Novel neo-clerodane diterpenoids from Teucrium quadrifarium and their anti-ferroptosis effect
This work was financially supported by the National Natural Science Foundation of China (32100322, 32460112, 32060100); and the Science and Technology Department of Guizhou Province (QKHZC[2022]019).
Abstract
Teucrifarides A–D (1–4), four previously unreported neo-clerodane-type diterpenoids, combined with sixteen known analogs (5–20), were purified from Teucrium quadrifarium. The absolute forma of compounds 1–4 were determined via spectroscopic and ECD calculation methods, together with X-ray crystallography experiments. Among them, compound 1 possessed a 5, 20-epoxy ring featuring a unique cage-like 12-oxatricyclo [5.3.2.01, 6]undecane skeleton. Meanwhile, 2 incorporated a 6, 20-epoxy ring with a novel 12-oxatricyclo [6.2.2.02, 7]undecane skeleton. Compounds 1 and 12 exhibited significant inhibitory effects against HT-22 cells ferroptosis induced by RSL3, with EC50 values of 11.8±1.0 μM, and 4.52±1.24 μM, respectively. Moreover, ROS accumulation in HT22 cells treated with compound 1 was also observed.Graphical Abstract
Keywords
Teucrium quadrifarium Lamiaceae Neo-clerodane Ferroptosis inhibitory activity ROS1 Introduction
The genus Teucrium (Lamiaceae) comprises shrubs and herbs, and the majority predominantly distributed in southwest regions of China [1]. The phytochemical research on this genus revealed that diterpenes, flavonoids, and triterpenes were the main metabolites of this plant [2]. Diterpenoids are well known as one of the primary secondary metabolites within the Lamiaceae family, and several complex diterpenoid scaffolds have been reported from the genus Teucrium [3-5]. Especially, abietane and clerodane exhibit interesting biological activities, including anti-ferroptosis [6], anti-inflammatory [7], anti-neuroinflammation [8], antifeedant [9], and cytotoxic activities [10], which have drawn the interest of pharmacologist.
Teucrium quadrifarium, a plant of the genus Teucrium, is a folk medicine to treat wind heat syndrome, and nosotoxicosis [11, 12]. However, to date, the phytochemical investigation of T. quadrifarium has yielded only a few natural compounds, such as flavonoids [13] and neo-clerodane diterpenoids [14]. As part of our efforts to obtain structurally intriguing and biologically significant clerodane-diterpenoids, a phytochemical study of T. quadrifarium was undertaken. As a result, twenty clerodane diterpenoids, including four previously undescribed neo-clerodane diterpenoids (1–4) were obtained. Herein, the structural elucidation, and anti-ferroptosis effects of these isolates were reported.
2 Results and discussion
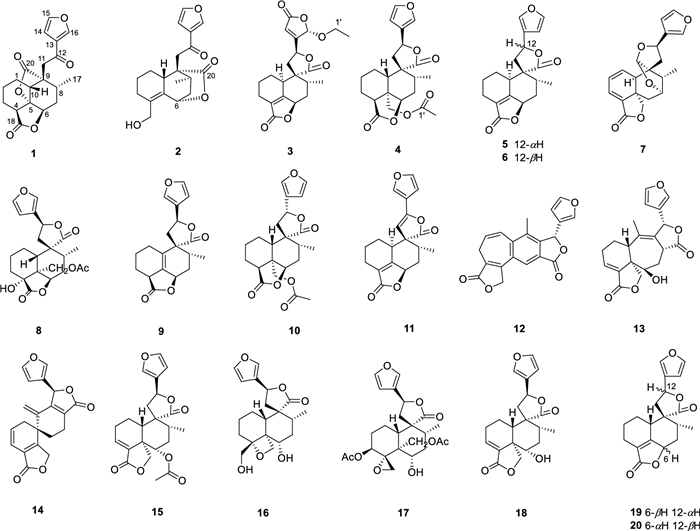
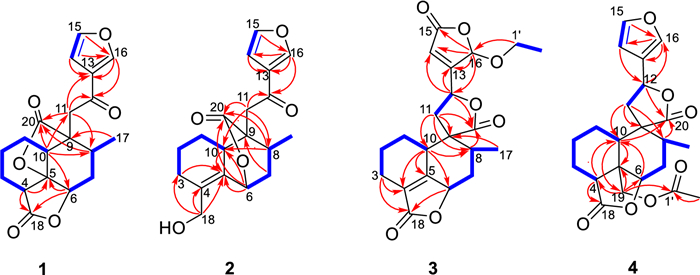
