Widely targeted metabolomics analysis reveals differences in volatile metabolites among four Angelica species
Abstract
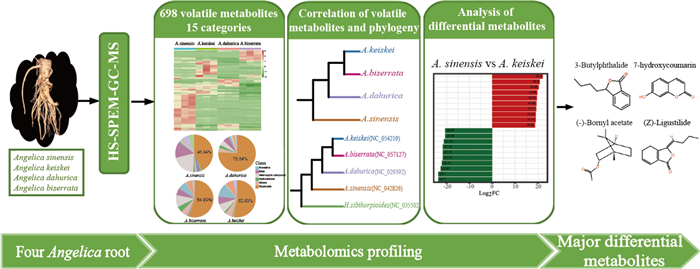
Angelica L. has attracted global interest for its traditional medicinal uses and commercial values. However, few studies have focused on the metabolomic differences among the Angelica species. In this study, widely targeted metabolomics based on gas chromatography-tandem mass spectrometry was employed to analyze the metabolomes of four Angelica species (Angelica sinensis (Oliv.) Diels (A. sinensis), Angelica biserrata (R.H.Shan & Yuan) C.Q.Yuan & R.H.Shan (A. biserrata), Angelica dahurica (Hoffm.) Benth. & Hook.f. ex Franch. & Sav. (A. dahurica) and Angelica keiskei Koidz. (A. keiskei)). A total of 698 volatile metabolites were identified and classified into fifteen different categories. The metabolomic analysis indicated that 7-hydroxycoumarin and Z-ligustilide accumulated at significantly higher levels in A. sinensis, whereas bornyl acetate showed the opposite pattern. Furthermore, a high correspondence between the dendrogram of metabolite contents and phylogenetic positions of the four species. This study provides a comprehensive biochemical map for the exploitation, application and development of the Angelica species as medicinal plants or health-related dietary supplements.Graphical Abstract
Keywords
Angelica Volatile metabolites Chinese traditional medicine Phylogeny1 Introduction
Angelica L., a genus in the family Apiaceae, comprises 90 herbaceous species widespread in north-temperate regions, especially Eurasia [1, 2]. Many plants within the genus have long been used in traditional Chinese medicine (TCM) [3], especially the dried roots of Angelica, which are valued for their blood-nourishing, menstruation -regulating, and analgesic properties [1, 4]. Various herbal preparations containing Angelica species are available over the counter, not only in China, but also in Europe and American countries [5, 6]. Besides its medicinal value, Angelica species are also highly appreciated in various industrial applications such as the dietary supplements, perfumery, and cosmetics [1, 7, 8]. Previous studies have demonstrated that the pharmacological activity of aromatic and medicinal plants is attributed to its effective volatile components [9]. Angelica species are particularly rich in secondary metabolites, including coumarins, flavonoids, terpenoids, as well as volatiles oils (VOs) [1, 3]. VOs are complex mixture of low molecular weight volatile compounds isolated from the raw plant material through distillation [10], and they have been reported to treat serious health diseases, involving gynecological diseases, fever, and arthritis [1, 11]. Modern medical research has indicated that the VOs composition is mainly responsible for the medicinal properties of the Angelica genus [12]. For example, phthalides in Angelica sinensis (Oliv.) Diels (A. sinensis) are one of the highly effective VOs to analgesic and sedative activities [6, 13], while Angelica biserrata (R.H.Shan & Yuan) C.Q.Yuan & R.H.Shan (A. biserrata) contains active ingredients such as oxygenates, terpenoids, ketones and esters with analgesic and anti-inflammatory effects [14]. However, most current studies have focused on a limited number of targeted compounds within a single Angelica species. There is a lack of comprehensive comparative studies examining the volatile metabolites of multiple Angelica species, which poses a significant obstacle to the application and exploitation of these medicinal plants.
A. sinensis, known as female ginseng, is the most well-known medicinal plant within Angelica [15]. However, in terms of phylogenetic groups, A. sinensis belongs to the Sinodielsia clade, which is phylogenetically distant from core Angelica group [16]. In contrast, Angelica dahurica (Hoffm.) Benth. & Hook.f. ex Franch. & Sav. (A. dahurica) and A. biserrata are important TCMs located within core Angelica group, primarily used for expelling wind, removing dampness, relieving pain and treating inflammation [14, 17, 18]. A significant proportion of the compounds isolated from these two herbs are coumarins and volatile oils [18]. Angelica keiskei Koidz. has recently gained interest as a herbal medicine, dietary supplement, and health food in Asian countries [19]. It is known as "Myeong-Il Yeob" in Korea and "Ashitaba" in Japan, both of which mean "tomorrow's leaf". The leaves are used to make tea, which has been reported to lower blood pressure [20]. The medicinal parts of A. sinensis, A. dahurica and A. biserrata are roots, whereas that of A. keiskei are leaves. Additionally, these species have distinct geographical distributions. A. biserrata, A. dahurica, and A. sinensis are mainly found in China, while A. keiskei is predominantly distributed in Japan. Therefore, understanding the differences in the accumulation of medicinally important metabolites among these widely used species is crucial. This knowledge could provide a biochemical map for the development of unexploited wild species as medicinal plants or dietary supplements boosting health and may also facilitate the exploration of the evolutionary mechanisms underlying their biosynthetic pathways.
With the development of metabolomics, high-throughput and high-resolution methods such as headspace solid phase micro-extraction gas chromatography-mass spectrometry (HS–SPME–GC–MS) have been widely used to identify metabolite profiles and detect differences in the biochemical compositions of aromatic and medicinal plants [12, 21, 22]. In this study, volatile metabolites from four Angelica species A. sinensis, A. dahurica, A. biserrata and A. keiskei were identified and quantified using widely targeted metabolomics. The objective was to elucidate the differences in the accumulation of medicinally important metabolites among the four species. This study provides elucidate information on the chemical composition of Angelica plants and may facilitate the identification of the biologically active substances responsible for the pharmacological activity.
