Occurrence of D-amino acids in natural products
Robert A. Welch Foundation (Y-0026)
Abstract
Since the identified standard genetic code contains 61 triplet codons of three bases for the 20 L-proteinogenic amino acids (AAs), no D-AA should be found in natural products. This is not what is observed in the living world. D-AAs are found in numerous natural compounds produced by bacteria, algae, fungi, or marine animals, and even vertebrates. A review of the literature indicated the existence of at least 132 peptide natural compounds in which D-AAs are an essential part of their structure. All compounds are listed, numbered and described herein. The two biosynthetic routes leading to the presence of D-AA in natural products are: non-ribosomal peptide synthesis (NRPS), and ribosomally synthesized and post-translationally modified peptide (RiPP) synthesis which are described. The methods used to identify the AA chirality within naturally occurring peptides are briefly discussed. The biological activity of an all-L synthetic peptide is most often completely different from that of the D-containing natural compounds. Analyzing the selected natural compounds showed that D-Ala, D-Val, D-Leu and D-Ser are the most commonly encountered D-AAs closely followed by the non-proteinogenic D-allo-Thr. D-Lys and D-Met were the least prevalent D-AAs in naturally occurring compounds.Graphical Abstract

Keywords
D-amino acid Chirality Biogenesis Natural products1 Introduction
Amino acids (AA) are very simple chemicals containing both an amine and a carboxylic acid group [1]. In alpha-AAs, the amine and carboxylic acid groups are attached to the same carbon atom. Hydrogen is always the third substituent, hence, when the fourth and last substituent is not hydrogen (glycine), the α-AA is chiral. These α-AAs are the most important building blocks of living organisms given their ability to combine to form proteins. It is not yet well understood why the L-enantiomers are predominantly found in proteins that consist of no more than 19 L-α-AAs [2]. However, as early as 1894, Emil Fisher discovered enzymes, that he called Invertin and Emuslin, able to change the chirality of sugars [3]. Carrying on with amino acids, he identified their two possible D- and L-forms [4]. For years, D-AAs were believed to be present only in unicellular living forms such as viruses or bacteria with a variety of D-AAs found in peptides and antibiotics such as penicillin, the first antibiotic [5]. However, in 1981, D-Ala was unambiguously established in the sequence of dermorphin1 {129}, a peptide extracted from the skin of the tree frog Phyllomedusa sauvagine [6]. Since then, numerous occurrences of D-AA have been found at different positions of bioactive peptides or proteins and in natural products [7]. Smaller amounts of free D-AAs are found in essentially all biological systems [8], sometimes playing vital roles and frequently being disease biomarkers [9].
1 The number in bold and italic refers to the number assigned to the compound in the first column of the tables.
This review focuses on D-AAs in natural products. What is their origin, how were they identified, and what is their function? D-aspartic acid was found in several tissues (teeth, bones, skin, lung, lens) of ageing living bodies, and D-serine was found, in addition to D-aspartic acid, in β-amyloid of Alzheimer patients [9, 10]. A wide variety of D-AAs can be found in the proteins of biological systems animal or vegetal, including mammal and humans as recently reviewed [8, 11]. These particular peptides or altered proteins will not be considered in this study. D-amino acids are also key building blocks in the biosynthesis of polyketide-nonribosomal hybrid peptides that found great interest in recent years [12–14]. To maintain a focused and manageable size for this review, these and other hybrid structures were not included. Rather, the large number of natural peptide compounds containing at least one D-AA is reviewed. These compounds were arbitrary sorted by their source: prokaryote bacteria and algae, eukaryote fungi, and multicellular invertebrate and vertebrate animals. Since the universal standard genetic code encodes only L-AAs, the presence of D-AAs in peptides and proteins is most frequently explained as the result of post-translational modifications or non-ribosomal biosynthesis. Non-proteinogenic AAs of the D- or L- configuration also must be considered in this context.
2 Defining D-amino acids
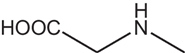
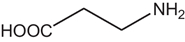
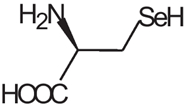
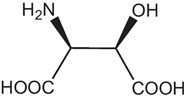
D-AAs are the opposite (mirror image) enantiomeric forms of the 19 natural chiral proteinogenic α-AAs found in natural products. However, there are numerous non-proteinogenic amino acids that also have a stereogenic center. Table 1 lists a selection of unusual AAs encountered when searching for D-AAs in natural products. Several rare methylated or hydroxylated variants of these amino acids are not included in this table. These non-proteogenic AAs will be listed in the subsequent "natural compound" tables since they were commonly found associated with proteogenic D-AA containing peptides.
Non proteogenic amino acids found in natural peptides sorted by increasing carbon number with L- or S- configuration shown.
3 Identifying D-amino acids
Due to the identical physicochemical properties of enantiomers in isotropical environments, except for their chiroptical qualities, the presence of D-AAs is not easy to determine and can often be overlooked. Fisher was the pioneer especially interested in the chirality of molecules establishing the D and L nomenclature and testing his isolated compounds by artificially synthesizing them and using optical rotation to detect their chirality [3, 4]. Spectroscopic, chemical, and especially separation methods are used to characterize D-AAs [9].
3.1 Spectroscopic methods
The spectroscopic methods using light for D-AA identification are optical rotation, called polarimetry, and circular dichroism spectroscopies. Raman optical activity is a new technique under development [15]. NMR spectroscopy uses very high frequency magnetic fields. 1H and 13C NMR spectra give structural information on the atom organization of the compound studied. Nuclear Overhauser Effect Spectroscopy (NOESY) is a powerful 2D NMR measurement giving information on the three-dimensional structure of biomolecules. Heteronuclear Single Quantum Coherence (HSQC) NMR gives correlations between carbons and protons that are separated by less than four bonds. Differences in NOESY or HSQC NMR assignments are seen between epimeric peptides differing in the chirality of amino acids. However, NMR methods are unable to determine the chirality of free AAs. Mass spectrometry analyses in vacuum detect charged compounds or fragments accelerated by electrical and magnetic fields. Fast-atom bombardment mass spectrometry (FAB-MS) uses a beam of neutral atomic gas (argon or xenon) bearing a high kinetic energy for the soft ionization of relatively large nonvolatile molecules with molecular weight up to 5000–6000 daltons and creation of charged fragments. Modern MS equipment can reach a m/z precision as low as 0.0001 Dalton allowing differentiation between a CH2 methylene group, m/z = 14.0156 Da, and a nitrogen atom, m/z = 14.0031. This was not possible with FAB-MS instruments but L- and D-enantiomers still cannot be differentiated by MS as they have the same exact mass.
The oldest and still very commonly used spectroscopic methods include polarimetry and circular dichroism methods [9]. Polarimetry measures the degree of rotation of a plane-polarized monochromatic light passing through a sample. Circular dichroism measures the absorption of left- and right-circularly polarized light of different wavelengths by a sample and displays the difference which is not nil for a chiral compound [9]. Polarimetry being linked to the solute refractive index is not very sensitive while circular dichroism can be somewhat more sensitive for solutes containing chromophores.
It was found that the optical properties of chiral molecules could be enhanced by orders of magnitude when adsorbed onto specific surfaces. Surface-enhanced polarimetry, circular dichroism and Raman optical activity methods using plasmonic nanostructures have potential in detecting smaller amounts of chiral molecules, but still are under developments [9].
3.2 Chemical methods
Chemical methods include Edman degradation of the peptide chain, Marfey analysis, and chemical synthesis of the identified compounds for a direct comparison of their biological effects. The classic Edman degradation process involves reacting the N-terminal amino acid with phenylisothiocyanate, cleaving it with trifluoroacetic acid, and converting the resulting thiazolinone in aqueous acid to a stable phenylthiohydantoin (PTH) amino acid which is identified. The process is repeated for the next amino acid and the PTH derivatives, that have a strong UV absorption, are analyzed by HPLC. Edman degradation gives the amino acid sequence of a protein or peptide but cannot distinguish between D and L-forms unless a chiral column is used for the HPLC analysis [16].
The Marfey method is more specifically designed to identify the amino acid chirality. Its first step is to completely degrade the peptide in 6N HCl media. The second step consists in reacting the hydrolysate with Nα-(2,4-dinitro-5-fluorophenyl)-L-alaninamide (L-FDAA) or with its enantiomer D-FDAA to form UV absorbing diastereoisomers that can be separated by a classical C18 column and the peak position are compared with those of known FDAA derivatives of amino acid standards [17]. However, the strong acid hydrolysis step can partially racemize each chiral AA, and convert asparagine and glutamine to aspartic acid and glutamic acid, respectively, among other undesired effects.
Synthesizing amino acids by classical organic chemistry produces racemic mixtures, however pure D-AAs can be obtained by several different means. Feeding living organisms with a racemic amino acid mixture will leave a residue of D-AAs since only the L-forms are consumed by the organisms. Once the amino acid sequence of a natural product is established, this sequence is reproduced using L-AAs and comparing the properties of the synthesized product with those of the natural one. The D-Ala in Dermorphin {129} was identified by this method. Dermorphin has an analgesic potency two to three orders of magnitude higher than morphine. The synthetic all-L dermorphin lacked any analgesic activity [6].
3.3 Separation methods
Separation methods are mainly chromatographic methods that separate compounds owing to their different affinities toward a stationary phase when they are carried by a mobile phase. Preparative liquid chromatography on low pressure columns usually is first used to isolate and purify the natural compounds. Thin layer chromatography (TLC), gas chromatography (GC), and high performance liquid chromatography (HPLC) were used to characterize the natural products or their amino acid constituents. The oldest approach to separate enantiomers is to derivatize them with an enantiomerically pure reagent prior to the chromatographic analysis. This is the principle of the Marfey method [17]. Classical HPLC, TLC, or GC for volatile derivatized compounds are able to separate the diastereoisomers obtained [9, 18]. In modern chiral studies, the derivatization approach has been supplanted by the use of chiral stationary phases (CSPs) which directly separate native molecules, hence avoiding several sample preparation steps.
