Recent advances in the application of [2 + 2] cycloaddition in the chemical synthesis of cyclobutane-containing natural products
This work was financially supported by the National Science Fund for Distinguished Young Scholars (82325047), Second Tibetan Plateau Scientific Expedition and Research (STEP) program (2019QZKK0502), NSFC-Joint Foundation of Yunnan Province (U2002221)
Abstract
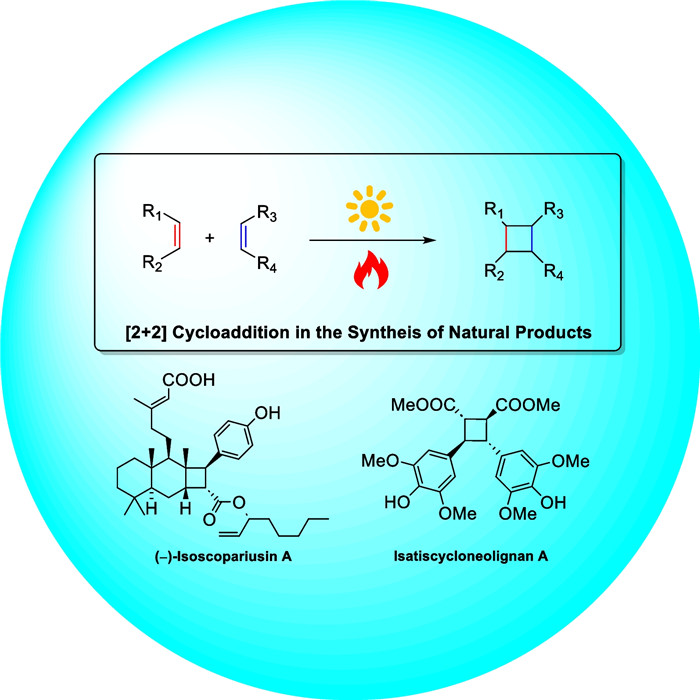
Cyclobutanes are distributed widely in a large class of natural products featuring diverse pharmaceutical activities and intricate structural frameworks. The [2 + 2] cycloaddition is unequivocally the primary and most commonly used method for synthesizing cyclobutanes. In this review, we have summarized the application of the [2 + 2] cycloaddition with different reaction mechanisms in the chemical synthesis of selected cyclobutane-containing natural products over the past decade.Graphical Abstract
Keywords
[2 + 2] Cycloaddition Total synthesis Natural products Cyclobutane1 Introduction
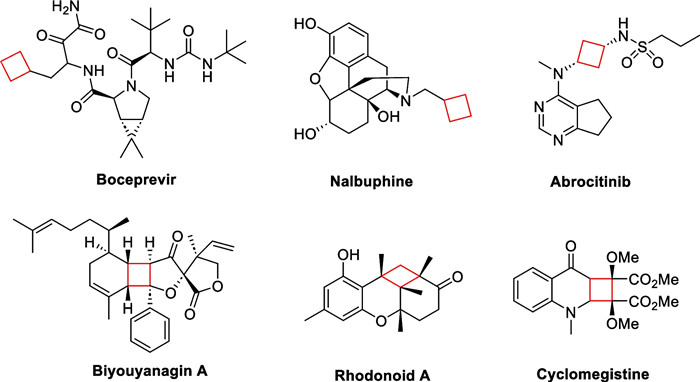
Natural products are an important source for the discovery of candidates of bioactive drug molecules and lead compounds. The cyclobutane subunit is prevalent in various drugs and drug prototypes such as boceprevir [1, 2], nalbuphine, exhibiting distinctive structures and broad bioactivities such as antibacterial, anti-viral, and immunosuppressant properties. In recent years, a variety of natural products containing cyclobutane motifs have been identified as terpenoids, meroterpenoids, and alkaloids. Notably, the cyclobutane-containing secondary metabolites are biosynthesized by a range of organisms from land plants to marine life forms, which indicates the importance of cyclobutane in biological evolution. Furthermore, these structures exhibit diverse biological activities with potential medicinal value (Scheme 1).
Representative drugs and natural products containing cyclobutane motifs
Cyclobutane motifs serve as crucial synthetic building blocks and functional groups in the modulation and design of structures in medicinal chemistry. To enhance clinical efficacy and improve ADMET properties, many pharmaceuticals have incorporated cyclobutane motifs. In 2012, Pfizer successfully launched Xeljanz, a highly potent medication targeting the JAK1/JAK3 receptors for the treatment of rheumatoid arthritis. Abrocitinib, a sulphonamide derivative approved by FDA for marketing in 2023, was developed by replacing the piperidine structure in Xeljanz with a 1, 3-disubstituted cyclobutane structure, which was found to increase the selectivity by 28 times towards the JAK1 receptor in the treatment of JAK1-mediated autoimmune diseases.
Several methodologies are employed for synthesizing cyclobutane-containing natural products, such as direct intramolecular ring closure [3-8], free radical cyclization [4, 5, 9-15], [2 + 2] cycloaddition reactions [16-21], ring-expansion approaches [22, 23], ring-contraction strategies [24-26], and rearrangement reactions. These methods are proven to be effective and reliable in producing high-quality results. Among the strategies, [2 + 2] cycloaddition reactions, widely employed in organic synthesis, have emerged as the approach for cyclobutane synthesis. In recent years, significant advancements in reaction conditions have led to milder reaction conditions and improved compatibility with a broader range of substrates. This progress has expanded the scope of cyclobutane derivatives, facilitating access to a diverse array of molecules. In this paper, we make a classification of [2 + 2] cycloaddition reactions by mechanism and showcase recent successful applications of this strategy in the total synthesis of cyclobutane-containing natural products and divide them into four categories.
2 [2 + 2] Cycloaddition reactions under thermal conditions
Pericyclic reactions, which are governed by the principles of molecular orbital symmetry, maximum overlap, and energy similarity, are well-understood thanks to the Woodard-Hoffmann rule and front-line orbital theory (FMO) [27]. Energetically, the two olefinic substrates are in the lowest state, where the molecular orbitals are symmetrically distributed around the carbon–carbon double bond. When coming in close proximity, cis-addition is a forbidden pathway in symmetry, resulting in an uneven reaction [28]. In contrast, the trans-addition approach can meet the requirements in symmetry, but the distorted stereochemistry still makes this issue a dilemma. In recent years, the emergence of substrates with orthogonal π-orbitals has reversed this trend [20, 29-31], allowing for reactions to occur under standard conditions with confidence. Currently, substituted cyclobutane motifs can be synthesized through two main methods in this condition. One is the addition of olefinic substrates to ketene [19, 32-40] or keteniminium ions [41-49] to produce cyclobutanone, followed by multi-stage functional group transformations. This approach has been proven to be highly effective and reliable. The other is the addition of olefinic substrates to allenes, leading to the efficient formation of cyclobutane adducts [50-56].
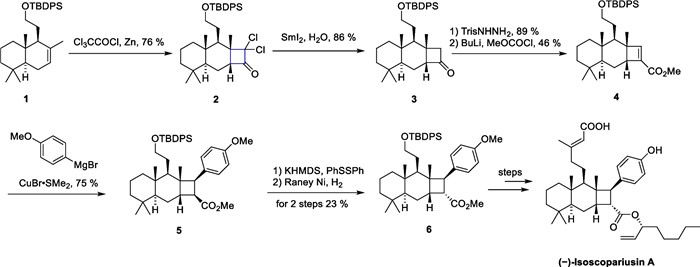
In 2021, Puno's group successfully completed the total synthesis of (−)-isoscopariusin A [57], a meroditerpenoid isolated from the aerial parts of Isodon scoparius. This compound features a unique 6/6/4 tricyclic skeleton and a tetrasubstituted cyclobutane motif. Due to the crowded cyclobutane in geometry and numerous stereochemical centers, the synthesis of this molecule is challenging. Bioactivity studies suggest that this molecule has significant immune-suppressive activity in T-cell proliferation. The synthetic route commenced with sclareolide as the starting material, leading to the production of compound 1 through six steps of reactions. Subsequently, thermal [2 + 2] cycloaddtion between 1 and dichloroketene, in situ generated by zinc dust and trichloroacetyl chloride, resulted in the formation of dichlorocyclobutanone 2. Treatment of 2 with samarium diiodide removes the chlorine atom, yielding the cyclobutanone 3, followed by the Shapiro reaction to give the unsaturated ester 4. Compound 4 reacted with Grignard reagent to introduce an aryl side-chain into the cyclobutane motif, yielding compound 5 and completing the overall synthesis of the molecule in 24 steps involving configuration inversion, Ni-catalyzed cross-coupling and deprotection.
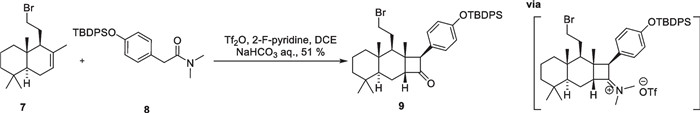
In the first route for the synthesis of 6/6/4 tricyclic core, configuration inversion of cyclobutane with mismatched C-8′ significantly lowered the overall efficiency. The authors have successfully developed a strategy to synthesize an α-arylcyclobutanone followed by face-selective homologation to access the key tricyclic core (Scheme 2). Inspired by the pioneering work of Ghosez et al. on the development of keteniminium ions chemistry [46-48 58, 59], compound 8 was obtained in a single step by an intermolecular [2 + 2] thermal cycloaddition between alkene 7 and keteniminium salts. This intermediate was subsequently transformed through carbon chain extension, Ni-catalyzed cross-coupling, and other functional group transformations, ultimately leading to a 12-step synthesis of (−)-isoscopariusin A on a gram scale. The [2 + 2] cycloaddition of olefins with ketene or keteniminium salts shows notable reactiveness, robustness, and applicability, demonstrating the potential for broad application (Scheme 3).
[2 + 2] cycloaddition between alkene and ketene in the synthesis of (−)-isoscopariusin A
[2 + 2] cycloaddition between alkene and keteniminium ions in the synthesis of (−)-isoscopariusin A
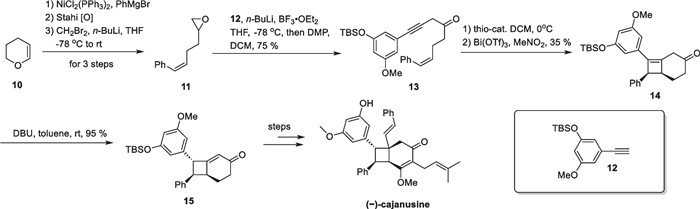
(−)-Cajanusine, a styrene-type compound with a fully substituted cyclobutane, was isolated from the leaves of Cajanus cajan by Ye's group [59]. The molecule exhibits significant cytotoxic activity against HepG2 and HepG2/ADM cells. Brown's group completed its first asymmetric total synthesis in 2020 [60] (Scheme 4).
Intramolecular [2 + 2] cycloaddition between alkene and allene in the synthesis of (−)-cajanusine
They employed an enantioselective [2 + 2]-cycloaddition strategy to construct a cyclobutane motif from allene and olefin. Starting with dihydropyran 10, the authors conducted a coupling reaction with phenyl Grignard reagent under Ni-catalyzed conditions [61]. In the presence of butyl lithium, the acetylide was obtained from compound 12 and then underwent addition to epoxide 11, followed by oxidation to produce compound 13 in 75% yield. Employing a chiral thiourea catalyst enables compound 13 to undergo an enantioselective isomerization reaction, generating an allenic ketone in situ, which undergoes a [2 + 2] cycloaddition reaction with an intramolecular double bond to give compound 14, with Bi(OTf)3 activating the carbonyl group near to the allene [62].