2 Results
2.1 Metabolomics profiling of four Angelica species
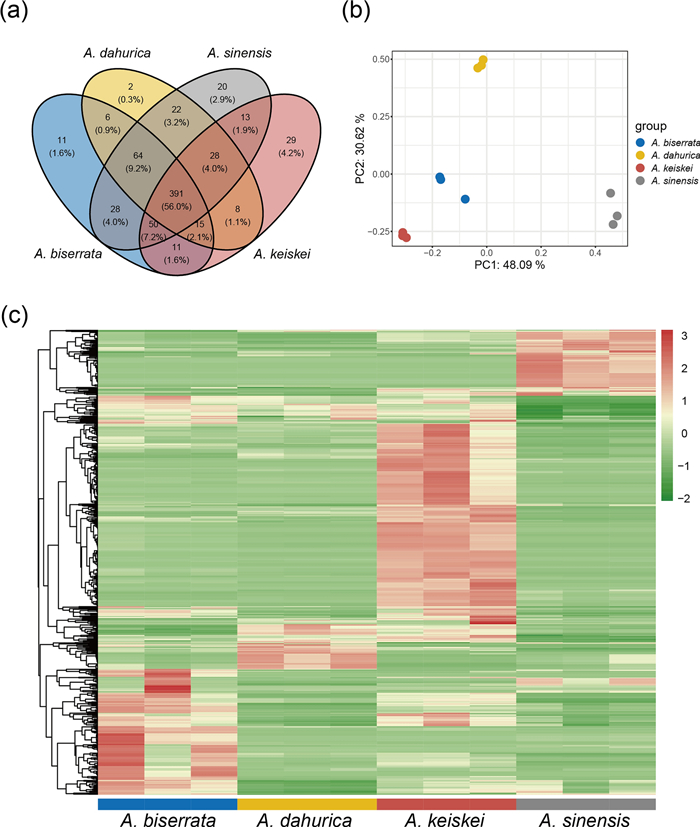
Widely targeted metabolomics provides a promising approach for the chemical screening of volatile metabolites, enabling the characterization of new metabolites in Angelica [12]. In this study, the root metabolomics data were generated to investigate the differences in volatile metabolites among four Angelica species. A total of 698 non-redundant volatile metabolites were qualified and quantified based on GC–MS (Table S1), with 616, 536, 576, and 545 metabolites detected in A. sinensis, A. dahurica, A. biserrata, A. keiskei, respectively. Of these, 391 metabolites were commonly detected in the roots of all four species (Fig. 1a).
An overview of volatile metabolites among four Angelica species. a Venn diagram showing the number of common and specific metabolites in the four species. b PCA of volatile metabolites for the four species with three biological replicates. c Heatmap clustering of volatile metabolites identified from the four species. Volatile metabolite abundance was Z-score transformed. The color-coded scale grading from green to red corresponds to the content of volatile metabolites shifting from low to high
Principal component analysis (PCA) of the metabolome data, transformed with Hellinger transformation method, divided the samples into four distinct groups corresponding to the four species. The three biological replicates were clustered closely, indicating high reproducibility and reliability. The PCA plot showed that PC1 and PC2 explained 48.09% and 30.62% of the total variance, respectively. Of the four clusters, PC1 mainly differentiated A. sinensis from the other Angelica species, while PC2 primarily segregated A. dahurica from the other Angelica species (Fig. 1b).
The abundances of volatile metabolites were normalized using Z-score and subjected to hierarchical clustering analysis (Fig. 1c). The results showed that significant differences among the four species. Of these, the abundance of volatile metabolites in A. keiskei was the highest.
2.2 Identification of differential metabolites in four Angelica species
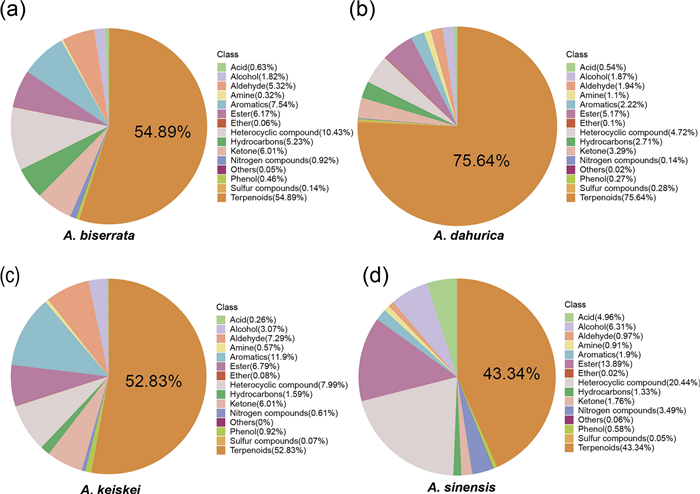
To explore the metabolite composition of the four species, 698 volatile metabolites were classified into 15 different categories, including terpenoids, ester, heterocyclic, aromatics and 11 others (Fig. 2). Terpenoids represented the largest proportion of all volatile metabolites across all four Angelica species, followed by heterocyclic compounds, eater, and aromatics. Notably, A. sinensis contained a relatively lower proportion of terpenoids (43.34%) compared to the other Angelica species, exhibiting a more balanced metabolite composition. In contrast, terpenoids amount accounted for over half of the total volatile metabolites in A. dahurica, A. biserrata, and A. keiskei, especially in A. dahurica, its proportion reached up to 75.64%.