Teucrifaride A (1) possesses the molecular formula of C19H20O6, based on the HR-ESI-MS ion at m/z 367.1149 [M + Na]+ (cacld 367.1152). In the 1H NMR spectrum (Table 1), one methyl (δH 1.03) and three olefinic protons (δH 6.75, 7.46, 8.09) were observed. Moreover, in the 13C NMR (Table 2) spectrum, 19 carbon resonances were assigned to one methyl, five methylenes, seven methines, and six non-protonated carbons, respectively. Moreover, a characteristic furan ring [δH/δC 6.75 (d, J = 2.4 Hz)/108.4 (C-14), 7.46 (t, J = 1.8 Hz)/144.7 (C-15), 8.09 (s)/147.6 (C-16), and δC 127.8 (C-13)] was deduced based on the 1D NMR data. The 1H-1H COSY correlations of H2−1/H2−2/H2−3/H-4, H-10/H2−1, and H-6/H2−7/H-8, H-8/H3−17, combined with the HMBCs of H-4 (δH 3.12) with C-5, C-18, of H-6 (δH 4.50) with C-5, C-18, of H-10 (δH 2.90) with C-6, C-8, C-11, C-20, of H3−17 (δH 1.03) with C-9, and of H-11, H-13, and H-16 with C-12 (δC 191.8) suggested that compound 1 was a characteristic neo-clerodane diterpenoid analogue, with one γ-lactonic ring unit [15]. In addition, an additional γ-lactonic ring fragment constructed via C-5–C-20 according to the key HMBC correlations of H-10, H-8, and H-11 with C-20, combined with the downfield characteristic at C-5 (δC 83.9). Thus, as shown in Fig. 1, the planner structure of 1 was constructed.
1H NMR (600 MHz) data of compounds 1–4 in CDCl3 (δ in ppm, J in Hz)
13C NMR (150 MHz) data of compounds 1–4 in CDCl3
Key 1H-1H COSY and HMBC correlations of compounds 1–4
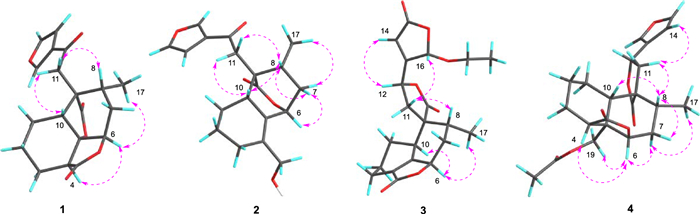
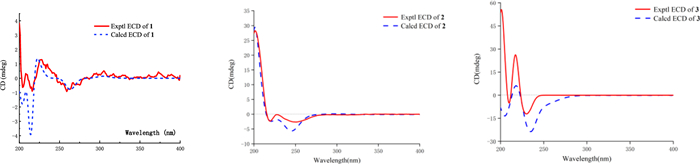
The NOESY data was used for analyzing the relative configuration of 1 (Fig. 2). The signals of CH3−17/H-6/H-4, assigned these groups were as α-orientation. On the contrary, the correlations of H-8/H-10/H-11 demonstrated these protons were β-orientation. Ultimately, the structure of 1 was fixed to be 4R, 5R, 6S, 8S, 9S, and 10S, through ECD calculation method (Fig. 3).
Key NOESY correlations of compounds 1–4
Experimental and Calculated ECD curves of 1–3
Teucrifaride B (2) with a molecular formula C19H22O5 in line with its HR-ESI-MS ion at m/z 353.1346 [M + Na]+ (cacld 353.1359). The NMR data of 2 closely resembled those of 1, indicated these two compounds share the almost same neo-clerodane skeleton. The main difference was a methylene [δH 4.14 (H-18), δC 61.6 (C-18)] in 2 replace of a carbonyl carbon δC 173.3 (C-18) in 1. Besides, an unusual δ-lactone ring was formed via C-6–C-7–C-8–C-9–C-20 based on the HMBC correlations of H-6, H-8, H-10, and H-11 with C-20, combined with the signals of H-6/H2−7/H-8/CH3−17 in the COSY spectrum (Fig. 1). Therefore, the planner structure of 2 was elucidated. Furthermore, the NOESY correlations (Fig. 2) of H-10/H-8/H-11, and H-8/H-7β/H-6, manifested that H-10, H-8, H-6, and H-11 were β-orientation. Similarly, CH3−17 was α-oriented deduced by the NOESY signals of CH3−17/H-7α. Subsequently, the structure of 2 was determined to be 6R, 8S, 9S, 10R, according to the calculated ECD curve was the same with that of experimental ECD curves in the range of 200–400 nm (Fig. 3).
