Discovery of a parallel family of euglenatide analogs in Euglena gracilis
This work was supported by a grant from the National Institutes of Health (R35 GM144076 to R.A.B.).
Abstract
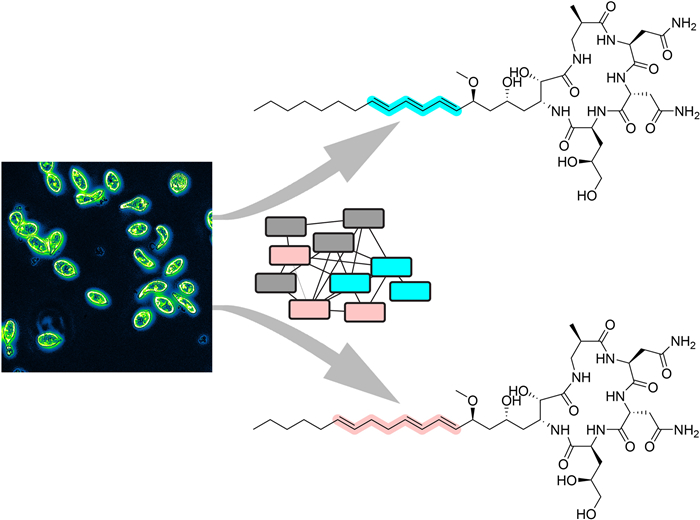
The euglenatides are a family of hybrid polyketide-nonribosomal peptides produced by the unicellular algae Euglena gracilis. These compounds have antiproliferative activity against fungal pathogens and mammalian cancer cell lines. Analysis of E. gracilis extracts revealed that the algae produce not only the euglenatides, but also a corresponding family of analogs that have the same molecular weights as the euglenatides, but are lacking the characteristic triene chromophore. In comparison to the euglenatides, the activity of these analogs is greatly reduced in a mammalian cytotoxicity assay, indicating that the triene is critical to the biological activity of the euglenatides.Graphical Abstract
Keywords
Euglena gracilis Euglenatide Natural products Polyketide Nonribosomal peptide1 Introduction
Polyketides and nonribosomal peptides are complex natural products that are produced on large enzymatic assembly lines known as polyketide synthases (PKSs) and nonribosomal peptide synthetases (NRPSs), respectively [1, 2]. Many of these natural products have been developed into important therapeutics, and thus discovery of structurally novel polyketides and nonribosomal peptides from underexplored sources is of interest. One of these potential sources is the unicellular flagellate algae Euglena gracilis, which can grow through photoautotrophic growth, using light as energy, heterotrophic feeding, using an external carbon source as energy, or a mixture of the two growth modes [3]. Although E. gracilis has been studied extensively as a potential source of food and biofuel, relatively little is known about its natural product biosynthetic potential. Even though the genome of E. gracilis has been only partially sequenced, these efforts indicate that the genome includes a large number of PKSs and NRPSs and other enzymes involved in secondary metabolism [4, 5].
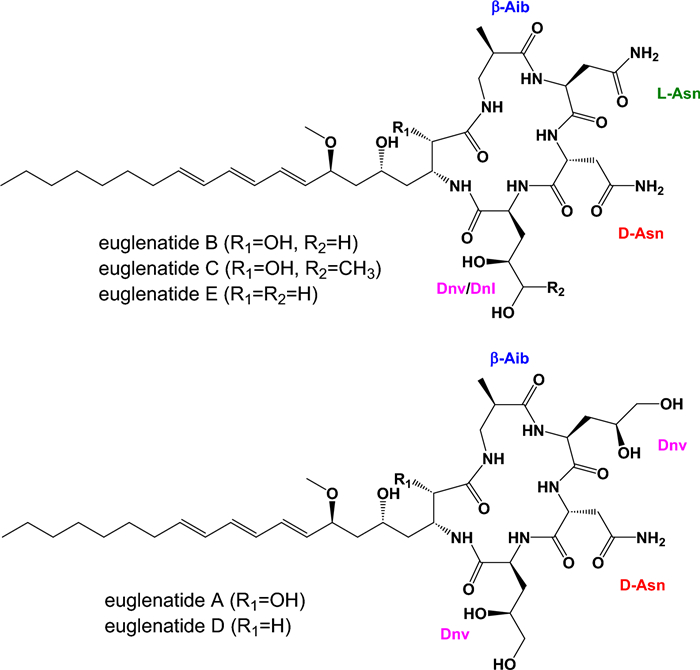
Recently, E. gracilis was shown to produce a family of hybrid polyketide-nonribosomal peptides, euglenatides A–E, with euglenatide B being the most abundantly produced (Fig. 1) [6]. Interestingly, the euglenatides are structurally similar to a family of hybrid polyketide-nonribosomal peptides previously identified in the nematode Caenorhabditis elegans [7-9]. The euglenatides have antiproliferative activity against multiple cancer cell lines in the mid-nanomolar range and several fungal pathogens in the low micromolar range, but have no activity against bacteria [6]. A survey of additional Euglena species suggests that they also produce similar natural products. Interestingly, production of the eugenatides did not occur in E. gracilis grown in rich medium and only occurred in E. gracilis grown in minimal medium containing specific amino acids, either glutamate or asparagine, under photosynthetic conditions [6].