Classification and proportion of volatile metabolites detected in the four Angelica species. a A. biserrata, b A. dahurica, c A. keiskei, d A. sinensis
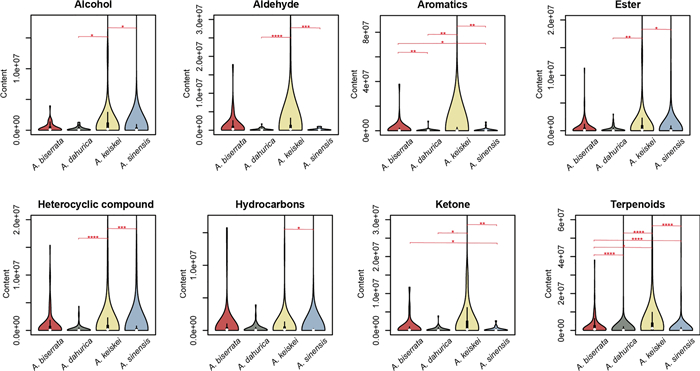
In addition, the relative abundance of each metabolite category in the four species were compared with Kruskal-Wallis test. Bonferroni-corrected p-values indicated significant difference in eight categories among the four species, including alcohol, aldehyde, aromatics, ester, heterocyclic compounds, hydrocarbons, ketone and terpenoids (Fig. 3, Figure S3; p-values were shown in the Table S2). Among these, terpenoids showed the most pronounced variation between species. In each of the eight categories, A. sinensis and A. keiskei differed significantly from at least one other species. These findings suggest that while the overall qualitative composition of volatile metabolites is similar, the individual components vary significantly between the Angelica species.
Comparison for the relative abundance of seven categories (alcohol, aromatics, aldehyde, ester, heterocyclic compounds, ketone and terpenoids) with significant differences in the four Angelica species
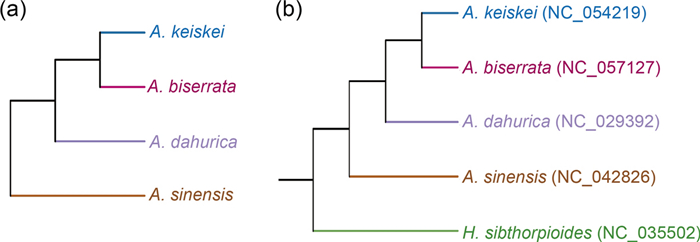
In this study, hierarchical clustering analysis was conducted using Bray–Curtis's dissimilarity distances to assess the composition and abundance of volatile metabolites across the four Angelica species. The resulting dendrogram (Fig. 4a) showed high correspondence with the phylogenetic tree (Fig. 4b) based on chloroplast sequences, indicating a correlation relationship between the volatile metabolites and the phylogenetic relationships.
Hierarchical clustering based on the similarity of volatile metabolites (a) and phylogenetic tree of the four Angelica species and H. sibthorpioides (b). The chloroplast sequences above were available in GenBank of NCBI
2.3 Differential metabolites between A. sinensis and the three other Angelica species
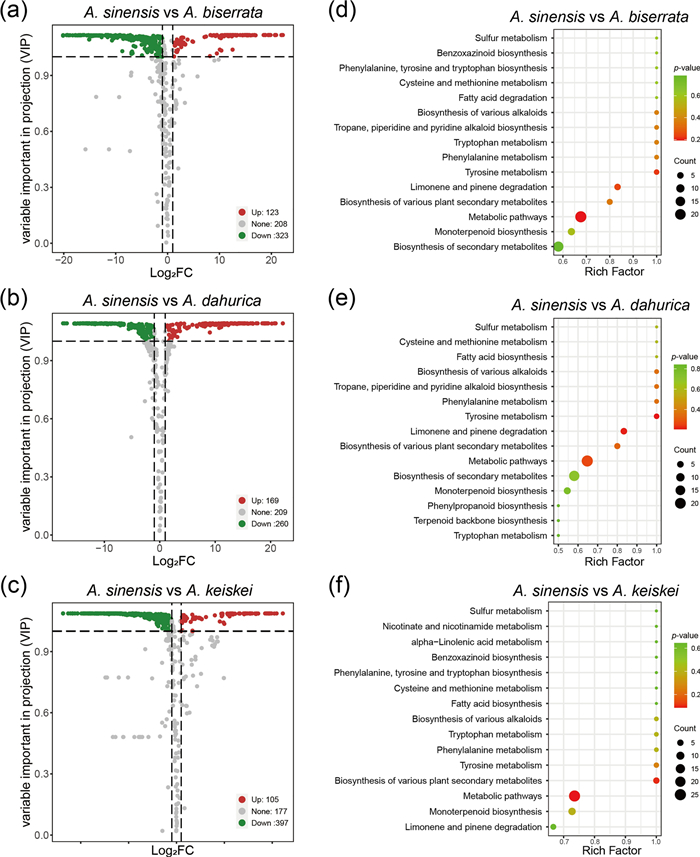
To further identify the metabolites responsible for differences among the four Angelica species, significantly different accumulated metabolites between groups were screened by |Log2FC|≥ 1 and VIP ≥ 1. A. sinensis belongs to Sinodielsia clade in Angelica genus, that was phylogenetically distant from core Angelica group, including A. biserrata, A. dahurica, A. keiskei [2, 23]. Moreover, PC1 mainly differentiated A. sinensis from the other three species (Fig. 1b), therefore, A. sinensis was used to comparison to other three species. Interestingly, A. sinensis exhibited fewer up-regulated metabolites compared to the other species. Additionally, no significantly enriched pathways were detected in the KEGG enrichment results of these differential metabolites, which could be a bias caused by the small dataset. In comparison to A. biserrata, 446 significantly differential metabolites were screened in A. sinensis, with 123 up-regulated and 323 down-regulated (Fig. 5a). The top 3 enrichment pathways were metabolic pathways (23 metabolites with p = 0.19), tyrosine metabolism (3 metabolites with p = 0.22) and limonene and pinene degradation (5 metabolites with p = 0.24) (Fig. 5d). When compared with A. dahurica, 429 significantly differential metabolites were detected in A. sinensis, including 169 up-regulated and 260 down-regulated (Fig. 5b). The top 3 enrichment pathways were tyrosine metabolism (3 metabolites with p = 0.21), limonene and pinene degradation (5 metabolites with p = 0.22) and metabolic pathways (22 metabolites with p = 0.26) (Fig. 5e). Finally, in comparison to A. keiskei, 502 significantly differential metabolites were identified in A. sinensis, with 105 up-regulated and 397 down-regulated (Fig. 5c), representing the largest number of differential metabolites among the groups. The top 3 enrichment pathways of these metabolites were metabolic pathways (25 metabolites with p = 0.09), biosynthesis of various plant secondary metabolites (5 metabolites with p = 0.10), and tyrosine metabolism (3 metabolites with p = 0.26) (Fig. 5f).