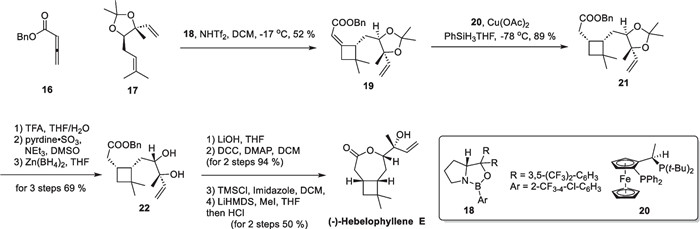
(−)-Hebelophyllene E, a novel sesquiterpene containing cyclobutane motif, was isolated from the ectomycorrhizal fungus Hebeloma longicaudum in 1999 [63]. Notably, determining the absolute configuration of the tertiary alcohol structure on the side chain using conventional spectroscopic methods proved to be challenging. In 2018, Brown successfully carried out the total synthesis and structural elucidation [52] (Scheme 5). The synthesis commenced with two prepared reaction substrates, compounds 16 and 17, on a decagram scale. Through extensive screening of various reaction conditions, a cyclobutane adduct 19 was assembled by an intermolecular asymmetric [2 + 2] cycloaddition reaction using a chiral CBS catalyst. Subsequent reduction of the unsaturated ester in a one-step MHAT reaction [64] afforded 21 with preserved stereochemistry in the use of ligand 20. Trifluoroacetic acid was employed to remove the protection group of 21, followed by stereochemical reversal of the hydroxyl group through a tandem redox process and lactonization reaction, enabling a successful total synthesis of (−)-hebelophyllene E in 10 steps.
Total synthesis of (−)-hebelophyllene E
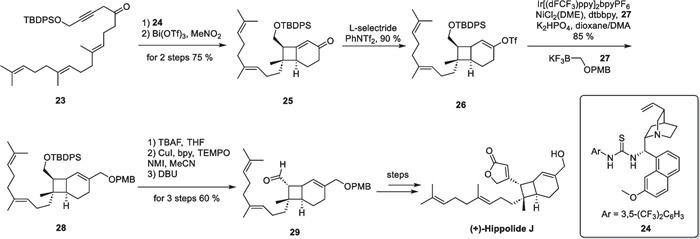
(+)-Hippolide J, a marine natural product with potent antifungal activity, was discovered in 2017 by Lin's group from the marine sponge Hippospongia lachne [65]. The fully substituted cyclobutane motif presents significant challenges from a synthetic standpoint. In 2020, Brown's group accomplished the first asymmetric total synthesis of this molecule utilizing an intramolecular [2 + 2] cycloaddition reaction between allene and alkene [66] (Scheme 6). Compound 23 was prepared in five steps, starting from farnesyl acetone before being subjected to isomerization in the presence of thiourea catalyst 24, resulting in an enantioselective yield of an allene intermediate. The intermediate then underwent an intramolecular [2 + 2] cycloaddition reaction, under the catalysis of Bi(OTf)3, ultimately producing compound 25. Subsequent reduction of 25 followed by being captured by the Comins reagent to produce 26. The authors utilized the reaction conditions of photoredox and nickel dual catalysis developed by Molander and co-workers [67, 68]. Compound 28 was synthesized through a one-step coupling reaction between compounds 26 and 27. Finally, the adduct was deprotected and subjected to oxidative tandem lactonization to complete the total synthesis of (+)-hippolide J.
Total synthesis of (+)-hippolide J
3 [2 + 2] Cycloaddition reactions under photocatalytic conditions
3.1 Direct photosynthesis
E.J. Corey discovered the generation of cyclobutane fragments during the synthesis of the sesquiterpene compound caryophyllene [69]. In the presence of excess isobutene, the α, β-unsaturated ketone experienced a direct intermolecular [2 + 2] reaction to produce a cyclobutane linked to cyclohexane under photocatalytic conditions. Despite the presence of regional isomers of "head-to-head" and "head-to-tail" adducts and the low yields, this study has revealed the potential applications of photochemical [2 + 2] cycloaddition reactions in total synthesis.
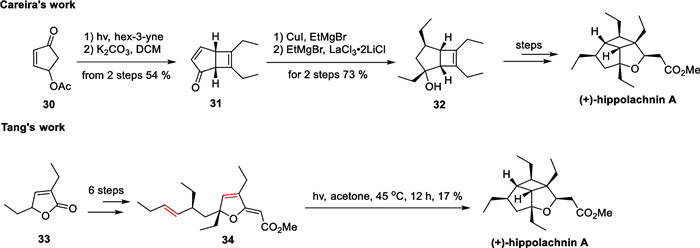
Hippolachnin A, a polyketide natural product with a distinctive 5/5/4 tricyclic bowl structure, was isolated from a sponge in the South China Sea by Lin's group in 2013 [70]. Structurally, this molecule possesses six consecutive stereocenters densely distributed on the backbone, four of which bear an ethyl substituent that protrudes in the convex direction, giving rise to a solid cage-like skeleton. Since its discovery, many synthetic chemists have pursued the total synthesis of this molecule. Since 2015, Carreira [71], Tang [72], and Trauner [73] have completed the total synthesis study of this molecule. The construction of the poly-substituted cyclobutane fragment was accomplished using the photocatalytic [2 + 2] reaction as a key step. Ding et al. have covered the synthetic efforts towards this molecule in their review [74] (Scheme 7).
Total synthesis of hippolachnin A
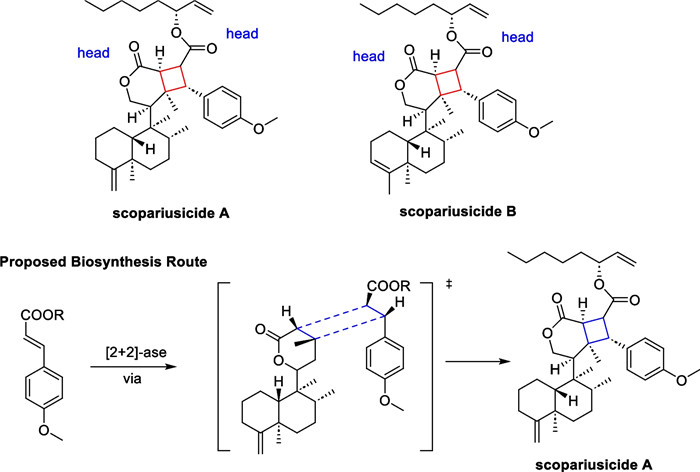
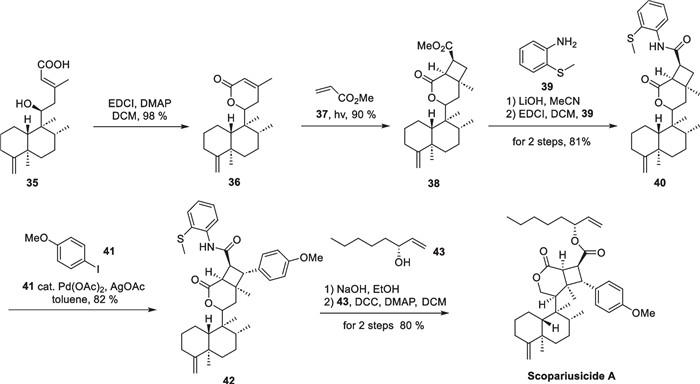
In the course of systematic studies of Isodon scoparius, Puno's group discovered two cyclobutane-containing meroterpenoid scopariusicides A and B in 2015 [75]. The two molecules represent a new class of meroditerpenoids with unsymmetrical cyclobutane motifs and ten stereochemical centers, three of which are quaternary (Scheme 8). These structural features give the cyclobutane structure a high degree of ring tension as well as spatial site resistance, making the synthesis of this compound more challenging. In the bioactive studies, scopariusicide A also showed excellent inhibition against human T cell proliferation in vitro at a very low IC50 (20.7 μM), which is significantly better than the positive control BD750. The authors proposed a biogenetic pathway for scopariusicides via a crossed "head-to-head" intermolecular [2 + 2] cycloaddition between an ent-clerodane diterpenoid 35, a high-content constituent in I. scoparius, and an unusual derivative of trans-4-hydroxycinnamic acid.
Scopariusicides A, B and its proposed biosynthesis
In the biomimetic synthesis of scopariusicide A (Scheme 9), compound 35, an abundant ent-clerodane in I. scoparius, was taken as the starting material, and underwent lactonization and isomerization to give 36. Subsequently, 36 underwent an intermolecular [2 + 2] cycloaddition with methyl acrylate to yield the adduct 38 in a "head to head" form under direct UV irradiation. Compound 38 underwent immediate hydrolysis under alkaline conditions to give the corresponding carboxylic acid product, followed by amidation to give compound 40. The amide group in 40 served as a substrate for Pd-catalyzed C-H activation to introduce an aryl ring in the cyclobutane fragment. Daugulis succeeded in arylation and alkylation of sp2 and sp3 C-H bonds using the amide group as the directing group and Pd(OAc)2 as the metal reagent [76, 77] in 2005 and 2010. Subsequently, Baran [78], Maimone [79], and Chen [80] et al. applied this strategy to the total synthesis of complex natural products. In this work, the introduction of aryl fragment of cyclobutane was also carried out using the strategy of C-H activation. In the synthesis of compound 42, the amide carbonyl group of 40 directed palladium acetate to the C-H bond in the β-position, which was subsequently followed by transmetallation and reductive elimination with compound 41 to introduce an aryl side chain, exhibiting excellent chemoselectivity and stereoselectivity. Subsequent removal of the directing group of 42 and an esterification reaction with (R)-1-octene-3-ol 43 allowed for a successful total synthesis of scopariusicide A. The combination of the crossed intermolecular [2 + 2] cycloaddition and the directing Pd-catalyzed C-H activation provides an important foundation for the total synthesis of other natural products with polysubstituted cyclobutane moieties.
Total synthesis of scopariusicide A
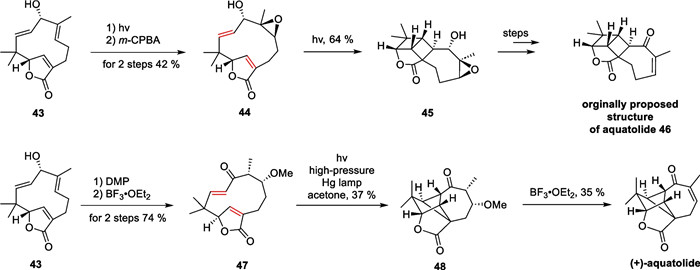
Aquatolide is a representative sesquiterpenoid isolated from Asteriscuc aquaticus in 1989 by San Feliciano et al. [81] Structurally, the compound possesses a specialized bicyclo[5.1.1]nonane and bicyclo[2.1.1]hexane backbone, which presents a very significant synthesis challenge. In 2019, Takao et al. accomplished a biomimetic synthesis of quatolide with a late-stage intramolecular transannular [2 + 2] cycloaddition as a key strategy [82] (Scheme 10). This synthetic work commenced with a starting material 43, which can be transformed into the initially proposed structure, through multiple steps including direct intramolecular [2 + 2] cycloaddition in 1989. Further oxidation of compound 43 leads to yield compound 47. Afterward, a [2 + 2] cycloaddition reaction was carried out, followed by the removal of the protecting group, resulting in the formation of aquatolide [83].