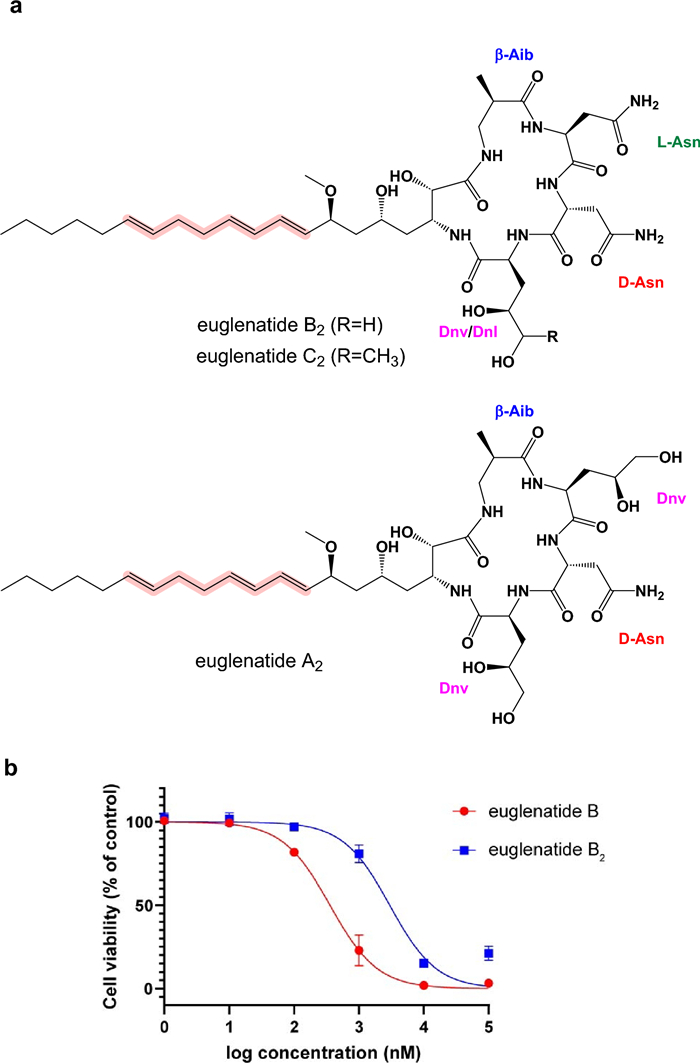
Previously reported structures of euglenatides A–E. The unusual amino acids found in the euglenatides include β-aminoisobutyric acid (β-Aib), 4, 5-dihydroxynorvaline (Dnv), and 4, 5-dihydroxynorleucine (Dnl)
Here, in analyzing extracts from E. gracilis, we discovered a new, parallel family of analogs that are produced in roughly equivalent amounts to euglenatides A–C. In comparison to the euglenatides, these analogs have the same molecular weights. However, the triene that is present in the euglenatides is disrupted in the analogs in that one of the double bonds is shifted by two carbons. The analogs are tenfold less bioactive against A549 lung adenocarcinoma cells than the euglenatides, indicating that the triene motif is important for activity.
