Antifungal alkaloids from Mahonia fortunei against pathogens of postharvest fruit
Abstract
Postharvest pathogens can affect a wide range of fresh fruit and vegetables, including grapes, resulting in significant profit loss. Isoquinoline alkaloids of Mahonia fortunei, a Chinese herbal medicine, have been used to treat infectious microbes, which might be effective against postharvest pathogens. The phytochemical and bioactive investigation of this plant led to the isolation of 18 alkaloids, of which 9 compounds inhibited the growth of Botrytis cinerea and 4 compounds against Penicillium italicum. The antifungal alkaloids could change the mycelium morphology, the total lipid content, and leak the cell contents of B. cinerea. Furthermore, the two most potent antifungal alkaloids, berberine (13) completely inhibited effect on gray mold of table grape at 512 mg L−1, while jatrorrhizine (18) exhibited an inhibition rate > 90% on grape rot at the same concentration, with lower cytotoxicity and residue than chlorothalonil, which suggested that ingredients of M. fortunei might be a low-toxicity, low-residue, eco-friendly botanical fungicide against postharvest pathogens.Graphical Abstract
Keywords
Mahonia fortunei Chemical constituents Botrytis cinerea Penicillium italicum Anti-postharvest pathogens1 Introduction
The major factor affecting the yield of fresh fruit and vegetables is postharvest fungal disease, such as Botrytis cinerea and Penicillium italicum [1], which usually endangers the three critical periods of flowering, maturation and storage of grapes, tomatoes, citrus and other fruit and vegetables. This results in loss of berry shedding and causes rot and loss during storage and transportation [2], accounting for 20–30% of crop losses during postharvest annually and even up to 50% in severity [3]. To date, application of chemical fungicides and low-temperature are widely used in controlling postharvest diseases. However, the characteristics of toxicity to animals, and the difficulty in degrading chemical fungicides have evoked environmental concerns all over the world, and their residues in fruit and vegetables have led to the ban. Furthermore, resistance to fungicides has been widely developed, although fungicides such as dimethylimide can prevent the damage of P. italicum and B. cinerea to vegetables and fruit [4]. A large number of botanical fungicides are generally regarded as green, pollution-free and low-toxic substances, and substitutes of fungicides can be sought from plants to control postharvest diseases of fruit and vegetables [5].
Isoquinoline alkaloids were a group of natural products that were primarily isolated from Papaveraceae, Berberidaceae and Ranunculaceae and were known for their antitumor, antifungal and analgesic activities [6]. Among berberine has antifungal activity against Colletotrichum capsici [7], while sanguinarine and chelerythrine inhibit Alternaria alternate, Curvularia lunata and Valsa mali [8]. Mahonia fortunei, widely distributed in America and Asia, is a traditional Chinese medicine with abundant isoquinoline alkaloids [9, 10]. It had pharmacological effects on Staphylococcus aureus, Candida albicans [11, 12], and Xanthomonas oryzae [13]. However, the lack of reports on its extract and compounds in phytopathogens control encouraged us to test antifungal bioactivity of M. fortunei against P. italicum and B. cinerea. As a result, its extracts showed inhibitory effects on two plant pathogens, and then the bio-guided isolation led to the identification of anti-fungal alkaloids from M. fortunei. Finally, antifungal effects of bioactive isoquinoline were examined in vitro and in vivo and the possible antifungal mechanism was studied.
2 Materials and methods
2.1 Experimental
Column chromatography (CC) was performed with silica gel (200–300 mesh), RP-C18 (40–63 μM) Sephadex LH-20 and Agilent 1260 Infinity Ⅱ (USA) semipreparative RP-C18. Compounds were identified by ESI–MS spectra (Agilent 1290/6545Q-TOF, USA) and NMR spectra (BRUKER Avance Ⅲ, 400 MHz, Switzerland). Ethyl acetate, petroleum ether, ethanol, chloroform, dichloromethane, methanol, acetone, Tween 80 and glucose were obtained from Tianjin Chemical Reagents (Tianjin, China). Chlorothalonil and MTT were obtained from Macklin Reagents (Shanghai, China).
2.2 Fungal pathogen and fruit
Pure cultures of B. cinerea and P. italicum in this study were from the State Key Laboratory for Conservation and Utilization of Bio-Resources in Kunming, Yunnan Province, P. R. China. The fungal strains were stored on potato dextrose agar (Medium) (PDA) slants and kept out of light at 4 ℃. The fungal spore suspension was harvested by submerging the PDA plate with 5 mL of potato dextrose broth (PDB) and filtering, and adjusted to 1 × 105 CFU mL−1 by a hemocytometer. Grapes (Vitis vinifera) with uniform size, maturity and color and no obvious disease were purchased from the fruit supermarket of Yunnan University.
2.3 Plant materials
M. fortunei was purchased from the Wanyao Store, Kunming, Yunnan Province, China. The plant was identified by Professor Li-Xing Zhao from Yunnan University. The specimen (No. YD-281) was deposited in the Key Laboratory of Medicinal Chemistry for Natural Resource, Ministry of Education and Yunnan Province, School of Chemical Science and Technology, Yunnan University, Kunming, P. R. China.
2.4 Extraction and isolation
The pulverized stems of M. fortunei (10 kg) were extracted by refluxing with 75% ethanol solution (2 h × 4) at 60 ℃. The obtained crude extract was diluted in HCl (pH 2–3). Next, the solution was filtered, the pH of the filtrate was adjusted to 9–10 with ammonia water, and it was continuously distributed with ethyl acetate to obtain non-alkaloid, small polar alkaloids and large polar alkaloids moieties [14]. The small polar alkaloids moiety (84 g, MIC B. cinerea = 200 mg/L, MIC P. italicum = 200 mg/L) was chromatographed for analysis using a silica gel column and CHCl3-MeOH gradient (45:1, 30:1, 15:1, 5:1, 0:1 v/v) to obtain seven fractions (Frs. Ⅰ–Ⅶ), the detailed method was presented in the Additional file 1.