Since enantiomers have identical properties in isotropic media, a chiral selector is needed to introduce some anisotropy and to allow for enantiomer differentiation. The chiral selector can make two transient diastereoisomers with both enantiomers. Once separated by the chromatographic process, the two enantiomers can be recovered independently. In chromatography, the chiral selector could be added to the mobile phase or attached to the stationary phase. Since chiral mobile phases need a continuous supply of expensive and pure chiral selector that must not disturb the detection method, CSPs are, by far, the method used in chromatography. Now, specific CSPs can routinely separate amino acid enantiomers that can be detected by MS or a variety of other detectors [19]. CSPs are more expensive chromatographic columns than classical columns. However, since the chiral selector is attached to the stationary phase, when used properly, CSP-containing columns can be used for hundreds or even thousands of enantiomer separations making the cost per separation insignificant [9]. The functionalized-carbohydrate CSPs based on bonded derivatized cellulose or amylose sugars cannot separate underivatized AAs or small peptides [19]. The macrocyclic glycopeptides based CSPs make chiral columns that are especially efficient in separating both native and N-blocked amino acids [20]. Recently introduced superficially porous particles bonded with chiral selectors allowed to obtain enantiomer separation in a very short time using minute amount of mobile phases [8, 9, 18, 19]. Associating achiral and chiral columns in 2D-HPLC greatly extended the capacity of the chromatographic methods allowing detecting trace amounts of chiral compounds [9, 18].
Peptide epimers that differ in the chirality of a single amino acid within the peptide chain are not enantiomers. They can be separated by achiral columns as demonstrated by the recent analysis of β-amyloids implicated in Alzheimer's disease [20]. However, CSPs [21] and especially macrocyclic glycopeptide bonded CSPs are particularly efficient in separating a wide variety of peptide epimers with minimum sample preparation [22, 23].
Capillary electrophoresis (CE) is a "micro-separation" method. However CE is not a chromatographic method and it separates compounds by their size to charge ratios using an electrical field created applying a high voltage in a capillary tube filled by the appropriate electrolyte. The CE technique requires that a chiral mobile-phase additive is dissolved in the running buffer, in order to separate any enantiomers, and especially D and L-AAs [9, 19]. CE provides high separation efficiency in relatively short electromigration times. The drawbacks are a mediocre reproducibility and sensitivity, and absence of any preparative capability.
4 Origin/biosynthesis of D-amino acids in natural products
Feeding cultures of Penicillium chrysogenium with 14C marked D or L-Valine, Arnstein and Margreiter obtained penicillin {28} containing 14C D-Valine only when the mycelium was fed with L-Valine [24]. They demonstrated that only L-AAs were processed by the mycelium and that the antibiotic synthesis necessarily involved racemase or epimerase enzymes. The standard genetic code contains six different codons of three DNA bases for L-Arg, L-Leu, and L-Ser, four codons of three bases for L-Ala, L-Gly, L-Pro, L-Thr, and L-Val, three codons, all starting with AU: AUA, AUC, and AUG for L-Ile, two codons for L-Asn, L-Asp, L-Cys, L-Gln, L-Glu, L-His, L-Lys, L-Phe, and L-Tyr, with the single codon, AUG, encoding L-Met, and UGG encoding L-Trp. This genetic code is almost universal for all known living organisms animal or vegetal. However, so far, there is no identified codon for D-AAs. Hence, the synthesis of D-containing peptides or proteins cannot come directly from the translation of DNA nucleotide sequences in ribosomal peptide synthesis. Non-ribosomal peptide synthesis (NRPS) is the main biosynthetic approach that produces the unique structural features observed in natural products containing D-AAs [25].
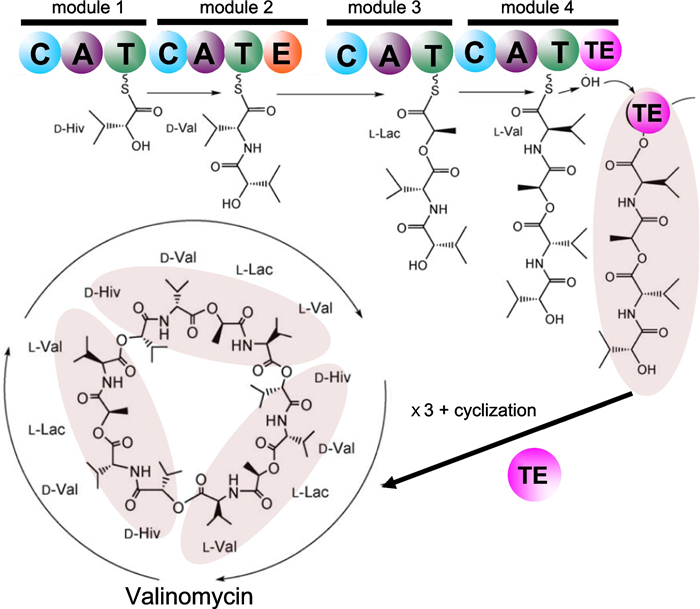
Nonribosomally assembled peptides can be altered by subsequent chemical peptide synthesis or enzyme catalysis in a connected ballet between multi-domain NRPS proteins and polyketide synthases. NRPS enzymes are the largest known enzymes with molecular weights passing 2.3 MDa or more than 21,000 residues [26]. The NRPS structure was found to be made by successive modules, each containing a condensation domain, C, an adenylation domain, A, and thiolation domain, T. As an example, the production of valinomycin {43} by the bacteria Streptomyces tsusimaensis was fully described [27]. Four NRPS modules were identified to incorporate successively D-α-hydroxy isovaleric acid (Hiv), L-valine, L-lactic acid, and another L-valine. An epimerase domain, E, and an iterative terminal domain, TE, were also identified (Fig. 1). The E domain converts the first L-valine into D-valine, and the TE domain cleaves the tetrapeptide and catalyzes a head-to-tail cyclization to give valinomycin {43} after coupling three tetrapeptides. Advanced modern bioinformatics deciphered the Streptomyces tsusimaensis gene responsible for the NRPS valinomycin {43} production. This gene of 39,266 base pairs (bp) contains eighteen open reading frames (ORN) producing different proteins. From these 18 ORNs, the valinomycin (vlm) cluster was identified as ORN16 and ORN17. ORN16 was called vlm1 of 10,286 bps between bp 19,526 and bp 29,812, and ORN17 was called vlm2 of 7967 bps between bp 29,835 and 37,802 [27]. Both ORNs contained two modules making the four modules responsible for the valinomycin {43} production (Fig. 1). This example shows that racemase, epimerase or isomerase enzymes encoded in the NRPS processes are responsible for the occurrence of D-AAs in natural products as obtained from their L-counterparts.
Simplified scheme of the valinomycin {43} biosynthesis by the NRPS subprotein of the bacteria Streptomyces tsusimaensis that was dissected into four modules containing a total number of fourteen domains (colored circles): C condensation, A adenylation, T thiolation, E epimerization, and TE C-terminal iterative domain. D-Hiv D-α-hydroxy isovaleric acid, L-Lac L-lactic acid. Adapted from [27]
The NRPS route is not the only natural process able to produce non-proteogenic and D-AA containing compounds. In the ribosomally-synthesized and post-translationally modified peptide (RiPP) synthesis route, organisms can synthesize compounds classically: normal codons for L-AAs are present in the mRNA at the position where the D-residue is found in the studied peptide. The L-AA is processed by a post-translational modification involving peptidyl-amino acyl L-D isomerization [8, 10]. Several mechanisms are possible. Simple epimerases just change a particular L-amino acid into its D-form using a deprotonation-protonation mechanism. The chiral AA hydrogen of a particular AA is removed to form an intermediate flat carbanion. The proton is reintroduced to the opposite side changing the chirality of the AA [28]. Hydroxylases or methylases are other enzymes that post-translationally modify L-AAs creating non-proteogenic AAs by converting the initial L-AA form into a D-form with hydroxyl or methyl groups added, respectively [29]. Also, specific enzymes are able to perform posttranslational conversion of a L-AA into another D-AA. For example, a lantibiotic synthetase found in Lactococcus lactis is able to change L-serine into D-alanine removing the hydroxyl group and changing the chirality in the production of the powerful lantibiotic 3147 {21} [30]. Replacing D-alanine by L-alanine in lantibiotic 3147 {21} reduced its bioactivity by 94% demonstrating that the type of AA and its chirality are both critical [30].
5 Occurence of D-amino acids in natural compounds of various origins
5.1 Prokaryotes
Prokaryotes are single-cell organisms lacking a nucleus. The domain of bacteria is of particular interest. They are also the first and most prevalent source of D-AA containing natural products. D-AAs were found in antibiotics, biosurfactants, toxins and siderophores produced by bacteria. All of these compounds facilitate bacterial life and survival. Antibiotics and toxins can limit or help eliminate competing bacteria or predator organisms. Biosurfactants facilitate bacterial spread in the medium or host. Iron is a critical element for bacterial development. Siderophores are very powerful iron-complexing compounds that selectively capture iron, releasing it specifically to the siderophore-producing strain. Table 2 lists D-AA containing compounds produced by prokaryotes, mainly bacteria [31–97]. The antibiotics are numbered and listed alphabetically with the bracketed number also used within this text after the compound name to facilitate reading. Compound names and numbers in the tables are followed by the name of the associated prokaryote, the structural formula, and molecular weight. The number of amino acids is given along with the number and proportion of D-AAs in each peptide. Also, the type of D-AAs and non-proteinogenic AAs are given in Table 2. The non-proteinogenic amino acids are listed using the Table 1 codes and adding the D- prefix if the D-form was found (Table 2).