Teucrifaride C (3) was yield as a white powder. The HR-ESI-MS data (m/z 411.1408 [M + Na]+; cacld for 411.1414) suggested a molecular of C21H24O7. By comparison of 1D NMR data, the structure of 3 is similar to the structure of teucvidin (5) [16], except for an ethoxy group [δH (1.26, t, J = 7.2 Hz), 3.78(1H, m), 3.99 (1H, m); δC 15.2, 67.4] and an α, β-unsaturated-γ-lactone fragment (δC 169.1, 163.3, 119.0, 101.9) in 3. Furthermore, the ethoxy unit was fixed at C-16 due to the 1H-1H COSY correlations of H3−2′/H2−1ʹ (Fig. 1), together with the HMBC correlations (Fig. 1) from H2−1′ (δH 3.78, 3.99) to C-16. Besides, the HMBC correlations from H-16 to C-15, C-13, C-14, and from H-12 to C-13, C-14, C-16, further constructed an a, b- unsaturated-γ-lactone fragment at C-12. Furthermore, the signals of H-10/H-6/H3−17 in the NOESY spectrum revealed that they were α-oriented. In contrast, the NOESY correlations of H-8/H-11b/H-16, and H-12/H-14 were deserved indicating that H-8, H-16 were β-oriented (Fig. 1). The test ECD curve of 3 was well matched with that of calculated ECD spectra (Fig. 3), and the structure of 3 was assigned as Fig. 4.
The chemical structures of compounds 1–20
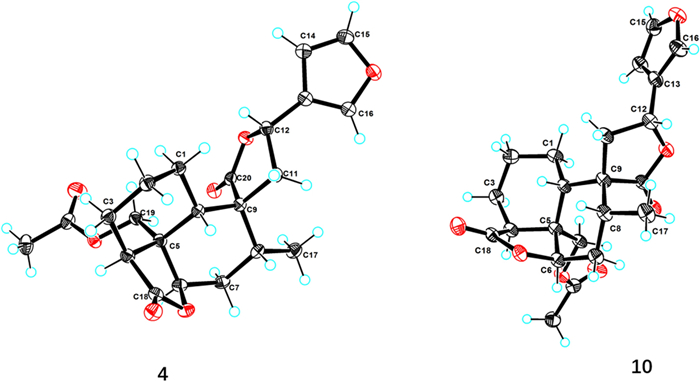
Teucrifaride D (4), a colorless acicular crystal, with the molecular formula of C22H26O7 based on the HR-ESI-MS ion at m/z of 425.1559 [M + Na]+ (C22H26O7Na, cacld for 425.1571) and 13C NMR data. After careful analysis of 1D NMR data of 4 (Tables 1 and 2), we deduced that 4 possesses the same structure as that of teucvisin B (10) [17]. Moreover, the NOESY correlations of H-10/H-7β/H-8/H-11/H-14, combined with the opposite signals of H-6/H-7α/CH3−17, further suggested H-12 was α-oriented. Finally, the structure of 4 was undoubtedly confirmed to be 4S, 5R, 6R, 8R, 9R, 10S, 12R through the signal-crystal X-ray diffraction experiment (Fig. 5).
X-ray ORTEP drawing of compounds 4 and 10
Sixteen known diterpenoids were determined to be teucvidin (5) [16], 12-epiteucvidin (6) [16], salvifaricin (7) [18], 19-acetyl-teuspinin (8) [19], isoteuflin (9) [20], teucvisin B (10) [17], crotocaudin (11) [21], isosalvipuberulin (12) [22], de-O-acetylsalvigenolide (13) [23], salvileucalin A (14) [18], 6a-acetoxyteuscordin (15) [24], montanin D (16) [25], teumicropodin (17) [26], 6-a-hydroxy-teuscordin (18) [27], teucvin (19) [28] and 12-epiteuflin (20) [16] through comparison of the NMR data with those of literatures.
