Natural approaches for the management of ulcerative colitis: evidence of preclinical and clinical investigations
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors
Abstract
Ulcerative colitis (UC) is a recurring autoimmune disorder characterized by persistent inflammation in the mucosal lining of the lower part of the large intestine. Conventional treatment options such as salicylates, corticosteroids, and immunosuppressants often come with severe side effects, limited bioavailability, and the development of drug resistance, which hampers their therapeutic effectiveness. Therefore, it is imperative to explore natural strategies as safe and alternative treatments for UC. Currently, around 40% of UC patients find relief through natural constituents, which can help reduce toxic side effects and maintain clinical remission. This review aims to provide a summary of both preclinical and clinical evidence supporting the efficacy of various natural substances in the prophylaxis of UC. These natural options include plant extracts, essential oils, nutraceuticals, and phytochemicals. Furthermore, we will delve into the potential mechanisms that underlie the protective and curative actions of these novel herbal agents. In summary, this review will explore the effectiveness of natural remedies for UC, shedding light on their preclinical and clinical findings and the mechanisms behind their therapeutic actions. These alternatives offer hope for improved treatment outcomes and reduced side effects for individuals suffering from this challenging autoimmune condition.Graphical Abstract
Keywords
Ulcerative colitis Pathophysiology Plant extracts Essential oils Nutraceuticals Phytocompounds1 Introduction
Globally, the prevalence of ulcerative colitis (UC) has seen a significant rise, particularly in Northern Europe and North America, with two distinct age peaks—one between 30 to 40 years and the other occurring in the 5th to 8th decades of life. This condition affects individuals of all genders and its exact cause remains unknown [1, 2]. The increasing incidence of UC is a growing concern in developed countries, such as Europe and the USA, according to epidemiological data [3, 4]. UC, also known as spastic colon, is a persistent auto inflammatory disorder presenting inflammation in the mucosal lining of the large intestine, primarily affecting the distal colon and rectum. It belongs to the group of inflammatory bowel diseases (IBD) and manifests as a chronic inflammatory process in the bowel, marked by periods of clinical relapse and remission [5–7].
The predominant form of IBD is UC, which presents with abdominal pain and rectal bleeding [8]. Severe cases may involve weight loss, tachycardia, fever, blood loss, and bowel enlargement [9]. Various factors, including infectious diseases (such as C. difficile and CMV), toxic reactions (linked to specific antibiotics and NSAIDs), mesenteric ischemia, or bowel malignancies, can contribute to enteritis and should be ruled out before initiating therapy [10, 11]. Patients taking immunosuppressants or steroids should also be screened for opportunistic UC include hematochezia (bloody stools), fecal incontinence, rectal tenesmus (the urge to defecate), and varying degrees of abdominal cramps and pain that are relieved after defecation [12, 13]. While the exact cause of UC remains elusive, recent research has pointed to factors such as abnormal immune responses, intestinal dysbiosis, environmental influences, and genetic susceptibility as potential contributors [14].
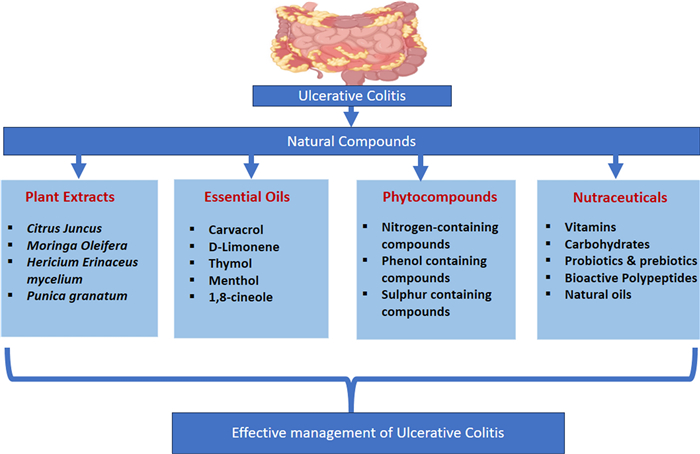
In recent decades, numerous investigations demonstrated the protective, medicinal, preventive, and mitigating effects of natural compounds on colonic inflammation [15, 16]. Currently, 40% of UC patients have found relief through the use of natural compounds, which not only reduce toxic side effects but also help maintain clinical remission [17]. These natural compounds often possess oxido-inflammatory and immunomodulatory activities, rendering them potential candidates for the treatment of UC [18, 19]. Therefore, in this article, we have provided an insight into the importance of natural or herbal substances; including plant extracts essential oils, nutraceuticals, and phytochemicals, along with the underlying mechanisms that contribute to their efficacy in preventing or treating UC. Various clinical trials using natural compounds in management of UC have been summarized in Table 1.
Clinical trials related to herbal drugs for the prophylaxis of Ulcerative colitis
2 Materials and methods
This study was performed by reviewing extensive details from preclinical and clinical investigations on Natural Compounds in managing Ulcerative Colitis. A comprehensive approach was adopted, utilizing international scientific databases like PubMed, Scopus, and specialized resources such as ClinicalTrials.gov to assimilate the latest research findings and clinical trial data.
3 Pathology of UC
UC progression is attributed to the erosion of mucosal barriers integrity, influenced by environmental and genetic factors, as well as abnormal immune responses that modify complex proteins within tight junctions, leading to increased apoptosis [20, 21]. Human epithelial homeostasis relies on a symbiotic flora, and any disruption in the composition of the intestinal microecosystem can trigger inflammatory responses. In this context, the delicate balance of the gut microbiota plays a crucial role in maintaining mucosal integrity. In UC patients, there is a decline in beneficial bacteria that aid in intestinal mucosa healing, coupled with an increase in the permeability of their intestinal epithelial barriers [22, 23].
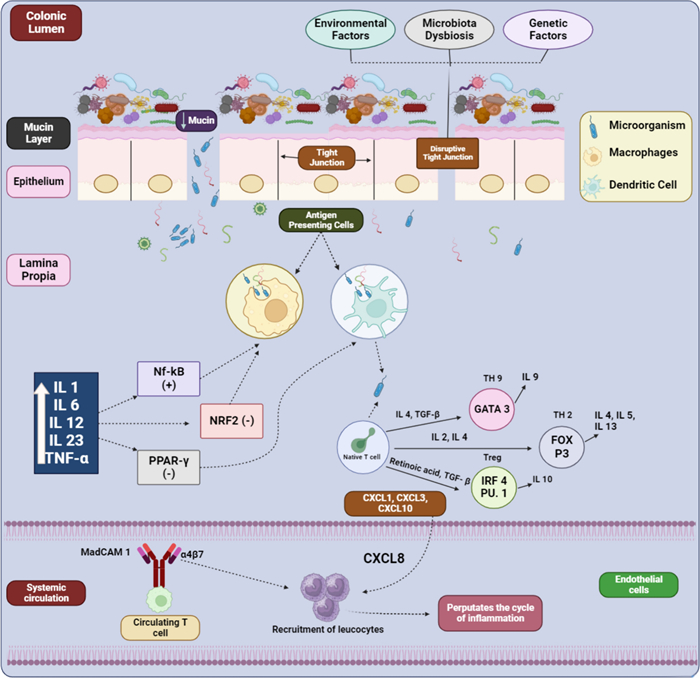
Local and systemic cytokines viz. TNF, IFN, and IL-13, produced by NK-cells and helper T-cells, compromise the integrity of tight junctions, resulting in the destruction of this protein complex (Fig. 1). Consequently, an increase in intestinal permeability occurs, allowing more intraluminal antigens to enter and induce inflammatory responses [24, 25].
Schematic illustration of pathogenic mechanisms of UC (Created by BioRender)
Antigen-presenting cells (macrophages and dendritic cells), are activated by symbiotic flora through toll-like receptors [26, 27]. The activation of dendritic cells triggers the differentiation of CD4+ T cells into various effector T cell subsets, viz. Th2, Th9, and regulatory T cells (Treg), influenced by IL-2, IL-4, TGF-beta, and retinoic acid [28, 29]. This immune activation leads to a persistent inflammatory state within the colonic mucosa, contributing to the chronic nature of UC. Moreover, the dominance of FOX P3, GATA binding protein 3 (GATA3), and IRF4 PU.1 in the lamina propia of UC patients promotes inflammation. Consequently, CD4+ T cells in the colonic mucosa are considered a key element in the etiology of UC [30, 31].
The continuous recruitment and activation of immune cells result in the release of various pro-inflammatory mediators, perpetuating the cycle of inflammation and tissue damage. In the luminal epithelium, macrophages play a crucial role as principal effector cells, which is vital for maintaining immune system and intestinal mucosal homeostasis [32, 33]. Activation of macrophages, in turn dysregulates the immune-inflammatory pathways viz. nuclear factor transcription and inflammasome pathways, which induces oxidative stress and inflammation [34, 35]. This sustained inflammatory response leads to the formation of ulcers and erosion of the colonic mucosa, characteristic of UC pathology.
4 Diagnosis of UC
The diagnosis of UC involves a multifaceted approach, wherein clinical evaluation, a comprehensive medical history assessment, and diagnostic tests are done [36]. It typically begins with a detailed discussion of the medical history, during which the healthcare provider will inquire about the symptoms, their duration, and any relevant family history of inflammatory bowel diseases. Following this, a comprehensive physical examination is conducted, aimed at identifying signs of inflammation, abdominal tenderness, and any other physical findings that may suggest UC [37, 38]. Laboratory tests play a significant role in the diagnostic process. Blood tests, such as CBC, ESR, and CRPare often carried out to evaluate indicators of inflammation [39]. Stool samples are analyzed to rule out infections or other gastrointestinal conditions that can present with symptoms similar to UC [40]. These tests help in differentiating UC from other potential causes of similar clinical presentations.
Imaging studies can also be helpful in diagnosis [41]. A key procedure is a colonoscopy, where endoscope (flexible tube with a camera) is introduced through the rectum and advanced via colon. This enables direct visualization of the colon and rectum's lining, allowing the physician to identify signs of inflammation and ulcerations, as well as to take biopsies for further examination [42, 43]. This visual assessment is crucial for confirming UC and assessing its severity. Biopsies are crucial for confirming the diagnosis of UC and distinguishing it from other gastrointestinal conditions. During endoscopy, small tissue samples are taken from inflamed areas of the colon and rectum. These biopsies are then sent to a pathology laboratory for microscopic evaluation [44, 45]. A flexible sigmoidoscopy is a similar procedure, but it focuses on the lower part of the colon (sigmoid colon) and the rectum. In some instances, an abdominal X-ray or a CT scan may be used to assess the extent and severity of inflammation [46].
Differential diagnosis is a critical step as UC shares symptoms with other conditions like infectious colitis, Crohn's disease or IBS (irritable bowel syndrome). Serological markers and fecal calprotectin levels can further assist in distinguishing UC from these other conditions. Careful evaluation, along with a combination of clinical assessment and diagnostic tests, helps differentiate UC from these conditions. Following a confirmed diagnosis, healthcare providers assess the extent and severity of UC, which guides treatment decisions. This assessment may involve using established scoring systems such as the Mayo Score or UCDAI (Ulcerative Colitis Disease Activity Index). Regular follow-up appointments and monitoring are crucial for evaluating disease progression, response to treatment, and the potential development of complications [47, 48].
In conclusion, diagnosing UC is a complex process that requires collaboration among various medical professionals, including gastroenterologists, radiologists, and pathologists. An early and precise diagnosis is paramount for effective management and improving the patient's lifestyle. Multidisciplinary care teams play a vital role in ensuring comprehensive evaluation and ongoing management of UC. Treatment strategies may differ based on disease extent and severity, depicting the importance of an accurate diagnosis for tailoring an appropriate management plan [49, 50].
5 Present clinical regimens of UC
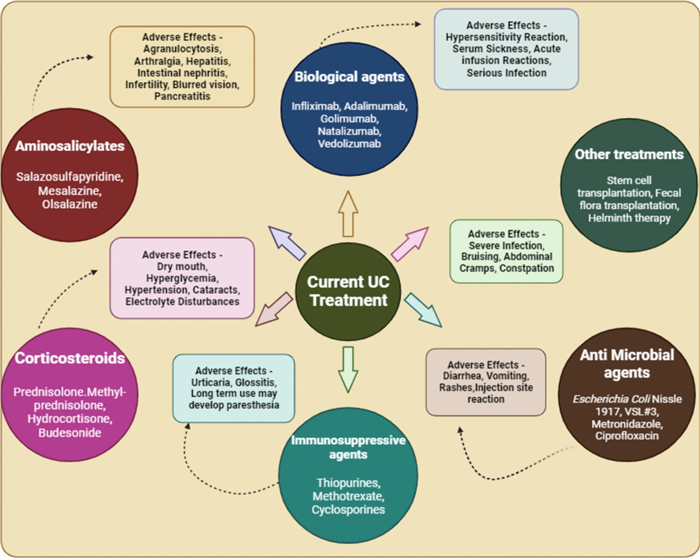
Treatment regimens for UC aim for inducing and maintaining clinical remission as well as to prevent long term complications such as colorectal cancer, disability and colectomy. Main objective of remission is to resolve the clinical symptoms which include amelioration of bowel movements, stoppage of rectal bleeding and mucosal healing [51]. Present clinical regimens for UC typically involve a combination of medication, lifestyle modifications, and sometimes surgery. The chief treatment options for UC are aminosalicylates, calcineurin inhibitors, immunomodulators, Janus kinase (JAK) inhibitors and corticosteroids (Fig. 2) [52, 53]. However, these regimens have shortcomings like medication associated issues (susceptibility to infections, liver problems, bone density loss, and allergic reactions), limited efficacy (frequent flare-ups), biologic therapy risks (infusion reactions, may reduce the effectiveness of antibodies), surgery considerations (may cause potential complications), cost and accessibility (biologics and JAK inhibitors), psychological impact etc. [54–56].
