The chemical structures and biological activities of indole diterpenoids
Abstract
Indole diterpenoids (IDTs) are an essential class of structurally diverse fungal secondary metabolites, that generally appear to be restricted to a limited number of fungi, such as Penicillium, Aspergillus, Claviceps, and Epichloe species, etc. These compounds share a typical core structure consisting of a cyclic diterpene skeleton of geranylgeranyl diphosphate (GGPP) and an indole ring moiety derived from indole-3-glycerol phosphate (IGP). 3-geranylgeranylindole (3-GGI) is the common precursor of all IDTs. On this basis, it is modified by cyclization, oxidation, and prenylation to generate a large class of compounds with complex structures. These compounds exhibit antibacterial, anti-insect, and ion channel inhibitory activities. We summarized 204 compounds of IDTs discovered from various fungi over the past 50 years, these compounds were reclassified, and their biological activities were summarized. This review will help to understand the structural diversity of IDTs and provide help for their physiological activities.Graphical Abstract
Keywords
Indole diterpenoids Structural classification Physiological activity Fungus1 Introduction
IDTs are a structurally diverse class of fungal secondary metabolites, all sharing a common core structure consisting of indole, and a diterpene carbon backbone derived from four mevalonate-derived isoprene units [1]. The molecular complexity of these compounds is achieved by adding more isoprene units to the core structure and by various modifications such as oxidation, cyclization, and halogenation. IDTs are ubiquitous in the natural environment, and moldy food produced by Penicillium sp. is a common source of these compounds. For example, the fungus P. tularense found in tomatoes has metabolites such as janthitrems, paspalinine, paxilline, etc. [2]. P. crustosum, which produces the shivering mycotoxin penitrems, is a common food-borne fungus that can cause the spoilage of many foods [3].
Most of the IDTs are potent tremor mammalian mycotoxins [4], that also exhibit excellent biological activities, including cytotoxic, antibacterial, antiviral, and protein tyrosine phosphatase inhibitory activities [5]. To date, some IDT compounds have been used for drug discovery. For example, as BK channel blockers, IDTs have been shown to reduce intraocular pressure and have been used to treat glaucoma [6]. The H1N1 virus is an invasive strain of influenza virus that can cause death in humans, and many IDTs have also shown significant activity against the H1N1 virus, especially emndole SB [7]. In agricultural settings, there has been a general trend toward using tremor-free IDTs as pesticides, such as 20, 25-dihydroxyaflavinine [8].
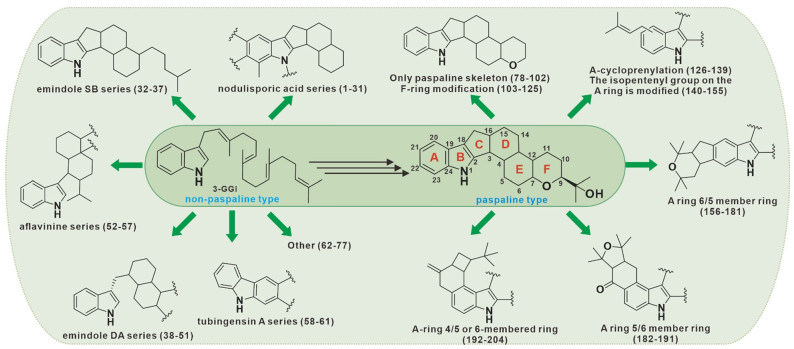
According to previous reports, 3-GGI is a common precursor compound of all IDTs. The origin of the indole ring in its structure was clarified in 1983, when Jesus et al. studied the biosynthesis of penitrem A, the results of isotope labeling experiments showed that the indole part was derived from the IGP precursor of tryptophan[9]. In 2013, Tagami et al. analyzed a pentenyltransfer PaxC in paxilline biosynthetic gene cluster, and proved that indole ring derived from IGP through in vitro enzyme activity experiment [10]. Next, on this basis, IDT can be further divided into two types, namely the paspaline type with a large proportion and a small part of the non-paspaline type. There are some reviews related to IDTs have been presented. For example, the synthesis and activity of paspaline-type compounds [11, 12], the structural diversity and biological activity [5], biosynthesis of IDTs are described [13-15]. However, considering that these reviews do not classify these two types of compounds uniformly, and exclude the cover of the latest IDT compounds in recent years. So here, we renamed the IDT skeleton rings A, B, C, D, E, and F, and 77 non-paspaline skeleton and 127 paspaline skeleton IDTs were uniformly reclassified and summarized according to their structures and oxidative modifications. This review will contribute to the scientific community's comprehensive and compact understanding of the complex and diverse IDTs.