2.5 Antifungal activity test in vitro
2.5.1 Inhibition of spore germination
The minimum inhibitory concentrations (MIC) and minimum fungicidal concentrations (MFC) of the compounds were determined by the double dilution method in 96-well plates [15]. Briefly, the initial series concentrations of the components and compounds were added separately, and the volumes were 50 μL. Afterwards, 50 μL of fungal spore suspension (1.0 × 105 CFU mL−1) was added to obtain five concentration gradients of compounds (128, 64, 32, 16, 8, 4, 2 mg L−1). Finally, 96-well plates were cultured in the darkness at 24 ℃ for 48 h, and the spore germination was measured under a microscope. Those without any germination were known as the MIC [16]. An aliquot (10 μL) of each well at concentrations above the MIC was inoculated on PDA and cultured at 24 ℃ for 48 h. The wells without any germination were known as the MFC. A positive control (chlorothalonil), blank control were incubated in PDB containing 1% DMSO [17]. The experiment was repeated 3 times.
2.5.2 Inhibition on the mycelial growth
Two major bioactive compounds berberine (13) and jatrorrhizine (18) were mixed into the PDA culture medium to obtain concentration ranges (512, 256, 128, 64 and 32 mg L−1), and then 5 mL of PDA was evenly transferred into sterilized petri dishes (inner diameter 60 mm) [18]. A mycelium plug (5 mm in diameter) was inoculated on the PDA culture dish in the center of the dish and each group was repeated 3 times. The culture dishes were sealed with paraffin film and cultured in the darkness at 24 ℃ for 48 h. The radial growth of hyphae was measured and the inhibitory effect was expressed as EC50 (50% concentration of the maximum effect). The EC50 value was calculated based on the relationship between the concentrations of the compounds and mycelial radial growth. First and foremost, the following formula was used to determine the relative inhibition rate (%) of mycelium growth [19]:
| $ \mathrm{Mycelial \; growth \; inhibition \; }(\mathrm{\%})=(\mathrm{C}-\mathrm{T})/\mathrm{C}\times 100 $ |
Here C and T were the mean diameter (mm) of mycelia of the blank control and treatment groups, respectively. Next, the inhibition rate was converted into a probability value (Y) and the compound concentrations (X) were log-transformed. Then, linear regression (Y = a + bx) was performed to estimate the coefficient (R2) from the computer-generated compound concentration and relative inhibition plots.
2.5.3 Scanning electron microscopy (SEM)
The spore suspensions of B. cinerea and P. italicum were placed in PDB medium, and cultured for 2 d on a shaker at 24 ℃ and 150 g [20]. The mycelium was collected and washed three times with phosphate buffered solution (PBS, pH = 6.8). Subsequently, compounds were added to treat B. cinerea at concentrations of 0, 1/2 × MIC, 1 × MIC and 2 × MIC, with three replicate wells set. After 120 min of treatment, the mycelium were fixed with 4% paraformaldehyde for 12 h at 4 ℃. Next, the samples were shifted to a string of ethanol solutions (30%, 50%, 70% and 90%) for 15 min every time, and then treated with 100% ethanol for 20 min. Thereafter, the treated mycelium was subjected to vacuum freeze-drying and SEM observation.
2.5.4 Determination of release of cell constituents
The release of cellular components was determined by gauging the absorbance (260 nm) of a B. cinerea suspension [21]. In brief, the mycelium of B. cinerea from 500 μL PDB of three-day-old was collected by centrifugation at 2950 g for 20 min, washed with PBS 3 times, and resuspended in 2 mL PBS. In the presence of 1 × MIC and 2 × MIC compounds, respectively, they were incubated on a shaking table (150 g) at 24 ℃ for 0, 60, 180 and 240 min. Subsequently, 2 mL samples were collected, centrifuged at 12, 000 g for 2 min, and the absorbance at 260 nm was measured.
2.5.5 Determination of total lipid content
The total lipid content of cells was established by the vanillin phosphate method [22]. The mycelium of three-day-old B. cinerea from 25 mL of PDB was collected and incubated with different concentrations (0, 1 × MIC and 2 × MIC) of the compounds at 24 ℃ for 4 h. The mycelium was dried with a vacuum freeze dryer for 8 h, and 0.01 g of the dried mycelium was shaken vigorously in 4.0 mL of methanol-chloroform-water mixed solvent (2:1:0.8, v/v) for 30 min. The lipid-containing lower phase was mixed well with 0.2 mL of the salt solution and centrifuged at 4000 g. Aliquots of 0.1 mL of the lipid mixture were then moved to new tubes, and 0.2 mL of sulfuric acid was added, and heated in a boiling water bath for 10 min. Thereafter, 3 mL of vanillin phosphate was added and reacted for 10 min. The absorbance was measured at 520 nm and the total lipid content was calculated based on the standard curve for cholesterol.
2.6 Protection against B. cinerea on grapes
First, the grapes were soaked in 75% ethanol for 3 min and then washed with distilled water to remove the ethanol residue [23]. Second, the fruit were soaked in compounds including 1% Tween 80 at concentrations of 512, 256, 128, 64 and 32 mg L−1 for 3 min. Finally, a sterile needle was used to puncture three points at the equator of each fruit, and afterwards, 10 μL of the fungal spore suspension diluted to 1.0 × 106 CFU mL−1 was applied to the wound. The treated fruit were stored in a humidified 1 L airtight container at 24 ℃, with 10 fruit per treatment. When the pathogen absolutely overspread the surface of the blank control fruit, the damaged area of the treated fruit were measured with Vernier caliper. The process was performed 3 times. The decay rate was calculated based on the following formula:
| $ \mathrm{Ratio \; of \; decay \; }(\mathrm{mm}^{2})=\pi \times \mathrm{a}\times \mathrm{b} $ |
| $ \mathrm{Inhibition \; rate }\left(\mathrm{\%}\right)=\left(\mathrm{C}-\mathrm{T}\right)/\mathrm{C}\times 100 $ |
Here, a and b represent act the transverse and radial damage radii (mm) of the inoculum, respectively, and C and T represent the average proportion of rotting lesions (mm2) in the control group and treatment groups, respectively.