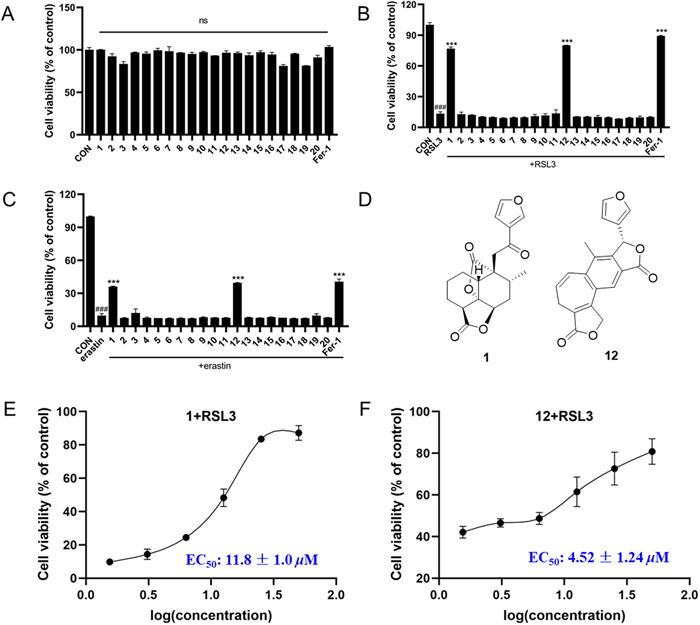
Considering that clerodane-diterpenoids were reported a remarkable anti-ferroptosis effect [6, 29], we evaluated the ferroptosis inhibitory effect of all isolates in HT22 cells. Firstly, their cytotoxicity was assayed, and none of them exhibited cytotoxicity on HT22 cells (Fig. 6A) at 40 μM. Then, the anti-ferroptosis effect of the isolates on HT22 cells deduced by RSL3 and erastin as depicted in Fig. 6B and Fig. 6C. Notably, of them, compounds 1 and 12 indicated obvious inhibitory effect against RSL3-induced ferroptosis, and potent ferroptosis inhibition on erastin-induced model, respectively. And the EC50 values of 1 and 12 were 11.8 ± 1.0 μM, and 4.52 ± 1.24 μM, respectively, for above two different induced-models (Fig. 6D).
Effects of compounds 1–20 on RSL3-induced HT22 cells ferroptosis. A Cell viability of HT22 cells treated with 40 μM isolated compounds and 1 μM Fer-1. B Cell viability of HT22 cells co-treated with 1 μM RSL3 and 40 μM isolated compounds and 1 μM Fer-1 for 24 h determined by an MTT assay. C Cell viability of HT22 cells co-treated with 1 μM erastin and 40 μM isolated compounds and 1 μM Fer-1 for 24 h determined by an MTT assay. D Chemical structures of 1 and 12. Dose–response relationship of compounds 1 (E), and 12 (F) inhibiting RSL3-induced ferroptosis in the HT22 cells. Data represent the means ± SEM from triplicate experiments. ###P < 0.0001 compared with control cell; ***P < 0.0001 compared with cells treated with RSL3
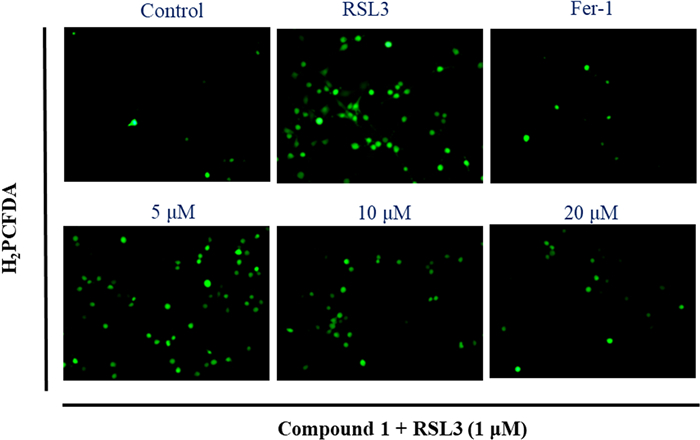
The increase of intracellular reactive oxygen species (ROS) was characteristic biomarkers of ferroptosis. To further explore the ferroptosis inhibition of new compound 1 against RSL3-reduced HT22 cells, the intracellular ROS levels by fluorescence microscopy was detected. As shown in Fig. 7, at the concentrations of 5, 10, and 20 μM, compound 1 can prominently inhibit intracellular ROS accumulation in a dose-dependent manner. The above results preliminarily uncovered that new compound 1 exerts anti-ferroptosis activity should be related to target mitochondria. However, to date, there is no clear mechanism for ferroptosis. Thus, compound 1 should be further researched for its anti-ferroptosis effective mechanism in the future.
