Newly isolated terpenoids (covering 2019–2024) from Aspergillus species and their potential for the discovery of novel antimicrobials
Abstract
The rapid emergence of drug-resistant microbial pathogens has posed challenges to global health in the twenty-first century. This development has significantly made most antibiotics ineffective in the treatment of infections they cause, resulting in increasing treatment costs and annual death rates. To address the challenge posed by these pathogens, we explore the potential of secondary metabolites from Aspergillus species as a source of new and effective therapeutic agents to treat drug-resistant infections. Terpenoids, a distinct group of natural products, are extensively distributed in plants and fungi, and have been attributed with significant antibacterial, anticancer, and antiviral activities. In this review, we present an overview of Aspergillus species, and review the novel terpenoids isolated from them from 2019 to April 2024, highlighting anti-infective activity against members of the ESKAPE pathogens. We further focus on the strategies through which the structural framework of these new terpenoids could be modified and/or optimized to feed a pipeline of new lead compounds targeting microbial pathogens. Overall, this review provides insight into the therapeutic applications of terpenoids sourced from Aspergillus species and the potential for the discovery of new compounds from these fungi to combat antimicrobial resistance.Graphical Abstract
Keywords
Antimicrobial resistance ESKAPE pathogens Fungi Secondary metabolites Anti-infective activities1 Introduction
Antimicrobial resistance (AMR) unequivocally remains a major threat to global health in the twenty-first century. AMR occurs when microbes, such as bacteria, fungi, viruses, and parasites acquire resistance to one or more drugs [1]. Although AMR is an evolutionary process that happens with time as a result of alteration in the genetic materials of microorganisms [2, 3], the human "action and inaction" in the excessive and inappropriate use of antibiotics has immensely contributed to the evolution and continuous emergency of AMR [4]. Antimicrobial resistance has been noticed to almost all the clinical drugs that have been developed [3, 5, 6]. It has been estimated that AMR will be responsible for the death of 10 million people by 2050, and worse still, it will economically cost more than US$ 100 trillion annually if drastic and coordinated actions are not taken to curb it [7]. Among the microorganisms that have been implicated in antimicrobial resistance, ESKAPE pathogens (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species) are popular owing to their built-in ability to resist a large class of antibiotics [1, 8, 9]. The World Health Organization (WHO), in its first ever documentation on antibiotic-resistant "priority pathogens", identified and accredited them as the priority pathogens in the fight against AMR [10]. Controlling the AMR of these bacteria is predicated on developing and designing new antimicrobial drugs and/or alternative strategies.
From the time immemorial, nature has been regarded as a reservoir of bioactive molecules. Natural products (NPs) are biological molecules with low molecular weights, and can be obtained from marine or terrestrial plants, animals, and microorganisms [11]. Natural products (or secondary metabolites) are an unprecedented source of chemical diversity, with unique pharmacological properties [11, 12]. Natural products and derivatives have historically been acting as an alternative, invaluable source of therapeutic agents towards several ailments [13, 14]. More than 50% of clinically approved antibiotics currently on the market are sourced from nature [13]. It is noteworthy that the first blockbuster antibiotic, penicillin, isolated by Sir Alexander Fleming from a green fungal mold Penicillium notatum in 1928 changed the course of history, and saved millions of lives during World War II [15].
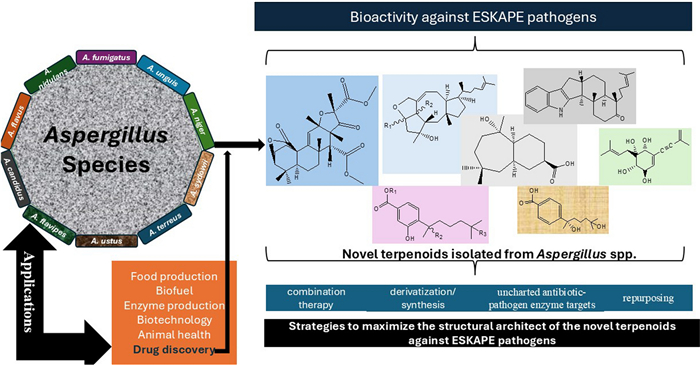
In nature, fungi are widely distributed and recognized as an emerging source of anti-infective agents, with unparallel and unmatched modes of actions [16, 17]. The genus Aspergillus is one of the largest genera in the fungal kingdom [18]. Aspergillus species has become a research hotspot recently among the scientific community due to their potent ability to biosynthesize unusual, rich, and matchless bioactive molecules [19-22]. Moreover, the past five years of research on the secondary metabolites from the Aspergillus has ushered in several new compounds, including peptides [23], alkaloids [24], and terpenoids [25]. These new natural products have been linked with several pharmacological actions, including activities against ESKAPE pathogen members [26-29]. In the absence of comprehensive, holistic, and standalone review on Aspergillus-derived terpenoids in the past five years [30-33], the central purpose of this work is to review and document newly reported terpenoids isolated from the Aspergillus in different habitats (covering 2019–April 2024), and examine their pharmacological actions against ESKAPE pathogens. We also delve into different ways these novel frameworks of terpenoids could be maximally or optimally utilized. Overall, the current work would afford us to gain further insight into the therapeutic roles of these terpenoids in the fight against AMR and open new research paradigm in future studies.
2 Methodology
2.1 Search strategy and inclusion criteria
The databases of Google Scholar, ScienceDirect, Springer, Scopus, and PubMed were utilized in the search for literature in this review using single or combination of key words—"Aspergillus", "Aspergillus AND natural products", Aspergillus secondary metabolites", Aspergillus AND terpenoids", Aspergillus natural products AND ESKAPE pathogens, "Aspergillus species AND secondary metabolites", and "Aspergillus AND bioactive compounds". The search covered publications from January 2019–April 2024. The search was refined to include only research studies that reported new terpenoids from the Aspergillus and/or their pharmacological actions against any ESKAPE pathogen. Only articles published in English language were included in the data search. Data was thoroughly screened, and relevant papers (those reported newly isolated terpenoids) were collated using Zotero reference manager.
2.2 Exclusion criteria
Abstracts, conference proceedings, commentaries, editorial and case reports that did not meet the inclusion criteria were excluded. Similarly, new terpenoids isolated from the co-culture of Aspergillus species with other organisms were excluded as shown in a previous study [34]. This is to document the new terpenoids specifically derived from Aspergillus species. Besides, we noticed some co-culture experiments lack controls during our data collection to ascertain whether the metabolites were really produced by the Aspergillus species.
3 The ESKAPE pathogens: to what extent have they "escaped"?
The ESKAPE pathogens account for the majority of resistant nosocomial infections worldwide [35, 36] and the incidence of resistance in these pathogens is ever-increasing. The acronym "ESKAPE" was coined by Louis B. Rice in 2008 [35] to highlight Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species based on their ability to "escape" the pharmacological actions of most current antibiotics [35]. Non-susceptible strains can be resistant to (ⅰ) at least one antimicrobial drug in three or more antimicrobial categories (multiple drug resistance; MDR), (ⅱ) all antimicrobial agents except in two or less antimicrobial categories (extensive drug resistance; XDR), or (ⅲ) all antimicrobial agents in all antimicrobial categories (pandrug resistance; PDR) [36]. The core resistance mechanisms involve modification of antibiotic targets, antibiotic influx prevention, biochemical modification of the antibiotics, and overexpression of efflux pumps, and have been comprehensively reviewed elsewhere [37-39]. The increase in drug-resistance in ESKAPE pathogens has resulted in diverse variants. Among them are carbapenem-resistant K. pneumoniae (CRKP) [40, 41], carbapenem-resistant A. baumanii (CRAB) [40], carbapenem-resistant P. aeruginosa (CRPA) [41], carbapenem-resistant Enterobacteriaceae (CRE), and multi-drug-resistant A. baumannii [42]. Others are vancomycin-resistant Enterococci (VRE), vancomycin-intermediate S. aureus (VISA) [43], vancomycin-resistant E. faecium (VRE), vancomycin-resistant S. aureus (VRSA), methicillin-resistant S. aureus (MRSA), and fluoroquinolone-resistant P. aeruginosa (FRPA) [44] to mention a few.
4 Aspergillus species: an overview of a genus of 'friends', or 'foes' with benefits
Aspergillus is a genus of spore-forming mold first described in 1729 by an Italian botanist, Pier Antonio Micheli. This genus includes more than 200 identified species that are characterized by the morphology of a conidophore (a long-chained projection of conidia or spores) [45]. Aspergillus is widely distributed across the globe and species are mainly found in the soil as saprophytes, where they survive on dead organic matter and play an important role in the recycling of elemental carbon and nitrogen through the environment. However, some species live in water, air and on vegetation [45].
The genus comprises species of positive and negative economic importance in medicine, agriculture, and industry. Anxiously, the world is medically sensing a threat currently posed by Aspergillus infections. About 12% of the species are known as major causes of life-threatening infections, including aspergilloma, invasive aspergillosis, and otomycosis in humans. The most representative culprits are A. flavus, A. terreus, A. felis, A. fumigatus, A. niger, and A. nidulans [45]. It is estimated that more than 300, 000 people develop aspergillosis yearly, with about 30 million people at risk [46]. The agricultural sector is not left out from the negative impact of Aspergillus species. They threaten global food security and safety initiatives. For instance, Aspergillus species contaminates agricultural commodities, such as cereal grains with toxins (aflatoxins and ochratoxins), leading to economic loss and food shortage [47]. It is more worrisome that consumption of aflatoxin-contaminated foods has great health implications. There is risk of developing cancers, especially liver cancers in human due to mycotoxin exposure, although no statistically significant link/association has been documented in the literature [48].
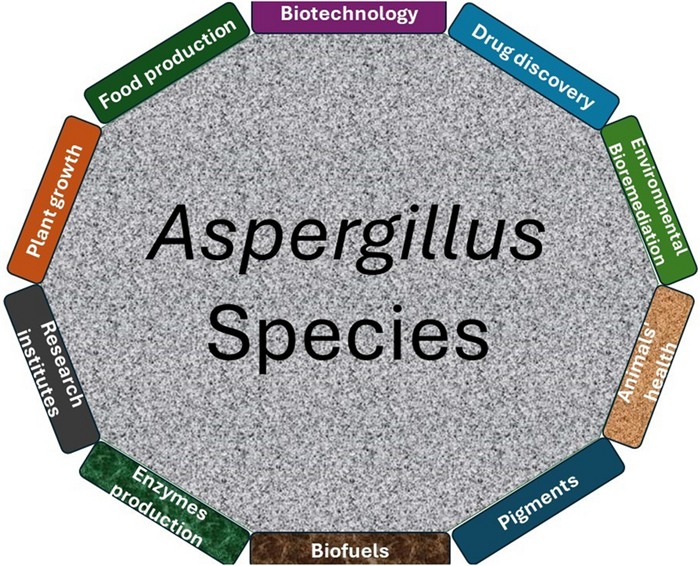
Regardless of the pathogenic role of Aspergillus species in our ecosystem, the genus has shaped biotechnology, food, and medical industries over the past 100 years (Fig. 1). The culprit A. terreus, for instance, has been utilized industrially in bio-based production of itaconic acid and the drug lovastatin. A. niger has gained industrial application in fermentation-based production of proteins and enzymes. These species, in addition to other culprits, are indeed human 'enemies' with enormous benefits [49]. Today, the impact of Aspergillus species in drug discovery has been much pronounced with respect to the number of bioactive secondary metabolites, including terpenoids isolated from the genus.
