Disclosing the potential of Cupressus leylandii A.B. Jacks & Dallim, Eucalyptus globulus Labill., Aloysia citrodora Paláu, and Melissa officinalis L. hydrosols as eco-friendly antimicrobial agents
Abstract
Antimicrobial resistance is a major global health concern, threatening the effective prevention and treatment of infections caused by microorganisms. These factors boosted the study of safe and green alternatives, with hydrosols, the by-products of essential oils extraction, emerging as promising natural antimicrobial agents. In this context, four hydrosols obtained from Cupressus leylandii A.B. Jacks & Dallim, Eucalyptus globulus Labill., Aloysia citrodora Paláu and Melissa officinalis L. were studied. Their chemical composition comprises neral, geranial, 1,8-cineole, terpinen-4-ol, and oplopanonyl acetate, compounds with recognised antimicrobial activity. Concerning antimicrobial activity, significant differences were found using different hydrosol concentrations (10–20% v/v) in comparison to a control (without hydrosol), showing the potential of the tested hydrosols to inhibit the microbial growth of Escherichia coli, Staphylococcus aureus, and Candida albicans. A. citrodora hydrosol was the most effective one, inhibiting 90% of E. coli growth and 80% of C. albicans growth, for both hydrosol concentrations (p < 0.0001). With hydrosol concentration increase, it was possible to observe an improved antimicrobial activity with significant reductions (p < 0.0001). The findings of this work indicate the viability of reusing and valuing the hydrosols, encouraging the development of green applications for different fields (e.g., food, agriculture, pharmaceuticals, and cosmetics).Graphical Abstract
Keywords
Hydrosols Essential oil by-products Chemical composition Antimicrobial activity Natural preservatives Waste valorisation1 Introduction
Antimicrobial resistance is a serious threat to public health worldwide, making it harder to effectively prevent and treat chronic illnesses due to the microorganism's capacity to survive and stay viable in the presence of antibiotics. In addition to the increased morbidity and mortality, this phenomenon may impact different areas, including global health, food sustainability and security, environmental wellness, and socio-economic development [1, 2]. In this scenario, alternative greener, non-toxic, and natural antimicrobial agents with bioactive capacity are needed.
Natural products, especially those derived from plants, have long been used in traditional medicine due to their preservative and therapeutic properties. Their complex chemical composition, which includes alkaloids, flavonoids, phenols, glycosides, steroids, saponins, and terpenoids, wides the sources of molecules with potential antimicrobial capacity. These molecules play a key role in inhibiting the microorganisms, presenting distinctive mechanisms of action that can cause modifications at their metabolic and physiological levels [1, 3, 4]. Several plants have been described as having bioactive properties, including Melissa officinalis, a widely used edible medicinal herb from the Lamiaceae family. M. officinalis has a phytochemical composition rich in bioactive compounds with pharmacological effects, including antioxidant, antimicrobial, and cytotoxic activities [5]. Aloysia citrodora, a species of the Verbenaceae family, often used for medical, cosmetic, and aromatic purposes, has remarkable biological properties like antioxidant, antimicrobial, and antiproliferative activities [6]. In another study [7], the plant genus Eucalyptus, which belongs to the Myrtaceae family, is referred to as a valuable source of bioactive components with antioxidant and antimicrobial capacity, making it a useful natural preservative for the pharmaceutical, cosmetical and food applications. The Cupressaceae, also known as the cypress family, is a genus of conifers that are found all over the world. Despite having a few species scarcely studied, they are reported to contain important volatile and phenolic compounds in their essential oils (EOs), extracts, and derived compounds. Insecticidal, antibacterial, and antifungal capacities dominate the reported biological activities [8]. These plants, among many others, are known worldwide and used at the industrial level to produce EOs.
Pathogens, including Staphylococcus aureus (Gram-positive spherical bacteria), Escherichia coli (Gram-negative bacillary bacteria), and Candida albicans (yeast), can proliferate in many different niches, allowing them to multiply and spread easily. These commensal microbes potentially cause a wide range of illnesses. For example, E. coli may lead to gastrointestinal and extra-intestinal infections, while S. aureus and C. albicans may affect the skin and mucosae of their hosts, causing systemic infections. In some cases, gastrointestinal intoxications and infections are caused by the development of enterotoxins (S. aureus), and Shiga-toxin (E. coli), which are ingested through contaminated water, food, and beverages [9–13].
In this scenario, hydrosols, also known as hydrolats, the secondary products of aromatic plant distillation, have raised attention as natural antimicrobials due to their eco-friendly characteristics and bioactive properties. They are a heterogeneous mixture of polar, oxygenated, hydrophilic, and volatile oil components forming hydrogen bonds with water. They contain bioactive hydrophilic substances and few hydrophobic components from the respective EOs, exhibiting bioactivities associated with their chemical composition, namely components holding different functional groups, e.g., methyl, hydroxyl, carbonyl, and carboxyl groups [14–16]. Despite coming from the same process, the composition and efficacy of the two distillation products (oil and hydrosol) vary. EOs application needs caution since several terpene molecules are particularly toxic, irritating skin upon contact. Moreover, they present a strong aroma, which might induce an unpleasant sensation and headaches. For these reasons, they are not typically consumed or used topically. Contrarily, hydrosols, which correspond to dilute terpenic solutions, are less harmful and thus more attractive for these applications [14, 17].
Hydrosols have recently started to receive a lot of credit in a variety of areas, such as food (e.g., flavouring, preservatives, and sanitisers) [18, 19], cosmetic and perfumery [20], aromatherapy [21], agriculture (e.g., biopesticides and repellents) [22], pharmaceutical (e.g., natural antibiotics, antioxidants and anti-inflammatory agents) [17, 23], and medical (e.g., antimicrobial and antitumor agents) [24]. According to circular economy principles, using industrial by-products can be a sustainable way to address the environmental problems caused by waste discharging. This is particularly interesting when they have promising biological properties such as antioxidant, anti-inflammatory, and antimicrobial activities [25–27].