The overall distribution and KEGG enrichment analysis of differential metabolites between A. sinensis and the three other Angelica species. a–c Volcano plots for differential metabolites between A. sinensis and the three other Angelica species. a A. sinensis vs A. biserrata. b A. sinensis vs A. dahurica. c A. sinensis vs A. keiskei. Colors of metabolites indicated significant differences (red, upregulated; green, downregulated). d–f KEGG pathway enrichment analysis of differential metabolites for A. sinensis vs A. biserrata (d), A. sinensis vs A. dahurica (e) and A. sinensis vs A. keiskei (f). Color of the bubbles represented statistical significance of the enriched terms, and the size of the bubbles represented number of differentially enriched metabolites. The pathway of "Biosynthesis of various plant secondary metabolites" including: crocin biosynthesis, cannabidiol biosynthesis, mugineic acid biosynthesis, pentagalloylglucose biosynthesis, benzoxazinoid biosynthesis, gramine biosynthesis, coumarin biosynthesis, furanocoumarin biosynthesis, hordatine biosynthesis, podophyllotoxin biosynthesis
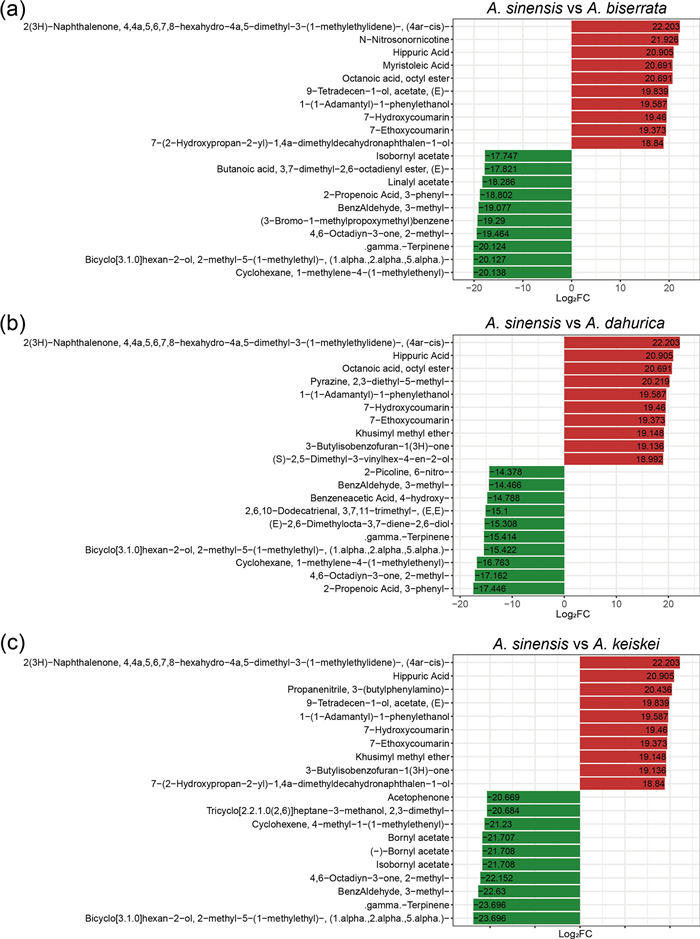
In order to delve into the details of the volatile metabolite difference between A. sinensis and the other three species, the most significantly twenty metabolites (the top 10 for up-regulation and down-regulation, respectively) were selected (Fig. 6). Hippuric acid, 7-hydroxycoumarin and 7-ethoxycoumarin were found to be more abundant in A. sinensis than the three other Angelica species. In addition, the abundance of 3-butylisobenzofuran-1(3H)-one was substantially higher A. sinensis than in A. dahurica and A. keiskei (log2FC > 19). In contrast, γ-terpinene and bornyl acetate were present in high abundance in A. dahurica, A. keiskei and A. biserrata, but were found in lower concentrations A. sinensis.
The top 20 metabolites of significantly differential volatiles between A. sinensis and three other Angelica species. Red indicates the more abundant metabolites in A. sinensis compared to A. biserrata (a), A. dahurica (b), A. keiskei (c). Green indicates the lower levels of metabolites in A. sinensis than that in other species
2.4 Differential metabolites between A. keiskei and the three other Angelica species
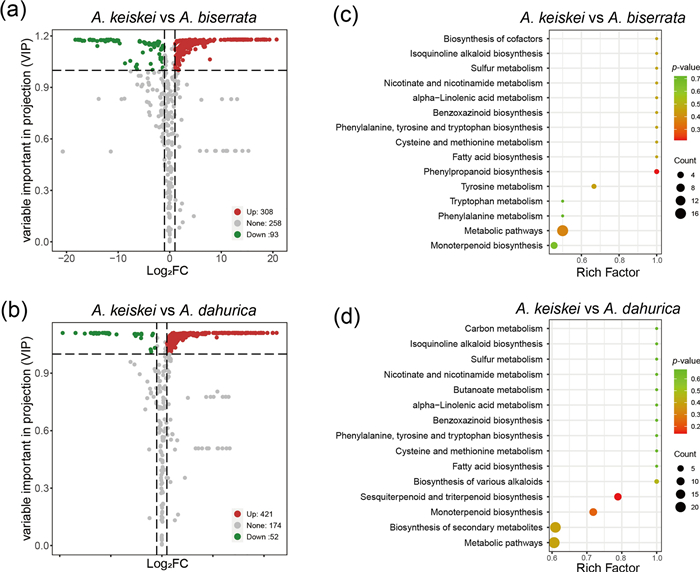
The abundance of volatile metabolites in the roots of A. keiskei was the highest among the four species, suggesting that even the non-medicinal parts of A. keiskei have potential for practical applications. To further investigate, the differences of metabolites between A. keiskei and the other Angelica species were compared. The volcanic map visually showed the overall distribution of differential metabolites in each comparison. In the comparison between A. keiskei and A. biserrata, 401 significantly different metabolites were detected, with 308 up-regulated and 93 down-regulated (Fig. 7a). These metabolites were associated with phenylpropanoid biosynthesis (2 metabolites with p = 0.21), metabolic pathways (17 metabolites with p = 0.38) and tyrosine metabolism (3 metabolites with p = 0.45) (Fig. 7c). In the comparison between A. keiskei and A. dahurica, 473 significantly different metabolites were detected, with 421 up-regulated and 52 down-regulated (Fig. 7b), which were associated with sesquiterpenoid and triterpenoid biosynthesis (8 metabolites with p = 0.14), monoterpenoid biosynthesis (9 metabolites with p = 0.23) and biosynthesis of secondary metabolites (22 metabolites with p = 0.39) (Fig. 7d).