An overview of some of the applications of the Aspergillus in medicine, agriculture, biotechnology, ecosystem, and industries
5 Terpenoids
5.1 Basic chemical classifications of terpenoids and their biosynthesis
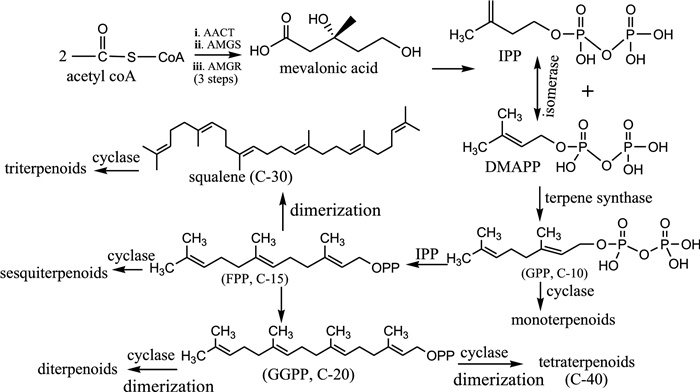
Terpenoids form a specialized group of organic compounds biosynthesized by most plants, animals, and microorganisms, including bacteria and fungi. There are over 90, 000 known terpenoids with diverse biological activities. The name "terpene" was first used by August Kekulé in 1863 to describe a chemical compound, turpentine/terpentine, derived from tree plant Pistacia terebinthus [50]. All terpenoids are formed biosynthetically from isopentenyl pyrophosphate (IPP) and its structurally distinct isomer, dimethylallyl pyrophosphate (DMAPP), via either a mevalonic acid pathway (MVA) or a 2-C-methylerythritol phosphate (MEP) (also called, non-mevalonate) pathway [51]. While MVA pathway is common in eukaryotes and the archaea, MEP is utilized by higher plants, eubacteria, and algae. Here, we summarize the MVA pathway as it is commonly utilized by most organisms.
Firstly, IPP and its isomer DMAPP are joined in a "head-to-tail" manner under the influence of an enzyme (terpene synthase) to initiate the formation of geranyl diphosphate (GPP, C-10) chain. When GPP combines with a unit of IPP, farnesyl diphosphate (FPP, C-15) is produced. Then, FPP initiates the formation of geranyl–geranyl- diphosphate (GGPP, C-20) when joined with another unit of IPP. The biosynthesized GGPP can enzymatically dimerize to produce C-40 chain length, from where carotenoids (tetraterpenoids) are formed. Likewise, enzymatic dimerization of FPP (C-15) can initiate the formation of squalene (C-30), a precursor of triterpenoids. Under the influence of enzyme cyclases, these varying chain lengths of carbon atoms cyclize to form different ring sizes and numbers of various terpenoids. Other biochemical transformations, including oxidation, substitution, de-substitution and rearrangement reactions can occur, leading to diverse terpenoid natural products (Fig. 2). Based on the isoprene principle, C-5 molecule, as proposed by Croatian-Swiss scientist, Leopold Ružicka [52], terpenoids could be distinctively classified into several groups, namely hemi-terpenoids (C-5), monoterpenoids (C-10), sesquiterpenoids (C-15), diterpenoids (C-20), triterpenoids (C-30), and tetraterpenoids (C-40).
Biosynthetic pathway of terpenoids via mevalonic acid [51]. AACT acetoactl-CoA thiolase, HMGS hydroxymethylglutaryl-CoA synthase, HMGR hydroxymethylglutaryl-CoA reductase, OPP diphosphate moieties, IPP isopentenyl pyrophosphate, DMAPP dimethylallyl pyrophosphate, GPP geranyl diphosphate, FPP farnesyl diphosphate, GGPP geranyl–geranyl-diphosphate
5.2 Quantification of terpenoids isolated from the Aspergillus species
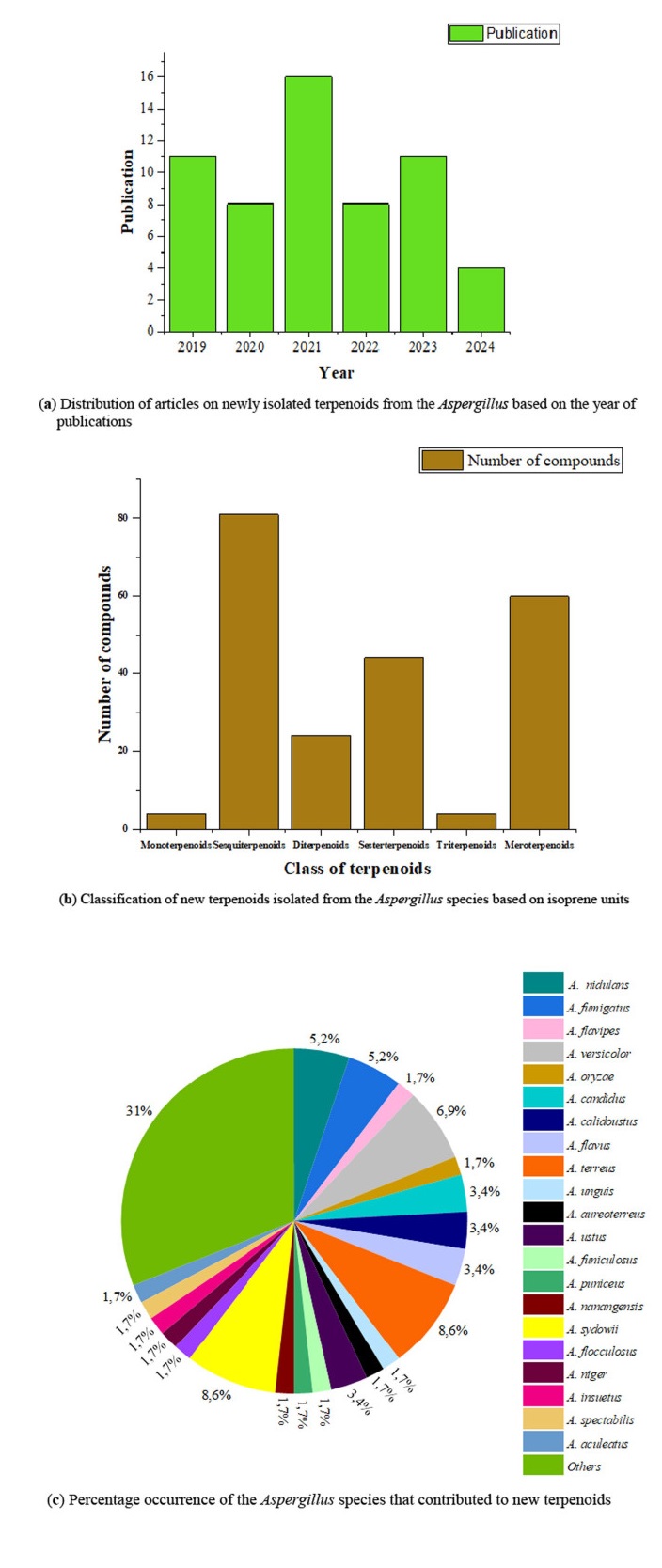
After extensive search and careful screening of the published articles from 2019 to April 2024 using the named databases, a total of 58 articles met our selection criteria for newly isolated terpenoids from the Aspergillus species. As shown in Fig. 3a, majority of the articles were published in 2021, followed by 2019 and 2023. During these studied periods, a total of 217 new terpenoids were isolated from the Aspergillus species and unambiguously characterized using spectroscopic methods. The most newly isolated terpenoids belong to the class sesquiterpenoids, followed by the meroterpenoids, and only a few monoterpenoids and triterpenoids were reported (Fig. 3b). More than 22 species of Aspergillus contributed to these new terpenoids. Notably, A. terreus (8.62%) and A. sydowii (8.62%) are the most studied Aspergillus species as shown by the number of occurrences, followed by A. versocolor (6.90%). However, most members of the genus (denoted as 'Others') are not fully characterized taxonomically at the species level as shown in Fig. 3c. Hence, full identification of these Aspergillus 'species' (denoted as 'Others') offers many research possibilities from the taxonomic perspective (Fig. 3c).
a–c Quantification of new terpenoids and the Aspergillus species that produced them
6 Chemical diversity of terpenoids isolated from the Aspergillus species
Several novel frameworks of terpenoids (Figs. 4, 5, 6, 7, 8, 9) have been isolated from the Aspergillus species. In this section, the novel molecules identified in each of the classes of terpenoids, including meroterpenoids are discussed.
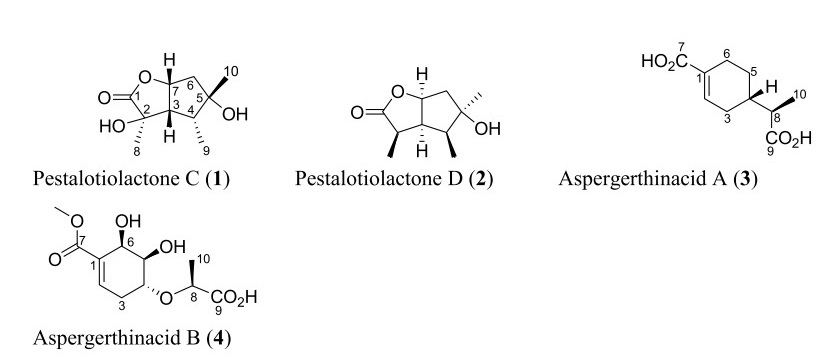
Structures of monoterpenoids isolated from Aspergillus species
Structures of sesquiterpenoids isolated from Aspergillus species
Structures of diterpenoids isolated from Aspergillus species
Structures of sesterterpenoids isolated from Aspergillus species
Structures of triterpenoids isolated from Aspergillus species
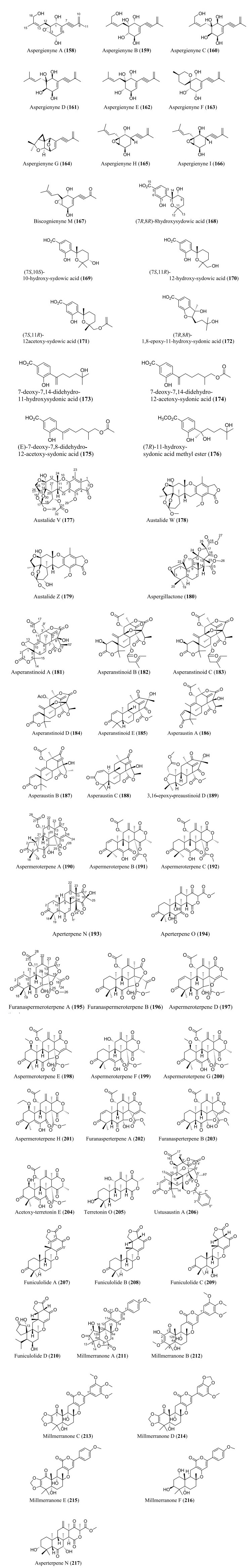
Structures of meroterpenoids isolated from Aspergillus species
6.1 Monoterpenoids
Only four monoterpenoids were isolated from the Aspergillus species in the past five years as shown in Fig. 4. These include pestalotiolactones C-D (1–2) which are two new butyrolactone-skeleton monoterpenoids isolated from deep-sea fungus A. versicolor SD-330 [53]. Aspergerthinacids A and B (3–4) are the other two new monoterpenes identified from the fungal strain Aspergillus sp. CYH26 isolated from the Chinese plant Cynanchum bungei Decne [54].