To reduce the lack of knowledge regarding hydrosols, the present work aimed to study the chemical composition of four hydrosols (Cupressus leylandii A.B. Jacks & Dallim, Eucalyptus globulus Labill., Aloysia citrodora Paláu and Melissa officinalis L.), obtained by hydro-distillation, and determine the antimicrobial properties against three pathogenic microorganisms, Staphylococcus aureus, Escherichia coli, and Candida albicans. It is expected to contribute to the recovery and use of these by-products finding eco-friendly applications as natural antimicrobial agents.
2 Results and discussion
2.1 Visual, olfactory, and acidity attributes of hydrosols
Hydrosols are mentioned in the literature as diluted solutions with an acidic character, presenting different characteristics such as aroma, colour, and chemical composition [16, 28]. In this work, all hydrosols were identified as a colourless liquid with mild to strong aroma and acidic pH as described in Table 1.
pH and sensorial properties of hydrosols
The pH values for the studied hydrosols ranged from 2.9 to 4.1, which agrees with other published works [16, 29] reporting pHs between 2.2 and 5.5, thus corroborating the predominantly acidic nature of these products. Jakubczyk and co-workers [30], who investigated the 17 most popular hydrosols for the cosmetic market, found a pH value of 3.34 for Melissa officinalis hydrosol, similar to the value obtained in this work (3.2). pH is an important parameter affecting hydrosols' final application, including its therapeutic effects [31].
2.2 Chemical composition of hydrosols
Table 2 provides the complete chemical composition of the studied hydrosols, where the identified components are mainly oxygenated monoterpenes. Following EOs extraction, the oil phase enters in contact with the water phase, allowing different polar hydrophilic volatile compounds to form hydrogen bonds and disperse in the water phase (hydrosol). The degree of hydrogen bonding of the components with water molecules is determined by the component's chemical structure (polarity factor), explaining why the oxygenated compounds present relatively higher solubility in water (when compared to hydrocarbons, for example), thus appearing as major components in the studied hydrosols [28, 32]. Besides that, a range of factors, including environmental (e.g., temperature, rainfall), geographical origin, harvesting conditions (e.g., season, growth stage), and plant material post-harvesting processing (e.g., drying, extraction methods, distillation conditions), influences the composition of the essential oil and respective by-products (content and quality) [28, 33].
Chemical composition of volatile compounds present in the hydrosols extracted from C. leylandii, E. globulus, A. citrodora, and M. officinalis, by hydro-distillation (mean ± SD, n = 3)
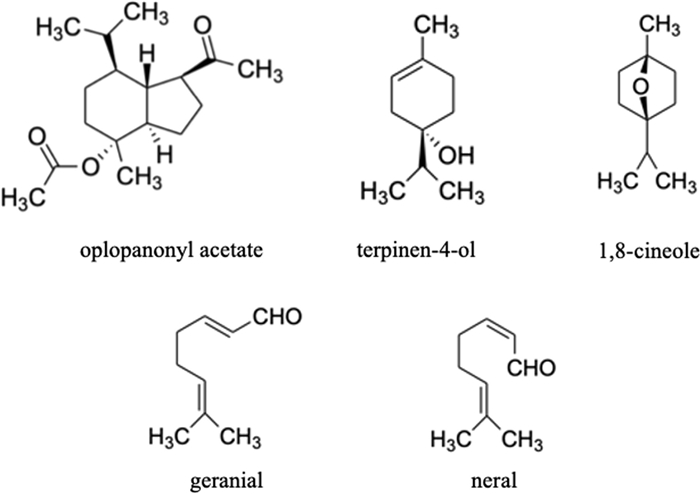
In C. leylandii hydrosol, 88.1% of the compounds were identified, with terpinen-4-ol (36.2%) and oplopanonyl acetate (12.8%) as the two major components. Concerning the hydrosol of E. globulus, 99.7% of the constituents were identified, with 1,8-cineole (90.1%) as the predominant one. The main components of the A. citrodora and M. officinalis hydrosols (with 96.6% and 99.4% of the total compounds identified, respectively) were citral isomers known as geranial (38.9% and 50.1% for A. citrodora and M. officinalis, respectively) and neral (39.0% and 42.0% for A. citrodora and M. officinalis, respectively). Low concentrations of less than 10% were determined for all the other components identified in the studied hydrosols. Figure 1 presents the chemical structure of the major compounds present in the hydrosols (e.g., oxygen-containing sesquiterpenes (oplopanonyl acetate) and oxygen-containing monoterpenes (terpinen-4-ol, 1,8-cineole, geranial, and neral)).
Chemical structures of the major compounds identified in the studied hydrosols (C. leylandii, E. globulus, A. citrodora, and M. officinalis)
Although the composition of C. leylandii and A. citrodora essential oils can be found in the literature, no previous works evaluated their hydrosol's chemical composition, with this data being reported for the first time in this work; even though C. leylandii hydrosol presents the same main component (terpinen-4-ol) as the hydrosols of the cypress family, such as the ones obtained from the C. lusitanica and C. sempervirens species [34, 35]. Moreover, the main components of A. citrodora hydrosols (neral and geranial) are likewise found in the corresponding essential oils [36, 37]. In other studies [38–40], the volatile composition of hydrosols from Eucalyptus species was studied, being found that oxygenated monoterpenes, particularly 1,8-cineole, are present in most of these hydrosols. This fact was also verified in this work, where this compound represents 90.1% of the E. globulus hydrosol composition. Petrakis and collaborators [41], who analysed the composition of M. officinalis hydrosols, reported that their major compounds were carvacrol, neral and geranial, analogously to this work, except for carvacrol, whose absence may be related to environmental factors and geographical origin of the studied plant species.
Studying a hydrosol's chemotype is fundamental to understanding the biological mechanisms underlying its bioactivity and directing its use to a particular application [14]. Although, currently, there are no quality standards for this class of natural products in the global pharmacopoeias, their standardisation (chemical and biological characterisation) will help ensure their quality, prospective uses, and safety [25, 27].
