Hypecotumines A-D, new isoquinoline alkaloids with potential PCSK9 inhibition activity from Hypecoum erectum L.
Abstract
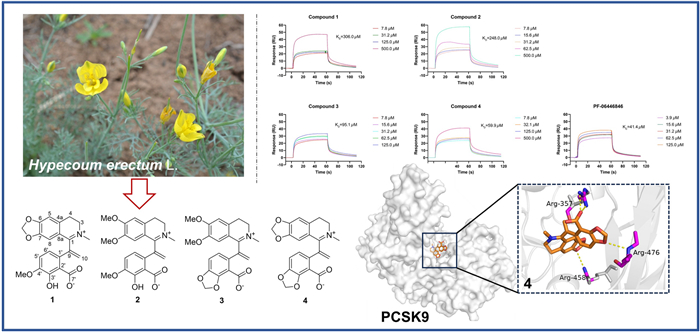
Four new isoquinoline alkaloids, hypecotumines A-D (1–4), were isolated and identified from the whole herbs of Hypecoum erectum L. Their structures were determined by a combination of HRESIMS, NMR, and X-ray diffraction analysis methods. Compounds 1–4 were characterized by a terminal double bond at C-9 and their plausible biosynthetic pathway was hypothesized. Since PCSK9 plays a key role in the development of cardiovascular disease (CVD), exploration of PCSK inhibitors from natural products are beneficial for drug discovery of CVD treatment. SPR and Western blot assays showed compound 4 had PCSK9 inhibition activity with KD value of 59.9 µM and thus elevated the LDLR level. Further molecular docking studies demonstrated that 4 and PCSK9 could form stable interactions via key hydrogen bonds.Graphical Abstract
Keywords
Hypecoum erectum L. Isoquinoline alkaloids Hypecotumines A-D PCSK9 inhibition1 Introduction
Cardiovascular disease (CVD) accounts for 32% of all global deaths and remains a major threat to human health [1]. Elevated level of low-density lipoprotein cholesterol (LDL-C) is a major risk factor for atherosclerosis and cardiovascular diseases [2]. Current therapies primarily focus on reducing LDL-C levels to mitigate the risk of cardiovascular disease [2]. Statins effectively lower LDL-C levels and reduce dyslipidemia in approximately 50% of patients [3, 4]. Furthermore, a significant number of patients have exhibited intolerance and resistance to statins [5]. The discovery of functional mutations in PCSK9, which cause familial hypercholesterolemia, and the crystal structure of PCSK9 have identified it as a distinct therapeutic target for cardiovascular disease [6, 7]. PCSK9 binding to low-density lipoprotein receptor (LDL-R) disrupts the recirculation of LDL-R, thereby impairing the liver's capacity to clear LDL-C from the bloodstream, resulting in elevated levels of LDL-C and the increased susceptibility to cardiovascular disease [8]. Hence, the intervention of PCSK9 have been identified as novel therapeutic strategies to potentially attenuate the risk of cardiovascular diseases [9].
Hypecoum erectum L. is an annual herb of Papaveraceae, which is used as an ethnomedicine and local medicine. H. erectum is utilized as antipyretic, analgesic and anti-inflammatory substance, specifically for the treatment of fever, pharyngitis and painful conditions [10]. Current pharmaceutical studies are primarily focused on anti-inflammatory and antimicrobial aspects [11, 12], and the activity regarding cardiovascular diseases has not been reported. As our ongoing search for structural interesting and bioactive quinoline alkaloids from H. erectum [13], four new isoquinoline alkaloids featured terminal double bond at C-9 (Fig. 1) were isolated and identified from the whole herbs of H. erectum. Their affinity with PCSK9 protein were evaluated and the results suggested their potential as LDL-C lowering agents. This paper herein reported the isolation, structure elucidation, and PCSK9 inhibition activities.