Current recommended treatments for UC along with their drawbacks (Created by BioRender)
6 Novel herbal approaches for UC
Novel herbal approaches for UC are gaining huge attention as complementary or alternative therapies to conventional medications [57]. Numerous herbs and natural compounds have demonstrated promising 'antioxidant, anti-inflammatory and immunomodulatory' actions both in preclinical and clinical studies [58]. These days' researchers are exploring bioactive compounds from botanical sources, such as dietary agents, plant extracts, essential oils, nutraceuticals and isolated phytochemicals, which possess additional immune modulating properties for the management of UC. While further research is necessary to establish their safety and effectiveness, these herbal approaches offer a promising avenue for individuals seeking alternative or adjunctive treatments for UC [59, 60].
6.1 Mechanisms underlying treatment of UC
The treatment of UC involves the modulation of various immunoinflammatory pathways, including the activation and suppression of key mechanisms such as PPARγ, Nrf2, NF-kB, and macrophages, along with the regulation of the Th17/Treg balance [61]. These multifaceted processes play pivotal roles in ameliorating the pathogenesis of UC.
Targeting on the regulation of Th17/Treg cells to precise their imbalances is an effective strategy to prevent the occurrence of UC. Many researchers have reported that monoclonal antibodies blocks or inhibits the Th17 cell differentiation effectively reducing the colonic inflammation by targeting various receptors or pathways. Considering the high cost of antibodies, present research has been focused on natural constituents with pharmacological activities that can also modulate Th17/Treg imbalances by regulating "transcription factors (RORγt, STAT3, Foxp3 and STAT5); the signal molecules (mTOR, PI3K/Akt, AMPK, Nrf-2/HO-1, HIF-1α, Notch, RhoA and GSK-3β); and the receptors (AhR, ERβ, PPARγ and TNFR2)" [62–64]. These natural constituents offer a cost-effective and accessible means of targeting the immune system to alleviate UC symptoms. Studies have shown plant products activates PPARγ factors by inhibiting activation of MAPK, adhesion marker-1 and E-selectin expressions, thereby reducing colonic inflammation [65–67]. Herbal products can be used as therapeutic adjuvants in UC and colorectal cancer associated with UC as they can regulate Nrf2 and, thereby reduces colonic inflammation via regulating various genes involved in cellular redox, protein degradation, DNA repair, xenobiotic metabolism, and apoptosis. They act by inhibiting the NF-kB activation pathways [68, 69]. As NF-kB activation plays a major role in the occurrence and development of UC by activating the release of proinflammatory cytokines. The modulatory mechanisms involved in NF-kB regulation by phytochemicals result in reduction of TNF-α, IL-1β, IL-6, IFN-γ, and COX-2 levels and augmented occludin, claudin-1, zonula occludens-1, and IL-10 expression. Thus, herbals by inhibiting of NF-kB activation pathway at the first stage, positively affects the progression of disease [70, 71]. Since, the activation of macrophages by extracellular pathogens leads to the activation of NF-KB and PPAR gamma, resulting in the release of inflammatory mediators inside the mucosa, leading to the development of the disease. Therefore, the main target to inhibit the progression of UC is to transfer the polarization of macrophage in to M2 phenotype. The related modulatory mechanisms namely regulating Hadhb-mediated FAO, the Sirt1/NF-kB signaling pathway, and mTORC2/PPAR-g signaling and other pathways are involved [72, 73]. This comprehensive understanding of the underlying mechanisms allows for the development of targeted herbal therapies that can more effectively manage UC.
In summary, the integration of novel herbal approaches into the treatment plan for UC offers a multifaceted strategy that addresses the complex immune-inflammatory pathways involved in the disease. These approaches provide a promising avenue for enhancing the efficacy of existing treatments while minimizing side effects and improving patient outcomes.
6.2 Plants
Natural products such as plant extracts have shown therapeutic, preventive as well as protective effect against UC in various chemically induced animal models (Table 2). Plant extracts act by multiple defense mechanisms via suppression of inflammatory biomarkers, elevation of anti-oxidant enzyme expressions in the colon tissue or by maintaining the homeostasis of epithelium [74].
Various extracts and their potential benefits for the management of Ulcerative colitis
6.2.1 Citrus junos
Citrus junos, commonly known as Yuzu and part of the Rutaceae family, is thought to possess anti-colitic properties. Numerous studies have indicated its potential advantages in mitigating intestinal inflammation. For instance, Abe et al., 2018 investigated the efficacy of Yuzu (Citrus junos) extract in a murine model induced with DSS (dextran sulphate sodium). They assessed the oxido-inflammatory balance in mice stimulated with lipids in vivo. The findings revealed a significant suppression of the macrophage cell line and proinflammatory cytokines, via inhibition of the NK pathway suggesting that the peel extract holds promise for mitigating the symptoms associated with UC [75]. In 2020, Mina Lee and colleagues isolated 13 limonoids from Citrus junos seed extract, among which sudachinoid A, a novel compound, and Ichangensin showed promise in treating UC. Researchers observed the anti-inflammatory efficacy of these phytocompounds, employing ELISA and Western blot analysis on mouse macrophages via downregulation of pro-inflammatory cytokines [76]. Another study demonstrated beneficial effects of Yuzu extract via inhibition of cox-2 and suppression of monocyte chemotactic protein, TNF, interleukins, p38 phosphorylation and nitric oxide synthase in RAW264.7 cells. Additionally, the positive outcomes were consistent in a murine colitis model, where histological examinations revealed a notable inhibition of disease activity index (DAI) in the treatment groups that received Yuzu peels extract. These promising results suggest the potential therapeutic efficacy of Yuzu extract in mitigating inflammation and supporting the management of colitis [77].
6.2.2 Moringa oleifera
Moringa oleifera, a member of the Moringaceae family, is renowned for its diverse medicinal properties effective against numerous ailments. Its anti-inflammatory properties have shown to mitigate inflammation and modulate gut microbiota homeostasis. For example, an investigation performed by Youjin et al. investigated the oxidoinflammatory mechanisms of isothiocyanate isolated from M. oleifera seed extract both in acute and chronic ulcer models. Utilizing DSS aggravated UC models, the research outcomes revealed a significant upregulation of antioxidant markers and epithelial barrier proteins, along with the inhibition of inflammatory keratinocyte-derived cytokines and nitric oxide levels. Importantly, the results demonstrated that this extract exerts its therapeutic effects by activating Nrf2 signaling pathways. This activation subsequently led to the suppression of interleukins, inducible nitric oxide synthase (NO), fecal lipocalin-2, and the upregulation of GSTP-1, claudin-1, and ZO-1 in the intestine. These results highlight valuable insights into the complex molecular mechanisms through which this extract mitigates inflammation and oxidative stress in ulcerative conditions [78]. Husien et al., conducted a study to investigate the underlying mechanisms of polysaccharides from M. oleifera by assessing inflammatory scores, DAI, tight junction proteins, interleukins, and mRNA expressions in the colonic mucosa in a mouse model induced with DSS. Results provided evidence for the preventive actions of this extract by activation of PPAR-γ and downregulation of TLR-4 pathway activation [79]. Hong et al. demonstrated the anticolitic activity of an isolated peptide from M. oleifera in a DSS-aggravated colitis model. Through a comprehensive assessment using RNA sequencing, the study revealed that peptide treatment exhibited the ability to inhibit microbial dysbiosis and the JAK-STAT activation pathway. This inhibition, in turn, led to the remodeling of the colon mucosal barrier and its associated metabolites demonstrating its therapeutic potential in maintaining the integrity of the colon mucosa [80]. In another investigation, Zhang et al., explored the anti-colitis potential of polyphenolic compounds extracted from M. oleifera using a DSS-induced model. Following oral administration, a noteworthy reduction in cell infiltration, inflammatory mediators, and alterations in protein expressions were observed. Additionally, in vivo assessments demonstrated a reversal of histological changes via downregulation of the NF-κB signaling pathway. All these findings highlight the promising therapeutic effects of M. oleiferapolyphenolic compounds in mitigating colitis-related inflammation [81].
6.2.3 Hericiumerinaceus mycelium
Hericium erinaceus, commonly known as Lion's Mane and belonging to the Hericiaceae family, has demonstrated the ability to alleviate symptoms of ulcerative colitis (UC) by exerting anti-inflammatory effects. For instance, Shaoand et al. evaluated the ameliorative actions of the unique polysaccharide EP-1, isolated from H. erinaceus extract, against acetic acid (A.A) treated UC model of rats. Employing IlluminaMiSeq and immunoblot analysis, the study revealed noteworthy alterations in intestinal structure, an increase in fatty acids, and a significant reduction in G-protein receptors in the epithelium [82]. Similarly, Wang et al., performed a research to explore the potential of the EP-1 polysaccharide against UC, utilizing Caco-2 cells in a rat model. Authors noticed that, treatment with EP-1 led to an increase in antioxidant markers, improvementsin mitochondrial function, and a reduction in apoptosis in the colon epithelium. These findings, in conjunction with the research by Shao et al., further support the therapeutic potential of EP-1 in ameliorating UC. The collective evidence from both studies highlights the multifaceted benefits of EP-1, indicating its potential as a complementary herbal medicine for alleviating the symptoms and underlying mechanisms of UC [83]. The potential ameliorative effects of HEP10, an another low-weight polysaccharide derived from H. erinaceus, were observed in both RAW264.7 murine macrophage cells and a DSS-induced mice model. Their investigation revealed that the beneficial impact of HEP10 is due to the suppression of NLRP3 activation and the suppression of other signalling pathways. Furthermore, these mechanisms were shown to modulate the gut micro biota. This suggests that HEP10 may have a positive influence on inflammatory responses and gut health through its regulatory effects on key cellular pathways and the microbiome [84]. Durmus et al. evaluated the prophylactic efficacy of H. erinaceus in the context of UC by assessing microscopic and macroscopic indices in a TNBS-induced animal model. The study revealed that treatment with H. erinaceus resulted in a notable reduction in various markers, including NO, malondialdehyde, myeloperoxidase, and other elevated mechanisms when compared to the control group [85]. A comprehensive investigation was conducted on the anticolitic potential of an HBQ-complex, comprising Hericium erinaceus, berberine, quercetin, niacin, and biotin. The study focused on its effects against ex vivo inflamed colon mucosa. The findings demonstrated a consistent and progressive reduction in pro-inflammatory cytokines along with an elevation in IL-10 both at mRNA and protein levels within the inflamed tissue [86].
6.2.4 Punica granatum
Punica granatum, often referred to as pomegranate and a member of the Lythraceae family, exhibits anti-colitic properties and demonstrates promise in alleviating symptoms, potentially enhancing gut health for individuals with UC. For instance, in an investigation performed by Shah et al., the researchers assessed the potential impact of P. granatum juice and purified punicalagin in inhibiting NF-κBsignaling pathways using a dinitro benzoic acid-aggravated colitis model. The dinitrobenzoic acid-injected model exhibited elevated levels of proinflammatory cytokines, inflammatory mediators, neutrophil infiltrations, and decreased antioxidant markers in the colon epithelium. However, the administration of juice and punicalagin reversed these effects.Remarkably, the ameliorative effect of P. granatum juice was found to be more effective compared to purified punicalagin. This suggests that the combination of compounds present in the pomegranate juice may contribute to a more pronounced reversal of colitis-related markers and highlights the potential superiority of the whole juice over isolated components in mitigating inflammatory responses in the studied colitis model [87]. Kamali et al. performed a clinical trial (randomized, placebo-controlled) to investigate the effectiveness of Punica granatum peel extract in managing symptoms of colitis, assessed through the Lichtiger Colitis Activity Index. The results demonstrated a no table increase in the clinical response, when compared to the placebo group over the four-week study period. This suggests that the anti-inflammatory activity of P. granatum extract may be advantageous in the complementary management of UC [88].
6.2.5 Miscellaneous extracts
Various other extracts have also been reported in literature having profound activity against UC. For example, a pre-clinical investigation of the prophylactic potential of Zanthoxylum alatum seed extract for the management of UC in mice was reported by Kalyankumarraju et al. The findings of in vitro and in vivo studies using NF-κB-luciferase translocation assay and DSS-induced UC model, respectively demonstrated that hydroalcoholic extract of ZA seed could be used as a prophylactic as well as effective treatment option for stress aggravated UC [89]. Zaware et al., explored the therapeutic potential of Mimosa pudica extract has also been explored by assessing colonic injury biomarkers in an A.A-induced colitis model, comparing its efficacy with the standard drug prednisolone. The ethanolic extracts of Mimosa pudica, enriched with flavonoid derivatives, demonstrated notable efficacy in alleviating UC symptoms in rats. This was evidenced by significant inhibition of key oxidative and inflammatory mediators, including myeloperoxidase and malondialdehyde levels. The findings highlight the promising role of Mimosa pudica extract to regulate pathological alterations in colonic tissue [90].