2.3 Antimicrobial activity of hydrosols
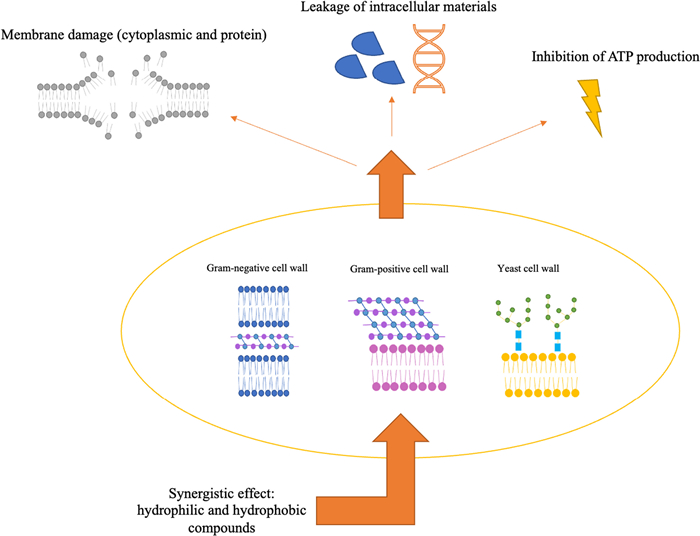
The chemical composition of a hydrosol indicates whether it has the potential to suppress microbial growth or pro-oxidant and inflammatory processes. Even though the potential mechanisms behind plant antimicrobial effects are not fully understood, these processes are attributed to the synergistic interaction established by compounds in their composition, which might have unique functional groups, polarities, and bioactivities. The interaction between these components and the bacterial cell membrane defines how antimicrobial activity occurs through different action mechanisms (Fig. 2) [43–45]. Depending on whether the bacteria is Gram-positive or Gram-negative, different areas of the microbial cells might be involved. Their susceptibility differs since Gram-positive bacteria contain a thick peptidoglycan layer connected to other hydrophobic compounds. This hydrophobic layer surrounding Gram-positive bacteria may facilitate the entrance of hydrophobic compounds. Gram-negative bacteria, conversely, have a more intricate cell wall, consisting of an outer membrane linked by lipoproteins to the inner peptidoglycan layer, increasing the resistance to the crossing of hydrophobic compounds [46, 47].
Mechanisms of action of hydrosol components on different cell walls (Gram-negative, Gram-positive, and yeast cells) bosting antimicrobial activity
According to the literature, the major components identified in the studied hydrosols (neral, geranial, 1,8-cineole, terpinen-4-ol, and oplopanonyl acetate) are associated with antimicrobial activity [48–50], corroborating the results of the performed antimicrobial assays. In this work, the statistical analysis enabled determining when there was a significant difference between the samples containing the hydrosol and a control (a sample prepared without adding hydrosol), proving whether the hydrosols can inhibit microbial growth. These differences are evident in Figs. 3, 4 and 5, where the effect of the studied hydrosols on microbial growth is represented. Analysing the susceptibility of S. aureus to the studied hydrosols (Fig. 3), it was verified that the concentration of 10% led to a reduction of 29.9% (p < 0.01) when using C. leylandii hydrosol, with no significant reduction when the other hydrosols were used. For 20%, the effect against S. aureus was increased, namely by lowering the microbial growth by 70.0% (with a significance of p < 0.0001) and 33.7% (p < 0.05) for the M. officinalis and A. citrodora hydrosols, respectively, compared to the control.
Quantification of S. aureus (CFU/mL) in the studied hydrosols, where (A) 10% hydrosol concentration and (B) 20% hydrosol concentration. ****p < 0.0001; ***p < 0.001; **p < 0.01; *p < 0.05; ns means not significant
Quantification of E. coli (CFU/mL) in the different studied hydrosols, where (A) 10% of hydrosol concentration and (B) 20% hydrosol concentration. ****p < 0.0001; ***p < 0.001; **p < 0.01; *p < 0.05; ns means not significant
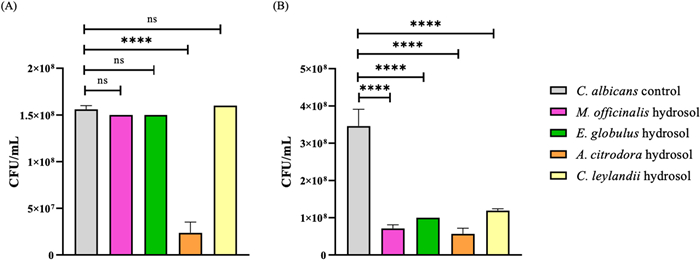
Quantification of C. albicans (CFU/mL) in the different studied hydrosols, where (A) 10% of hydrosol concentration and (B) 20% hydrosol concentration. ****p < 0.0001; ***p < 0.001; **p < 0.01; *p < 0.05; ns means not significant
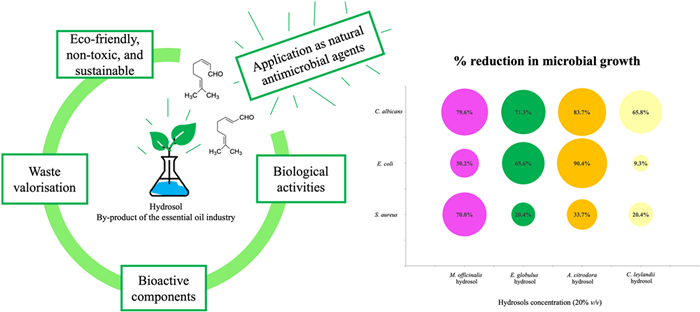
The A. citrodora hydrosol was the most effective against E. coli, with reductions of 90.8% (at 10% concentration) and 90.4% (at 20% concentration) with a significant difference of p < 0.0001 for both concentrations, which represents a reduction of 1 log10. The effect of this hydrosol was followed by E. globulus with cuts of 60% (at 10% concentration with p < 0.001) and 65.6% (at 20% concentration with p < 0.0001), and M. officinalis, which was able to reduce 30.2% when using 20% concentration (p < 0.0001) (Fig. 4).
A. citrodora hydrosol was also the most effective in inhibiting yeast growth, with reductions of 84.8% (at 10% concentration) and 83.7% (at 20% concentration) for C. albicans, with a significance of p < 0.0001 for both concentrations. The other hydrosols only showed this bioactivity for 20% concentration, with reductions of 79.6% for M. officinalis, 71.3% for E. globulus, and 65.8% for C. leylandii (Fig. 5). A significant difference of p < 0.0001 was observed for all hydrosols compared to the C. albicans control.