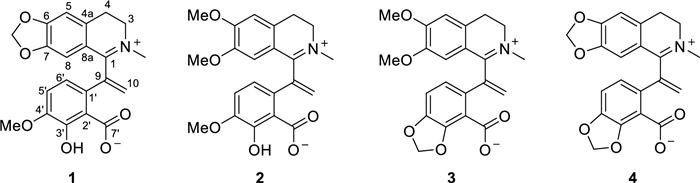
Molecular structures of compounds 1–4
2 Results and discussion
Hypecotumine A (1) was obtained as colorless crystals in MeOH. It has the molecular formula of C21H19NO6, with 13 degrees of unsaturation determined by HRESIMS (m/z 382.1283 [M + H]+, calcd 382.1285) data. The IR absorption bands at 3432, 1630, and 1588 cm−1 indicated the presence of hydroxyl and ester carbonyl functionalities. Interpreting the 1H and 13C NMR spectra easily revealed 21 carbon atoms, which were categorized into 11 sp2 quaternary carbons, four sp2 methines, four methylenes (one sp2 methylene), and two methyl groups. These signals suggested that compound 1 was a typical isoquinoline alkaloid and had a similarity with leptopidine [14]. The distinctive differences were the presence of an additional terminal double bond (δC 143.4, 126.4; δH 5.42, 6.20) in 1. The key HMBC correlations of H-5′ (δH 6.85) and H-6′ (δH 6.91) to δC 143.4 illustrated that the terminal double bond was located at C-9. The observation of HMBC cross-peaks of δH 6.20 and 5.42 to C-1 (δC 165.1) and C-1' (δC 130.7) further confirmed the above elucidation. 2D NMR (HSQC, HMBC, and 1H–1H COSY) experiments established the other parts of the molecule of 1 were the same as leptopidine, the planar structure of 1 was thereby established as shown in Fig. 1.
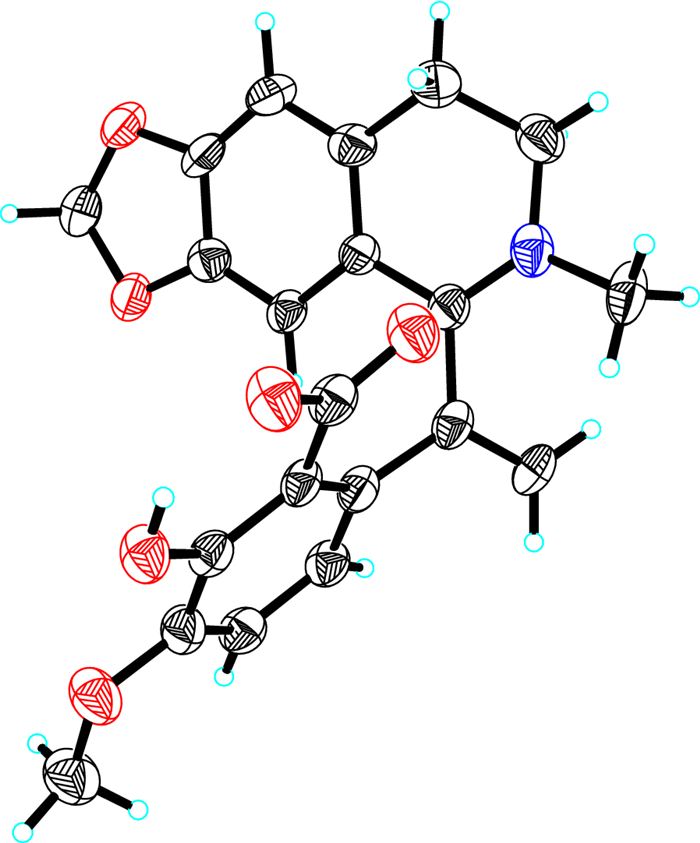
The existence of zwitterion in 1 was verified by X-single crystal diffraction analysis (CCDC 2348263) (Fig. 2), which unambiguously established the final structure of 1.
The X-ray single crystal structure of compound 1
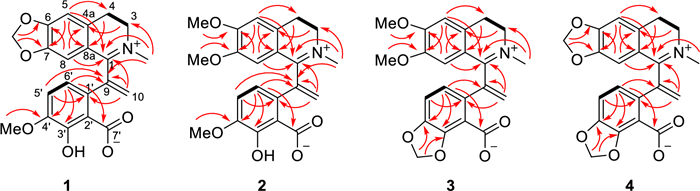
Hypecotumine B (2) was obtained as colorless needle crystals in MeOH and has the molecular formula of C22H23NO6, which was established by HRESIMS (m/z 398.1594 [M + H]+, calcd 398.1598) and 13C NMR data. Analyzing the 1H and 13C NMR spectroscopic data implied that compound 2 had the same structural skeleton with 1, and the major differences were the replacement of methylenedioxy group by two methoxy groups (δC 55.9, 56.3; δH 3.56, 3.90) in 1. The HMBC correlations of δH 3.56 and 3.90 to δC 147.4 and 154.5, respectively, suggested that the two methoxy groups were located at C-6 and C-7, respectively. Thus, the structure of 2 was assigned as shown in Fig. 1, which was verified by further 2D NMR (HSQC, HMBC, and 1H–1H COSY) experiments (Fig. 3).