D-amino acids found in natural compounds coming from prokaryotic organisms (non-photosynthetic bacteria)
The syringopeptin antibiotics {36}, {37} and {38} from the bacteria Pseudomonas syringae are among the larger peptides listed, containing up to 25 amino acids with up to 16 in the D-form [66–68]. Jessenipeptin {20} from Pseudomonas sp.QS1027 is another large antibiotic with 19 AAs, 13 of which are the D-antipodes (Fig. 2) [50]. The first and best-known penicillin {28} antibiotic from Penicillium chysogenum and the siderophore chrysobactin {47} from Erwinia chrysanthemi are made up of only two AAs, one being in its D-form: respectively D-glutamine [58] and D-lysine [76] (Fig. 2). There is no direct or obvious relationship between the total number of amino acids in a compound and the number of which are of the D-configuration although larger peptides could accommodate more D-AAs (Table 2). The phytotoxin fuscopeptin A {60} from Pseudomonas fuscovaginae consists of 19 AAs with 74% or 14 in the D-form (Fig. 2) [89]. The small cytotoxic burdolhac A {57} from Burkholderia thilandensis has a similar D-proportion with only four amino acids, three of which are the D-configuration [86]. On the other hand, the antibiotics daptomycin {11} from Sterptomyces roseosporus or mattacin {24} from Paenibacillus kobensis contain respectively 14 and 10 total AAs but only respectively three (21%) [36] and one (10%) [48] are in the D-form.
Structure of D-amino acid containing natural compounds produced by bacteria. The bracketed numbers refer to the Table 2 number codes along with additional information
The small Staphylopine {55} produced by the MRSA Staphylococcus aureus is a metallophore that is extremely efficient in complexing zinc. This property explains its high pathogenicity since the host defense, called nutritional immunity, consists in restricting zinc availability critical to bacterial development [84]. Staphylopine {55} contains a D-histidine unit that acts as the metal chelating group.
Most of the listed antibiotics were synthesized by bacteria using a NRPS process. Bottromycins {7} are powerful heptapeptides produced by Spectromyces bottropensis that were extensively studied. It was found that they were produced following a RiPP pathway with a ribosomally produced core peptide that is post-translationally modified by tailoring enzymes [36]. Lacticin 3147A2 {21} is a larger antibiotic with 29 amino acids and only 2 D-AAs. It is synthesized by Lactococcus lactis also via a RiPP process [44]. Lacticin 3147A2 {21} is a lantibiotic compound, meaning that it contains the pseudo-amino acid lanthionine which is enzymatically created by connecting two alanines, or aminobutyric acid and alanine via a sulfur atom. Lacticin A2 {21} synergistically works with its other RiPP produced lacticitin 3142A1 compound inducing pore formation in bacterial walls [29, 30, 51]. Lantibiotics are much more powerful than antibiotics and offer hope in overcoming antibiotic resistant Staphilococcus aureus and other lethal bacteria [51].
5.2 Photosynthetic microorganisms (prokaryote cyanobacteria and eukariote algae)
Prokaryotic cyanobacteria and eukaryotic algae contain pigments that allow them to perform photosynthesis producing oxygen associating them to the plant kingdom. Table 3 lists a selection of D-amino containing compounds produced by cyanobacteria or algae [98–107]. These relatively small compounds were sought for their biological activity as indicated in this table. Amphibactin {70} from the protobacterum Vibrio sp.R-10 is an amphiphilic siderophore that collects the rare iron ions present in the marine environment [98]. All the other listed marine natural products that contain D-AAs are cytotoxins (Table 3). Microcyctin {78} produced by the algae Nostoc sp.152 is a particularly virulent hepatotoxin [104].
Photosynthetic micro-organisms producing D-amino acid containing natural products: prokaryotic cyanobacteria and eukaryotic algae and plankton
5.3 Fungi
Fungi are eukaryotic multi-cellular organisms that are unable to directly synthesize essential nutrients. They are heterotrophic living entities that depend on other organisms for their sustenance. Fungi- produced peptides and depsipeptides that have been found to contain D-AAs are listed in Table 4 [108–119]. They have various biological effects, most helping the fungus to survive. The cytotoxic fungal compounds were found to be pharmacological interesting, having anticancer, antimalarial, or antibiotic properties (Table 4). Malformins {90–92}, a plant toxin produced by the fungus Aspergillus niger, was extensively studied, presenting numerous variations [115–118]. Studying the activity of artificially synthesized malmorfin compounds, it was demonstrated that the D-Cys and D-Leu amino acids found in the structure were critical for their biological activity (Fig. 3) [118]. D-His is rarely encountered in natural products. However, the ergot fungus Verticilium kibiense produces a polypeptide associating five L-Arg-D-His dipeptides [119]. This decapeptide was the sole natural compound reported to date in the literature that contained the rare D-histidine moiety.
D-containing peptides and depsipeptides produced by fungi, either unicellular yeast or multicellular mushrooms
Structure of Malformin A2 {90}, B {91} and C {92} produced by the fungus Aspergillus niger showing the constant position of the three D-amino acids. The bracketed numbers refer to the Table 4 number codes along with additional information
5.4 Multicellular organisms
Table 5 lists the D-AA containing natural compounds found in both invertebrate and vertebrate animals [101, 102, 120–150]
D-amino acid containing natural products found in invertebrate and vertebrate animals
5.4.1 Invertebrates
Most of the invertebrates that were found to produce D-AA containing compounds were marine mollusks, sponges and snails. The peptide and most often depsipeptide compounds found in invertebrates were relatively small with less than 15 amino acids and molecular weights lower than 1800 Da and were produced by NRPS processes. Kahalalides, depsipeptides from the herbivorous Elysia marine mollusk family and their algal diet Bryopsis pennata were extensively studied and presented wide structural variations and promising pharmacological properties. Kahalalide D {98} and V {104} are small tripeptides each containing a D-Trp unit (Table 5). Kahalalide R {103} is a tridecapeptide containing five D-AAs. Kahalalides are cytotoxic and active against cancer cells or AIDS opportunistic infectious bacteria [101, 102, 120, 121]. All reported kahalalides were produced by NRPS processes [101].
An interesting exception in the making of invertebrate natural compounds is the largest compound in Table 5; polytheonamide A {125} with 48 amino acids wherein 18 are in the D-form (37.5%) with a total mass of 5029 Da [143]. Polytheonamide A {125} is produced by the sponge Theonella swinhoei. It was demonstrated that polytheonamide {125} is a RiPP produced compound: it was initially synthesized by the normal ribosome pathway with all proteinogenic L-AAs and then modified through numerous epimerisations. It contains 18 different D-AAs (Table 5). Also, specific N-methyltransferase reactions introduced several non-proteogenic D-amino acids [29, 151]. A single epimerase, named PoyD, generated all D-AA residues acting by protonation-deprotonation of the α-hydrogen of the L-AA [29]. This post-translationally modified compound has cytotoxic capabilities by creating membrane channels acting on cation circulation [143, 151].
5.4.2 Vertebrates
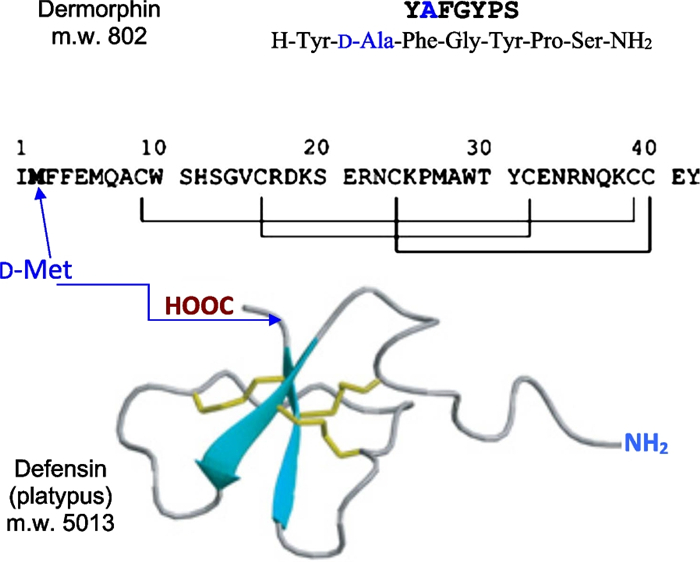
Since the discovery of D-alanine in dermorphin {129} produced by the frog Phyllomedusa bicolor [6] (Fig. 4), a few more natural compounds containing D-AAs were reported in vertebrates as listed in Table 5. Defensin DLP-2 {132} was found in the venom of the mammal Ornithorynchus anatinus, the platypus of Australia. Out of the 42 AAs found in this defensive venomous natural product only one had the D-configuration (Table 5 and Fig. 4). It is interesting to note that the synthesis of the all-L version of the venom has the same biological activity as the natural D-methionine containing, compounds. However, the natural and synthetic isomeric peptides showed significant differences in their liquid chromatography reversed-phase retention times [152]. An isomerase, glycoprotein of 52 kDa was found in frog skin secretion [151]. It could convert the L-form of the second amino acid residue into its D-form suggesting a RiPP synthesis of the observed D-AA containing compound {129} found in the frog [152].
Top: the 7 amino acid sequence of dermorphin {129}, the first natural compound containing a D-amino acid found in vertebrates. Bottom: the 42 amino acid sequence of the platypus defensin {132} all L, except D-methionine in position 2. The calculated ribbon quaternary structure of the large peptide shows the disulfide connectivities in yellow and secondary-structural element attractions in blue [150]. The bracketed numbers refer to the Table 5 number codes along with additional information
6 Outlook/overview of D-amino acid containing natural products
The selection of 132 D-containing natural products presented in this work consisted of 69 compounds found in eukaryotic bacteria (Table 2), 13 compounds found in marine eukaryotic cyanobacteria and prokaryotic algae (Table 3), 12 compounds found in fungi (Table 4), and 38 compounds found in invertebrate and vertebrate animals (Table 5). These 132 compounds contained a cumulated number of 1166 amino acids of which 442 had the D-configuration (37.9%).