2.7 Cytotoxicity assay
The cytotoxic effects of compounds on HacaT cells from Yunnan University of Chinese Medicine were detected by the MTT method [24]. The cell line was incubated in DMEM with the addition of fetal bovine serum (10%) and penicillin and streptomycin (1%) containing carbon dioxide (5%) at 37 ℃. A total of 5 × 103 cells/well were added to a 96-well plate for culture for 24 h. Then, 16 mg L−1, 32 mg L−1 compounds and chlorothalonil were added and cultured for 72 h, with three repeat wells. The supernatant was collected by centrifugation at 1500 g for 15 min and incubated with MTT solution (10%) for 3 h. Next, the precipitate was dissolved in DMSO (150 μL) and the absorbance at 595 nm was measured.
2.8 Residue of sample in grapes
Extraction and purification of grape samples was performed by the QuEChERS method [25], with slight modifications. Each grape was treated with 0.2 mL 512 mg L−1 berberine, jatrorrhizine and chlorothalonil. Fifteen gram grape samples were placed into polypropylene centrifuge tubes, 15 mL of acetonitrile and 6 g of MgSO4 were added, and the samples were centrifuged at 5000 g for 3 min. Next, 2 mL of extract and 3 + 1 (w/w) MgSO4-N-propyl ethylenediamine solid phase adsorbent (200 g L−1 extract) were mixed and centrifuged. The final extract was analyzed by LCMS.
3 Results
3.1 Compounds from the stems of M. fortunei
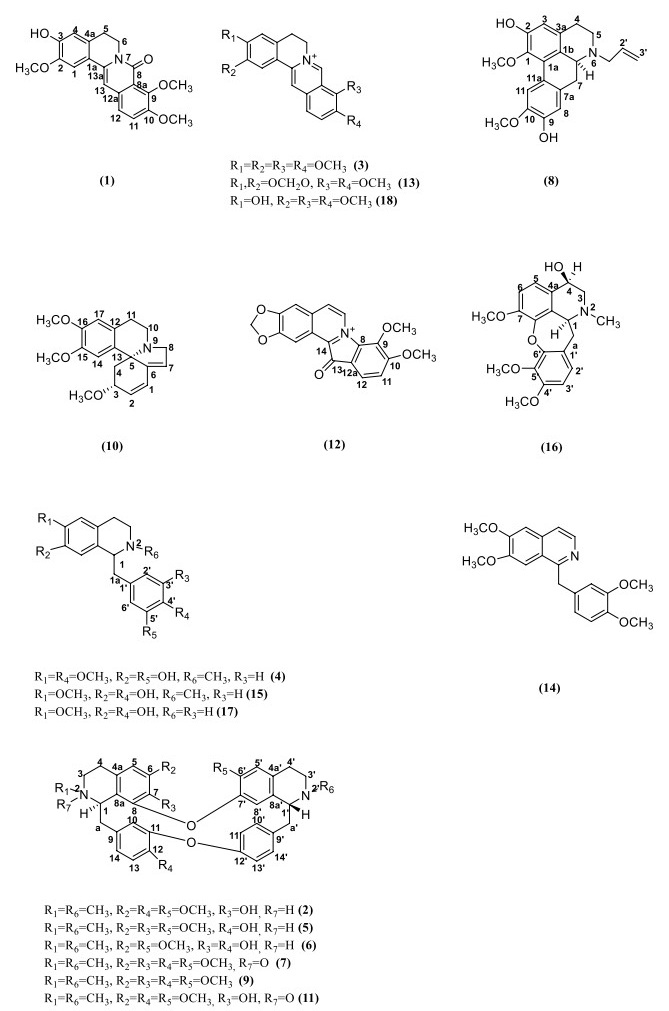
Eighteen known alkaloids sinotumine Ⅰ (1) [26], limacine (2) [27], palmatine (3) [28], reticuline (4) [29], berbamine (5) [30], obamegine (6) [30], fenfangjine A (7) [31], N-allyllaurolitsine (8) [32], isotetrandrine (9) [33], erysotrine (10) [34], fenfangjine P (11) [35], coptisonine (12) [36], berberine (13) [28], papaverine (14) [37], N-methycoclaurine (15) [38], (+)-4-Hydroxysarcocapnine (16) [39], coclaurine (17) [40], and jatrorrhizine (18) [28] were isolated from M. fortunei, and their structures were identified by comparison with the literatures (Fig. 1).
Structure of compounds
3.2 Antifungal assays in vitro
3.2.1 Inhibition of spore germination
The MICs and MFCs were carried out on the anti-B. cinerea and P. italicum bioactivities of all compounds. The results showed that palmatine (3), berbamine (5), berberine (13) and jatrorrhizine (18) inhibited the spore germination of P. italicum. The spore germination of B. cinerea could be inhibited by sinotumine Ⅰ (1), limacine (2), palmatine (3), reticuline (4), berbamine (5), fenfangjine A (7), erysotrine (10), berberine (13) and jatrorrhizine (18). Compounds 1 and 13 had the same MIC and MFC values, which were 16 mg L−1 and 128 mg L−1, respectively. Among them, the anti-B. cinerea bioactivities of compounds 1, 2, 3, 4, 5, 7 and 10 were reported for the first time (Table 1).
