Structure–function insights of natural Ganoderma polysaccharides: advances in biosynthesis and functional food applications
Abstract
Ganoderma polysaccharides (GPs), derived from various species of the Ganoderma genus, exhibit diverse bioactivities, including immune modulation, anti-tumor effects, and gut microbiota regulation. These properties position GPs as dual-purpose agents for medicinal and functional food development. This review comprehensively explores the structural complexity of six key GPs and their specific mechanisms of action, such as TLR signaling in immune modulation, apoptosis pathways in anti-tumor activity, and their prebiotic effects on gut microbiota. Additionally, the structure–activity relationships (SARs) of GPs are highlighted to elucidate their biological efficacy. Advances in green extraction techniques, including ultrasonic-assisted and enzymatic methods, are discussed for their roles in enhancing yield and aligning with sustainable production principles. Furthermore, the review addresses biotechnological innovations in polysaccharide biosynthesis, improving production efficiency and making large-scale production feasible. These insights, combined with ongoing research into their bioactivity, provide a solid foundation for developing health-promoting functional food products that incorporate GPs. Furthermore, future research directions are suggested to optimize biosynthesis pathways and fully harness the health benefits of these polysaccharides.Graphical Abstract

Keywords
Ganoderma polysaccharides extraction techniques structural characteristics Bioactivity biosynthetic pathways Functional food applications1 Introduction
Ganoderma spp., a medicinal fungus belonging to the Basidiomycota division and Polyporaceae family, has been extensively studied due to its remarkable pharmacological properties. In traditional Chinese medicine (TCM), Ganoderma has long been regarded as a valuable medicinal herb, with records in ancient texts such as Shen Nong Ben Cao Jing (The Divine Farmer's Materia Medica), where it is noted for its ability to alleviate fatigue, treat respiratory ailments, and improve gastrointestinal health. In recent years, as the global influence of TCM has expanded, particularly in Europe and North America, the therapeutic value of Ganoderma has gained increasing recognition beyond Asia [1]. Modern pharmacological research has revealed that Ganoderma contains a wide range of bioactive compounds, including polysaccharides, triterpenes, sterols, peptides, and fatty acids [2]. These compounds exhibit a broad spectrum of biological activities, further reinforcing the global prominence of Ganoderma in pharmacopeias [3]. Among these, GPs have garnered significant attention due to their complex structures and diverse bioactivities, including immune modulation, anti-tumor effects, regulation of gut microbiota, and antioxidant properties [4]. GPs are now considered critical components in modern medicine and nutrition. According to recent market research, the global Ganoderma extract market is projected to reach USD 6.86 billion by 2024, with an estimated increase to USD 10.58 billion by 2029, representing a compound annual growth rate (CAGR) of 9.04% (Mordor Intelligence, 2024). This significant market growth reflects the rising demand for natural health-promoting products and highlights the immense potential of GPs for applications in functional foods, positioning them as promising candidates for dietary supplements, functional beverages, and health snacks [5].
The Ganoderma genus consists of over 200 species worldwide, with G. lucidum, G. sinense, G. tsugae, G. applanatum, G. atrum, and G. leucocontextum being the most studied for their polysaccharide [6]. Polysaccharides from these species are extracted using a variety of techniques, including hot water extraction, enzymatic extraction, ultrasonic-assisted extraction, and supercritical fluid extraction, resulting in diverse polysaccharide structures [7]. These polysaccharides exhibit complex structural characteristics, including high molecular weights ranging from several thousand to several million daltons, diverse monosaccharide compositions, various glycosidic linkages, and intricate spatial conformations [8, 9]. The complexity of these structures primarily arises from the biosynthetic pathways of GPs, where glycosyltransferases and glycosyl hydrolases polymerize nucleotide-sugar precursors to form the polysaccharide backbone, which is then modified by the addition of different monosaccharide units and branching structures through the action of other related enzymes [10]. The diversity in structure directly contributes to the wide range of biological activities exhibited by GPs, including immunomodulatory, anti-tumor, gut microbiota regulation, antioxidant, anti-inflammatory, and hepatoprotective effects [11–16]. Of all the species, G. lucidum has garnered the most attention, with reviews systematically summarizing its polysaccharide structures and biological functions, making it the most comprehensively studied species in the genus [17–19]. While reviews have made significant contributions, they tend to focus on specific aspects, such as polysaccharide structures or broad biological activity listings, lacking a comprehensive integration of structural characteristics, structure–activity relationships, biosynthetic pathways, and their potential applications in medicinal and functional food development.
A comprehensive investigation into the chemical structures, biological activities, biosynthetic pathways, and potential applications of polysaccharides derived from six Ganoderma species is essential for driving progress in this field. This review explores their mechanisms of interaction in immunomodulation, gut health regulation, and anti-tumor activity, alongside their structure–activity relationships. Moreover, the review systematically addresses recent biotechnological advancements, particularly the breakthroughs in overexpression of key biosynthetic genes, which have significantly enhanced the production capacity of Ganoderma polysaccharides, making large-scale application in both medicinal and functional food development.
2 Extraction and structural characteristics of GPs
2.1 Extraction of GPs
2.1.1 Content variation of GPs
G. lucidum is rich in various chemical constituents, including 0.7–1.8% ash, 21.83–27.78% crude fiber, 3.0–5.8% fat, 13.3–23.6% protein, 42.8–82.3% carbohydrates [20]. The contents of GPs undergo substantial dynamic variations depending on the growth stage and the specific part of the organism, including the mycelium, fruiting body, and spores. Mid-infrared (Mid-IR) and near-infrared (NIR) spectroscopy were used to assess polysaccharide content in Ganoderma mycelium from various sources, with contents ranging from 0.6% to 11.3% [21]. Four different Ganoderma strains grown on various substrates were investigated, and the polysaccharide content was found to range from 18.45 to 112.82 mg/g of dry weight [22]. Subsequently, The total polysaccharide content of G. lucidum at six different growth stages was systematically assessed using the total carbohydrate assay kit [23]. The results indicated that the total polysaccharide content gradually increased from stages S1 to S3 (2.5–3.2%), suggesting that this stage is critical for polysaccharide accumulation. The highest polysaccharide content in G. tsugae was observed during the early developmental stage, particularly at primordium formation (S1, 1.5%), with the content gradually decreasing as the growth stages progressed [24]. These findings suggest that the early growth stages of Ganoderma, particularly stages S1 to S3, are crucial for polysaccharide accumulation. Moreover, Ganoderma samples from different regions in China showed that regional differences significantly influence the structure and content of their structural types. Specifically, β-1,3-glucan and β-1,3,6-glucan were identified as the primary structural components, with polysaccharide content ranging from 5.5 to 18.5 mg/g dry weight [25]. Recent studies employing hyperspectral imaging (HSI) technology in conjunction with machine learning models have facilitated the non-destructive detection of polysaccharide content in Ganoderma fruiting bodies [26]. These technologies not only improve the accuracy of polysaccharide content prediction but also provide real-time monitoring tools for determining the optimal harvest time for Ganoderma.
2.1.2 Hot water and alkaline extraction
Polysaccharides serve as a crucial bioactive component within Ganoderma, thereby underscoring the necessity for their efficient extraction for subsequent functional applications. The detailed extraction procedures are presented in Table 1. Hot water extraction (HWE) is one of the most common methods for extracting GPs. HWE for polysaccharides involves using high temperatures to disrupt the cell walls through thermal expansion and contraction [27]. This process allows the polysaccharides to be released from the cell matrix and dissolved into the surrounding hot water. The extraction process of GPs, typically conducted at 50 ℃–100 ℃ for 1.5–5 h with 2–3 repetitions, is a relatively simple method conducive to large-scale production [28]. However, this process is characterized by its low efficiency, with yields ranging from 0.21% to 2.36% [25, 29]. While optimizing extraction conditions (e.g. elevated temperature, extended duration) can augment yield, it simultaneously results in increased energy consumption. Additionally, temperatures above 150 ℃ for more than 15 min induce a conformational change in the β-glucan structure that may result in reduced biological activity [7, 30]. Alkaline extraction (AE) uses chemical reagents, including NaOH, KOH, to break down cell walls and increase the yield of polysaccharides [31]. This procedure is commonly conducted after HWE. Alkali-soluble polysaccharides from Ganoderma were extracted using 6–8% NaOH, resulting in a significant yield increase ranging from 2.1% to 8.2% [32, 33]. Scanning electron microscopy (SEM) observations revealed that alkaline treatment disrupts the fibrous structure of Ganoderma cell walls, facilitating the release of polysaccharides [34, 35].
Extraction methods and parameters of polysaccharides extraction from Ganoderma spp
2.1.3 Ultrasonic extraction
Ultrasonic extraction, a contemporary technique characterized by its efficiency, speed, low-temperature requirements, and solvent conservation, is frequently employed for the extraction of GPs. The ultrasonic-assisted extraction (UAE) method, optimized with extraction parameters of 40–81 ℃ and 590–600 W, effectively balances yield and antioxidant activity, producing GPs yields ranging from 0.52% to 2.7% [36–39]. Furthermore, In vitro antioxidant activity assays revealed that the polysaccharides obtained through UAE demonstrate notable DPPH radical scavenging activity, comparable to that of the traditional antioxidant vitamin C, and surpass those extracted using HWE. Moreover, UAE is often used in conjunction with other biochemical reagents to enhance yields. For instance, Ultrasonic-assisted enzymatic extraction (UAEE) was used to extract polysaccharides from Vietnamese red reishi mushrooms. [40]. The optimal conditions were identified as pH 5.5, 45 ℃ for 30 min, and 480 W, employing Viscozyme and Chitinase enzymes, which resulted in a yield of 3.2%. An ultrasonic circulation extraction technique combined with ultrafine grinding (UCE) was developed, yielding a concentration of Ganoderma lucidum polysaccharides (GLPs) at 47.87 mg/mL [41]. In tests assessing antioxidant activity, this technique demonstrated a notable DPPH radical scavenging rate of 53.63%, suggesting its considerable potential for antioxidant applications. Additionally, an ultrasonic/microwave-assisted extraction (UMAE) method was developed for GLPs. Under optimal conditions, UMAE markedly enhanced the extraction efficiency of these polysaccharides, yielding a 115.6% increase over traditional HWE and a 27.7% rise compared to UAE [42].
2.1.4 Other extraction methods
With the advancement of extraction technologies, a growing number of new methods have been applied to the extraction of GPs, leading to increased efficiency and yield [27]. A mechanochemical-assisted extraction (MCAE) technique was developed for efficiently extracting polysaccharides from Ganoderma spores [43]. This method, in comparison to the hot reflux extraction approach, not only markedly enhanced the extraction yield to 5.92% at a reduced temperature but also diminished the extraction duration by approximately 45.8%. Continuous phase transition extraction (CPTE) technology significantly outperforms traditional methods, achieving a polysaccharide extraction rate that is 3.34 times higher than HWE and 2.68 times higher than UAE [44]. Furthermore, the GPs extracted through CPTE demonstrate a higher molecular weight and significant immunoregulatory activity. Microwave-assisted extraction (MAE) has also emerged as a highly efficient method for extracting GPs, producing notable results in shorter timeframes. Studies show that MAE can enhance the extraction yield of GPs to approximately 7.7%, with extraction times reduced to nearly 90% of those required by conventional HWE [45]. The ternary deep eutectic solvents (DESs) system, composed of choline chloride, guaiacol, and lactic acid in a specific molar ratio, was optimized under carefully controlled conditions, leading to an impressive extraction efficiency for GPs, with the yield reaching as high as 9.5% [46].
The extraction of GPs is essential for their functional applications. Contemporary techniques, including UAE, Subcritical water extraction (SWE), and CPTE, markedly enhance both the yield and purity of the extracted polysaccharides compared to conventional HWE. These techniques also reduce extraction duration and minimize structural damage to maintain bioactivity, underscoring their significant potential for broader applications.
2.2 Structural characteristics of GPs
Polysaccharides have been extensively studied in various parts of Ganoderma species, including fruiting bodies, mycelium, spores, and fermentation broth [51]. These polysaccharides are primarily crude extracts, often containing heterogeneous mixtures of polysaccharides, glycoproteins, and other macromolecules [52, 53]. Among these, only a subset has been purified and structurally characterized as homogeneous polysaccharides with defined chemical structures.
As summarized in Table 2 and Fig. 1, homogeneous polysaccharides derived from Ganoderma encompass diverse structural types, such as β-D-glucans, α-D-glucans, α-D-galactans, and heteropolysaccharides [54]. Despite their structural diversity, many Ganoderma polysaccharides share conserved features, including β-(1 → 3)-D-glucan backbones with β-(1 → 6)-linked branching, which are commonly observed across different species [55]. In addition, conserved galactan backbones composed of α-(1 → 6)-linked galactose residues are also present in certain polysaccharides. These conserved motifs play critical roles in their immunomodulatory and antitumor activities [56]. However, structural diversity arises from variations in monosaccharide composition, glycosidic linkages, molecular weights, branching patterns, and stereochemical configurations. For example, polysaccharides often include both α- and β-glycosidic linkages (e.g., α-D-Glcp and β-D-Glcp), which contribute to their conformational flexibility and biological activities [57]. These structural features, both conserved motifs and diverse modifications, collectively contribute to their broad spectrum of biological activities.