The intracellular ROS levels was detected by fluorescence microscopy: HT22 cells induced by RSL3 and treated with an indicated concentrations of compound 1 (5, 10, and 20 μM) or Fer-1(1 μM) for 4 h under a fluorescence microscope after H2PCFDA staining (20x)
3 Conclusion
In conclusion, twenty neo-clerodane isloates were isolated from T. quadrifarium, including four new (1–4) and sixteen known ones (5–20). Among them, fifteen compounds were isolated from this plant for the first time, and the results enriches the chemical components of this plant. Additionally, new compound 1 exhibited a significant ferroptosis inhibitory effect against RSL3-induced HT22 cells.
4 Experimental section
4.1 General experimental procedures
XtaLAB Pro diffractometer was used to determine X-ray crystallography. HR-ESI–MS, IR, UV, NMR spectra were determined as described previously [30].
4.2 Plant material
The whole plants of T. quadrifarium were collected in Pingtang County, Guizhou Province, China, in August, 2021, and identified by Prof. Ni Zhang. A specimen voucher (No. TZC20210806) is stored in the Natural Products Research Center of Guizhou Province.
4.3 Extraction and isolation
The air-dried material (20.0 kg) was smashed and extracted with 95% aqueous EtOH (3 × 70 L, each 2 h) under reflux. The crude EtOH extract was obtained by evaporation under vacuum conditions. The crude samples were suspended in H2O and then leached with petroleum ether (PE), and ethyl acetate (EA), respectively. The PE part (1047.4 g) and EA part (819.9 g) were evaporated to dryness. The PE layer was purified with silica gel chromatography, employing stepwise gradient elution with PE/EA mixture ranging from.
100:0 to 0:100, to afford seven fractions (Fr. A–G), according to TLC plates. Fr. D (74.5 g) was isolated on CC (MeOH/H2O, from 50:50 to 95:5) to yield eight fractions (Fr. D1–D8). Fr. D5 (13.0 g) was further purified via Sephadex LH-20 (CH2Cl2/MeOH, 1:1) to yield compound 5 (32.3 mg), 6 (18.4 mg). Compound 1 (MeOH/H2O, 57:43, tR 19.5 min, 9.3 mg), and 9 (MeCN/H2O, 49:51, tR 26.0 min, 9.1 mg) were obtained from Fr. D6 via semi-preparative HPLC. And compounds 4 (MeCN/H2O, 45:55, tR 53.0 min, 5.6 mg), 10 (MeCN/H2O, 45:55, tR 47.0 min, 3.6 mg) isolated from Fr. D6 by semi–preparative HPLC. Fr. D4 was isolated through Sephadex LH-20 (CH2Cl2/MeOH, 1:1) and semi-preparative HPLC to give compounds 11 (MeOH/H2O, 64:36, tR 27.5 min, 9.6 mg), and 12 (MeOH/H2O, 48:52, tR 31.0 min, 1.9 mg). Fr. D3 was repeatedly separated on CC, and subsequently purified to have 7 (13.2 mg) and 8 (6.3 mg) via Sephadex LH-20 (CH2Cl2/MeOH, 1:1). Fr. F (87.5 g) was chromatographed on CC (MeOH/H2O, from 30:70 to 95:5) to give eight fractions (Fr. F1–Fr. F8). Fr. F2 (17.2 g) further afforded six fractions (Fr. F2a–Fr. F2f) after purified by silica gel (CH2Cl2/MeOH, from 100:1 to 0:100). Compounds 13 (MeCN/H2O, 35:65, tR 27.0 min, 2.8 mg), 14 (MeCN/H2O, 35:65, tR 40.0 min, 3.3 mg), and 15 (MeOH/H2O, 48:52, tR 48.0 min, 17.9 mg) were obtained from Fr. F2c, and compounds 16 (MeCN/H2O, 40:60, tR 36.0 min, 17.8 mg), 17 (MeOH/H2O, 48:52, tR 39.0 min, 12.5 mg), 18 (MeCN/H2O, 48:52, tR 26.0 min, 9.8 mg) were yield from Fr.F2d, by semi-preparative HPLC.