2 Results and discussion
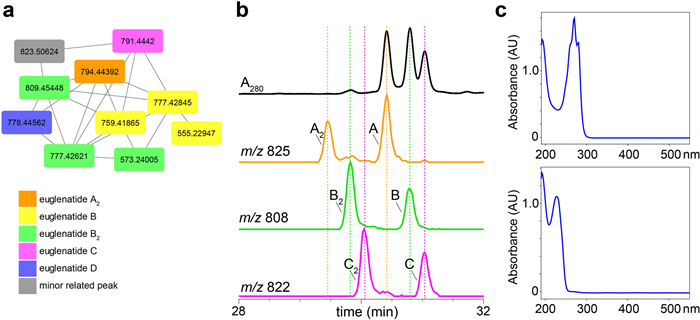
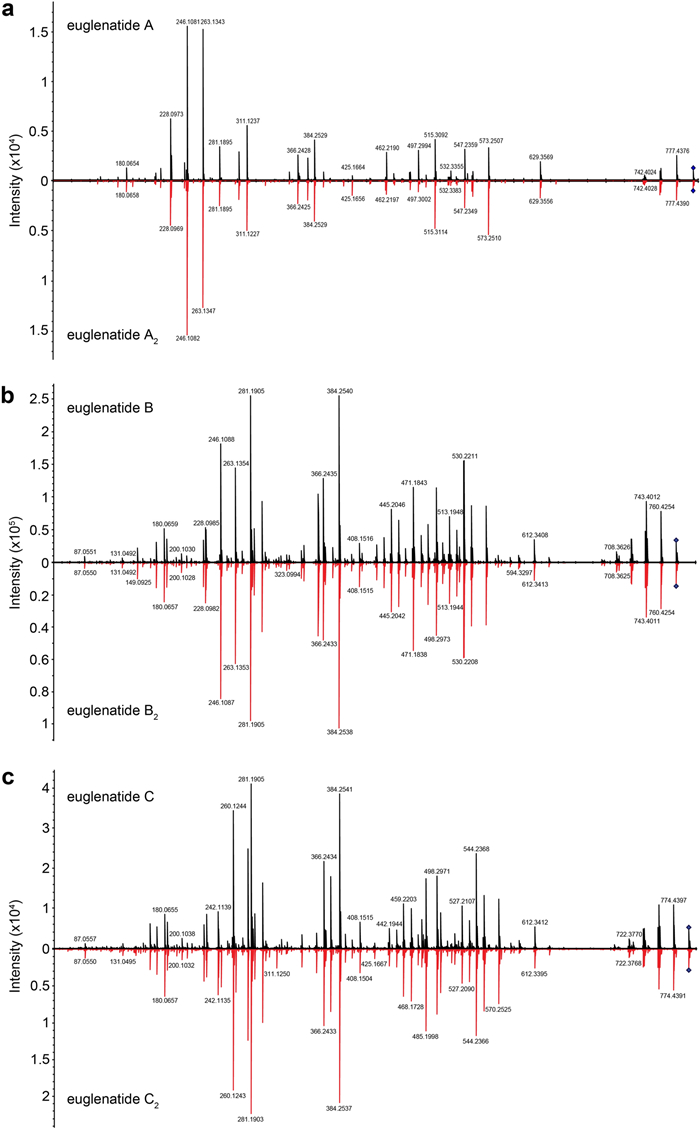
In order to determine whether E. gracilis produces additional euglenatides or euglenatide biosynthetic intermediates, we cultured E. gracilis using similar methods to those reported by Aldholmi et al. [6]; that is, we used minimal medium in which the carbon source was sodium acetate and the nitrogen source was glutamate, and we cultured the algae under photosynthetic conditions. In our hands, euglenatide B was more than tenfold more abundant than euglenatides A and C, euglenatide D was only minorly present, and euglenatide E was not detectable. Using molecular networking and LC–MS analysis of E. gracilis crude extracts, we noted that E. gracilis produces not only the euglenatides, but also a corresponding set of euglenatide analogs that we named euglenatides A2-C2 (Fig. 2) [10, 11]. These analogs have the same exact masses and MS fragmentation patterns as euglenatides A-C (Fig. 3, Fig. S1), but are slightly more polar based on their retention times (Fig. 2b). Unlike euglenatides A–C, which have a UV–Vis chromophore characteristic of a conjugated triene, euglenatides A2–C2 do not absorb at 280 nm and do not possess a conjugated triene (Fig. 2b, c). Each euglenatide is produced at roughly similar levels as its corresponding euglenatide analog. We have not been able to enhance the production of the euglenatide analogs relative to the euglenatides by harvesting the E. gracilis culture at a particular time point or utilizing a particular nitrogen source (glutamate versus asparagine).