The key HMBC (arrows) and 1H-1H COSY (bold lines) correlations of compounds 1–4
Hypecotumine C (3) was purified as yellow oil. It had the molecular formula of C22H21NO6 with 13 degrees of unsaturation determined by 13C NMR and HRESIMS (m/z 396.1443 [M + H]+, calcd 396.1442) data. Comprehensive analysis of 1H and 13C NMR spectra suggested that compounds 3 and 2 had the same basic skeleton, and the visible distinction was the presence of a typical methylenedioxy moiety (δH 6.06; δC 102.3). The HMBC correlations of δH 6.06 to C-4′ (δC 146.1) and C-3′ (δC 149.3) assigned the methylenedioxy was located at C-3' and C-4'. Further analysis of HSQC, HMBC, and 1H–1H COSY spectra finally established the structure of 3 was shown in Figs. 1 and 3.
Hypecotumine D (4) was inferred to possess the molecular formula of C21H17NO6 via HRESIMS (m/z 380.1134 [M + H]+, calcd 380.1129) and 13C NMR data. Inspection of NMR data indicated that 4 was the analogue of compounds 1–3. Two characteristic methylenedioxy moieties (δH 5.99, 6.09; δC 102.0, 101.9) situated at C-6/C-7 and C-3′/C-4′ in 4 were elucidated by HMBC correlations of δH 5.99 to C-6 (δC 151.6) and C-7 (δC 147.3), and of δH 6.09 to C-4′ (δC 146.7) and C-3′ (δC 149.3). The structure of 4 was finally confirmed by HSQC, HMBC, 1H–1H COSY spectra, as shown in Figs. 1 and 3.
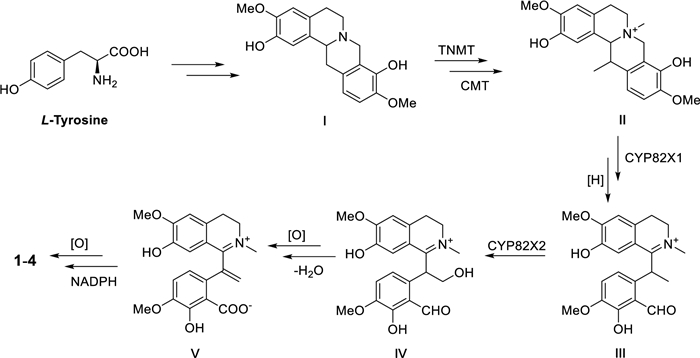
Since compounds 1–4 shared a common structural skeleton, they are likely derived from the same biosynthetic precursor (Scheme 1). L-tyrosine as a starting building block could form precursor I through multi-step reactions [15]. The subsequent process may be catalyzed by tetrahydroprotoberberine N-methyltransferase (TNMT), C-methyltransferase (CMT), and CYP82X1-like cytochrome P450 enzymes yielded key intermediate III [15-17]. Intermediate V then could be generated under the catalyzation of cytochrome p450 enzymes like CYP82X2 as the key step. Finally, the methylenedioxy moiety in compounds 1–4 could be formed under oxidation and NADPH as the critical steps [18].
Hypothesis biogenetic pathway for compounds 1–4
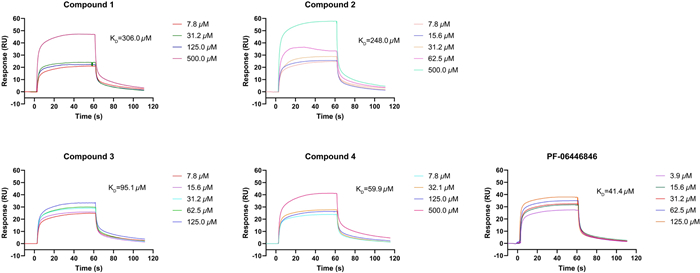
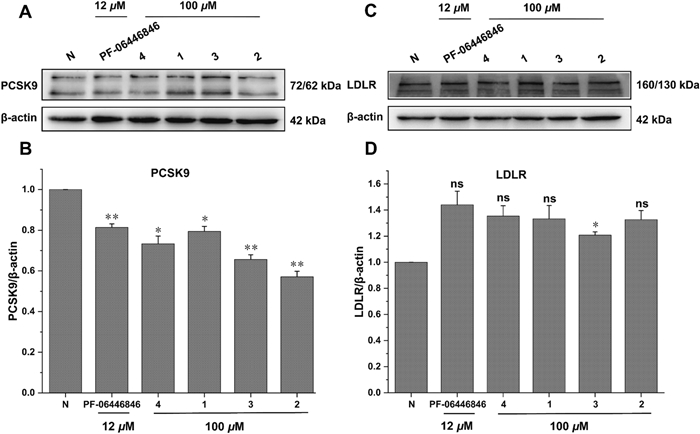
All the isolates were assessed for the affinity with PCSK9 protein by surface plasmon resonance (SPR) analysis and PF-06446846 was used as the positive control [19]. The interactions were tested as the concentrations of compounds from 7.8125 to 500.0 µM with a flow rate of 10 μL/min. It was found that compounds 1 and 2 had weak affinity with PCSK9, meanwhile compounds 3 and 4 had moderate affinity strength with PCSK9 compared to the positive