Total synthesis of aquatolide by Takao
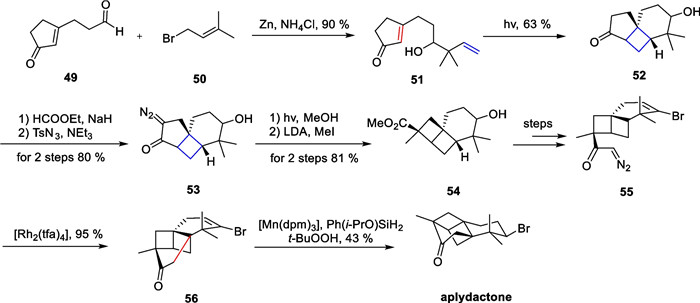
Marine natural products have emerged as a research hotspot in recent years, attracting the interest of chemists worldwide. In 2001, Stonik and co-workers isolated a halogenated sesquiterpene named aplydactone [84], which possessed an extremely tensile cyclobutane motif and a bromine-containing chiral center. In addition, the three quaternary carbon centers of the molecule are located at the junctions of the four-membered ring, which results in the susceptibility of the molecule to ring system rearrangements under acidic conditions. The true structure of the compound was not determined until 15 years later using single-crystal diffraction. Therefore, this molecule is extremely challenging to synthesize. In 2017, Zhang and co-workers from Xiamen University accomplished the total synthesis of this molecule, using intramolecular [2 + 2] cycloaddition and carbene insertion reactions as key strategies [85] (Scheme 11).
Total synthesis of aplydactone by Zhang
A selective allylation of 49 with a nucleophilic attack by 50 afforded compound 51. Subsequently, an intramolecular [2 + 2] cycloaddition reaction can be carried out under photocatalytic conditions to yield compound 52. A formylation/diazotransfer process produces the α-diazoketone 53, followed by a Wolff rearrangement leading to the ring contraction to produce compound 54. In the presence of [Rh2(tfa)4], the diazoketone structure of compound 55 is denitrated to produce carbenes intermediate, which then interact with the catalyst to form a metal carbene intermediate, undergoing a one-step carbene insertion reaction on the neighboring C-H to construct the high-tensile bridged ring. The brominated double bond in the obtained 56 was reduced by the MHAT reaction [64] to produce the natural product aplydactone.
3.2 Photocatalytic energy transfer strategy (EnT)
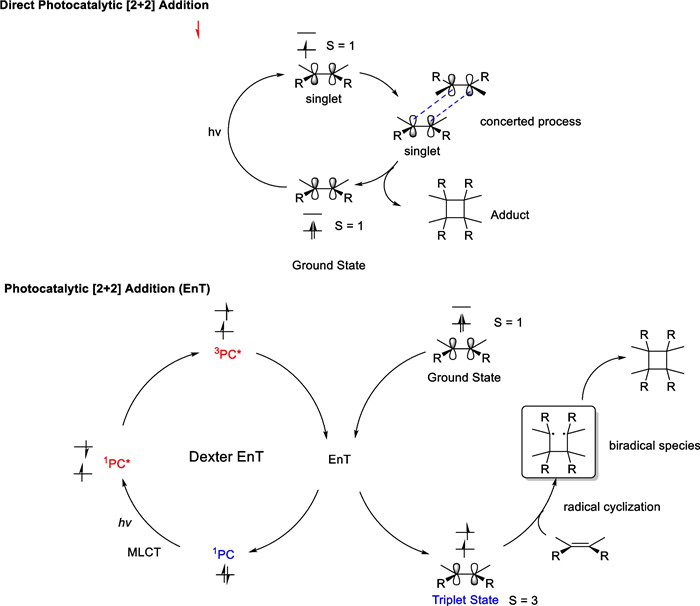
As discussed earlier, direct photosynthesis has broad applications in synthesizing natural products containing cyclobutane. However, this strategy has certain limitations. First, the light source is mainly a high-pressure mercury lamp and a short-wavelength UV light source to ensure smooth excitation of the carbon–carbon double bond in one of the substrates from the ground state to the spin singlet state. The high energy required for the excitation process not only promotes the electrons on the carbon–carbon double bond to high energy levels but also induces side reactions, such as geometrical configuration distortion and functional group cleavage. Second, direct photochemical [2 + 2] addition reactions tend to occur between electron-rich neutral olefins and electron-deficient olefins with a narrow substrate range. Therefore, energy transfer strategies for [2 + 2] cycloaddition reactions serve as a valuable complement to direct photochemical reactions.
The mechanism between energy transfer strategy and direct photochemical [2 + 2] addition is quite different [86-89] (Scheme 12). In the EnT process, visible light or near-ultraviolet light is used to excite the photosensitizer to the spin-triplet state in two steps, which activates the alkenes to the triplet state in Dexter energy transfer. In recent years, transition metal complex, represented by Ir and Ru, have flourished and shown strong applications in organic chemical reactions with energy transfer strategies.
Mechanism of the direct photocatalytic [2 + 2] cycloaddition and EnT pathway
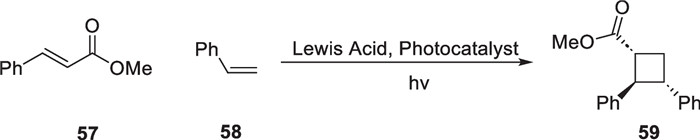
In 2019, Yoon's group reported using Lewis-catalyzed energy transfer strategy to mediate intermolecular [2 + 2] cycloaddition reactions between cinnamate derivatives and styrene substrates [90], which expanded the application of this strategy to natural product synthesis (Scheme 13). In 2023, Puno and co-workers conducted a semisynthetic study of the natural products (+)-isoscopariusins B and C [91], with EnT mediated [2 + 2] reaction as a key transformation (Scheme 14).
EnT mediated [2 + 2] cycloaddition by T.P.Yoon
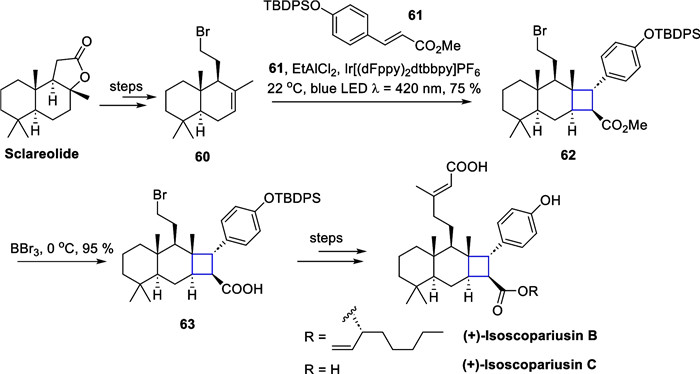
EnT mediated [2 + 2] cycloaddition in the total synthesis of (+)-isoscopariusins B and C
Starting from (+)-sclareolide, a commercially available chiral source, 60 was obtained through hydrolysis of the lactone, reduction, bromination, and MHAT reactions developed by the Shenvi's group [64]. Building on the groundwork of Yoon's group in 2019 [90], screening of conditions and optimization of an intermolecular crossed [2 + 2] cycloaddition reaction were made with methyl cinnamate derivatives, affording a single diastereoisomer 62 under 420 nm blue LED illumination with dichloroethylaluminum as a Lewis acid [19]. Selective elimination of the methyl group through boron tribromide conditions yielded the carboxylic acid 63. An esterification with 63 and a Ni-catalyzed cross-coupling reaction culminated in the synthesis of (+)-isoscopariusins B and C. Notably, the [2 + 2] cycloaddition reaction, mediated by the EnT strategy, allows for the preparation of tetrasubstituted cyclobutane substrates on a gram scale, while preserving the halogen atoms and other functional groups. This approach offers broad substrate adaptability, compatibility with various functional groups, and mild reaction conditions.
Among the various natural products containing cyclobutane, artochamin J and piperarborenine B are two representative molecules. In scheme, Wang et al. discovered a fascinating structure named artochamin J [92], in this class from a root extract of the plant. The molecule has bicyclo[3.2.0]heptane carbon frameworks, as well as four consecutive stereocenters on cyclobutane motif, which greatly increase the synthetic challenges. The molecule was isolated and obtained as a pair of enantiomers, suggesting that the formation of cyclobutane may occur through a non-enzymatic reaction. Piperarborenin B, an amide compound, contains a densely packed and fully substituted cyclobutane motif [93]. In 2022, Brown introduced a [2 + 2] reaction methodology in a transient coordination mode with high functional group compatibility and high reaction yields, facilitating the total synthesis of these two molecules [94] (Scheme 15).
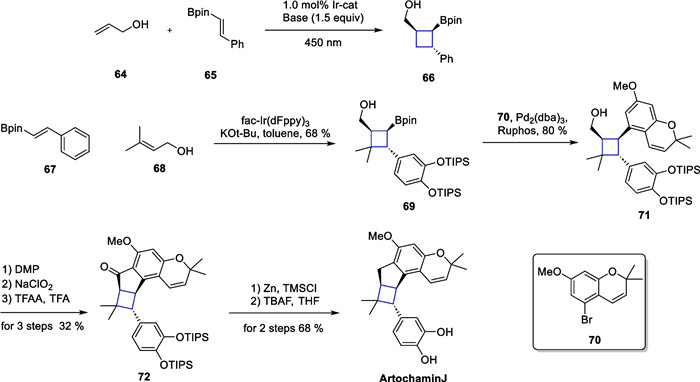
Photocatalytic [2 + 2] cycloaddition in the total synthesis of artochamin J
In this study, the authors utilized compounds 64 and 65 as template substrates. They screened photocatalysts, bases, and reaction solvents to find the optimal reaction conditions. As a result, compound 66 was prepared rapidly in a yield of up to 89% and a diastereoselectivity exceeding 20:1. After successfully realizing the transformation of the template reaction, an extension of the substrate scope was pursued. The authors screened more than thirty substrates, all of which demonstrated the robustness and universality of the method. Moreover, the authors also provide insights into the reaction mechanisms. Under alkaline conditions, the hydroxyl group can be deprotonated to form an alkoxy-negative ion, and this species can subsequently coordinate with the boron atom. This transient coordination spatially closes the distance between the two substrates, and the photocatalyst then sensitizes the conjugated olefin to the triplet state, where an intermolecular [2 + 2] cycloaddition reaction takes place. The authors synthesized compound 69 using compounds 67 and 68 through an intermolecular [2 + 2] cycloaddition mediated by the energy transfer strategy (EnT). Subsequently, a Suzuki reaction of 69 with compound 70 produced compound 71. Further oxidation and Clemmensen reduction in tandem remove the TIPS protecting group to give artochamin J.
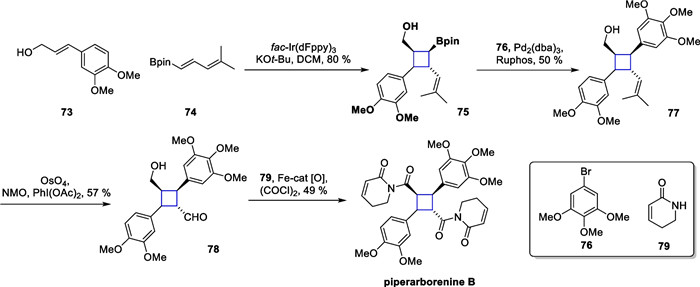
The method was further utilized to synthesize piperarborenine B. Commercially available compounds 73 and 74 were subjected to light to produce adduct 75, which subsequently underwent a Suzuki coupling reaction with 76 to produce bis-aryl-substituted product 77. Compound 78 was formed through a cleavage reaction by OsO4, followed immediately by an oxidative tandem amidation process, culminating in the total synthesis (Scheme 16).