Another study explored the oxidoinflammatory effects of a sicilian variety of garlic extract in an ex vivo experimental model using mouse specimens treated with lipopolysaccharide. The investigation revealed a notable reduction in inflammatory mediators, enzymes, and gene expressions induced by LPS. Both hydroalcoholic and water extracts of garlic exhibited protective effects on the colon, with the hydroalcoholic extract demonstrating a more potent anti-inflammatory effect. These findings highlight the effectiveness of garlic extracts in the management of UC, pointing towards their beneficial impact on mitigating ulcerogenic scores in the colon [91]. Obiri et al. investigated the therapeutic potential of Cordia vignei leaf extract in ameliorating A.A trearted UC model of rats. Histological examinations depicted a significant reduction in inflammatory scores, indicating the efficacy of this leaf extract in preventing colon tissue damage. Moreover, C. vignei administration significantly upregulated the antioxidant markers & suppressed serum inflammatory levels in the A.A-induced colonic environment [92]. A study was conducted to evaluate the effectiveness of Alstonia boonei extract in alleviating symptoms in comparison to the standard drug prednisolone, using a UC model treated with DSS. Researchers demonstrated a noteworthy inhibition of inflammatory secretions and prostaglandins, along with an elevation of antioxidant markers in colon epithelium. Histological findings further supported these outcomes, revealing an increase in body weight, which had been decreased by the induction of colitis. These collective results strongly suggest a protective role for A. boonei extract in inflammatory bowel disease [93]. Qin et al. investigated the potential mechanisms underlying the efficacy of Rubia cordifolia L. extract against the management of UC using a mice model treated with DSS. In vivo study revealed a dual inhibitory effect on both the formation of the NLRP3 and the activation of the JAK-STAT pathway inside the colon. This dual inhibition resulted in decrease in mortality, decreased release of inflammatory cytokines & alleviation of clinical symptoms [94]. An investigation reported the anticolitic effects of Sesbania grandiflora extract in a mice model of UC induced by A.A. The study demonstrated significant alterations in the DAI, restoration of biochemical parameters, and inhibition of free fatty acids in the diseased model, particularly when treated with S. grandiflora extract. Additionally, histological findings indicated that extract could effectively prevent cellular infiltration, necrosis, and other ulcerative signs in the colon mucosa [95]. Silva et al. explored the efficacy of lutein, a carotenoid extracted from Tagetes erecta L, for its anticolitic activity in an experimental UC model. The study revealed significant reductions in disease severity, achieved by downregulating the secretion of ulcerative biomarkers and enhancing endogenous antioxidant markers in nude mice treated with DSS. Lutein demonstrated the capacity to attenuate histological alterations, counteract oxidative stress, and increase mucin staining in the colon [96]. A preclinical investigation conducted to evaluate the potency of Ziziphusspina (ZS) extract in mitigating colon inflammation, using a rat model treated with A.A. Researchers employed HPLC and histochemical methods to analyze the effects of this extract. Findings of their study demonstrated that pretreatment of ZS fruit extract was associated with inhibition of pathological injury scores, proinflammatory cytokines & oxidative stress, alterations in body weight, mucin concentration, and apoptosis. Moreover, pre-administration with ZS extract modulated the Nrf2, HO-1 &p38 MAPK expression in A.A administered UC model, as demonstrated by quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR) analysis. These results highlight that Ziziphusspina fruit extract could serve as an additive or alternative medication for the management of UC, emphasizing its potential as a therapeutic agent in combating UC-related symptoms and pathology [97]. A study explored the antioxidant defense mechanism of Averrhoa bilimbi fruit extract (ABFE) in comparison to the standard drug sulfasalazine, for prophylaxis against UC in A.A-treated rats. The protective effects of ABFE were proved as its administration led to significant changes in mucosal injury markers, spleen and colon tissue weight, oxido-inflammatory markers, and colon histology in treated groups compared to the control group. Findings from antioxidant & anti-inflammatory studies suggest that ABFE could serve as a potential candidate in the treatment of ulcerative colitis [98]. Chen et al. explored the protective role of B. xiexin decoction against a UC model treated with DSS, employing ELISA &immunohistochemistry (IHC). The findings demonstrated that B. xiexin decoction treatment led to decreased disease activity index, ulcer scores, levels of 8-oxoguanine, interleukins, and the results demonstrated a notable increase in the clinical response when compared to the placebo group over the four-week study period. Additionally it also inhibited the levels of (myeloperoxidase) & MDA (malondialdehyde) in the mice epithelium. Furthermore, activation of Nrf2 was observed in response to B. xiexin decoction treatment. These results collectively highlight the potential oxidoinflammatory properties of B. xiexin decoction in preventing and treating UC. The modulation of key markers and pathways associated with inflammation and oxidative stress suggests B. xiexin decoction as a potential candidate for therapeutic intervention in treating UC [99]. Several medicinal plants have also been explored. For instance, Lie et al., have demonstrated anti-ulcerative potential of Saussurea Pulchella and their research involved analyzing the chemical composition of the extract, studying its effects on UC symptoms in mice (Adult male BALB/c mice). The findings revealed that the extract reduced UC symptoms, inflammation, and oxidative stress while improving colon health. Key metabolites, biological targets, and metabolisms related to extract effectiveness against UC were identified. Overall, the study suggests that this extract could serve as a promising natural treatment for UC [100]. In another study, Rehman et al., aimed to explore the potential of Calliandra haematocephala in treating UC by assessing its impact on inflammatory mediators and oxidative stress markers using rat models. Results showed that both extracts mitigated inflammation, reduced colon ulceration, normalized oxidative stress markers, and modulated gene expression related to inflammation [101]. Devi et al., investigated role of Bacopa monnieri in management of A.A.-induced UC in mice. Acetic acid infusion caused severe colon inflammation and increased myeloperoxidase activity. Treatment with B. monnieri extract and its saponin-rich fraction significantly suppressed inflammation, myeloperoxidase levels, and disease activity score in a dose-dependent manner [102]. Jia et al., have evaluated the potential of ethanolic extract of Limonium bicolor (a traditional Chinese medicine) in both cell and mouse models of UC. Outcomes showed that the extract suppressed cytokine secretion in macrophages and exhibited protective effects against UC in mice, reducing disease severity and restoring colon health. Additionally, it reversed microbial imbalances associated with UC. Network pharmacology analysis identified key compounds in extract linked to inflammatory genes, enhancing understanding of its therapeutic potential and paving the way for UC treatment development [103]. Abdellatif et al. aimed to evaluate the total phenolic and flavonoid contents, antioxidant properties, chemical composition, and anticoloitis potential of Cassia fistula leaf extract in A.A induced UC in male rats. The extract demonstrated dose-dependent efficacy in treating UC in rats, improving hematological parameters, liver biomarkers, oxidative stress, and histopathological features. Molecular docking studies revealed the potential anti-inflammatory and antiapoptotic activities of C. fistula compounds, particularly emodin and isorhamnetin, which exhibited strong binding affinities to COX-2 and caspase-3 proteins, respectively [104]. Cumulative results of all findings highlight the effectiveness of various extracts in the management of UC and this could act as a potential candidate for management of UC.
6.3 Essential oils
Several preclinical studies demonstrated that essential oils can enhance the equilibrium of GIT immunity through their 'anti-inflammatory activity' and the downregulation of pro-inflammatory mediators (Table 4) [105]. Also essential oils can lead to suppression or activation of various protein expressions and translocation of signaling pathways and thus mitigating UC [106].
6.3.1 Carvacol
An investigation is carried out using a colitis model induced with A.A to evaluate the antioxidant and anti-inflammatory efficacy of a phenolic monoterpene, carvacol revealed that pretreatment with carvacol showed decreased level of inflammatory mediators, DAI, macroscopic and microscopic damages and also significant elevation of antioxidant enzymes in the colon tissue [107]. Another investigation demonstrated the anticolitic effect of carvacol is associated with the protection of mucosal barrier through reducing translocation of NF-κB pathway and inhibiting TLR4 receptor activation pathway in DSS induced colitis model. It also ameliorates UC in the colon by inhibiting inflammatory scores and cell apoptosis caused by induction of LPS (Liu et al. 2022). The results of this study highlight that, carvacol could be a prophylactic intervention in the management of UC [108].
6.3.2 D-Limonene
An in vivo analysis demonstrated the 'anticolitic' effects of D-limonene for the prevention of UC against a TNBS administered colitis model. The induction of colitis with TNBS caused elevated levels of pathological scores via activation of NF-κB translocation in the colon epithelium which has been reversed by treatment with D-limonene [109]. Lihua et al. 2016 demonstrated the potential oxidoinflammatory activity of D-limonene for the management of UC using rats treated with DSS. Their findings indicated a notable suppression of matrix metalloproteinase, TGF-β expression and increase in antioxidant markers in limonene treated rats, suggesting the protective role of D-limonene in the management of UC [110]. Another in vivo study was carried out by Estrella et al. to investigate the ameliorative action of D-limone in oxazolone treated colitis model. Prevention of mucosal damage, hyperalgesia, pathological biomarkers and major antinociceptive activity was observed in limonene treated rats. The cumulative results from above mentioned studies demonstrated the curative potential of D-limonene for the prophylaxis of UC [111].
6.3.3 Thymol
A colitis model treated with DSS was employed to investigate the mechanism and protective efficacy of thymol in treating UC. Thymol treatment demonstrates a beneficial impact on experimental colitis by effectively reducing pathological scores, mitigating mucosal damage, and suppressing pro-inflammatory cytokines. Additionally, it elevates antioxidant markers via inhibition of nuclear factor pathway activation in mice [112]. Another study explored the anticolitic activity of thymol against the management of colitis, using a rat model treated with A.A. The colon mucosa of rats showed marked inhibition of histological damages, pro-inflammatory cytokines and elevation of protein (pNFκB p65) via down regulation of NF-κB pathway [113]. The more study demonstrated the effectiveness of thymol in same colitis model by comparing it with a reference compound (prednisolone). Treatment with thymol exhibited better anticolitic effect as compared to prednisolone group by reducing all pathological scores in the rat colon cells. The above mechanism depicts the potential activity of thymol in the prophylaxis of UC [114].
6.3.4 Menthol
The oxidoinflammatory efficacy of menthol evaluated in A.A treated rats for the management of UC revealed that pre-treatment with menthol significantly inhibited mucosal damage, inflammatory levels and histopathogical scores in the colon cells at medium to higher dose as compared to a standard drug dexamethasone [115]. Another study has demonstrated the anticolitic activity of the menthol by using A.A induced model in rats. Due to A.A administration, a significant reduction in ulcer scores, antioxidant markers, glutathione levels and also a raised level of malondialdehyde, lipid peroxidation activity were observed in rats. Treatment with menthol attenuates all the above ulcerogenic scores in the colon mucosa [116]. Jing et al. reported the ameliorating efficacy of menthol for the treatment of colitis-associated caner by using AOM-DSS/AD (azoxymethane combined with dextran sulphate sodium) mouse model. Administration of menthol to the mouse causes significant reduction of histopathological scores, DAI, proliferation biomarkers and inflammatory mediators in the colon epithelium. In addition menthol supplement diet also improves the tumorigenesis, growth of butyrate producing bacteria in AD induced mouse model [117]. The above reports depicted that menthol may be an advantageous therapeutic target in the management and prevention of UC.
6.3.5 1, 8-cineole (Eucalyptol)
An in vitro and histopathological study was conducted, to evaluate the targets and 'anti-inflammatory' mechanisms of 1, 8-cineole (Eucalyptol) by using UC model treated with DSS. In vitro studies revealed, 1, 8-cineole (Eucalyptol) inhibits the macrophage polarization by affecting the formation of HSP90- SGT1-NLRP3 complex. Authors have also evaluated that 1, 8-cineole (Eucalyptol) also improved the epithelial barrier disruption and pathological symptoms in DSS induced mice [118]. Venkataraman et al. conducted a molecular docking analysis to assess the agonistic activity of eucalyptol towards the PPARγ protein, employing both in vitro (TNF-α-stimulated HT-29 cells) and in vivo approaches (DSS-induced colitis model). Authors reported that, eucalyptol could exert potent anticolitic activity by activating PPARγ protein and Nrf2 translocation inside the epithelium [119]. In an another study Sandeep et al. have demonstrated the anticolitic effect of 1, 8-cineole (Eucalyptol) using LPS-stimulated RAW macrophages and DSS induced experimental model. Pretreatment with 1, 8-cineole (Eucalyptol) demonstrated a dose responsive inhibition of DAI; pro inflammatory cytokines and other pathological symptoms in DSS treated mice. Furthermore, it alleviated ulcer scores in the colon epithelium by suppressing the expression of NF-κB and COX-2 in both the experimental models [120]. The above findings suggested that 1, 8-cineole could be a valuable candidate for the management of UC.
6.4 Phytocompounds
Medicinal plants contain numerous chemical compounds (phytocompounds), which are known for their promising therapeutic effect in colon inflammation and are used since years [121, 122]. Several researchers have evaluated the potential of these phytocompounds (Table 3) and underlying anti ulcerative mechanisms using various animal models that have been summarized below:
Anticolitic potential of Essential oil and Phytocompounds
6.4.1 Nitrogen containing compounds
Nitrogen containing secondary metabolites, especially derived from medicinal plants exhibits a potent anti-inflammatory and immune regulating properties against UC [123]. For instance Zhang et al. considered an alkaloidal compound Corynoline, extracted from Corydalis bungeana Turcz, to evaluate its 'anti-inflammatory' activity against UC mice treated with DSS. Their findings demonstrated, corynoline treatment reduced all the ulcerogenic markers in DSS administered coitic mice and also down-regulated the inflammatory cytokines, oxidative stress markers in the intestinal tissue by restraining the activated NF-κB translocation [124]. Another study evaluated the mechanism and curative effect of coptisine, using DSS treated mice for the treatment of UC. The authors demonstrated, coptisine exhibits an inhibitory effect on macrophage polarization by regulating m6A methylation of tuberous sclerosis complex. In addition, coptisine treatment ameliorated the colitic symptoms and inhibited the inflamed lesions in mice administered with DSS [125]. Zhang et al. investigated the immunomodulatory action of Cepharanthine by conducting both in vitro experiments on LPS-administered RAW264.7 macrophages and in vivo experiments on a colitis model administered with DSS. Their analysis demonstrated that, Cepharanthine inhibited the Aconitate decarboxylase 1 over-expression, macrophage cell infiltration and also maintains the gut microbiota homeostasis to combat UC [126]. Anti inflammatory activity of an isoquinoline alkaloid, protropine was evaluated by Yue et al. using DSS administered colitis model. The study demonstrated that, protropine administration effectively ameliorated UC by maintaining gut microbiota dysbiosis, inhibiting inflammatory gene expressions and modulating intestinal mucosal barrier integrity significantly [127]. In another investigation, Liu et al. evaluated the mechanism and impact of Oxysophocarpine on UC by using DSS treated mouse model. Oxysophocarpine administration alleviated colitis by suppressing the level of phosphorylation of nuclear factor-κB and theTNF receptor-associated Factor 6 (TRAF6) in DSS treated mouse. Moreover, Oxysophocarpine reduced DAI, inflammatory cell infiltration, colon injury and oxidative stress in the colon tissue [128].