6.2 Sesquiterpenoids
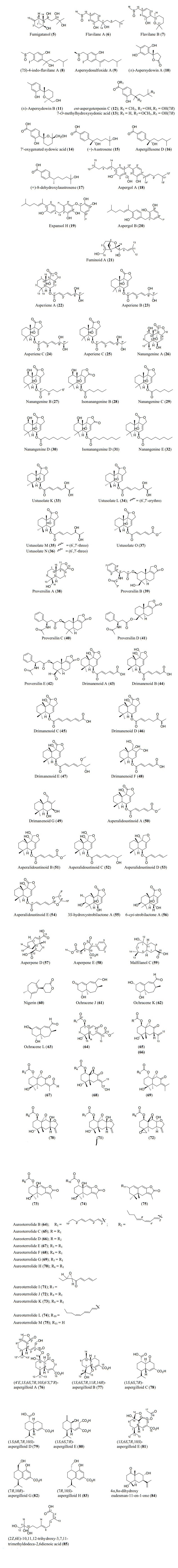
Sesquiterpenoids are abundant in the genus Aspergillus and 81 compounds were isolated. They belong to different sesquiterpenoid skeletons, including bergamotane-type (5), bisabolane-type (6–21), drimane-type (22–56), humulane-derived (61–63), eremophilane-type (64–75), cadinene-type (76–83), and eudesmane-type sesquiterpenoid (84) among others (Fig. 5).
To start with, one previously undescribed compound, fumigatanol (5), was recently reported from the fungus A. fumigatus M1 isolated from the plant Aconitum brevicalcaratum Diels. [55]. In a study published recently, a pair of new enantiomers (±)-flavilane A (6) together with a new derivative flavilane B (7) was identified from marine-derived fungus, A. flavipes 297 isolated from the fresh seawater in China. Compounds (6) and (7) are rare cases of bisabolane-type sesquiterpenoids with methylsulfinyl moiety [56]. In addition to these flavilanes, an iodo-derivative of flavilane A was recently reported from cold-seep fungus A. sydowii 10–31 collected in Taiwan Island. The compound (7S)-4-iodo-flavilane A (8) adds to the sesquiterpenoid molecular diversity, bearing both sulphur and iodine [57]. Similarly, a new sulphur-containing bisabolane sesquiterpenoid has been identified. The compound, aspersydosulfoxide A (9), was isolated from the marine-derived A. sydowii LW09 [58]. (±)-aspersydowin A (10) and (±)-aspersydowin B (11) were isolated from the fungus A. sydowii. The two compounds are enantiomers [59]. Likewise, ent-aspergoterpenin C and 7-O-methylhydroxysydonic acid (12–13) were isolated from deep-sea sediment-derived fungus A. versicolor SD-330 collected in South China Sea [53]. Compounds 7′‑oxygenated sydowic acid and (−)-austrosene (14–15) were discovered from the fermented culture of Aspergillus sp. SCSIO06786 obtained from the deep-sea sediment [60]. Meanwhile, after much scrutiny, compound (14) was reported a year after as a new isolated bisabolene sesquiterpenoid obtained from A. versicolor SD-330 [61]. Aspergillusene D (16) was discovered from the fermented culture of sponge-derived fungus A. sydowii SCSIO 41301 harvested from the Xisha Islands in China [62]. (+)-8-dehydroxylaustrosene (17), a new bisabolane sesquiterpenoid, was reportedly isolated and characterized from marine fungus A. sydowii BTBU20213012 [63]. Aspergol A (18), expansol H (19) and aspergol B (20) featuring bisabolene carbon framework were isolated from marine-derived Aspergillus sp. MCCC 3A00392 collected at central Pacific Ocean [64]. Fuminoid A (21), a new compound with a bicyclo[3.2.1]octane ring isolated from the Huperzia serrata-derived fungus A. fumigatus, represents an unusual bisabolane scaffold via the transformation of the methyl [65].
In addition to these sesquiterpenoids, four drimane sesquiterpene esters were isolated from the strain A. flavus derived from the marine environment. Notably, the asperienes A–D (22–25) are epimers, and differed only in the side chain configuration at C-6′, and C-7′. Structurally, compounds 22 and 23 possess erythro configuration (6′R, 7′R or 6′S, 7′S) at C-6′ and C-7′ while compounds 24 and 25 have threo configuration (6′S, 7′R or 6′R, 7′S) at C-6′ and C-7′ [66]. Similarly, seven new drimane sesquiterpenoids (26–32) have been reported from the fermented culture of a novel Australian fungus A. nanangensis. Structurally, nanangenine A (26) features as trihydroxylated drimane lactone. While nanangenines B-E (27, 29–30, 32) bear C6′/C8′ acyl side chains, isonanangenines B and D (28, 31) bear isomeric lactone rings [67]. Ustusolates K–O (33–37) represent five sesquiterpenoids with drimane skeleton recently isolated from Aspergillus sp. RR-YLW-12 derived from marine organism, Rhodomela confervoides. Ustusolate K features 6′, 7′-diol in the side chains in addition to two double bonds at C2′ and C4′. However, ustusolates M and N (35–36) were obtained as inseparable epimers [68]. Proversilins A−E (38–42) form new sets of drimane-type sesquiterpenoids discovered from A. versicolor F210 isolated from the plant Lycoris radiata bulbs. Structurally, proversilins B-E distinctively possess N-acetyl-β-phenylalanine moiety, representing first examples of natural products with such moiety [69]. Drimanenoids A−G (43–49) were recently discovered from Aspergillus sp. NF2396 ethyl acetate extracted derived from an earwig. These compounds structurally feature an unsaturated fatty acid side chain at C-6′ with conjugated double bond ranging from 1–3, and a terminal carboxyl or methyl group [70]. Asperalidoustinoids A–E (50–54) were isolated from wetland soil-derived fungus A. calidoustus. Asperalidoustinoid E (54) distinctively features a rare dioxolane moiety [71]. Compounds (55–56), reported as new drimane sesquiterpenoids, were discovered from the fermented culture of algae-derived Aspergillus sp. RR-YLW-12 isolated from the strain Rhodomela confervoides [25].
Again, asperpenes D and E (57–58) were chromatographed and identified from the fungal strain Aspergillus sp. SCS-KFD66 ethyl acetate extract isolated from bivalve mollusk, Sanguinolaria chinensis [72]. Bicyclic malfilanol C (59) was identified from ethyl acetate extract of A. puniceus A2 derived from deep-sea sediment [73]. Chemical profiling of marine sponge-derived A. niger 164, 117 collected in the South China Sea resulted in nigerin (60) and ochracenes J-L (61–63). While ochracenes J-L form new sets of humulane-derived sesquiterpenoids, the nigerin represents a new sesquiterpene scaffold, featuring an unprecedented 1-(3-n-pentyl)-2, 5, 6-trimethyl-cycloheptane ring system. [74]. Highly oxygenated twelve compounds, characterized as unusual eremophilane sesquiterpenes with an oxidized C-4 and named as aureoterrolides B-M (64–75), were obtained from the culture of the fungus A. aureoterreus. Isolate aureoterrolide J (72) represents first pentacyclic furanoeremophilane, comprising a 3/6/6/5/3 and a 3, 6-spiro ring system [75]. Compounds (76–83) possessing cadinene-sesquiterpene skeleton were identified and elucidated from fungus A. flavus isolated from the toxic plant, Tylophora ovata [76]. Similarly, one novel eudesmane-sesquiterpene, 4α, 8α-dihydroxyeudesman-11-en-1-one (84), has been reportedly isolated from endophytic fungus A. flavus [76]. Meanwhile, chemical investigation of ethyl acetate extract obtained from fungal strain Aspergillus sp. SCSIO 41029 isolated from deep sea gave only linear sesquiterpene (85) identified in these reviewing years [77].
6.3 Diterpenoids
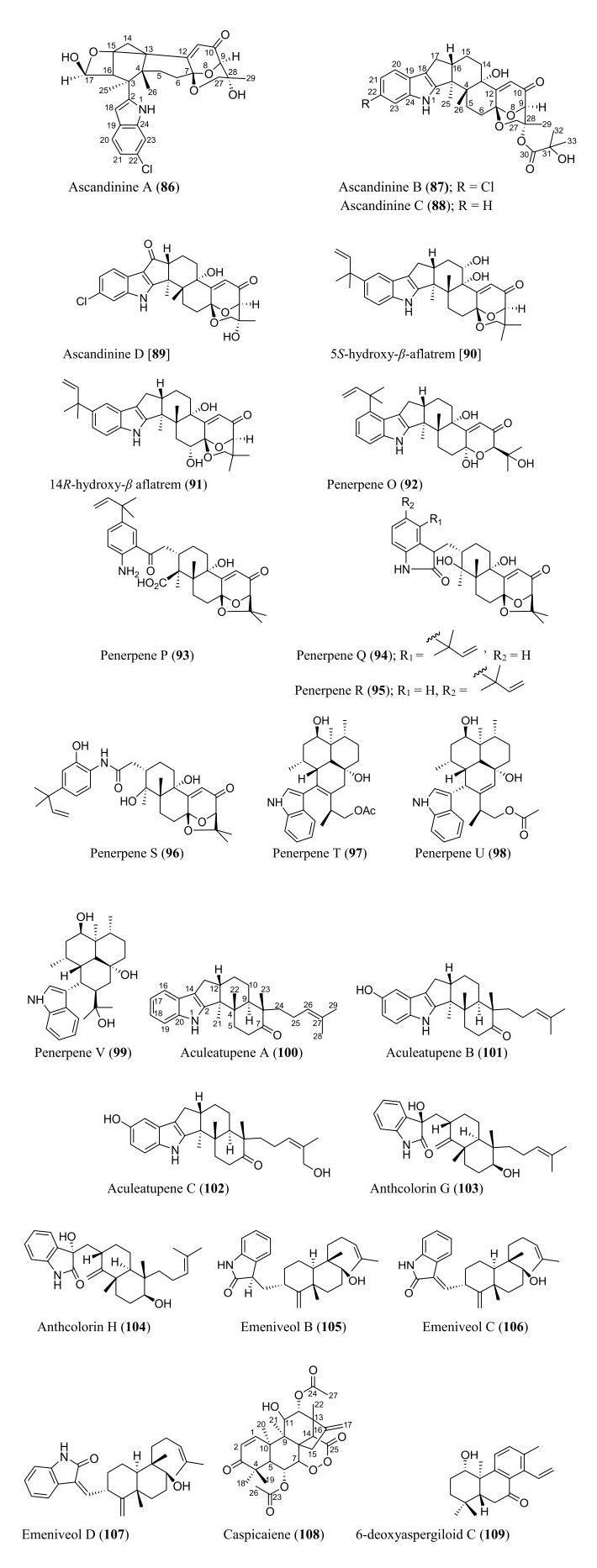
Diterpenoids form an integral part of the Aspergillus secondary metabolites as revealed in the data obtained. Statistically, a total of twenty-four diterpenoids were isolated. Most of the isolated diterpenoids possess an indole skeleton (Fig. 6). Ascandinines A−D (86–89) are new indole diterpenoids isolated from an Antarctic sponge-derived fungus A. candidus HDN15-152. Ascandinine A (86) possesses a 6/6/6/6/6 pentacyclic ring system with an indole substitute and a 2oxabicyclo[2.2.2]octan-3-ol motif, while ascandinines B−D (87–89) possess a rare carbon skeleton, featuring 6/5/5/6/6/6/6-fused ring system [78]. Heptacyclic 5S-hydroxy-β-aflatrem (90) and 14R-hydroxy-β aflatrem (91), paxilline-type indole diterpenes, were identified from Sphagneticola trilobata-derived fungus Aspergillus sp. PQJ-1 [79]. Similarly, eight new indole-diterpenoids, penerpenes O–V (92–99), were recently isolated from Aspergillus sp. ZF-104 derived from marine soft coral. Penerpenes R and S (95–96) possess an unusual indolin-2-one units in their structures [80]. Three indole diterpenoids were obtained from A. aculeatus KKU-CT2 derived from a contaminated laboratory agar plate. The new pentacyclic diterpenoids, aculeatupenes A–C (100–102), feature a 6/5/5/6/6 ring structure [81]. Anthcolorins G–H (103–104), oxoindolo-diterpene epimers, were discovered from a culture of the endophytic fungus A. versicolor isolated from mangrove Avicennia marina fruit [82]. Three new oxoindolo-type diterpenoids have been recently reported. The emeniveol B–D (105–107) were isolated from marine-derived Aspergillus sp. MCCC 3A00392 collected at the central Pacific Ocean. Notably, emeniveol C is the cis-isomer of emeniveol D [64]. Caspicaiene (108) possessing a kaurene diterpenoid nucleus with 6/6/6/5/6 ring system structure was reported from the culture of endophytic Aspergillus N830 isolated from the plant Gleditsia caspia desf. [83]. A cleistanthane diterpenoid, 6-deoxyaspergiloid C (109), was isolated from the Aspergillus candidus [84].