Total synthesis of piperarborenine B
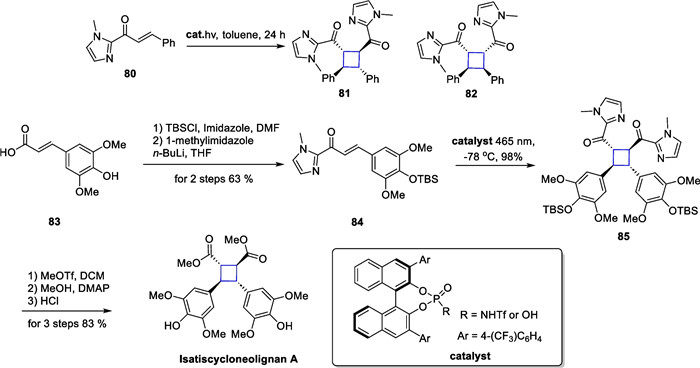
Truxinates are a large class of natural products consisting of cinnamate dimers, with more than 100 reported compounds. Isatiscycloneolignan A, a highly oxidized truxinate-type compound, was isolated from the leaves of Isatis indigotica [95]. This compound exhibited excellent neuroprotective activity. Barbarumamide C from Lycium barbarum is also a representative compound of the truxinates family [96]. Biologically, this molecule shows superior anti-AD activity compared to resveratrol and is expected to be used as an adjuvant drug in the treatment of AD. In 2023, Yoon reported an intermolecular asymmetric [2 + 2] cycloaddition catalyzed by chiral Bronsted acids and completed the total synthesis of these two natural products by this approach [97] (Scheme 17).
Total synthesis of isatiscycloneolignan A
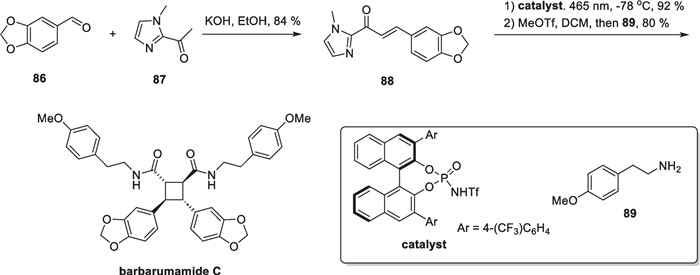
The authors designed compound 80 as a substrate for the template reaction. Compound 80 yielded two addition products, 81 and 82, with different relative configuration under light. Through optimization of the reaction conditions and screening of bronsted acids, the addition product could be obtained in 87% yield with very high selectivity (99% e.e., d.r. = 11:1). Control experiments demonstrated that in the absence of a chiral bronsted acid, the addition reaction occurred with low yields and poor selectivity. The authors expanded the substrates and found that site-blocking and electronic effects had minimal impact on the reaction. The reaction was generally feasible and dominated by all-trans addition products. After demonstrating the feasibility of this strategy, the authors used it as a general method to synthesize more complex and biologically active members of this class. Compound 84 can be obtained from starting material 83 by TBS protection and subsequent amidation. Subsequently, the adduct 85 can be obtained through self-dimerization using this strategy. A subsequent 3-step transformation culminated in the total synthesis of isatiscycloneolignan A. Additionally, the authors also successfully synthesized another cinnamate derivative 88, using an aldol reaction between compounds 86 and 87, and then completed the total synthesis of barbarumamide C using this same strategy (Scheme 18).
Total synthesis of barbarumamide C
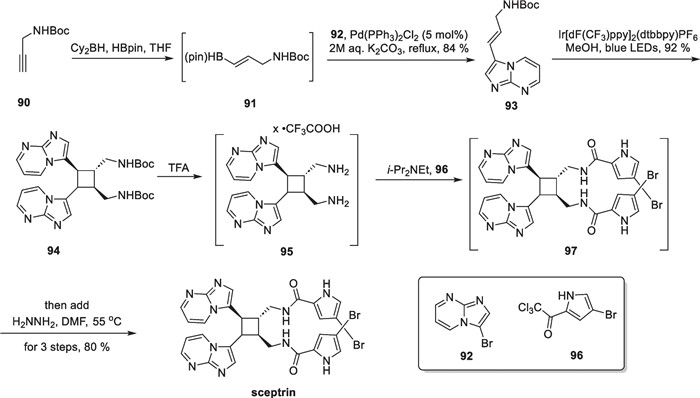
Sceptrin, a unique pyrrole-imidazole alkaloid, was initially isolated from the Caribbean sponge Agelas sceptrum by Clardy, Faulkner et al. in 1981 [98], possesing a tetrasubstituted cyclobutane core. It is likely that the molecule is formed through a "head-to-head" intermolecular [2 + 2] reaction of the monomer hymenidin [99]. After its discovery, scientists conducted semi-synthetic and biomimetic synthetic studies on sceptrin. In 2020, Jamison reported the shortest synthetic route to date with a 4-step synthesis of the compound [100] (Scheme 19).
EnT mediated [2 + 2] cycloaddition in the total synthesis of sceptrin
The commercially available N-Boc-propargylamine 90 was used to synthesize compound 91 through a B-H insertion reaction. They then performed a one-pot Suzuki reaction of compound 91 with compound 92 using Pd(PPh3)2Cl2 as a catalyst to produce 93 without purification in two tandem reactions. Compound 93 is made up of disubstituted olefins and heterocyclic fragment that can form conjugations with low excitation energies. Various photocatalysts and solvents were initially tested, leading to the discovery that Ir[dF(CF3)ppy]2(dtbbpy)PF6 can stimulate compound 93 to the triplet state, facilitating an intermolecular [2 + 2] cycloaddition to afford 94. With compound 94 in hand, the Boc groups are removed using trifluoroacetic acid, resulting in the primary amine structure, which is then subjected to an amidation reaction with compound 96 to produce amide 97. Compound 97 was synthesized by a one-step hydrazinolysis reaction, culminating in the synthesis of sceptrin. It is noteworthy that the synthetic route only involved three times of purification, and many chemical transformations were conducted using a one-pot method. The [2 + 2] cycloaddition, mediated by the energy transfer strategy, showed excellent reaction efficiency and broad functional group compatibility.
3.3 Electron transfer strategy (SET)
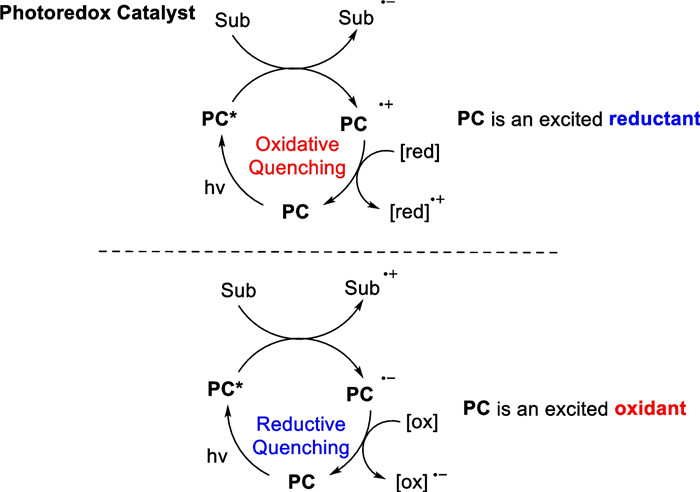
Both the direct photocatalytic [2 + 2] cycloaddition reaction and the [2 + 2] cycloaddition reaction mediated by energy transfer strategy involve interactions between an excited state substrate and a ground state substrate. Although the former is a synergistic process in terms of the reaction mechanism, the latter is a step-wise process of tandem cycloaddition of free radicals, both strategies involve electroneutral intermediates that mainly occur between neutral olefins and electron-deficient olefins. For the [2 + 2] cycloaddition reactions of electron-rich olefinic substrates and electron-deficient substrates, the visible light-catalyzed electron transfer strategy provides a powerful complement [101-103]. The electron transfer approach involves using the high redox potential of the excited state transition metal complex to oxidize a single electron in the electron-rich olefinic substrate. This electron-deficient radical cation is susceptible to nucleophilic attack by another substrate double bond, leading to the formation of carbon–carbon bonds through a reductive quenching process. The electron transfer strategy can also utilize the excited state transition metal complexes as electron donors to transfer their own single electrons to the LUMO orbitals of the electron-deficient olefin to produce an active radical anion. This electron-rich species can readily undergo nucleophilic attack on another electron-deficient olefinic substrate and subsequently loses a single electron, completing the catalytic cycle to form a carbon–carbon bond [102-107] (Scheme 20).
Mechanism of the [2 + 2] cycloaddition mediated by SET
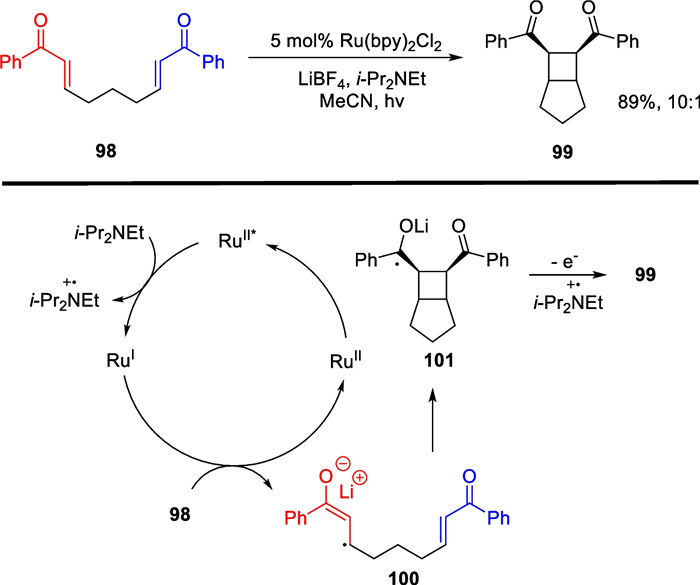
In 2008, Yoon reported visible light-catalyzed intramolecular [2 + 2] cycloaddition reactions of double electron-deficient alkenes [108] (Scheme 21). The authors achieved a remarkable yield up to 89% and 10:1 diastereoselectivity in generating tetrasubstituted cyclobutane fragments. Using Ru(bpy)3 2+ as a photocatalyst and DIPEA as an electron donor, the valence of the excited state photocatalyst was lowered, which initiated a one-electron reduction process of the photocatalyst to the electron-deficient substrate. This resulted in the production of a radical anion that underwent an intramolecular cyclization process. Yoon's group successfully filled a gap in electron-rich olefin substrate reactions by the discovery of a [2 + 2] cycloaddition reaction between intermolecular dual electron-rich alkenes four years later [109].