The EA layer was purified with silica gel chromatography and afforded eight fractions (Fr. EA-Fr. EH) according to TLC plates. Fr. EB (26.8 g) was further chromatographed on CC (MCI, MeOH/H2O, from 30:70 to 95:5) to give nine fractions (Fr. EB1–EB9). Compounds 2 (MeOH/H2O, 56:44, tR 27.0 min, 12.5 mg), 3 (MeCN/H2O, 45:55, tR 37.0 min, 7.0 mg), 19 (MeOH/H2O, 40:60, tR 48.0 min, 2.5 mg), and 20 (MeOH/H2O, 40:60, tR 57.0 min, 4.2 mg) were purified from Fr. EB5 through semi-preparative HPLC.
Teucrifaride A (1): Yellow oil; UV (MeOH) λmax (log ε): 200 nm: (2.87);
Teucrifaride B (2): Yellow oil; UV (MeOH) λmax (log ε): 200 (2.87) nm, 254 (2.00) nm;
Teucrifaride C (3): White and amorphous powder; UV (MeOH) λmax (log ε): 206 nm: (3.28);
Teucrifaride D (4): Colorless acicular crystal; mp 151–152 ℃; UV (MeOH) λmax (log ε): 208 nm: (3.68);
4.4 Single-crystal X-ray diffraction analysis of 4 and 10
A suitable crystal of compounds 4 and 10 were chosen and subjected to X-ray diffraction analysis on a XtaLAB AFC12 single X-ray diffractometer. The crystal was maintained at 100.00 (10) K for 4, and at 99.99 (10) K for 10, during data collection. The structural analysis was initiated using the Olex2 software, where the crystal structures were determined by the SHELXT program with intrinsic phasing. Subsequently, the structures were refined using the SHELXL refinement package, employing least squares minimization techniques [30]. The crystallographic data (CCDC.2383165 for 4 and CCDC.2383166 for 10) can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html.
4.5 ECD calculations
ECD calculation methods were the same with previous reports [30].
4.6 Cell viability assay
HT22 cells were grown in a culture medium consisting of DMEM (from HyCyte) supplemented with 1% penicillin/streptomycin (ECOTOP) and 10% fetal bovine serum (HyCyte). The culture was maintained in a 5% CO2 environment at a temperature of 37 ℃ within an incubator. HT22 cells were plated at a density of 3000 cells per well in 96-well plates and allowed to attach for 24 h. Subsequently, RSL3 (1 μM) or erastin (1 μM) along with different concentrations of compounds were introduced. After 24 h, MTT reagent (10 μL, 5 mg/mL) was introduced to each well and incubated for an additional 4 h. Following this, the formazan crystals were solubilized using 150 μL of DMSO. The plates were softly shaken for 15 min at ambient temperature, and then tested the absorbance at 490 nm using a microplate reader.
4.7 Intracellular reactive oxygen species detection
HT22 cells were cultured at 300, 000 cells/well in 6-well plates for 24 h. RSL3 (1 μM) and three different concentrations (5, 10, 20 μM) of compound 1 and Fer-1 (1 μM) were added to plates. After a 4 h reaction, the supernatant medium was aspirated off and H2PCFDA (1 mL, 10 μM) was added in an incubator and incubated for 40 min (37 ℃), then washed twice with serum-free medium and photographed with a fluorescence microscope under dark conditions.
Notes
Author contributions
Huan Wang and Han-Fei Liu: Writing-original draft, methodology, investigation. Xiao-Qiao Yang: Methodology, investigation, formal analysis. Yu-Qiong Liao: Formal analysis, investigation. Fen-Cong Pan: Formal analysis, investigation. Jin-Yu Li: Supervision, Project administration, Writing-original draft. Hua-Yong Lou: Design experiment, Writing – review & editing, Project administration, Funding acquisition. Wei-Dong Pan: Writing-review, editing, project administration. All authors read and approved the final submission.