Structural characterization from Ganoderma spp
The proposed chemical structures of the homopolysaccharides and heteropolysaccharides of Ganoderma spp
2.2.1 Glucans and heteroglucans
Glucans are a major class of GPs, accounting for more than 40% of the total polysaccharide content [37]. The intricate structure of glucans is driven by several key factors, including molecular weight, glycosidic types, degree of branching (DB), and spatial conformation [9]. β-D-glucans are the most common polysaccharides found in Ganoderma, with molecular weight ranging from 1 × 103 Da to 5.5 × 106 Da, primarily forming triple-helical and linear conformations, and the glycosidic types include β-(1 → 3), β-(1 → 4), and β-(1 → 6) linkages [58, 59]. The (1 → 3)-β-D-glucopyranosyl residues, which form the backbone of β-glucan, are frequently characterized. Typically, these main chains consist of 2–6 repeating units, with an average of 1–3 branches at the O-6 positions. The branching patterns include various forms of β-(1 →), β-(1 → 3), β-(1 → 4), and β-(1 → 6) glucans with 1–7 repeat units, as well as other glycosidic linkages such as β-(4 →)-D-GlcpA-(1 →) and terminal (T)-α-D-Glcp. These components collectively constitute highly branched β-D-glucans and β-D-heteroglucans [60, 61]. For instance, the GLP20 has a backbone composed of three (1 → 3)-β-D-Glcp units with branches linked at the O-6 position, forming a triple-helix conformation in water [62]. In addition to the 1,3-β-D-Glcp backbone, β-D-glucans are widely distributed in various combinations with (1 → 3), (1 → 4), and (1 → 6) linkages, exhibiting significant structural diversity and complexity. For example, the main chain of GLSWA-Ⅰ consists of 1,3-β-D-Glcp, 1,4-β-D-Glcp, and 1,6-β-D-Glcp residues, with branches composed of β-D-Glcp-(1 → 4)-β-D-Glcp-(→ and β-D-Glcp-(→ residues attached at the O-6 and O-4 positions, resulting in a DB of 0.44 [63]. The backbone of GLSA50-1B is composed of 1,6-β-D-Glcp units, interspersed with 1–7 repeating 1,4-β-D-Glcp units as side chains linked at the O-4 position [64]. Additionally, glucans comprising mixed β-D- and α-D-glucan residues have also been identified including GAP-2 and GLP-3 [57, 65]. The backbone of GLP-3 is primarily composed of α-D-glucan and β-D-glucan residues in a molar ratio of 18:1, whereas GAP-2 contains these residues in a molar ratio of 3:8. Heteroglucans, primarily composed of Glc with minor sugars such as Ara, Gal, and Xyl, have been identified in Ganoderma. A notable example is GLP-1, which features a main chain of 1,3-β-D-Glcp and 1,4-β-D-Glcp with an inserted 1,6-α-D-Galp residue. This sequence is further complicated by the presence of two intricate side chains at the O-2 and O-4 positions of the Galp residues [66]. The structural diversity of glucans, encompassing mixed β-D- and α-D-glucan residues, heteroglucans, as well as variations in glycosidic linkages, space formation, and molar ratios, highlights the intricate architecture of GPs. These intricate structural features are key to defining their unique biological activities and potential applications.
2.2.2 Galactans and heterogalactans
Galactans and heterogalactans constitute another prominent structural class of polysaccharides found in Ganoderma. The primary structural characteristic of these polysaccharides is a backbone composed of 1,6-α-D-Galp units, with side chains including various non-reducing sugars, such as T-α-L-Fuc, T-α-D-Galp, T-α-D-Manp, and T-α-D-Galp-(1 → 6)-α-D-Galp-(1 → 4)-β-D-Glcp-(1 →), attached to the O-2 positions [56, 67, 68]. A homogeneous galactan from Ganoderma fruiting bodies, identified as LZ-D-1, has a molecular weight of 2.80 × 104 Da and a backbone composed of five 1,6-α-D-Galp repeat units with T-α-L-Fucp side chains attached at the O-2 position [69]. In vitro cell assays have demonstrated that LZ-D-1 stimulates the proliferation of mouse spleen lymphocytes, indicating its potential to enhance immune activity. LZ-C-1 was characterized by a main chain of 1,3-β-D-Glcp, 1,4,6-β-D-Glcp, and 1,6-α-D-Galp residues, with T-α-L-Fucp linkages at the O-2 position of the 1,6-α-D-Galp residues forming branches [70]. Additionally, the glycopeptides found in Ganoderma feature sugar chains containing heterogalactans, with main chains composed of 1,6-α-D-Galp, 1,4,6-β-D-Glcp, and 1,4-β-D-Glcp. Examples include GL-PWQ3 and GLPCW-Ⅱ, which have molecular weights of 2.4 × 104 Da and 1.2 × 104 Da, respectively [56, 71]. The polysaccharide portion of GL-PWQ3 mainly consists of 1,6-α-D-Galp, 1,6-β-D-Glcp, and 1,4-β-D-Glcp residues, with side branches linked at the O-3 position with T-Glcp and 1,3-Glcp, and at the O-2 position with T-Fucp, T-Manp, or T-Glcp. The pronounced immunomodulatory and antioxidant properties of GL-PWQ3 highlight its therapeutic potential, further emphasizing the importance of Ganoderma-derived galactans and heterogalactans in health promotion and disease prevention.
2.2.3 Other types of polysaccharides
Galactoglucomannan was discovered with a molecular weight of 8.0 × 104 Da from the fruiting bodies of G. lucidum, which they named GLP70-1–2 [72]. GLP70-1–2 possesses a complex main chain structure, consisting of → 6)-α-D-Glcp-(1 → 6)-β-D-Galp-(1 → [6)-β-D-Manp-(1]3 → 4)-α-D-Glcp-(1 → 6)-α-D-Glcp-(1 → 2)-β-D-Galp-(1 → [4)-α-D-Glcp-(1 → 6)-β-D-Manp-(1 → 2)-β-D-Galp-(1]2 → 6)-β-D-Glcp-(1 → 6)-β-D-Glcp-(1 →, with two highly complex side chains attached at the O-4 and O-3 positions of the 1,6-β-D-Galp residues. Another homogeneous polysaccharide, a fucoxylomannan with a molecular weight of 3.5 × 104 Da, was identified from the fruiting bodies of G. lucidum using alkaline extraction methods [73]. The structure of fucoxylomannan is characterized by a main chain composed of 1,4-α-D-Manp repeating units. These units are flanked by side chains of T-α-D-Fucp-(1 → 2)-β-D-Xylp-(1 →, which are attached at the O-6 positions of the 1,4-α-D-Manp units. Additionally, from G. sinense, a polysaccharide named GSPB70-S was identified, with a main chain consisting of → 3)-β-D-Glcp-(1 → 4)-α-D-GlcpNAc-(1 → 4)-α-D-Manp-(1 → 3)-β-D-Glcp-(1 → [74]. GSPB70-S exhibits multiple biological activities, including antioxidant, immunomodulatory, and α-glucosidase inhibitory effects, indicating its potential application in diabetes treatment.
GPs display a multifaceted array of structural types, including glucans, heteroglucans, galactans, heterogalactans, galactoglucomannans, fucoxylomannans, and others. These polysaccharides exhibit a high level of complexity and diversity in their primary structures, as well as a range of biological activities including immunomodulation, anti-tumor, anti-oxidant, and regulating the intestinal flora. The structural characteristics of GPs are closely related to their biological functions, underscoring their significant potential in medicinal value and biomedical research. These results offer a rigorous scientific foundation and novel pathways for further exploration into the pharmacological mechanisms of Ganoderma, as well as the creation of functional products derived from this fungus.
2.2.4 Structural characteristics of GPs
The comprehensive analysis of polysaccharides from six Ganoderma species reveals shared structural characteristics, including β-(1 → 3)-D-glucan backbones with β-(1 → 6) branching, α-(1 → 6)-linked galactose residues, and molecular weights ranging from 2 to 4000 kDa. These polysaccharides predominantly exhibit triple-helical or spherical conformations and consist mainly of glucans, galactoglucans, and heteropolysaccharides.
Notable species-specific features include G. lucidum's predominant β-D-glucans (GLP20, GLPs) with triple-helical conformation, G. sinense's unique GSPB70-S sequence containing N-acetylglucosamine, and G. leucocontextum's distinctive α-D/β-D-glucan ratio (17.5:1.5) in GLP-3. G. atrum produces PSG-1-F0.2 with a characteristic (1 → 3)-β-D-Glcp backbone and high glucuronic acid content (15.3%), while G. applanatum synthesizes GAP-2 with a unique α/β-mixed backbone structure and diverse monosaccharide composition (Glc: Man: Gal: GlcA: Xyl = 82.0:5.6:4.3:4.2:3.9).
These structural variations reflect evolutionary adaptations among Ganoderma species, contributing to the diverse pharmacological activities observed across different polysaccharide types. Further exploration of structure–activity relationships (SARs) is warranted to better understand their biological roles and support the targeted utilization of GPs in medicinal and functional food applications.
3 Biological activity of GPs
3.1 The relationships between chemical structure and bioactivity
The structure–activity relationship of GPs demonstrates a significant dependence on various structural features, including monosaccharide composition, glycosidic bond types, molecular weight (Mw), branching patterns, and spatial conformation. Initially, variations in monosaccharide composition have been observed to impact the activity of GPs. Mannose-rich GPs have been found to bind to mannose receptors (MR) on macrophages, which promotes macrophage activation and enhances their phagocytic capabilities [84, 85]. For instance, GPs containing more than 5% mannose have been shown to activate macrophages more effectively, resulting in increased secretion of TNF-α compared to GPs with only 1.6% mannose [59]. Additionally, studies suggest that an increased mannose content also enhances natural killer (NK) cell activation, thereby improving their tumor-killing capabilities [86, 87].
Besides monosaccharide composition, the glycosidic linkage patterns play a crucial role in determining the bioactivity of GPs. Among various glycosidic types, the β-(1 → 3)-D-glucan backbone forms the primary structural basis for the immunomodulatory and antitumor effects of GPs [88]. The incorporation of β-(1 → 6)-D-glucan branches significantly enhances the bioactivity of β-(1 → 3)-D-glucans, suggesting the importance of branching patterns in function optimization [77, 89]. In particular, β-glucans with a branching ratio between 0.2 (1:5 branching) and 0.33 (1:3 branching) are recognized as the most potent immunomodulators, displaying enhanced capacity to regulate immune responses and inhibit tumor growth [90–92].
The Mw and configuration of polysaccharides are critical factors in determining their bioactivity. High Mw β-glucans, particularly those exhibiting triple-helix structures, are well-known for their potent immunomodulatory and antitumor activities [93]. This superior bioactivity is attributed to their complex three-dimensional conformations, which enable more effective binding to binding to pattern recognition receptors (PRRs). For example, the formation of a β-(1 → 3)-glucan triple-helix has been shown to significantly enhance the activity of immune cells, including macrophages [94]. Additionally, high Mw polysaccharides exhibit extended half-lives in vivo, leading to prolonged biological effects. While a high Mw is not an absolute requirement for immunostimulatory activity, its combination with specific structural features can synergistically enhance biological efficacy. Notably, lower molecular weight β-glucans have also demonstrated significant immunostimulatory potential, indicating that the interaction between Mw and structural properties is crucial for determining overall bioactivity [9].
The spatial conformation of β-glucans, which exists in three primary forms—triple-helix, single-helix, and random coil—is determined by the arrangement of sugar residues, Mw, and hydrogen bonding interactions between and within chains [95–97]. While early studies showed inconsistent correlations between β-glucan conformation and immunomodulatory effects, recent evidence strongly supports the superior immunoactivity of triple-helix structures [98–101]. Specifically, these glucans stimulate monocytes and macrophages to release pro-inflammatory cytokines such as IL-6, IL-1β, and TNF-α, thereby enhancing immune responses and inhibiting tumor growth, even with a relatively loose helical configuration. Conversely, single-helix β-glucans, while also immunoactive, exhibit lower stability and reduced efficacy in modulating immune responses and suppressing tumors [88, 102, 103]. Overall, while triple-helix structures demonstrate optimal immunomodulatory and antitumor effects, single-helix and random coil configurations also contribute to specific biological responses, suggesting a sophisticated recognition system within the host for different β-glucan conformations [95, 104].
3.2 Immunomodulation activity
GPs have attracted increasing attention for their multifunctional roles in immune regulation. As shown in Fig. 2 and Table 3, GPs have emerged as a research focal point, given their ability to modulate the immune system through various mechanisms. Studies have demonstrated that GPs bind with high affinity to critical immune receptors, including Dectin-1, mannose receptor (MR), complement receptor (CR), toll-like receptor (TLR) 2, and TLR4, suggesting their potential use in immunomodulatory applications [105].
Possible immunomodulatory mechanisms of GPs. GPs interact with key immune receptors, such as Dectin-1, MR, CR3 and TLRs, triggering downstream signaling pathways, including NF-κB, MAPKs, and PI3K/Akt [120]. These pathways lead to the activation of various immune cells, including macrophages, dendritic cells, T cells, B cells, NK cells, and neutrophils. Upon activation, these cells secrete pro-inflammatory cytokines such as TNF-α, IL-1β, IL-6, and IFN-γ, which promote immune cell proliferation, activation, and enhance phagocytosis. These cytokines also inhibit tumor cell proliferation by inducing apoptosis and reducing angiogenesis. For example, Gl-BSP derived from G. lucidum significantly increases NK cell and T cell cytotoxicity, contributing to tumor inhibition by upregulating IFN-γ and TNF-α. IFN-γ activates macrophages and NK cells, enhancing their cytotoxicity, while TNF-α induces apoptosis in tumor cells and disrupts the tumor vasculature. In parallel, the PI3K/Akt pathway enhances T cell and B cell survival and proliferation, while mTORC1 activation supports immune cell growth and function. Collectively, these pathways create a robust immune response, modulate the tumor microenvironment, and enhance antitumor immunity
Bioactive polysaccharides from six Ganoderma
Dectin-1, a C-type lectin-like receptor expressed on immune cells such as macrophages, dendritic cells, neutrophils, and monocytes, plays a pivotal role in recognizing and binding β-glucans with β-(1,3) and/or β-(1,6) glycosidic linkages [106, 107]. Upon activation of Dectin-1, downstream Syk kinase undergoes phosphorylation, subsequently activating PLCγ, which subsequently triggers the activation of protein kinase C (PKC) and the production of reactive oxygen species (ROS) [108]. These signaling events lead to the activation of two key pathways. The first involves the activation of PKC, which triggers the Card9/Bcl10/Malt1 complex, promoting the downstream activation of NF-κB and MAPK pathways, driving the production of pro-inflammatory cytokines [109, 110]. The second pathway involves the generation of ROS, which acts as a signaling molecule to further enhance immune responses by activating the inflammasome, leading to the maturation of cytokines such as IL-1β and amplifying the inflammatory response [111].
Recent studies identified a β-1,3-D-glucan (GSG) derived from Ganoderma spores, characterized by a β-1,3-glucan backbone with β-1,6-glucose side chains. GSG binds to Dectin-1 and activates the MAPK signaling pathway, resulting in significant immunomodulatory effects [112]. Other polysaccharides with similar structural characteristics also enhance the activation and maturation of immune cells via pattern recognition receptors, including Dectin-1, scavenger receptors, CR3, and TLR4. These interactions activate downstream signaling molecules such as Syk, JNK, p38, ERK, and NF-κB [113–115]. Furthermore, GLP-3, a water-soluble polysaccharide isolated from G. leucocontextum, with a molecular weight of 159.7 kDa and an α-D-1,4-glucose backbone, exhibits notable immunomodulatory activity [57]. GLP-3 enhances macrophage phagocytosis and pinocytosis while promoting cytokine production through TLR2-mediated activation of MAPKs (JNK, ERK, and p38), PI3K/Akt, and NF-κB pathways.