control. The results also indicated the methylenedioxy moiety at C-3' and C-4' made a greater contribution to their affinity strength. The compound-protein interaction curves are shown in Fig. 4, and the KD values of compounds 1–4 interacting with PCSK9 were 306.0, 248.0, 95.1, and 59.9 μM, respectively. Besides, Western blot assay was conducted to evaluate the effect of compounds 1–4 on protein levels of PKSC9 and LDL-R [20, 21]. The results showed that compounds 1–4 could downregulate the PCSK9 protein (72/62 kDa) level and thus upregulate the LDL-R protein (160/130 kDa) (Fig. 5). The results were consistent with the SPR analysis in general, which indicated compounds 1–4 have the potential lowering activity of LDL-C.
The binding affinity of compounds 1–4 with PCSK9 by SPR (concentrations of compounds are ranging from 7.8 to 500 µM)
Effects of compounds 1–4 on PCSK9 and LDLR in HepG2 cells. (A and C) Western blotting assays showing the protein levels of PCSK9 and LDLR in HepG2 cells treated with or without compounds. (B and D) Quantification of protein levels PCSK9 and LDLR in HepG2 cells. 3 independent experiments were presented and PF-06446846 was used as a positive control. ns, not significant; p < 0.05, **p < 0.01. One-way ANOVA with the Dunnett's t-test. Bars represent mean ± S.E.M
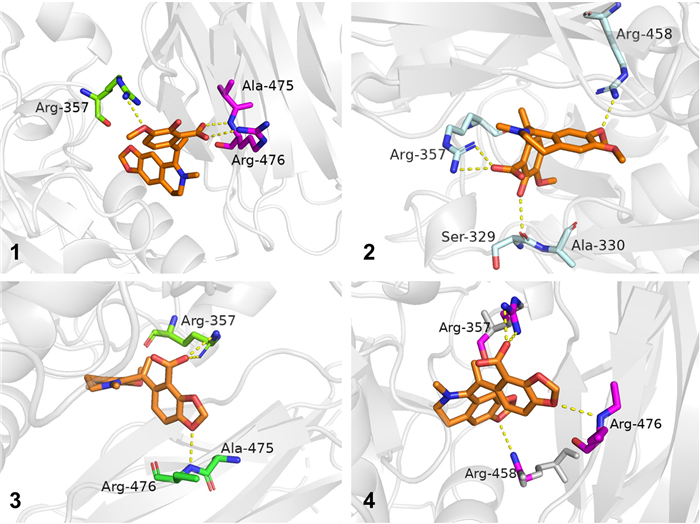
To further investigate the binding positions of compounds with the PCSK9 protein, all the compounds were docked with the crystal structure of PCSK9 protein (PDB ID: 6U3X, resolution: 2.64 Å; https://www.rcsb.org) with the software Schrodinger 2012.1, and the docking results were further processed with Pymol 3.0 [22]. The docking results revealed that all four compounds exhibited a variety of interaction forces with PCSK9, such as hydrogen bond, salt bridge and pi-cation interaction. Some of the key interactions between compounds and amino acid residues (e.g. ARG-357 and ARG-458) were same as those of C4 [22]. Comparing to docking result of PF-06446846, there were also pi-cation interaction with ARG-458 and salt bridge with ASP-360 in compound 4. However, these interactions were absent in compounds 1–3, which might be the reason that 4 had better affinity with PCSK9 (Figure S5). Docking results are shown in Fig. 6, the yellow dotted lines represent interaction forces.
Docking results of compounds 1–4 with PCSK9 (PDB ID: 6U3X)
3 Experimental
3.1 General experimental procedures
SPR analysis were performed on Biacore T200. X-ray data were recorded using a Bruker APEX DUO instrument. NMR, HRESIMS, UV, and IR spectra were measured as described previously [13].