E. gracilis produces the euglenatide analogs, euglenatides A2, B2, and C2. a Molecular networking analysis of crude E. gracilis extracts analyzed in positive (ESI+) mode. The orange node represents the [M-CH4O + H]+ ion for A2, the yellow nodes represent the [M-CH4O + H]+, [M-CH6O2 + H]+, and fragment ions of B, the green nodes represent the [M + H]+, [M-CH4O + H]+ and fragment ions of B2, the pink node represents the [M-CH4O + H]+ ion of C, and the purple node represents the [M-CH4O + H]+ ion of D. b Extracted ion chromatograms (EICs) of euglenatides A–C and their analogs A2–C2 analyzed in negative (ESI-) mode, along with the absorbance at 280 nm. The fraction analyzed here has roughly equal amounts of the different euglenatides and their analogs. However, in the crude extracts, euglenatide B and B2 are much more abundant than euglenatides A, A2, C, and C2. c UV–Vis spectrum of euglenatide B (top) and euglenatide B2 (bottom)
MS–MS spectra of the euglenatides and their analogs. Mirrored MS–MS spectra for euglenatides A and A2 (a), euglenatides B and B2 (b), euglenatides C and C2 (c)
To further characterize the chemical structures of the euglenatide analogs, we purified the most abundant one, euglenatide B2, as well as euglenatide B for comparison purposes. Chromatographic fractionation of defatted methanolic extracts of E. gracilis by reversed phase C18 flash chromatography, LH-20 Sephadex chromatography, and semipreparative HPLC allowed the purification of the two euglenatides (Fig. S2). Analysis of the 1D and 2D NMR (DMSO-d6) data for euglenatide B (Table 1; Table S1 and Figs. S3, S4) in comparison to literature [6] confirmed its identification (Fig. 1).
1D and 2D NMR (DMSO-d6) data for euglenatide B and its analog euglenatide B2
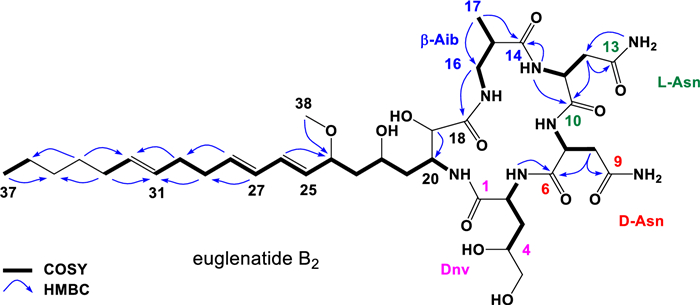
Comparison of the 1D NMR (DMSO-d6) data for euglenatide B2 with that of euglenatide B disclosed common peptide and polyketide substructures, with the significant differences being the presence of two extra allylic methylene resonances in euglenatide B2 at C-29 (δ2H 2.10, m; δC 32.2) and C-30 (δ2H 2.04, m; δC 31.7), accompanied by a change in the pattern of olefinic methine resonances (Fig. 4, Table 1; Table S2, and Figs. S5–S13). These NMR changes in euglenatide B2 suggested that one double bond (Δ31) is separated from the diene (Δ25 and Δ27) by two methylenes at C-29 and C-30, which explains the absence of the conjugated triene chromophore in the UV–Vis spectrum. Diagnostic 2D NMR correlations (shown in Fig. 4) confirmed the double bond shift and the molecular connectivity of euglenatide B2, while NMR similarities with euglenatide B, including comparable J values, and biosynthetic considerations suggested a common absolute configuration. Finally, Double Quantum Filtered (DQF)-COSY NMR analysis of euglenatide B2 allowed the assignment of the Δ31 double bond geometry as E (trans) based on a coupling constant (J31, 32) of 16.2 Hz (Figs. S10, S11). This assignment is further supported with the upfield chemical shifts of the allylic methylenes at C-30 (δC 31.7) and C-33 (δC 31.9) [12-15]. Thus, we conclude that the double bond at C-29 in euglenatide B has been shifted by two carbons to C-31 in euglenatide B2 (Fig. 5a).