GPs exhibit remarkable immunomodulatory potential by engaging with critical immune receptors and activating essential signaling pathways, such as NF-κB, MAPK, and PI3K. These mechanisms highlight the potential of GPs as promising therapeutic candidates for immune regulation. Future studies should explore their clinical applications and potential synergistic effects with existing immunotherapies to address inflammatory and autoimmune diseases.
3.3 Immune-mediated antitumor activities
GPs activate immune cells by PRRs on the surface of immune cells, enhancing their phagocytic and stimulating the secretion of pro-inflammatory cytokines including TNF-α, IL-1, and IL-6. These cytokines inhibit tumor cell proliferation and promote apoptosis while modulating the tumor microenvironment (TME) by facilitating the recruitment and activation of additional immune effector cells, thus enhancing overall antitumor immunity.
Gl-BSP, derived from G. lucidum broken spore, exerts significant antitumor effects by enhancing the activity of NK cells, T cells, and macrophages [116]. The results showed that neutralization with antibodies against TNF-α and IFN-γ significantly reduced the tumor-inhibitory effects of Gl-BSP on S180 and PG tumor cells. Similarly, GL-PS has been shown to inhibit glioma growth by increasing serum IL-2, TNF-α, and IFN-γ, which enhances the cytotoxic activity of NK cells and T cells and promotes dendritic cell maturation [117]. SBSGL downregulated PD-1/PD-L1, enhancing Th1 immune responses, with increased TNF-α and IL-2 and reduced IL-10 and IL-6 levels, indicating stronger antitumor immunity and suppression of Th2 responses [118]. Additionally, GLPs enhance CD8+T cell secretion of IFN-γ and perforin, strengthening antitumor immunity in the tumor microenvironment [119]. Furthermore, combining GLP with anti-PD-1 antibodies significantly improves the efficacy of anti-PD-1 immunotherapy, underscoring its potential to boost immunotherapeutic outcomes.
In summary, GPs exert significant immunomodulatory and immune-mediated antitumor activities by influencing immune-related cells such as B cells, T cells, DCs, macrophages, and NK cells. Their antitumor effects are primarily mediated through immunoregulation, anti-angiogenesis, and cytotoxic mechanisms. These findings suggest the presence of a complex biological system within the host capable of recognizing and responding to different glucan structures, offering new perspectives for further exploration and the development of functional food and therapeutic applications.
3.4 Non-immune antitumor activities
GPs demonstrate significant antitumor activities not only by enhancing host immune responses but also through direct effects on tumor cells via multiple mechanisms [89, 121]. As shown in Fig. 3 and Table 3, these mechanisms include: (1) apoptosis induction through both mitochondrial pathway (disrupting mitochondrial membrane potential, promoting cytochrome c release, and activating caspase-3/caspase-9 cascade) and death receptor pathway (upregulating Fas/TRAIL-R expression and activating caspase-8-dependent pathway), ultimately leading to PARP cleavage and programmed cell death; (2) proliferation and metastasis inhibition through regulation of cell cycle proteins (p21, CDK2) and key signaling pathways (PI3K/Akt, MAPK, FAK), as well as EMT-related proteins (E-cadherin, N-cadherin, Vimentin, Snail1, and ZEB1) and growth factor receptors (EGFR, TGFβR); and (3) autophagy modulation via regulation of key proteins (LC3-Ⅱ, p62, RACK1) and disruption of autophagosome-lysosome fusion, affecting cellular stress responses and survival. These molecular mechanisms work synergistically at multiple cellular levels to achieve comprehensive antitumor effects.
The antitumor mechanisms of GPs via multiple signaling pathways. 1. Mitochondrial apoptosis pathway: GPs disrupt mitochondrial membrane potential, leading to cytochrome c release, which activates downstream caspase-9 and caspase-3, ultimately resulting in apoptosis. 2. Death receptor pathway: GPs upregulate death receptors such as Fas and TRAIL-R, along with their ligands, activating the extrinsic apoptotic pathway. This leads to the formation of the death-inducing signaling complex (DISC) and activates caspase-8 and caspase-3, which subsequently induce apoptosis. GPs promote apoptosis in colon cancer cells such as HCT-116 and LoVo through this Fas-mediated, caspase-dependent pathway. 4. FAK/PI3K/Akt and MAPK pathways: GPs inhibit the PI3K/Akt pathway, which downregulates anti-apoptotic proteins such as Bcl-2 and Bcl-xL, promoting apoptosis. Additionally, GPs obstruct cancer cell proliferation and migration by inhibiting the FAK/Src/Rac/Cdc42 pathway, which is essential for cell motility. GPs also regulate the MAPK pathway, inhibiting ERK signaling, which suppresses cell proliferation. 5. Autophagy Modulation: GPs regulate autophagy by inhibiting the Akt/mTOR pathway, resulting in the activation of autophagy-related proteins, including Beclin-1, LC3-Ⅰ, and LC3-Ⅱ, and promoting p62 degradation. This modulation leads to enhanced autophagy, which culminates in cancer cell death. Compounds such as RSGLP increase autophagosome accumulation and disrupt autophagic flux, contributing to the inhibition of cancer cell proliferation. 6. EMT Inhibition: GPs suppress the EMT by upregulating E-cadherin and downregulating mesenchymal markers such as N-cadherin and Vimentin. This reduces tumor cell migration and invasion. The polysaccharide GSBP-2 inhibits EMT by downregulating mesenchymal markers and blocking the PI3K/Akt pathway, further inhibiting cancer cell metastasis. 7. Cell Cycle Regulation: GPs induce cell cycle arrest by upregulating p21, which suppresses the activity of CDK2 and Cyclin D1, leading to a halt at the G1/S transition. GPs also downregulate PRMT6, reducing the activity of CDK2, FAK, and SRC, which enhances cell cycle arrest and promotes apoptosis. This mechanism is crucial for inhibiting tumor cell proliferation
The apoptotic effects of GPs are primarily mediated through both intrinsic and extrinsic pathways, which converge at the activation of caspase-3, thereby amplifying the apoptotic response. SeGLP-2B-1, a selenium-enriched heteropolysaccharide (1.06 × 106 Da) with a β-1,3-glucan backbone and β-1,6-glucan side chains, demonstrates this mechanism by inducing mitochondrial membrane potential disruption and triggering the caspase cascade activation [122, 123]. Similarly, BSGLWE from Ganoderma spores induces apoptosis by modulating Bcl-2 levels and activating caspase-3/9 [124, 125]. The combination of GLPs (Mw > 10 kDa) with 5-fluorouracil further demonstrates this mechanism by reactivating mutant p53 and enhancing mitochondria-mediated apoptosis [126], while crude GLPs induce Fas-mediated apoptosis in colon cancer cells [127, 128].
GPs exhibit potent anti-proliferative and anti-metastatic effects through modulation of multiple interconnected signaling pathways. GAP-2 (21.3 kDa) from G. lucidum significantly inhibits various cancer cell lines including A549, SKOV3, and SMMC-7721, with a survival rate of 60.9% in A549 cells at 600 μg/mL [65]. WSG, a water-soluble glucan with an Mw of approximately 1000 kDa, suppresses cancer cell growth and migration by inhibiting ERK, AKT, FAK, and TGFβR signaling pathways [129–131]. The complex polysaccharide GLP, composed of Ara, Gal, Glc, and Xyl in a molar ratio of 4:2:10:1, arrests prostate cancer cell cycle through PRMT6 pathway regulation [132, 133]. GSBP-2 (11.5 kDa) prevents breast cancer metastasis by inhibiting EMT through PI3K/Akt pathway modulation and regulating EMT-related proteins including E-cadherin, N-cadherin, Vimentin, Snail1, and ZEB1 [82].
The autophagy-regulating effects of GPs represent another crucial mechanism in their antitumor activity, functioning as both a tumor suppressor and a modulator of apoptotic responses. RSGLP, at a concentration of 200 µg/ml, effectively disrupts autophagic flux by modulating LC3-Ⅱ and p62 expression, leading to autophagosome accumulation and subsequent cancer cell death [134]. SBSGL, isolated from G. lucidum spore broken powder, demonstrates similar effects by inhibiting hepatoblastoma progression through RACK1-mediated autophagy regulation, specifically by reducing RACK1 protein expression through O-GlcNAc modification inhibition, disrupting autophagosome-lysosome fusion, and modulating the LC3-Ⅱ/LC3-Ⅰ ratio [118].
This comprehensive antitumor activity of GPs, encompassing apoptosis induction, proliferation inhibition, and autophagy regulation, along with their well-characterized molecular mechanisms, positions them as promising candidates for cancer therapy. The diverse mechanisms through which GPs exert their antitumor effects suggest potential advantages in targeting multiple aspects of tumor development and progression simultaneously.
3.5 Regulate the gut microbiota
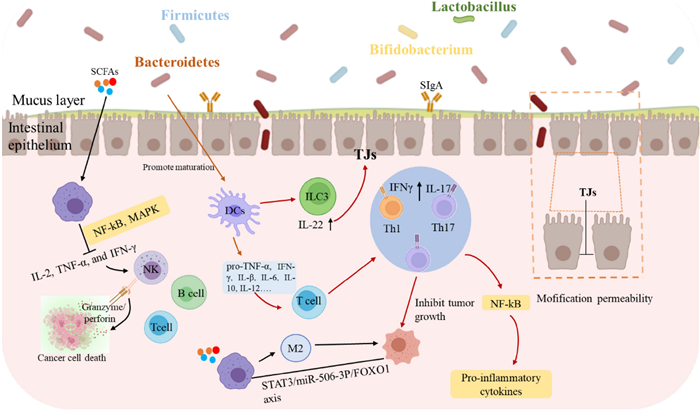
GPs regulate intestinal homeostasis through multiple mechanisms and pathways [11]. As shown in Fig. 4 and Table 3, these mechanisms include: (1) enhancement of intestinal barrier function through upregulation of tight junction proteins and modulation of signaling pathways; (2) modulation of gut microbiota composition and metabolite production, particularly SCFAs; and (3) regulation of immune responses, inflammatory processes, and anti-tumor through multiple pathways, ultimately leading to improved intestinal health.
Regulatory Effects of GPs on gut microbiota and immune system: implications for tumor suppression and intestinal health [11]
The complex structures of GPs render them impervious to direct digestion and absorption by the digestive system of the host, facilitating their transit to the colon. During simulated gastric and intestinal digestion, GPs exhibited remarkable stability with molecular weight only decreasing from 198.0 kDa to 147.1 kDa [135]. In the colon, polysaccharides from Ganoderma mycelium exemplify this mechanism by strengthening multiple barrier components: mechanical barrier (upregulating occludin), immune barrier (increasing IL-2, IL-4, and SIgA levels), and biological barrier function [136]. This protective effect is particularly evident in chemotherapy-induced intestinal injury, where co-administration of homogeneous GPs (SGP, Mw 3.6 kDa) with paclitaxel effectively mitigates barrier damage through upregulation of tight junction proteins (TJs) including ZO-1, E-cadherin, β-catenin, and occluding [137–139]. Additionally, studies have shown that GPs may enhance tight junction dynamics by modulating signaling pathways such as PI3K/Akt /mTOR and NF-κB, both of which play key roles in regulating the expression of TJs [138, 139].
GPs exhibit potent modulatory effects on gut microbiota composition and metabolite production. In the colon, GPs significantly modulate gut microbiota by selectively promoting beneficial bacteria (Bifidobacteria and Lactobacilli) while suppressing pathogenic species (such as E. coli and Clostridium perfringens). G. lucidum spore polysaccharides (CPGS and RPGS) demonstrate distinct effects on microbial composition, with CPGS increasing Verrucomicrobia and Proteobacteria, while RPGS enhances Actinobacteria populations, both significantly enriching immune-regulatory bacterial genera, including Adlercreutzia, Prevotella, and unclassified Desulfovibrionaceae [136]. These beneficial bacteria efficiently utilize GPs, particularly β-D-glucan-rich heteropolysaccharides, to produce short-chain fatty acids (SCFAs), including acetate, propionate, and butyrate, through microbial fermentation [140, 141].
The immune-modulatory and anti-inflammatory effects of GPs are mediated through multiple mechanisms. SCFAs, especially butyrate, exhibit significant anti-inflammatory effects through dual mechanisms: activation of G-protein coupled receptors (GPR43) and inhibition of histone deacetylases (HDACs) [142]. This leads to reduced production of pro-inflammatory cytokines such as IL-6 and IL-8 [143], and decreased IL-23 expression in intestinal epithelial cells through reduced STAT1 levels [144]. Additionally, β-D-glucans from Ganoderma effectively restore Th17/Treg cell balance, while GLPs demonstrate significant effects in inhibiting colitis and tumor development through modulation of immune cell function [145]. The modulatory effects of gut microbiota on immune regulation and tumor development are further evidenced by specific bacterial populations. Studies have shown that increased abundance of Alistipes is associated with tumor growth inhibition [146]. Moreover, Spearman correlation analysis revealed a significant negative correlation between Ruminococcus abundance and fructose-6-phosphate levels in tumors, suggesting a close relationship between gut microbiota modulation and tumor metabolism [147].
These findings demonstrate the complex interplay between GPs-mediated immune regulation and gut microbiota modulation, where SCFAs serve as key mediators in anti-inflammatory responses, while specific bacterial populations contribute to both immune homeostasis and tumor suppression through distinct metabolic pathways.
4 The biosynthesis of GPs
The cell wall of G. lucidum is primarily composed of various polysaccharides, which play a critical role in maintaining the structural integrity of the cell wall and conferring unique biological activities. The basic framework of the cell wall is made up of chitin, β-glucans, and α-glucans [171–174]. The biosynthesis of these polysaccharides is a multi-stage process involving the cytoplasm, endoplasmic reticulum, and Golgi apparatus [10]. During this process, nucleotide sugar precursors are polymerized into short chains, which are subsequently elongated into long chains and modified by branching. These polysaccharides are then transported to the cell wall via ABC transporter-dependent and Wzy-dependent pathways, where they are assembled into a complete structure [175]. The polysaccharide-rich cell wall not only provides physical robustness but also imparts a variety of functional properties, enabling Ganoderma to exhibit significant immunomodulatory, anti-tumor, and regulate gut microbiota.