3.2 Plant material
H. erectum (whole herbs) was bought from Eastern medicinal materials market of Hebei Province in September 2023. The voucher specimen (No. KIBZY20230702) was identified by Dr. Chunlei Xiang and preserved at Kunming Institute of Botany.
3.3 Extraction and isolation
Crude alkaloids (800 g) were obtained from H. erectum whole herbs (40.0 kg) by following the published procedures [13], which were separated into five fractions (I-V) by silica gel CC eluted with CH2Cl2/MeOH (60:1 → 0:1, v/v). Fr. V (30.0 g) was eluted with CH2Cl2/MeOH (40:1 → 0:1, v/v) to give five subfractions (V1-5). Fr. V5 (6.0 g) was subjected to Sephadex LH-20 (MeOH), followed by semipreparative high-performance using Waters X-Bridge C18 (10 × 150 mm, 5 μm) column with CH3CN/H2O (32:68, 0.1%, v/v, formic acid) to afford compound 3 (8.5 mg, tR = 23.0 min). Fr. V4 (12.3 g) was subjected to gradient elution (CH2Cl2/MeOH: 30:1 → 0:1, v/v) and followed by semi-preparative high performance liquid chromatography (HPLC) separation using Waters X-Bridge C18 (10 × 150 mm, 5 μm) column, CH3CN/H2O (38:62, 0.1%, v/v, formic acid) to afford compound 4 (5.1 mg, tR = 42.0 min). Fr. IV (80.0 g) was eluted with CH2Cl2/MeOH (50:1 → 0:1, v/v) to give five subfractions (IV1-5). IV3 (15.3 g) was then subjected to gradient elution (CH2Cl2/MeOH: 30:1 → 0:1, v/v), purified using semi-preparative HPLC with Waters X-Bridge C18 (10 × 150 mm, 5 μm) column CH3CN/H2O (48:52, 0.1%, v/v, formic acid), which finally gave compounds 1 (6.2 mg, tR = 32.6 min), and 2 (26.8 mg, tR = 17.5 min).
3.4 Compound characterization
Hypecotumine A (1): white crystal; UV (MeOH) λmax (log ε) 210.0 (5.38), 241.5 (5.12), 312.0 (4.78), 357.5 (4.75) nm; IR (KBr) νmax 3432, 2912, 1630, 1588, 1504, 1485, 1394 and 1366 cm−1; ESIMS m/z 382 [M + H]+; HRESIMS m/z 382.1283 [M + H]+ (calcd for C21H19NO6, 382.1285); 1H and 13C NMR data, see Tables 1 and 2, respectively.
1H NMR (500 MHz) spectroscopic data for compounds 1–4 (δ in ppm, J in Hz)
13C NMR (125 MHz) spectroscopic data for compounds 1–4 (δ in ppm)
Hypecotumine B (2): white needle-like crystal, amorphous powder; UV (MeOH) λmax (log ε) 208.5 (5.23), 240.0 (4.95), 314.0 (4.69), 355.0 (4.59) nm; IR (KBr) νmax 3432, 2937, 1626, 1606, 1564, 1519, 1481 and 1461 cm−1; ESIMS m/z 398 [M + H]+; HRESIMS m/z 398.1594 [M + H]+ (calcd for C22H23NO6, 398.1598); 1H and 13C NMR data, see Tables 1 and 2, respectively.
Hypecotumine C (3): yellow oil; UV (MeOH) λmax (log ε) 204.5 (5.06), 242.5 (4.69), 304.5 (4.43), 372.0 (4.30) nm; IR (KBr) νmax 3432, 2932, 1709, 1604, 1564, 1519, 1444 and 1382 cm−1; ESIMS m/z 396 [M + H]+; HRESIMS m/z 396.1443 [M + H]+ (calcd for C22H21NO6, 396.1442); 1H and 13C NMR data, see Tables 1 and 2, respectively.
Hypecotumine D (4): yellow oil; UV (MeOH) λmax (log ε) 206.0 (5.49), 245.5 (5.12), 300.5 (4.84), 373.0 (4.66) nm; IR (KBr) νmax 3434, 2924, 1630, 1607, 1503, 1486 1465 and 1384 cm−1; ESIMS m/z 380 [M + H]+; HRESIMS m/z 380.1134 [M + H]+ (calcd for C21H17NO6, 380.1129); 1H and 13C NMR data, see Tables 1 and 2, respectively.