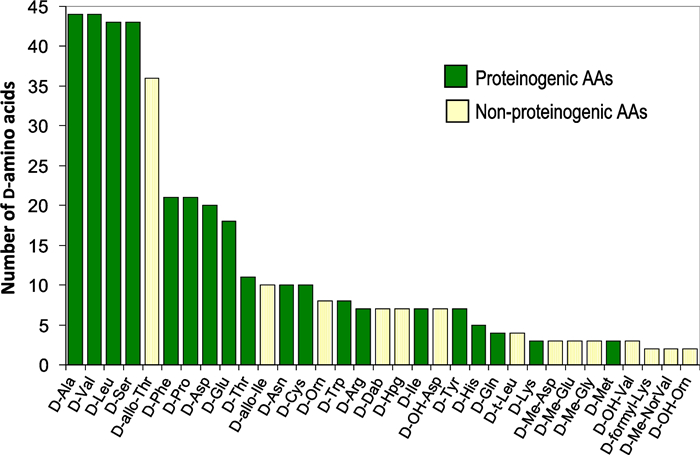
Figure 5 presents the occurrence of D-AAs found in the analyzed set of 132 compounds. The non-proteinogenic amino acids are shown with light yellow bars. 329 proteinogenic D-AAs (green bars) covering the whole set of the 19 amino acids having a stereogenic carbons were counted. The two most encountered D-proteinogenic amino acids were D-Ala and D-Val closely followed by D-Leu and D-Ser. D-allo-threonine is next being the most encountered D-non-proteogenic amino acid of the 113 found (Tables 2, 3, 4, 5). It is pointed out that threonine has two asymmetric centers, hence four enantiomeric forms. L- and D-threonine have respectively the (S-R) and (R-S) configurations and L- and D-allo-threonine are the (S-S) and (R-R) enantiomers. A similar stereochemistry is found with isoleucine. All the proteinogenic L-AAs are of the S-configuration except for cysteine (and selenocysteine) in which the sulfur (or selenium) heavier atom alters the sequence making it of the R-configuration according to the Cahn-Ingold-Prelog rules [1, 2].
Sixteen D-non-proteinogenic AAs were not shown in Fig. 2 because they were encountered only in one compound. These D-non-proteinogenic AAs included D-citrulline found in the siderophore azobactin {46} (Table 2) or D-kynurenine found in discodermin E {113} (Table 5). Likely because there are a large variety of biologically modified AAs (Table 1), the number of encountered non-proteinogenic D-AAs (light yellow bars in Fig. 5) is significantly lower than that of proteinogenic D-AAs (green bars). Including all found D-AAs, proteinogenic and non-proteinogenic, there is a trend that larger peptides with more AAs also contain more D-AAs. Figure 6 plots the number of D-AAs found in natural peptides presented in Tables 2, 3, 4, 5 versus the total number of AAs in the compound to see if there is a trend. 70% of the natural compounds listed in Tables 2, 3, 4, 5 are small peptides containing a maximum of 10 AAs. The linear regression obtained for the relation [n D-AAs] versus [total AAs] is: number of D-AA = 0.42 × total AA number (Fig. 6 green dotted line). The 0.42 slope means that, on average, in D-AA containing natural products, two D-AAs are encountered for each five AAs (Fig. 6). This linear trend has a loose 0.73 regression coefficient with the 30% larger compounds having more weight than the 70% smaller ones. However, an outlier with 48 AAs, polytheonamide {125} (Table 5), is predicted to contain 19 D-AAs. It contains only 18, a close value. On the other hand, cyclosporine A {86} is an undecapeptide, it should contain two or even three D-AAs. Only one was found (Table 4). Considering polytheonamide A {125} as an outlier and excluding it, a quadratic regression provides a slightly better fit of the Fig. 3 points (purple dashed line and regression equation in Fig. 6). The regression coefficient is similar. The quadratic equation predicts that a 20 AA peptide should contain 11 D-amino-acids when the linear fit predicts only 8. Jessineptin {20} (Table 2) contains 19 AAs in which 13 are D-AAs. The quadratic fit is better than the linear one for large peptides. Neither fit predicts well the number of D-AAs in small peptides. However, a trend is perceptible: peptides with more than 10 AAs will contain several D-AAs.
7 Conclusions
The presence of unusual D-amino acids in natural peptide products was first identified by natural product chemists and biochemists. For years, they were the only researchers discovering and identifying an increasing number of D-AA containing natural products. The protocol was to purify a new natural peptide product, then analyze its AA sequence to obtain its formula and then synthesize the all L-AA containing peptide for biological activity. This step was often the one detecting AA chirality when the all L-AA synthesized compound was inactive (e.g. dermorphin {129}) implying the presence of D-AAs.
The occurrence of D-AAs in natural products having significant biological activities was demonstrated in a wide variety of cases involving monocellular organisms, bacteria, fungi or algae. For a long period of time, D-AAs were not sought in vertebrates that were unquestionably regarded as made exclusively of L-AAs. That may explain why the D-AA occurrence was not reported in organized living forms until recently. The all L-AA dogma was destroyed when D-AAs were found in compounds secreted by invertebrates, vertebrate amphibians and even in one particular mammal: the Australian platypus. Since the presence of D-AAs in more and more natural and biological compounds is detected as the technologies improve, an increasing part of the scientific community has become aware of the need to know the chiral status of AAs in natural products as well as in proteomic studies and obviously in peptide-based pharmaceutical compounds. If D-AAs are sought after, they may be found in many more compounds that what is now known [11]. A powerful method to detect D-AAs in peptide was recently proposed [153].
The function of the known D-containing natural compounds generated by living organisms is to favor the survival and development of the producing entity either by impairing the growth of competing organisms (antibiotics, cytotoxics, siderophores), or by facilitating the entity's expansion (surfactants). These natural products were sought for their bioactivity that could have important applications in medicine, pharmacy and agriculture. It was also demonstrated that free D-AAs were occasionally used by complex eco-systems [152].
Since the standard genetic code encodes only the 19 proteinogenic L-AAs, natural compounds containing D-AAs are mostly produced by NRPS routes. To date, there is no evidence that ribosomes can directly incorporate D-AAs in a peptide chain [152]. However, RiPP post-tranlational modifications of ribosomic all L-AA peptides into D-containing epimeric forms are known and may not be as rare as once thought. With the massive progress made in bioinformatic scanning of the genetic code for a vastly increasing number of living animals and plants, the biosynthetic gene cluster code needed to produce a given peptide or even protein can be calculated and searched within databases in a short time [150]. The software antiSMASH searching for antibiotics and secondary metabolite biosynthetic gene clusters is freely available at https://antismash.secondarymetabolites.org/ [154].
In most cases, the biological activity of the D-containing natural products is completely different of that of the analogous all-L containing stereoisomer. This greatly helps in locating the D-AA(s) in the peptide chain using organic synthesis followed by biological tests. However, there are a few cases where little or no difference in the biological activity was observed between the D- and L-containing analogous natural products. Since these cases may render the D-forms unnoticed, they may be more common than have been reported [10]. The interesting questions that arise for these rare cases are: why does Nature incorporate such D-amino acids? and does resistance to protease degradation play a role in such cases [155]?
Notes
Author Contribution
Daniel W. Armstrong: Conceptualization; Formal analysis; Methodology; Project administration; Visualization; Writing - original draft; Writing - review & editing. Alan Berthod: Investigation; Formal analysis; Writing – original draft; Writing- review & editing. All authors read and approved the final manuscript.
Data availability
Since this is a review article, there is no new experimentl data that was generated by the authors.
Declarations
Competing interests
The authors declare that they do not have any competing interests.