The MICs and MFCs of the individual compounds of M. fortunei against spore germination of B. cinerea and P. italicum
3.2.2 Inhibition on the mycelial growth
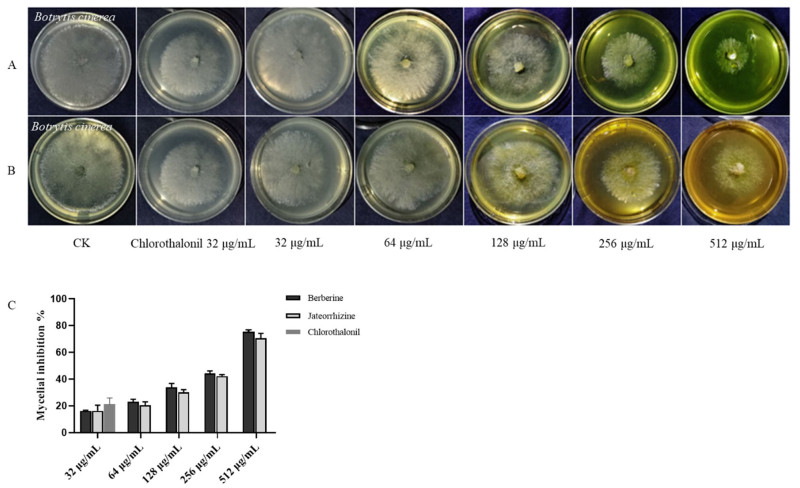
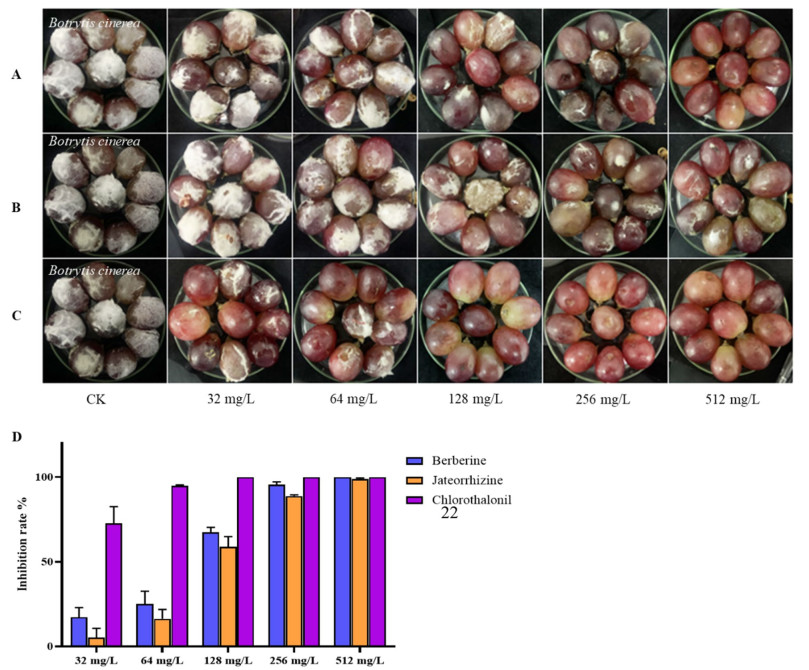
The antifungal activities and EC50 values of the compounds against B. cinerea in vitro were shown in Fig. 2 and Table 2. The compounds against hyphal growth of B. cinerea presented a quantity-effect relationship. At a concentration of 32 mg L−1, berberine (13) and jatrorrhizine (18) had similar inhibitory effects on mycelial growth, and the inhibition rate was only approximately 15%. However, at 512 mg L−1, the inhibition rate of berberine was 75%.
The compounds inhibited mycelial growth of B. cinerea. Treatment with (A) berberine; (B) jateorrhizine; (C) inhibition rate of mycelia growth
EC50 values of the compounds inhibited mycelial growth of B. cinerea
3.2.3 Scanning electron microscopy (SEM)
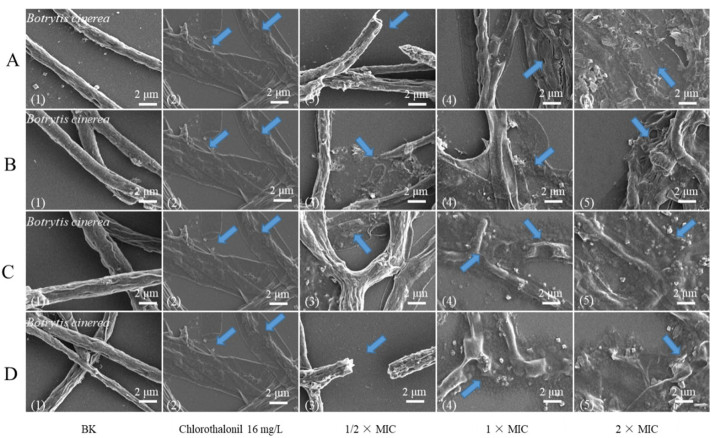
The mycelia of B. cinerea and P. italicum were treated with the active compounds at concentrations of 1/2 × MIC, 1 × MIC and 2 × MIC for 3 h, respectively (Fig. 3). The morphological changes of mycelium of P. italicum were shown in the Additional file 1. The untreated mycelium surface of B. cinerea (Fig. 3A1, D1) presents a typical linear uniform, complete, smooth and regular surface. After contact with 1/2 × MIC jateorrhizine (16 mg L−1) and berberine (8 mg L−1) (Fig. 3B3, FC3), most hyphae became irregular and shriveled. Similarly, after treatment with the concentration of 2 × MIC sinotumine Ⅰ (32 mg L−1) (Fig.3A5), the morphology of fungal hyphae changed and deformed obviously, even leading to leakage of contents. After exposure to the positive control drug chlorothalonil at 16 mg L−1 (Fig. 3A2), the mycelium morphology of fungi became concave and irregular and contracted. This result indicated that the fungal toxicity of these compounds may be achieved by destroying cell membranes and further blocking cell growth.