6.4 Sesterterpenoids
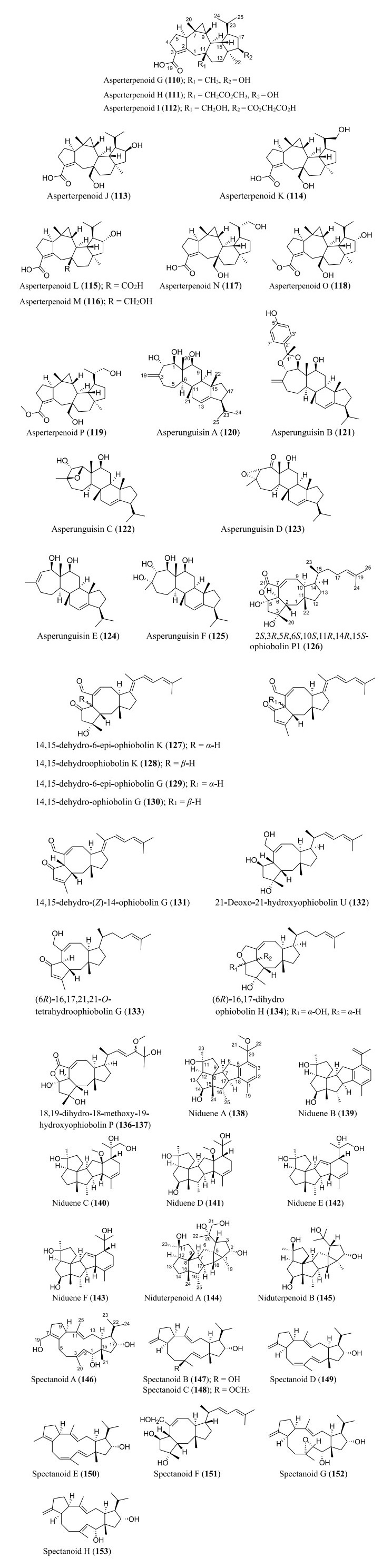
Several new sesterterpenoids have been identified from Aspergillus species. Specifically, forty-four new metabolites were recorded as depicted in Fig. 7. For instance, ten new sesterterpenoids were isolated from A. oryzae. The asperterpenoids G-P (110–119) feature pentacyclic skeleton with an unusual 5/7/3/6/5 ring pattern [85]. Asperunguisins A−F (120–125), asperane class of sesterterpenoids, were identified from a culture of endolichenic fungus A. unguis isolated from the lichen Xanthoria sp. These asperunguisins were uniquely characterised by hydroxylated 7/6/6/5 tetracyclic system [86]. Ophiobolin P1 (126) was recently reported from a wetland soil-derived fungus A. calidoustus [71]. Similarly, five previously undescribed ophiobolin-group sesterterpenoids (127–131) have been reported from the marine fungal strain A. flocculosus isolated from the seaweed Padina sp. [87]. 21-Deoxo-21-hydroxyophiobolin U (132) was recently reported from fungus Aspergillus sp. RR-YLW-12 derived from marine red algae, Rhodomela confervoides [68]. In addition to these ophiobolins, three new compounds (133–135) belonging to this type of sesterterpenoids were identified from a deep sea-derived fungus A. insuetus SD-512 extract obtained from cold seep sediments [88]. Compound (136) and its isomer (137) were newly ophiobolin sesterterpenoids discovered from the fermented culture of algae-derived Aspergillus sp. RR-YLW-12 isolated from the strain Rhodomela confervoides. Notably, compound (137) is a C18 epimer of 18, 19-dihydro-18-methoxy-19-hydroxyophiobolin P (136) [25]. Six functionalized sesterterpenoids named niduenes A−F (138–143) were discovered from endophytic fungus A. nidulans. These new niduenes are characterized by an unprecedented 5/5/5/5/6 pentacarbocyclic ring system. Intriguingly, niduenes A and B (138–139) feature the first pentacarbocyclic sesterterpenoids with aromatic nucleus [89]. Likewise, two highly congested compounds (144–145) with hexacarbocyclic 5/5/5/5/3/5 ring system have been reported from solid cultivation of A. nidulans. The carbon skeleton of the two compounds structurally devoid of unsaturated functional groups. Notably, the two isolated compounds represent the first set of hexacyclic sesterterpenoids [90]. Spectanoids A–H (146–153) were isolated from the fungus Aspergillus spectabilis obtained from Artemisia grassland. Spectanoids A-G consist of tricarbocyclic skeleton, with a rare 5/12/5 ring arrangement [91].
6.5 Triterpenoids
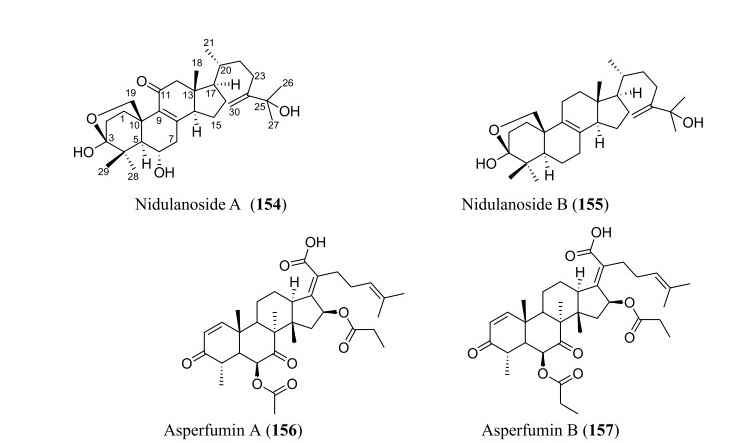
Triterpenoids, though few, are parts of the secondary metabolites isolated from Aspergillus species as revealed by the analyzed data. In total, four new triterpenoids were introduced to the chemical diversity of the genus. These include two new 30-norlanostane triterpenoids, nidulanosides A (154) and B (155), which were isolated from fungus A. nidulan ethyl acetate extract. These compounds represent the first naturally occurring 30-norlanostane triterpenoids with a C9 side chain attachment at C-17, and a hemi-acetal formed between C-3 and C-19 [92] (Fig. 8). The other two, asperfumins A and B (156–157), were obtained from a chemical study on A. fumigatu, an endophytic fungus isolated from Cleidion brevipetiolatum fresh root [93].
6.6 Meroterpenoids
Meroterpenoids are specialized forms of terpenoids with exceptionally diverse structures, ranging from simple to complex ones. Unlike their 'normal' terpenoid counterparts, they are characterized by mixed biosynthetic pathways, and broadly grouped as polyketide-terpenoids or nonpolyketide-terpenoids pathways [30]. Meroterpenoids are well distributed in Aspergillus (Fig. 9). They include aspergienynes A–I (158–166), diisoprenyl-cyclohexene class of meroterpenoids, that were recently isolated from mangrove-derived endophytic fungus Aspergillus sp. GXNU-Y65 [94]. Biscognienyne M (167), a new member of diisoprenyl-cyclohexene class of meroterpenoids, was isolated from the mangrove endophytic fungus Aspergillus QG1a [95]. Nine novel meroterpenoids (168–176) were discovered from solid rice culture of the endophytic fungus A. versicolor isolated from mangrove Avicennia marina fruit [82].
Austalide meroterpenoids V and W (177–178) were derived from A. ustus VKM F-4692 isolated from the building stone. Interestingly, these two compounds are the first sets of austalides with a 5/6/6/6/6/5/5 heptacyclic ring system [96]. Recently, austalide Z (179) was reported from soft coral-derived ethyl acetate extract of Aspergillus sp. [97]. A new 3, 5-dimethylorsellinic acid (DMOA)-based meroterpenoid, aspergillactone (180), was reportedly identified from extracts of Aspergillus sp. CSYZ-1 [98]. Similarly, five (DMOA)-based meroterpenoids, trivially named as asperanstinoids A–E (181–185), were recently reported from soil-derived fungus A. calidoustus. Asperanstinoid A (181) exemplifies second form of (DMOA)-based meroterpenoid in nature, featuring an unusual 6/5/6/6/6/5-fused hexacycles and an uncommon "1, 13-epoxy" moiety [99]. Asperaustins A−C (186–188) represent three new DMOA-derived meroterpenoids isolated from Aspergillus sp. fungus ZYH026 obtained from marine brown alga Saccharina cichorioides. Structurally, asperaustin A (186) with a unique 5/6/6/6/5 pentacarbocyclic skeleton features an unprecedented spiro[4.5]deca-3, 6-dien-2-one attachment [100]. 3, 16-epoxy-preaustinoid D (189), a new DMOA-based meroterpenoid, was isolated from wetland soil-derived fungus A. calidoustus [71]. Aspermeroterpene A–C (190–192) were obtained from the marine fungus A. terreus GZU31-1. Compound (190) represents first unprecedented and highly congested hexacyclic meroterpene with 5/3/6/6/6/5 carbon skeleton [101]. Aperterpenes N and O (193–194) were identified from the ethyl acetate extract of the fermented culture of endophytic fungal strain A. terreus EN-539 isolated from marine alga, Laurencia okamurai [102].