6.4.2 Phenol containing compounds
Polyphenolics or flavanoids are important among all the secondary metabolites that possess a strong 'anti-oxidant and anti-inflammatory' properties and also been reported to have efficacious against UC [17]. For instance, Mahsa et al. used a colitis model induced with A.A, to investigate the anticolitic efficacy of two phenolic derivatives i.e. coumaric acid and syringic acid. A notable suppression of inflammatory cytokines and upegulation of NQO1 mRNA, HO-1, and Nrf2 expression was observed in the epithelial colonic tissue, as compared to the A.A group [129]. Prakash et al. have investigated in vivo anticolitic effects of wedelolactone using DSS administered UC model of female albino wistar rats by targeting interleukin-6/signal transducer pathway. From 'anti-inflammatory' study, it was demonstrated that, pro inflammatory cytokines expression was reduced significantly and blockage of IL-6/STAT3 pathway preserved after exposure of wedelolactone [130]. An in vivo investigation in C57BL/6J mice was performed to evaluate the anti-inflammatory effects of Puerarin in colitis. Researchers observed that Puenarin treatment alleviated the crypt deformation, inflammatory cell infiltration and reduced the pro-inflammatory cytokine markers in the colon mucosa of mice and well act as a potent therapeutic agent that could be used in UC for maintaining gut microbiota homeostasis [131]. A natural flavonoid Galangin has been demonstrated to have protective effect on UC in an in vivo animal model. Molecular biology techniques demonstrated that, galangin can ameliorate colitis by inhibiting HSP90β oligomerization and restraining inflammasome activation in the colon tissue [132]. The anticolitic effect of Cardamonin via necroptosis inhibition by using lipopolysaccharide administered HT29, L929, or RAW264.7 cell have been reported. Shen et al. have reported that, Cardamonin prevents the necrosome formation by blocking RIPK1/3 phosphorylation in the colon tissues. Furthermore, oral treatment of Cardamonin alleviated the colitis symptoms, restrains the colon damage and inhibits necroinflammation in DSS administered mice suggesting that Cardamonin could serves as a novel inhibitor of necroptosis and holds promise as a potential candidate for treating UC [133]. Another study investigated the therapeutic effects of another phenolic compound i.e. Genistein against UC by using rat model treated with A.A. Administration with a dose of 25-mg/kg, genistein ameliorated the pathological symptoms, colon damage and cell infiltration in A.A injected rats. A remarkable balance of gene and protein expressions has been observed in the colon tissue. Moreover, antioxidant potential of this phenolic compound increases mitochondrial biogenesis followed by reducing cell apoptosis, suggesting its beneficial role [134].
6.4.3 Sulphur containing compounds
Sulphur containing compounds isolated from natural sources has been verified for its excellent anti-inflammatory activity in various preclinical models against the treatment of UC. They exert their anti-inflammatory action by inhibiting several signaling pathways [135]. For instance, anti-inflammatory potential of N-acetylcysteinehas been investigated on remission maintenance in 168 UC patients in a clinical trial (double blind randomized control) via grouping them as NAC (N-acetylcysteine) group & placebo group. Findings of this study revealed a significant reduction of remission in NAC group as compared to the placebo group [136]. In another investigation, Zhang et al. evaluated the colonic mucosal healing effect of diallyltrisulfide in mice after induction of colitis using DSS. Their findings demonstrated that diallyltrisulfide improved cell migration, wound healing, focal adhesion kinase phosphorylation in the intestinal epithelial cells by promoting endocytosis of integrin β1 and thereby promoting mucosal healing [137]. Lohning et al. used a chemically induced murine model and performed in silico techniques, to investigate the pathways by which 6-MITC (6- (methylsulfinyl)hexyl isothiocyanate) restrains inflammation in the epithelial cells. It was observed, 6-MITC treatment ameliorates the intestinal inflammation by suppressing NF-kB signaling through GSK-3b (glycogen synthase kinase 3 beta) inhibition and also improves colonic alterations in DSS induced mouse [138]. Similarly, Zhang et al. explored the anti-inflammatory activity of sulforaphane in UC mice treated with DSS. After DSS induction, the mice showed, decreased body weight and increased colon damage, intestinal dysbiosis, myeloperoxidase activity in the colon tissues. Meanwhile, administration of sulforaphane reversed all the pathological symptoms and modulated the gut homeostasis in DSS treated mice [139]. The protective role of a sulphur containing aminoacid, ergothionene against UC was studied in DSS treated animal model. Authors reported that ergothionene alleviates colon damage; pathological symptoms in DSS induced rats. Furthermore, it inhibited the expression of TLR4/MyD88/NF-κB pathways and exhibited potent antioxidant activity. [140].
6.5 Nutraceuticals
Nutraceuticals play a vital role in treating UC via modulating gut microbiota composition, regulating immune system and maintenance of remission [141, 142]. Enormous studies have been reported where nutraceuticals (Table 4) such as vitamins, carbohydrates, bioactive polypeptides, prebiotics, probiotics and natural oils have exhibited potential preventive effect in UC as summarized below:
The protective effect of nutraceuticals in the management of ulcerative colitis
6.5.1 Vitamins
A randomized controlled clinical trial to calculate DAI was done by using mayo clinic score on 150 patients. Results demonstrated that intake of Vit-A at a dose of 25000 IU/day could improve mucosal damage and disease activity index [143]. A study was carried out in DSS administered mouse, to examine the ameliorative action of Vit-C against UC. The elevated levels of inflammatory cytokines and reduction in fibroblast, collagen expressions in the colon cells were reversed by treatment of high dose Vit-C in DSS induced mouse [144]. Another study has demonstrated that, combination of Vit-C with Vit-D3 could attenuate intestinal barrier, tight junction proteins by regulating Notch-1 signaling pathway in DSS induced guinea pig model facilitating treatment of UC [145]. Guo et al. conducted a meta analysis to evaluate clinical safety and effectiveness of Vit-D in treating UC by using RevMan 5.4 software. Results demonstrated that treatment with Vit-D was more effective than conventional treatment groups in ameliorating mayo score and epithelial barrier function, and downregulating inflammatory mediators in the patients [146]. The protective effect of alpha- and gamma-tocopherol against a DSS induced colitis model was also observed. The treatment with tocopherol attenuated the symptoms caused by DSS administration and also modulated gut microbiota [147].
6.5.2 Carbohydrates
Carbohydrates have also demonstrated ameliorative effect in UC [148]. For instance, Yan et al. carried out both in vitro and in vivo analysis to explore the potential effectiveness of dandelion polysaccharides (DP) in IEC-6 cells by using UC model treated with DSS. In vitro findings highlighted the efficacy of DP in suppressing inflammation, ferroptosis, and oxidative stress via activation of Nrf2pathway. Additionally, DP maintained intestinal permeability and restored mitochondrial damage by enhancing iron transport. Moreover, it ameliorates the DAI and inflammatory scores in mice treated with DSS [149]. Polysaccharides from Atractylodes macrocephala (AMP) also regulated the gut homeostasis by inhibiting the production of short-chain fatty acids and bile acids as demonstrated by Feng et al. Administration of AMP significantly reduced the mucosal injury and histopathological scores in DSS treated colitis model [150]. The anti inflammatory activity of polysaccharides (PLS), extracted from Morindacitrifolihas also been observed in a UC model induced with A.A. Treatment with PLS was demonstrated to reduce oxidative stress, lower inflammatory scores, and decrease cox-2 expression, suggesting its promising potential in treating UC [151]. A recent research has demonstrated the anticolitic effect of polysaccharides from Rheum tanguticum via modulating gut microbiota dysbiosis in a mice model induced with DSS. The heteropolysaccharide present in RTP improved the pathological symptoms, reduced oxidative stress, proinflammatory cytokines and modulates the gene expressions related to NF-κB signaling pathway in DSS treated mice [152].
6.5.3 Probiotics and prebiotics
An investigation aimed to evaluate the mechanism of Lactobacillus acidophilusPIN7 supplement diet in modulating the gut microbiota employing DSS aggravated colitis model. The PIN7 groups treated with lysozyme and heat-killed variants demonstrated elevated colonic expressions of toll-like receptors and epithelial junction proteins, along with reduced expressions of pro-inflammatory mediators, p-IκBα and mucosal injury scores in the colons of colitis mice [153]. Zhang et al. evaluated the anticolitic efficacy of Bifidobacterium strains (B. longum FBJ20M1, B. longum FGDLZ8M1, B. longum FGSZY16M3, and B. longum FJSWXJ2M1) by using DSS induced colitis model. All strains were found to be effective in ameliorating disease scores, colon injury and epithelial permeability and the B. longum FGDLZ8M1 strain exhibited a greater efficacy as compared to other three strains [154].
Various prebiotics have also show beneficial effects against UC [155]. In a recent study, the anticolitic potential of alginate oligosaccharides was observed i.e. alleviation of DAI and intestinal barrier damage in a DSS induced model. The alginate oligosaccharides relieve UC by modulating intestinal homeostasis, reducing Bax protein expressions and elevating Bcl-2 protein expressions in colon mucosa [156]. In the year 2023 Chen et al. performed an in vitro study to investigate the modulating effects of fructooligosaccharides-short chain fatty acid (FOS) esters i.e. butyrylated FOS and propionylated FOS in UC patients by assessing the short-chain fatty acid levels during fermentation. Both esters demonstrated efficacy in modulating intestinal homoeostasis and inflammation [157].
6.5.4 Bioactive polypeptides
Mota et al. used a functional food lupin protein concentrate, to evaluate its anicolitic efficacy against TNBS and A.A induced ulcer model. An oligomer, deflamin present in lupin seeds have demonstrated to be efficacious in ameliorating the pathological symptoms of UC in both the models by inhibiting MMP-9 inflammatory pathway [158]. Another bioactive peptide, foxtail millet protein hydrolysates (FMPH) also reduced inflammatory markers in DSS aggravated UC mice by inhibiting DAI, inflammasome activation, NF-κB phosphorylation through NLRP3/ASC/caspase-1 pathway [159]. Tuna bioactive peptide also depicts anti-inflammatory activity against UC model treated with DSS. Administration of tuna bioactive peptide, significantly improves the morphological and ulcerogenic scores in UC mice. It acts by increasing antioxidant markers, tight junction proteins and short-chain fatty acid levels [160].
6.5.5 Natural oils
Numerous studies have demonstrated that incorporating natural oils into the diet or using them topically could potentially have therapeutic benefits for UC, as they can modulate the gut microbiota, inhibit inflammation and improve intestinal homeostasis [161]. For instance, Bartoszek et al. performed an in vivo study to investigate the anticolitic effect of walnut oil in mouse. The presence of linoleic acid and linolenic acids in the walnut oil suppress the inflammatory cytokines and improves colon damage, intestinal permeability by restoring tight junction proteins [162]. The anticolitis effect of extravirgin olive oil (EVOO) has also been observed in DSS induced rats. The study evaluated the ameliorative effect of this oil by examining DAI, histological scores and biochemical markers in the colon of rats and found promising outcomes [163]. The administration of flaxseed oil, obtained from Linum usitatissimum Lin DSS induced rats reversed the ulcerogenic scores such as lowered the DAI and reduced the oxidative stress [164]. Another study using cottonseed oil demonstrated the anticolitic activity of this oil by using DSS aggravated colitis model. The animal model showed significant decrease in DAI, biochemical markers, inflammatory scores including 8-hydroxyguanosine and nitrotyrosine in colon mucosa which were aggravated by DSS administration. In addition, treatment with cotton seed oil reduced alpha-smooth muscle actin and type I collagen in order to inhibit intestinal fibrosis in the DSS induced model [165]. The above studies provide beneficial therapeutic effects of natural oils in the prevention of UC.
7 Clinical research
While numerous herbal treatments for UC are explored, their safety for human use must be ensured. In recent decade, clinical trials in UC have evolved (Table 1). For instance, in a double-blind clinical trial study Uchiyama et al., evaluated the efficacy of Indigo naturalis, extracted from Assam indigo. In their study, 46 UC patients received either Indigo naturalis or placebo for 2 weeks. Results showed significant improvement in the patients receiving Indigo naturalis compared to the placebo group. Short-term Indigo naturalis use appears effective and safe for UC treatment [166]. Papada et al., conducted a Randomised, Double-Blind, Placebo-Controlled Trial to examine the impact of Pistacia lentiscussupplement on oxidative stress markers and plasma-free amino acids in active IBD patients. Pistacia lentiscus supplementation led to significant reductions in LDL levels and ratios in the intervention group, suggesting its potential as a non-pharmacological intervention to mitigate oxidative stress in IBD. Additionally, Pistacia lentiscus supplementation ameliorated decreases in plasma-free amino acids observed in patients with UC receiving placebo, indicating a potential role in improving metabolic profiles in UC [167]. Similar trial was performed by Baghizadeh and his co-authors to investigate the efficacy of Plantago major seed supplementation on UC symptoms. Sixty-one subjects received either 3600 mg/day of roasted Plantago major seed or roasted wheat flour for 8 weeks alongside standard medications. The Lichtiger Colitis Activity Index was used to assess variables at baseline, week 4, and week 8. Results showed that abdominal tenderness, gastroesophageal reflux, gastric pain, visible blood in stool, distension, and anal pain were significantly reduced in the Plantago major group compared to the placebo group [168].