4.1 Biosynthesis of the nucleotide sugar
Nucleotide sugars are indispensable precursors in the biosynthesis of complex polysaccharides, such as glucans, mannans, and galactofucans, which are essential components of fungal and plant cell walls. These nucleotide sugars act as activated donors of sugar residues, facilitating the elongation of polysaccharide chains through the action of glycosyltransferases. While the nucleotide sugar biosynthesis pathways are relatively well-defined and conserved across various organisms, the biosynthesis of GPs exhibits unique regulatory mechanisms and enzymatic specificity.
In GPs production, several enzymes involved in phosphosugar metabolism and glycosyltransferases play pivotal roles. Key enzymes such as phosphoglucomutase (PGM) and phosphomannomutase (PMM) are critical in the production of nucleotide sugars like UDP-glucose and GDP-mannose, which are essential for polysaccharide biosynthesis [176]. These enzymes not only influence intracellular and extracellular polysaccharide yields but also affect the structural composition of the polysaccharides. For instance, silencing of PGM reduces extracellular polysaccharide (EPS) production while increasing intracellular polysaccharide (IPS) levels, indicating its significant role in modulating polysaccharide distribution [177, 178]. Similar studies found that overexpression of PGM resulted in a 40–44% increase in polysaccharide yield and significant upregulation of related genes [179].
Transcriptional regulation also plays a crucial role in nucleotide sugar biosynthesis in Ganoderma. The transcription factor GlbHLH has been shown to regulate the expression of key genes involved in polysaccharide synthesis, such as PGM and UDP-glucose pyrophosphorylase (UGP) [180]. Silencing GlbHLH results in reduced polysaccharide production and altered cell wall composition, highlighting its importance in regulating the biosynthesis of nucleotide sugar precursors. Overexpression of GlbHLH or related genes significantly enhances polysaccharide yield, particularly through the upregulation of glycosyltransferase activity.
Genetic modifications, such as the overexpression of PMM1 or the introduction of heterologous genes like VHb, have been successfully employed to increase polysaccharide production in Ganoderma [181]. For example, overexpression of PMM1 resulted in a 1.41-fold increase in extracellular polysaccharide production, with a corresponding increase in mannose content, which enhanced the immunomodulatory properties of the polysaccharides [182]. Furthermore, the use of symbiotic fungal inducers has been shown to activate biosynthetic pathway genes, leading to a 3.4-fold increase in polysaccharide production and significant alterations in sugar composition, particularly a decrease in glucose content and an increase in mannose, galactose, and other sugars [183].
In summary, nucleotide sugar biosynthesis in Ganoderma is regulated by both enzymatic and transcriptional mechanisms. Through genetic engineering and biological induction strategies, both polysaccharide yields and their bioactivity have been significantly enhanced, providing valuable approaches for industrial GPs production and applications.
4.2 GPs elongation, modification, and the role of enzymes
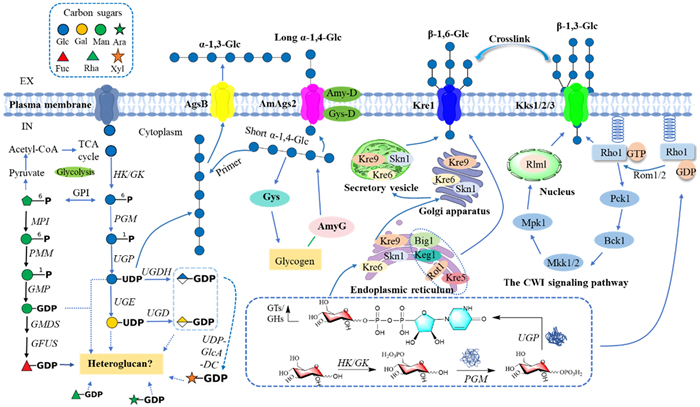
The biosynthesis of polysaccharides in Ganoderma is a complex process involving the elongation, branching, and modification of polysaccharide chains, primarily facilitated by glycosyltransferases (GTs) and glycoside hydrolases (GHs). GTs are responsible for recognizing and transferring activated nucleotide sugars to acceptor molecules, leading to the gradual elongation of polysaccharide chains [184, 185]. Genomic studies have identified 16 nucleotide sugar biosynthesis enzymes and 80 GT-encoding genes in G. lucidum mycelium, highlighting the diverse range of enzymes involved in polysaccharide biosynthesis [186]. Among these enzymes, the synthesis of β-(1,3)-glucan is performed by a plasma membrane-bound glucan synthase complex that uses UDP-glucose as a substrate to polymerize glucose monomers into β-(1,3)-glucan chains [187]. These chains are extruded through the plasma membrane and integrated into the cell wall matrix, where they undergo cross-linking and modification by transglycosylases, forming a stable network structure. The glucan synthase complex consists of two key proteins: a catalytic subunit encoded by the FKS/GSC gene, responsible for polysaccharide chain synthesis, and a regulatory subunit encoded by the RHO1 gene, which controls glucan synthase activity by cycling between inactive GDP-bound and active GTP-bound states [188]. Other polysaccharides, such as xylomannans and fucogalactans, are synthesized through pathways regulated by key genes identified via genomic sequence comparison and functional annotation, including Och1p, Van1p, Anp1p, and Mnn9p, all of which are potentially involved in the synthesis of these complex heteropolysaccharides [189].
In addition, proteins with the SKN1 domain play crucial roles in the biosynthesis of β-(1,6)-glucans, which are essential for the branching and structural integrity of fungal cell walls [190]. These proteins have been well-characterized in Saccharomyces cerevisiae, where they contribute to the formation of branched glucan structures that enhance cell wall flexibility and strength [191]. GHs play a pivotal role in the hydrolysis and remodeling of polysaccharides, including branch formation and side chain elongation. For example, The GH72 family of β-1,3-glucan transferases extends β-1,6-glucan chains by incorporating branches, which are essential for enhancing immunomodulatory and antitumor activities [176]. GH16 and GH17 families further support cell wall integrity by cross-linking β-glucans and chitin [192, 193]. In Saccharomyces cerevisiae and Aspergillus fumigatus, GH16 enzymes (e.g., Crh1p, Crh2p) and GH17 enzymes (e.g., Bgl2p) facilitate β-1,6-glucan and chitin cross-linking, maintaining cell wall rigidity and flexibility [194, 195]. Specifically, Bgl2p cleaves and transfers β-1,3-glucan in S. cerevisiae, forming curved polymers, while AfBgt1p and AfBgt2p in A. fumigatus generate curved and branched glucans, enhancing cell wall complexity.
As shwon in Fig. 5, polysaccharide elongation, modification, and structural assembly in Ganoderma are facilitated by a diverse set of glycosyltransferases and glycoside hydrolases. These enzymes work in tandem to create complex, branched polysaccharides that form the fungal cell wall, with GTs responsible for polymerization and GHs for modification and cross-linking. Understanding these enzymatic processes provides key insights into the regulation of polysaccharide biosynthesis and the potential for applications in functional food development.
Biosynthesis of polysaccharides in Ganoderma [176]. The biosynthesis of homopolysaccharides (e.g., β-(1,3)-glucans, β-(1,6)-glucans, α-glucans) and heteropolysaccharides (e.g., glucomannans, galactofucans) in Ganoderma begins with monosaccharide uptake, followed by phosphorylation and conversion into nucleotide sugars including UDP-glucose, GDP-mannose, and UDP-glucuronic acid. GTs elongate these nucleotide sugars into complex polysaccharides, which are subsequently modified by transglycosylases and glycoside hydrolases (GHs). The glucan synthase complex (FKS and RHO1) plays a pivotal role in β-(1,3)-glucan synthesis. This intricate assembly enhances cell wall integrity and contributes to the immunomodulatory, antioxidant, and antitumor activities of Ganoderma
5 Functional food applications of GPs
GPs, with their diverse bioactive properties, have demonstrated significant potential in the functional food industry. Comprehensive reviews emphasize that their immune-modulating, anti-tumor, and prebiotic characteristics have led to extensive commercial applications in this field. GPs are widely incorporated into functional beverages, such as herbal teas and energy drinks, especially in the Asian market, where their immune-boosting and fatigue-reducing effects are highly regarded [196]. These products capitalize on GPs' capacity to activate macrophages, natural killer (NK) cells, and T cells, providing a natural means of supporting immune health [197]. Additionally, GPs hold a prominent place in the global dietary supplement market, especially in the US and Europe. Here, they are formulated into capsules, tablets, or powders, marketed for their immune-regulatory, anti-inflammatory, and tumor-suppressing effects [146]. Further expanding their application, GPs are increasingly integrated into functional snacks and food additives, such as energy bars and cereals, often in combination with probiotics and fiber to enhance gut health [198] Acting as prebiotics, GPs foster the growth of beneficial gut bacteria, such as Lactobacillus and Bifidobacterium, promoting digestive health and supporting overall immune function [199]. In recent years, GPs have also gained traction in the beauty and health food markets due to their potent antioxidant and anti-tumor properties, finding applications in anti-aging and cancer-preventive food products [200]. Collectively, the multifunctional properties of GPs position them as a versatile and valuable ingredient in the functional food market, meeting the increasing consumer demand for immune support, gut health, and disease prevention.
Despite the widespread commercial application of GPs in functional foods, several technical and production challenges hinder their optimal utilization in health products. To address these challenges, several approaches can be adopted. First, the low extraction yield of water-soluble GPs—typically ranging between 1 and 3%—remains a major concern. To improve extraction efficiency and purity, and thus reduce production costs, biotechnological fermentation has emerged as a promising solution, as highlighted in numerous studies. Second, further research is needed to explore the bioactivity of GPs under different processing conditions, particularly focusing on their stability during food production and storage. This can be achieved through the development of microencapsulation techniques or the incorporation of antioxidants to protect GPs from environmental degradation. Moreover, improving consumer acceptance remains a key priority. The intrinsic bitterness of GPs poses a challenge in food formulations, necessitating the development of flavor-masking techniques or the combination of GPs with other ingredients to enhance taste profiles. Additionally, the market for GPs health products is highly diverse, yet the lack of activity-based quality standards results in significant variability in product quality and efficacy. Therefore, implementing activity-oriented quality control measures, such as the quantification of specific bioactive components like β-glucans, is crucial to ensuring product consistency and therapeutic effectiveness across the global market.
6 Conclusion and future perspectives
GPs have emerged as bioactive compounds with significant pharmacological potential, owing to their complex structural features, such as β-(1 → 3)-D-glucan backbones, β-(1 → 6)-D-glucan branching, and triple-helix conformations. These structural variations, along with diverse monosaccharide compositions, influence their immunomodulatory, anti-tumor, and gut microbiota-regulating properties. Specifically, β-(1 → 6) branching and high molecular weight have been linked to enhanced immune responses by activating PRRs such as Dectin-1 and TLRs, promoting cytokine production and immune cell activation. Additionally, structural elements like triple-helix conformations and functional groups, including acetyl and carboxyl groups, may influence bioactivity, contributing to the observed pharmacological effects.
While previous reviews have advanced the understanding of GPs, they often focus on isolated aspects, such as specific structural features or generalized biological activities, without comprehensively examining the interconnections between structural characteristics, biosynthetic pathways, and functional applications. This review addresses that gap by providing a holistic synthesis of GP structural complexity, SARs, biosynthesis mechanisms, and their applications in medicinal and functional food development. Such an integrative approach offers a clearer understanding of how structural features, such as glycosidic linkage patterns and branching ratios, determine biological activity, while emphasizing the potential of genetic engineering and advanced extraction techniques for optimizing GP yield and functionality.
However, significant challenges remain unresolved. The structural heterogeneity of GPs and variability in extraction methods often lead to inconsistencies in bioactivity reports. Traditional techniques, such as hot water and alkaline extraction, risk degrading polysaccharide structures, compromising biological efficacy. Furthermore, the limited control over biosynthetic pathways, particularly glycosyltransferase activity and branching patterns, hinders large-scale production of GPs with consistent structural features and bioactivity. Addressing these challenges requires innovations in extraction, structural characterization, and biosynthetic optimization to fully realize the therapeutic potential of GPs.
Future research should prioritize several strategies. The adoption of green extraction technologies, including mechanochemical-assisted extraction and deep eutectic solvents, can enhance GP yield while maintaining structural integrity. Genetic engineering approaches, such as overexpressing glycosyltransferase enzymes, hold promise for improving biosynthesis and structural uniformity. Exploring the synergistic effects of GPs with probiotics could uncover novel mechanisms for gut microbiota modulation, enhancing immune health and cancer prevention. Finally, standardizing analytical methods, such as molecular weight profiling and glycosidic linkage analysis, is crucial for ensuring reproducibility and consistency in both research and commercial applications.
By addressing these gaps and building on the comprehensive framework presented in this review, Ganoderma polysaccharides hold immense promise for developing functional foods, pharmaceuticals, and personalized health management strategies. This integrative approach not only deepens the scientific understanding of GPs but also bridges traditional herbal medicine with evidence-based modern health sciences.
Notes
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Nos. 82373762, 31872675), Major Special Programe of science and technology of Yunnan (202402AA310032, 202305AH340005), and the Cooperation Project with DR PLANT Company (2023), and the Foundation of the State Key Laboratory of Phytochemistry and Plant Resources in West China (Nos. P2020-KF02, P2022-KF10).
Author contributions
Zhou-Wei Wu: Conceptualization, Investigation, Formal analysis, Methodology, Data Curation, Visualization, Writing—Original Draft. Xue-Fang Zhao: Investigation, Data Curation. Chen-Xi Quan: Investigation, Visualization. Xiao-Cui Liu: Visualization. Xin-Yu Tao: Investigation. Yu-jie Li: Investigation. Xing-Rong: Formal analysis, Visualization. Ming-Hua Qiu: Supervision, Writing—Review and Editing, Project administration, Funding acquisition.
Data availability
No data was used for the research described in the article.
Declarations
Competing interests
No potential conflict of interest was reported by the authors.