Two furanaspermeroterpenes A and B (195–196) were co-isolated in addition to five previously undescribed congeners aspermeroterpenes D–H (197–201) from fungal strain A. terreus GZU-31-1 derived from marine environment. While the two furanaspermeroterpenes A and B have unusual pentacyclic skeleton, and feature the first sets of DMOA-derived meroterpenoids in which five-membered D/E rings were coupled, aspermeroterpene D (197) possess a rare cis-fused A/B ring [103]. In addition to this, a study by the same author(s) had earlier reported the isolation of furanasperterpenes A and B (202–203) along with 11-acetoxy-terretonin E (204) from the same fungus strain A. terreus GZU-31-1. Structurally, the difference between these furanasperterpenes and the 11-acetoxy-terretonin E arises from furan ring formation between C-7 and C-15 in furanasperterpenes A and B [104]. Similarly, terretonin O (205) has been identified from methanolic extracts of marine A. terreus LGO13 and thermophilic A. terreus TM8 [105]. Ustusaustin A (206), a novel austin-type meroterpene, was isolated from the culture extract of A. ustus TK-5. Ustusaustin A features as the first example of 1′-nor-austin analogues with the possession of a rare 7-benzoylation [106]. Funiculolides A−D (207–210), four new fungal meroterpenoids, were reported from A. funiculosus CBS 116.56 by utilizing the heterologous expression of a cryptic gene cluster in the fungal strain. Intriguingly, these compounds unexpectedly and biosynthetically utilized 5-methylorsellinic acid (5-MOA) as against the DMOA [107]. Fermented culture of Australian fungus Aspergillus sp. CMB-MRF324 derived from pasture soil reportedly yielded six millmerranones A−F (211–216). Millmerranone A (211) distinguishably features a rare carbon ring bearing a unique cyclic carbonate, thereby making it an unusual meroterpenoid [108]. Asperterpene N (217) was discovered from the fermented culture of endophytic fungus Aspergillus sp. derived from Tripterygium wilfordii. Compound (217) is a unique DMOA-based meroterpenoid, featuring a cis-fused C/ D ring [109].
7 Pharmacological activities against ESKAPE pathogens
A few pharmacological activities have been reported for some of the new terpenoids against at least one member of the ESKAPE pathogens. However, much work needs to be done to assay other compounds. Aspergillactone (180) was reported to display selective but effective antibacterial activity against four clinical strains of methicillin-resistant S. aureus (USA300, BKS231, BKS233 and ATCC 25923), with minimum inhibitory concentrations (MICs) of 2, 4, 8 and 16 μg/mL, respectively. However, it inhibited A. baumannii, E. faecium, P. aeruginosa, E. faecalis, and K. pneumonia at MICs > 32 μg/mL than the positive control [98]. Compound (5) inhibited the strain of S. aureus at a concentration of 256 μg/mL [55]. Flavilanes A and B (6–7) possess antimicrobial action against the human pathogen, S. aureus, with MICs of 64 and 32 μg/mL, respectively [56]. Compound (14) isolated from A. versicolor had inhibition against zoonotic human bacterium P. aeruginosa QDIO-6 (8.0 μg/mL), though with less activity compared to the antibiotic chloramphenicol (2.0 μg/mL) [61]. Similarly, ent-aspergoterpenin C (12) and 7-O-methylhydroxysydonic acid (13) were also reported to inhibit P. aeruginosa with MIC values of 8.0 and 32.0 μg/mL respectively. However, the monoterpenoid compounds (1) and (2) showed no antibacterial action against the pathogen [53]. In the same vein, other newly isolated monoterpenoids, aspergerthinacids A and B (3–4), were reportedly inactive against S. aureus [54]. While compounds (133) and (134) did not exhibit strong activity (MIC > 32 μg/mL), (5S, 6S)-16, 17-dihydroophiobolin H (135) exhibited inhibitory effects against P. aeruginosa at a concentration of 8.0 μg/mL. Notably, the β-H at C-6 is critical for the stronger activity of ophiobolin (135) compared to (134) [88]. Isolate (59) obtained from the ethyl acetate extract of A. puniceus A2 weakly inhibited S. aureus ATCC 29213 [73]. Millmerranones A−F (211–216) displayed no growth inhibition against S. aureus ATCC 25923, with half inhibitory concentration (IC50) > 30 μM [108]. Compound (17) did not exhibit activity against S. aureus ATCC 25923 at the highest tested concentration of 200.0 μg/mL compared to the control methicillin (MIC, 0.5 μg/mL) [63]. Antibacterial activities of compound (15) against the pathogenic strains of S. aureus (ATCC 29213), K. pneumonia (ATCC 13883), E. faecalis (ATCC 29212), A. baumannii (ATCC 19606), and methicillin-resistant S. aureus have also been reported [60]. Drimanenoids C–D (45–46) and F (48) showed inhibition against methicillin-resistant S. aureus, with inhibition diameters of 2.66±0.47, 4.66±0.47, and 5.00±0.00 mm respectively [70]. The aperterpenes (193–194) had no inhibitory activity against S. aureus and P. aeruginosa at a concentration > 32 μg/mL [102]. 6-deoxyaspergiloid C (109) exhibited no activity against the growth of S. aureus (MIC, > 100 μg/mL) [84]. The aculeatupenes (100) and (101) showed antibacterial effects against S. aureus ATCC 25923 and P. aeruginosa ATCC 27853, with MIC of 128 μg/mL [81].
8 Strategies to maximize the structural architect of the new terpenoids against ESKAPE pathogens
Aspergillus species, undoubtedly, remain sources of terpenoids with intriguing, structural carbon frameworks. It has been stressed that terpenoids possess potential as substitutes in overcoming AMR [110, 111]. These unique new secondary metabolites have not been fully maximized in the quest for new therapeutic agents against ESKAPE pathogens. A critical analysis of the obtained data revealed that only a few of the newly isolated terpenoids have been screened or channeled for antibacterial activities against these pathogens. While some displayed no activity, a number of them showed appreciable antibacterial effects against the tested pathogens. There are many ways of maximizing and/or optimizing the potential therapeutic values of these vast arrays of unique compounds. One approach is to utilize combinatory therapy of these terpenoids with antibiotics. Combinatory therapy is a new weapon to fight against multidrug resistance [112]. It does not only foster the optimization of antibacterial efficacy, but also counters development of resistance. It has been shown as an effective means to increase the potency of treatment against multidrug-resistant bacteria. A study reported that natural terpene S-limonene decreased the MIC from 16 to 0.237 µg/mL when combined with antibiotic, rifampicin [113]. Diterpenoids salvipisone and aethiopinone reportedly enhanced the activity of the antibiotic oxacillin against S. aureus. They reduced S. aureus biofilm by 40% in combination with oxacillin [114].
Chemical derivatization or synthesis of analogues of these terpenoids with unusual carbon framework is another innovative strategy. Some common structural optimization strategies include (ⅰ) altering existing functional groups or introducing new ones to improve interactions with biological targets, (ⅱ) changing the core structure of the terpenoids to explore different biological activities, (ⅲ) modifying the spatial arrangement of atoms to enhance selectivity and potency, and (ⅳ) conjugating a sugar unit to improve solubility and transport across membranes. In the case of fumigatanol (5), modifying hydroxyl groups to esters (–COOCH₃) or ethers (–OCH₃) could improve membrane permeability. Also, by expanding the 6-membered ring in Malfilanol C (59) to a 7-membered ring could improve its binding affinity with the biological targets. In drug development and modern rational medicinal chemistry, this approach does not only save costs, but also reduces the time in developing terpene-based therapeutics for these bacteria [115, 116]. Undertaking detailed mechanistic studies on the new terpenoids that showed antibacterial actions will drive the development of target therapeutic intervention. It is one thing is for these terpenoids to show bactericidal activity, and another to know how they exert the bactericidal actions. While there are many enzyme targets/mechanisms attributed to the current clinical antibiotics/drugs, it will be great to explore uncharted paths in antibiotic-pathogen enzyme targets [117-119]. Moreover, new drugs/antibiotics are more prone to bacterial resistance when they have targets/mode of action similar to those currently being managed to treat infections. This pivotal approach offers one advantage to combat multidrug resistant infections caused by ESKAPE pathogens. Another 'unpopular' alternative intervention that might be profitable is to 'repurpose' other terpenoids that have not been studied for their anti-ESKAPE pathogen activities. The bulk of these new terpenoids as revealed from the available data has been driven by anticancer/cytotoxic research. Thus, massive antibacterial screening of this chemical library of novel terpenoids against ESKAPE members might result in lead/hit identification, optimization, and development.
9 Concluding remarks and future outlook
The rise in antimicrobial resistance (AMR) from ESKAPE pathogens coupled with the need for new and effective drugs has necessitated developing and designing new antimicrobial drugs and/or alternative strategies for curbing these pathogens. Recent investigations on the natural products from the Aspergillus species offer the possibility of finding promising lead drug candidates. In this paper, we systematically synthesized and holistically documented newly isolated terpenoids from this genus (covering 2019–April 2024). We also examined their therapeutic prospects in the fight against ESKAPE members. The main Aspergillus species involved in the production of these terpenoids include the "culprits" A. terreus, A. sydowii, A. versicolor, A. fumigatus, A. nidulans, and A. flavus among others. In total, 217 new terpenoids were isolated. Among which, 81 are sesquiterpenoids, 60 are meroterpenoids, and 44 are sesterterpenoids. Pharmacologically, a few of these terpenoids exhibited appreciable in vitro antibacterial effects against ESKAPE pathogens. However, in vivo efficacy testing as well as the mechanism of their antibacterial drug targets is necessary for comprehensive evaluation of their drug-like potency. Importantly, a larger percentage of the compounds have not been fully characterized for their antibacterial activity. Thus, there is a need for these compounds to be subjected to in vitro and in vivo testing for their activity against ESKAPE pathogens and others that are being added to the WHO list of pathogens of concern. Additionally, innovative strategies, including chemical derivatization and combination therapy should be employed to optimize the bioactivity of compounds with antimicrobial activity, particularly those that have new biological targets providing in-depth understanding of their mechanisms of action, and opening new opportunities for antimicrobial development in the future.
Notes
Abbreviations
AMR:
Antimicrobial resistance
ATCC:
American type culture collection
DMAPP:
Dimethylallyl pyrophosphate
DMOA:
3, 5-Dimethylorsellinic acid
ESKAPE:
Enterococcus faecium, & nbsp; Staphylococcus aureus, & nbsp; Klebsiella pneumoniae, & nbsp; Acinetobacter baumannii, & nbsp; Pseudomonas aeruginosa, and & nbsp; Enterobacter & nbsp; species
FPP:
Farnesyl diphosphate (FPP
GGPP:
Geranyl-geranyl- diphosphate
IPP:
Isopentenyl pyrophosphate
MDR:
Multidrug resistance
MEP:
2-C-methylerythritol phosphate
MIC:
Minimum inhibitory concentration
MVA:
Mevalonic acid
MRSA:
Methicillin-resistant & nbsp; S. aureus
NPs:
Natural products
PDR:
Pandrug-resistance
WHO:
World Health Organization
XDR:
Extensive drug resistance
Acknowledgement
We are grateful to Rhodes University, South Africa for providing access to data used in this study.
Author contributions
OO, IN and DA conceptualized the work. OO was responsible for data collection and analysis and wrote the first draft. IN, DA, TS, RLH and RAD reviewed and edited the manuscript. RAD supervised and acquired funding.