References
-
1.Berg JM, Tymoczko JL, Stryer JM. Biochemistry.. 6th ed. New York, NY: Freeman publishers, 2007. PubMed Google Scholar
-
2.Delvin MD. Textbook of biochemistry with clinical correlations. Hoboken, NJ: Wiley, 2011. PubMed Google Scholar
-
3.Fisher E. Einfluss der Configuration auf die Wirkung der Enzyme (Influence of configuration on the action of enzymes). Berich Deutsch Chem Gesell. 1894;27(3): 2985-93. CrossRef PubMed Google Scholar
-
4.Fisher E. Untersuchungen über aminosäuren, polypeptide und proteïne. Berlin: Verlag Von Julius Spinger, 1906. PubMed Google Scholar
-
5.Abraham EP. Biochemistry of some peptides and steroid antibiotics. New York: Wiley, 1957. PubMed Google Scholar
-
6.Montecucchi PC, De Castiglione R, Piani S, Gozzini L, Erspamer V. Amino acid composition and sequence of Dermorphin, a novel opiate-like peptide from the skin of Phyllomedusa Sauvagei. Int J Pept Prot Res. 1981;17(3): 275-83. CrossRef PubMed Google Scholar
-
7.Jilek A, Kreil G. D-amino acids in animal peptides. Monatsheffe für Chemie. 2008;139: 1-5. CrossRef PubMed Google Scholar
-
8.Du S, Wey M, Armstrong DW. D-amino acids in biological systems. Chirality. 2023. CrossRef PubMed Google Scholar
-
9.Liu Y, Wu Z, Armstrong DW, Wolosker H, Zheng Y. Detection and analysis of chiral molecules as disease biomarkers. Nat Rev Chem. 2023;7: 355-73. CrossRef PubMed Google Scholar
-
10.Ollivaux C, Soyez D, Toullec JY. Biogenesis of D-amino acid containing peptides/proteins: where, when, and how? J Peptide Sci. 2014;20: 595-612. CrossRef PubMed Google Scholar
-
11.Mast DH, Checco JW, Sweedler JV. Advancing D-amino acid-containing peptide discovery in the metazoan. BBA Proteins Proteom. 2021;1869: 140553. CrossRef PubMed Google Scholar
-
12.Walsh CT. Polyketide and nonribosomal peptide antibiotics: modularity and versatility. Science. 2004;303: 1805-10. CrossRef PubMed Google Scholar
-
13.Walsh CT, O'Briaen RV, Khosla C. Nonproteinogenic amino-acids building blocks for nonribosomal peptide and hybrid polyketide scaffolds. Angew Chem Int Ed. 2013;52: 7098-124. CrossRef PubMed Google Scholar
-
14.Wang H, Fewer DP, Holm L, Rouhiainen L, Sivonen K. Atlas of nonribosomal peptide and polyketide biosynthetic pathways reveals common occurrence of nonmodular enzymes. PNAS. 2014;111: 9259-64. CrossRef PubMed Google Scholar
-
15.Barron LD, Hecht L, McColl IH, Blanch EW. Raman optical activity comes to age. Mol Phys. 2004;102: 731-44. CrossRef PubMed Google Scholar
-
16.Schlesinger DH. Proteins, traditional methods of sequence determination. In Worsfold P, Townsend A, Poole C (Eds) Encyclopedia of analytical science, 3rd edn., Vol. 8, pp. 352–357. https://doi.org/10.1016/B0-12-369397-7/00497-0 PubMed Google Scholar
-
17.Marfey P. Determination of D-amino acids. Ⅱ. Use of a bifunctional reagent, 1,5-difluoro-2,4-dinitrobenzene. Carlsberg Res Comm. 1984;49(6): 591-6. CrossRef PubMed Google Scholar
-
18.Sung YS, Berthod A, Roy D, Armstrong DW. A closer examination of 6-aminoquinolyl-N-hydroxysuccinimudyl carbamate amino acid derivation in HPLC with multiple detection modes. Chromatographia. 2021;84: 719-27. CrossRef PubMed Google Scholar
-
19.Stalcup AM. Chiral separations. Ann Rev Anal Chem. 2010;3: 341-63. CrossRef PubMed Google Scholar
-
20.Readel ER, Wey M, Armstrong DW. Rapid and selective separation of amyloid beta from its stereoisomeric point mutations implicated in neurodegenerative Alzheimer disease. Anal Chim Acta. 2021;1163: 338506. CrossRef PubMed Google Scholar
-
21.Du S, Readel ER, Wey M, Armstrong DW. Complete identification of all 20 relevant epimeric peptides in β-amyloid: a new HPLC-MS based analytical strategy for Alzheimer's research. Chem Commun. 2020;56(10): 1537-40. CrossRef PubMed Google Scholar
-
22.Berthod A, Liu Y, Bagwill C, Armstrong DW. Facile LC enantioresolution of native amino acids and peptides using a teicoplanin chiral stationary phase. J Chromatogr A. 1996;731: 123-37. CrossRef PubMed Google Scholar
-
23.Wimalasinghe R, Breitbach ZS, Lee JT, Armstrong DW. Separation of peptides on superficially porous particles based macrocyclic glycopeptide liquid stationary phases: consideration of fast separations. Anal Bioanal Chem. 2017;409: 2437-47. CrossRef PubMed Google Scholar
-
24.Arnstein HRV, Margreiter H. The biosynthesis of penicillin. Biochem J. 1958;68: 339-48. CrossRef PubMed Google Scholar
-
25.Mahariel MA, Essen LO. Nonribosomal peptide synthetases: mechanistic and structural aspects of essential domains. Meth Enzymol. 2009;458: 337-51. PubMed Google Scholar
-
26.Raush C, Hoof I, Weber T, Wohlleben W, Huson D. Phylogenetic analysis of condensation domains in NRPS sheds light on their functional evolution. BMC Evolution Biol. 2007;7: 78-92. CrossRef PubMed Google Scholar
-
27.Cheng YQ. Deciphering the biosynthetic codes for the potent anti-SARS-CoV cyclodepsipeptideValinomycin in Streptomyces tsusimaensis ATCC 15141. ChemBioChem. 2006;7: 471-7. CrossRef PubMed Google Scholar
-
28.Allard STM, Giraud MF, Naismith JH. Epimerases; structure, function and mechanism. Cell Mol Life Sci. 2001;58: 1650-5. CrossRef PubMed Google Scholar
-
29.Arnison PG, Bibb MJ, Bierbaum G, et al. Ribosomally synthesized and post-translationally modified peptide natural products: overview and recommendations for a universal nomenclature. Nat Prod Rep. 2013;30: 108-60. CrossRef PubMed Google Scholar
-
30.Cotter PD, O'Connor PM, Draper LA, Lawton EM, Deegan LH, Hill C, Ross RP. Posttranslational conversion of L-serines to D-alanines is vital for optimal production and activity of the lantibiotic lacticin 3147. Proc Natl Acad Sci USA. 2005;102: 18584-9. CrossRef PubMed Google Scholar
-
31.Miao V, Brost R, Chapple J, et al. The lipopeptide antibiotic A54145 biosynthetic gene cluster from Streptomyces fradiae. J Ind Microbiol Biotechnol. 2006;33: 129-40. CrossRef PubMed Google Scholar
-
32.Ciferri O, Albertini A, Cassani G. Origin of the sarcosine molecules of actinomycins. Biochem J. 1965;96: 853-61. CrossRef PubMed Google Scholar
-
33.Höltzel A, Schmid DG, Nicholson GJ, et al. Arylomycins A and B, new biaryl-bridged lipopeptide antibiotics produced by Streptomyces sp. Tü 6075. Ⅱ. Structure elucidation. J Antibiot. 2002;55: 571-7. CrossRef PubMed Google Scholar
-
34.Peypoux F, Pommier MT, Das BC, et al. Structures of bacillomycin D and bacillomycin L peptidolipid antibiotics from Bacillus subtilis. J Antibiot. 1984;37: 1600-4. CrossRef PubMed Google Scholar
-
35.Ikai Y, Oka H, Hayakawa J, et al. Total structures and antimicrobial activity of bacitracin minor components. J Antibiot. 1995;48: 233-42. CrossRef PubMed Google Scholar
-
36.Franz J, Kazmaier U, Truman AW, Koehnke J. Bottromycins—biosynthesis, synthesis and activity. Nat Prod Rep. 2021;38: 1659-83. CrossRef PubMed Google Scholar
-
37.Epperson JD, Ming LJ. Proton NMR studies of Co(ⅱ) complexes of the peptide antibiotic bacitracin and analogues: Insight into structure−activity relationship. Biochemistry. 2000;39: 4037-45. CrossRef PubMed Google Scholar
-
38.Wu X, Ballard J, Jiang YW. Structure and biosynthesis of the BT peptide antibiotic from Brevibacillus texasporus. Appl Environ Microbiol. 2005;71: 8519-30. CrossRef PubMed Google Scholar
-
39.Hojati Z, Milne C, Harvey B, et al. Structure, biosynthetic origin, and engineered biosynthesis of calcium-dependent antibiotics from Streptomyces coelicolor. Chem Biol. 2002;9: 1175-87. CrossRef PubMed Google Scholar
-
40.Ramesh S, Govender T, Kruger HG, Alberico F, Dela Torre BG. An improved and efficient strategy for the total synthesis of a colistin-like peptide. Tetrahedron Lett. 2016;57: 1885-8. CrossRef PubMed Google Scholar
-
41.Baltz RH, Miao V, Wrigley SK. Natural products to drugs: daptomycin and related lipopeptide antibiotics. Nat Prod Rep. 2005;22: 717-41. CrossRef PubMed Google Scholar
-
42.Debono M, Barnhart M, Carrell CB, et al. A21978C, a complex of new acidic peptide antibiotics: isolation, chemistry, and mass spectral structure elucidation. J Antibiot. 1987;40: 761-77. CrossRef PubMed Google Scholar
-
43.Miao V, Coeffet-Legal MF, Brian P, et al. Daptomycin biosynthesis in Streptomyces roseosporus: cloning and analysis of the gene cluster and revision of peptide stereochemistry. Microbiology. 2005;151: 1507-23. CrossRef PubMed Google Scholar