8 Conclusion and future perspectives
The chronic, relapsing nature of UC and the rising global prevalence of UC, especially in developed nations, necessitate the urgent need for effective therapeutic strategies. The present review has highlighted the significance of natural compounds, including plant extracts, essential oils, nutraceuticals, and phytochemicals, in offering protective, therapeutic, preventive, and ameliorative effects on colonic inflammation. The diverse array of natural compounds reviewed in this article, with their oxidoinflammatory properties, represents a promising avenue for the management of UC. These compounds not only provide relief to patients but also offer potential advantages in terms of reduced toxic side effects, contributing to enhanced overall well-being. Evidence supporting the efficacy of natural substances encourages further research, particularly rigorous clinical trials and translational studies, to validate their effectiveness across diverse patient populations. A deeper understanding of their underlying mechanisms is crucial for optimizing treatment strategies. Personalized medicine approaches, which consider genetic and environmental factors, may enhance the precision and effectiveness of natural compound-based interventions.Moreover, ongoing research into the intricate interplay between the autoimmunity, gut microbiota, and environmental factors in the context of UC will provide valuable insights.Future research should focus on the interplay between autoimmunity, gut microbiota, and environmental factors in UC. This knowledge will guide the development of novel prophylactic interventions that leverage the synergistic effects of natural compounds, targeting specific pathways involved in UC pathogenesis. Furthermore, exploring novel delivery systems, such as nanoparticles or targeted drug delivery techniques, could improve the bioavailability and therapeutic potential of natural compounds, enhancing their efficacy in UC treatment.Overall, integrating natural compounds into comprehensive treatment approaches holds promise for revolutionizing the management of UC and warrants continued investigation.
Notes
Abbreviations
A.A
Acetic acid
DAI
Disease activity index
DSS
Dextran sulphate sodium
IBD
Inflammatory bowel disease
JAK-STAT
Janus kinase/signal transducers and activators of transcription
NF-κB
Nuclear factor kappa B
Nrf2
Nuclear factor erythroid 2–related factor 2
NLRP3
Nucleotide-binding domain, leucine-rich–containing family, pyrin domain–containing protein-3
NO
Nitric oxide synthase
PPAR-γ
Peroxisome proliferator-activated receptor gamma
TLR
Toll like receptor
TNBS
2, 4, 6-Trinitrobenzene sulfonic acid
UC
Ulcerative colitis
Acknowledgements
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author contributions
Rudra Narayan Subudhi and Neelam Poonia were involved in writing original draft; Dilpreet Singhand Vimal Arora were involved in reviewing and editing the manuscript.
Availability of data and materials
Authors agree to share the data whenever required.
Declarations
Competing interests
The authors declare that there are no conflicts of interest regarding the publication of this paper.
References
-
1.Buie MJ, Quan J, Windsor JW, Coward S, Hansen TM, King JA, Kotze PG, Gearry RB, Ng SC, Mak JW, Abreu MT. Global hospitalization trends for Crohn’s disease and ulcerative colitis in the 21st century: a systematic review with temporal analyses. Clin Gastroenterol Hepatol. 2023;21(9): 2211-21. CrossRef PubMed Google Scholar
-
2.Wei SC, Sollano J, Hui YT, Yu W, Santos Estrella PV, Llamado LJ, Koram N. Epidemiology, burden of disease, and unmet needs in the treatment of ulcerative colitis in Asia. Expert Rev Gastroenterol Hepatol. 2021;15(3): 275-89. CrossRef PubMed Google Scholar
-
3.Aniwan S, Santiago P, Loftus EV Jr, Park SH. The epidemiology of inflammatory bowel disease in Asia and Asian immigrants to Western countries. United Eur Gastroenterol J. 2022;10(10): 1063-76. CrossRef PubMed Google Scholar
-
4.Lewis JD, Parlett LE, Funk ML, Brensinger C, Pate V, Wu Q, Dawwas GK, Weiss A, Constant BD, McCauley M, Haynes K. Incidence, prevalence, and racial and ethnic distribution of inflammatory bowel disease in the United States. Gastroenterology. 2023;165(5): 1197-205. CrossRef PubMed Google Scholar
-
5.Weber F, Eger KI, March C, Croner RS, Meyer F. Manifestation of acute appendicitis as known but paradox visceral side effect of ulcerative colitis anti-inflammatory therapy with januskinase-inhibitor Tofacitinib (Xeljanz™). Pathol Res Pract. 2023;248: 154333. CrossRef PubMed Google Scholar
-
6.Gros B, Kaplan GG. Ulcerative colitis in adults: a review. JAMA. 2023;330(10): 951-65. CrossRef PubMed Google Scholar
-
7.Sandborn WJ, Danese S, Leszczyszyn J, Romatowski J, Altintas E, Peeva E, Hassan-Zahraee M, Vincent MS, Reddy PS, Banfield C, Salganik M. Oral ritlecitinib and brepocitinib for moderate-to-severe ulcerative colitis: results from a randomized, phase 2b study. Clin Gastroenterol Hepatol. 2023. CrossRef PubMed Google Scholar
-
8.Lim J, Rezaie A. Irritable bowel syndrome-like symptoms in quiescent inflammatory bowel disease: a practical approach to diagnosis and treatment of organic causes. Dig Dis Sci. 2023;68(11): 4081-97. CrossRef PubMed Google Scholar
-
9.Singh S, Dulai PS. Ulcerative colitis: clinical manifestations and management. Yamada's Textbook of Gastroenterology. 2022, 1248-93. CrossRef PubMed Google Scholar
-
10.Kotze PG, Heuthorst L, Lightner AL, Damião AO, Bemelman WA. New insights on the surgical management of ulcerative colitis in the 21st century. Lancet Gastroenterol Hepatol. 2022. CrossRef PubMed Google Scholar
-
11.Raine T, Bonovas S, Burisch J, Kucharzik T, Adamina M, Annese V, Bachmann O, Bettenworth D, Chaparro M, Czuber-Dochan W, Eder P. ECCO guidelines on therapeutics in ulcerative colitis: medical treatment. J Crohns Colitis. 2022;16(1): 2-17. CrossRef PubMed Google Scholar
-
12.Guo XY, Liu XJ, Hao JY. Gut microbiota in ulcerative colitis: insights on pathogenesis and treatment. J Dig Dis. 2020;21(3): 147-59. CrossRef PubMed Google Scholar
-
13.Amiot A, Bouguen G, Bonnaud G, Bouhnik Y, Hagege H, Peyrin-Biroulet L, Abitbol V, Malamut G, Amiot A, Boruchowicz A, Siproudhis L. Clinical guidelines for the management of inflammatory bowel disease: update of a French national consensus. Dig Liver Dis. 2021;53(1): 35-43. CrossRef PubMed Google Scholar
-
14.Liu S, Eisenstein S. State-of-the-art surgery for ulcerative colitis. Langenbeck’s Arch Surg. 2021;406(6): 1751-61. CrossRef PubMed Google Scholar
-
15.Lu L, Dong J, Liu Y, Qian Y, Zhang G, Zhou W, Zhao A, Ji G, Xu H. New insights into natural products that target the gut microbiota: effects on the prevention and treatment of colorectal cancer. Front Pharmacol. 2022;13: 964793. CrossRef PubMed Google Scholar
-
16.Salibay CC, Mahboob T, Verma AK, San Sebastian JS, Tabo HA, Raju CS, Nissapatorn V. Natural product–derived drugs for the treatment of inflammatory bowel diseases (IBD). Inflamm Nat Prod. 2021. CrossRef PubMed Google Scholar
-
17.Xue JC, Yuan S, Meng H, Hou XT, Li J, Zhang HM, Chen LL, Zhang CH, Zhang QG. The role and mechanism of flavonoid herbal natural products in ulcerative colitis. Biomed Pharmacother. 2023;158: 114086. CrossRef PubMed Google Scholar
-
18.Duan L, Cheng S, Li L, Liu Y, Wang D, Liu G. Natural anti-inflammatory compounds as drug candidates for inflammatory bowel disease. Front Pharmacol. 2021;14(12): 684486. CrossRef PubMed Google Scholar
-
19.Akkol EK, Karpuz B, Sobarzo-Sánchez E, Khan H. A phytopharmacological overview of medicinal plants used for prophylactic and treatment of colitis. Food Chem Toxicol. 2020;144: 111628. CrossRef PubMed Google Scholar
-
20.Dunleavy KA, Raffals LE, Camilleri M. Intestinal barrier dysfunction in inflammatory bowel disease: underpinning pathogenesis and therapeutics. Digest Dis Sci. 2023;68(12): 4306-20. CrossRef PubMed Google Scholar
-
21.Liu Z, Zhang Y, Jin T, Yi C, Ocansey DK, Mao F. The role of NOD2 in intestinal immune response and microbiota modulation: a therapeutic target in inflammatory bowel disease. Int Immunopharmacol. 2022;113: 109466. CrossRef PubMed Google Scholar
-
22.Fu Q, Song T, Ma X, Cui J. Research progress on the relationship between intestinal microecology and intestinal bowel disease. Anim Models Exp Med. 2022;5(4): 297-310. CrossRef PubMed Google Scholar
-
23.Chu J, Feng S, Guo C, Xue B, He K, Li L. Immunological mechanisms of inflammatory diseases caused by gut microbiota dysbiosis: s review. Biomed Pharmacother. 2023;164: 114985. CrossRef PubMed Google Scholar
-
24.Mahapatro M, Erkert L, Becker C. Cytokine-mediated crosstalk between immune cells and epithelial cells in the gut. Cells. 2021;10(1): 111. CrossRef PubMed Google Scholar
-
25.Nakase H, Sato N, Mizuno N, Ikawa Y. The influence of cytokines on the complex pathology of ulcerative colitis. Autoimmunity Rev. 2022;21(3): 103017. CrossRef PubMed Google Scholar
-
26.Du L, Ha C. Epidemiology and pathogenesis of ulcerative colitis. Gastroenterol Clin. 2020;49(4): 643-54. CrossRef PubMed Google Scholar
-
27.Kaur A, Goggolidou P. Ulcerative colitis: understanding its cellular pathology could provide insights into novel therapies. J Inflamm. 2020;17: 1-8. CrossRef PubMed Google Scholar
-
28.Kałużna A, Olczyk P, Komosińska-Vassev K. The role of innate and adaptive immune cells in the pathogenesis and development of the inflammatory response in ulcerative colitis. J Clin Med. 2022;11(2): 400. CrossRef PubMed Google Scholar
-
29.Gomez-Bris R, Saez A, Herrero-Fernandez B, Rius C, Sanchez-Martinez H, Gonzalez-Granado JM. CD4 T-cell subsets and the pathophysiology of inflammatory bowel disease. Int J Mol Sci. 2023;24(3): 2696. CrossRef PubMed Google Scholar
-
30.Tindemans I, Joosse ME, Samsom JN. Dissecting the heterogeneity in T-cell mediated inflammation in IBD. Cells. 2020;9(1): 110. CrossRef PubMed Google Scholar
-
31.Lu Q, Yang MF, Liang YJ, Xu J, Xu HM, Nie YQ, Wang LS, Yao J, Li DF. Immunology of inflammatory bowel disease: molecular mechanisms and therapeutics. J Inflamm Res. 2022;15: 1825-44. CrossRef PubMed Google Scholar
-
32.Moreira Lopes TC, Mosser DM, Gonçalves R. Macrophage polarization in intestinal inflammation and gut homeostasis. Inflamm Res. 2020;69: 1163-72. CrossRef PubMed Google Scholar
-
33.Yip JL, Balasuriya GK, Spencer SJ, Hill-Yardin EL. The role of intestinal macrophages in gastrointestinal homeostasis: heterogeneity and implications in disease. Cell Mol Gastroenterol Hepatol. 2021;12(5): 1701-18. CrossRef PubMed Google Scholar
-
34.Xiang C, Liu M, Lu Q, Fan C, Lu H, Feng C, Yang X, Li H, Tang W. Blockade of TLRs-triggered macrophage activation by caffeic acid exerted protective effects on experimental ulcerative colitis. Cell Immunol. 2021;365: 104364. CrossRef PubMed Google Scholar
-
35.Dharmasiri S, Garrido-Martin EM, Harris RJ, Bateman AC, Collins JE, Cummings JF, Sanchez-Elsner T. Human intestinal macrophages are involved in the pathology of both ulcerative colitis and Crohn disease. Inflamm Bowel Dis. 2021;27(10): 1641-52. CrossRef PubMed Google Scholar
-
36.Gohil S, Majd Z, Sheneman JC, Abughosh SM. Interventions to improve medication adherence in inflammatory bowel disease: a systematic review. Patient Educ Counsel. 2022;105(7): 1731-42. CrossRef PubMed Google Scholar
-
37.Gajendran M, Loganathan P, Jimenez G, Catinella AP, Ng N, Umapathy C, Ziade N, Hashash JG. A comprehensive review and update on ulcerative colitis. Dis Mon. 2019;65(12): 100851. CrossRef PubMed Google Scholar