SET mediated intramolecular [2 + 2] cycloaddition by T.P. Yoon
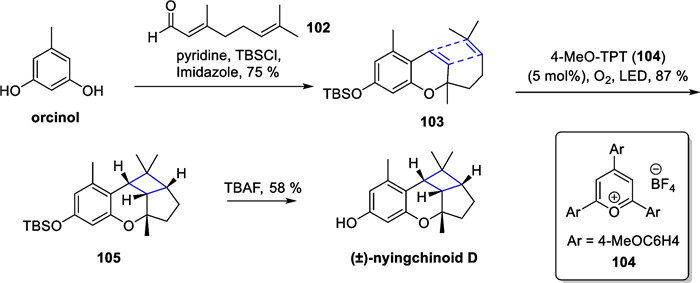
In 2018, Hou reported eight new polycyclic meroterpenoids molecules as enantiomers from Azalea ninghaiensis. Among them, (±)-nyingchinoid D [110] featured a ring system with a fused cyclobutane scaffold. Later, George's group successfully synthesized nyingchinoid D and its homologs [111] (Scheme 22). Starting from orcinol, the authors obtained a bis-olefinic substrate 103 with tandem TBS protection by Friedel–Crafts alkylation reaction. With 4-MeO-TPT 104 as the photocatalyst and oxygen as the oxidative quencher of the photocatalyst, compound 105 was obtained in 87% yield under light. Subsequent removal of the TBS group delivered (±)-nyingchinoid D.
SET mediated intramolecular [2 + 2] cycloaddition in the total synthesis of (±)-nyingchinoid D by George
In 2007, Ronald J. Quinn and his team discovered a new lignan, endiandrin A, from the roots of Endiandra anthropophagorum using a bioactivity-directed isolation method [112]. Endiandrin A is supposed to comprise two monomeric molecules formed by a "head-to-head" fashion, forming a cyclobutane with all-trans substituents. This unique backbone structure is very rare in lignans. The authors conducted activity tests and structural derivatization of the molecule at the beginning of the isolation. Both this compound and its methylated derivatives, di-O-methylendiandrin A, showed specific antagonism against the glucocorticoid receptor and were considered a promising new class of adjuvant drugs for treating rheumatoid arthritis. Therefore, the total synthesis of this molecule is of paramount practical significance.
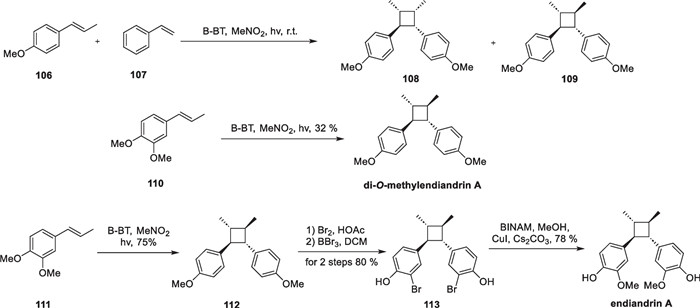
In 2017, Zhang developed a heterogenetic photocatalyst-mediated intermolecular crossed [2 + 2] cycloaddition reaction [113]. This strategy was employed to achieve the enantioselective total synthesis of endiandrin A and di-O-methylendiandrin A. Conventional organic photocatalysts, such as aromatic ketones and organometallic complexes, often coexist with the reaction substrate in the solution during the reaction. Although they can provide better energy transfer efficiencies by achieving fuller and closer contact, the substrates and products are hard to separate from these catalysts. Therefore, additional purification means are often required. To tackle this issue, the authors used a photocatalyst based on a conjugated microporous polymer network, expanding the scope and type of photocatalysis (Scheme 23).
Total Synthesis of endiandrin A and di-O-methylendiandrin A
The synthesized polymer-attached photocatalysts B-BT were characterized using scanning electron microscopy (SEM), transmission electron microscopy (TEM), and solid-state NMR techniques. The results showed that the catalysts were uniformly arranged in the form of fibers. Subsequently, the authors established a template reaction for the cycloaddition reaction using 106 and 107 as substrates and B-BT as the photocatalyst. Through screening of reaction conditions, compounds 108 and 109 were obtained in a good yield under optimal conditions. After establishing the robust reaction conditions, the authors achieved the one-step total synthesis of di-O-methylendiandrin A from compound 110. Compound 111 underwent dimerization under light conditions to yield compound 112, which was then subjected to bromination and copper-catalyzed cross-coupling, affording the target endiandrin A.
4 Transition metal-catalyzed [2 + 2] cycloaddition strategy
As previously mentioned, the [2 + 2] cycloaddition reaction involves the formation of carbon–carbon bonds between a ground state olefinic substrate and an excited state olefinic substrate or radical ions. This process occurs in a synergistic or stepwise manner, as the two substrates are brought into close proximity and their molecular orbitals overlap. In addition, the substrates used for cyclization reactions require the use of olefinic substrates with extended conjugation, whether by direct excitation, triplet state sensitized activation mode or based on radical ions generated by photoredox strategies. Unconjugated aliphatic olefins generally exhibit transitions with short wavelengths (< 200 nm), higher-energy triplet excited states, and redox potentials that are beyond the scope of most common photocatalysts. For the [2 + 2] cycloaddition reaction of bis-aliphatic olefin substrates, transition metal catalysis plays a particularly important role.
In 1973, Kochi and Salomon reported that Cu(Ⅰ) salts, particularly CuOTf, which has a better catalysis effect on the [2 + 2] cycloaddition process among many transition metals [114]. Under light irradiation, the Cu-olefin complex undergoes either a metal-to-olefin ligand charge transfer (MLCT) or an olefin ligand-to-Cu charge transfer (LMCT) process, which in turn induces cyclization to form a cyclobutane structure. Although the substrate scope is limited, the CuOTf-catalyzed photochemical [2 + 2] reaction successfully yielded cyclobutane-containing natural products.
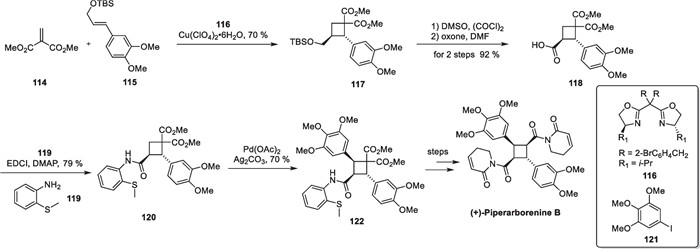
In 2021, Yoon's group accomplished the complete synthesis of advanced intermediates using this strategy [116]. Piperarborenin B is an amide compound that was isolated by Chen et al. in 2004 from the stems of Piper arborescens [94]. It contains a densely packed, tetrasubstituted cyclobutane fragment and exhibits excellent inhibitory effects against various cancer cell lines. This molecule is of great interest both structurally and biologically. Once reported, the molecule attracted the attention of many organic synthetic chemists. Baran's group elegantly achieved the first total synthesis of piperarborenine B in 2011 [78]. In 2016, Hu employed a transition metal-catalyzed [2 + 2] cycloaddition to complete an asymmetric total synthesis of the molecule [115] (Scheme 24).
Cu(ClO4)2·6H2O mediated intramolecular [2 + 2] cycloaddition in the total synthesis of (+)-piperarborenine B
Hu and co-workers developed a Cu(ClO4)2-catalyzed intermolecular [2 + 2] cycloaddition reaction paired with 116 as a chiral ligand, starting with compounds 114 and 115 to prepare compound 117 asymmetrically. The use of chiral ligands not only stabilizes organometallic complexes but also introduces a chiral environment during the cyclization process, expanding the application of this strategy to the construction of cyclobutanes. The TBS protection group is removed from adduct 117, and then it undergoes tandem oxidation and reaction with compound 119 to introduce a directing group, resulting in the formation of compound 120. Compound 120 subsequently undergoes a C-H arylation reaction under Pd(OAc)2 to introduce trimethoxyphenyl, which completes the total synthesis of (+)-piperarborenine B after several steps of functional group transformations.
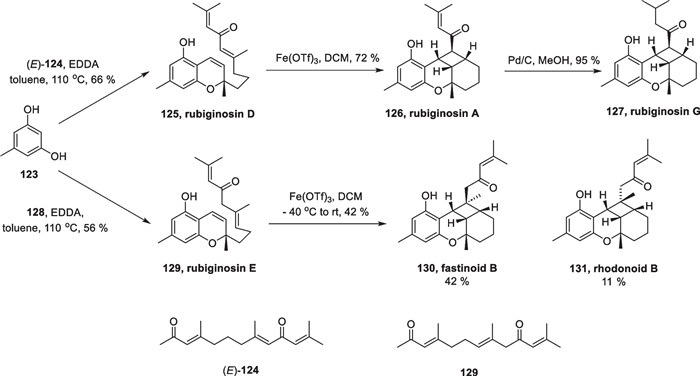
Besides copper catalyzed [2 + 2] cycloaddition, Fe-catalyzed reactions also play an important role in total synthesis. In 2021, George completed six meroterpenoids rubiginosins A, D, E, and G, fastinoid B, and rhodonoid B. using this method [117] (Scheme 25).
Total Synthesis of six meroterpenoids by Fe-catalyzed [2 + 2] cycloaddition
5 Non-classical [2 + 2] cycloaddition reactions
During the construction of cyclobutane fragments using [2 + 2] cycloaddition reactions, two olefinic substrate fragments typically interact with each other. This reaction is typically a [2π + 2π] process, where the formation of the two carbon–carbon bonds of the cyclobutane fragment is coupled to each other by interactions between the π electrons. The electrons are then paired to form the σ bonds. In recent years, a novel non-classical [2 + 2] cycloaddition reaction has been widely reported in addition to this bonding mode. The cycloaddition process involves a reaction mechanism where weak chemical bonds within the substrate are broken, releasing the energy of tension. This results in the formation of two alkyl radicals that later undergo a cyclization reaction with another substrate, ultimately leading to the formation of a cyclobutane structure.
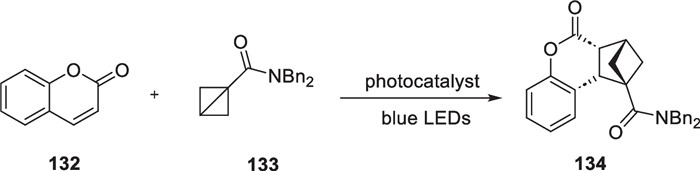
In 2022, Frank Glorius reported that they successfully carried out intermolecular [2π + 2σ] cycloaddition reactions between coumarin analogs and high-tensile bicyclo[1.1.0]butanes under light conditions [118]. This reaction leds to the production of a series of bicyclo[2.1.1]hexane compounds with notable medicinal properties (Scheme 26).
Non-classic intermolecular [2 + 2] cycloaddition between coumarin analogs and high-tensile bicyclo[1.1.0]butane derivatives
The authors observed that bicyclo[1.1.0]butane derivatives possessed multiple high-strength ring systems. Additionally, the sp3 carbon atoms have lost their tetrahedral structure, resulting in a significant degree of carbon–carbon bond bending. As a result, these bonds could be easily broken by external effects. Coumarins have a significant electron delocalization system between the benzene ring and the unsaturated lactone ring. When exposed to ultraviolet light, they can emit fluorescence. The presence of hydroxyl and other chromophores on the benzene ring further enhances the fluorescence. When the compounds are exposed to light in the presence of a photocatalyst, the resulting triplet species have an extended excited state lifetime, facilitating interaction with high-strength rings and tandem cyclization. Through a screening of photocatalysts, solvents and reaction conditions, the compound 134 was obtained by intermolecular [2π + 2σ] cycloaddition reaction. They also synthesized a range of derivatives with different substituent types using this reaction.