-
44.Sugawara K, Numata K, Konishi M, Kawaguchi H. Empedopeptin (BMY-28117), a new depsipeptide antibiotic. Ⅱ. Structure determination. J Antibiot. 1984;37: 958-64. CrossRef PubMed Google Scholar
-
45.Yin X, Zabriskie TM. The enduracidin biosynthetic gene cluster from Streptomyces fungicidicus. Microbiology. 2006;152: 2969-83. CrossRef PubMed Google Scholar
-
46.Li J, Jensen SE. Nonribosomal biosynthesis of fusaricidins by Paenibacillus polixyma PKB1 involves direct activation of D-amino acid. Chem Biochem. 2008;15: 118-27. PubMed Google Scholar
-
47.Govaerts C, Orwa J, Schepdael AV, Roets E, Hoogmartens J. Structure elucidation of four related substances in gramicidin with liquid chromatography/mass spectrometry. Rapid Com Mass Spec. 2001;15: 128-34. CrossRef PubMed Google Scholar
-
48.Tamaki M, Takimoto M, Sofuku S, Muramatsu I. Synthetic studies on gratisin.Ⅱ. J Antibiot. 1983;36: 751-2. CrossRef PubMed Google Scholar
-
49.Besson F, Hourdou ML, Michel G. Studies on the biosynthesis of iturin, an antibiotic of Bacillus subtilis, and a lipopeptide containing beta-hydroxy fatty acids. Biochim Biophys Acta. 1990;1036: 101-6. CrossRef PubMed Google Scholar
-
50.Martin NI, Sprules T, Carpenter MR, Cotter PD, Hill C, Ross RP, Vederas JC. Structural characterization of Lactisin 3147, a two-peptide lantibiotic with synergistic activity. Biochemistry. 2004;43: 3049-56. CrossRef PubMed Google Scholar
-
51.Arp J, Götze S, Mukherji R, et al. Synergistic activity of cosecreted natural products from amoebae-associated bacteria. Proc Natl Acad Sci USA. 2018;115: 3758-63. CrossRef PubMed Google Scholar
-
52.O'Sullivan J, McCullough JE, Tymiak AA, Kirsch DR, Trejo WH, Principe PA. Lysobactin, a novel antibacterial agent produced by Lysobacter sp. Ⅰ. Taxonomy, isolation and partial characterization. J Antibiot. 1988;41: 1740-4. CrossRef PubMed Google Scholar
-
53.Gerard J, Lloyd R, Barsby T, Haden P, Kelly MT, Andersen RJ. Massetolides A-H, antimycobacterial cyclic depsipeptides produced by two pseudomonads isolated from marine habitats. J Nat Prod. 1997;60: 223-9. CrossRef PubMed Google Scholar
-
54.Martin NI, Hu H, Moake MM, et al. Isolation, structural characterization, and properties of Mattacin (Polymyxin M), a cyclic peptide antibiotic produced by Paenibacillus kobensis M. J Biol Chem. 2003;278: 13124-32. CrossRef PubMed Google Scholar
-
55.Schlusselhuber M, Godard J, Sebban M, et al. Characterization of Milkisin, a novel lipopeptide with antimicrobial properties produced by pseudomonas sp. ucma 17988 isolated from bovine raw milk. Front Microbiol. 2018;9: 1030. CrossRef PubMed Google Scholar
-
56.Sengupta S, Banerjee AB, Bose SK. Gamma-glutamyl and D- or L-peptide linkages in mycobacillin, a cyclic peptide antibiotic. Biochem J. 1971;121: 839-46. CrossRef PubMed Google Scholar
-
57.Okumura Y, Sakamoto M, Takei T, Ishikura T, Fukagawa Y. Controlled biosynthesis of neoviridogriseins, new homologues of viridogrisein. Ⅳ. In vitro synergism between neoviridogrisein Ⅱ and the antibiotics of the mikamycin A group. J Antibiot. 1979;32: 1130-6. CrossRef PubMed Google Scholar
-
58.Queener SW. Molecular biology of penicillin and cephalosporin biosynthesis. Antimicrob Agents Chemother. 1990;34: 943-8. CrossRef PubMed Google Scholar
-
59.Shoji J, Hinoo H, Katayama T, Nakagawa Y, Ikenishi Y, Iwatani K, Yoshida T. Structures of new peptide antibiotics, plusbacins A1–A4 and B1–B4. J Antibiot. 1992;45: 824-31. CrossRef PubMed Google Scholar
-
60.Govaerts C, Orwa J, Schepdael AV, Roets E, Hoogmartens J. Characterization of polypeptide antibiotics of the polymyxin series by LC-ESI-ion trap tandem MS. J Pept Sci. 2002;8: 45-55. CrossRef PubMed Google Scholar
-
61.Bhattacharjya S, David SA, Mathan VI, Balaram P. Polymyxin B nonapeptide: conformation in water and in the lipopolysaccharide-bound state determined by 2D-NMR and molecular dynamics. Biopol. 1997;41: 251-65. CrossRef PubMed Google Scholar
-
62.de Crécy-Lagard V, Saurin W, Thibaut D, Gil P, Naudin L, Crouzet J, Blanc V. Streptogramin B biosynthesis in Streptomyces pristinaespiralis and Streptomyces virginiae: molecular characterization of the last structural peptide synthetase gene. Antimicrob Agents Chemother. 1997;41: 1904-9. CrossRef PubMed Google Scholar
-
63.Martin DG, Mizsak SA, Biles C, Stewart JC, Meulman PA. Structure of quinomycin antibiotics. J Antibiot. 1975;28: 332-6. CrossRef PubMed Google Scholar
-
64.McCafferty DG, Cudic P, Frankel BA, Barkallah S, Kruger RG, Li W. Chemistry and biology of the ramoplanin family of peptide antibiotics. Biopolymers. 2002;66: 261-84. CrossRef PubMed Google Scholar
-
65.Harris CM, Kibby JJ, Fehlner JR, Raabe JR, Barber TA, Harris TM. Amino acid constituents of Ristocetin A. J Am Chem Soc. 1979;101(2): 437-45. CrossRef PubMed Google Scholar
-
66.Segre A, Bachmann RC, Ballio A, et al. The structure of syringomycins A1, E and G. FEBS Lett. 1989;255: 27-31. CrossRef PubMed Google Scholar
-
67.Grgurina I, Mariotti F. Biosynthetic origin of syringomycin and syringopeptin 22, toxic secondary metabolites of the phytopathogenic bacterium Pseudomonas syringae pv. FEBS Lett. 1999;462: 151-4. CrossRef PubMed Google Scholar
-
68.Carpaneto A, Dalla Serra M, Menestrina G, Fogliano V, Gambale F. The phytotoxic lipodepsipeptide Syringopeptin 25A from Pseudomonas syringae pv syringae forms ion channels in sugar beet vacuoles. J Membr Biol. 2002;188: 237-48. CrossRef PubMed Google Scholar
-
69.Berthod A. Macrocyclic glycopeptides chiral stationary phases. In Reference Collection in Chemistry, Molecular Sciences and Chemical Engineering, 2022, Elsevier, Amsterdam. https://doi.org/10.1016/B978-0-32-390644-9.00004-4. PubMed Google Scholar
-
70.Bassarello C, Lazzaroni S, Bifulco G, et al. Tolaasins A−E, five new lipodepsipeptides produced by Pseudomonas tolaasii. J Nat Prod. 2004;67: 811-6. CrossRef PubMed Google Scholar
-
71.Otsuka H, Shoji J, Kawano K, Kyogoku Y. Structure confirmation of triostin a by 1H and 13C magnetic resonance. J Antibiot. 1976;29: 107-10. CrossRef PubMed Google Scholar
-
72.Mootz HD, Marahiel MA. The tyrocidine biosynthesis operon of Bacillus brevis: complete nucleotide sequence and biochemical characterization of functional internal adenylation domains. J Bacteriol. 1997;179: 6843-50. CrossRef PubMed Google Scholar
-
73.Cheng YQ. Deciphering the biosynthetic codes for the potent anti-SARS-CoV cyclodepsipeptide valinomycin in Streptomyces tsusimaensis ATCC 15141. ChemBioChem. 2006;7: 471-7. CrossRef PubMed Google Scholar
-
74.Zhou Q, Grundmann F, Kaiser M, et al. Structure and biosynthesis of Xenoamicins from Entomopathogenic xenorhabdus. Chem Eur J. 2013;19: 16772. CrossRef PubMed Google Scholar
-
75.Demange P, Bateman A, Dell A, Abdallah MA. Structure of azotobactin D, a siderophore of Azotobacter vinelandii strain D (CCM 289). Biochemistry. 1988;27: 2745-52. CrossRef PubMed Google Scholar
-
76.Persmark M, Expert D, Neilands JB. Isolation, characterization, and synthesis of chrysobactin, a compound with siderophore activity from Erwinia chrysanthemi. J Biol Chem. 1989;264: 3187-93. CrossRef PubMed Google Scholar
-
77.Risse D, Beiderbeck H, Taraz K, Budzikiewicz H, Gustine D. Corrugatin, a lipopeptide siderophore from Pseudomonas corrugata. Zeitschrift für Naturforschung C. 1998;53: 295-304. CrossRef PubMed Google Scholar
-
78.Xu G, Martinez JS, Groves JT, Butler A. Membrane affinity of the amphiphilic Marinobactin siderophores. J Am Chem Soc. 2002;124: 13408-15. CrossRef PubMed Google Scholar
-
79.Persmark M, Frejd T, Mattiasson B. Purification, characterization, and structure of pseudobactin 589 A, a siderophore from a plant growth promoting Pseudomonas. Biochemistry. 1990;29: 7348-56. CrossRef PubMed Google Scholar
-
80.Georgias H, Taraz K, Budzikiewicz H, Geoffroy V, Meyer J-M. The structure of the pyoverdin from Pseudomonas fluorescens 1.3. Structural and biological relationships of pyoverdins from different strains. Zeitschrift für Naturforschung C. 1999;54: 301-8. CrossRef PubMed Google Scholar
-