-
38.Smith RL, Taylor KM, Friedman AB, Gibson RN, Gibson PR. Systematic review: clinical utility of gastrointestinal ultrasound in the diagnosis, assessment and management of patients with ulcerative colitis. J Crohns Colitis. 2020;14(4): 465-79. CrossRef PubMed Google Scholar
-
39.Kucharzik T, Koletzko S, Kannengiesser K, Dignass A. Ulcerative colitis—diagnostic and therapeutic algorithms. Dtsch Arztebl Int. 2020;117(33–34): 564. CrossRef PubMed Google Scholar
-
40.Axelrad JE, Olén O, Askling J, Lebwohl B, Khalili H, Sachs MC, Ludvigsson JF. Gastrointestinal infection increases odds of inflammatory bowel disease in a nationwide case–control study. Clin Gastroenterol Hepatol. 2019;17(7): 1311-22. CrossRef PubMed Google Scholar
-
41.Frickenstein AN, Jones MA, Behkam B, McNally LR. Imaging inflammation and infection in the gastrointestinal tract. Int J Mol Sci. 2019;21(1): 243. CrossRef PubMed Google Scholar
-
42.Takenaka K, Fujii T, Kawamoto A, Suzuki K, Shimizu H, Maeyashiki C, Yamaji O, Motobayashi M, Igarashi A, Hanazawa R, Hibiya S. Deep neural network for video colonoscopy of ulcerative colitis: a cross-sectional study. Lancet Gastroenterol Hepatol. 2022;7(3): 230-7. CrossRef PubMed Google Scholar
-
43.Mokter MF, Oh J, Tavanapong W, Wong J, de Groen PC. Classification of ulcerative colitis severity in colonoscopy videos using vascular pattern detection. InMachine Learning in Medical Imaging: 11th International Workshop, MLMI 2020, Held in Conjunction with MICCAI 2020, Lima, Peru, October 4, 2020, Proceedings 11 2020 (pp. 552–562). Springer International Publishing. https://doi.org/10.1007/978-3-030-59861-7_56 PubMed Google Scholar
-
44.Linggi B, Jairath V, Zou G, Shackelton LM, McGovern DP, Salas A, Verstockt B, Silverberg MS, Nayeri S, Feagan BG, van de Casteele N. Meta-analysis of gene expression disease signatures in colonic biopsy tissue from patients with ulcerative colitis. Sci Rep. 2021;11(1): 18243. CrossRef PubMed Google Scholar
-
45.Battat R, Vande Casteele N, Pai RK, Wang Z, Zou G, McDonald JW, Duijvestein M, Jeyarajah J, Parker CE, Van Viegen T, Nelson SA. Evaluating the optimum number of biopsies to assess histological inflammation in ulcerative colitis: a retrospective cohort study. Aliment Pharmacol Ther. 2020;52(10): 1574-82. CrossRef PubMed Google Scholar
-
46.Park SB, Kim SJ, Lee J, Lee YJ, Baek DH, Seo GS, Kim ES, Kim SW, Kim SY. Efficacy of sigmoidoscopy for evaluating disease activity in patients with ulcerative colitis. BMC Gastroenterol. 2022;22(1): 1-7. CrossRef PubMed Google Scholar
-
47.Chen H, Wu L, Wang M, Shao B, Ye L, Zhang Y, Cao Q. Use of the ulcerative colitis endoscopic index of severity and Mayo endoscopic score for predicting the therapeutic effect of mesalazine in patients with ulcerative colitis. Laparosc Endosc Robot Surg. 2021;4(2): 33-9. CrossRef PubMed Google Scholar
-
48.Pagnini C, Di Paolo MC, Mariani BM, Urgesi R, Pallotta L, Vitale MA, Villotti G, d’Alba L, De Cesare MA, Di Giulio E, Graziani MG. Mayo endoscopic score and ulcerative colitis endoscopic index are equally effective for endoscopic activity evaluation in ulcerative colitis patients in a real-life setting. Gastroenterol Insights. 2021;12(2): 217-24. CrossRef PubMed Google Scholar
-
49.Xiao BH, Ma XD, Lv JJ, Yang T, Liu XJ, An LY, Qi YX, Lu ML, Duan YQ, Sun DL. Systematic evaluation of the diagnostic approach of inflammatory bowel disease guidelines. Int J Clin Pract. 2021;75(10): 14365. CrossRef PubMed Google Scholar
-
50.Shaban N, Hoad CL, Naim I, Alshammari M, Radford SJ, Clarke C, Marciani L, Moran G. Imaging in inflammatory bowel disease: current and future perspectives. Frontline Gastroenterol. 2022. CrossRef PubMed Google Scholar
-
51.Le Berre C, Ananthakrishnan AN, Danese S, Singh S, Peyrin-Biroulet L. Ulcerative colitis and Crohn’s disease have similar burden and goals for treatment. Clin Gastroenterol Hepatol. 2020;18(1): 14-23. CrossRef PubMed Google Scholar
-
52.D’Amico F, Fasulo E, Jairath V, Paridaens K, Peyrin-Biroulet L, Danese S. Management and treatment optimization of patients with mild to moderate ulcerative colitis. Expert Rev Clin Immunol. 2023;13: 1-4. CrossRef PubMed Google Scholar
-
53.Cai Z, Wang S, Li J. Treatment of inflammatory bowel disease: a comprehensive review. Front Med. 2021;8: 765474. CrossRef PubMed Google Scholar
-
54.Chen M, Lan H, Jin K, Chen Y. Responsive nanosystems for targeted therapy of ulcerative colitis: current practices and future perspectives. Drug Deliv. 2023;30(1): 2219427. CrossRef PubMed Google Scholar
-
55.Ferretti F, Cannatelli R, Monico MC, Maconi G, Ardizzone S. An update on current pharmacotherapeutic options for the treatment of ulcerative colitis. J Clin Med. 2022;11(9): 2302. CrossRef PubMed Google Scholar
-
56.Alsoud D, Verstockt B, Fiocchi C, Vermeire S. Breaking the therapeutic ceiling in drug development in ulcerative colitis. Lancet Gastroenterol Hepatol. 2021;6(7): 589-95. CrossRef PubMed Google Scholar
-
57.Gupta M, Mishra V, Gulati M, Kapoor B, Kaur A, Gupta R, Tambuwala MM. Natural compounds as safe therapeutic options for ulcerative colitis. Inflammopharmacology. 2022;30(2): 397-434. CrossRef PubMed Google Scholar
-
58.Moudgil KD, Venkatesha SH. The anti-inflammatory and immunomodulatory activities of natural products to control autoimmune inflammation. Int J Mol Sci. 2022;24(1): 95. CrossRef PubMed Google Scholar
-
59.El Menyiy N, El Allam A, Aboulaghras S, Jaouadi I, Bakrim S, El Omari N, Shariati MA, Miftakhutdinov A, Wilairatana P, Mubarak MS, Bouyahya A. Inflammatory auto-immune diseases of the intestine and their management by natural bioactive compounds. Biomed Pharmacother. 2022;1(151): 113158. CrossRef PubMed Google Scholar
-
60.Davila MM, Papada E. The role of plant-derived natural products in the management of inflammatory bowel disease—what is the clinical evidence so far? Life. 2023;13(8): 1703. CrossRef PubMed Google Scholar
-
61.Guo N, Lv LL. Mechanistic insights into the role of probiotics in modulating immune cells in ulcerative colitis. Immunity, Inflamm Dis. 2023;11(10): e1045. CrossRef PubMed Google Scholar
-
62.Chang Y, Zhai L, Peng J, Wu H, Bian Z, Xiao H. Phytochemicals as regulators of Th17/Treg balance in inflammatory bowel diseases. Biomed Pharmacother. 2021;1(141): 111931. CrossRef PubMed Google Scholar
-
63.Chen L, Ruan G, Cheng Y, Yi A, Chen D, Wei Y. The role of Th17 cells in inflammatory bowel disease and the research progress. Front Immunol. 2023;9(13): 1055914. CrossRef PubMed Google Scholar
-
64.Zhao J, Lu Q, Liu Y, Shi Z, Hu L, Zeng Z, Tu Y, Xiao Z, Xu Q. Th17 cells in inflammatory bowel disease: cytokines, plasticity, and therapies. J Immunol Res. 2021;22: 2021. CrossRef PubMed Google Scholar
-
65.Caioni G, Viscido A, Angelo M, Panella G, Castelli V, Merola C, Frieri G, Latella G, Cimini A, Benedetti E. Inflammatory bowel disease: new insights into the interplay between environmental factors and PPARγ. Int J Mol Sci. 2021;22(3): 985. CrossRef PubMed Google Scholar
-
66.Villarroel-Vicente C, Gutierrez-Palomo S, Ferri J, Cortes D, Cabedo N. Natural products and analogs as preventive agents for metabolic syndrome via peroxisome proliferator-activated receptors: an overview. Eur J Med Chem. 2021;5(221): 113535. CrossRef PubMed Google Scholar
-
67.Venkataraman B, Ojha S, Belur PD, Bhongade B, Raj V, Collin PD, Adrian TE, Subramanya SB. Phytochemical drug candidates for the modulation of peroxisome proliferator-activated receptor γ in inflammatory bowel diseases. Phytother Res. 2020;34(7): 1530-49. CrossRef PubMed Google Scholar
-
68.Laurindo LF, de Maio MC, Minniti G, de Góes CN, Barbalho SM, Quesada K, Guiguer EL, Sloan KP, Detregiachi CR, Araújo AC, de Alvares GR. Effects of medicinal plants and phytochemicals in Nrf2 pathways during inflammatory bowel diseases and related colorectal cancer: a comprehensive review. Metabolites. 2023;13(2): 243. CrossRef PubMed Google Scholar
-
69.Liu H, Johnston LJ, Wang F, Ma X. Triggers for the nrf2/are signaling pathway and its nutritional regulation: Potential therapeutic applications of ulcerative colitis. Int J Mol Sci. 2021;22(21): 11411. CrossRef PubMed Google Scholar
-
70.Laurindo LF, Santos AR, Carvalho AC, Bechara MD, Guiguer EL, Goulart RD, Vargas Sinatora R, Araújo AC, Barbalho SM. Phytochemicals and regulation of NF-kB in inflammatory bowel diseases: an overview of in vitro and in vivo effects. Metabolites. 2023;13(1): 96. CrossRef PubMed Google Scholar
-
71.Yu C, Wang D, Yang Z, Wang T. Pharmacological effects of polyphenol phytochemicals on the intestinal inflammation via targeting TLR4/NF-κB signaling pathway. Int J Mol Sci. 2022;23(13): 6939. CrossRef PubMed Google Scholar
-
72.Wang K, Mao T, Lu X, Wang M, Yun Y, Jia Z, Shi L, Jiang H, Li J, Shi R. A potential therapeutic approach for ulcerative colitis: targeted regulation of macrophage polarization through phytochemicals. Front Immunol. 2023;1(14): 1155077. CrossRef PubMed Google Scholar
-
73.Du Y, Rong L, Cong Y, Shen L, Zhang N, Wang B. Macrophage polarization: an effective approach to targeted therapy of inflammatory bowel disease. Expert Opin Ther Targets. 2021;25(3): 191-209. CrossRef PubMed Google Scholar
-
74.do Nascimento RD, da FonsecaMachado AP, Galvez J, Cazarin CB, Junior MR. Ulcerative colitis: gut microbiota, immunopathogenesis and application of natural products in animal models. Life Sci. 2020;258: 118129. CrossRef PubMed Google Scholar
-
75.Abe H, Ishioka M, Fujita Y, Umeno A, Yasunaga M, Sato A, Ohnishi S, Suzuki S, Ishida N, Shichiri M, Yoshida Y. Yuzu (Citrus junos Tanaka) peel attenuates dextran sulfate sodium-induced murine experimental colitis. J Oleo Sci. 2018;67(3): 335-44. CrossRef PubMed Google Scholar
-
76.Shin J, Song HY, Lee M. Sudachinoid-and ichangensin-type limonoids from Citrus junos downregulate pro-inflammatory cytokines. Int J Mol Sci. 2020;21(18): 6963. CrossRef PubMed Google Scholar
-
77.Kim SH, Shin EJ, Hur HJ, Park JH, Sung MJ, Kwon DY, Hwang JT. Citrus junos Tanaka peel extract attenuates experimental colitis and inhibits tumour growth in a mouse xenograft model. J Funct Foods. 2014;8: 301-8. CrossRef PubMed Google Scholar
-
78.Kim Y, Wu AG, Jaja-Chimedza A, Graf BL, Waterman C, Verzi MP, Raskin I. Isothiocyanate-enriched moringa seed extract alleviates ulcerative colitis symptoms in mice. PLoS ONE. 2017;12(9): e0184709. CrossRef PubMed Google Scholar
-
79.Mohamed Husien H, Peng W, Su H, Zhou R, Tao Y, Huang J, Liu M, Bo R, Li J. Moringa oleifera leaf polysaccharide alleviates experimental colitis by inhibiting inflammation and maintaining intestinal barrier. Front Nutr. 2022;10(9): 1055791. CrossRef PubMed Google Scholar
-
80.Hong ZS, Xie J, Wang XF, Dai JJ, Mao JY, Bai YY, Sheng J, Tian Y. Moringa oleifera Lam. peptide remodels intestinal mucosal barrier by inhibiting JAK-STAT activation and modulating gut microbiota in colitis. Front Immunol. 2022;13: 924178. CrossRef PubMed Google Scholar
-
81.Zhang Y, Peng L, Li W, Dai T, Nie L, Xie J, Ai Y, Li L, Tian Y, Sheng J. Polyphenol extract of Moringa oleifera leaves alleviates colonic inflammation in dextran sulfate sodium-treated mice. Evid Based Complement Altern Med. 2020. CrossRef PubMed Google Scholar
-
82.Shao S, Wang D, Zheng W, Li X, Zhang H, Zhao D, Wang M. A unique polysaccharide from Hericium erinaceus mycelium ameliorates acetic acid-induced ulcerative colitis rats by modulating the composition of the gut microbiota, short chain fatty acids levels and GPR41/43 respectors. Int Immunopharmacol. 2019;1(71): 411-22. CrossRef PubMed Google Scholar