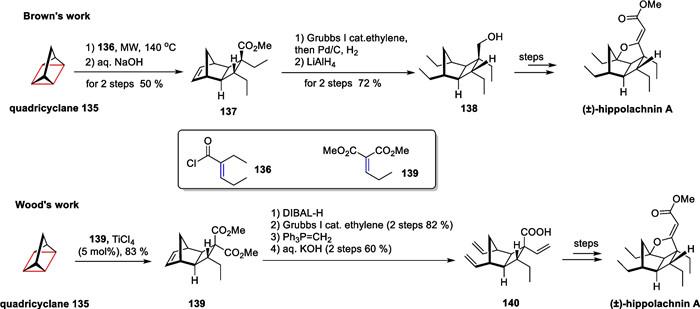
Hippolachnin A has gathered global attention for its unique properties and characteristics [70]. In comparison to Carreira's previous synthesis approach for hippolachnin A [71], Prof. Wood from Baylor University and Associate Prof. Brown from Indiana University, respectively, developed a novel method using a high-strength ring to successfully complete the total synthesis of this compound [119] (Scheme 27).
Non-classic intermolecular [2 + 2] cycloaddition in the total synthesis of hippolachnin A
Both Wood and Brown utilized tetracyclic [2.2.1.02, 6.03, 5]heptane as the initial material in the synthesis. They constructed a cyclobutane fragment in the core skeleton of hippolachnin A by performing an intermolecular [2π + 2σ] cycloaddition with polysubstituted olefinic substrates. This process involved breaking weak bonds in the tensile ring under visible light-catalyzed conditions, enabling the creation of four chiral centers in a single step. Subsequent steps included alkene complex metathesis, reductive hydrogenation, and a bridgehead sp3 C-H oxidation, ultimately leading to the successfully total synthesis.
6 Conclusions
A variety of cyclobutane-containing natural products featuring peculiar skeletons and diverse activities have been successfully synthesized in recent years. The [2 + 2] cycloaddition is employed as a dominant approach to assemble the cyclobutane scaffolds. Advancements in the field of visible light catalysis, organic catalysis, and transition metal catalysis have significantly enhanced the modes of [2 + 2] cycloaddition reactions. These progressions have led to improved reaction efficiency, increased functional group compatibility, and expanded application scopes for this reaction. The integration of computational chemistry and AI techniques is one of ongoing interest focus for investigating reaction mechanisms. These techniques can be employed to study the reaction mechanism of a specific double bond, estimate the necessary energy for excitation, rationalize photocatalyst screening, and augment stereoselectivity and regioselectivity. With advancements in the [2 + 2] cycloaddition methodology and a deeper comprehension of the cyclobutane chemistry, we anticipate that more efficient synthesis will be developed to assess the structurally complex cyclobutane-containing natural products, thereby facilitating expanded exploration of their chemical space and biological activity.
Notes
Acknowledgements
We acknowledge the authors cited in references for their contributions to the research on chemical synthesis of cyclobutane-containing natural products.
Author contributions
All the authors read and approved the manuscript.
Availability of data and materials
All the data and materials provided in the manuscript are obtained from included references and available upon request.
Declarations
Ethics approval and consent to participate
Ethical declaration is not applicable for this review.
Competing interests
All the authors declare that there is no competitive interest related to this work.
References
-
1.Rotella DP. The discovery and development of boceprevir. Expert Opin Drug Discov. 2013;8: 1439-47. CrossRef PubMed Google Scholar
-
2.Rizza SA, Talwani R, Nehra V, Temesgen Z. Boceprevir. Drugs Today Barc Spain. 2011;47: 743-51. CrossRef PubMed Google Scholar
-
3.Wolleb H, Carreira EM. Total Synthesis of (+)-Dendrowardol C. Angew Chem Int Ed. 2017;56: 10890-3. CrossRef PubMed Google Scholar
-
4.Zhao N, Xie S, Tian P, Tong R, Ning C, Xu J. Asymmetric total synthesis of (+)-astellatol and (−)-astellatene. Org Chem Front. 2019;6: 2014-22. CrossRef PubMed Google Scholar
-
5.Chuang H-Y, Isobe M. Novel synthesis of right segment of solanoeclepin A org. Lett. 2014;16: 4166-9. PubMed Google Scholar
-
6.Komada T, Adachi M, Nishikawa T. A concise synthesis of a highly strained cyclobutane in solanoeclepin A by radical cyclization. Chem Lett. 2012;41: 287-9. CrossRef PubMed Google Scholar
-
7.Gao M, Wang Y-C, Yang K-R, He W, Yang X-L, Yao Z-J. Enantioselective total synthesis of (++)-plumisclerin A. Angew Chem Int Ed. 2018;57: 13313-8. CrossRef PubMed Google Scholar
-
8.Tsao K-W, Cheng C-Y, Isobe M. Cobalt-mediated synthesis of the tricyclo[5.2.1.01, 6]decene framework in solanoeclepin A. Org Lett. 2012;14: 5274-7. CrossRef PubMed Google Scholar
-
9.Zhang W. Discovery and development of cyclobutanone-based free radical ring expansion and annulation reactions. Curr Org Chem. 2002;6: 1015-29. CrossRef PubMed Google Scholar
-
10.Zhao N, Yin S, Xie S, Yan H, Ren P, Chen G, Chen F, Xu J. Total synthesis of astellatol. Angew Chem Int Ed. 2018;57: 3386-90. CrossRef PubMed Google Scholar
-
11.Nugent WA, RajanBabu TV. Transition-metal-centered radicals in organic synthesis. Titanium(Ⅲ)-induced cyclization of epoxy olefins. J Am Chem Soc. 1988;110: 8561-2. CrossRef PubMed Google Scholar
-
12.RajanBabu TV, Nugent WA. Intermolecular addition of epoxides to activated olefins: a new reaction. J Am Chem Soc. 1989;111: 4525-7. CrossRef PubMed Google Scholar
-
13.RajanBabu TV, Nugent WA. Selective generation of free radicals from epoxides using a transition-metal radical. A powerful new tool for organic synthesis. J Am Chem Soc. 1994;116: 986-97. CrossRef PubMed Google Scholar
-
14.Gansäuer A, Greb A, Huth I, Worgull D, Knebel K. Formal total synthesis of (±)-fragranol via template catalyzed 4-exo cyclization. Tetrahedron. 2009;65: 10791-6. CrossRef PubMed Google Scholar
-
15.Knölker H-J, Schmitt O, Wanzl G, Baum G. Stereoselective total synthesis of (±)-fragranol by TiCl4 promoted [2+2] cycloaddition of allyl-tert-butyldiphenylsilane and methyl methacrylate. Chem Commun. 1999. CrossRef PubMed Google Scholar
-
16.Lu P, Bach T. Total synthesis of (+)-lactiflorin by an intramolecular [2+2] photocycloaddition. Angew Chem Int Ed. 2012;51: 1261-4. CrossRef PubMed Google Scholar
-
17.Sarkar D, Bera N, Ghosh S. [2+2] Photochemical cycloaddition in organic synthesis. Eur J Org Chem. 2020;2020: 1310-26. CrossRef PubMed Google Scholar
-
18.Bell JD, Murphy JA. Recent advances in visible light-activated radical coupling reactions triggered by (ⅰ) ruthenium, (ⅱ) iridium and (ⅲ) organic photoredox agents. Chem Soc Rev. 2021;50: 9540-685. CrossRef PubMed Google Scholar
-
19.Guo R, Brown MK. Lewis acid-promoted [2+2] cycloadditions of allenes and ketenes: versatile methods for natural product synthesis. Acc Chem Res. 2023;56: 2253-64. CrossRef PubMed Google Scholar
-
20.Alcaide B, Almendros P, Aragoncillo C. Exploiting [2+2] cycloaddition chemistry: achievements with allenes. Chem Soc Rev. 2010;39: 783-816. CrossRef PubMed Google Scholar
-
21.Richardson AD, Vogel TR, Traficante EF, Glover KJ, Schindler CS. Total synthesis of (+)-cochlearol B by an approach based on a catellani reaction and visible-light-enabled [2+2] cycloaddition. Angew Chem Int Ed. 2022;134: e202201213. CrossRef PubMed Google Scholar
-
22.Liu R, Zhang M, Wyche TP, Winston-McPherson GN, Bugni TS, Tang W. Stereoselective preparation of cyclobutanes with four different substituents: total synthesis and structural revision of pipercyclobutanamide A and piperchabamide G. Angew Chem Int Ed. 2012;124: 7621-4. CrossRef PubMed Google Scholar
-
23.Panish RA, Chintala SR, Fox JM. A Mixed-ligand chiral rhodium(Ⅱ) catalyst enables the enantioselective total synthesis of piperarborenine B. Angew Chem Int Ed. 2016;128: 5067-71. CrossRef PubMed Google Scholar
-
24.Fu C, Zhang Y, Xuan J, Zhu C, Wang B, Ding H. Diastereoselective total synthesis of salvileucalin C. Org Lett. 2014;16: 3376-9. CrossRef PubMed Google Scholar
-
25.Drew SL, Lawrence AL, Sherburn MS. Total synthesis of kingianins A, D, and F. Angew Chem Int Ed. 2013;52: 4221-4. CrossRef PubMed Google Scholar
-
26.Baran PS, Richter JM. Enantioselective total syntheses of welwitindolinone A and fischerindoles I and G. J Am Chem Soc. 2005;127: 15394-6. CrossRef PubMed Google Scholar
-
27.Woodward RB, Hoffmann R. The conservation of orbital symmetry. Angew Chem Int Ed. 1969;8: 781-853. CrossRef PubMed Google Scholar
-
28.Burke LA. Theoretical study of [2+2] cycloadditions. Ketene with ethylene. J Org Chem. 1985;50: 3149-55. CrossRef PubMed Google Scholar
-
29.McCaleb KL, Halcomb RL. Intramolecular ketene−allene cycloadditions. Org Lett. 2000;2: 2631-4. CrossRef PubMed Google Scholar
-
30.Wang Y, Wei D, Li Z, Zhu Y, Tang M. DFT study on the mechanisms and diastereoselectivities of Lewis acid-promoted ketene-alkene [2+2] cycloadditions: what is the role of lewis acid in the ketene and C=X (X = O, CH2, and NH) [2+2] cycloaddition reactions. J Phys Chem A. 2014;118: 4288-300. CrossRef PubMed Google Scholar
-
31.Huang W, Tidwell TT. Allenylketenes: versatile substrates in nucleophilic, electrophilic, and cycloaddition reactions. Synthesis. 2000;2000: 457-70. CrossRef PubMed Google Scholar
-
32.Fu N, Tidwell TT. Preparation of β-lactams by [2+2] cycloaddition of ketenes and imines. Tetrahedron. 2008;64: 10465-96. CrossRef PubMed Google Scholar
-