81.Fernández DU, Fuchs R, Schäfer M, Budzikiewicz H, Meyer JM. The pyoverdin of Pseudomonas fluorescens G173, a novel structural type accompanied by unexpected natural derivatives of the corresponding ferribactin. Zeitschrift fur Naturforschung C. 2003;58: 1-10. CrossRef PubMed Google Scholar
-
82.Grim KP, Francisco BS, Radin JN, et al. The metallophore Staphylopine enables Staphylococcus aureus to compete with the host for Zinc and overcome nutritional immunity. MBio. 2017;8: e01281-e1317. CrossRef PubMed Google Scholar
-
83.Gwose I, Taraz K. Pyoverdins from Pseudomonas putida. Zeitschrift fur Naturforschung C. 1992;47: 487-502. CrossRef PubMed Google Scholar
-
84.Schalk IJ, Rigouin C, Godet J. An overview of siderophore biosynthesis among fluorescent Pseudomonas and new insights into their complex cellular organization. Env Microbiol. 2020;22: 1447-53. CrossRef PubMed Google Scholar
-
85.Sørensen D, Nielsen TH, Christophersen C, Sørensen J, Gajhede M. Cyclic lipoundecapeptide amphisin from Pseudomonas sp. strain DSS73. Acta Crystal C Crystal Struct Comm. 2001;57: 1123-4. CrossRef PubMed Google Scholar
-
86.Fukui Y, Narita K, Dan S, et al. Total synthesis of burkholdacs A and B and 5,6,20-tri-epi-burkholdac A. Eur J Med Chem. 2014;76: 301-13. CrossRef PubMed Google Scholar
-
87.Ehling-Schulz M, Fricker M, Grallert H, Rieck P, Wagner M, Scherer S. Cereulide synthetase gene cluster from emetic Bacillus cereus: structure and location on a mega virulence plasmid related to Bacillus anthracis toxin plasmid pXO1. BMC Microbiol. 2006;6: 20-30. CrossRef PubMed Google Scholar
-
88.Vanittanakom N, Loeffler W, Koch U, Jung G. Fengycin—a novel antifungal lipopeptide antibiotic produced by Bacillus subtilis F-29-3. J Antibiot. 1986;39: 888-901. CrossRef PubMed Google Scholar
-
89.Ballio A, Bossa F, Camoni L, et al. Structure of fuscopeptins, phytotoxic metabolites of Pseudomonas fuscovaginae. FEBS Lett. 1996;381: 213-6. CrossRef PubMed Google Scholar
-
90.Pettit GR, Tan R, Melody N, et al. Antineoplastic agents. Part 409: isolation and structure of montanastatin from a terrestrial actinomycete. Bioorg Med Chem. 1999;7: 895-9. CrossRef PubMed Google Scholar
-
91.Gross H, Stockwell VO, Henkels MD, Nowak-Thompson B, Loper JE, Gerwick WH. The genomisotopic approach: a systematic method to isolate products of orphan biosynthetic gene clusters. Chem Biol. 2007;14: 53-63. CrossRef PubMed Google Scholar
-
92.Quail JW, Ismail N, Pedras MS, Boyetchko SM. Pseudophomins A and B, a class of cyclic lipodepsipeptides isolated from a Pseudomonas species. Acta Crystal C, Crystal Struct Comm. 2002;58: 268-71. CrossRef PubMed Google Scholar
-
93.Morikawa M, Daido H, Takao T, Murata S, Shimonishi Y, Imanaka S. A new lipopeptide biosurfactant produced by Arthrobacter sp. strain MIS38. J Bacteriol. 1993;175: 6459-66. CrossRef PubMed Google Scholar
-
94.Béchet M, Caradec T, Hussein W, et al. Structure, biosynthesis, and properties of kurstakins, nonribosomal lipopeptides from Bacillus spp. Appl Microbiol Biotechnol. 2012;95: 593-600. CrossRef PubMed Google Scholar
-
95.Matsuyama T, Kaneda K, Nakagawa Y, Isa K, Hara-Hotta H, Yano I. A novel extracellular cyclic lipopeptide which promotes flagellum-dependent and -independent spreading growth of Serratia marcescens. J Bacteriol. 1992;174: 1769-76. CrossRef PubMed Google Scholar
-
96.Henriksen A, Anthoni U, Nielsen TH, Sorensen J, Christophersen C, Gajhede M. Cyclic lipoundecapeptide tensin from Pseudomonas fluorescens strain 96.578. Acta Crystal C Crystal Struct Comm. 2000;56: 113-5. CrossRef PubMed Google Scholar
-
97.Laycock MV, Hildebrand PD, Thibault P, Walter JA, Wright JLC. Viscosin, a potent peptidolipid biosurfactant and phytopathogenic mediator produced by a pectolytic strain of Pseudomonas fluorescens. J Agric Food Chem. 1991;39: 483-9. CrossRef PubMed Google Scholar
-
98.Martinez JS, Carter-Franklin JN, Mann EL, Martin JD, Haygood MG, Butler A. Structure and membrane affinity of a suite of amphiphilic siderophores produced by a marine bacterium. Proc Nat Acad Sci. 2003;100: 3754-9. CrossRef PubMed Google Scholar
-
99.Itou Y, Suzuki S, Ishida K, Murakami M. Anabaenopeptins G and H, potent carboxypeptidase A inhibitors from the cyanobacterium Oscillatoria agardhii (NIES-595). Bioorg Med Chem Lett. 1999;9: 1243-6. CrossRef PubMed Google Scholar
-
100.Williams DE, Craig M, Holmes CFB, Andersen RJ. Ferintoic acids A and B, new cyclic hexapeptides from the freshwater cyanobacterium Microcystis aeruginosa. J Nat Prod. 1996;59: 570-5. CrossRef PubMed Google Scholar
-
101.Gao J, Hamann MT. Chemistry and biology of Kahalalides. Chem Rev. 2011;111: 3208-35. CrossRef PubMed Google Scholar
-
102.Kan Y, Fujita T, Sakamoto B, Hokama Y, Nagai H, Kahalalide K. A new cyclic depsipeptide from the hawaiian green alga bryopsis species. J Nat Prod. 1999;62: 1169-72. CrossRef PubMed Google Scholar
-
103.Namikoshi M, Rinehart KL, Sakai R, et al. Identification of 12 hepatotoxins from a Homer Lake bloom of the cyanobacteria Microcystis aeruginosa, Microcystis viridis, and Microcystis wesenbergii: nine new microcystins. J Org Chem. 1992;57: 866-72. CrossRef PubMed Google Scholar
-
104.Sivonen K, Namikoshi M, Evans WR, Färdig M, Carmichael WW, Rinehart KL. Three new microcystins, cyclic heptapeptide hepatotoxins, from Nostoc sp. strain 152. Chem Res Toxicol. 1992;5: 464-9. CrossRef PubMed Google Scholar
-
105.Mazur-Marzec H, Meriluoto J, Pliński M, Szafranek J. Characterization of nodularin variants in Nodularia spumigena from the Baltic Sea using LC/MS/MS. Rapid Commun Mass Spectrom. 2006;20: 2023-32. CrossRef PubMed Google Scholar
-
106.Hanessian S, Tremblay M, Petersen JFW. The N-Acyloxyiminium ion aza-prins route to octahydroindoles: total synthesis and structural confirmation of the antithrombotic marine natural product Oscillarin. J Am Chem Soc. 2004;126: 6064-71. CrossRef PubMed Google Scholar
-
107.Meriluoto JA, Sandström A, Eriksson JE, Remaud G, Grey Craig A, Chattopadhyaya J. Structure and toxicity of a peptide hepatotoxin from the cyanobacterium Oscillatoria agardhii. Toxicon. 1989;27: 1021-34. CrossRef PubMed Google Scholar
-
108.Singh SB, Zink DL, Liesch JM, et al. Structure and chemistry of Apicidins, a class of novel cyclic tetrapeptides without a terminal α-keto epoxide as inhibitors of histone deacetylase with potent antiprotozoal activities. J Org Chem. 2002;67: 815-25. CrossRef PubMed Google Scholar
-
109.Kuzma M, Jegorov A, Kačer P, Havlíček V. Sequencing of new beauverolides by HPLC/MS. J Mass Spectrom. 2001;36: 1108-15. CrossRef PubMed Google Scholar
-
110.Closse A, Huguenin R. Isolierung und strukturaufklärung von Chlamydocin. Helv Chim Acta. 1974;57: 533-45. CrossRef PubMed Google Scholar
-
111.Lawen A, Zocher R. Cyclosporin synthetase. The most complex peptide synthesizing multienzyme polypeptide so far described. J Biol Chem. 1990;265: 11355-60. CrossRef PubMed Google Scholar
-
112.Scott-Craig JS, Panaccione DG, Pocard JA, Walton JD. The cyclic peptide synthetase catalyzing HC-toxin production in the filamentous fungus Cochliobolus carbonum is encoded by a 15.7-kilobase open reading frame. J Biol Chem. 1992;267: 26044-9. CrossRef PubMed Google Scholar
-
113.Walton JD. HC-toxin. Phytochemistry. 2005;67: 1406-13. CrossRef PubMed Google Scholar
-
114.Baute R, Deffieux G, Merlet D, Baute MA, Neveu A. New insecticidal cyclodepsipeptides from the fungus Isaria feline. Ⅱ. Structure elucidation of isariins B. C and D. J Antibiot. 1981;34: 1266-70. CrossRef PubMed Google Scholar
-
115.Sugawara F, Kim KW, Uzawa J, Yoshida S, Takahashi N, Curtis RW. Structure of malformin A2, reinvestigation of phytotoxic metabolites produced by aspergillus niger. Tetrahedron Lett. 1990;31: 4337-40. CrossRef PubMed Google Scholar
-
116.Kim KW, Sugawara F, Yoshida S, Murofushi N, Takahashi N, Curtis RW. Structure of malformin B, a phytotoxic metabolite produced by Aspergillus niger. Biosci Biotechnol Biochem. 1993;57: 787-91. CrossRef PubMed Google Scholar
-
117.Anderegg RJ, Biemann K, Büchi G, Cushman M. Malformin C, a new metabolite of Aspergillus niger. J Am Chem Soc. 1976;98: 3365-70. CrossRef PubMed Google Scholar
-
118.Bodanszky M, Bednarek MA, Yiotakis AE, Curtis RW. Allomalformin. Int J Pept Protein Res. 1982;20: 16-25. CrossRef PubMed Google Scholar
-