-
83.Wang D, Zhang Y, Yang S, Zhao D, Wang M. A polysaccharide from cultured mycelium of Hericium erinaceus relieves ulcerative colitis by counteracting oxidative stress and improving mitochondrial function. Int J Biol Macromol. 2019;125: 572-9. CrossRef PubMed Google Scholar
-
84.Ren Y, Sun Q, Gao R, Sheng Y, Guan T, Li W, Zhou L, Liu C, Li H, Lu Z, Yu L. Low weight polysaccharide of Hericium erinaceus ameliorates colitis via inhibiting the NLRP3 inflammasome activation in association with gut microbiota modulation. Nutrients. 2023;15(3): 739. CrossRef PubMed Google Scholar
-
85.Durmus A, Durmus I, Bender O, Karatepe O. The effect of Hericium erinaceum on the prevention of chemically induced experimental colitis in rats. Korean J Intern Med. 2021;36(Suppl 1): S44. CrossRef PubMed Google Scholar
-
86.Gravina AG, Pellegrino R, Palladino G, Coppola A, Brandimarte G, Tuccillo C, Ciardiello F, Romano M, Federico A. Hericium erinaceus, in combination with natural flavonoid/alkaloid and B3/B8 vitamins, can improve inflammatory burden in Inflammatory bowel diseases tissue: an ex vivo study. Front Immunol. 2023. CrossRef PubMed Google Scholar
-
87.Shah TA, Parikh M, Patel KV, Patel KG, Joshi CG, Gandhi TR. Evaluation of the effect of Punica granatum juice and punicalagin on NFκB modulation in inflammatory bowel disease. Mol Cell Biochem. 2016;419: 65-74. CrossRef PubMed Google Scholar
-
88.Kamali M, Tavakoli H, Khodadoost M, Daghaghzadeh H, Kamalinejad M, Gachkar L, Mansourian M, Adibi P. Efficacy of the Punica granatum peels aqueous extract for symptom management in ulcerative colitis patients. A randomized, placebo-controlled, clinical trial. Complement Ther Clin Pract. 2015;21(3): 141-6. CrossRef PubMed Google Scholar
-
89.Kalyankumarraju M, Puppala ER, Ahmed S, Kumar GJ, Tene K, Syamprasad NP, Sahu BD, Barua CC, Naidu VG. Zanthoxylum alatum Roxb. seed extract ameliorates stress aggravated DSS-induced ulcerative colitis in mice: plausible role on NF-κB signaling axis. J Ethnopharmacol. 2021;279: 114385. CrossRef PubMed Google Scholar
-
90.Zaware B, Gilhotra R, Chaudhari SR. Potential of Mimosa pudica leaf in the treatment of ulcerative colitis in rat. Bangladesh J Pharmacol. 2018;13(3): 241-7. CrossRef PubMed Google Scholar
-
91.Recinella L, Gorica E, Chiavaroli A, Fraschetti C, Filippi A, Cesa S, Cairone F, Martelli A, Calderone V, Veschi S, Lanuti P. Anti-inflammatory and antioxidant effects induced by Allium sativum L. extracts on an ex vivo experimental model of ulcerative colitis. Foods. 2022;11(22): 3559. CrossRef PubMed Google Scholar
-
92.Owusu G, Obiri DD, Ainooson GK, Osafo N, Antwi AO, Duduyemi BM, Ansah C. Acetic acid-induced ulcerative colitis in Sprague Dawley rats is suppressed by hydroethanolic extract of Cordia vignei leaves through reduced serum levels of TNF-α and IL-6. Int J Chronic Dis. 2020;6: 2020. CrossRef PubMed Google Scholar
-
93.Adjouzem CF, Gilbert A, Mbiantcha M, Yousseu Nana W, Matah Marthe Mba V, Djuichou Nguemnang SF, Tsafack EG, Atsamo AD. Effects of aqueous and methanolic extracts of stem bark of Ansonia bonnie De wild (A polynucleate) on dextran sodium sulfate-induced ulcerative colitis in Wistar rats. Evid Based Complement Altern Med. 2020. CrossRef PubMed Google Scholar
-
94.Qin W, Luo H, Yang L, Hu D, Jiang SP, Peng DY, Hu JM, Liu SJ. Rubia cordifolia L. ameliorates DSS-induced ulcerative colitis in mice through dual inhibition of NLRP3 inflammasome and IL-6/JAK2/STAT3 pathways. Heliyon. 2022. CrossRef PubMed Google Scholar
-
95.Gupta RA, Motiwala MN, Mahajan UN, Sabre SG. Protective effect of Sesbania grandiflora on acetic acid induced ulcerative colitis in mice by inhibition of TNF-α and IL-6. J Ethnopharmacol. 2018;12(219): 222-32. CrossRef PubMed Google Scholar
-
96.Meurer MC, Mees M, Mariano LN, Boeing T, Somensi LB, Mariott M, Dos Santos AC, Longo B, França TC, Klein-Júnior LC, de Souza P. Hydroalcoholic extract of Tagetes erecta L. flowers, rich in the carotenoid lutein, attenuates inflammatory cytokine secretion and improves the oxidative stress in an animal model of ulcerative colitis. Nutr Res. 2019;66: 95-106. CrossRef PubMed Google Scholar
-
97.Almeer RS, Mahmoud SM, Amin HK, Moneim AE. Ziziphus spina-christi fruit extract suppresses oxidative stress and p38 MAPK expression in ulcerative colitis in rats via induction of Nrf2 and HO-1 expression. Food Chem Toxicol. 2018;1(115): 49-62. CrossRef PubMed Google Scholar
-
98.Suluvoy JK, Sakthivel KM, Guruvayoorappan C, Grace VB. Protective effect of Averrhoa bilimbi L. fruit extract on ulcerative colitis in wistar rats via regulation of inflammatory mediators and cytokines. Biomed Pharmacother. 2017;91: 1113-21. CrossRef PubMed Google Scholar
-
99.Chen G, Yang Y, Liu M, Teng Z, Ye J, Xu Y, Cai X, Cheng X, Yang J, Hu C, Wang M. Banxia xiexin decoction protects against dextran sulfate sodium-induced chronic ulcerative colitis in mice. J Ethnopharmacol. 2015;26(166): 149-56. CrossRef PubMed Google Scholar
-
100.Liu Y, Wang C, Wu J, Tan L, Gao P, Wu S, Tang D, Wang Q, Wang C, Li P, Liu J. Study on the comprehensive phytochemicals and the anti-ulcerative colitis effect of Saussurea pulchella. Molecules. 2023;28(4): 1526. CrossRef PubMed Google Scholar
-
101.Rehman IU, Saleem M, Raza SA, Bashir S, Muhammad T, Asghar S, Qamar MU, Shah TA, Bin Jardan YA, Mekonnen AB, Bourhia M. Anti-ulcerative colitis effects of chemically characterized extracts from Calliandra haematocephala in acetic acid-induced ulcerative colitis. Front Chem. 2024;27(12): 1291230. CrossRef PubMed Google Scholar
-
102.Devi K, Bali A, Bhatia P, Singh N, Jaggi AS. Exploring the ameliorative potential of Bacopa monnieri in acetic acid induced ulcerative colitis in mice. Nat Prod Res. 2024;38(12): 2105-10. CrossRef PubMed Google Scholar
-
103.Jia W, Yu S, Liu X, Le Q, He X, Yu L, He J, Yang L, Gao H. Ethanol extract of limonium bicolor improves dextran sulfate sodium-induced ulcerative colitis by alleviating inflammation and restoring gut microbiota dysbiosis in mice. Mar Drugs. 2024;22(4): 175. CrossRef PubMed Google Scholar
-
104.Abdellatif NA, Eltamany EE, El-Shenawy NS, Nafie MS, Hassan YM, Al-Eisa RA, Badr JM, Abdelhameed RF. Cassia fistula leaves extract profiling and its emphasis on induced ulcerative colitis in male rats through inhibition of caspase 3 and cyclooxygenase-2. Arab J Chem. 2024;16: 105672. CrossRef PubMed Google Scholar
-
105.Zhao Q, Zhu L, Wang S, Gao Y, Jin F. Molecular mechanism of the anti-inflammatory effects of plant essential oils: a systematic review. J Ethnopharmacol. 2023;30(301): 115829. CrossRef PubMed Google Scholar
-
106.Li J, Zhang X, Luan F, Duan J, Zou J, Sun J, Shi Y, Guo D, Wang C, Wang X. Therapeutic potential of essential oils against ulcerative colitis: a review. J Inflamm Res. 2024;17: 3527. CrossRef PubMed Google Scholar
-
107.de Santana Souza MT, Teixeira DF, de Oliveira JP, Oliveira AS, Quintans-Junior LJ, Correa CB, Camargo EA. Protective effect of carvacrol on acetic acid-induced colitis. Biomed Pharmacother. 2017;1(96): 313-9. CrossRef PubMed Google Scholar
-
108.Liu M, Mao G, Zhou X, Wan X, Zhang F, Dai L, Chen Y, Dai N, Zhang Y, Du Q. Carvacrol ameliorates DSS-induced intestinal inflammation in rats by suppressing the TLR4/NF-κB pathway. Nat Prod Commun. 2022. CrossRef PubMed Google Scholar
-
109.d’Alessio PA, Ostan R, Bisson JF, Schulzke JD, Ursini MV, Béné MC. Oral administration of d-limonene controls inflammation in rat colitis and displays anti-inflammatory properties as diet supplementation in humans. Life Sci. 2013;92(24–26): 1151-6. CrossRef PubMed Google Scholar
-
110.Yu L, Yan J, Sun Z. D-limonene exhibits anti-inflammatory and antioxidant properties in an ulcerative colitis rat model via regulation of iNOS, COX-2, PGE2 and ERK signaling pathways. Mol Med Rep. 2017;15(4): 2339-46. CrossRef PubMed Google Scholar
-
111.Estrella GR, Eva GT, Alberto HL, Guadalupe VD, Azucena CV, Sandra OS, Noé AV, Javier LM. Limonene from Agastache mexicana essential oil produces antinociceptive effects, gastrointestinal protection and improves experimental ulcerative colitis. J Ethnopharmacol. 2021;15(280): 114462. CrossRef PubMed Google Scholar
-
112.Liu DM, Zhou CY, Meng XL, Wang P, Li W. Thymol exerts anti-inflammatory effect in dextran sulfate sodium-induced experimental murine colitis. Trop J Pharm Res. 2018;17(9): 1803-10. CrossRef PubMed Google Scholar
-
113.Chamanara M, Abdollahi A, Rezayat SM, Ghazi-Khansari M, Dehpour A, Nassireslami E, Rashidian A. Thymol reduces acetic acid-induced inflammatory response through inhibition of NF-kB signaling pathway in rat colon tissue. Inflammopharmacology. 2019;27: 1275-83. CrossRef PubMed Google Scholar
-
114.Tahmasebi P, Froushani SM, Ahangaran NA. Thymol has beneficial effects on the experimental model of ulcerative colitis. Avicenna J Phytomed. 2019;9(6): 538. CrossRef PubMed Google Scholar
-
115.Ghasemi-Pirbaluti M, Motaghi E, Bozorgi H. The effect of menthol on acute experimental colitis in rats. Eur J Pharmacol. 2017;15(805): 101-7. CrossRef PubMed Google Scholar
-
116.Bastaki SM, Adeghate E, Amir N, Ojha S, Oz M. Menthol inhibits oxidative stress and inflammation in acetic acid-induced colitis in rat colonic mucosa. Am J Transl Res. 2018;10(12): 4210. PubMed Google Scholar
-
117.Luo L, Yan J, Chen B, Luo Y, Liu L, Sun Z, Lu Y. The effect of menthol supplement diet on colitis-induced colon tumorigenesis and intestinal microbiota. Am J Transl Res. 2021;13(1): 38. PubMed Google Scholar
-
118.Ma S, Yang B, Du Y, Lv Y, Liu J, Shi Y, Huang T, Xu H, Deng L, Chen X. 1, 8-cineole ameliorates colon injury by downregulating macrophage M1 polarization via inhibiting the HSP90-NLRP3-SGT1 complex. J Pharm Anal. 2023;13(9): 984-98. CrossRef PubMed Google Scholar
-
119.Venkataraman B, Almarzooqi S, Raj V, Bhongade BA, Patil RB, Subramanian VS, Attoub S, Rizvi TA, Adrian TE, Subramanya SB. Molecular docking identifies 1, 8-Cineole (Eucalyptol) as a novel PPARγ agonist that alleviates colon inflammation. Int J Mol Sci. 2023;24(7): 6160. CrossRef PubMed Google Scholar
-
120.Subramanya SB, Venkataraman B, Almarzooqi S, Raj V, Subramanian VS, Bhongade BA. 1, 8-Cineole, a bioactive monoterpenoid, mitigates colon inflammation by stimulating colon PPAR-γ transcription factor. FASEB J. 2022. CrossRef PubMed Google Scholar
-
121.Hossen I, Hua W, Ting L, Mehmood A, Jingyi S, Duoxia X, Yanping C, Hongqing W, Zhipeng G, Kaiqi Z, Fang Y. Phytochemicals and inflammatory bowel disease: a review. Crit Rev Food Sci Nutr. 2022;60(8): 1321-45. CrossRef PubMed Google Scholar
-
122.Moon SY, Kim KD, Yoo J, Lee JH, Hwangbo C. Phytochemicals targeting JAK–STAT pathways in inflammatory bowel disease: insights from animal models. Molecules. 2021;26(9): 2824. CrossRef PubMed Google Scholar
-
123.Zhang J, Sun S, Chen H, Feng Y, Li Y, Dong Z. Advances in natural compound-based nanomedicine and the interaction with gut microbiota in ulcerative colitis therapy. Front Pharmacol. 2023. CrossRef PubMed Google Scholar
-
124.Zhang H, Lang W, Li S, Xu C, Wang X, Li Y, Zhang Z, Wu T, Feng M. Corynoline ameliorates dextran sulfate sodium-induced colitis in mice by modulating Nrf2/NF-κB pathway. Immunopharmacol Immunotoxicol. 2023;45(1): 26-34. CrossRef PubMed Google Scholar
-