Key 2D NMR correlations for the euglenatide B analog, euglenatide B2
Chemical structures of euglenatides A2, B2, and C2 (a) and the antiproliferative activity of euglenatides B and B2 against A549 cells (b)
Similarly, our data suggest that euglenatides A2 and C2 are exactly the same as euglenatides A and C, but one of the double bonds in the triene has been shifted by two carbons (Fig. 5a). Specifically, our data show that (1) euglenatides A-C and A2-C2 have the same exact masses and MS/MS fragmentation patterns (Fig. 3, Fig. S1), (2) the conjugated triene chromophore in euglenatides A-C is absent in A2-C2 (Fig. 2b, c), and (3) the euglenatides and their analogs have regularly spaced elution times by LC–MS (Fig. 2b). Several trials were made to purify euglenatides A, A2, C and C2 for NMR analysis, but unfortunately their low abundance and co-elution with other metabolites hindered their purification.
Previously, it was shown that euglenatide B had antiproliferative activity in the mid-nanomolar range against mammalian cancer cell lines, including A549 lung adenocarcinoma cells. To test the antiproliferative activity of the euglenatide analogs and thus the importance of the conjugated triene motif, we tested the activity of both euglenatides B and B2 against A549 cells (Fig. 5b). Our data confirm the potency of euglenatide B in the mid-nanomolar range, with an IC50 of 358 nM, in comparison to the previously reported IC50 of 773 nM. The data also show that euglenatide B2 is about tenfold less potent than euglenatide B, with an IC50 of 2987 nM. Thus, the conjugated triene is quite important for the activity of the euglenatides, and this information should inform future efforts to characterize the structure–activity relationships of the euglenatides.
3 Conclusions
Here, we show that E. gracilis produces not only the previously reported euglenatides A–C, but also a related family of euglenatide analogs, euglenatides A2–C2, in roughly equal amounts. In the euglenatide analogs, one of the double bonds in the conjugated triene has been shifted by two carbons, thereby disrupting the chromophore. The euglenatide B2 analog was fully characterized by NMR spectroscopy and mass spectrometry and shown to have antiproliferative activity that is roughly tenfold less than euglenatide B against A549 lung adenocarcinoma cells, indicating that the conjugated triene is important to the activity of the euglenatides. This information helps to characterize the structure–activity relationships of the euglenatides.
4 General experimental procedures
4.1 Cultivation of E. gracilis
E. gracilis var. saccharophila (CCAP 1224/5T) was obtained from the Culture Collection of Algae and Protozoa (United Kingdom). An axenic stock was generated by plating the algae on plates containing a 1:1 mix of E. gracilis medium (EG) and Jaworski's medium (JM) and 15 g/L of agar and picking a single colony. EG and JM were made using a previously described method [6]. E. gracilis was seeded typically in a 1:10 ratio into EG: JM medium in an Erlenmeyer flask and incubated at room temperature under a daylight lamp for 7d on a 150 rpm shaker. The lamp was set at a 12 h light/12 h dark cycle with an intensity of 2000 lumens.
4.2 Large scale cultivation of E. gracilis for euglenatide production
The EG: JM culture of E. gracilis was adjusted to 4 g/L (Abs 0.1 at 740 nm). 75 mL of this suspension was harvested by centrifugation at 3000 g at 4 ℃ for 20 min, washed three times with sterile Milli-Q water to remove any trace components of EG: JM medium, and seeded into 1.5L of synthetic medium (JM + 1 g/L sodium acetate trihydrate + 0.1 g/L calcium chloride) with 30 mM Glu (pH adjusted to 4.8 prior to autoclaving) in each bioreactor. The culture was incubated at room temperature under a daylight lamp at 12 h light/12 h dark cycle for 10d before collecting the cells for further extraction. Approximately 12 L of culture was generated in all. The cultures were centrifuged at 3000 g for 20 min, and the culture medium was discarded.