119.Nishikawa M, Ogawa K. Occurrence of D-histidine residues in antimicrobial poly(arg-his) conferring resistance to enzymatic hydrolysis. FEMS Microb Lett. 2004;239: 255-9. CrossRef PubMed Google Scholar
-
120.Goetz G, Nakao Y, Scheuer PJ. Two acyclic Kahalalides from the Sacoglossan mollusk Elysia rufescens. J Nat Prod. 1997;60: 562-7. CrossRef PubMed Google Scholar
-
121.Tilvi S, Naik CG. Tandem mass spectrometry of kahalalides: identification of two new cyclic depsipeptides, kahalalide R and S from Elysia grandifolia. J Mass Spectrom. 2007;42: 70-80. CrossRef PubMed Google Scholar
-
122.Rao KV, Na M, Cook JC, Peng J, Matsumoto R, Hamann MT. Kahalalides V-Y isolated from a Hawaiian collection of the sacoglossan mollusk Elysia rufescens. J Nat Prod. 2008;71: 772-8. CrossRef PubMed Google Scholar
-
123.Fernández R, Rodriguez J, Quiñoa E, et al. Onchidin B: a new cyclodepsipeptide from the mollusc Onchidium sp.. J Am Chem Soc. 1996;118: 11635-43. CrossRef PubMed Google Scholar
-
124.Katamani Y, Minakata H, Kenny PTM, et al. Achatin Ⅰ, an endogenous neuroexitatory tetrapeptide for Achata fulica containing a D-amino acid residue. Biochem Biophys Res Comm. 1989;160: 1015-20. CrossRef PubMed Google Scholar
-
125.Roy MC, Ohtani Ⅱ, Tanaka J, Higa T, Satari R. Barangamide A, a new cyclic peptide from the Indonesian sponge Theonella swinhoei. Tetrahedron Lett. 1999;40: 5373-6. CrossRef PubMed Google Scholar
-
126.Trevisi L, Bova G, Cargnelli G, et al. Callipeltin A, a cyclic depsipeptide inhibitor of the cardiac sodium-calcium exchanger and positive inotropic agent. Biochem Biophys Res Commun. 2000;279: 219-22. CrossRef PubMed Google Scholar
-
127.Calimsiz S, Morales Ramos AI, Lipton MA. Solid-phase synthesis and configurational reassigment of callipeltin E. Implications for the structures of callipeltins A and B. J Org Chem. 2006;71: 6351-6. CrossRef PubMed Google Scholar
-
128.D'Auria MV, Sepe V, D'Orsi R, Bellotta F, Debitus C, Zampella A, et al. Isolation and structural elucidation of callipeltins J–M: antifungal peptides from the marine sponge Latrunculia sp. Tetrahedron. 2007;63: 131-40. CrossRef PubMed Google Scholar
-
129.Bonnington LS, Tabaka J, Higa T, Kimura J, Yoshimura Y, Nakao Y, Scheuer PJ. Cupolamide A: a cytotoxic cyclic heptapeptide from two samples of the sponge Theonella cupola. J Org Chem. 1997;62: 7765-7. CrossRef PubMed Google Scholar
-
130.Nakao Y, Matsunaga S, Fusetani N. Three more cyclotheonamides, C, D, and E, potent thrombin inhibitors from the marine sponge Theonella swinhoei. Biorg Med Chem. 1995;3: 1115-22. CrossRef PubMed Google Scholar
-
131.Sato K, Horibe K, Amano K, et al. Membrane permeabilization induced by discodermin A, a novel marine bioactive peptide. Toxicon. 2001;39: 259-64. CrossRef PubMed Google Scholar
-
132.Li HY, Matsunaga S, Fusetani N. Halicylindramides A-C, antifungal and cytotoxic depsipeptides from the marine sponge Halichondria cylindrata. J Med Chem. 1995;38: 338-43. CrossRef PubMed Google Scholar
-
133.Kobayashi JI, Itagaki F, Shigemori I, Takao T, Shimonishi Y. Keramamides E, G, H, and J, new cyclic peptides containing an oxazole or a thiazole ring from a Theonella sponge. Tetrahedron. 1995;51: 2525-32. CrossRef PubMed Google Scholar
-
134.Araki T, Matsunaga S, Fusetani N. Koshikamide A2, a cytotoxic linear undecapeptide from a marine sponge of Theonella sp. Biosci Biotech Biochem. 2005;69: 1318-22. CrossRef PubMed Google Scholar
-
135.Qureshi A, Colin PL, Faulkner DJ. Microsclerodermins F-I, antitumor and antifungal cyclic peptides from the lithistid sponge Microscleroderma sp. Tetrahedron. 2000;56: 3679-85. CrossRef PubMed Google Scholar
-
136.Rashid MA, Gustafson KR, Cartner LK, Shigematsu N, Pannell LK, Boyd MR. Microspinosamide, a new HIV-inhibitory cyclic depsipeptide from the marine sponge Sidonops microspinosa. J Nat Prod. 2001;64: 117-21. CrossRef PubMed Google Scholar
-
137.de Silva ED, Williams DE, Andersen RJ, Klix H, Holmes FB, Allen TM. Motuporin, A potent protein phosphatase inhibitor isolated from the papua new guinea sponge Theonella swinhoei Gray. Tetrahedron Lett. 1992;33: 1561-4. CrossRef PubMed Google Scholar
-
138.Schmidt EW, Harper MK, Faulkner DJ. Mozamides A and B, cyclic peptides from a Theonellid sponge from Mozambique. J Nat Prod. 1997;60: 779-82. CrossRef PubMed Google Scholar
-
139.Oku N, Gustafson KR, Cartner LK, et al. Neamphamide A, a new HIV-inhibitory depsipeptide from the Papua New Guinea marine sponge Neamphius huxleyi. J Nat Prod. 2004;67: 1407-11. CrossRef PubMed Google Scholar
-
140.Capon RJ, Ford J, Lacey E, Gill JH, Heiland K, Friedel T. Phoriospongin A and B: two new nematocidal depsipeptides from the Australian marine sponges Phoriospongia sp. and Callyspongia bilamellata. J Nat Prod. 2002;65: 358-63. CrossRef PubMed Google Scholar
-
141.Gulavita NK, Gunasekera SP, Pomponi SA, Robinson EV. Polydiscamide A: a new bioactive depsipeptide from the marine sponge Discodermia sp. J Org Chem. 1992;57: 1767-72. CrossRef PubMed Google Scholar
-
142.Feng Y, Carroll AR, Pass DM, Archbold JK, Avery VM, Quinn RJ. Polydiscamides B-D from a marine sponge Ircinia sp. as potent human sensory neuron-specific G protein coupled receptor agonists. J Nat Prod. 2008;71: 8-11. CrossRef PubMed Google Scholar
-
143.Hamada T, Matsunaga S, Yano G, Fusetani N. Polytheonamides A and B, highly cytotoxic, linear polypeptides with unprecedented structural features, from the marine sponge, Theonella swinhoei. J Am Chem Soc. 2005;127: 110-8. CrossRef PubMed Google Scholar
-
144.Nakao Y, Masuda A, Matsunaga S, Fusetani N. Pseudotheonamides, serine protease inhibitors from the marine sponge Theonella swinhoei 1. J Am Chem Soc. 1999;121: 2425-31. CrossRef PubMed Google Scholar
-
145.Roy MC, Ohtani Ⅱ, Ichiba T, Tanaka J, Satari R, Higa T. New cyclic peptides from the Indonesian sponge Theonella swinhoei. Tetrahedron. 2000;56: 9079-92. CrossRef PubMed Google Scholar
-
146.Li S, Dumdei EJ, Blunt JW, Munro MHG, Robinson WT, Pannell LK. Theonellapeptolide IIIe, a new cyclic peptolide from the New Zealand deep water sponge, Lamellomorpha strongylata. J Nat Prod. 1998;61: 724-8. CrossRef PubMed Google Scholar
-
147.Keppel-Hesselink JM, Schatman ME. Rediscovery of old drug: the forgotten case of Dermorphin for postoperative pain and palliation. J Pain Res. 2018;11: 2991-5. CrossRef PubMed Google Scholar
-
148.Misicka A, Lipkowski AW, Horvath R, Davis P, Kramer TH, Hruby VJ. Topographical requirements for delta opioid ligands: common structural features of dermenkephalin and deltorphin. Life Sci. 1992;51: 1025-32. CrossRef PubMed Google Scholar
-
149.Mignogna G, Simmaco M, Kreil G, Barra D. Antibacterial and haemolytic peptides containing D-alloisoleucine from the skin of Bombina variegata. EMBO J. 1993;12: 4829-32. CrossRef PubMed Google Scholar
-
150.Torres AM, Tsampazi C, Geraghty DP, Bansal PS, Alewood PF. D-Amino acid residue in a defensin-like peptide from platypus venom: effect on structure and chromatographic properties. Biochem J. 2005;391: 215-20. CrossRef PubMed Google Scholar
-
151.Freeman MF, Vagstadt AL, Piel J. Polytheonamide biosynthesis showcasing the metabolic potential of sponge-associated uncultivated Entotheonella bacteria. Curr Op Chem Biol. 2016;31: 8-14. CrossRef PubMed Google Scholar
-
152.Aliashkevich A, Alvarez L, Cava F. New insights into the mechanisms and biological roles of D-amino acids in complex eco-systems. Front Microbiol. 2018;9: 683-93. CrossRef PubMed Google Scholar
-
153.Livnat I, Tai HC, Jansson ET, Bai L, Romanova EV, Chen TT, Liu DD, Weiss KR, Jing J, Sweedler JV. A D-amino acid containing neuropeptide discovery funnel. Anal Chem. 2016;88: 11868-76. CrossRef PubMed Google Scholar
-
154.Blin K, Shaw S, Augustijn HE, et al. AntiSMASH 7.0: new and improved predictions for detection, regulation, chemical structures, and visualization. Nucl Acids Res. 2023;51: gkad344. CrossRef PubMed Google Scholar
-
155.Sung YS, Khvalbota L, Dhaubhadel U, Spanik I, Armstrong DW. Teicoplanin aglycone media and carboxypeptidase Y: tools for finding low-abundance D-amino acids and epimeric peptides. Chirality. 2023;35: 449-504. CrossRef PubMed Google Scholar
Copyright information
© The Author(s) 2023
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.