The findings of this work reveal that the antimicrobial activity of hydrosols often rises with concentration, with A. citrodora and M. officinalis as the most promising ones, followed by E. globulus and C. leylandii hydrosols. In this way, analysing the chemical composition of the two most promising hydrosols, their antimicrobial capacity may be associated with the main components' composition, the isomers of citral, which have been shown to have both biofilm-preventing and antimicrobial properties [51, 52]. According to Viktovorá and collaborators [53], who investigated the microbial cells' resistance to citral, found MIC (minimal inhibition concentration) values of 110 and 92 μL/L on C. albicans and S. aureus, respectively, being able to inhibit both bacteria and yeast growth. Chueca et al. [54] reported that a concentration of 300 μL/L of citral could inactivate at least 2.5 log10 cycles of exponentially growing cells of E. coli. Results reported by Somolinos et al. [55] showed that citral treatment caused sublethal injury to the cytoplasmic and outer membranes of E. coli cells. Citral mode of action may involve the penetration in phospholipid membranes, physical disruption of structural and functional characteristics, interruption of electron transfer across membranes, and oxidative stress culminating in membrane lipid peroxidation (leading to a burst on reactive oxidative species) [52, 56]. Thus, the present data may support the efficacy found in this work for the A. citrodora and M. officinalis hydrosols against the studied microorganisms. However, it is important to note that this major compound is diluted in a mixture of the hydrosol compounds. The antimicrobial potential may come from a synergistic effect among this by-product's different compounds and citral.
Regarding hydrosols' bioactivities, in the study of Hung et al., [26], L. cubeba fruit hydrosol containing neral and geranial as major components, inhibited the proliferation of C. albicans and revealed a fungicidal activity by applying it at 10 and 40% (v/v) concentration, respectively. These findings corroborate the results of this work where C. albicans was inhibited by approximately 80% with a significance of p < 0.0001 by applying 10–20% (v/v) of A. citrodora hydrosol, and 20% (v/v) of M. officinalis hydrosol, both hydrosols holding the main components reported for the L. cubeba hydrosol.
1,8-cineole has also been reported as a strong antimicrobial bioactive [48, 49]. This property can be related to its mode of action, which involves irreversible damage to the cell membrane leading to a decrease in ATP (adenosine triphosphate), protein, and DNA (deoxyribonucleic acid), as well as to cytoplasmic leakage [57]. Moreover, in the study by Khalaf and co-workers [58], Eucalyptus calmadulensis hydrosols, which present 1,8-cineole as the main compound, showed to inhibit different bacteria, among them E. coli, P. aeruginosa, S. epidermidis, S. mutans, K. pneumoniae, P. vulgaris (when applied directly, 100% concentration), and P. syogenes (with 25% concentration). In the present work, it was possible to reduce 60–65% of E. coli growth using lower concentrations (10–20% (v/v)) of E. globulus hydrosol, indicating a promising result for more dilute applications of this by-product. Even presenting the lowest found bioactivity, some authors have reported that the main compounds of C. leylandii hydrosol (terpinen-4-ol and oplopanonyl acetate) also have antimicrobial potential [48, 50]. These compounds can penetrate cell walls and membranes, causing internal osmotic pressure, weakening and rupturing the membrane and, subsequently, losing the cytoplasmic material [59]. Although the precise mechanisms underlying the oxygen-containing terpene groups' antimicrobial potential remain unclear, their lipophilic nature often results in cellular membrane expansion and damage, causing an increase in permeability, disruption of membrane-bound proteins, respiration suppression, and altered ion transport [60].
Various factors can influence a compound's biological activity, with functional groups holding different impacts by playing a role in polarity, solubility, and hydrogen bonding capacity, among others. The bioactivity of oxygenated molecules and hydrocarbons follows the following order: phenols > aldehydes > ketones > alcohols > ethers > hydrocarbons [61, 62]. In this regard, hydrosols' antimicrobial activity might be favoured by their hydrophilic environment, which increases the volatiles' bioavailability for interaction with bacteria and fungi. Particularising, a compound's polarity will affect its capacity to permeate and/or disrupt membranes. As a result, cellular targets of more hydrophobic compounds (acting on membrane disruption) differ from those of less hydrophobic molecules (acting on interactions with proteins) [17, 62]. In the study of Buccioni et al. [63], L. monocytogenes cells treated with 500 μL/mL of Coridothymus capitatus hydrosols, showed a diffuse aggregation and cell damage in response to the implied stress. These factors may indicate a synergistic effect among hydrosol components able to promote cellular stress, even when they show, individually, low antimicrobial activity. These findings point out a promising use of hydrosols in inhibiting target microorganisms in different environments.
3 Conclusions
Chemical and antimicrobial characterisation of hydrosols derived from plants used in EOs industries (C. leylandii, E. globulus, A. citrodora, and M. officinalis) was performed. Their main components were identified, and the associated antimicrobial potential was disclosed, considering the use of hydrosols as natural preservatives. Among the studied hydrosols, it was possible to recognise the most promising ones as A. citrodora > M. officinalis > E. globulus > C. leylandii, based on the antimicrobial capacity evaluation, which showed significant differences compared to the control (sample with no added hydrosol).
A. citrodora hydrosol used at 10% and 20% (v/v) concentrations was able to inhibit 90% of E. coli and 80% of C. albicans growth (with p < 0.0001), indicating its potential as an antimicrobial agent. The findings of this work revealed that the antimicrobial activity of hydrosols increased with concentration, presenting significant reductions (p < 0.0001), namely of 70% and 79.6% on S. aureus and C. albicans growth, respectively, with M. officinalis hydrosol, and 71.3% on C. albicans with E. globulus hydrosol.
Overall, hydrosols from aromatic plants, and even forestry biomass, may add value to productive chains, contributing to the consolidation of a biobased circular economy, by transforming this by-product into a green, non-toxic, and valuable ingredient for numerous applications in areas such as food, agriculture, pharmaceuticals, and cosmetics. Hydrosols are thus emerging as relevant candidates for antimicrobial applications, finding a direct use without prior pre-treatments, which also conforms with the principles of green chemistry.