4.3 Purification of euglenatides
12L worth of cells were extracted with 2L of 90% MeOH. The 90% MeOH was extracted with 2L of hexanes. The MeOH layer was evaporated to yield the methanolic extractives (1.6 g) that were further fractionated on a C18 reversed phase flash column (300 mm × 46 mm, 80 g silica) with a 10% (200 mL) step-wise gradient elution from 20 to 100% MeCN. HPLC–DAD-MS analysis revealed the fraction eluting with 60% MeCN to be rich in euglenatides (Fig. S2). This fraction (80 mg) was chromatographed on an LH-20 Sephadex gel column, eluting with MeOH. Based on HPLC–DAD-MS analysis, subfractions containing mainly metabolites of m/z(–) 808 (euglenatide B and its analog) were combined (25 mg) and subjected to repeated semi-preparative reversed phase HPLC (HS C18-10, 250 mm × 10 mm, 10 μm, 3 mL/min) with an isocratic elution over 35 min with 34% MeCN/H2O to yield euglenatide B (3 mg) and its new isomer, euglenatide B2 (3 mg) (Fig. S2).
4.4 Euglenatide B
White powder; NMR (600 MHz, DMSO-d6) see Table 1 and Table S1 and Figs. S3, S4; ESI(−)MS m/z 808 [M − H]− and m/z 844 [M − H + Cl]−; HRESI(+)MS m/z 810.4618 [M + H]+ (calcd for C38H64N7O12, 810.4613).
4.5 Euglenatide B2
White powder; NMR (600 MHz, DMSO-d6) see Table 1 and Table S2 and Figures S5-S13; ESI(−)MS m/z 808 [M − H]− and m/z 844 [M − H + Cl]−; HRESI(+)MS m/z 810.4617 [M + H]+ (calcd for C38H64N7O12, 810.4613).
4.6 Antiproliferation assay
A549 cells were cultured in DMEM medium supplemented with 10% FBS, 100 U/mL penicillin and 100 μg/μL streptomycin. 6 × 103 A549 cells per 100 μL were seeded in a 96-well plate and incubated at 37℃ with 5% CO2. After 22 h of incubation, each well was treated with 1 μL of dimethyl sulfoxide (DMSO) or 1 μL of euglenatide B or euglenatide B2 in DMSO at final concentration of 105, 104, 103, 102, 10, or 1 nM. Each concentration was tested in triplicate. The plate was incubated for 72 h, and cell viability was tested with the MTS assay (Promega). IC50 values were calculated using GraphPad. Wells with only culture media were used as a blank, and wells seeded with A549 cells and treated with 1% DMSO were used as a negative control. The viability of treated A549 cells relative to the negative control was calculated after a blank correction.
Notes
Acknowledgements
The authors would like to thank J. Rocca for his assistance in acquiring NMR spectra, I. Ghiviriga for additional NMR advice, and R. Yu for help preparing samples. NMR spectra were acquired in the McKnight Brain Institute at the National High Magnetic Field Laboratory's Advanced Magnetic Resonance Imaging and Spectroscopy (AMRIS) Facility, which is supported by National Science Foundation Cooperative Agreement DMR-2128556 and the State of Florida. Mass spectrometry analysis was performed at the University of Florida's Mass Spectrometry Research and Education Center, which is supported in part by the National Institutes of Health (S10 OD030250-01A1). This work was supported by a grant from the National Institutes of Health (R35 GM144076 to R.A.B.).
Author contributions
A.H.E. purified compounds and elucidated their structures. X.K. cultured algae, purified compounds, and performed mammalian cell culture assays. S.R. tested growth conditions for enhancing analog production. D.V.P. performed mass spectrometry and molecular networking analysis. R.A.B. conceived the study, and R.A.B. and A.H.E. wrote the manuscript, which was approved by all authors.
Availability of data and materials
The authors will provide access to all datasets upon request.
Declarations
Competing interests
The authors declare no conflict of interest.