125.Zhao M, Li P, Qiao D, Hua S, Yue Q, Dai Y, Huang Y, Jiang J, Yin H, Li M, Ding Y. N6-methyladenosine modification of TSC1 mRNA contributes to macrophage polarization regulated by Coptisine in DSS-induced ulcerative colitis. Phytomedicine. 2024;1(122): 155153. CrossRef PubMed Google Scholar
-
126.Zhang MN, Xie R, Wang HG, Wen X, Wang JY, He L, Zhang MH, Yang XZ. Cepharanthine alleviates DSS-induced ulcerative colitis via regulating aconitate decarboxylase 1 expression and macrophage infiltration. Molecules. 2023;28(3): 1060. CrossRef PubMed Google Scholar
-
127.Yue M, Huang J, Ma X, Huang P, Liu Y, Zeng J. Protopine alleviates dextran sodium sulfate-induced ulcerative colitis by improving intestinal barrier function and regulating intestinal microbiota. Molecules. 2023;28(13): 5277. CrossRef PubMed Google Scholar
-
128.Liu C, Wang R, Jiao X, Zhang J, Zhang C, Wang Z. Oxysophocarpine suppresses TRAF6 level to ameliorate oxidative stress and inflammatory factors secretion in mice with dextran sulphate sodium (DSS) induced-ulcerative colitis. Microb Pathog. 2023;1(182): 106244. CrossRef PubMed Google Scholar
-
129.Ekhtiar M, Ghasemi-Dehnoo M, Mirzaei Y, Azadegan-Dehkordi F, Amini-Khoei H, Lorigooini Z, Samiei-Sefat A, Bagheri N. The coumaric acid and syringic acid ameliorate acetic acid-induced ulcerative colitis in rats via modulator of Nrf2/HO-1 and pro-inflammatory cytokines. Int Immunopharmacol. 2023;1(120): 110309. CrossRef PubMed Google Scholar
-
130.Prakash T, Janadri S. Anti-inflammatory effect of wedelolactone on DSS induced colitis in rats: IL-6/STAT3 signaling pathway. J Ayurveda Integr Med. 2023;14(2): 100544. CrossRef PubMed Google Scholar
-
131.Zou Y, Ding W, Wu Y, Chen T, Ruan Z. Puerarin alleviates inflammation and pathological damage in colitis mice by regulating metabolism and gut microbiota. Front Microbiol. 2023. CrossRef PubMed Google Scholar
-
132.Yang L, Ma XY, Mu KX, Dai Y, Xia YF, Wei ZF. Galangin targets HSP90β to alleviate ulcerative colitis by controlling fatty acid synthesis and subsequent NLRP3 inflammasome activation. Mol Nutr Food Res. 2023;67(11): 2200755. CrossRef PubMed Google Scholar
-
133.Shen X, Chen H, Wen T, Liu L, Yang Y, Xie F, Wang L. A natural chalcone cardamonin inhibits necroptosis and ameliorates dextran sulfate sodium (DSS)-induced colitis by targeting RIPK1/3 kinases. Eur J Pharmacol. 2023;5(954): 175840. CrossRef PubMed Google Scholar
-
134.Alharbi TS, Alshammari ZS, Alanzi ZN, Althobaiti F, Elewa MA, Hashem KS, Al-Gayyar MM. Therapeutic effects of genistein in experimentally induced ulcerative colitis in rats via affecting mitochondrial biogenesis. Mol Cell Biochem. 2023;21: 1-4. CrossRef PubMed Google Scholar
-
135.Cao X, Cao L, Zhang W, Lu R, Bian JS, Nie X. Therapeutic potential of sulfur-containing natural products in inflammatory diseases. Pharmacol Ther. 2020;1(216): 107687. CrossRef PubMed Google Scholar
-
136.Shirazi KM, Sotoudeh S, Shirazi AM, Moaddab SY, Nourpanah Z, Nikniaz Z. Effect of N-acetylcysteine on remission maintenance in patients with ulcerative colitis: a randomized, double-blind controlled clinical trial. Clin Res Hepatol Gastroenterol. 2021;45(4): 101532. CrossRef PubMed Google Scholar
-
137.Zhang Y, Guo Y, Zhang Q, Zhu Y, Xia Y, Wei Z, Dai Y. Diallyl trisulfide, a major bioactive constituent of garlic, promotes colonic mucosal healing in ulcerative colitis through accelerating focal adhesion assembly and consequent epithelial cell migration via the Rab21-Integrin β1-Fak pathway. Mol Nutr Food Res. 2023;20: 2200784. CrossRef PubMed Google Scholar
-
138.Lohning A, Kidachi Y, Kamiie K, Sasaki K, Ryoyama K, Yamaguchi H. 6-(methylsulfinyl) hexyl isothiocyanate (6-MITC) from Wasabia japonica alleviates inflammatory bowel disease (IBD) by potential inhibition of glycogen synthase kinase 3 beta (GSK-3β). Eur J Med Chem. 2021;15(216): 113250. CrossRef PubMed Google Scholar
-
139.Zhang Y, Tan L, Li C, Wu H, Ran D, Zhang Z. Sulforaphane alter the microbiota and mitigate colitis severity on mice ulcerative colitis induced by DSS. AMB Express. 2020;10(1): 119. CrossRef PubMed Google Scholar
-
140.Pang L, Wang T, Liao Q, Cheng Y, Wang D, Li J, Fu C, Zhang C, Zhang J. Protective role of ergothioneine isolated from Pleurotus ostreatus against dextran sulfate sodium-induced ulcerative colitis in rat model. J Food Sci. 2022;87(1): 415-26. CrossRef PubMed Google Scholar
-
141.Maio AC, Basile G, Iacopetta D, Catalano A, Ceramella J, Cafaro D, Saturnino C, Sinicropi MS. The significant role of nutraceutical compounds in ulcerative colitis treatment. Curr Med Chem. 2022;29(24): 4216-34. CrossRef PubMed Google Scholar
-
142.Parian AM, Limnetic BN, Shah ND, Mullin GE. Nutraceutical supplements for inflammatory bowel disease. Nutr Clin Pract. 2015;30(4): 551-8. CrossRef PubMed Google Scholar
-
143.Shirazi KM, Nikniaz Z, Shirazi AM, Rohani M. Vitamin A supplementation decreases disease activity index in patients with ulcerative colitis: a randomized controlled clinical trial. Complement Ther Med. 2018;1(41): 215-9. CrossRef PubMed Google Scholar
-
144.Kondo K, Hiramoto K, Yamate Y, Goto K, Sekijima H, Ooi K. Ameliorative effect of high-dose vitamin C administration on dextran sulfate sodium-induced colitis mouse model. Biol Pharm Bull. 2019;42(6): 954-9. CrossRef PubMed Google Scholar
-
145.Qiu F, Zhang Z, Yang L, Li R, Ma Y. Combined effect of vitamin C and vitamin D3 on intestinal epithelial barrier by regulating Notch signaling pathway. Nutr Metab. 2021;18(1): 49. CrossRef PubMed Google Scholar
-
146.Guo X, Liu C, Huang Y. Efficacy and safety of vitamin d adjuvant therapy for ulcerative colitis: a meta-analysis. Comput Math Methods Med. 2022;20: 2022. CrossRef PubMed Google Scholar
-
147.Liu KY, Nakatsu CH, Jones-Hall Y, Kozik A, Jiang Q. Vitamin E alpha-and gamma-tocopherol mitigate colitis, protect intestinal barrier function and modulate the gut microbiota in mice. Free Radical Biol Med. 2021;1(163): 180-9. CrossRef PubMed Google Scholar
-
148.Niu W, Chen X, Xu R, Dong H, Yang F, Wang Y, Zhang Z, Ju J. Polysaccharides from natural resources exhibit great potential in the treatment of ulcerative colitis: a review. Carbohyd Polym. 2021;15(254): 117189. CrossRef PubMed Google Scholar
-
149.Yan S, Yin L, Dong R. Inhibition of IEC-6 cell proliferation and the mechanism of ulcerative colitis in C57BL/6 Mice by Dandelion root polysaccharides. Foods. 2023;12(20): 3800. CrossRef PubMed Google Scholar
-
150.Feng W, Liu J, Tan Y, Ao H, Wang J, Peng C. Polysaccharides from Atractylodes macrocephala Koidz Ameliorate ulcerative colitis via extensive modification of gut microbiota and host metabolism. Food Res Int. 2020;138: 109777. CrossRef PubMed Google Scholar
-
151.Batista JA, de Aguiar Magalhães D, Sousa SG, dos Santos FJ, Pereira CM, do Nascimento Lima JV, de Albuquerque IF, Bezerra NL, de Brito TV, da Silva Monteiro CE, Franco AX. Polysaccharides derived from Morinda citrifolia Linn. reduce inflammatory markers during experimental colitis. J Ethnopharmacol. 2020;10(248): 112303. CrossRef PubMed Google Scholar
-
152.Zhang Y, Liu Y, Luo J, Liu Y, Yu S, Liu J. Rheum tanguticum polysaccharide alleviates DSS-induced ulcerative colitis and regulates intestinal microbiota in mice. Food Biosci. 2023;53: 102788. CrossRef PubMed Google Scholar
-
153.Kye YJ, Lee SY, Kim HR, Lee BH, Park JH, Park MS, Ji GE, Sung MK. Lactobacillus acidophilus PIN7 paraprobiotic supplementation ameliorates DSS-induced colitis through anti-inflammatory and immune regulatory effects. J Appl Microbiol. 2022;132(4): 3189-200. CrossRef PubMed Google Scholar
-
154.Zhang C, Zhao Y, Jiang J, Yu L, Tian F, Zhao J, Zhang H, Chen W, Zhai Q. Identification of the key characteristics of Bifidobacterium longum strains for the alleviation of ulcerative colitis. Food Funct. 2021;12(8): 3476-92. CrossRef PubMed Google Scholar
-
155.Liu N, Wang H, Yang Z, Zhao K, Li S, He N. The role of functional oligosaccharides as prebiotics in ulcerative colitis. Food Funct. 2022;13(13): 6875-93. CrossRef PubMed Google Scholar
-
156.Wu A, Gao Y, Kan R, Ren P, Xue C, Kong B, Tang Q. Alginate oligosaccharides prevent dextran-sulfate-sodium-induced ulcerative colitis via enhancing intestinal barrier function and modulating gut microbiota. Foods. 2023;12(1): 220. CrossRef PubMed Google Scholar
-
157.Chen W, Tan D, Yang Z, Tang J, Bai W, Tian L. Fermentation patterns of prebiotics fructooligosaccharides-SCFA esters inoculated with fecal microbiota from ulcerative colitis patients. Food Chem Toxicol. 2023;1(180): 114009. CrossRef PubMed Google Scholar
-
158.Mota J, Casimiro S, Fernandes J, Hartmann RM, Schemitt E, Picada J, Costa L, Marroni N, Raymundo A, Lima A, Ferreira RB. Lupin protein concentrate as a novel functional food additive that can reduce colitis-induced inflammation and oxidative stress. Nutrients. 2022;14(10): 2102. CrossRef PubMed Google Scholar
-
159.Zhang B, Xu Y, Zhao C, Zhang Y, Lv H, Ji X, Wang J, Pang W, Wang X, Wang S. Protective effects of bioactive peptides in foxtail millet protein hydrolysates against experimental colitis in mice. Food Funct. 2022;13(5): 2594-605. CrossRef PubMed Google Scholar
-
160.Xiang XW, Zhou XL, Wang R, Shu CH, Zhou YF, Ying XG, Zheng B. Protective effect of tuna bioactive peptide on dextran sulfate sodium-induced colitis in mice. Mar Drugs. 2021;19(3): 127. CrossRef PubMed Google Scholar
-
161.Zhou Y, Wang D, Duan H, Zhou S, Guo J, Yan W. The potential of natural oils to improve inflammatory bowel disease. Nutrients. 2023;15(11): 2606. CrossRef PubMed Google Scholar
-
162.Bartoszek A, Makaro A, Bartoszek A, Kordek R, Fichna J, Salaga M. Walnut oil alleviates intestinal inflammation and restores intestinal barrier function in mice. Nutrients. 2020;12(5): 1302. CrossRef PubMed Google Scholar
-
163.Takashima T, Sakata Y, Iwakiri R, Shiraishi R, Oda Y, Inoue N, Nakayama A, Toda S, Fujimoto K. Feeding with olive oil attenuates inflammation in dextran sulfate sodium-induced colitis in rat. J Nutr Biochem. 2014;25(2): 186-92. CrossRef PubMed Google Scholar
-
164.Zhou Q, Ma L, Zhao W, Zhao W, Han X, Niu J, Li R, Zhao C. Flaxseed oil alleviates dextran sulphate sodium-induced ulcerative colitis in rats. J Funct Foods. 2020;64: 103602. CrossRef PubMed Google Scholar
-
165.Park JS, Choi J, Hwang SH, Kim JK, Kim EK, Lee SY, Lee BI, Park SH, Cho ML. Cottonseed oil protects against intestinal inflammation in dextran sodium sulfate-induced inflammatory bowel disease. J Med Food. 2019;22(7): 672-9. CrossRef PubMed Google Scholar
-
166.Uchiyama K, Takami S, Suzuki H, Umeki K, Mochizuki S, Kakinoki N, Iwamoto J, Hoshino Y, Omori J, Fujimori S, Yanaka A. Efficacy and safety of short-term therapy with indigo naturalis for ulcerative colitis: an investigator-initiated multicenter double-blind clinical trial. PLoS ONE. 2020;15(11): e0241337. CrossRef PubMed Google Scholar
-
167.Papada E, Forbes A, Amerikanou C, Torović L, Kalogeropoulos N, Tzavara C, Triantafillidis JK, Kaliora AC. Antioxidative efficacy of a Pistacia lentiscus supplement and its effect on the plasma amino acid profile in inflammatory bowel disease: a randomised, double-blind, Placebo-Controlled Trial. Nutrients. 2018;10(11): 1779. CrossRef PubMed Google Scholar
-
168.Baghizadeh A, Davati A, Heidarloo AJ, Emadi F, Aliasl J. Efficacy of Plantago major seed in management of ulcerative colitis symptoms: a randomized, placebo controlled, clinical trial. Complement Ther Clin Pract. 2021;1(44): 101444. CrossRef PubMed Google Scholar
Copyright information
© The Author(s) 2024
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.