4 Experimental section
4.1 General experimental procedures
Four different plants (Cupressus leylandii A.B. Jacks & Dallim, Eucalyptus globulus Labill., Aloysia citrodora Paláu and Melissa officinalis L.) supplied by Deifil Technology Lda (Póvoa de Lanhoso, Portugal), were received fresh, cut (leaves, branches, and flowers), frozen, and stored at − 20 ℃ (Hotpoint-Ariston, Italy). HPLC grade n-hexane (CarloErba Reagents, France) and anhydrous sodium sulphate (Sigma-Aldrich, Germany) were used in sample preparation for the chemical analysis. The microbial cultures (bacteria, yeast) selected in this study (Staphylococcus aureus ATCC 6538, Escherichia coli ATCC 8739, and Candida albicans ATCC 10231) were purchased from Mistracon (Spain). The microorganisms' substrates, brain–heart infusion (BHI) broth and nutrient agar were purchased from Liofilchem (Italy). The used water was distilled water.
4.2 Hydrosols obtainment
The hydrosols were obtained by hydro-distillation using a plant mass-to-water ratio of 1:1 (w/w), following an adapted procedure [64]. Briefly, 50 g of the plant were weighed and charged to the distillation vessel, then added with 50 mL of distilled water. The procedure comprised a first heating period to reach the water's boiling point. From this point, the hydrosols were collected for 10 min. After cooling, the final hydrosols were stored under refrigerated conditions at 4 ℃ before analysis. Right after production, the hydrosols were examined. The visual and olfactory inspection, done by one individual, was performed to preliminary access colour and odour sensory parameters. The pH was evaluated using a pH meter (InoLab, WTW Series pH 720, Weilheim, Germany).
4.3 Hydrosols chemical composition
The chemical composition of hydrosols was characterised by gas chromatography-mass spectrometry (GC–MS, Shimadzu, Japan) analysis. The sample preparation comprised a liquid–liquid extraction (LLE), where the hydrosol samples (15 mL) were vigorously mixed with 5 mL of n-hexane in a separating funnel for approximately 10 min. After phase separation, the lower-density liquid (n-hexane phase) was collected, added with anhydrous sodium to remove water, and filtered (Whatman filter n°4). The used gas chromatography conditions followed the ones previously described in [65] using an SH-RXi-5 ms column system (30 m × 0.25 mm × 0.25 μm). The injector temperature was set at 260 ℃. The oven temperature programming was as follows: 40 ℃ for 4 min, raised to 175 ℃ at a rate of 3 ℃/min, then to 300 ℃ at a rate of 15 ℃/min and held for 10 min. The sampling method used a split ratio of 1:10, and the injection volume was 1 μL. Helium was applied as the carrier gas adjusted to a linear velocity of 30 cm/s. The ionisation energy was 70 eV, and a scan range of 35–500 u with a scan time of 0.3 s was used. The compounds were identified by comparing the linear retention index (LRI) and the mass spectra with the NIST17 mass spectral Library data (considering a similarity > 90%). LRI determination was based on the retention times obtained from a mixture of n-alkanes (C8–C40, ref. 40147-U, Supelco) analysed under identical conditions. Comparisons with commercial standard compounds and published data were also used when possible. The different compounds were quantified as a relative percentage of total volatiles using relative peak area values obtained from the total ion current (TIC) values.
4.4 Hydrosols antimicrobial activity
4.4.1 Microbial strains and growth conditions
The microbial cultures of Staphylococcus aureus ATCC 6538, Escherichia coli ATCC 8739, and Candida albicans ATCC 10231, stored in an ultra-freezer (ThermoFisher, STP, AS) at − 70 ℃ were activated in BHI broth and incubated in a bacteriological oven (Raypa, Incutterm, Barcelona, Spain) at 37 ℃ for 24 h. Subsequently, the inoculum was prepared in BHI broth by standardising the cell density suspension in a densitometer (DEN-1 McFarland densitometer, Grant-bio, UK) at a wavelength of 550 nm, to have a final cell density of 1.5 × 108 cells/mL.
4.4.2 Antimicrobial activity assays
The susceptibility of the chosen strains to C. leylandii, E. globulus, A. citrodora, and M. officinalis hydrosols was performed using the viable cell counting method, colony forming units (CFU), as described in [66]. In brief, to prepare the samples, different concentrations of hydrosols (0.5 and 1.0 mL, representing 10 and 20% (v/v) of the total volume of the culture medium) were added to 4.5 mL of BHI broth with 10% of the standardised inoculum (1.5 × 108 cells/mL). A control was prepared by replacing the hydrosol with sterile distilled water. The tubes were incubated at 37 ℃ for 24 h, followed by serial dilutions and plating on nutrient agar, for cell counting.
4.5 Statistical analysis
The results were analysed using ANOVA statistical test with Tukey's multiple comparison post-test using the GraphPad Prism® 8.0 software (San Diego-CA, USA).
Notes
Acknowledgements
Financial support through national funds FCT/MCTES (PIDDAC) to LSRE-LCM (UIDB/50020/2020 and UIDP/00690/2020), ALiCE (LA/P/0045/2020), CIMO (UIDB/00690/2020 and UIDP/00690/2020), and SusTEC (LA/P/0007/2021). FCT for the SFRH/BD/148124/2019 Heloísa Helena Scorsato de Almeida research grant. Pedro Crugeira thanks OleaChain (NORTE-06-3559-FSE14 000188) for his research contract. GreenHealth project (Norte-01-0145-FEDER-000042). Deifil Technology Lda (www.deifil.pt) for supplying the studied plants.
Author contributions
HHSA: Conceptualization, Methodology, Investigation, Writing—Original Draft preparation. PJLC: Methodology, Formal Analysis. JSA: Methodology, Formal Analysis, Writing—Reviewing and Editing. AER: Supervision, Writing—Reviewing and Editing. M-FB: Conceptualization, Supervision, Resources, Writing— Reviewing and Editing.
Availability of data and materials
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Declarations
Competing interests
The authors declare that they have no conflict of interest.