The overall distribution and KEGG enrichment analysis of differential metabolites between A. keiskei and A. biserrata (a, c), A. keiskei and A. dahurica (b, d). a, b Volcano plots for differential metabolites. The colors of metabolites indicated significant differences (red, upregulated; green, downregulated). c, d KEGG pathway enrichment analysis of differential metabolites. Color of the bubbles represented statistical significance of the enriched terms, and the size of the bubbles represented number of differential metabolites
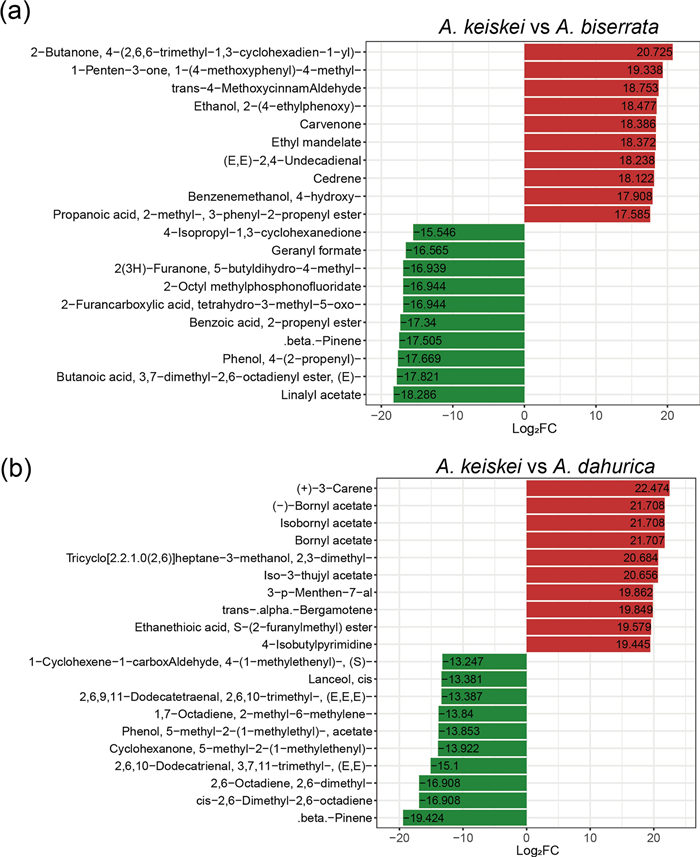
Moreover, to further investigate the differences of volatile metabolites between A. keiskei and other Angelica species, twenty of the most differentiated metabolites were selected for comparison (Fig. 8). The terpenoids metabolites, carvenone and cedrene were more abundant in A. keiskei than in A. biserrate, while carene, bornyl acetate and isobornyl acetate were the most enriched in A. keiskei compared to A. dahurica. Conversely, β-pinene was found in higher concentrations in A. dahurica and A. biserrata than in A. keiskei.
Top 20 metabolites with significant difference between A. keiskei and A. biserrata (a), A. keiskei and A. dahurica (b). Red and green represent up-regulated and down-regulated metabolites in A. keiskei, respectively
3 Discussion
Widely targeted metabolomics offers a promising way for screening volatile metabolites and enables the identification of new volatile metabolites in Angelica [12]. Using the method, a total of 698 volatile metabolites were identified and classified into 15 categories, including terpenoids, ester, heterocyclic, aromatics and 11 others (Fig. 2). A clustering heat map of the metabolites revealed significant difference among the four species, with A. keiskei having the highest number and abundance of volatile metabolites. Consistent with the previous reports, terpenoids were the largest and most diverse class of volatile metabolites in the four Angelica species [1, 2]. Pairwise comparisons between species revealed fewer up-regulated metabolites in A. sinensis compared to the other three species (A. dahurica, A. keiskei, A. biserrata), while most differential metabolites were down-regulated in A. dahurica and A. biserrata relative to A. keiskei. These findings demonstrate that the analyzing differential metabolites is valuable for understanding the differences of chemical properties among the four species.
A. sinensis has long been used to treat various gynecological conditions [5, 6, 24], with phthalides believed to be responsible for its bioactive properties [6, 25]. This study found that 3-butylisobenzofuran-1(3H)-one and Z-ligustilide were present in all four species, with relatively higher concentrations in A. sinensis, consistent with previous studies [5]. In addition, coumarin and its derivatives, important heterocyclic metabolites [26], are known for their anti-HIV, anticancer activity agents, and anticoagulant activities [27, 28]. The results show that A. sinensis contained significantly higher levels of 7-hydroxycoumarin and 7-ethoxycoumarin than A. dahurica, A. biserrata, and A. keiskei (Fig. 2). Notably, 7-hydroxycoumarin, a precursor to furocoumarins and pyranocoumarins, plays a critical role in the biosynthesis of these compounds, which are key contributors to the herb's pharmaceutical activity [15, 29, 30]. Its higher abundance in A. sinensis was probably associated with this biosynthetic process. Nevertheless, in ancient Chinese medical systems, the pharmacological effect of medicinal plants depends not only on the high abundance of a single compound, but also on the synergy of multiple active ingredients [31, 32]. Furthermore, this study revealed that the proportion of various metabolites in A. sinensis was more balanced compared to the other species (Fig. 2), which may explain the widespread and versatile use in TCM.