Funding
This work was supported by grants awarded to RAD by the South African Medical Research Council (SAMRC), with funds received from the South African National Department of Health, and the UK Medical Research Council, with funds received from the UK Government's Newton Fund (Grant No.: 96185) and by South African National Research Foundation (NRF) through the DSI/NRF South African Research Chair Initiative (NRF UID 87583). OO was supported by a Rhodes University Post-Doctoral Fellowship, while IN and TS received Post-Doctoral Fellowships from the SAMRC. DA was supported by DAAD Scholarship (Reference number: 91758998). The authors' opinions and conclusions as expressed in this paper are their own and should not be attributed to any of the funding bodies itemized above.
Availability of data and materials
All the data in the manuscript are obtained from included references and available upon request.
Declarations
Ethics approval and consent to participate
None of the authors of this article has performed studies involving animals in this article. Therefore, ethical declaration is not applicable for this work.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
-
1.Kalpana S, Lin W-Y, Wang Y-C, Fu Y, Lakshmi A, Wang H-Y. Antibiotic resistance diagnosis in ESKAPE pathogens—a review on proteomic perspective. Diagnostics 2023;13: 1014. CrossRef PubMed Google Scholar
-
2.Smith WPJ, Wucher BR, Nadell CD, Foster KR. Bacterial defences: mechanisms, evolution and antimicrobial resistance. Nat Rev Microbiol 2023;21: 519-34. CrossRef PubMed Google Scholar
-
3.Christaki E, Marcou M, Tofarides A. Antimicrobial resistance in bacteria: mechanisms, evolution, and persistence. J Mol Evol 2020;88: 26-40. CrossRef PubMed Google Scholar
-
4.Bungau S, Tit DM, Behl T, Aleya L, Zaha DC. Aspects of excessive antibiotic consumption and environmental influences correlated with the occurrence of resistance to antimicrobial agents. Curr Opin in Environ Sci Health 2021;19: 100224. CrossRef PubMed Google Scholar
-
5.Lee Y, Puumala E, Robbins N, Cowen LE. Antifungal drug resistance: molecular mechanisms in Candida albicans and beyond. Chem Rev 2021;121: 3390-411. CrossRef PubMed Google Scholar
-
6.Ippolito MM, Moser KA, Kabuya J-BB, Cunningham C, Juliano JJ. Antimalarial drug resistance and implications for the WHO global technical strategy. Curr Epidemiol Rep 2021;8: 46-62. CrossRef PubMed Google Scholar
-
7.Dadgostar P. Antimicrobial resistance: implications and costs. Infect Drug Resist 2019;12: 3903-10. CrossRef PubMed Google Scholar
-
8.De Oliveira DMP, Forde BM, Kidd TJ, Harris PNA, Schembri MA, Beatson SA, et al. Antimicrobial resistance in ESKAPE pathogens. Clin Microbiol Rev. 2020. https://doi.org/10.1128/cmr.00181-19. PubMed Google Scholar
-
9.Zhen X, Lundborg CS, Sun X, Hu X, Dong H. Economic burden of antibiotic resistance in ESKAPE organisms: a systematic review. Antimicrob Resist Infect Control 2019;8: 137. CrossRef PubMed Google Scholar
-
10.Mancuso G, Midiri A, Gerace E, Biondo C. Bacterial antibiotic resistance: the most critical pathogens. Pathogens 2021;10: 1310. CrossRef PubMed Google Scholar
-
11.Thomford NE, Senthebane DA, Rowe A, Munro D, Seele P, Maroyi A, et al. Natural products for drug discovery in the 21st century: innovations for novel drug discovery. Int J Mol Sci 2018;19: 1578. CrossRef PubMed Google Scholar
-
12.Bernardini S, Tiezzi A, Laghezza Masci V, Ovidi E. Natural products for human health: an historical overview of the drug discovery approaches. Nat Prod Res 2018;32: 1926-50. CrossRef PubMed Google Scholar
-
13.Newman DJ, Cragg GM. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J Nat Prod 2020;83: 770-803. CrossRef PubMed Google Scholar
-
14.Atanasov AG, Zotchev SB, Dirsch VM, Supuran CT. Natural products in drug discovery: advances and opportunities. Nat Rev Drug Discov 2021;20: 200-16. CrossRef PubMed Google Scholar
-
15.Lalchhandama K. History of penicillin. Wiki J Med 2021;8: 1-16. CrossRef PubMed Google Scholar
-
16.Tiwari P, Bae H. Endophytic fungi: key insights, emerging prospects, and challenges in natural product drug discovery. Microorganisms 2022;10: 360. CrossRef PubMed Google Scholar
-
17.Gakuubi MM, Munusamy M, Liang Z-X, Ng SB. Fungal endophytes: a promising frontier for discovery of novel bioactive compounds. J Fungi 2021;7: 786. CrossRef PubMed Google Scholar
-
18.Frisvad JC, Larsen TO. Chemodiversity in the genus Aspergillus. Appl Microbiol Biotechnol 2015;99: 7859-77. CrossRef PubMed Google Scholar
-
19.Zhang X, Li Z, Gao J. Chemistry and biology of secondary metabolites from Aspergillus genus. Nat Prod J 2018;8: 275-304. CrossRef PubMed Google Scholar
-
20.Boruta T, Milczarek I, Bizukojc M. Evaluating the outcomes of submerged co-cultivation: production of lovastatin and other secondary metabolites by Aspergillus terreus in fungal co-cultures. Appl Microbiol Biotechnol 2019;103: 5593-605. CrossRef PubMed Google Scholar
-
21.El-hawary SS, Moawad AS, Bahr HS, Ramadan Abdelmohsen U, Mohammed R. Natural product diversity from the endophytic fungi of the genus Aspergillus. RSC Adv 2020;10: 22058-79. CrossRef PubMed Google Scholar
-
22.Sun Y, Liu W-C, Shi X, Zheng H-Z, Zheng Z-H, Lu X-H, et al. Inducing secondary metabolite production of Aspergillus sydowii through microbial co-culture with Bacillus subtilis. Microb Cell Fact 2021;20: 42. CrossRef PubMed Google Scholar
-
23.Yao F-H, Liang X, Cheng X, Ling J, Dong J-D, Qi S-H. Antifungal peptides from the marine gorgonian-associated fungus Aspergillus sp. SCSIO41501. Phytochemistry 2021;192: 112967. CrossRef PubMed Google Scholar
-
24.He W, Xu Y, Wu D, Wang D, Gao H, Wang L, et al. New alkaloids from the diversity-enhanced extracts of an endophytic fungus Aspergillus flavus GZWMJZ-288. Bioorg Chem 2021;107: 104623. CrossRef PubMed Google Scholar
-
25.Fang S-T, Liu X-H, Yan B-F, Miao F-P, Yin X-L, Li W-Z, et al. Terpenoids from the marine-derived fungus Aspergillus sp. RR-YLW-12, associated with the red alga Rhodomela confervoides. J Nat Prod 2021;84: 1763-71. CrossRef PubMed Google Scholar
-
26.Yang W, Chen X, Li Y, Guo S, Wang Z, Yu X. Advances in pharmacological activities of terpenoids. Nat Prod Comm 2020;15: 1934578X20903555. CrossRef PubMed Google Scholar
-
27.Dong L-M, Huang L-L, Dai H, Xu Q-L, Ouyang J-K, Jia X-C, et al. Anti-MRSA sesquiterpenes from the semi-mangrove plant Myoporum bontioides A Gray. Mar Drugs 2018;16: 438. CrossRef PubMed Google Scholar
-
28.Azuama OC, Ortiz S, Quirós-Guerrero L, Bouffartigues E, Tortuel D, Maillot O, et al. Tackling Pseudomonas aeruginosa virulence by mulinane-like diterpenoids from Azorella atacamensis. Biomolecules 2020;10: 1626. CrossRef PubMed Google Scholar
-
29.Johansen B, Duval RE, Sergere J-C. First evidence of a combination of terpinen-4-ol and α-terpineol as a promising tool against ESKAPE pathogens. Molecules 2022;27: 7472. CrossRef PubMed Google Scholar
-
30.Jiang M, Wu Z, Liu L, Chen S. The chemistry and biology of fungal meroterpenoids (2009–2019). Org Biomol Chem 2021;19: 1644-704. CrossRef PubMed Google Scholar
-
31.Gozari M, Alborz M, El-Seedi HR, Jassbi AR. Chemistry, biosynthesis and biological activity of terpenoids and meroterpenoids in bacteria and fungi isolated from different marine habitats. Eur J Med Chem 2021;210: 112957. CrossRef PubMed Google Scholar
-
32.Sun L, Wang H, Yan M, Sai C, Zhang Z. Research advances of bioactive sesquiterpenoids isolated from marine-derived Aspergillus sp. Molecules 2022;27: 7376. CrossRef PubMed Google Scholar
-
33.Zhao W-Y, Yi J, Chang Y-B, Sun C-P, Ma X-C. Recent studies on terpenoids in Aspergillus fungi: chemical diversity, biosynthesis, and bioactivity. Phytochemistry 2022;193: 113011. CrossRef PubMed Google Scholar
-
34.Abdel-Wahab NM, Scharf S, Özkaya FC, Kurtán T, Mándi A, Fouad MA, et al. Induction of secondary metabolites from the marine-derived fungus Aspergillus versicolor through co-cultivation with Bacillus subtilis. Planta Med 2019;85: 503-12. CrossRef PubMed Google Scholar
-
35.Aloke C, Achilonu I. Coping with the ESKAPE pathogens: evolving strategies, challenges and future prospects. Microb Pathog 2023;175: 105963. CrossRef PubMed Google Scholar
-
36.Garcia J, Rodrigues F, Castro F, Aires A, Marques G, Saavedra MJ. Antimicrobial, antibiofilm, and antioxidant properties of Boletus edulis and Neoboletus luridiformis against multidrug-resistant ESKAPE pathogens. Front Nutr. 2022. https://doi.org/10.3389/fnut.2021.773346. PubMed Google Scholar
-
37.Jadimurthy R, Mayegowda SB, Nayak SC, Mohan CD, Rangappa KS. Escaping mechanisms of ESKAPE pathogens from antibiotics and their targeting by natural compounds. Biotechnol Rep 2022;34: e00728. CrossRef PubMed Google Scholar
-
38.Venkateswaran P, Vasudevan S, David H, Shaktivel A, Shanmugam K, Neelakantan P, et al. Revisiting ESKAPE pathogens: virulence, resistance, and combating strategies focusing on quorum sensing. Front Cell Infect Microbiol 2023;13: 1159798. CrossRef PubMed Google Scholar
-