SEM photos of B. cinerea untreated mycelium (A1–D1); mycelia treated with sinotumine Ⅰ (A), reticuline (B), berberine (C) and jatrorrhizine (D) at 1/2 × MIC, 1 × MIC, 2 × MIC (A3–D5); mycelium treated with chlorothalonil at 16 mg L−1 (A2–D2). The blue arrow represents the observed morphological change
3.2.4 Determination of release of cell constituents and total lipid content
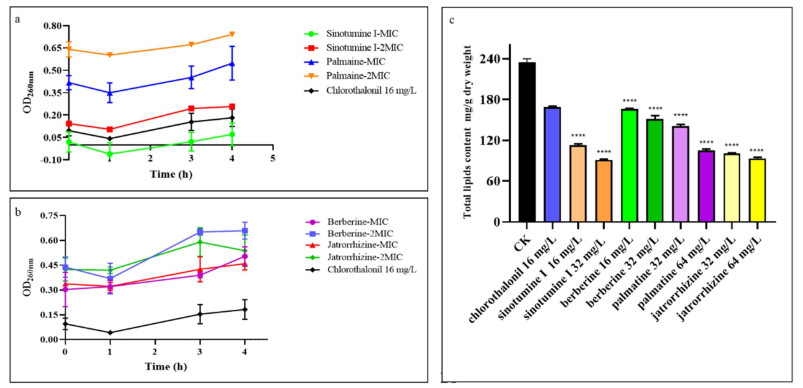
Although the OD260 of the suspension treated with active compounds decreased slightly after 60 min of exposure, the absorbance of the suspension changed significantly in the subsequent cell treatment. The OD260 of the suspension treated with jatrorrhizine at 1 × MIC (32 mg L−1) for 180 min was 0.43, which was obviously higher than that of the control group (0.34) (Fig. 4b). After 180 min of exposure, the OD260 values of sinotumine Ⅰ at 2 × MIC (32 mg L−1) and palmatine at 1 × MIC (32 mg L−1) were 0.24 and 0.45, respectively, slightly higher than those of the control group (0.14 and 0.42). After positive chlorothalonil treatment for 180 min, the OD260 value was 0.15, slightly higher than that of the control group (0.09).
Effects of compounds at MIC and 2 MIC on the release of 260 nm absorption substances of B. cinerea treated with sinotumine Ⅰ and palmatine (a); berberine and jatrorrhizine (b); total lipid content of B. cinerea cells in the presence of different concentrations of the compounds sinotumine Ⅰ, palmatine, berberine and jatrorrhizine (c). Data displayed were the average value of the aggregated data. ****P < 0.0001 versus CK
The effect of the compounds on the total lipid content of B. cinerea was shown in Fig. 4c After incubation with compound sinotumine Ⅰ (1) at 1 × MIC (16 mg L−1) for 4 h, the total lipid content was 112.7 mg g−1 dry weight, which was remarkably lower than that of the control group (234.9 mg g−1 dry weight) and chlorothalonil treatment group (168.9 mg g−1 dry weight). The total lipid content (151.2 mg g−1 dry weight) of the cells after B. cinerea exposure to berberine (13) at 2 × MIC (32 mg L−1) was also lower than that in the control group. Palmatine (3) and jatrorrhizine (18) both reduced the cell lipid content after treatment with B. cinerea cells.
3.3 Protection against B. cinerea on grapes
The antifungal effects of the alkaloid moiety of M. fortunei on grapes were shown in the Additional file 1. With berberine (13) at 128 mg L−1, there was a 50% reduction in the decay of grapes compared with untreated samples. At 512 mg L−1, berberine completely inhibited grapes rot. Jatrorrhizine (18) produced a 99% reduction in the decay of grapes at 512 mg L−1 (Fig. 5). This meant that berberine and jatrorrhizine could inhibit gray mold on grapes.
Prevention of against B. cinerea of grapes with two compounds after 10 d. Treatment with (A) berberine; (B) jateorrhizine; (C) chlorothalonil; (D) inhibition rate on grape rot
3.4 Residue of sample in grapes
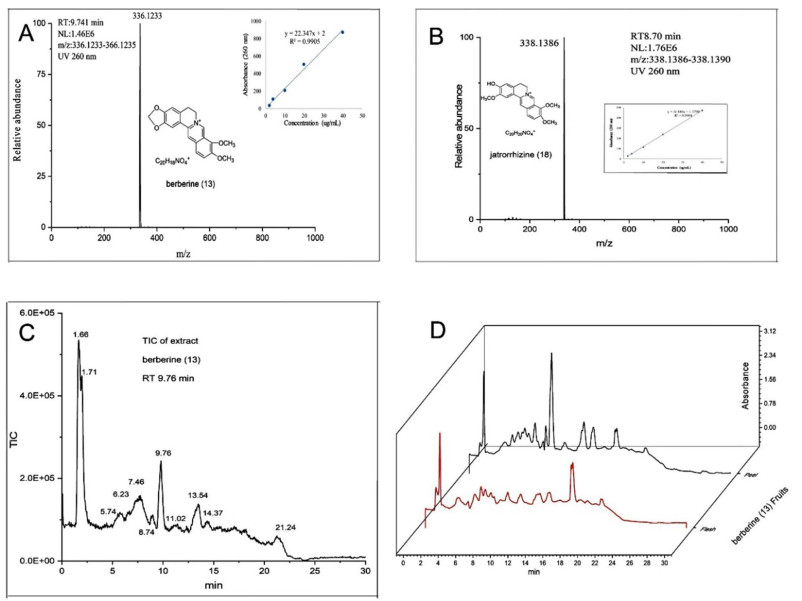
The residues of grapes treated with berberine and jatrorrhizine and chlorothalonil as positive controls were detected by LC–MS (Fig. 6, Table 3). There was almost no residue in the peel and flesh of grape treated with jatrorrhizine, and the residue was 0.004% (the residue was the total residual as a percentage of the total amount used). Berberine residue was found in both grape peel and grape flesh, but its toxicity was relatively low. The residue of chlorothalonil on grape peel was 0.35%, much higher than the jatrorrhizine residue.