Moreover, A. keiskei, known as ashitaba in Japanese, has traditionally been used for its leaves as herbs, food and spices [20, 33]. A. keiskei has been utilized both as a medicine and food due to its abundant pharmacological effects, including anti-cancer, lowering blood sugar and blood lipids, and improving human immunity [20, 34]. Currently, A. keiskei is primarily used in raw forms, such as tea and cosmetics, which limits its broader medicinal and clinical applications [33, 35]. Interestingly, bornyl acetate, a terpenoid previously unmentioned in A. keiskei, was detected with high expression levels in its root. Bornyl acetate is known for its antibacterial, insecticidal, and anesthetic effects, especially when combined with other aromatic metabolites in the VOs [36]. This finding supports the potential development of A. keiskei as a source of active ingredients for health-related dietary supplements. Overall, this study greatly enriches the chemical composition database of A. keiskei and suggests that its potential for medicinal and health-related applications.
Previous studies have verified that plants with closer phylogenetic relationship often exhibit similarities not only in morphology but also in chemical composition and curative effects [37-39]. This study indicated the high correspondence between the volatile metabolites and the phylogenetic relationships. Although more extensive sampling and deeper investigations would be necessary to establish more reliable correlations, the study implied that phylogenetic relationships could serve as a window to coarsely apprehend the unknown biochemical diversity of some plants based on the known biochemical map of phylogenetically related species. This finding may offer a great tool for searching replacements of medicinal plant resources that are endangered with closely related non-endangered species.
4 Conclusions
This study investigated the metabolites of four Angelica species using widely targeted metabolomics and identified differences in the accumulation of medicinally important metabolites among species. For example, high levels of bornyl acetate metabolites were detected in A. keiskei, while coumarins and phthalides were significantly lower in A. keiskei compared to A. sinensis. Additionally, the high correspondence between the dendrogram of metabolite contents and the phylogenetic tree suggested a potential correlation between volatile metabolites and phylogenetic relationships. Taken all together, this study provides a comprehensive biochemical map for the exploitation, application, and development of the Angelica species as TCM or health-related dietary supplements.
5 Experimental section
5.1 Plant samples
Four species in genus Angelica, including A. sinensis, A. dahurica, A. biserrata, and A. keiskei, were analyzed in this study. The A. sinensis plants were collected in Minxian (104° 3′ 23″ E, 35° 2′ 16″ N), Gansu Province, China. The A. dahurica, A. biserrata, and A. keiskei plants were collected from the experimental field of the Agricultural Genomics Institute at Shenzhen (Chinese Academy of Agricultural Sciences, China). The specimens of the four species were deposited at Agricultural Genomics Institute at Shenzhen, Chinese Academy of Agricultural Sciences and identified by Prof. Li Wang (A. sinensis (202011002), A. dahurica (202008001), A. biserrata (202106003) and A. keiskei (202109004)).
Roots of each species were sampled with three biological replicates. The collected roots were washed, naturally dried, frozen in liquid nitrogen, and then stored at -80 ℃ for further analysis.
5.2 Solid phase microextraction (SPEM) extraction
The samples were ground into powder in liquid nitrogen. Powdered samples (1 g) were weighed and transferred immediately to a 20 mL head-space vial (Agilent, Palo Alto, CA, USA), containing NaCl saturated solution to inhibit potential enzyme reactions. The headspace vials were sealed using crimp-top caps. As for SPME analysis, each vial was placed in 60 ℃ for 5 min, and then a 120 µm DVB/CWR/PDMS fiber (Agilent, Palo Alto, CA, USA) was exposed to the headspace of the sample for 15 min at 100 ℃. DVB/CWR/PDMS are three-phase fiber heads, which were confirmed to be able to extract more volatile metabolites than other fiber headers [40]. The quality control (mix) sample was prepared by mixing equal volumes of samples into a single tube, and during the instrumental analysis, a quality control sample was inserted into each of the 10 test samples to check the repeatability of the analysis process (Figure S1 and Table S1).
5.3 GC–MS analysis
After the extraction procedure, the fiber was transferred to the injection port of the GC–MS system (Model 8890; Agilent, Palo Alto, CA, USA). The SPME fiber was desorbed and maintained in the injection port at 250 ℃ for 5 min in the split-less mode. The identification and quantification of volatile metabolites was carried out using an Agilent Model 8890 GC and a 7000 D mass spectrometer (Agilent, Palo Alto, CA, USA), equipped with a 30 m × 0.25 mm × 0.25 μm DB-5MS (5% phenyl-polymethylsiloxane) capillary column. Helium was used as the carrier gas at a linear velocity of 1.2 mL/min. The injector temperature was kept at 250 ℃ and the detector at 280 ℃. The oven temperature was programmed as followings: 40 ℃ (3.5 min), increasing at 10 ℃ min−1 to 100 ℃, 7 ℃ min−1 to 180 ℃, 25 ℃ min−1 to 280 ℃ and hold for 5 min. Mass spectra was recorded in electron impact ionization mode at 70 eV. The quadrupole mass detector, ion source and transfer line temperatures were set, respectively, at 150, 230 and 280 ℃. For the identification and quantification of analytes, the MS was selected ion monitoring mode.
5.4 Qualitative and quantitative analysis
The qualitative analysis of primary and secondary mass spectrometry data, as well as the quantification of widely targeted metabolites, was performed by MetWare Biotechnology (Wuhan, China) with the self-built database MWDB and publicly available metabolite databases. Following the mass spectrometry analysis, all raw data were processed with Qualitative Analysis Workflows B.08.00 (Agilent, Palo Alto, CA, USA), and chromatographic peaks were integrated and corrected with MassHunter quantitative.