References
-
1.Wu S, Zhang S, Peng B, Tan D, Wu M, Wei J, et al. Ganoderma lucidum: a comprehensive review of phytochemistry, efficacy, safety and clinical study. Food Sci Hum Wellness. 2024;13(2): 568-96. CrossRef PubMed Google Scholar
-
2.Zeng P, Guo Z, Zeng X, Hao C, Zhang Y, Zhang M, et al. Ganoderma lucidum polysaccharide: chemical, biochemical, preclinical and clinical studies as an approved drug for treating myopathy and other diseases in China. J Cell Mol Med. 2018;22(7): 3278-97. CrossRef PubMed Google Scholar
-
3.Zhang J, Liu Y, Tang Q, Zhou S, Feng J, Chen H. Polysaccharide of Ganoderma and its bioactivities. In: Lin Z, Yang B, editors. Ganoderma and Health: Biology, Chemistry and Industry. 2019. pp. 107–134. PubMed Google Scholar
-
4.Hsu K, Cheng K. From nutraceutical to clinical trial: frontiers in Ganoderma development. Appl Microbiol Biotechnol. 2018;102(21): 9037-51. CrossRef PubMed Google Scholar
-
5.Seweryn E, Ziala A, Gamian A. Health-promoting of polysaccharides extracted from Ganoderma lucidum. Nutrients. 2021. CrossRef PubMed Google Scholar
-
6.Sulkowska-Ziaja K, Balik M, Szczepkowski A, Trepa M, Zengin G, Kala K, et al. A review of chemical composition and bioactivity studies of the most promising species of Ganoderma spp. Diversity. 2023. CrossRef PubMed Google Scholar
-
7.Leong YK, Yang F-C, Chang J-S. Extraction of polysaccharides from edible mushrooms: emerging technologies and recent advances. Carbohydr Polym. 2021;251: 117006. CrossRef PubMed Google Scholar
-
8.Liu Y, Tang Q, Zhang J, Xia Y, Yang Y, Wu D, et al. Triple helix conformation of β-d-glucan from Ganoderma lucidum and effect of molecular weight on its immunostimulatory activity. Int J Biol Macromol. 2018;114: 1064-70. CrossRef PubMed Google Scholar
-
9.Lu J, He R, Sun P, Zhang F, Linhardt RJ, Zhang A. Molecular mechanisms of bioactive polysaccharides from Ganoderma lucidum (Lingzhi), a review. Int J Biol Macromol. 2020;150: 765-74. CrossRef PubMed Google Scholar
-
10.Zhang Z, Cui F, Sun L, Zan X, Sun W. Recent advances in Ganoderma lucidum polysaccharides: structures/bioactivities, biosynthesis and regulation. Food Biosci. 2023;56: 103281. CrossRef PubMed Google Scholar
-
11.Zheng M, Pi X, Li H, Cheng S, Su Y, Zhang Y, et al. Ganoderma spp. polysaccharides are potential prebiotics: a review. Crit Rev Food Sci Nutr. 2024;64(4): 909-27. CrossRef PubMed Google Scholar
-
12.Qin X, Fang Z, Zhang J, Zhao W, Zheng N, Wang X. Regulatory effect of Ganoderma lucidum and its active components on gut flora in diseases. Front Microbiol. 2024;15: 1362479. CrossRef PubMed Google Scholar
-
13.Zhang H, Zhang J, Liu Y, Tang C. Recent advances in the preparation, structure, and biological activities of β-glucan from Ganoderma species: a review. Foods. 2023;12(15): 12975. CrossRef PubMed Google Scholar
-
14.Ye T, Ge Y, Jiang X, Song H, Peng C, Liu B. A review of anti-tumour effects of Ganoderma lucidum in gastrointestinal cancer. Chin Med. 2023;18(1): 811. CrossRef PubMed Google Scholar
-
15.Ahmad MF, Ahmad FA, Zeyaullah M, Alsayegh AA, Mahmood SE, AlShahrani AM, et al. Ganoderma lucidum: novel insight into hepatoprotective potential with mechanisms of action. Nutrients. 2023;15(8): 1874. CrossRef PubMed Google Scholar
-
16.Andrejc DC, Knez Z, Marevci MK. Antioxidant, antibacterial, antitumor, antifungal, antiviral, anti-inflammatory, and neuro-protective activity of Ganoderma lucidum: an overview. Front Pharmacol. 2022;13: 934982. CrossRef PubMed Google Scholar
-
17.Liu Y, Wu J, Hao H. Antitumor immunostimulatory activity of the traditional Chinese medicine polysaccharide on hepatocellular carcinoma. Front Immunol. 2024;15: 1369110. CrossRef PubMed Google Scholar
-
18.Ahmad MF, Ahmad FA, Hasan N, Alsayegh AA, Hakami O, Bantun F, et al. Ganoderma lucidum: multifaceted mechanisms to combat diabetes through polysaccharides and triterpenoids: a comprehensive review. Int J Biol Macromol. 2024;268: 131644. CrossRef PubMed Google Scholar
-
19.Peng H, Zhong L, Cheng L, Chen L, Tong R, Shi J, et al. Ganoderma lucidum: current advancements of characteristic components and experimental progress in anti-liver fibrosis. Front Pharmacol. 2022;13: 1094405. CrossRef PubMed Google Scholar
-
20.El Sheikha AF. Nutritional profile and health benefits of Ganoderma lucidum “Lingzhi, Reishi, or Mannentake” as functional foods: current scenario and future perspectives. Foods. 2022;11(7): 1030. CrossRef PubMed Google Scholar
-
21.Ma Y, He H, Wu J, Wang C, Chao K, Huang Q. Assessment of polysaccharides from mycelia of genus Ganoderma by mid-infrared and near-infrared spectroscopy. Sci Rep. 2018. CrossRef PubMed Google Scholar
-
22.Skalicka-Woźniak K, Szypowski J, Łoś R, Siwulski M, Sobieralski K, Głowniak K, et al. Evaluation of polysaccharides content in fruit bodies and their antimicrobial activity of four Ganoderma lucidum (W Curt.: Fr.) P. Karst. strains culitvated on different wooden substrates. Acta Soc Bot Pol. 2012;81: 17-21. CrossRef PubMed Google Scholar
-
23.Nakagawa T, Zhu Q, Tamrakar S, Amen Y, Mori Y, Suhara H, et al. Changes in content of triterpenoids and polysaccharides in Ganoderma lingzhi at different growth stages. J Nat Med. 2018;72(3): 734-44. CrossRef PubMed Google Scholar
-
24.Xu A, Yang X, Li Y, Jacob MS, Zhang B, Li X. Fresh-eating Lingzhi becomes possible: comparative evaluation of nutritional and taste profile of Ganoderma tsugae at different fruiting body morphogenesis stages. LWT. 2024;201: 116234. CrossRef PubMed Google Scholar
-
25.Zheng W, Lan S, Zhang W, Nie B, Zhu K, Ye X, et al. Polysaccharide structure evaluation of Ganoderma lucidum from different regions in China based on an innovative extraction strategy. Carbohydr Polym. 2024;335: 122079. CrossRef PubMed Google Scholar
-
26.Liu Y, Long Y, Liu H, Lan Y, Long T, Kuang R, et al. Polysaccharide prediction in Ganoderma lucidum fruiting body by hyperspectral imaging. Food Chem X. 2022;13: 100199. CrossRef PubMed Google Scholar
-
27.Guo Q, Liang S, Ge C, Xiao Z. Research progress on extraction technology and biological activity of polysaccharides from edible fungi: a review. Food Rev Int. 2023;39(8): 4909-40. CrossRef PubMed Google Scholar
-
28.Nguyen T, Ngo T, Do Dat T, Nguyen D, Hoang M, Mai P, et al. Effects of extraction technology on bioactivities from polysaccharide-rich Ganoderma lucidum. Minist Sci Technol Vietnam. 2022;64: 32-7. CrossRef PubMed Google Scholar
-
29.Feng J, Feng N, Tang Q, Liu Y, Yang Y, Liu F, et al. Optimization of Ganoderma lucidum polysaccharides fermentation process for large-scale production. Appl Biochem Biotechnol. 2019;189: 972-86. CrossRef PubMed Google Scholar
-
30.Lyu F, Xu X, Zhang L. Natural polysaccharides with different conformations: extraction, structure and anti-tumor activity. J Mater Chem B. 2020;8(42): 9652-67. CrossRef PubMed Google Scholar
-
31.Wiater A, Paduch R, Choma A, Pleszczynska M, Siwulski M, Dominik J, et al. Biological study on carboxymethylated (1→3)-α-D-glucans from fruiting bodies of Ganoderma lucidum. Int J Biol Macromol. 2012;51(5): 1014-23. CrossRef PubMed Google Scholar
-
32.Sood G, Sharma S, Kapoor S, Khanna P. Optimization of extraction and characterization of polysaccharides from medicinal mushroom Ganoderma lucidum using response surface methodology. J Med Plant Res. 2013;7(31): 2323-9. CrossRef PubMed Google Scholar
-
33.Peng Y, Zhang L. Chain conformation of an alkali-soluble polysaccharide from mycelium of Ganoderma tsugae. J Macromol Sci Phys. 2005;B44(4): 445-53. CrossRef PubMed Google Scholar
-
34.Huang S, Li J, Wang Z, Pan H, Chen J, Ning Z. Optimization of alkaline extraction of polysaccharides from Ganoderma lucidum and their effect on immune function in mice. Molecules. 2010;15(5): 3694-708. CrossRef PubMed Google Scholar
-
35.Chen X, Wang W, Li S, Xue J, Fan L, Sheng Z, et al. Optimization of ultrasound-assisted extraction of Lingzhi polysaccharides using response surface methodology and its inhibitory effect on cervical cancer cells. Carbohydr Polym. 2010;80(3): 944-8. CrossRef PubMed Google Scholar
-
36.Alzorqi I, Singh A, Manickam S, Al-Qrimli HF. Optimization of ultrasound-assisted extraction (UAE) of β-D-glucan polysaccharides from Ganoderma lucidum for prospective scale-up. Resour Eff Technol. 2017;3(1): 46-54. CrossRef PubMed Google Scholar
-
37.Alzorqi I, Sudheer S, Lu T-J, Manickam S. Ultrasonically extracted β-d-glucan from artificially cultivated mushroom: characteristic properties and antioxidant activity. Ultrason Sonochem. 2017;35: 531-40. CrossRef PubMed Google Scholar
-
38.Ma C, Feng M, Zhai X, Hu M, You L, Luo W, et al. Optimization for the extraction of polysaccharides from Ganoderma lucidum and their antioxidant and antiproliferative activities. J Taiwan Inst Chem Eng. 2013;44(6): 886-94. CrossRef PubMed Google Scholar
-
39.Zheng S, Zhang W, Liu S. Optimization of ultrasonic-assisted extraction of polysaccharides and triterpenoids from the medicinal mushroom Ganoderma lucidum and evaluation of their in vitro antioxidant capacities. PLoS ONE. 2021;15(12): e0244749. CrossRef PubMed Google Scholar
-
40.Do DT, Lam DH, Nguyen T, Mai TTP, Vuong HT, Nguyen DV, et al. Utilization of response surface methodology in optimization of polysaccharides extraction from Vietnamese red Ganoderma lucidum by ultrasound-assisted enzymatic method and examination of bioactivities of the extract. Sci World J. 2021;2021: 7594092. CrossRef PubMed Google Scholar
-
41.Chen T, Wu Y, Wu J, Ma L, Dong Z, Wu J. Efficient extraction technology of antioxidant crude polysaccharides from Ganoderma lucidum (Lingzhi), ultrasonic-circulating extraction integrating with superfine-pulverization. J Taiwan Inst Chem Eng. 2014;45(1): 57-62. CrossRef PubMed Google Scholar
-
42.Papinutti L. Effects of nutrients, pH and water potential on exopolysaccharides production by a fungal strain belonging to Ganoderma lucidum complex. Bioresour Technol. 2010;101(6): 1941-6. CrossRef PubMed Google Scholar
-
43.Zhu X, Chen X, Xie J, Wang P, Su W. Mechanochemical-assisted extraction and antioxidant activity of polysaccharides from Ganoderma lucidum spores. Int J Food Sci Technol. 2012;47(5): 927-32. CrossRef PubMed Google Scholar
-
44.Liu G, Zhang J, Hou T, An S, Guo B, Liu C, et al. Extraction kinetics, physicochemical properties and immunomodulatory activity of the novel continuous phase transition extraction of polysaccharides from Ganoderma lucidum. Food Funct. 2021;12(20): 9708-18. CrossRef PubMed Google Scholar
-
45.Smiderle FR, Morales D, Gil-Ramírez A, de Jesus LI, Gilbert-López B, Iacomini M, et al. Evaluation of microwave-assisted and pressurized liquid extractions to obtain β-d-glucans from mushrooms. Carbohydr Polym. 2017;156: 165-74. CrossRef PubMed Google Scholar
-
46.Li R, Shi G, Chen L, Liu Y. Polysaccharides extraction from Ganoderma lucidum using a ternary deep eutectic solvents of choline chloride/guaiacol/lactic acid. Int J Biol Macromol. 2024;263: 130263. CrossRef PubMed Google Scholar
-
47.Kan Y, Chen T, Wu Y, Wu J. Antioxidant activity of polysaccharide extracted from Ganoderma lucidum using response surface methodology. Int J Biol Macromol. 2015;72: 151-7. CrossRef PubMed Google Scholar
-
48.Pan K, Jiang Q, Liu G, Miao X, Zhong D. Optimization extraction of Ganoderma lucidum polysaccharides and its immunity and antioxidant activities. Int J Biol Macromol. 2013;55: 301-6. CrossRef PubMed Google Scholar
-
49.Matsunaga Y, Machmudah S, Wahyudiono KH, Sasaki M, Goto M. Subcritical water extraction and direct formation of microparticulate polysaccharide powders from Ganoderma lucidum. Int J Technol. 2014;5(1): 40-50. CrossRef PubMed Google Scholar
-
50.Matsunaga Y, Wahyudiono, Machmudah S, Sasaki M, Goto M. Hot compressed water extraction of polysaccharides from Ganoderma lucidum using a semibatch reactor. Asia-Pac J Chem Eng. 2014;9(1): 125-33. CrossRef PubMed Google Scholar
-
51.Gong T, Yan R, Kang J, Chen R. Chemical components of Ganoderma. In: Lin Z, Yang B, editors. Ganoderma and health: biology, chemistry and industry. 1st ed. 2019. pp. 59–106. PubMed Google Scholar