References
-
1.Nivina A, Yuet KP, Hsu J, Khosla C. Evolution and diversity of assembly-line polyketide synthases. Chem Rev 2019;119: 12524-47. CrossRef PubMed Google Scholar
-
2.Miyanaga A, Kudo F, Eguchi T. Protein-protein interactions in polyketide synthase-nonribosomal peptide synthetase hybrid assembly lines. Nat Prod Rep 2018;35: 1185-209. CrossRef PubMed Google Scholar
-
3.Inwongwan S, Kruger NJ, Ratcliffe RG, O'Neill EC. Euglena central metabolic pathways and their subcellular locations. Metabolites 2019;9: 115. CrossRef PubMed Google Scholar
-
4.Ebenezer TE, Zoltner M, Burrell A, Nenarokova A, Novak Vanclova AMG, Prasad B, Soukal P, Santana-Molina C, O'Neill E, Nankissoor NN, et al. Transcriptome, proteome and draft genome of Euglena gracilis. BMC Biol 2019;17: 11. CrossRef PubMed Google Scholar
-
5.O'Neill EC, Trick M, Hill L, Rejzek M, Dusi RG, Hamilton CJ, Zimba PV, Henrissat B, Field RA. The transcriptome of Euglena gracilis reveals unexpected metabolic capabilities for carbohydrate and natural product biochemistry. Mol Biosyst 2015;11: 2808-20. CrossRef PubMed Google Scholar
-
6.Aldholmi M, Ahmad R, Carretero-Molina D, Perez-Victoria I, Martin J, Reyes F, Genilloud O, Gourbeyre L, Gefflaut T, Carlsson H, et al. Euglenatides, potent antiproliferative cyclic peptides isolated from the freshwater photosynthetic microalga Euglena gracilis. Angew Chem Int Ed Engl 2022;61: e202203175. CrossRef PubMed Google Scholar
-
7.Shou Q, Feng L, Long Y, Han J, Nunnery JK, Powell DH, Butcher RA. A hybrid polyketide-nonribosomal peptide in nematodes that promotes larval survival. Nat Chem Biol 2016;12: 770-2. CrossRef PubMed Google Scholar
-
8.Butcher RA. Small-molecule pheromones and hormones controlling nematode development. Nat Chem Biol 2017;13: 577-86. CrossRef PubMed Google Scholar
-
9.Feng L, Gordon MT, Liu Y, Basso KB, Butcher RA. Mapping the biosynthetic pathway of a hybrid polyketide-nonribosomal peptide in a metazoan. Nat Commun 2021;600: 472-7. CrossRef PubMed Google Scholar
-
10.Wang M, Carver JJ, Phelan VV, Sanchez LM, Garg N, Peng Y, Nguyen DD, Watrous J, Kapono CA, Luzzatto-Knaan T, et al. Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nat Biotechnol 2016;34: 828-37. CrossRef PubMed Google Scholar
-
11.Aron AT, Gentry EC, McPhail KL, Nothias LF, Nothias-Esposito M, Bouslimani A, Petras D, Gauglitz JM, Sikora N, Vargas F, et al. Reproducible molecular networking of untargeted mass spectrometry data using GNPS. Nat Protoc 2020;15: 1954-91. CrossRef PubMed Google Scholar
-
12.Willis RH, de Vries DJ. BRS1, a C30 bis-amino, bis-hydroxy polyunsaturated lipid from an Australian calcareous sponge that inhibits protein kinase C. Toxicon 1997;35: 1125-9. CrossRef PubMed Google Scholar
-
13.Won TH, You M, Lee SH, Rho BJ, Oh DC, Oh KB, Shin J. Amino alcohols from the ascidian Pseudodistoma sp. Mar Drugs 2014;12: 3754-69. CrossRef PubMed Google Scholar
-
14.Ciavatta ML, Manzo E, Nuzzo G, Villani G, Varcamonti M, Gavagnin M. Crucigasterins A-E, antimicrobial amino alcohols from the Mediterranean colonial ascidian. Tetrahedron 2010;66: 7533-8. CrossRef PubMed Google Scholar
-
15.Garrido L, Zubía E, Ortega MJ, Naranjo S, Salvá J. Obscuraminols, new unsaturated amino alcohols from the tunicate: structure and absolute configuration. Tetrahedron 2001;57: 4579-88. CrossRef PubMed Google Scholar
Copyright information
© The Author(s) 2025.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.