5.5 Statistical analysis
After the metabolite data was transformed with Hellinger transformation, principal component analysis (PCA) was performed using the function rda in the R package vegan v 2.6-2 [41]. In addition, the data set that was log10 transform and mean centering were imported into the R package MetaboAnalystR v5.0 [42] to conduct orthogonal partial least squares-discriminant analysis (OPLS-DA) and extract the variable important in projection (VIP) value from the analysis results to evaluate the variance importance of compounds. The values of R2X, R2Y, and Q2 for OPLS-DA models were showed in Figure S2. In order to avoid overfitting, a permutation test (100 permutations) was performed. Based on Bray–Curtis's dissimilarity distances of the composition and abundance of all volatile metabolites after ln transform, which were calculated using the function vegdist built in vegan, hierarchical clustering was visualized with the R package factoextra v.1.0.7 [43].
The chloroplast sequence alignments of A. sinensis, A. dahurica, A. biserrata, and A. keiskei were generated using MAFFT v7.475 [44]. Phylogenetic trees were constructed by maximum likelihood using IQ-TREE v 2.1.2 [45] with Hydrocotyle sibthorpioides Lam. as an outgroup.
All identified metabolites were annotated with KEGG database (http://www.kegg.jp/kegg/compound/) and further subjected to KEGG enrichment analyses with the R package clusterProfiler v. 4.4.4 [46].
Notes
Acknowledgements
This work was supported by the National Science Foundation of China, Grant 32470245.
Author contributions
Jiaojiao Ji and Lanlan Zang wrote the main manuscript text and prepared all the figures and tables. Jiaojiao Ji, Lanlan Zang and Tingting Lu collected plant material. Xiaoxu Han, Cheng Li, Soo-Rang Lee and Li Wang revised and supervised the manuscript. All authors have read and agreed to the published version of the manuscript.
Data availability
The data presented in this study are available on request from the corresponding author.
Declarations
Competing interests
The authors declare no conflicts of interest.
References
-
1.Sowndhararajan K, et al. A review of the composition of the essential oils and biological activities of angelica species. Sci Pharm 2017;85(3): 33. CrossRef PubMed Google Scholar
-
2.Feng T, et al. Molecular systematics of Angelica and allied genera (Apiaceae) from the Hengduan Mountains of China based on nrDNA ITS sequences: phylogenetic affinities and biogeographic implications. J Plant Res 2009;122(4): 403-14. CrossRef PubMed Google Scholar
-
3.Sarker S, Nahar L. Natural medicine: the genus Angelica. Curr Med Chem 2004;11(11): 1479-500. CrossRef PubMed Google Scholar
-
4.Dong H, et al. Cool temperature enhances growth, ferulic acid and flavonoid biosynthesis while inhibiting polysaccharide biosynthesis in Angelica sinensis. Molecules 2022;27(1): 320. CrossRef PubMed Google Scholar
-
5.Hook ILI. Danggui to Angelica sinensis root: are potential benefits to European women lost in translation. A review. J Ethnopharmacol 2014;152(1): 1-13. CrossRef PubMed Google Scholar
-
6.Wei WL, et al. Angelica sinensis in China-a review of botanical profile, ethnopharmacology, phytochemistry and chemical analysis. J Ethnopharmacol 2016;190: 116-41. CrossRef PubMed Google Scholar
-
7.Alkan Turkucar S, Aktas Karacelik A, Karakose M. Phenolic compounds, essential oil composition, and antioxidant activity of Angelica purpurascens (Ave-Lall.) Gill. Turk J Chem 2021;45(3): 956-66. CrossRef PubMed Google Scholar
-
8.Zhang HY, et al. Angelica sinensis (Oliv.) diels in China: distribution, cultivation, utilization and variation. Genet Resourc Crop Evol 2012;59(4): 607-13. CrossRef PubMed Google Scholar
-
9.Pandey AK et al. Distribution of aromatic plants in the world and their properties. Feed Additives. 2020, United States: Elsevier Science Publishing Co Inc. 89–114. PubMed Google Scholar
-
10.Sadgrove NJ, Padilla-Gonzalez GF, Phumthum M. Fundamental chemistry of essential oils and volatile organic compounds, methods of analysis and authentication. Plants 2022;11(6): 789. CrossRef PubMed Google Scholar
-
11.Perveen A, Ijaz S, Ghaffar N. Comparative phytochemical and physicochemical study of seeds of the genus Angelica L. from Neelum valley Azad Kashmir, Pakistan. Pak J Bot 2020;52(1): 257-60. CrossRef PubMed Google Scholar
-
12.Kumar P, Rana V, Singh AN. Angelica glauca Edgew. - a comprehensive review. J Appl Res Med Aromat Plants. 2022;31: 100397. PubMed Google Scholar
-
13.Du J, et al. Ligustilide inhibits spontaneous and agonists-or K+ depolarization-induced contraction of rat uterus. J Ethnopharmacol 2006;108(1): 54-8. CrossRef PubMed Google Scholar
-
14.Ma J, et al. The ethnopharmacology, phytochemistry and pharmacology of Angelica biserrata - a review. J Ethnopharmacol 2019;231: 152-69. CrossRef PubMed Google Scholar
-
15.Han X, et al. The chromosome-level genome of female ginseng (Angelica sinensis) provides insights into molecular mechanisms and evolution of coumarin biosynthesis. Plant J 2022;112(5): 1224-37. CrossRef PubMed Google Scholar
-
16.Wen J, et al. A transcriptome-based study on the phylogeny and evolution of the taxonomically controversial subfamily Apioideae (Apiaceae). Ann Bot 2020;125(6): 937-53. CrossRef PubMed Google Scholar
-
17.Zhao H, et al. The Angelica dahurica: a review of traditional uses. Phytochem Pharmacol Front Pharmacol 2022;13: 896637. CrossRef PubMed Google Scholar
-