39.David S, Reuter S, Harris SR, Glasner C, Feltwell T, Argimon S, et al. Epidemic of carbapenem-resistant Klebsiella pneumoniae in Europe is driven by nosocomial spread. Nat Microbiol 2019;4: 1919-29. CrossRef PubMed Google Scholar
-
40.Hamidian M, Nigro SJ. Emergence, molecular mechanisms and global spread of carbapenem-resistant Acinetobacter baumannii. Microbial Genomics 2019;5: e000306. CrossRef PubMed Google Scholar
-
41.Lin K-Y, Lauderdale T-L, Wang J-T, Chang S-C. Carbapenem-resistant Pseudomonas aeruginosa in Taiwan: prevalence, risk factors, and impact on outcome of infections. J Microbiol Immunol Infect 2016;49: 52-9. CrossRef PubMed Google Scholar
-
42.Hsu L-Y, Apisarnthanarak A, Khan E, Suwantarat N, Ghafur A, Tambyah PA. Carbapenem-resistant Acinetobacter baumannii and Enterobacteriaceae in South and Southeast Asia. Clin Microbiol Rev 2016;30: 1-22. CrossRef PubMed Google Scholar
-
43.Cheng X, Ma L, Wang Y, Sun W, Su J. Prevalence and molecular characteristics of heterogeneous vancomycin intermediate Staphylococcus aureus in a tertiary care center of northern China. Diagn Microbiol Infect Dis 2024;108: 116180. CrossRef PubMed Google Scholar
-
44.Khan M, Summers S, Rice SA, Stapleton F, Willcox MDP, Subedi D. Acquired fluoroquinolone resistance genes in corneal isolates of Pseudomonas aeruginosa. Infect Genet Evol 2020;85: 104574. CrossRef PubMed Google Scholar
-
45.Steinbach WJ. Aspergillus species. In: Principles and practice of pediatric infectious diseases. Elsevier; 2023. p. 1262- 1268. e2. https://doi.org/10.1016/B978-0-323-75608-2.00244-5. PubMed Google Scholar
-
46.Fisher MC, Gurr SJ, Cuomo CA, Blehert DS, Jin H, Stukenbrock EH, et al. Threats posed by the fungal kingdom to humans, wildlife, and agriculture. MBio. 2020. https://doi.org/10.1128/mbio.00449-20. PubMed Google Scholar
-
47.Taniwaki MH, Pitt JI, Magan N. Aspergillus species and mycotoxins: occurrence and importance in major food commodities. Curr Opin Food Sci 2018;23: 38-43. CrossRef PubMed Google Scholar
-
48.Claeys L, Romano C, De Ruyck K, Wilson H, Fervers B, Korenjak M, et al. Mycotoxin exposure and human cancer risk: a systematic review of epidemiological studies. Comprehensive Rev Food Sci Food Safety 2020;19: 1449-64. CrossRef PubMed Google Scholar
-
49.Cairns TC, Nai C, Meyer V. How a fungus shapes biotechnology: 100 years of Aspergillus niger research. Fungal Biol Biotechnol 2018;5: 13. CrossRef PubMed Google Scholar
-
50.Hillier SG, Lathe R. Terpenes, hormones and life: isoprene rule revisited. J Endocrinol 2019;242: R9-22. CrossRef PubMed Google Scholar
-
51.Luo P, Huang J-H, Lv J-M, Wang G-Q, Hu D, Gao H. Biosynthesis of fungal terpenoids. Nat Prod Rep. 2024. https://doi.org/10.1039/D3NP00052D. PubMed Google Scholar
-
52.Ruzicka L. The isoprene rule and the biogenesis of terpenic compounds. Experientia 1953;9: 357-67. CrossRef PubMed Google Scholar
-
53.Li X-D, Li X-M, Yin X-L, Li X, Wang B-G. Antimicrobial sesquiterpenoid derivatives and monoterpenoids from the deep-sea sediment-derived fungus Aspergillus versicolor SD-330. Mar Drugs 2019;17: 563. CrossRef PubMed Google Scholar
-
54.Pan G, Li Y, Che X, Tian D, Han W, Wang Z, et al. New thio-compounds and monoterpenes with anti-inflammatory activities from the fungus Aspergillus sp. CYH26. Front Microbiol. 2021. https://doi.org/10.3389/fmicb.2021.668938. PubMed Google Scholar
-
55.Ding H, Wang J-P, Deng S-P, Gan J-L, Li B-X, Yao L-L, et al. A new sesquiterpenoid from the aconitum-derived fungus Aspergillus fumigatus M1. Nat Prod Res 2023;37: 3443-51. CrossRef PubMed Google Scholar
-
56.Chen Y, Zhu H-Y, Xu L-C, Wang S-P, Liu S, Liu G-D, et al. Antimicrobial and cytotoxic phenolic bisabolane sesquiterpenoids from the fungus Aspergillus flavipes 297. Fitoterapia 2021;155: 105038. CrossRef PubMed Google Scholar
-
57.Liu Y-P, Fang S-T, Wang B-G, Ji N-Y. Phenol derivatives from the cold-seep fungus Aspergillus sydowii 10–31. Phytochem Lett 2022;52: 63-6. CrossRef PubMed Google Scholar
-
58.Yang X, Yu H, Ren J, Cai L, Xu L, Liu L. Sulfoxide-containing bisabolane sesquiterpenoids with antimicrobial and nematicidal activities from the marine-derived fungus Aspergillus sydowii LW09. J Fungi 2023;9: 347. CrossRef PubMed Google Scholar
-
59.Jiang Y, Cui C, Chen C, Wang N, Liao H, Li Q, et al. Two pairs of new bisabolane-type sesquiterpenoids from Aspergillus sydowii. Chem Biodivers 2023;20: e202301047. CrossRef PubMed Google Scholar
-
60.Pang X, Lin X, Zhou X, Yang B, Tian X, Wang J, et al. New quinoline alkaloid and bisabolane-type sesquiterpenoid derivatives from the deep-sea-derived fungus Aspergillus sp. SCSIO06786. Fitoterapia 2020;140: 104406. CrossRef PubMed Google Scholar
-
61.Li X-D, Li X, Li X-M, Yin X-L, Wang B-G. Antimicrobial bisabolane-type sesquiterpenoids from the deep-sea sediment-derived fungus Aspergillus versicolor SD-330. Nat Prod Res 2021;35: 4265-71. CrossRef PubMed Google Scholar
-
62.Liu N, Peng S, Yang J, Cong Z, Lin X, Liao S, et al. Structurally diverse sesquiterpenoids and polyketides from a sponge-associated fungus Aspergillus sydowii SCSIO41301. Fitoterapia 2019;135: 27-32. CrossRef PubMed Google Scholar
-
63.Zhang X, Dong Y, Liu X, Wang R, Lu J, Song F. New bisabolane-type sesquiterpenoid from Aspergillus sydowii BTBU20213012. Nat Prod Res. 2023. https://doi.org/10.1080/14786419.2023.2236764. PubMed Google Scholar
-
64.Yu H-Y, Chen Y-S, Wang Y, Zou Z-B, Xie M-M, Li Y, et al. Anti-necroptosis and anti-ferroptosis compounds from the deep-sea-derived fungus Aspergillus sp. MCCC 3A00392. Bioorg Chem 2024;144: 107175. CrossRef PubMed Google Scholar
-
65.Jiang Y, Chen C, Zhu H, Li Q, Mao L, Liao H, et al. An indole diketopiperazine alkaloid and a bisabolane sesquiterpenoid with unprecedented skeletons from Aspergillus fumigatus. Org Biomol Chem 2023;21: 2236-42. CrossRef PubMed Google Scholar
-
66.Liu Y-F, Yue Y-F, Feng L-X, Zhu H-J, Cao F. Asperienes A-D, Bioactive sesquiterpenes from the marine-derived fungus Aspergillus flavus. Mar Drugs 2019;17: 550. CrossRef PubMed Google Scholar
-
67.Lacey HJ, Gilchrist CLM, Crombie A, Kalaitzis JA, Vuong D, Rutledge PJ, et al. Nanangenines: drimane sesquiterpenoids as the dominant metabolite cohort of a novel Australian fungus, Aspergillus nanangensis. Beilstein J Org Chem 2019;15: 2631-43. CrossRef PubMed Google Scholar
-
68.Fang S-T, Shi Z-Z, Song Y-P, Yin X-L, Ji N-Y. New ophiobolin sesterterpenoid and drimane sesquiterpenoids from a marine-alga-derived fungus Aspergillus sp. Fitoterapia 2023;170: 105659. CrossRef PubMed Google Scholar
-
69.Li H, Zhang R, Cao F, Wang J, Hu Z, Zhang Y. Proversilins A-E, drimane-type sesquiterpenoids from the endophytic Aspergillus versicolor. J Nat Prod 2020;83: 2200-6. CrossRef PubMed Google Scholar
-
70.Salman K, Zhu H, Sun Z, Li Y, Wang L, Wang R, et al. Seven drimane-type sesquiterpenoids from an earwig-associated Aspergillus sp. Chin J Nat Med 2023;21: 58-64. CrossRef PubMed Google Scholar
-
71.Li F, Mo S, Yin J, Zhang S, Gu S, Ye Z, et al. Structurally diverse metabolites from a soil-derived fungus Aspergillus calidoustus. Bioorg Chem 2022;127: 105988. CrossRef PubMed Google Scholar
-
72.An C-L, Kong F-D, Li Y, Ma Q-Y, Xie Q-Y, Yuan J-Z, et al. Asperpenes D and E from the fungus Aspergillus sp. SCS-KFD66 isolated from a bivalve mollusk, Sanguinolaria chinensis. J Asian Nat Prod Res 2021;23: 117-22. CrossRef PubMed Google Scholar
-
73.Niu S, Huang S, Wang J, He J, Chen M, Zhang G, et al. Malfilanol C, a new sesquiterpenoid isolated from the deep-sea-derived Aspergillus puniceus A2 fungus. Nat Prod Res 2024;38: 1362-8. CrossRef PubMed Google Scholar
-
74.Shang R-Y, Cui J, Li J-X, Miao X-X, Zhang L, Xie D-D, et al. Nigerin and ochracenes J−L, new sesquiterpenoids from the marine sponge symbiotic fungus Aspergillus niger. Tetrahedron 2022;104: 132599. CrossRef PubMed Google Scholar
-
75.Zang Y, Zhou B, Wei M, Shi Z, Feng G, Deng M, et al. Aureoterrolides B-M: eremophilane-type sesquiterpenoids isolated from Aspergillus aureoterreus and their cytotoxicity. Phytochem 2022;202: 113294. CrossRef PubMed Google Scholar
-
76.Liu Z, Zhao J-Y, Sun S-F, Li Y, Qu J, Liu H-T, et al. Sesquiterpenes from an endophytic Aspergillus flavus. J Nat Prod 2019;82: 1063-71. CrossRef PubMed Google Scholar
-
77.Chen W, Chen C, Long J, Lan S, Lin X, Liao S, et al. Bioactive secondary metabolites from the deep-sea derived fungus Aspergillus sp. SCSIO 41029. J Antibiot 2021;74: 156-9. CrossRef PubMed Google Scholar
-
78.Zhou G, Sun C, Hou X, Che Q, Zhang G, Gu Q, et al. Ascandinines A-D, indole diterpenoids, from the sponge-derived fungus Aspergillus candidus HDN15-152. J Org Chem 2021;86: 2431-6. CrossRef PubMed Google Scholar
-
79.Li W, Yi G, Lin K, Chen G, Hui Y, Chen W. Cytotoxic indole diterpenoids from a Sphagneticola trilobata-derived fungus Aspergillus sp. PQJ-1. Molecules 2023;28: 7003. CrossRef PubMed Google Scholar
-
80.Zhang F, Yang L, Xie Q-Y, Guo J-C, Ma Q-Y, Dai L-T, et al. Diverse indole-diterpenoids with protein tyrosine phosphatase 1B inhibitory activities from the marine coral-derived fungus Aspergillus sp. ZF-104. Phytochem 2023;216: 113888. CrossRef PubMed Google Scholar