UHPLC/ESI Q-Orbitrap MS chromatograms and spectra: qualitative compounds by LC–MS characteristics berberine (A); jatrorrhizine (B); total ion chromatogram of crude fruit extracts (C); UV (260 nm) absorption curve of crude fruit extracts (D)
HPLC/MS detection sample residue experiment
4 Discussion
One of the most potent bioactive compounds berberine, produced on a large scale in industry with antibacterial, anti-cancer [41], anti-inflammatory [42] and obesity prevention bioactivity [43], showed antifungal bioactivity in vitro against B. cinerea [44]. Now, the protective effect against B. cinerea on grapes of berberine and jatrorrhizine in vivo further verified their prevention and treatment of postharvest gray mold for the first time. Moreover, one of the main advantages of M. fortunei is that its residue on the product is very low and has low toxicity, which will facilitate the development of alternatives to synthetic fungicides. And then SEM micrographs indicated that the bioactive alkaloids of M. fortunei could alter cellular morphology and destroy membrane of B. cinerea, and the hyphae even collapsed and fractured after incubation with higher concentrations of bioactive alkaloids. In fact, the integrity of the cytoplasmic membrane is the key factor in fungal growth, material exchange and information transmission between cells and the outside world, as well as in maintaining the stability of the intracellular environment [45]. Therefore, damage to the membrane can lead to the leakage of cell contents [46]. Measuring the release of cellular material is an indicator of cell lysis, and significant leakage of cytoplasmic matter often demonstrates severe and irreversible impairment to the cytoplasmic membrane and plasma membrane [47]. The decrease in lipid content also suggests that membrane stability may be reduced [48]. Here, an obvious leakage of the contents of the mycelia was observed after treatment. Further exposure of B. cinerea to the bioactive alkaloids resulted in a decrease in lipid content, the main component of biofilm. Therefore, we infer that it may cause damage to the cell membrane system and show antifungal activity.
Abbreviations
CC:
Column chromatography
MIC:
Minimum inhibitory concentration
MFC:
Minimum fungicidal concentration
PBS:
Phosphate buffered solution
SEM:
Scanning electron microscopy
TIC:
Total ion chromatograms
PDA:
Potato dextrose agar
PDB:
Potato dextrose broth
MTT:
3-(4,5-Dimethylthiazole-2-yl)-2,5-diphenyltetrazolium-bromide
NMR:
Nuclear magnetic resonance spectroscopy
MPLC:
Medium-pressure liquid chromatography
Notes
Acknowledgements
This work was supported by NSFC (32170405), the High-level Talent Promotion and Training Project of Kunming (2022SCP003), Project of Yunnan Characteristic Plant Screening and R & D Service CXO Platform (2022YKZY001), and Scientific and Technological Innovation Team of Yunnan Province(202105AE160006). We thank the Advanced Analysis and Measurement Center of Yunnan University for technical support.
Author contributions
X-DL conceived the projects, revised the manuscript and provided financial support. X-NW finished the isolation and identification studies; X-NW, Z-JW, YZ, HW and M-LX implemented the antifungal studies; Y-YL completed cytotoxic experiment; X-DL and L-XZ revised the manuscript; All authors read and approved the final manuscript.
Funding
NSFC, 32170405, Xiao-Dong Luo, the High-level Talent Promotion and Training Project of Kunming, 2022SCP003, Xiao-Dong Luo, Project of Yunnan Characteristic Plant Screening and R & D Service CXO Platform, 2022YKZY001, Xiao-Dong Luo, Scientific and Technological Innovation Team of Yunnan Province, 202105AE160006, Xiao-Dong Luo.
Data availability
Relevant data from this study had been submitted to the Science Data Bank. (DOI:10.57760/sciencedb.06816).
Declarations
Competing interests
The authors declare no conflicts of interest regarding the publication of this paper.
References
-
1.An B, Li BQ, Li H, Zhang ZQ, Qin GZ, Tian SP, Aquaporin8 regulates cellular development and reactive oxygen species production, a critical component of virulence in Botrytis cinerea[J]. New Phytol. 209(4), 1668-80 (2016) CrossRef PubMed Google Scholar
-
2.Shen Y, Yang H, Effect of preharvest chitosan-g-salicylic acid treatment on postharvest table grape quality, shelf life, and resistance to Botrytis cinerea-induced spoilage[J]. Sci Hortic. 224, 367-73 (2017) CrossRef PubMed Google Scholar
-
3.Shao X, Cheng S, Wang H, Yu D, Mungai C, The possible mechanism of antifungal action of tea tree oil on Botrytis cinerea[J]. J Appl Microbiol. 114(6), 1642-9 (2013) CrossRef PubMed Google Scholar
-
4.Yourman LF, Jeffers SN, Dean RA, Genetic analysis of isolates of Botrytis cinerea sensitive and resistant to benzimidazole and dicarboximide fungicides[J]. Phytopathology. 90(8), 851-9 (2000) CrossRef PubMed Google Scholar
-
