleurotus ostreatus through genome mining and coculture optimization. J Agric Food Chem 2023;71: 11110-23. CrossRef PubMed Google Scholar
-
35.Shen XT, Mo XH, Zhu LP, Tan LL, Du FY, Wang QW, Zhou YM, Yuan XJ, Qiao B, Yang S. Unusual and highly bioactive sesterterpenes synthesized by Pleurotus ostreatus during coculture with Trametes robiniophila Murr. Appl Environ Microbiol 2019;85: e00293-e319. PubMed Google Scholar
-
36.Xiong J, Zhou PJ, Jiang HW, Huang T, He YH, Zhao ZY, Zang Y, Choo YM, Wang X, Chittiboyina AG, Pandey P, Hamann MT, Li J, Hu JF. Forrestiacids A and B, pentaterpene inhibitors of acl and lipogenesis: extending the limits of computational NMR methods in the structure assignment of complex natural products. Angew Chem Int Ed 2021;60: 22270-5. CrossRef PubMed Google Scholar
-
37.Chen HP, Jiang MY, Zhao ZZ, Feng T, Li ZH, Liu JK. Vibralactone biogenesis-associated analogues from submerged cultures of the fungus Boreostereum vibrans. Nat Prod Bioprospect 2018;8: 37-45. CrossRef PubMed Google Scholar
Copyright information
© The Author(s) 2025
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.