3.5 Crystal data for Hypecotumine A (1)
Hypecotumine A (1): C21H19NO6•3(H2O), M = 435.42, a = 40.812(3) Å, b = 13.2173(9) Å, c = 15.3426(9) Å, α = 90°, β = 90.934(7)°, γ = 90°, V = 8275.1(9) Å3, T = 150.(2) K, space group C12/c1, Z = 16, μ(Cu Kα) = 0.931 mm−1, 36, 414 reflections measured, 7593 independent reflections (Rint = 0.3067). The final R1 values were 0.1195 (I > 2σ(I)). The final wR(F2) values were 0.3090 (I > 2σ(I)). The final R1 values were 0.1990 (all data). The final wR(F2) values were 0.3769 (all data). The goodness of fit on F2 was 0.996. Crystallographic data (excluding structure factor tables) for compound 1 have been deposited with the Cambridge Crystallographic Data Center as supplementary publication (deposit number CCDC 2348263). Copies of the data can be obtained free of charge by application to CCDC, 12 Union Road, Cambridge CB 1EZ, UK [fax: Int. + 44 (0) (1223) 336033; e-mail: deposit@ccdc.cam.ac.uk.
3.6 Experimental methods
Protein preparation: The protein concentration of PCSK9 was 40 µg/mL. Ligand pre-enrichment: ligand pre-enrichment was performed by manual injection to test the pre-enrichment of PCSK9 at a concentration of 40 µg/mL in 10 mM sodium acetate solution at pH 4.5 and 5.0 (pH 4.5 was selected for coupling). The proteins were immobilized in the Fc-4 channel of CM5 chip using amino coupling, respectively. And the Fc-1 channel was activated and closed as the reference channel of the Fc-4 channel, respectively [23].
3.7 Compounds interaction analysis with protein PCSK9
Biacore T200 was used to characterize interactions between small molecules and protein. The compounds 1–4 were formulated in a concentration gradient using running buffer to prepare a series of concentrations ranging from 500.0, 250.0, 125.0, 62.5, 31.25, 15.625, and 7.8125 µM. Flow through Fc1-Fc4 and determine the interaction between compounds and PCSK9 immobilized on the chip surface. Operation parameter setting: flow path 4–1 (contact time 80 s, flow rate 30 μL/min, dissociation time 80 s), 25 ℃.
3.8 Cell culture
Human hepatocellular liver cell, HepG2 was obtained from ATCC Cell Bank and cultured 12–24 h in advance in RMPI1640 medium using culture medium containing 10% fetal bovine serum. Positive drug and compounds were solubilized using DMSO and diluted to 12 µmol and 100 µmol individually. Then the compounds were added to the cells 6 well plates, incubated in a 37 ℃ cell culture incubator at 5% CO2 for 24 h.
3.9 Western blot assay
HepG2 cells were washed two times in pre-cooled PBS and lysed using RIPA buffer (Thermo fisher CN) on ice. The cells were centrifuged for 10 ~ 15 s per 10 min to fully lyse, continuing 60 min, and detected the protein concentration with BCA protein concentration assay kit. Proteins were separated by 12% sodium dodecyl sulfate polyacrylamide gel (SDS-PAGE) with electrophoresis and transferred to activated PVDF membrane (Millipore USA). Then, PVDF membrane were treated with 5% milk for 1.5 h. After that, the membrane was incubated with primary antibody (1:500) overnight at 4 ℃ and with secondary antibody (1:5000) for 60 min at room temperature. The band pictures were got by Gel imaging and chemiluminescence imaging system (UVITEC UK).
3.10 Statistical analysis
The experiment was repeated five times and the results were expressed by mean ± S.E.M. Significance level was fixed by one-way analysis of variance (ANOVA) and Dunnett's t-test. P represented the degree of significance [20].
3.11 Molecular docking
Schrodinger 2012.1 soft was used for molecular docking. The docking results were processed by Pymol. The PDB of PCSK9 protein is 6U3X (resolution: 2.64 Å) (https://www.rcsb.org). The process of protein preparation includes addition of hydrogens, addition of missing side chains, removal of waters, assigning protonation states and energy minimization [24]. Ligands were prepared by generating 3D coordinates, using OPLS3e force field, assigning protonation states, and energy minimization formation. The position and size of the docking box was determined by the position and size of the original ligand. The SP docking mode was used to generate docking poses.
4 Concluding remarks
Four new isoquinoline alkaloids, hypecotumines A-D (1–4), featured terminal double bond at C-9 were isolated and identified from H. erectum. These alkaloids were evaluated for their affinity with PCSK9 by SPR assay and were further verified by Western blot assay, the results showed alkaloids 1–4 had potential PCSK9 inhibition activity. Further molecular docking verified the intimate interaction of alkaloids 1–4 and PCSK9, which demonstrated that alkaloids 1–4 are expected to be lead compound for LDL-C lowering.