-
81.Chaiyosang B, Kanokmedhakul K, Yodsing N, Boonlue S, Yang J-X, Wang YA, et al. Three new indole diterpenoids from Aspergillus aculeatus KKU-CT2. Nat Prod Res 2022;36: 4973-81. CrossRef PubMed Google Scholar
-
82.Elsbaey M, Tanaka C, Miyamoto T. New secondary metabolites from the mangrove endophytic fungus Aspergillus versicolor. Phytochem Lett 2019;32: 70-6. CrossRef PubMed Google Scholar
-
83.Moussa AY, Sobhy HA, Eldahshan OA, Singab ANB. Caspicaiene: a new kaurene diterpene with anti-tubercular activity from an Aspergillus endophytic isolate in Gleditsia caspia desf. Nat Prod Res 2021;35: 5653-64. CrossRef PubMed Google Scholar
-
84.Han J, Lu F, Bao L, Wang H, Chen B, Li E, et al. Terphenyl derivatives and terpenoids from a wheat-born mold Aspergillus candidus. J Antibiot 2020;73: 189-93. CrossRef PubMed Google Scholar
-
85.Yang W, Chen T, Chen Y, Tan Q, Ou Y, Li G, et al. Antiplasmodial asperterpenoids from two Aspergillus oryzae transformants with heterologous expression of sesterterpene genes. J Org Chem 2022;87: 16807-19. CrossRef PubMed Google Scholar
-
86.Li Y-L, Gao Y, Liu C-Y, Sun C-J, Zhao Z-T, Lou H-X. Asperunguisins A-F, cytotoxic asperane sesterterpenoids from the endolichenic fungus Aspergillus unguis. J Nat Prod 2019;82: 1527-34. CrossRef PubMed Google Scholar
-
87.Choi B-K, Trinh PTH, Lee H-S, Choi B-W, Kang JS, Ngoc NTD, et al. New ophiobolin derivatives from the marine fungus Aspergillus flocculosus and their cytotoxicities against cancer cells. Mar Drugs 2019;17: 346. CrossRef PubMed Google Scholar
-
88.Chi L-P, Li X-M, Wan Y-P, Li X, Wang B-G. Ophiobolin sesterterpenoids and farnesylated phthalide derivatives from the deep sea cold-seep-derived fungus Aspergillus insuetus SD-512. J Nat Prod 2020;83: 3652-60. CrossRef PubMed Google Scholar
-
89.Fu A, Chen C, Li Q, Ding N, Dong J, Chen Y, et al. Niduenes A−F, six functionalized sesterterpenoids with a pentacyclic 5/5/5/5/6 skeleton from endophytic fungus Aspergillus nidulans. Chin Chem Lett. 2023. https://doi.org/10.1016/j.cclet.2023.109100. PubMed Google Scholar
-
90.Li Q, Chen C, Wei M, Dai C, Cheng L, Tao J, et al. Niduterpenoids A and B: two sesterterpenoids with a highly congested hexacyclic 5/5/5/5/3/5 ring system from the fungus Aspergillus nidulans. Org Lett 2019;21: 2290-3. CrossRef PubMed Google Scholar
-
91.Wei M, Zhou P, Huang L, Yin J, Li Q, Dai C, et al. Spectanoids A−H: eight undescribed sesterterpenoids from Aspergillus spectabilis. Phytochem 2021;191: 112910. CrossRef PubMed Google Scholar
-
92.Li Q, Zheng Y, Fu A, Wei M, Kang X, Chen C, et al. 30-norlanostane triterpenoids and steroid derivatives from the endophytic fungus Aspergillus nidulans. Phytochem 2022;201: 113257. CrossRef PubMed Google Scholar
-
93.Liu L, Ni H-F, Qiu X, Wan L, Zhao M. Cytotoxic tetracyclic triterpenes from the endophytic fungus Aspergillus fumigatus of Cleidion brevipetiolatum. Phytochem Lett 2021;44: 87-9. CrossRef PubMed Google Scholar
-
94.Qin F, Luo L, Liu Y-C, Bo X-L, Wu F-R, Wang F-F, et al. Diisoprenyl-cyclohexene-type meroterpenoids from a mangrove endophytic fungus Aspergillus sp. GXNU-Y65 and their anti-nonalcoholic steatohepatitis activity in AML12 cells. Phytochem 2024;218: 113955. CrossRef PubMed Google Scholar
-
95.Bo X, Zhao Y, Qin F, Wu F, Tan M, Ju S, et al. Cytotoxic metabolites from the mangrove endophytic fungus Aspergillus sp. GXNU QG1a. Nat Prod Res. 2024. https://doi.org/10.1080/14786419.2024.2303601. PubMed Google Scholar
-
96.Antipova TV, Zaitsev KV, Oprunenko YF, Ya. Zherebker A, Rystsov GK, Zemskova MY, et al. Austalides V and W, new meroterpenoids from the fungus Aspergillus ustus and their antitumor activities. Bioorg Med Chem Lett 2019;29: 126708. CrossRef PubMed Google Scholar
-
97.Elnaggar M, Elissawy AM, Youssef FS, Kicsák M, Kurtán T, Singab ANB, et al. Austalide derivative from marine-derived Aspergillus sp. and evaluation of its cytotoxic and ADME/TOPKAT properties. RSC Adv 2023;13: 16480-7. CrossRef PubMed Google Scholar
-
98.Cen S, Jia J, Ge Y, Ma Y, Li X, Wei J, et al. A new antibacterial 3, 5-dimethylorsellinic acid-based meroterpene from the marine fungus Aspergillus sp. CSYZ-1. Fitoterapia 2021;152: 104908. CrossRef PubMed Google Scholar
-
99.Mo S, Yin J, Ye Z, Li F, Lin S, Zhang S, et al. Asperanstinoids A-E: undescribed 3, 5-dimethylorsellinic acid-based meroterpenoids from Aspergillus calidoustus. Phytochem 2021;190: 112892. CrossRef PubMed Google Scholar
-
100.Wen H, Yang X, Liu Q, Li S, Li Q, Zang Y, et al. Structurally diverse meroterpenoids from a marine-derived Aspergillus sp. fungus. J Nat Prod 2020;83: 99-104. CrossRef PubMed Google Scholar
-
101.Tang Y, Liu Y, Ruan Q, Zhao M, Zhao Z, Cui H. Aspermeroterpenes A-C: three meroterpenoids from the marine-derived fungus Aspergillus terreus GZU-31-1. Org Lett 2020;22: 1336-9. CrossRef PubMed Google Scholar
-
102.Li H-L, Li X-M, Li X, Yang S-Q, Wang B-G. Structure, absolute configuration and biological evaluation of polyoxygenated meroterpenoids from the marine algal-derived Aspergillus terreus EN-539. Phytochem Lett 2019;32: 138-42. CrossRef PubMed Google Scholar
-
103.Tang Y, Chen X, Zhou Y, Zhao M, He J, Liu Y, et al. Furanaspermeroterpenes A and B, two unusual meroterpenoids with a unique 6/6/6/5/5 pentacyclic skeleton from the marine-derived fungus Aspergillus terreus GZU-31-1. Bioorg Chem 2021;114: 105111. CrossRef PubMed Google Scholar
-
104.Tang Y, Li Y, Zhao M, Ruan Q, Liu Y, Li C, et al. Furanasperterpenes A and B, two meroterpenoids with a novel 6/6/6/6/5 ring system from the marine-derived fungus Aspergillus terreus GZU-31-1. Bioorg Chem 2020;100: 103968. CrossRef PubMed Google Scholar
-
105.Hamed A, Abdel-Razek AS, Omran DA, El-Metwally MM, El-Hosari DG, Frese M, et al. Terretonin O: a new meroterpenoid from Aspergillus terreus. Nat Prod Res 2020;34: 965-74. CrossRef PubMed Google Scholar
-
106.Li X, Li L, Li X-M, Li H-L, Konuklugil B, Wang B-G. Ustusaustin A: a new neuraminidase inhibitory meroterpene from the ascidian-derived endophytic fungus Aspergillus ustus TK-5. Nat Prod Res 2021;35: 4939-44. CrossRef PubMed Google Scholar
-
107.Yan D, Matsuda Y. Genome mining-driven discovery of 5-methylorsellinate-derived meroterpenoids from Aspergillus funiculosus. Org Lett 2021;23: 3211-5. CrossRef PubMed Google Scholar
-
108.Wu T, Salim AA, Capon RJ. Millmerranones A-F: a Meroterpene cyclic carbonate and related metabolites from the Australian fungus Aspergillus sp. CMB-MRF324. Org Lett 2021;23: 8424-8. CrossRef PubMed Google Scholar
-
109.Deng M, Tan X, Qiao Y, Sun W, Xie S, Shi Z, et al. New secondary metabolites from the endophytic fungus Aspergillus sp. from Tripterygium wilfordii. Nat Prod Res 2022;36: 3544-52. CrossRef PubMed Google Scholar
-
110.Mahizan NA, Yang S-K, Moo C-L, Song AA-L, Chong C-M, Chong C-W, et al. Terpene derivatives as a potential agent against antimicrobial resistance (AMR) pathogens. Molecules 2019;24: 2631. CrossRef PubMed Google Scholar
-
111.Sharma A, Biharee A, Kumar A, Jaitak V. Antimicrobial terpenoids as a potential substitute in overcoming antimicrobial resistance. Curr Drug Targets 2020;21: 1476-94. CrossRef PubMed Google Scholar
-
112.Aghaei Gharehbolagh S, Izadi A, Talebi M, Sadeghi F, Zarrinnia A, Zarei F, et al. New weapons to fight a new enemy: a systematic review of drug combinations against the drug-resistant fungus Candida auris. Mycoses 2021;64: 1308-16. CrossRef PubMed Google Scholar
-
113.Sieniawska E, Swatko-Ossor M, Sawicki R, Skalicka-Woźniak K, Ginalska G. Natural terpenes influence the activity of antibiotics against isolated Mycobacterium tuberculosis. Med Princ Pract 2016;26: 108-12. CrossRef PubMed Google Scholar
-
114.Zacchino SA, Butassi E, Cordisco E, Svetaz LA. Hybrid combinations containing natural products and antimicrobial drugs that interfere with bacterial and fungal biofilms. Phytomed 2017;37: 14-26. CrossRef PubMed Google Scholar
-
115.Hong B, Luo T, Lei X. Late-stage diversification of natural products. ACS Cent Sci 2020;6: 622-35. CrossRef PubMed Google Scholar
-
116.Jansen DJ, Shenvi RA. Synthesis of medicinally relevant terpenes: reducing the cost and time of drug discovery. Future Med Chem 2014;6: 1127-48. CrossRef PubMed Google Scholar
-
117.Akunuri R, Unnissa T, Vadakattu M, Bujji S, Mahammad Ghouse S, Madhavi Yaddanapudi V, et al. Bacterial pyruvate kinase: a new potential target to combat drug-resistant Staphylococcus aureus infections. ChemistrySelect 2022;7: e202201403. CrossRef PubMed Google Scholar
-
118.Jackson E, Dowd C. Inhibition of 1-Deoxy-D-Xylulose-5-Phosphate Reductoisomerase (Dxr): a review of the synthesis and biological evaluation of recent inhibitors. Curr Topics Med Chem 2012;12: 706-28. CrossRef PubMed Google Scholar
-
119.Xiao B, Shi G, Chen X, Yan H, Ji X. Crystal structure of 6-hydroxymethyl-7, 8-dihydropterin pyrophosphokinase, a potential target for the development of novel antimicrobial agents. Structure 1999;7: 489-96. CrossRef PubMed Google Scholar
Copyright information
© The Author(s) 2025
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.