33.Salzner U, Bachrach SM. Cycloaddition reactions between cyclopentadiene and ketene. Ab initio examination of [2+2] and [4+2] pathways. J Org Chem. 1996;61: 237-42. CrossRef PubMed Google Scholar
-
34.Yamabe S, Kuwata K, Minato T. Frontier-orbital analyses of ketene [2+2] cycloadditions. Theor Chem Acc. 1999;102: 139-46. CrossRef PubMed Google Scholar
-
35.Rasik CM, Brown MK. Total synthesis of Gracilioether F: development and application of lewis acid promoted ketene-alkene [2+2] cycloadditions and late-stage C–H oxidation. Angew Chem Int Ed. 2014;53: 14522-6. CrossRef PubMed Google Scholar
-
36.Rullière P, Cannillo A, Grisel J, Cividino P, Carret S, Poisson JF. Total synthesis of proteasome inhibitor (−)-omuralide through asymmetric ketene [2+2]-cycloaddition. Org Lett. 2018;20: 4558-61. CrossRef PubMed Google Scholar
-
37.Snider BB, Beal RB. Total synthesis of sesquiterpenes via intramolecular ketene cycloadditions: isocomene and α-cis- and α-trans-bergamotenes, an approach to seychellene. J Org Chem. 1988;53: 4508-15. CrossRef PubMed Google Scholar
-
38.Boulton LT, Brick D, Fox ME, Jackson M, Lennon IC, McCague R, Parkin N, Rhodes D, Ruecroft G. Synthesis of the Potent Antiglaucoma Agent, Travoprost. Org Process Res Dev. 2002;6: 138-45. CrossRef PubMed Google Scholar
-
39.Wang Q, Chen C. An approach to the core skeleton of lancifodilactone F. Org Lett. 2008;10: 1223-6. CrossRef PubMed Google Scholar
-
40.Farcet JB, Himmelbauer M, Mulzer J. A non-photochemical approach to the bicyclo [3.2.0]heptane core of bielschowskysin. Org Lett. 2012;14: 2195-7. CrossRef PubMed Google Scholar
-
41.Snider BB. Intramolecular cycloaddition reactions of ketenes and keteniminium salts with alkenes. Chem Rev. 1988;88: 793-811. CrossRef PubMed Google Scholar
-
42.Kolleth A, Lumbroso A, Tanriver G, Catak S, Sulzer-Mossé S, De Mesmaeker A. Synthesis of amino-cyclobutanes via [2+2] cycloadditions involving keteniminium intermediates. Tetrahedron Lett. 2016;57: 2697-702. CrossRef PubMed Google Scholar
-
43.Ramirez M, Li W, Lam Y, Ghosez L, Houk KN. Mechanisms and conformational control of (4+2) and (2+2) cycloadditions of dienes to keteniminium cations. J Org Chem. 2020;85: 2597-606. CrossRef PubMed Google Scholar
-
44.Kolleth A, Lumbroso A, Tanriver G, Catak S, Sulzer-Mossé S, De Mesmaeker A. Synthesis of 4-membered ring alkaloid analogues via intramolecular [2+2] cycloaddition involving keteniminium salt intermediates. Tetrahedron Lett. 2017;58: 2904-9. CrossRef PubMed Google Scholar
-
45.Evano G, Lecomte M, Thilmany P, Theunissen C. Keteniminium ions: unique and versatile reactive intermediates for chemical synthesis. Synthesis. 2017;49: 3183-214. CrossRef PubMed Google Scholar
-
46.Chen L, Ghosez L. Study of chiral auxiliaries for the intramolecular [2+ 2] cycloaddition of a keteniminium salt to an olefinic double bond. A new asymmetric synthesis of cyclobutanones. Tetrahedron Lett. 1990;31: 4467-70. CrossRef PubMed Google Scholar
-
47.Urch CJ, Walter GC. [2+2] Cycloadditions of keteniminium ions and alkenes: a stereoselective synthesis of substituted cyclobutylamines. Tetrahedron Lett. 1988;29: 4309-12. CrossRef PubMed Google Scholar
-
48.Ghosez L, Marko I, Hesbain-Frisque A. Intramolecular cycloadditions of keteniminium salts. A novel approach toward prostaglandins. Tetrahedron Lett. 1986;27: 5211-4. CrossRef PubMed Google Scholar
-
49.Maskeri MA, Fernandes AJ, Di Mauro G, Maulide N, Houk KN. Taming keteniminium reactivity by steering reaction pathways: computational predictions and experimental validations. J Am Chem Soc. 2022;144: 23358-67. CrossRef PubMed Google Scholar
-
50.López-Carrillo V, Echavarren AM. Gold (Ⅰ)-catalyzed intermolecular [2+2] cycloaddition of alkynes with alkenes. J Am Chem Soc. 2010;132: 9292-4. CrossRef PubMed Google Scholar
-
51.Conner ML, Xu Y, Brown MK. Catalytic enantioselective allenoate–alkene [2+2] cycloadditions. J Am Chem Soc. 2015;137: 3482-5. CrossRef PubMed Google Scholar
-
52.Wahl JM, Conner ML, Brown MK. Synthesis of (−)-Hebelophyllene E: an entry to geminal dimethyl-cyclobutanes by [2+2] cycloaddition of alkenes and allenoates. Angew Chem Int Ed. 2018;57: 4647-51. CrossRef PubMed Google Scholar
-
53.Zheng W-F, Bora PP, Sun G-J, Kang Q. Rhodium-catalyzed regio-and stereoselective [2+2] cycloaddition of allenamides. Org Lett. 2016;18: 3694-7. CrossRef PubMed Google Scholar
-
54.Ohno H, Mizutani T, Kadoh Y, Aso A, Miyamura K, Fujii N, Tanaka T. A highly regio-and stereoselective formation of bicyclo [4.2.0] oct-5-ene derivatives through thermal intramolecular [2+2] cycloaddition of allenes. J Org Chem. 2007;72: 4378-89. CrossRef PubMed Google Scholar
-
55.Li X-X, Zhu L-L, Zhou W, Chen Z. Formal intermolecular [2+2] cycloaddition reaction of alleneamides with alkenes via gold catalysis. Org Lett. 2012;14: 436-9. CrossRef PubMed Google Scholar
-
56.Conner ML, Brown MK. Synthesis of 1, 3-substituted cyclobutanes by allenoate-alkene [2+2] cycloaddition. J Org Chem. 2016;81: 8050-60. CrossRef PubMed Google Scholar
-
57.Yan B, Zhou M, Li J, Li X, He S, Zuo J, Sun H, Li A, Puno P. (−)-Isoscopariusin A, a naturally occurring immunosuppressive meroditerpenoid: structure elucidation and scalable chemical synthesis. Angew Chem Int Ed. 2021;60: 12859-67. CrossRef PubMed Google Scholar
-
58.Marko I, Ronsmans B, Hesbain-Frisque AM, Dumas S, Ghosez L, Ernst B, Greuter H. Intramolecular [2+2] cycloadditions of ketenes and keteniminium salts to olefins. J Am Chem Soc. 1985;107: 2192-4. CrossRef PubMed Google Scholar
-
59.Li X-L, Zhao B-X, Huang X-J, Zhang D-M, Jiang R-W, Li Y-J, Jian Y-Q, Wang Y, Li Y-L, Ye W-C. (+)-and (−)-Cajanusine, a pair of new enantiomeric stilbene dimers with a new skeleton from the leaves of Cajanus cajan. Org Lett. 2014;16: 224-7. CrossRef PubMed Google Scholar
-
60.Guo R, Witherspoon BP, Brown MK. Evolution of a strategy for the enantioselective synthesis of (−)-cajanusine. J Am Chem Soc. 2020;142: 5002-6. CrossRef PubMed Google Scholar
-
61.Wenkert E, Michelotti EL, Swindell CS, Tingoli M. Transformation of carbon-oxygen into carbon-carbon bonds mediated by low-valent nickel species. J Org Chem. 1984;49: 4894-9. CrossRef PubMed Google Scholar
-
62.Line NJ, Witherspoon BP, Hancock EN, Brown MK. Synthesis of ent-[3]-ladderanol: development and application of intramolecular chirality transfer [2+2] cycloadditions of allenic ketones and alkenes. J Am Chem Soc. 2017;139: 14392-5. CrossRef PubMed Google Scholar
-
63.Wichlacz M, Ayer WA, Trifonov LS, Chakravarty P, Khasa D. A caryophyllene-related sesquiterpene and two 6, 7-seco-caryophyllenes from liquid cultures of Hebeloma longicaudum. J Nat Prod. 1999;62: 484-6. CrossRef PubMed Google Scholar
-
64.Wu J, Ma Z. Metal-hydride hydrogen atom transfer (MHAT) reactions in natural product synthesis. Org Chem Front. 2021;8: 7050-76. CrossRef PubMed Google Scholar
-
65.Jiao WH, Hong LL, Sun JB, Piao SJ, Chen GD, Deng H, Wang SP, Yang F, Lin HW. (±)-Hippolide J-a pair of unusual antifungal enantiomeric sesterterpenoids from the marine sponge Hippospongia lachne. Eur J Org Chem. 2017;24: 3421-6. CrossRef PubMed Google Scholar
-
66.Guo R, Beattie SR, Krysan DJ, Brown MK. Enantioselective synthesis of (+)-hippolide J and reevaluation of antifungal activity. Org Lett. 2020;22: 7743-6. CrossRef PubMed Google Scholar
-
67.Karakaya I, Primer DN, Molander GA. Photoredox cross-coupling: Ir/Ni dual catalysis for the synthesis of benzylic ethers. Org Lett. 2015;17: 3294-7. CrossRef PubMed Google Scholar
-
68.Karimi-Nami R, Tellis JC, Molander GA. Single-electron transmetalation: protecting-group-independent synthesis of secondary benzylic alcohol derivatives via photoredox/nickel dual catalysis. Org Lett. 2016;18: 2572-5. CrossRef PubMed Google Scholar
-
69.Corey EJ, Mitra RB, Uda H. Total synthesis of d, l-Caryophyllene and d, l-Isocaryophyllene1. J Am Chem Soc. 1964;86: 485-92. CrossRef PubMed Google Scholar
-
70.Piao S-J, Song Y-L, Jiao W-H, Yang F, Liu X-F, Chen W-S, Han B-N, Lin H-W. Hippolachnin A, a new antifungal polyketide from the South China sea sponge Hippospongia lachne. Org Lett. 2013;15: 3526-9. CrossRef PubMed Google Scholar
-
71.Ruider SA, Sandmeier T, Carreira EM. Total synthesis of (±)-Hippolachnin A. Angew Chem Int Ed. 2015;54: 2378-82. CrossRef PubMed Google Scholar
-
72.Li Q, Zhao K, Peuronen A, Rissanen K, Enders D, Tang Y. Enantioselective total syntheses of (+)-hippolachnin A, (+)-gracilioether A, (−)-gracilioether E, and (−)-gracilioether F. J Am Chem Soc. 2018;140: 1937-44. CrossRef PubMed Google Scholar
-
73.Winter N, Rupcic Z, Stadler M, Trauner D. Synthesis and biological evaluation of (±)-hippolachnin and analogs. J Antibiot (Tokyo). 2019;72: 375-83. CrossRef PubMed Google Scholar
-
74.Li J, Gao K, Bian M, Ding H. Recent advances in the total synthesis of cyclobutane-containing natural products. Org Chem Front. 2020;7: 136-54. CrossRef PubMed Google Scholar
-
75.Zhou M, Li X-R, Tang J-W, Liu Y, Li X-N, Wu B, Qin H-B, Du X, Li L-M, Wang W-G, Pu J-X, Sun H-D. Scopariusicides, novel unsymmetrical cyclobutanes: structural elucidation and concise synthesis by a combination of intermolecular [2+2] cycloaddition and C-H functionalization. Org Lett. 2015;17: 6062-5. CrossRef PubMed Google Scholar