2 Non-paspaline skeleton type
2.1 Nodulisporic acid series
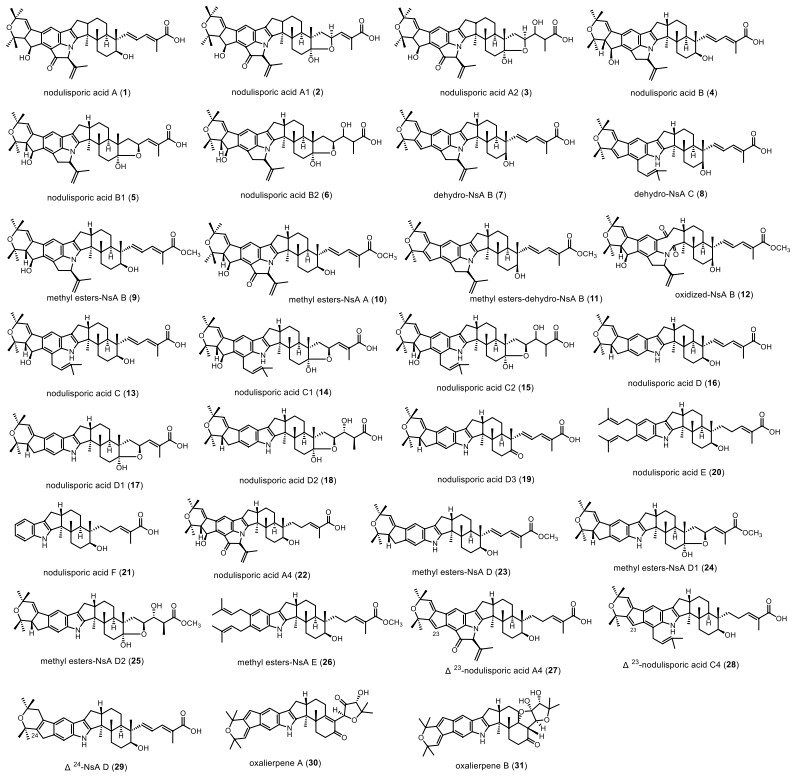
A significant feature of this series is a caproic acid attached to the E ring, which contains 31 kinds of IDT compounds (Fig. 1 and Table 1). Nodulisporic acid A (NsA A, 1) was discovered in Hypoxylon pulicicidum in 1992, and was first reported as a potent insecticide in 1997 [16]. It exhibits optimal activity with an LD90 (lethal dose 90%) of 1.5 μM in the flea assay and an IC50 (half maximal inhibitory concentration) of 0.00027 μM in the binding assay [17]. In 1999, Otto D et al. found the compounds nodulisporic acid A1 (NsA A1, 2) and nodulisporic acid A2 (NsA A2, 3) from Nodulisporium spp., the LD50 (lethal dose 50%) of 2 to green flies was 0.3–1 μg/mL, like compound 1. In the mosquito larvae assay, compound 2 was the strongest with an LD90 of 200 ng/mL [18], while 3 was slightly less active, with an LD50 of 0.6–1.5 μg/mL [19]. From Nodulisporium spp., nodulisporic acid B (NsA B, 4), nodulisporic acid B1 (NsA B1, 5), nodulisporic acid B2 (NsA B2, 6), dehydro-NsA B (7), dehydro-NsA C (8) and derivative-compound 9–12 were found in 2002, in which 4 was 100 fold less active on fleas than 1. 5 is slightly more active than 6. It was also found that while the methyl ester derivative of 11 was tenfold less active than the corresponding acid 1, the activity of 5 and its methyl ester 10 was similar. 10 might be slightly more potent than 4. However, compounds 5, 6, and 12 were inactive at 100 ppm (parts per million) [20]. In 2003, nodulisporic acid C (NsA C, 13), nodulisporic acid C1 (NsA C1, 14), nodulisporic acid C2 (NsA C2, 15) were found. 13 showed good activity against fleas, which was 10 times lower than 1, and the LD90 was 10 μg/ml; but compounds 14 and 15 had no activity in the flea test. The activity of compound 13 was significantly lower in mosquito larvae and fly maggot larvae assays (LD90 = 10, 000 ng/mL) [21]. In 2004, nodulisporic acid D (NsA D, 16), D1 (NsA D1, 17), D2 (NsA D2, 18), D3 (NsA D3, 19), E (NsA E, 20), F (NsA F, 21), A4 (NsA A4, 22) and compound 23–29 were successively discovered in the mutant strain Nodulisporium spp. In the flea assay test, compounds 16, 20, and 21 were 62, 12, and 30 fold less active than 1, respectively. Nodulisporic acid containing a dienoic acid chain showed better activity in its series. For example, 1 is more active than 2 and 3. However, in the NsA D series, the biological activity of 18 is significantly better than that of 16 and 17. No biological activity was detected for compounds 23–29, andΔ23 or 24-nodulisporic acids (27, 28, 29) were less active than corresponding nodulisporic acids of the same class [18]. In 2022, Zhang YH et al. isolated two specific compounds, oxalerpene A and B (30 and 31), from Penicillium oxalicum. 30 is the first IDT derivative with a 4-hydroxy-5, 5-dimethylhydrofuran-3-one in the five-membered side chain. 31 has a unique 6/5/6/6/6/6/6/5/5/5 ring system. Oxalerpene A and B have antiviral activity against H1N1 and respiratory syncytial virus (RSV) with IC50 values from 2.8 to 9.4 µM [22].