Notes
Acknowledgements
This work was financially supported by National Key R & D Program of China (2022YFF1100301), Major Science and Technology Project of Henan Province (231100310200), Yunnan Applied Basic Research Projects (202301AS070057), Yunnan Science and Technology Department (202305AH340005), and DR PLANT.
Author contributions
The authors read and approved the final manuscript.
Data availability
The data supporting the results of this study can be obtained from the corresponding author upon reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
References
-
1.World Health Organization. Cardiovascular diseases (CVDs) WHO. 2021. https://www.who.int/en/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds). PubMed Google Scholar
-
2.Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman MJ, De Backer GG, Delgado V, Ference BA, Graham IM, Halliday A, Landmesser U, Mihaylova B, Pedersen TR, Riccardi G, Richter DJ, Sabatine MS, Taskinen MR, Tokgozoglu L, Wiklund O. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 2020;41(1): 111-88. CrossRef PubMed Google Scholar
-
3.Mihaylova B, Emberson J, Blackwell L, Keech A, Simes J, Barnes EH, Voysey M, Gray A, Collins R, Baigent C. Cholesterol Treatment Trialists' (CTT) Collaborators: the effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet 2012;380(9841): 581-90. CrossRef PubMed Google Scholar
-
4.Koo BK. Statin for the primary prevention of cardiovascular disease in patients with diabetes mellitus. Diabetes Metab J 2014;38(1): 32-4. CrossRef PubMed Google Scholar
-
5.Pijlman AH, Huijgen R, Verhagen SN, Imholz BP, Liem AH, Kastelein JJ, Abbink EJ, Stalenhoef AF, Visseren FL. Evaluation of cholesterol lowering treatment of patients with familial hypercholesterolemia: a large cross-sectional study in The Netherlands. Atherosclerosis 2010;209(1): 189-94. CrossRef PubMed Google Scholar
-
6.Piper DE, Jackson S, Liu Q, Romanow WG, Shetterly S, Thibault ST, Shan B, Walker NPC. The crystal structure of PCSK9: a regulator of plasma LDL-cholesterol. Structure 2007;15: 545-52. CrossRef PubMed Google Scholar
-
7.Abifadel M, Varret M, Rabès JP, Allard D, Ouguerram K, Devillers M, Cruaud C, Benjannet S, Wickham L, Erlich D, Derré A, Villéger L, Farnier M, Beucler I, Bruckert E, Chambaz J, Chanu B, Lecerf JM, Luc G, Moulin P, Weissenbach J, Prat A, Krempf M, Junien C, Seidah NG, Boileau C. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet 2003;34(2): 154-6. CrossRef PubMed Google Scholar
-
8.Seidah NG, Benjannet S, Wickham L, Marcinkiewicz J, Jasmin SB, Stifani S, Basak A, Prat A, Chretien M. The secretory proprotein convertase neural apoptosis-regulated convertase 1 (NARC-1): liver regeneration and neuronal differentiation. Proc Natl Acad Sci U S A 2003;100(3): 928-33. CrossRef PubMed Google Scholar
-
9.Brandts J, Ray KK. Novel and future lipid-modulating therapies for the prevention of cardiovascular disease. Nat Rev Cardiol 2023;20: 600-16. CrossRef PubMed Google Scholar
-
10.Sun PT, Cao YG, Xue GM, Li M, Zhang CL, Zhao F, Cao ZY, Wang D, Gustafson KR, Zheng XK, Feng WS, Chen H. Hypeisoxazole A, a racemic pair of tetrahydroisoxazole-fused benzylisoquinoline alkaloids from Hypecoum erectum and structural revision of hypecoleptopine. Org Lett 2022;24(7): 1476-80. CrossRef PubMed Google Scholar
-
11.Wen H, Lei J, Zhang D, Yuan X, Dang J, Li JM, Shao YY, Tao Y. Anti-inflammatory activity of total alkaloids from Hypecoum leptocarpum hook. f. et Thoms. Pharmacogn Mag 2018;14(56): 397-403. CrossRef PubMed Google Scholar
-