References
-
1.Abushaheen MA, Muzaheed, Fatani AJ, Alosaimi M, Mansy W, George M, et al. Antimicrobial resistance, mechanisms and its clinical significance. Dis Month. 2020;66(6): 100971. CrossRef PubMed Google Scholar
-
2.Majumder MAA, Rahman S, Cohall D, Bharatha A, Singh K, Haque M, et al. Antimicrobial stewardship: fighting antimicrobial resistance and protecting global public health. Infect Drug Resist. 2020;13: 4713-38. CrossRef PubMed Google Scholar
-
3.de Melo ALF, Rossato L, dos Barbosa M, Palozi RAC, Alfredo TM, Antunes KA, et al. From the environment to the hospital: how plants can help to fight bacteria biofilm. Microbiol Res. 2022;261: 127074. CrossRef PubMed Google Scholar
-
4.Saeed F, Afzaal M, Tufail T, Ahmad A. Use of natural antimicrobial agents: a safe preservation approach. Active Antimicrobial Food Packaging. 2019, 1-18. PubMed Google Scholar
-
5.Petrisor G, Motelica L, Craciun LN, Oprea OC, Ficai D, Ficai A. Melissa officinalis: composition, pharmacological effects and derived release systems—a review. Int J Mol Sci. 2022;23: 3591. CrossRef PubMed Google Scholar
-
6.Rashid HM, Mahmod AI, Afifi FU, Talib WH. Antioxidant and antiproliferation activities of lemon verbena (Aloysia citrodora): an in vitro and in vivo study. Plants. 2022;11(6): 785. CrossRef PubMed Google Scholar
-
7.Čmiková N, Galovičová L, Schwarzová M, Vukic MD, Vukovic NL, Kowalczewski PŁ, et al. Chemical composition and biological activities of eucalyptus globulus essential oil. Plants. 2023;12(5): 1076. CrossRef PubMed Google Scholar
-
8.Frezza C, De Vita D, Sciubba F, Toniolo C, Tomassini L, Nicoletti M, et al. There is not only Cupressus sempervirens L.: a review on the phytochemistry and bioactivities of the other cupressus L. species. Appl Sci. 2022;12: 7353. CrossRef PubMed Google Scholar
-
9.Bencardino D, Amagliani G, Brandi G. Carriage of Staphylococcus aureus among food handlers: an ongoing challenge in public health. Food Control. 2021;130: 108362. CrossRef PubMed Google Scholar
-
10.Cheung GYC, Bae JS, Otto M. Pathogenicity and virulence of Staphylococcus aureus. Virulence. 2021;12: 547-69. CrossRef PubMed Google Scholar
-
11.Rani A, Ravindran VB, Surapaneni A, Mantri N, Ball AS. Review: trends in point-of-care diagnosis for Escherichia coli O157:H7 in food and water. Int J Food Microbiol. 2021;349: 109233. CrossRef PubMed Google Scholar
-
12.Riesute R, Salomskiene J, Moreno DS, Gustiene S. Effect of yeasts on food quality and safety and possibilities of their inhibition. Trends Food Sci Technol. 2021;108: 1-10. CrossRef PubMed Google Scholar
-
13.Talapko J, Juzbašić M, Matijević T, Pustijanac E, Bekić S, Kotris I, et al. Candida albicans-the virulence factors and clinical manifestations of infection. J Fungi. 2021;7(2): 1-19. CrossRef PubMed Google Scholar
-
14.D'Amato S, Serio A, López CC, Paparella A. Hydrosols: Biological activity and potential as antimicrobials for food applications. Food Control. 2018;86: 126-37. CrossRef PubMed Google Scholar
-
15.Šilha D, Švarcová K, Bajer T, Královec K, Tesařová E, Moučková K, et al. Chemical composition of natural hydrolates and their antimicrobial activity on arcobacter-like cells in comparison with other microorganisms. Molecules. 2020;25(23): 5654. CrossRef PubMed Google Scholar
-
16.Tavares CS, Gameiro JA, Roseiro LB, Figueiredo AC. Hydrolates: a review on their volatiles composition, biological properties and potential uses. Phytochem Rev. 2022;21: 1661. CrossRef PubMed Google Scholar
-
17.Di Vito M, Smolka A, Proto MR, Barbanti L, Gelmini F, Napoli E, et al. Is the antimicrobial activity of hydrolates lower than that of essential oils? Antibiotics. 2021;10(1): 88. CrossRef PubMed Google Scholar
-
18.İncegül Y, Çam M. Recovery of water-soluble materials after distillation of sage (Salvia officinalis L.) and the use of materials in the production of cake and ice cream. J Food Meas Charact. 2021;15: 2688. CrossRef PubMed Google Scholar
-
19.Rossi C, Maggio F, Chaves-López C, Valbonetti L, Berrettoni M, Paparella A, et al. Effectiveness of selected essential oils and one hydrolate to prevent and remove Listeria monocytogenes biofilms on polystyrene and stainless steel food-contact surfaces. J Appl Microbiol. 2021;132: 1866. CrossRef PubMed Google Scholar
-
20.Prusinowska R, Migielski K, Stobiecka A, Kunicka-Styczyńska A. Hydrolates from lavender (Lavandula angustifolia)—their chemical composition as well as aromatic, antimicrobial and antioxidant properties. Nat Prod Res. 2016;30(4): 386-93. CrossRef PubMed Google Scholar
-
21.Popa CL, Lupitu A, Mot MD, Copolovici L, Moisa C, Copolovici DM. Chemical and biochemical characterization of essential oils and their corresponding hydrolats from six species of the Lamiaceae family. Plants. 2021;10(11): 2489. CrossRef PubMed Google Scholar
-
22.Proto MR, Biondi E, Baldo D, Levoni M, Filippini G, Modesto M, et al. Essential oils and hydrolates: potential tools for defense against bacterial plant pathogens. Microorganisms. 2022;10(4): 702. CrossRef PubMed Google Scholar
-
23.Oliveira AS, Rolo J, Gaspar C, Cavaleiro C, Salgueiro L, Palmeira-de-Oliveira R, et al. Chemical characterization and bioactive potential of Thymus × citriodorus (Pers.) Schreb. preparations for anti-acne applications: antimicrobial, anti-biofilm, anti-inflammatory and safety profiles. J Ethnopharmacol. 2022;287: 114935. CrossRef PubMed Google Scholar