5.Breda CA, Gasperini AM, Garcia VL, Monteiro KM, Bataglion GA, Eberlin MN, Duarte MCT, Phytochemical analysis and antifungal activity of extracts from leaves and fruit residues of Brazilian savanna plants aiming its use as safe fungicides[J]. Nat Prod Bioprospect. 6(4), 195-204 (2016) CrossRef PubMed Google Scholar
-
6.Shang XF, Yang CJ, Morris-Natschke SL, Li JC, Yin XD, Liu YJ, Guo X, Peng JW, GoTo M, Zhang JY, Lee KH, Biologically active isoquinoline alkaloids covering 2014–2018[J]. Med Res Rev. 40, 1-78 (2020) CrossRef PubMed Google Scholar
-
7.Li Y, Wei J, Yang H, Dai J, Ge X, Molecular dynamics investigation of the interaction between Colletotrichum capsici cutinase and berberine suggested a mechanism for reduced enzyme activity[J]. PLoS ONE. 16(2), e0247236 (2021) CrossRef PubMed Google Scholar
-
8.Yang R, Gao ZF, Zhao JY, Li WB, Zhou L, Miao F, New class of 2-aryl-6-chloro-3, 4-dihydroisoquinolinium salts as potential antifungal agents for plant protection: synthesis, bioactivity and structure-activity relationships[J]. J Agric Food Chem. 63(7), 1906-14 (2015) CrossRef PubMed Google Scholar
-
9.Jae-Cheon S, Margarita PA, A new species of Atemelia (Lepidoptera, Yponomeutoidea, Praydidae) feeding on the ornamental shrub Mahonia (Ranunculales: Berberidaceae) in Chile[J]. Ann Entomol Soc Am. 107(2), 339-46 (2014) CrossRef PubMed Google Scholar
-
10.Li AR, Zhu Y, Li XN, Tian XJ, Antimicrobial activity of four species of Berberidaceae[J]. Fitoterapia. 78(7), 379-81 (2007) CrossRef PubMed Google Scholar
-
11.Pfoze NL, Kumar Y, Myrboh B, Bhagobaty RK, Joshi SR, In vitro antibacterial activity of alkaloid extract from stem bark of Mahonia manipurensis Takeda[J]. J Med Plant Res. 5(5), 859-61 (2011) PubMed Google Scholar
-
12.Slobodníková L, KoSt'álová D, Labudová D, Kotulová D, Kettmann V, Antimicrobial activity of Mahonia aquifoliumcrude extract and its major isolated alkaloids[J]. Phytother Res. 18(8), 674-6 (2004) CrossRef PubMed Google Scholar
-
13.Yuan GQ, Chen YY, Li FJ, Zhou RJ, Li QQ, Lin W, Huang LH, Isolation of an antibacterial substance from Mahonia fortunei and its biological activity against Xanthomonas oryzae pv. oryzicola[J]. J Phytopathol. 165(5), 289-96 (2017) CrossRef PubMed Google Scholar
-
14.Cai XH, Tan QG, Liu YP, Feng T, Du ZZ, Li WQ, Luo XD, A cage-monoterpene indole alkaloid from Alstonia scholaris[J]. Org Lett. 10(4), 577-80 (2008) CrossRef PubMed Google Scholar
-
15.Wang Z, Bai H, Lu C, Hou C, Qiu Y, Zhang P, Duan J, Mu H, Light controllable chitosan micelles with ROS generation and essential oil release for the treatment of bacterial biofilm[J]. Carbohyd Polym. 205, 533-9 (2018) CrossRef PubMed Google Scholar
-
16.Danac R, Mangalagiu II, Antimycobacterial activity of nitrogen heterocycles derivatives: bipyridine derivatives. Part Ⅲ [13,14][J]. Eur J Med Chem. 74, 664-70 (2014) CrossRef PubMed Google Scholar
-
17.Benincasa M, Zahariev S, Pelillo C, Milan A, Gennaro R, Scocchi M, PEGylation of the peptide Bac7(1–35) reduces renal clearance while retaining antibacterial activity and bacterial cell penetration capacity[J]. Eur J Med Chem. 95, 210-9 (2015) CrossRef PubMed Google Scholar
-
18.Wang Y, Liu X, Chen T, Xu Y, Tian S, Antifungal effects of hinokitiol on development of Botrytis cinerea in vitro and in vivo[J]. Postharvest Biol Technol. 159, 111038 (2020) CrossRef PubMed Google Scholar
-
19.Zhao Y, Yang YH, Ye M, Wang KB, Su FW, Chemical composition and antifungal activity of essential oil from Origanum vulgare against Botrytis cinerea[J]. Food Chem. 365(2), 130506 (2021) CrossRef PubMed Google Scholar
-
20.Zhao Y, Qin XJ, Wang ZJ, Jin Q, Wang XN, Chen SS, Luo XD, Amphotericin B and 5-flucytosine as fungicides against Penicillium italicum for citrus fruit rot[J]. Postharvest Biol Technol. 193, 112058 (2022) CrossRef PubMed Google Scholar
-
21.Paul S, Dubey RC, Maheswari DK, Kang SC, Trachyspermum ammi (L.) fruit essential oil influencing on membrane permeability and surface characteristics in inhibiting food-borne pathogens[J]. Food Control. 22(5), 725-31 (2011) CrossRef PubMed Google Scholar
-
22.Helal GA, Sarhan MM, Abu Shahla ANK, Abou El-Khair EKA, Effects of Cymbopogon citratus L. essential oil on the growth, lipid content and morphogenesis of Aspergillus niger ML2-strain[J]. J Basic Microbiol. 46(6), 456-69 (2010) CrossRef PubMed Google Scholar
-
23.Xu D, Deng Y, Han T, Jiang L, Xi P, Wang Q, Jiang Z, Gao L, In vitro and in vivo effectiveness of phenolic compounds for the control of postharvest gray mold of table grapes[J]. Postharvest Biol Technol. 139, 106-14 (2018) CrossRef PubMed Google Scholar
-
24.Lu YH, Chen MC, Liu F, Xu Z, Tian XT, Xie Y, Huang CG, Synthesis and cytotoxic activity of novel C-23-modified asiatic acid derivatives[J]. Molecules. 25(16), 3709 (2020) CrossRef PubMed Google Scholar
-
25.Wang J, Chow W, Leung D, Chang J, Application of ultrahigh-performance liquid chromatography and electrospray ionization quadrupole orbitrap high-resolution mass spectrometry for determination of 166 pesticides in fruits and vegetables[J]. J Agric Food Chem. 60(49), 12088-104 (2012) CrossRef PubMed Google Scholar
-