-
52.Ding L, Shangguan H, Wang X, Liu J, Shi Y, Xu X, et al. Extraction, purification, structural characterization, biological activity, mechanism of action and application of polysaccharides from Ganoderma lucidum: a review. Int J Biol Macromol. 2025;288: 138575. CrossRef PubMed Google Scholar
-
53.Kou F, Ge Y, Wang W, Mei Y, Cao L, Wei X, et al. A review of Ganoderma lucidum polysaccharides: health benefit, structure–activity relationship, modification, and nanoparticle encapsulation. Int J Biol Macromol. 2023;243: 125199. CrossRef PubMed Google Scholar
-
54.Lin S, Wang S, Wang L, Lin D. Research progress on chemistry of Ganoderma polysaccharides. J Fungal Res. 2024;22(1): 9-21. CrossRef PubMed Google Scholar
-
55.Yang L, Huang J, Huang N, Qin S, Chen Z, Xiao G, et al. Structure–activity relationship of synthesized glucans from Ganoderma lucidum with in vitro hypoglycemic activity. Int J Biol Macromol. 2025;288: 138586. CrossRef PubMed Google Scholar
-
56.Luo H, Zhang Y, Wang S, Lin S, Wang L, Lin Z, et al. Structural characterization and anti-oxidative activity for a glycopeptide from Ganoderma lucidum fruiting body. Int J Biol Macromol. 2024;261: 129793. CrossRef PubMed Google Scholar
-
57.Gao X, Qi J, Ho C, Li B, Mu J, Zhang Y, et al. Structural characterization and immunomodulatory activity of a water-soluble polysaccharide from Ganoderma leucocontextum fruiting bodies. Carbohydr Polym. 2020;249: 116874. CrossRef PubMed Google Scholar
-
58.Wen L, Sheng Z, Wang J, Jiang Y, Yang B. Structure of water-soluble polysaccharides in spore of Ganoderma lucidum and their anti-inflammatory activity. Food Chem. 2022;373(Pt A): 131374. CrossRef PubMed Google Scholar
-
59.Li J, Gu F, Cai C, Hu M, Fan L, Hao J, et al. Purification, structural characterization, and immunomodulatory activity of the polysaccharides from Ganoderma lucidum. Int J Biol Macromol. 2020;143: 806-13. CrossRef PubMed Google Scholar
-
60.Zhang H, Nie S, Cui SW, Xu M, Ding H, Xie M. Characterization of a bioactive polysaccharide from Ganoderma atrum: Re-elucidation of the fine structure. Carbohydr Polym. 2017;158: 58-67. CrossRef PubMed Google Scholar
-
61.Sheng Z, Wen L, Yang B. Structure identification of a polysaccharide in mushroom Lingzhi spore and its immunomodulatory activity. Carbohydr Polym. 2022;278: 118939. CrossRef PubMed Google Scholar
-
62.Liu Y, Zhang J, Tang Q, Yang Y, Guo Q, Wang Q, et al. Physicochemical characterization of a high molecular weight bioactive β-d-glucan from the fruiting bodies of Ganoderma lucidum. Carbohydr Polym. 2014;101: 968-74. CrossRef PubMed Google Scholar
-
63.Wang Y, Liu Y, Yu H, Zhou S, Zhang Z, Wu D, et al. Structural characterization and immuno-enhancing activity of a highly branched water-soluble β-glucan from the spores of Ganoderma lucidum. Carbohydr Polym. 2017;167: 337-44. CrossRef PubMed Google Scholar
-
64.Dong Q, Wang Y, Shi L, Yao J, Li J, Ma F, et al. A novel water-soluble β-d-glucan isolated from the spores of Ganoderma lucidum. Carbohydr Res. 2012;353: 100-5. CrossRef PubMed Google Scholar
-
65.Gong Z, Liu M, Liu H, Deng Z, Qin X, Nie J, et al. Structural features and in vitro antitumor activity of a water-extracted polysaccharide from Ganoderma applanatum. New J Chem. 2023;47: 13205-17. CrossRef PubMed Google Scholar
-
66.Gao X, Qi J, Ho CT, Li B, Xie Y, Chen S, et al. Purification, physicochemical properties, and antioxidant activities of two low-molecular-weight polysaccharides from Ganoderma leucocontextum fruiting bodies. Antioxidants. 2021;10: 1145. CrossRef PubMed Google Scholar
-
67.Zhou Y, Li L, Sun Z, Liu R, Zhu Y, Yi J, et al. Structural characterization and osteogenic differentiation-promoting activity of polysaccharide purified from Chroogomphus rutilus. Carbohydr Polym. 2024;328: 121709. CrossRef PubMed Google Scholar
-
68.Qu Y, Yan J, Zhang X, Song C, Zhang M, Mayo KH, et al. Structure and antioxidant activity of six mushroom-derived heterogalactans. Int J Biol Macromol. 2022;209: 1439-49. CrossRef PubMed Google Scholar
-
69.Ye L, Zhang J, Zhou K, Yang Y, Zhou S, Jia W, et al. Purification, NMR study and immunostimulating property of a fucogalactan from the fruiting bodies of Ganoderma lucidum. Planta Med. 2008;74: 1730-4. CrossRef PubMed Google Scholar
-
70.Ye L, Zhang J, Yang Y, Zhou S, Liu Y, Tang Q, et al. Structural characterisation of a heteropolysaccharide by NMR spectra. Food Chem. 2009;112: 962-6. CrossRef PubMed Google Scholar
-
71.Ye L, Zhang J, Ye X, Tang Q, Liu Y, Gong C, et al. Structural elucidation of the polysaccharide moiety of a glycopeptide (GLPCW-Ⅱ) from Ganoderma lucidum fruiting bodies. Carbohydr Res. 2008;343: 746-52. CrossRef PubMed Google Scholar
-
72.Su D, Lei A, Nie C, Chen Y. The protective effect of Ganoderma atrum polysaccharide on intestinal barrier function damage induced by acrylamide in mice through TLR4/MyD88/NF-κB based on the iTRAQ analysis. Food Chem Toxicol. 2023;171: 113548. CrossRef PubMed Google Scholar
-
73.da Silva MS, de Lima BD, Zavadinack M, Simas FF, Smiderle FR, de Santana-Filho AP, et al. Antimelanoma effect of a fucoxylomannan isolated from Ganoderma lucidum fruiting bodies. Carbohydr Polym. 2022;294: 119823. CrossRef PubMed Google Scholar
-
74.Chen Y, Ou X, Yang J, Bi S, Peng B, Wen Y, et al. Structural characterization and biological activities of a novel polysaccharide containing N-acetylglucosamine from Ganoderma sinense. Int J Biol Macromol. 2020;158: 1204-15. CrossRef PubMed Google Scholar
-
75.da Silva Milhorini S, Zavadinack M, Dos Santos JF, de Lara EL, Smiderle FR, Iacomini M. Structural variety of glucans from Ganoderma lucidum fruiting bodies. Carbohydr Res. 2024;538: 109099. CrossRef PubMed Google Scholar
-
76.Cao C, Liao Y, Yu Q, Zhang D, Huang J, Su Y, et al. Structural characterization of a galactoglucomannan with anti-neuroinflammatory activity from Ganoderma lucidum. Carbohydr Polym. 2024;334: 122030. CrossRef PubMed Google Scholar
-
77.Wang J, Yuan Y, Yue T. Immunostimulatory activities of β-d-glucan from Ganoderma lucidum. Carbohydr Polym. 2014;102: 47-54. CrossRef PubMed Google Scholar
-
78.Liu H, Cheng J, Wang X, Jiang Y, Ni J, Zhang Y, et al. Structure identification of Ganoderma lucidum spore polysaccharides and their antitumor activity in vivo. Molecules. 2024;29(10): 2348. CrossRef PubMed Google Scholar
-
79.Liu Y, Wang Y, Zhou S, Yan M, Tang Q, Zhang J. Structure and chain conformation of bioactive β-D-glucan purified from water extracts of Ganoderma lucidum unbroken spores. Int J Biol Macromol. 2021;180: 484-93. CrossRef PubMed Google Scholar
-
80.Fu Y, Shi L, Ding K. Structure elucidation and anti-tumor activity in vivo of a polysaccharide from spores of Ganoderma lucidum (Fr.) Karst. Int J Biol Macromol. 2019;141: 693-9. CrossRef PubMed Google Scholar
-
81.Li Y, Fang L, Zhang K. Structure and bioactivities of a galactose rich extracellular polysaccharide from submergedly cultured Ganoderma lucidum. Carbohydr Polym. 2007;68: 323-8. CrossRef PubMed Google Scholar
-
82.Xie Y, Su Y, Wang Y, Zhang D, Yu Q, Yan C. Structural clarification of mannoglucan GSBP-2 from Ganoderma sinense and its effects on triple-negative breast cancer migration and invasion. Int J Biol Macromol. 2024;269: 131903. CrossRef PubMed Google Scholar
-
83.Han X, Yue G, Yue R-Q, Dong C, Chan C, Ko C, et al. Structure elucidation and immunomodulatory activity of a beta glucan from the fruiting bodies of Ganoderma sinense. PLoS ONE. 2014;9: 100380. CrossRef PubMed Google Scholar
-
84.Li W, Tang X, Shuai X, Jiang C, Liu X, Wang L, et al. Mannose receptor mediates the immune response to Ganoderma atrum polysaccharides in macrophages. J Agric Food Chem. 2017;65: 348-57. CrossRef PubMed Google Scholar
-
85.Ying Y, Hao W. Immunomodulatory function and anti-tumor mechanism of natural polysaccharides: a review. Front Immunol. 2023;14: 1147641. CrossRef PubMed Google Scholar
-
86.Jaynes JM, Sable R, Ronzetti M, Bautista W, Knotts Z, Abisoye-Ogunniyan A, et al.·Mannose receptor (CD206) activation in tumor-associated macrophages enhances adaptive and innate antitumor immune responses.·Sci Transl Med.·2020;12(530):eaax6337. https://doi.org/10.1126/scitranslmed.aax6337 PubMed Google Scholar
-
87.Lemieszek MK, Nunes FM, Rzeski W. Branched mannans from the mushroom Cantharellus cibarius enhance the anticancer activity of natural killer cells against human cancers of lung and colon. Food Funct. 2019;10: 5816-26. CrossRef PubMed Google Scholar
-
88.Wu L, Zhao J, Zhang X, Liu S, Zhao C. Antitumor effect of soluble β-glucan as an immune stimulant. Int J Biol Macromol. 2021;179: 116-24. CrossRef PubMed Google Scholar
-
89.Gao X, Homayoonfal M. Exploring the anti-cancer potential of Ganoderma lucidum polysaccharides (GLPs) and their versatile role in enhancing drug delivery systems: a multifaceted approach to combat cancer. Cancer Cell Int. 2023;23: 31. CrossRef PubMed Google Scholar
-
90.Bae IY, Kim H, Yoo HJ, Kim ES, Lee S, Park DY, et al. Correlation of branching structure of mushroom β-glucan with its physiological activities. Food Res Int. 2013;51: 195-200. CrossRef PubMed Google Scholar
-
91.Cadar E, Negreanu-Pirjol T, Pascale C, Sirbu R, Prasacu I, Negreanu-Pirjol B-S, et al. Natural bio-compounds from Ganoderma lucidum and their beneficial biological actions for anticancer application: a review. Antioxidants. 2023;12: 11907. CrossRef PubMed Google Scholar
-
92.Xue H, Wang W, Bian J, Gao Y, Hao Z, Tan J. Recent advances in medicinal and edible homologous polysaccharides: extraction, purification, structure, modification, and biological activities. Int J Biol Macromol. 2022;222: 1110-26. CrossRef PubMed Google Scholar
-
93.Zhang H, Li Y, Fu Y, Jiao H, Wang X, Wang Q, et al. A structure-functionality insight into the bioactivity of microbial polysaccharides toward biomedical applications: a review. Carbohydr Polym. 2024;335: 122078. CrossRef PubMed Google Scholar
-
94.Stothers CL, Burelbach KR, Owen AM, Patil NK, McBride MA, Bohannon JK, et al. β-Glucan induces distinct and protective innate immune memory in differentiated macrophages. J Immunol. 2021;207: 2785-98. CrossRef PubMed Google Scholar
-
95.Kono H, Kondo N, Isono T, Ogata M, Hirabayashi K. Characterization of the secondary structure and order-disorder transition of a β-(1→3, 1→6)-glucan from Aureobasidium pullulans. Int J Biol Macromol. 2019;28: 104993. CrossRef PubMed Google Scholar
-
96.Okobira T, Miyoshi K, Uezu K, Sakurai K, Shinkai S. Molecular dynamics studies of side chain effect on the beta-1,3-D-glucan triple helix in aqueous solution. Biomacromol. 2008;9: 783-8. CrossRef PubMed Google Scholar
-
97.Chen H, Liu N, He F, Liu Q, Xu X. Specific β-glucans in chain conformations and their biological functions. Polym J. 2022;54: 427-53. CrossRef PubMed Google Scholar
-
98.Cao Y, Xu X, Liu S, Huang L, Gu J. Ganoderma: a cancer immunotherapy review. Front Pharmacol. 2018;9: 1217. CrossRef PubMed Google Scholar
-
99.Sohretoglu D, Huang S. Ganoderma lucidum polysaccharides as an anti-cancer agent. Anti-Cancer Agents Med Chem. 2018;18: 667-74. CrossRef PubMed Google Scholar
-
100.Falch BH, Espevik T, Ryan L, Stokke BT. The cytokine stimulating activity of (1→3)-beta-D-glucans is dependent on the triple helix conformation. Carbohydr Res. 2000;329: 587-96. CrossRef PubMed Google Scholar
-
101.Demir G, Klein HO, Mandel-Molinas N, Tuzuner N. Beta glucan induces proliferation and activation of monocytes in peripheral blood of patients with advanced breast cancer. Int Immunopharmacol. 2007;7: 113-6. CrossRef PubMed Google Scholar
-
102.Yanagihara S, Kasho N, Sasaki K, Shironaka N, Kitayama Y, Yuba E, et al. pH-sensitive branched β-glucan-modified liposomes for activation of antigen presenting cells and induction of antitumor immunity. J Mater Chem B. 2021;9: 7713-24. CrossRef PubMed Google Scholar
-
103.Su F, Song Q, Zhang C, Xu X, Li M, Yao D, et al. A β-1,3/1,6-glucan from Durvillaea Antarctica inhibits tumor progression in vivo as an immune stimulator. Carbohydr Polym. 2019;222: 114993. CrossRef PubMed Google Scholar