12.Yuan HL, Zhao YL, Qin XJ, Liu YP, Yang XW, Luo XD. Diverse isoquinolines with anti-inflammatory and analgesic bioactivities from Hypecoum erectum. J Ethnopharmacol 2021;270: 113811. CrossRef PubMed Google Scholar
-
13.Wei YL, Li S, Wen HY, Dong J, Liang ZZ, Li XY, Zhang Y. 1H NMR guided isolation of 3-arylisoquinoline alkaloids from Hypecoum erectum L. and their anti-inflammation activity. Phytochemistry 2024;222: 114093. CrossRef PubMed Google Scholar
-
14.Wen H, Yuan X, Li C, Li J, Yue H. Two new isoquinoline alkaloids from Hypecoum leptocarpum Hook. f. et Thoms. Nat Prod Res 2024;38(8): 1392-7. CrossRef PubMed Google Scholar
-
15.Singh A, Menéndez-Perdomo IM, Facchini PJ. Benzylisoquinoline alkaloid biosynthesis in opium poppy: an update. Phytochem Rev 2019;18: 1457-82. CrossRef PubMed Google Scholar
-
16.Dang TT, Chen X, Facchini P. Acetylation serves as a protective group in noscapine biosynthesis in opium poppy. Nat Chem Biol 2015;11: 104-6. CrossRef PubMed Google Scholar
-
17.Xu Z, Li Z, Ren F, Gao R, Wang Z, Zhang J, Zhao T, Ma X, Pu X, Xin T, Rombauts S, Sun W, Van de PY, Chen S, Song J. The genome of Corydalis reveals the evolution of benzylisoquinoline alkaloid biosynthesis in Ranunculales. Plant J 2022;111: 217-30. CrossRef PubMed Google Scholar
-
18.Dewick PM. Alkaloids. In medicinal natural products: a biosynthetic approach. Chichester: John Wiley & Sons Ltd; 2002. p. 7–38. PubMed Google Scholar
-
19.Lintner NG, McClure KF, Petersen D, Londregan AT, Piotrowski DW, Wei L, Xiao J, Bolt M, Loria PM, Maguire B, Geoghegan KF, Huang A, Rolph T, Liras S, Doudna JA, Dullea RG, Cate JH. Selective stalling of human translation through small-molecule engagement of the ribosome nascent chain. PLoS Biol 2018;16(4): e1002628. CrossRef PubMed Google Scholar
-
20.Won H, Son MG, Pel P, Nhoek P, An CY, Kim YM, Chae HS, Chin YW. Chemical constituents from Morus alba with proprotein convertase subtilisin/kexin type 9 expression and secretion inhibitory activity. Org Biomol Chem 2023;21(13): 2801-8. CrossRef PubMed Google Scholar
-
21.Guo P, Chen T, Hu X, Duan Y, Zheng L, Du G, Wang Q, Ding A, Qin G, Chen Y, Wang W. Lindenane sesquiterpenoid dimers from Chloranthus japonicus improve LDL uptake by regulating PCSK9 and LDLR. Bioorg Chem 2024;142: 106958. CrossRef PubMed Google Scholar
-
22.Petrilli WL, Adam GC, Erdmann RS, Abeywickrema P, Agnani V, Ai X, Baysarowich J, Byrne N, Caldwell JP, Chang W, DiNunzio E, Feng Z, Ford R, Ha S, Huang Y, Hubbard B, Johnston JM, Kavana M, Lisnock JM, Liang R, Lu J, Lu Z, Meng J, Orth P, Palyha O, Parthasarathy G, Salowe SP, Sharma S, Shipman J, Soisson SM, Strack AM, Youm H, Zhao K, Zink DL, Zokian H, Addona GH, Akinsanya K, Tata JR, Xiong Y, Imbriglio JE. From screening to targeted degradation: strategies for the discovery and optimization of small molecule ligands for PCSK9. Cell Chem Biol 2020;27(1): 32-40.e3. CrossRef PubMed Google Scholar
-
23.Huang M, Zhou Y, Duan D, Yang C, Zhou Z, Li F, Kong Y, Hsieh YC, Zhang R, Ding W, Xiao W, Puno P, Chen C. Targeting ubiquitin conjugating enzyme UbcH5b by a triterpenoid PC3-15 from Schisandra plants sensitizes triple-negative breast cancer cells to lapatinib. Cancer Lett 2021;504: 125-36. CrossRef PubMed Google Scholar
-
24.Chen C, Liu JW, Guo LL, Xiong F, Ran XQ, Guo YR, Yao YG, Hao XJ, Luo RC, Zhang Y. Monoterpenoid indole alkaloid dimers from Kopsia arborea inhibit cyclin-dependent kinase 5 and tau phosphorylation. Phytochemistry 2022;203: 113392. CrossRef PubMed Google Scholar
Copyright information
© The Author(s) 2024
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.