Data availability
The data that support the findings of this study are openly available in the Science Data Bank at https://doi.org/https://doi.org/
Declarations
Competing interests
There is no competing interest.
References
-
1.Flora of China Editorial Committee of Chinese Academy of Sciences. Flora of China. Beijing: Science Press, 1990. PubMed Google Scholar
-
2.Zhang K, Wang XL, Li YK, Li J, Xv KP, Tan GS. Chemical constituents and pharmacological activities of genus Teucrium. Central South Pharm 2016;14(7): 735-41. PubMed Google Scholar
-
3.Aydogan F, Boga M, Khan SI, Zulfiqar F, Khan IA, Ali Z. Phytochemical investigation of Teucrium pruinosum and biological potential assessment of the isolated diterpenoids. Biochem Syst Ecol 2022;105: 104545. CrossRef PubMed Google Scholar
-
4.Kurimoto S, Wakabayashi K, Sasaki YF, Nakamura T, Kubota T. Teuchamaedol A, a new neo-clerodane diterpenoid from the aerial parts of Teucrium chamaedrys. Tetrahedron Lett 2022;100: 153890. CrossRef PubMed Google Scholar
-
5.Lv HW, Luo JG, Zhu MD, Zhao HJ, Kong LY. neo-Clerodane diterpenoids from the aerial parts of Teucrium fruticans cultivated in China. Phytochemistry 2015;119: 26-31. CrossRef PubMed Google Scholar
-
6.Tan Q, Fang Y, Peng X, Zhou H, Xu J, Gu Q. A new ferroptosis inhibitor, isolated from Ajuga nipponensis, protects neuronal cells via activating NRF2-antioxidant response elements (AREs) pathway. Bioorg Chem 2021;115: 105177. CrossRef PubMed Google Scholar
-
7.Li S, Xu D, Jia J, Zou W, Liu J, Wang Y, Zhang K, Zheng X, Ma YY, Zhang X, Zhao DG. Structure and anti-inflammatory activity of neo-clerodane diterpenoids from Scutellaria barbata. Phytochemistry 2023;213: 113771. CrossRef PubMed Google Scholar
-
8.Peng X, Tan Q, Zhang Z, Wu D, Xu J, Zhou H, Gu Q. Discovery of neo-clerodane diterpenoids from Ajuga campylantha as neuroprotective agents against ferroptosis and neuroinflammation. J Nat Prod 2023;86(8): 2006-21. CrossRef PubMed Google Scholar
-
9.Krishna Kumari GN, Aravind S, Balachandran J, Ganesh MR, Soundarya Devi S, Rajan SS, Malathi R, Ravikumar K. Antifeedant neo-clerodanes from Teucrium tomentosum Heyne (Labiatae). Phytochemistry 2003. CrossRef PubMed Google Scholar
-
10.Kheawchaum S, Mahidol C, Thongnest S, Boonsombat J, Batsomboon P, Sitthimonchai S, Ruchirawat S, Prawat H. Ent-abietane diterpenoid lactone glycosides and a phenolic glycoside from Phlogacanthus pulcherrimus T Anderson with cytotoxic and cancer chemopreventive activities. Phytochemistry 2022;201: 113261. CrossRef PubMed Google Scholar
-
11.Hunan Academy of Chinese Medicine. Pharmaceutical Records of Hunan. Changsha: Hunan People's Publishing House, 1979. PubMed Google Scholar
-
12.National Institutes for Food and Drug Contro. Yunnan Institute for Drug Control. In: Annals of Chinese national medicine. Beijing: People's Medical Publishing House, 1984. PubMed Google Scholar
-
13.Xie N, Ming ZD, Zhao SX, Feng R. Flavones from Teurium quadrifarium. J China Pharm Univ 1991;22(4): 200-2. PubMed Google Scholar
-
14.Zhu YY, Li GY. Studies on the diterpenoids of Teucrium quadrifarium Buch-Ham. Acta Pharm Sin B 1993;28(9): 679-83. PubMed Google Scholar
-
15.Aydoğan F, Ali Z, Zulfiqar F, Karaalp C, Khan IA, Bedir E. neo-clerodanes from Teucrium divaricatum subsp. divaricatum and their biological activity assessment. Phytochem Lett 2023;54: 45-9. CrossRef PubMed Google Scholar