-
104.Yan X, Liu B, Ru G, Feng J. Preparation and characterization of curdlan with unique single-helical conformation and its assembly with Congo Red. Carbohydr Polym. 2021;263: 117985. CrossRef PubMed Google Scholar
-
105.Hsu T, Cheng S, Yang W, Chin S, Chen B, Huang M, et al. Profiling carbohydrate-receptor interaction with recombinant innate immunity receptor-Fc fusion proteins. J Biol Chem. 2009;284: 34479-89. CrossRef PubMed Google Scholar
-
106.Sylla B, Guégan JP, Wieruszeski JM, Nugier-Chauvin C, Legentil L, Daniellou R, et al. ·Probing β-(1→3)-D-glucans interactions with recombinant human receptors using high-resolution NMR studies. Carbohydr Res. 2011;346: 1490-1494. CrossRef PubMed Google Scholar
-
107.Brown G, Gordon S. Immune recognition. A new receptor for beta-glucans. Nature. 2001;413: 36-7. CrossRef PubMed Google Scholar
-
108.Goodridge HS, Reyes CN, Becker CA, Katsumoto TR, Ma J, Wolf AJ, et al. Activation of the innate immune receptor Dectin-1 upon formation of a ‘phagocytic synapse’. Nature. 2011;472: 471-5. CrossRef PubMed Google Scholar
-
109.Gross O, Grupp C, Steinberg C, Zimmermann S, Strasser D, Hannesschläger N, et al. Multiple ITAM-coupled NK-cell receptors engage the Bcl10/Malt1 complex via Carma1 for NF-κB and MAPK activation to selectively control cytokine production. Blood. 2008;112(6): 2421-8. CrossRef PubMed Google Scholar
-
110.Lee EJ, Brown BR, Vance EE, Snow PE, Silver PB, Heinrichs D, et al. Mincle activation and the Syk/Card9 signaling axis are central to the development of autoimmune disease of the eye. J Immunol. 2016;196(7): 3148-58. CrossRef PubMed Google Scholar
-
111.Harijith A, Ebenezer DL, Natarajan V. Reactive oxygen species at the crossroads of inflammasome and inflammation. Front Physiol. 2014;5: 352. CrossRef PubMed Google Scholar
-
112.Guo L, Xie JH, Ruan YY, Zhou L, Zhu HY, Yun XJ, et al. Characterization and immunostimulatory activity of a polysaccharide from the spores of Ganoderma lucidum. Int Immunopharmacol. 2009;9: 1175-82. CrossRef PubMed Google Scholar
-
113.Ahmad MF, Ahmad FA, Khan MI, Alsayegh AA, Wahab S, Alam MI, et al. Ganoderma lucidum: a potential source to surmount viral infections through β-glucans immunomodulatory and triterpenoids antiviral properties. Int J Biol Macromol. 2021;187: 769-79. CrossRef PubMed Google Scholar
-
114.Wang C, Lu C, Pi C, Zhuang Y, Chu C-L, Liu W, et al. Extracellular polysaccharides produced by Ganoderma formosanum stimulate macrophage activation via multiple pattern-recognition receptors. BMC Complement Altern Med. 2012;12: 119. CrossRef PubMed Google Scholar
-
115.Lin Y, Liang Y, Lee S, Chiang B. Polysaccharide purified from Ganoderma lucidum induced activation and maturation of human monocyte-derived dendritic cells by the NF-κB and p38 mitogen-activated protein kinase pathways. J Leukoc Biol. 2005;78(2): 533-43. CrossRef PubMed Google Scholar
-
116.Wang PY, Zhu XL, Lin ZB. Antitumor and immunomodulatory effects of polysaccharides from broken-spore of Ganoderma lucidum. Front Pharmacol. 2012;3: 135. CrossRef PubMed Google Scholar
-
117.Wang C, Shi S, Chen Q, Lin S, Wang R, Wang S, et al. Antitumor and immunomodulatory activities of Ganoderma lucidum polysaccharides in glioma-bearing rats. Integr Cancer Ther. 2018;17(3): 674-83. CrossRef PubMed Google Scholar
-
118.Shen R, Ge Y, Qin Y, Gao H, Yu H, Wu H, et al. Sporoderm-broken spores of Ganoderma lucidum modulate hepatoblastoma malignancy by regulating RACK1-mediated autophagy and tumour immunity. J Cell Mol Med. 2024;28(6): e18223. CrossRef PubMed Google Scholar
-
119.Li W, Zhou Q, Lv B, Li N, Bian X, Chen L, et al. Ganoderma lucidum polysaccharide supplementation significantly activates T-cell-mediated antitumor immunity and enhances anti-PD-1 immunotherapy efficacy in colorectal cancer. J Agric Food Chem. 2024;72(21): 12072-82. CrossRef PubMed Google Scholar
-
120.Ren L, Zhang J, Zhang T. Immunomodulatory activities of polysaccharides from Ganoderma on immune effector cells. Food Chem. 2021;340: 127933. CrossRef PubMed Google Scholar
-
121.Gao X, Homayoonfal M. Exploring the anti-cancer potential of Ganoderma lucidum polysaccharides (GLPs) and their versatile role in enhancing drug delivery systems: a multifaceted approach to combat cancer. Cancer Cell Int. 2023;23(1): 324. CrossRef PubMed Google Scholar
-
122.Shang D, Zhang J, Wen L, Li Y, Cui Q. Preparation, characterization, and antiproliferative activities of the Se-containing polysaccharide SeGLP-2B-1 from Se-enriched Ganoderma lucidum. J Agric Food Chem. 2009;57(17): 7737-42. CrossRef PubMed Google Scholar
-
123.Shang D, Li Y, Wang C, Wang X, Yu Z, Fu X. A novel polysaccharide from Se-enriched Ganoderma lucidum induces apoptosis of human breast cancer cells. Oncol Rep. 2011;25(1): 267-72. CrossRef PubMed Google Scholar
-
124.Zhang W, Lei Z, Meng J, Li G, Zhang Y, He J, et al. Water extract of sporoderm-broken spores of Ganoderma lucidum induces osteosarcoma apoptosis and restricts autophagic flux. Oncol Targets Ther. 2019;12: 11651-21165. CrossRef PubMed Google Scholar
-
125.Na K, Li K, Sang T, Wu K, Wang Y, Wang X. Anticarcinogenic effects of water extract of sporoderm-broken spores of Ganoderma lucidum on colorectal cancer in vitro and in vivo. Int J Oncol. 2017;50(5): 1541-54. CrossRef PubMed Google Scholar
-
126.Jiang D, Wang L, Zhao T, Zhang Z, Zhang R, Jin J, et al. Restoration of the tumor-suppressor function to mutant p53 by Ganoderma lucidum polysaccharides in colorectal cancer cells. Oncol Rep. 2017;37(1): 594-600. CrossRef PubMed Google Scholar
-
127.Liang Z, Guo YT, Yi YJ, Wang RC, Hu QL, Xiong XY. Ganoderma lucidum polysaccharides target a Fas/caspase dependent pathway to induce apoptosis in human colon cancer cells. Asian Pac J Cancer Prev. 2014;15(9): 3981-6. CrossRef PubMed Google Scholar
-
128.Liang Z, Yi Y, Guo Y, Wang R, Hu Q, Xiong X. Inhibition of migration and induction of apoptosis in LoVo human colon cancer cells by polysaccharides from Ganoderma lucidum. Mol Med Rep. 2015;12(5): 7629-36. CrossRef PubMed Google Scholar
-
129.Hsu W, Qiu W, Tsao S, Tseng A, Lu M, Hua W, et al. Effects of WSG, a polysaccharide from Ganoderma lucidum, on suppressing cell growth and mobility of lung cancer. Int J Biol Macromol. 2020;165: 1604-13. CrossRef PubMed Google Scholar
-
130.Hsu W, Hua W, Qiu W, Tseng A, Cheng H, Lin T. WSG, a glucose-enriched polysaccharide from Ganoderma lucidum, suppresses tongue cancer cells via inhibition of EGFR-mediated signaling and potentiates cisplatin-induced apoptosis. Int J Biol Macromol. 2021;193: 1201-8. CrossRef PubMed Google Scholar
-
131.Lo H, Lin T, Lin C, Wang W, Chen Y, Tsai P, et al. Targeting TGFβ receptor-mediated Snail and Twist: WSG, a polysaccharide from Ganoderma lucidum, and it-based dissolvable microneedle patch suppress melanoma cells. Carbohydr Polym. 2024;341: 122298. CrossRef PubMed Google Scholar
-
132.Zhao X, Zhou D, Liu Y, Li C, Zhao X, Li Y, et al. Ganoderma lucidum polysaccharide inhibits prostate cancer cell migration via the protein arginine methyltransferase 6 signaling pathway. Mol Med Rep. 2018;17(1): 147-57. CrossRef PubMed Google Scholar
-
133.Wang M, Yu F. Research progress on the anticancer activities and mechanisms of polysaccharides from Ganoderma. Front Pharmacol. 2022;13: 891171. CrossRef PubMed Google Scholar
-
134.Zhong J, Fang L, Chen R, Xu J, Guo D, Guo C, et al. Polysaccharides from sporoderm-removed spores of Ganoderma lucidum induce apoptosis in human gastric cancer cells via disruption of autophagic flux. Oncol Lett. 2021;21(5): 425. CrossRef PubMed Google Scholar
-
135.Yang K, Zhang Y, Cai M, Guan R, Neng J, Pi X, et al. In vitro prebiotic activities of oligosaccharides from the by-products in Ganoderma lucidum spore polysaccharide extraction. RSC Adv. 2020;10: 14794-802. CrossRef PubMed Google Scholar
-
136.Jin M, Zhu Y, Shao D, Zhao K, Xu C, Li Q, et al. Effects of polysaccharide from mycelia of Ganoderma lucidum on intestinal barrier functions of rats. Int J Biol Macromol. 2017;94: 1-9. CrossRef PubMed Google Scholar
-
137.Li D, Gao L, Li M, Luo Y, Xie Y, Luo T, et al. Polysaccharide from spore of Ganoderma lucidum ameliorates paclitaxel-induced intestinal barrier injury: apoptosis inhibition by reversing microtubule polymerization. Biomed Pharmacother. 2020;130: 110539. CrossRef PubMed Google Scholar
-
138.Sharma AR, Tirpude NV, Kumari M, Padwad YS. Rutin prevents inflammation-associated colon damage via inhibiting the p38/MAPKAPK2 and PI3K/Akt/GSK3β/NF-κB signalling axes and enhancing splenic Tregs in DSS-induced murine chronic colitis. Food Funct. 2021;12(18): 8492-506. CrossRef PubMed Google Scholar
-
139.Yu C, Jia G, Deng Q, Zhao H, Chen X, Liu G, et al. The effects of glucagon-like peptide-2 on the tight junction and barrier function in IPEC-J2 cells through phosphatidylinositol 3-kinase–protein kinase B–mammalian target of rapamycin signaling pathway. Asian-Australas J Anim Sci. 2016;29(5): 731-8. CrossRef PubMed Google Scholar
-
140.Xie J, Liu Y, Chen B, Zhang G, Ou S, Luo J, et al. Ganoderma lucidum polysaccharide improves rat DSS-induced colitis by altering cecal microbiota and gene expression of colonic epithelial cells. Food Nutr Res. 2019;63: e1559. CrossRef PubMed Google Scholar
-
141.Chen M, Xiao D, Liu W, Song Y, Zou B, Li L, et al. Intake of Ganoderma lucidum polysaccharides reverses the disturbed gut microbiota and metabolism in type 2 diabetic rats. Int J Biol Macromol. 2020;155: 890-902. CrossRef PubMed Google Scholar
-
142.Mann ER, Lam YK, Uhlig HH. Short-chain fatty acids: linking diet, the microbiome and immunity. Nat Rev Immunol. 2024;24(8): 577-95. CrossRef PubMed Google Scholar
-
143.Li M, van Esch BCAM, Henricks PAJ, Folkerts G, Garssen J. The anti-inflammatory effects of short chain fatty acids on lipopolysaccharide- or tumor necrosis factor α-stimulated endothelial cells via activation of GPR41/43 and inhibition of HDACs. Front Pharmacol. 2018;9: 533. CrossRef PubMed Google Scholar
-
144.Martin-Gallausiaux C, Larraufie P, Jarry A, Béguet-Crespel F, Marinelli L, Ledue F, et al. Butyrate produced by commensal bacteria down-regulates indoleamine 2,3-Dioxygenase 1 (IDO-1) expression via a dual mechanism in human intestinal epithelial cells. Front Immunol. 2018;9: 2838. CrossRef PubMed Google Scholar
-
145.Liu L, Feng J, Jiang S, Zhou S, Yan M, Zhang Z, et al. Anti-inflammatory and intestinal microbiota modulation properties of Ganoderma lucidum β-d-glucans with different molecular weight in an ulcerative colitis model. Int J Biol Macromol. 2023;251: 126351. CrossRef PubMed Google Scholar
-
146.Guo C, Guo D, Fang L, Sang T, Wu J, Guo C, et al. Ganoderma lucidum polysaccharide modulates gut microbiota and immune cell function to inhibit inflammation and tumorigenesis in colon. Carbohydr Polym. 2021;267: 118231. CrossRef PubMed Google Scholar
-
147.Su J, Li D, Chen Q, Li M, Su L, Luo T, et al. Anti-breast cancer enhancement of a polysaccharide from spore of Ganoderma lucidum with paclitaxel: Suppression on tumor metabolism with gut microbiota reshaping. Front Microbiol. 2018;9: 3099. CrossRef PubMed Google Scholar
-
148.Jin M, Zhu Y, Shao D, Zhao K, Xu C, Li Q, et al. Effects of polysaccharide from mycelia of Ganoderma lucidum on intestinal barrier functions of rats. Int J Biol Macromol. 2017;94: 1-9. CrossRef PubMed Google Scholar
-
149.Liu K-S, Zhang C, Dong H-L, Li K-K, Han Q-B, Wan Y, et al. GSP-2, a polysaccharide extracted from Ganoderma sinense, is a novel toll-like receptor 4 agonist. PLoS ONE. 2019;14(8): e0221636. CrossRef PubMed Google Scholar
-
150.Liu Y, Tang Q, Yang Y, Zhou S, Wu D, Tang C, et al. Characterization of polysaccharides from the fruiting bodies of two species of genus Ganoderma (Agaricomycetes) and determination of water-soluble β-D-glucan using high-performance liquid chromatography. Int J Med Mushrooms. 2017;19(1): 75-85. CrossRef PubMed Google Scholar