-
24.Ha SY, Jung JY, Lee DH, Yang JK. Anti-allergic and anti-inflammatory effects of hydrosol extracted from Zanthoxylum schinifolium branch. BioResources. 2021;16(3): 5721-32. CrossRef PubMed Google Scholar
-
25.Arsanjani ZN, Etemadfard H, Moein M. Comparative chemical evaluation of commercially available mint hydrosols produced in Fars province. Iran J Rep Pharm Sci. 2020;9(1): 52-8. CrossRef PubMed Google Scholar
-
26.Hung TT, Trang PT, Viet H, Lan NTM, Ngan LTM, Hieu TT. In vitro antimicrobial activity of hydrosol from Litsea cubeba (Lour.) Pers. against Helicobacter pylori and Candida albicans. Biomed Res Ther. 2020;7(6): 3819-28. CrossRef PubMed Google Scholar
-
27.Politi M, Menghini L, Conti B, Bedini S, Farina P, Cioni PL, et al. Reconsidering hydrosols as main products of aromatic plants manufactory: the Lavandin. Molecules. 2020;25: 1-19. CrossRef PubMed Google Scholar
-
28.Aćimović M, Tešević V, Smiljanić K, Cvetković M, Stanković J, Kiprovski B, et al. Hydrolates: By-products of essential oil distillation: chemical composition, biological activity and potential uses. Adv Technol. 2020;9(2): 54-70. CrossRef PubMed Google Scholar
-
29.Kahar M, Fauzi M, Mamat R. Properties and potential of agarwood hydrosol as a drink: a review 1. Food Res. 2021;5(3): 29-35. CrossRef PubMed Google Scholar
-
30.Jakubczyk K, Tuchowska A, Janda-Milczarek K. Plant hydrolates—antioxidant properties, chemical composition and potential applications. Biomed Pharmacother. 2021;1: 142. PubMed Google Scholar
-
31.Acheampong A, Borquaye LS, Acquaah SO, Osei-owusu J, Tuani GK. Antimicrobial activities of some leaves and fruit peels hydrosols. Int J Chem Biomol Sci. 2015;1(3): 158-62. PubMed Google Scholar
-
32.Politeo O, Popović M, Veršić Bratinčević M, Koceić P, Ninčević Runjić T, Mekinić IG. Conventional vs. microwave-assisted hydrodistillation: influence on the chemistry of sea fennel essential oil and its by-products. Plants. 2023;12(7): 1466. CrossRef PubMed Google Scholar
-
33.Řebíčková K, Bajer T, Šilha D, Ventura K, Bajerová P. Comparison of chemical composition and biological properties of essential oils obtained by hydrodistillation and steam distillation of Laurus nobilis L. Plant Foods Hum Nutr. 2020;75: 495-504. CrossRef PubMed Google Scholar
-
34.Politi M, Ferrante C, Menghini L, Angelini P, Flores GA, Muscatello B, et al. Hydrosols from Rosmarinus officinalis, Salvia officinalis, and Cupressus sempervirens: phytochemical analysis and bioactivity evaluation. Plants. 2022;11(3): 349. CrossRef PubMed Google Scholar
-
35.Tavares CS, Martins A, Faleiro ML, Miguel MG, Duarte LC, Gameiro JA, et al. Bioproducts from forest biomass: essential oils and hydrolates from wastes of Cupressus lusitanica Mill. and Cistus ladanifer L. Ind Crops Prod. 2020;144: 112034. CrossRef PubMed Google Scholar
-
36.Bahramsoltani R, Rostamiasrabadi P, Shahpiri Z, Marques AM, Rahimi R, Farzaei MH. Aloysia citrodora Paláu (Lemon verbena): a review of phytochemistry and pharmacology. J Ethnopharmacol. 2018;222: 34-51. CrossRef PubMed Google Scholar
-
37.Elechosa MA, Di Leo LP, Juárez MA, Viturro CI, Heit CI, Molina AC, et al. Essential oil chemotypes of Aloysia citrodora (Verbenaceae) in Northwestern Argentina. Biochem Syst Ecol. 2017;74: 19-29. CrossRef PubMed Google Scholar
-
38.Ieri F, Cecchi L, Giannini E, Clemente C, Romani A. GC-MS and HS-SPME-GC×GC-TOFMS determination of the volatile composition of essential oils and hydrosols (By-products) from four Eucalyptus species cultivated in Tuscany. Molecules. 2019;24(2): 1-15. CrossRef PubMed Google Scholar
-
39.Ndiaye EHB, Diop MB, Gueye MT, Ndiaye I, Diop SM, Fauconnier ML, et al. Characterization of essential oils and hydrosols from senegalese Eucalyptus camaldulensis Dehnh. J Essent Oil Res. 2018;30(2): 131-41. CrossRef PubMed Google Scholar
-
40.Ndiaye EHB, Gueye MT, Ndiaye I, Diop SM, Diop MB, Fauconnier ML, et al. Chemical composition of essential oils and hydrosols of three Eucalyptus species from Senegal: Eucalyptus alba Renv, Eucalyptus camaldulensis Dehnh and Eucalyptus tereticornis Hook. Am J Essent Oil Nat Prod. 2017;5(1): 1-7. PubMed Google Scholar
-
41.Petrakis EA, Kimbaris AC, Lykouressis DP, Polissiou MG, Perdikis DC. Hydrosols evaluation in pest control: insecticidal and settling inhibition potential against Myzus persicae (Sulzer). J Appl Entomol. 2015;139(4): 260-7. CrossRef PubMed Google Scholar
-
42.Adams RP. Identification of essential oil components by gas chromatography/mass spectroscopy. Allured Pub. Corp. 2017. PubMed Google Scholar
-
43.Baptista RC, Horita CN, Sant'Ana AS. Natural products with preservative properties for enhancing the microbiological safety and extending the shelf-life of seafood: a review. Food Res Int. 2020;127: 108762. CrossRef PubMed Google Scholar
-
44.Pinto T, Aires A, Cosme F, Bacelar E, Morais MC, Oliveira I, et al. Bioactive (Poly)phenols, volatile compounds from vegetables, medicinal and aromatic plants. Foods. 2021;10(1): 106. CrossRef PubMed Google Scholar
-
45.Valková V, Ďúranová H, Galovičová L, Borotová P, Vukovic NL, Vukic M, et al. Cymbopogon citratus essential oil: its application as an antimicrobial agent in food preservation. Agronomy. 2022;12(1): 155. CrossRef PubMed Google Scholar