26.Lyu HN, Zeng KW, Cao NK, Zhao MB, Jiang Y, Tu PF, Alkaloids from the stems and rhizomes of Sinomenium acutum from the Qinling Mountains, China[J]. Phytochemistry. 156, 241-9 (2018) CrossRef PubMed Google Scholar
-
27.Lin LZ, Shieh HL, Angerhofer CK, Pezzuto JM, Ruangrungsi N, Cytotoxic and antimalarial bisbenzylisoquinoline alkaloids from Cyclea barbata[J]. J Nat Prod. 56, 22-9 (1993) CrossRef PubMed Google Scholar
-
28.Cheng X, Wang D, Jiang L, Yang D, DNA topoisomerase I inhibitory alkaloids from Corydalis saxicola[J]. Chem Biodivers. 5(7), 1335-44 (2008) CrossRef PubMed Google Scholar
-
29.Cruz PEOD, Costa EV, Moraesa VRDS, Nogueiraa PCDL, Vendraminb ME, Chemical constituents from the bark of Annona salzmannii (Annonaceae)[J]. Biochem Syst Ecol. 39, 872-5 (2011) CrossRef PubMed Google Scholar
-
30.Hostalkova A, Marikova J, Opletal L, Korabecny J, Hulcova D, Kunes J, Novakova L, Perez DI, Jun D, Kucera T, Andrisano V, Siatka T, Cahlikova L, Isoquinoline alkaloids from Berberis vulgaris as potential lead compounds for the treatment of Alzheimer's disease[J]. J Nat Prod. 82(2), 239-48 (2019) CrossRef PubMed Google Scholar
-
31.Ogino T, Sato T, Sasaki H, Chin M, Mitsuhashi H, Four new bisbenzylisoquinoline alkaloids from the root of Stephania tetrandra (Fen-Fang Ji)[J]. Heterocycles. 27(5), 1149-54 (1988) CrossRef PubMed Google Scholar
-
32.Sulaiman SN, Mukhtar MR, Hadi AH, Awang K, Hazni H, Zahari A, Litaudon M, Zaima K, Morita H, Lancifoliaine, a new bisbenzylisoquinoline from the bark of Litsea lancifolia[J]. Molecules. 16(4), 3119-27 (2011) CrossRef PubMed Google Scholar
-
33.Tanahashi T, Su Y, Nagakura N, Nayeshiro H, Quaternary isoquinoline alkaloids from Stephania cepharantha[J]. Chem Pharm Bull. 48(3), 370-3 (2000) CrossRef PubMed Google Scholar
-
34.Chawla AS, Chunchatprasert S, Jackson AH, Studies of erythrina alkaloids: Ⅶ-13C NMR spectral studies of some erythina alkaloids[J]. Organ Magn Reson. 21(1), 39-41 (1983) CrossRef PubMed Google Scholar
-
35.Wang R, Liu Y, Shi G, Zhou J, Yu D, Bioactive bisbenzylisoquinoline alkaloids from the roots of Stephania tetrandra[J]. Bioorg Chem. 98, 103697 (2020) CrossRef PubMed Google Scholar
-
36.Yang TC, Chao HF, Shi LS, Chang TC, Lin HC, Chang WL, Alkaloids from Coptis chinensis root promote glucose uptake in C2C12 myotubes[J]. Fitoterapia. 93, 239-44 (2014) CrossRef PubMed Google Scholar
-
37.Janssen RHAM, Lousberg RJJC, Wijkens P, Kruk C, Theuns HG, Assignment of 1H and 13C NMR resonances of some isoquinoline alkaloids[J]. Phytochemistry. 28(10), 2833-9 (1989) CrossRef PubMed Google Scholar
-
38.Lee JK, Cho JG, Song MC, Yoo JS, Lee DY, Yang HJ, Han KM, Kim DH, Oh YJ, Jeong TS, Isolation of isoquinoline alkaloids from the tuber of Corydalis turtschaninovii and their inhibition activity on low density lipoprotein oxidation[J]. J Korean Soc Appl Biol Chem. 52(6), 646-54 (2009) CrossRef PubMed Google Scholar
-
39.Tojo E, Dominguez D, Castedo L, Alkaloids from Sarcocapnos-Enneaphylla[J]. Phytochemistry. 30(3), 1005-10 (1991) CrossRef PubMed Google Scholar
-
40.Lei Y, Wu LJ, Bi D, Sun JW, Tu PF, Isolation and identification of chemical constituents from stems of Miliusa balansae Fin. et Gag[J]. J Shenyang Pharm Univ. 26, 104-7 (2009) PubMed Google Scholar
-
41.Imenshahidi M, Hosseinzadeh H, Berberis vulgaris and berberine: an update review[J]. Phytother Res. 30, 1745-64 (2016) CrossRef PubMed Google Scholar
-
42.Hu Y, Chen X, Duan H, Hu Y, Mu X, Chinese herbal medicinal ingredients inhibit secretion of IL-6, IL-8, E-selectin and TXB2 in LPS-induced rat intestinal microvascular endothelial cells[J]. Immunopharmacol Immunotoxicol. 31(4), 550-5 (2009) CrossRef PubMed Google Scholar
-
43.Park HJ, Jung EY, Shim I, Berberine for appetite suppressant and prevention of obesity[J]. Biomed Res Int. 2020(4), 1-7 (2020) PubMed Google Scholar
-
44.Zhao ZM, Shang XF, Lawoe R, Liu YQ, Yang CJ, Anti-phytopathogenic activity and the possible mechanisms of action of isoquinoline alkaloid sanguinarine[J]. Pestic Biochem Physiol. 159, 51-8 (2019) CrossRef PubMed Google Scholar
-
45.Fisher K, Phillips C, Potential antimicrobial uses of essential oils in food: is citrus the answer[J]. Trends Food Sci Technol. 19(3), 156-64 (2008) CrossRef PubMed Google Scholar
-
46.Liu K, Zhou X, Fu M, Inhibiting effects of epsilon-poly-lysine (ε-PL) on Pencillium digitatum and its involved mechanism[J]. Postharvest Biol Technol. 123, 94-101 (2017) CrossRef PubMed Google Scholar
-
47.Tao N, Jia L, Zhou H, He X, Effect of octanal on the mycelial growth of Penicillium italicum and P. digitatum[J]. World J Microbiol Biotechnol. 30(4), 1169-75 (2013) PubMed Google Scholar
-
48.Helal GA, Sarhan MM, Abu Shahla ANK, Abou El-Khair EK, Effects of Cymbopogon citratus L. essential oil on the growth, morphogenesis and aflatoxin production of Aspergillus flavus ML2-strain[J]. J Basic Microbiol. 47, 5-15 (2007) CrossRef PubMed Google Scholar
Copyright information
© The Author(s) 2023
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.