18.Liu M, et al. Constructing a core collection of the medicinal plant Angelica biserrata using genetic and metabolic data. Front Plant Sci 2020;11: 600249. CrossRef PubMed Google Scholar
-
19.Ohkura N, et al. Possible antithrombotic effects of Angelica keiskei (Ashitaba). Pharmazie 2018;73(6): 315-7. PubMed Google Scholar
-
20.Kil YS, et al. Angelica keiskei, an emerging medicinal herb with various bioactive constituents and biological activities. Arch Pharm Res 2017;40(6): 655-75. CrossRef PubMed Google Scholar
-
21.Chen Q, et al. Characterisation of the flavour profile of dry fermented sausages with different NaCl substitutes using HS-SPME-GC-MS combined with electronic nose and electronic tongue. Meat Sci 2021;172: 108338. CrossRef PubMed Google Scholar
-
22.Hua YL, et al. Urinary metabolomics analysis reveals the effect of volatile oil from Angelica sinensis on LPS-induced inflammation rats. Biomed Chromatogr 2019;33(2): e4402. CrossRef PubMed Google Scholar
-
23.Liao CY, et al. A systematic study of North American Angelica species (Apiaceae) based on nrDNA ITS and cpDNA sequences and fruit morphology. J Syst Evol 2022;60(4): 789-808. CrossRef PubMed Google Scholar
-
24.Yeh JC, et al. The natural compound n-butylidenephthalide derived from the volatile oil of Radix Angelica sinensis inhibits angiogenesis in vitro and in vivo. Angiogenesis 2011;14(2): 187-97. CrossRef PubMed Google Scholar
-
25.Chen X-P, et al. Phytochemical and pharmacological studies on Radix Angelica sinensis. Chin J Nat Med 2013;11(6): 577-87. CrossRef PubMed Google Scholar
-
26.Wu L, et al. The structure and pharmacological functions of coumarins and their derivatives. Curr Med Chem 2009;16(32): 4236-60. CrossRef PubMed Google Scholar
-
27.Zhou D, et al. Biotransformation of neuro-inflammation inhibitor kellerin using Angelica sinensis (Oliv) Diels callus. RSC Adv 2016;6(99): 97302-12. CrossRef PubMed Google Scholar
-
28.Kim CY, et al. Emergence of a proton exchange-based isomerization and lactonization mechanism in the plant coumarin synthase COSY. Nat Commun 2023;14(1): 597. CrossRef PubMed Google Scholar
-
29.Mazimba O. Umbelliferone: sources, chemistry and bioactivities review. Bull Fac Pharm Cairo Univ 2017;55(2): 223-32. PubMed Google Scholar
-
30.Vanholme R, et al. COSY catalyses trans-cis isomerization and lactonization in the biosynthesis of coumarins. Nat Plants 2019;5(10): 1066-75. CrossRef PubMed Google Scholar
-
31.Liu P, et al. Bioactive equivalence of combinatorial components identified in screening of an herbal medicine. Pharm Res 2014;31(7): 1788-800. CrossRef PubMed Google Scholar
-
32.Song HP, et al. A chemical family-based strategy for uncovering hidden bioactive molecules and multicomponent interactions in herbal medicines. Sci Rep 2016;6: 23840. CrossRef PubMed Google Scholar
-
33.Rong Y, et al. Characterization of aroma, sensory and taste properties of Angelica keiskei tea. Eur Food Res Technol 2021;247(7): 1665-77. CrossRef PubMed Google Scholar
-
34.Guiné RP, Gonçalves FJ. Bioactive compounds in some culinary aromatic herbs and their effects on human health. Mini Rev Med Chem 2016;16(11): 855-66. CrossRef PubMed Google Scholar
-
35.Kim DW, et al. Quantitative analysis of phenolic metabolites from different parts of Angelica keiskei by HPLC-ESI MS/MS and their xanthine oxidase inhibition. Food Chem 2014;153: 20-7. CrossRef PubMed Google Scholar
-
36.Liang H, et al. Genome-wide identification of BAHD superfamily and functional characterization of bornyl acetyltransferases involved in the bornyl acetate biosynthesis in Wurfbainia villosa. Front Plant Sci 2022;13: 860152. CrossRef PubMed Google Scholar
-
37.Hao D-C, Xiao P-G. Pharmaceutical resource discovery from traditional medicinal plants: pharmacophylogeny and pharmacophylogenomics. Chin Herb Med 2020;12(2): 104-17. PubMed Google Scholar
-
38.Kang KB, et al. Comprehensive mass spectrometry-guided phenotyping of plant specialized metabolites reveals metabolic diversity in the cosmopolitan plant family Rhamnaceae. Plant J 2019;98(6): 1134-44. CrossRef PubMed Google Scholar
-
39.Saslis-Lagoudakis CH, et al. The use of phylogeny to interpret cross-cultural patterns in plant use and guide medicinal plant discovery: an example from Pterocarpus (Leguminosae). PLoS ONE 2011;6(7): e22275. CrossRef PubMed Google Scholar
-
40.Bertuzzi AS, et al. Detection of volatile compounds of cheese and their contribution to the flavor profile of surface-ripened cheese. Compr Rev Food Sci Food Saf 2018;17(2): 371-90. CrossRef PubMed Google Scholar
-
41.Oksanen J et al. Multivariate analysis of ecological communities in R: vegan tutorial. R package version 1.7. 2013. PubMed Google Scholar
-
42.Chong J, Xia J. MetaboAnalystR: an R package for flexible and reproducible analysis of metabolomics data. Bioinformatics 2018;34(24): 4313-4. CrossRef PubMed Google Scholar
-
43.Lê S, Josse J, Husson F. FactoMineR: an R package for multivariate analysis. J Stat Softw 2008;25(1): 1-18. CrossRef PubMed Google Scholar
-
44.Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 2013;30(4): 772-80. CrossRef PubMed Google Scholar
-
45.Nguyen L-T, et al. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol 2015;32(1): 268-74. CrossRef PubMed Google Scholar
-
46.Wu T, et al. clusterProfiler 4.0: a universal enrichment tool for interpreting omics data. The Innovation 2021;2(3): 100141. CrossRef PubMed Google Scholar
Copyright information
© The Author(s) 2025
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.