-
16.Simoes F, Rodríguez B, Bruno M, Piozzi F, Savona G, Arnold NA. neo-Clerodane diterpenoids from Teucrium kotschyanum. Phytochemistry 1989;28(10): 2763-8. CrossRef PubMed Google Scholar
-
17.Lv HW, Luo JG, Meng DZ, Shan SM, Kong LY. Teucvisins A-E, five new neo-clerodane diterpenes from Teucrium viscidum. Chem Pharm Bull 2014;62(5): 472-6. CrossRef PubMed Google Scholar
-
18.Aoyagi Y, Yamazaki A, Nakatsugawa C, Fukaya H, Takeya K, Kawauchi S, Izumi H. Salvileucalin B, a novel diterpenoid with an unprecedented rearranged neoclerodane skeleton from Salvia leucantha Cav. Org Lett 2008;10(20): 4429-32. CrossRef PubMed Google Scholar
-
19.Piozzi F, Savona G, Paternostro M, Rodriguez BP. New clerodane diterpenoids from Teucrium spinosum L. Heterocycles 1980;14: 193. CrossRef PubMed Google Scholar
-
20.Bruno M, Piozzi F, Savona G, De La Torre MC, Rodríguez B. neo-Clerodane diterpenoids from Teucrium canadense. Phytochemistry 1989;28(12): 3539-41. CrossRef PubMed Google Scholar
-
21.Li W, Wang RM, Pan YH, Zhao YY, Yuan FY, Huang D, Tang GH, Bi HC, Yin S. Crotonpenoids A and B, two highly modified clerodane diterpenoids with a tricyclo[7.2.1.02, 7]dodecane core from Croton yanhuii: isolation, structural elucidation, and biomimetic semisynthesis. Org Lett 2020;22(11): 4435-9. CrossRef PubMed Google Scholar
-
22.Xu G, Peng L, Niu X, Zhao Q, Li R, Sun H. Novel diterpenoids from Salvia dugesii. Helv Chim Acta 2004;87(4): 949-55. CrossRef PubMed Google Scholar
-
23.Jiang YJ, Su J, Shi X, Wu XD, Chen XQ, He J, Shao LD, Li XN, Peng LY, Li RT, Zhao QS. neo-Clerodanes from the aerial parts of Salvia leucantha. Tetrahedron 2016;72(35): 5507-14. CrossRef PubMed Google Scholar
-
24.Papanov GY, Malakov PY. New furanoid diterpenes from Teucrium scordium L. Zeitschrift für Naturforschung B 1981;36(1): 112-3. CrossRef PubMed Google Scholar
-
25.Ye D, Shu LP, Qiang Z, Xun L, Li SD. Clerodane diterpenoids from Kinostemon alborubrum. Helv Chim Acta 2002;85(8): 2547-52. CrossRef PubMed Google Scholar
-
26.Maria C, Rodríguez B, Bruno M, Savona G, Piozzi F, Servettaz O. neo-clerodane diterpenoids from Teucrium micropodioldes. Phytochemistry 1988;27(1): 213-6. CrossRef PubMed Google Scholar
-
27.Gacs-Baitz E, Kajtar M, Papanov G, Malakov P. Carbon-13 NMR spectra of some furanoid diterpenes from Teucrium species. Heterocycles 1982;19: 539-50. CrossRef PubMed Google Scholar
-
28.Mbwambo ZH, Foubert K, Chacha M, Kapingu MC, Magadula JJ, Moshi MM, Lemiere F, Goubitz K, Fraanje J, Peschar R, Vlietinck A, Apers S, Pieters L. New furanoditerpenoids from Croton jatrophoides. Planta Med 2009;75(3): 262-7. CrossRef PubMed Google Scholar
-
29.Shi HW, Jiang XF, Cao LD, Peng X, Tan QY, Teng XF, Gu Q, He L. Chemical constituents of Ajuga forrestii and their anti-ferroptosis activity. Fitoterapia 2023;166: 105461. CrossRef PubMed Google Scholar
-
30.Zhao X, Zheng ZP, Chen C, Wang H, Liu HF, Li JY, Sun C, Lou HY, Pan WD. New clerodane diterpenoids from Callicarpa pseudorubella and their antitumor proliferative activity. Fitoterapia 2024;174: 105878. CrossRef PubMed Google Scholar
Copyright information
© The Author(s) 2025.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.