-
76.Zaitsev VG, Shabashov D, Daugulis O. Highly regioselective arylation of sp3 C−H bonds catalyzed by palladium acetate. J Am Chem Soc. 2005;127: 13154-5. CrossRef PubMed Google Scholar
-
77.Shabashov D, Daugulis O. Auxiliary-assisted palladium-catalyzed arylation and alkylation of sp2 and sp3 carbon−hydrogen bonds. J Am Chem Soc. 2010;132: 3965-72. CrossRef PubMed Google Scholar
-
78.Gutekunst WR, Baran PS. Total synthesis and structural revision of the piperarborenines via sequential cyclobutane C-H arylation. J Am Chem Soc. 2011;133: 19076-9. CrossRef PubMed Google Scholar
-
79.Ting CP, Maimone TJ. C-H bond arylation in the synthesis of aryltetralin lignans: a short total synthesis of podophyllotoxin. Angew Chem Int Ed. 2014;53: 3115-9. CrossRef PubMed Google Scholar
-
80.Feng Y, Chen G. Total synthesis of celogentin C by stereoselective C-H activation. Angew Chem Int Ed. 2010;49: 958-61. CrossRef PubMed Google Scholar
-
81.San Feliciano A, Medarde M, Miguel del Corral JM, Aramburu A, Gordaliza M, Barrero AF. Aquatolide. A new type of humulane-related sesquiterpene lactone. Tetrahedron Lett. 1989;30: 2851-4. CrossRef PubMed Google Scholar
-
82.Takao K, Kai H, Yamada A, Fukushima Y, Komatsu D, Ogura A, Yoshida K. Total syntheses of (+)-aquatolide and related humulanolides. Angew Chem Int Ed. 2019;58: 9851-5. CrossRef PubMed Google Scholar
-
83.Lodewyk MW, Soldi C, Jones PB, Olmstead MM, Rita J, Shaw JT, Tantillo DJ. The correct structure of aquatolide—experimental validation of a theoretically-predicted structural revision. J Am Chem Soc. 2012;134: 18550-3. CrossRef PubMed Google Scholar
-
84.Fedorov SN, Radchenko OS, Shubina LK, Kalinovsky AI, Gerasimenko AV, Popov DY, Stonik VA. Aplydactone, a new sesquiterpenoid with an unprecedented carbon skeleton from the sea hare aplysia dactylomela, and its cargill-like rearrangement. J Am Chem Soc. 2001;123: 504-5. CrossRef PubMed Google Scholar
-
85.Liu C, Chen R, Shen Y, Liang Z, Hua Y, Zhang Y. Total synthesis of aplydactone by a conformationally controlled C−H functionalization. Angew Chem Int Ed. 2017;56: 8187-90. CrossRef PubMed Google Scholar
-
86.Strieth-Kalthoff F, Glorius F. Triplet energy transfer photocatalysis: unlocking the next level. Chem. 2020;6: 1888-903. CrossRef PubMed Google Scholar
-
87.Zhou Q-Q, Zou Y-Q, Lu L-Q, Xiao W-J. Visible-light-induced organic photochemical reactions through energy-transfer pathways. Angew Chem Int Ed. 2019;58: 1586-604. CrossRef PubMed Google Scholar
-
88.Bach T, Hehn JP. Photochemical reactions as key steps in natural product synthesis. Angew Chem Int Ed. 2011;50: 1000-45. CrossRef PubMed Google Scholar
-
89.Strieth-Kalthoff F, James MJ, Teders M, Pitzer L, Glorius F. Energy transfer catalysis mediated by visible light: principles, applications, directions. Chem Soc Rev. 2018;47: 7190-202. CrossRef PubMed Google Scholar
-
90.Daub ME, Jung H, Lee BJ, Won J, Baik M-H, Yoon TP. Enantioselective [2+2] cycloadditions of cinnamate esters: generalizing lewis acid catalysis of triplet energy transfer. J Am Chem Soc. 2019;141: 9543-7. CrossRef PubMed Google Scholar
-
91.Li M-X, Yan B-C, Zhou M, Li X-R, Li X, He S-J, Sun H-D, Puno P-T. Cyclobutane-containing meroditerpenoids, (+)-isoscopariusins B and C: structure elucidation and biomimetic synthesis. Org Lett. 2023;25: 2981-5. CrossRef PubMed Google Scholar
-
92.Wang YH, Hou AJ, Chen DF, Weiller M, Wendel A, Staples RJ. Prenylated stilbenes and their novel biogenetic derivatives from Artocarpus chama. EUR J Org Chem. 2006;15: 3457-63. CrossRef PubMed Google Scholar
-
93.Lee F-P, Chen Y-C, Chen J-J, Tsai I-L, Chen I-S. Cyclobutanoid amides from Piper arborescens. Helv Chim Acta. 2004;87: 463-8. CrossRef PubMed Google Scholar
-
94.Liu Y, Ni D, Brown MK. Boronic ester enabled [2+2]-cycloadditions by temporary coordination: synthesis of artochamin J and piperarborenine B. J Am Chem Soc. 2022;144: 18790-6. CrossRef PubMed Google Scholar
-
95.Xi Y-F, Liu S-F, Hong W, Song X-Y, Lou L-L, Zhou L, Yao G-D, Lin B, Wang X-B, Huang X-X, Song S-J. Discovery of cycloneolignan enantiomers from Isatis indigotica fortune with neuroprotective effects against MPP+-induced SH-SY5Y cell injury. Bioorganic Chem. 2019;88: 102926-32. CrossRef PubMed Google Scholar
-
96.Chai T, Zhang W-H, Jiao H, Qiang Y. Hydroxycinnamic acid amide dimers from goji berry and their potential anti-AD activity. Chem Biodivers. 2021;18: e2100436. CrossRef PubMed Google Scholar
-
97.Genzink MJ, Rossler MD, Recendiz H, Yoon TP. A general strategy for the synthesis of truxinate natural products enabled by enantioselective [2+2] photocycloadditions. J Am Chem Soc. 2023;145: 19182-8. CrossRef PubMed Google Scholar
-
98.Walker RP, Faulkner DJ, Van Engen D, Clardy J. Sceptrin, an antimicrobial agent from the sponge Agelas sceptrum. J Am Chem Soc. 1981;103: 6772-3. CrossRef PubMed Google Scholar
-
99.Kobayashi J, Ohizumi Y, Nakamura H, Hirata Y. A novel antagonist of serotonergic receptors, hymenidin, isolated from the Okinawan marine sponge Hymeniacidon sp.,. Experientia. 1986;42: 1176-7. CrossRef PubMed Google Scholar
-
100.Nguyen LV, Jamison TF. Total synthesis of (±)-sceptrin. Org Lett. 2020;22: 6698-702. CrossRef PubMed Google Scholar
-
101.Liu Y, Song R, Li J. The cycloaddition reaction using visible light photoredox catalysis. Sci China Chem. 2016;59: 161-70. CrossRef PubMed Google Scholar
-
102.Narayanam JMR, Stephenson CRJ. Visible light photoredox catalysis: applications in organic synthesis. Chem Soc Rev. 2011;40: 102-13. CrossRef PubMed Google Scholar
-
103.Zhang T, Zhang Y, Das S. Deal; photoredox catalysis for the cycloaddition reactions. ChemCatChem. 2020;12: 6173-85. CrossRef PubMed Google Scholar
-
104.Shaw MH, Twilton J, MacMillan DWC. Photoredox catalysis in organic chemistry. J Org Chem. 2016;81: 6898-926. CrossRef PubMed Google Scholar
-
105.Buzzetti L, Crisenza GEM, Melchiorre P. Mechanistic studies in photocatalysis. Angew Chem Int Ed. 2019;58: 3730-47. CrossRef PubMed Google Scholar
-
106.Cismesia MA, Yoon TP. Characterizing chain processes in visible light photoredox catalysis. Chem Sci. 2015;6: 5426-34. CrossRef PubMed Google Scholar
-
107.Marchini M, Bergamini G, Cozzi PG, Ceroni P, Balzani V. Photoredox catalysis: the need to elucidate the photochemical mechanism. Angew Chem. 2017;129: 12996-7. CrossRef PubMed Google Scholar
-
108.Ischay MA, Anzovino ME, Du J, Yoon TP. Efficient visible light photocatalysis of [2+2] enone cycloadditions. J Am Chem Soc. 2008;130: 12886-7. CrossRef PubMed Google Scholar
-
109.Ischay MA, Ament MS, Yoon TP. Crossed intermolecular [2+2] cycloaddition of styrenes by visible light photocatalysis. Chem Sci. 2012;3: 2807-11. CrossRef PubMed Google Scholar
-
110.Huang G-H, Hu Z, Lei C, Wang P-P, Yang J, Li J-Y, Li J, Hou A-J. Enantiomeric pairs of meroterpenoids with diverse heterocyclic systems from Rhododendron nyingchiense. J Nat Prod. 2018;81: 1810-8. CrossRef PubMed Google Scholar
-
111.Hart JD, Burchill L, Day AJ, Newton CG, Sumby CJ, Huang DM, George JH. Visible-light photoredox catalysis enables the biomimetic synthesis of nyingchinoids A, B, and D, and rasumatranin D. Angew Chem Int Ed. 2019;58: 2791-4. CrossRef PubMed Google Scholar
-
112.Davis RA, Carroll AR, Duffy S, Avery VM, Guymer GP, Forster PI, Quinn RJ. Endiandrin A, a potent glucocorticoid receptor binder isolated from the australian plant Endiandra anthropophagorum. J Nat Prod. 2007;70: 1118-21. CrossRef PubMed Google Scholar
-
113.Li R, Ma BC, Huang W, Wang L, Wang D, Lu H, Landfester K, Zhang KAI. Photocatalytic regioselective and stereoselective [2+2] cycloaddition of styrene derivatives using a heterogeneous organic photocatalyst. ACS Catal. 2017;7: 3097-101. CrossRef PubMed Google Scholar
-
114.Salomon RG, Kochi JK. Copper (Ⅰ) triflate: a superior catalyst for olefin photodimerization. Tetrahedron Lett. 1973;14: 2529-32. CrossRef PubMed Google Scholar
-
115.Hu J-L, Feng L-W, Wang L, Xie Z, Tang Y, Li X. Enantioselective construction of cyclobutanes: a new and concise approach to the total synthesis of (+)-piperarborenine B. J Am Chem Soc. 2016;138: 13151-4. CrossRef PubMed Google Scholar
-
116.Gravatt CS, Melecio-Zambrano L, Yoon TP. Olefin-supported cationic copper catalysts for photochemical synthesis of structurally complex cyclobutanes. Angew Chem Int Ed. 2021;60: 3989-93. CrossRef PubMed Google Scholar
-
117.Burchill L, Day AJ, Yahiaoui O, George JH. Biomimetic total synthesis of the rubiginosin meroterpenoids. Org Lett. 2021;23: 578-82. CrossRef PubMed Google Scholar
-
118.Kleinmans R, Pinkert T, Dutta S, Paulisch TO, Keum H, Daniliuc CG, Glorius F. Intermolecular [2π+2σ]-photocycloaddition enabled by triplet energy transfer. Nature. 2022;605: 477-82. CrossRef PubMed Google Scholar
-
119.Timmerman JC, Wood JL. Synthesis and biological evaluation of hippolachnin A analogues. Org Lett. 2018;20: 3788-92. CrossRef PubMed Google Scholar
Copyright information
© The Author(s) 2024
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.