-
46.Angane M, Swift S, Huang K, Butts CA, Quek SY. Essential oils and their major components: an updated review on antimicrobial activities, mechanism of action and their potential application in the food industry. Foods. 2022;11: 464. CrossRef PubMed Google Scholar
-
47.Pateiro M, Munekata PES, Sant'Ana AS, Domínguez R, Rodríguez-Lázaro D, Lorenzo JM. Application of essential oils as antimicrobial agents against spoilage and pathogenic microorganisms in meat products. Int J Food Microbiol. 2021;337: 108966. CrossRef PubMed Google Scholar
-
48.Taha ASM, Eldahshan OA. Chemical characteristics, antimicrobial, and cytotoxic activities of the essential oil of Egyptian Cinnamomum glanduliferum bark. Chem Biodivers. 2017;14(5). PubMed Google Scholar
-
49.Vazquez NM, Mariani F, Torres PS, Moreno S, Galvan EM. Cell death and biomass reduction in biofilms of multidrug resistant extended spectrum β-lactamase-producing uropathogenic Escherichia coli isolates by 1,8-cineole. PLoS ONE. 2020;15: e0241978. CrossRef PubMed Google Scholar
-
50.Smith ECJ, Williamson EM, Wareham N, Kaatz GW, Gibbons S. Antibacterials and modulators of bacterial resistance from the immature cones of Chamaecyparis lawsoniana. Phytochemistry. 2007;68(2): 210-7. CrossRef PubMed Google Scholar
-
51.Shi C, Song K, Zhang X, Sun Y, Sui Y, Chen Y, et al. Antimicrobial activity and possible mechanism of action of citral against cronobacter sakazakii. PLoS ONE. 2016;11(7): e0159006. CrossRef PubMed Google Scholar
-
52.Thielmann J, Muranyi P. Review on the chemical composition of Litsea cubeba essential oils and the bioactivity of its major constituents citral and limonene. J Essent Oil Res. 2019;31: 361-78. CrossRef PubMed Google Scholar
-
53.Viktorová J, Stupák M, Řehořová K, Dobiasová S, Hoang L, Hajšlová J, et al. Lemon grass essential oil does not modulate cancer cells multidrug resistance by citral—its dominant and strongly antimicrobial compound. Foods. 2020;9(5): 585. CrossRef PubMed Google Scholar
-
54.Chueca B, Pagán R, García-Gonzalo D. Oxygenated monoterpenes citral and carvacrol cause oxidative damage in Escherichia coli without the involvement of tricarboxylic acid cycle and Fenton reaction. Int J Food Microbiol. 2014;189: 126-31. CrossRef PubMed Google Scholar
-
55.Somolinos M, García D, Condón S, MacKey B, Pagán R. Inactivation of Escherichia coli by citral. J Appl Microbiol. 2010;108(6): 1928-39. CrossRef PubMed Google Scholar
-
56.Ju J, Xie Y, Yu H, Guo Y, Cheng Y, Zhang R, et al. Synergistic inhibition effect of citral and eugenol against Aspergillus niger and their application in bread preservation. Food Chem. 2020;310: 125974. CrossRef PubMed Google Scholar
-
57.Sobhy M, Ali SS, Cui H, Lin L, El-Sapagh S. Exploring the potential of 1,8-cineole from cardamom oil against food-borne pathogens: antibacterial mechanisms and its application in meat preservation. Microb Pathog. 2023;184: 106375. CrossRef PubMed Google Scholar
-
58.Khalaf ZZ, Zahra LA. Evaluation of the activity of essential oil and hydrosol from eucalyptus camaldulensis against some bacterial species. Iraqi J Sci. 2020;61(6): 1282-8. CrossRef PubMed Google Scholar
-
59.Bordini EAF, Tonon CC, Francisconi RS, Magalhães FAC, Huacho PMM, Bedran TL, et al. Antimicrobial effects of terpinen-4-ol against oral pathogens and its capacity for the modulation of gene expression. Biofouling. 2018;34: 815-25. CrossRef PubMed Google Scholar
-
60.Zengin H, Baysal AH. Antibacterial and antioxidant activity of essential oil terpenes against pathogenic and spoilage-forming bacteria and cell structure-activity relationships evaluated by SEM microscopy. Molecules. 2014;19: 17773-98. CrossRef PubMed Google Scholar
-
61.Nguyen HV, Meile JC, Lebrun M, Caruso D, Chu-Ky S, Sarter S. Litsea cubeba leaf essential oil from Vietnam: chemical diversity and its impacts on antibacterial activity. Lett Appl Microbiol. 2018;66(3): 207-14. CrossRef PubMed Google Scholar
-
62.Van de Vel E, Sampers I, Raes K. A review on influencing factors on the minimum inhibitory concentration of essential oils. Crit Rev Food Sci Nutr. 2019;59: 357-78. CrossRef PubMed Google Scholar
-
63.Buccioni F, Purgatorio C, Maggio F, Garzoli S, Rossi C, Valbonetti L, et al. Unraveling the antimicrobial effectiveness of coridothymus capitatus hydrolate against Listeria monocytogenes in environmental conditions encountered in foods: an in vitro study. Microorganisms. 2022;10(5): 920. CrossRef PubMed Google Scholar
-
64.Di Vito M, Bellardi MG, Mondello F, Modesto M, Michelozzi M, Bugli F, et al. Monarda citriodora hydrolate vs essential oil comparison in several anti-microbial applications. Ind Crops Prod. 2019;128: 206-12. CrossRef PubMed Google Scholar
-
65.Spréa RM, Fernandes Â, Calhelha RC, Pereira C, Pires TCSP, Alves MJ, et al. Chemical and bioactive characterization of the aromatic plant: Levisticum officinale W.D.J. Koch: a comprehensive study. Food Funct. 2020;11(2): 1292-303. CrossRef PubMed Google Scholar
-
66.Bari ML, Yeasmin S. Microbes Culture Methods. Infect Immun. 2022, 77-98. PubMed Google Scholar
Copyright information
© The Author(s) 2024
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.