-
151.Yu Q, Nie SP, Li WJ, Zheng WY, Yin PF, Gong D-M, et al. Macrophage immunomodulatory activity of a purified polysaccharide isolated from Ganoderma atrum. Phytother Res. 2013;27(2): 186-91. CrossRef PubMed Google Scholar
-
152.Yu Q, Nie SP, Wang JQ, Yin PF, Huang DF, Li WJ, et al. Toll-like receptor 4-mediated ROS signaling pathway involved in Ganoderma atrum polysaccharide-induced tumor necrosis factor-α secretion during macrophage activation. Food Chem Toxicol. 2014;66: 14-22. CrossRef PubMed Google Scholar
-
153.Yu Q, Nie SP, Wang JQ, Huang DF, Li WJ, Xie MY. Signaling pathway involved in the immunomodulatory effect of Ganoderma atrum polysaccharide in spleen lymphocytes. J Agric Food Chem. 2015;63(10): 2734-40. CrossRef PubMed Google Scholar
-
154.Yu Q, Nie SP, Wang JQ, Huang DF, Li WJ, Xie MY. Molecular mechanism underlying chemoprotective effects of Ganoderma atrum polysaccharide in cyclophosphamide-induced immunosuppressed mice. J Funct Foods. 2015;15: 52-60. CrossRef PubMed Google Scholar
-
155.Xiang QD, Yu Q, Wang H, Zhao MM, Liu SY, Nie SP, et al. Immunomodulatory activity of Ganoderma atrum polysaccharide on purified T lymphocytes through Ca2+/CaN and mitogen-activated protein kinase pathway based on RNA sequencing. J Agric Food Chem. 2017;65(26): 5306-15. CrossRef PubMed Google Scholar
-
156.Hou K, Yu Q, Hu X, Ding X, Hong J, Chen Y, et al. Protective effect of Ganoderma atrum polysaccharide on acrolein-induced macrophage injury via autophagy-dependent apoptosis pathway. Food Chem Toxicol. 2019;133: 110757. CrossRef PubMed Google Scholar
-
157.Zhang S, Nie S, Huang D, Li W, Xie M. Immunomodulatory effect of Ganoderma atrum polysaccharide on CT26 tumor-bearing mice. Food Chem. 2013;136(3): 1213-9. CrossRef PubMed Google Scholar
-
158.Zhang S, Nie S, Huang D, Huang J, Feng Y, Xie M. A polysaccharide from Ganoderma atrum inhibits tumor growth by induction of apoptosis and activation of immune response in CT26-bearing mice. J Agric Food Chem. 2014;62(38): 9296-304. CrossRef PubMed Google Scholar
-
159.Zhu KX, Nie SP, Li C, Gong D, Xie MY. Ganoderma atrum polysaccharide improves aortic relaxation in diabetic rats via PI3K/Akt pathway. Carbohydr Polym. 2014;103: 520-7. CrossRef PubMed Google Scholar
-
160.Zheng B, Ying M, Xie J, Chen Y, Wang Y, Ding X, et al. A Ganoderma atrum polysaccharide alleviated DSS-induced ulcerative colitis by protecting the apoptosis/autophagy-regulated physical barrier and the DC-related immune barrier. Food Funct. 2020;11(12): 10690-9. CrossRef PubMed Google Scholar
-
161.Wang Y, Chang X, Zheng B, Chen Y, Xie J, Shan J, et al. Protective effect of Ganoderma atrum polysaccharide on acrolein-induced apoptosis and autophagic flux in IEC-6 cells. Foods. 2022;11(2): 240. CrossRef PubMed Google Scholar
-
162.Lan YH, Lee PC, Lu YS, Adela Nathania E, Kuo CH, Takemoto L, et al. Exploring the anti-invasive effects of Ganoderma tsugae (Songshan Lingzhi) against metastatic melanoma: Insights from an in vivo study on oxidative stress modulation. J Funct Foods. 2024;116: 106169. CrossRef PubMed Google Scholar
-
163.Yuan J, Ding L, Han L, Pang L, Zhang P, Yang X, et al. Thermal/ultrasound-triggered release of liposomes loaded with Ganoderma applanatum polysaccharide from microbubbles for enhanced tumour ablation. J Control Release. 2023;363: 84-100. CrossRef PubMed Google Scholar
-
164.Li M, Yu L, Zhai Q, Liu B, Zhao J, Zhang H, et al. Ganoderma applanatum polysaccharides and ethanol extracts promote the recovery of colitis through intestinal barrier protection and gut microbiota modulations. Food Funct. 2022;13(2): 688-701. CrossRef PubMed Google Scholar
-
165.Xu H, Liu L, Ding M, Fan W, Chen C, Song H. Effect of Ganoderma applanatum polysaccharides on MAPK/ERK pathway affecting autophagy in breast cancer MCF-7 cells. Int J Biol Macromol. 2020;146: 353-62. CrossRef PubMed Google Scholar
-
166.Zhen D, Su L, Miao Y, Zhao F, Ren G, Mahfuz S, et al. Purification, partial characterization and inducing tumor cell apoptosis activity of a polysaccharide from Ganoderma applanatum. Int J Biol Macromol. 2018;115: 10-7. CrossRef PubMed Google Scholar
-
167.Gao Z, Yuan F, Li H, Feng Y, Zhang Y, Zhang C, et al. The ameliorations of Ganoderma applanatum residue polysaccharides against CCl4-induced liver injury. Int J Biol Macromol. 2019;137: 1130-40. CrossRef PubMed Google Scholar
-
168.Gao X, Zeng R, Qi J, Ho C-T, Li B, Chen Z, et al. Immunoregulatory activity of a low-molecular-weight heteropolysaccharide from Ganoderma leucocontextum fruiting bodies in vitro and in vivo. Food Chem X. 2022;14: 100321. CrossRef PubMed Google Scholar
-
169.Gao X, Qi J, Ho C-T, Li B, Xie Y, Chen S, et al. Purification, physicochemical properties, and antioxidant activities of two low-molecular-weight polysaccharides from Ganoderma leucocontextum fruiting bodies. Antioxidants. 2021;10(7): 1145. CrossRef PubMed Google Scholar
-
170.Gao X, Qi J, Ho C-T, Li B, Mu J, Zhang Y, et al. Structural characterization and immunomodulatory activity of a water-soluble polysaccharide from Ganoderma leucocontextum fruiting bodies. Carbohydr Polym. 2020;249: 116874. CrossRef PubMed Google Scholar
-
171.Gow NAR, Lenardon MD. Architecture of the dynamic fungal cell wall. Nat Rev Microbiol. 2023;21(4): 248-59. CrossRef PubMed Google Scholar
-
172.Zol-Hanlon MI, Schumann B. Open questions in chemical glycobiology. Commun Chem. 2020;3(1): 102. CrossRef PubMed Google Scholar
-
173.Lechat H, Amat M, Mazoyer J, Buléon A, Lahaye M. Structure and distribution of glucomannan and sulfated glucan in the cell walls of the red alga Kappaphycus alvarezii (Gigartinales, Rhodophyta). J Phycol. 2000;36(5): 891-902. CrossRef PubMed Google Scholar
-
174.Lee TH, Arai M, Murao S. Localization of glucomannan and fucogalactomannan in Rhodotorula glutinis cell wall and spheroplast formation of its living cell. Agric Biol Chem. 1981;45: 2343-5. CrossRef PubMed Google Scholar
-
175.Stephens Z, Wilson LFL, Zimmer J. Diverse mechanisms of polysaccharide biosynthesis, assembly and secretion across kingdoms. Curr Opin Struct Biol. 2023;79: 102564. CrossRef PubMed Google Scholar
-
176.Liu JJ, Hou YK, Wang X, Zhou XT, Yin JY, Nie SP. Recent advances in the biosynthesis of fungal glucan structural diversity. Carbohydr Polym. 2024;329: 121782. CrossRef PubMed Google Scholar
-
177.Xu J, Ji S-L, Li H, Zhou J, Duan Y, Dang L, et al. Increased polysaccharide production and biosynthetic gene expressions in a submerged culture of Ganoderma lucidum by the overexpression of the homologous α-phosphoglucomutase gene. Bioprocess Biosyst Eng. 2015;38(2): 399-405. CrossRef PubMed Google Scholar
-
178.Adil B, Xiang Q, He M, Wu Y, Asghar MA, Arshad M, et al. Effect of sodium and calcium on polysaccharide production and the activities of enzymes involved in the polysaccharide synthesis of Lentinus edodes. AMB Express. 2020;10(1): 47. CrossRef PubMed Google Scholar
-
179.Xu J, Ji S, Li H, Zhou J, Duan Y, Dang L, et al. Increased polysaccharide production and biosynthetic gene expressions in a submerged culture of Ganoderma lucidum by the overexpression of the homologous α-phosphoglucomutase gene. Bioprocess Biosyst Eng. 2015;38(2): 399-405. CrossRef PubMed Google Scholar
-
180.Yang Y, Zhang Y, He J, Wu Q, Li Y, Li W, et al. Transcription factor GlbHLH regulates hyphal growth, stress resistance, and polysaccharide biosynthesis in Ganoderma lucidum. J Basic Microbiol. 2022;62(1): 82-91. CrossRef PubMed Google Scholar
-
181.Li H, Zhang D, Yue T, Jiang L, Yu X, Zhao P, et al. Improved polysaccharide production in a submerged culture of Ganoderma lucidum by the heterologous expression of Vitreoscilla hemoglobin gene. J Biotechnol. 2016;217: 132-7. CrossRef PubMed Google Scholar
-
182.Zhao L, Cao Y, Luo Q, Xu Y, Li N, Wang C, et al. Overexpression of phosphomannomutase increases the production and bioactivities of Ganoderma exopolysaccharides. Carbohydr Polym. 2022;294: 119828. CrossRef PubMed Google Scholar
-
183.Xu J, Yan X, Jia X, Wang Y, Xu H, Yu H, et al. A new strategy to improve Ganoderma polysaccharides production by symbiotic fungi elicitors through activating the biosynthetic pathway. Int J Biol Macromol. 2023;235: 123798. CrossRef PubMed Google Scholar
-
184.Zabotina OA, Zhang N, Weerts R. Polysaccharide biosynthesis: glycosyltransferases and their complexes. Front Plant Sci. 2021;12: 720709. CrossRef PubMed Google Scholar
-
185.Breton C, Šnajdrová L, Jeanneau C, Koča J, Imberty A. Structures and mechanisms of glycosyltransferases. Glycobiology. 2006;16(2): 29R-37R. CrossRef PubMed Google Scholar
-
186.Wang Q, Xu M, Zhao L, Chen L, Ding Z. Novel insights into the mechanism underlying high polysaccharide yield in submerged culture of Ganoderma lucidum revealed by transcriptome and proteome analyses. Microorganisms. 2023;11(3): 772. CrossRef PubMed Google Scholar
-
187.Fu X, Zan X, Sun L, Tan M, Cui F, Liang Y-Y, et al. Functional characterization and structural basis of the β-1,3-glucan synthase CMGLS from mushroom Cordyceps militaris. J Agric Food Chem. 2022;70(28): 8725-37. CrossRef PubMed Google Scholar
-
188.Gow NAR, Latge J-P, Munro CA. The fungal cell wall: structure, biosynthesis, and function. In: Heitman J, Howlett BJ, Crous PW, Stukenbrock EH, James TY, Gow NAR, editors. The fungal kingdom. 2017. pp. 267–92. PubMed Google Scholar
-
189.Sonets Ⅳ, Dovidchenko NV, Ulianov SV, Yarina MS, Koshechkin SI, Razin SV, et al. Unraveling the polysaccharide biosynthesis potential of Ganoderma lucidum: a chromosome-level assembly using Hi-C sequencing. J Fungi (Basel). 2023;9(10): 1020. CrossRef PubMed Google Scholar
-
190.Chen S, Xu J, Liu C, Zhu Y, Nelson DR, Zhou S, et al. Genome sequence of the model medicinal mushroom Ganoderma lucidum. Nat Commun. 2012;3(1): 913. CrossRef PubMed Google Scholar
-
191.Shahinian S, Bussey H. β-1,6-Glucan synthesis in Saccharomyces cerevisiae. Mol Microbiol. 2000;35(3): 477-89. CrossRef PubMed Google Scholar
-
192.Patel P, Free SJ. Characterization of Neurospora crassa GH16, GH17, and GH72 gene families of cell wall crosslinking enzymes. Cell Surf. 2022;8: 100073. CrossRef PubMed Google Scholar
-
193.Patel PK, Free SJ. The genetics and biochemistry of cell wall structure and synthesis in Neurospora crassa, a model filamentous fungus. Front Microbiol. 2019;10: 2294. CrossRef PubMed Google Scholar
-
194.Aimanianda V, Simenel C, Garnaud C, Clavaud C, Tada R, Barbin L, et al. The dual activity responsible for the elongation and branching of β-(1,3)-glucan in the fungal cell wall. MBio. 2017;8(3): e00619-e717. CrossRef PubMed Google Scholar
-
195.Gastebois A, Mouyna I, Simenel C, Clavaud C, Coddeville B, Delepierre M, et al. Characterization of a new β-(1–3)-glucan branching activity of Aspergillus fumigatus. J Biol Chem. 2010;285(4): 2386-96. CrossRef PubMed Google Scholar
-
196.Lin YL, Lee S-S, Hou S-M, Chiang BL. Polysaccharide purified from Ganoderma lucidum induces gene expression changes in human dendritic cells and promotes T helper 1 immune response in BALB/c mice. Mol Pharmacol. 2006;70(2): 637-44. CrossRef PubMed Google Scholar
-
197.Zhu X, Chen A, Lin Z. Ganoderma lucidum polysaccharides enhance the function of immunological effector cells in immunosuppressed mice. J Ethnopharmacol. 2007;111(2): 219-26. CrossRef PubMed Google Scholar
-
198.Yanni A, Kourkoutas Y. Editorial: functional foods and bioactive compounds for improving and maintaining digestive health. Front Nutr. 2022;8: 815370. CrossRef PubMed Google Scholar
-
199.Su L, Li D, Su J, Zhang E, Chen S, Zheng C, et al. Polysaccharides of sporoderm-broken spore of Ganoderma lucidum modulate adaptive immune function via gut microbiota regulation. Evid Based Complement Alternat Med. 2021;2021: 8842062. CrossRef PubMed Google Scholar
-
200.Wang J, Cao B, Zhao H, Feng J. Emerging roles of Ganoderma lucidum in anti-aging. Aging Dis. 2017;8(6): 691-707. CrossRef PubMed Google Scholar
Copyright information
© The Author(s) 2025.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.