Chemical structures of nodulisporic acid series
Name, bioactivities and source of nodulisporic acid series
2.2 Emindole SB series
The emindole SB series is different from the Nodulisporic acid series in that there is no caproic acid on the E ring (Fig. 2 and Table 2). In 1966, the compound emindole SB (32) was isolated from Claviceps paspali, which was cytotoxic to cancer cell lines, and also showed antibacterial activity against Staphylococcus aureus ATCC 6538 and Bacillus subtilis ATCC 6633 [23-25]. In 2010, asporyzin C (33) was isolated from Aspergillus oryzae, and the antibacterial activity against E. coli as well as the antifungal activity against plant pathogens Colletotrichum lagenarium and Fusarium oxysporium were assayed. 33 exhibited intense activity against E. coli with an inhibitory diameter of 8.3 mm [25]. In 2020, the natural product penerpene J (34) was found in the fungus Penicillium sp. KFD28. This compound has inhibitory activity against both PTP1B (protein tyrosine phosphatase 1B) and TCPTP (protein tyrosine phosphatase), with IC50 values of 9.5 μM and 14.7 μM, respectively [26]. In 2021, Chaiyosang B et al. isolated three novel IDTs aculeatupenes A-C (35–37) from the mycelium of Aspergillus aculeatus KKU-CT2. Compounds 35 and 36 showed weak cytotoxicity against HelaS3, KB, HepG2, MCF-7, and A549 cancer cell lines with IC50 values of 11.12–67.81 μM. 37 showed weak cytotoxicity against the HelaS3 cell line with an IC50 value of 17.48 μM, but no cytotoxicity against the vero cell line. Moreover, it was also found to exhibit weak antifungal activity against Bacillus cereus [27].
Chemical structures of emindole SB series
Name, bioactivities and source of emindole SB series
2.3 Emindole DA series
The common feature of this series of compounds is that they contain a 6/6-membered ring linked to a methylene group at the 3-position of the indole ring (Fig. 3 and Table 3). In 1988, the X-ray molecular structures of emindole DA (38) and DB (39) from Emericella desertorum were reported, both of which are tremor toxic to mammals [28, 29]. In 1989, nominine (40) was isolated as the leading organic soluble component of the sclerotium of the fungus Aspergillus nomius NRRL 13, 137, which showed potent activity against the widespread crop pest Heliothis zea. When added to the standard test diet at 100 ppm dry weight, it resulted in 40% mortality and 97% weight loss relative to controls [30]. In 1992, compounds radarin A–D (41–44) were isolated from the fungus Aspergillus sulphureus. When added to a standard test diet of the corn worm Helicoverpa zea at 100 ppm, 41 reduced body weight gain by 52.7% relative to the control after 1 week. 43 also showed some activity at the same concentration, resulting in a 17.1% reduction in body weight gain. While 42 and 44 were inactive. Further biological evaluations were then performed to show that 41 was active against human lung cancer A549, breast cancer MCF7, and colon adenocarcinoma HT-29 cells with ED50 values of 2.5, 5.5, and 1.9 µg/mL, respectively. 42 was active in all three cell lines with ED50 (median effective dose) values of 2.0, 2.0, and 0.7 µg/mL, respectively [31]. In 1992, emeniveol (45) was isolated from Emericella nivea, and when the concentration was 100 mg/L, it could inhibit the germination of pine pollen and the growth of camellia pollen by about 35.5% [32]. In 2006, three IDTs were isolated from the mycelium of Emericella purpurea, namely emindoles PA (46), PB (47), and PC (48), among which 47 has strong anti-cancer activity [33]. Later, it found that its precursor compound preemindole PA (49) [13]. Liu L et al. isolated the compound penicindopene A (50) from Penicillium sp. YPCMAC1 in 2019, that showed moderate cytotoxicity to A549 and HeLa cell lines, with IC50 values of 15.2 and 20.5 µM, respectively [34]. In 2021, the compound penerpenes M (51) was discovered from the fungus Penicillium sp. KFD28. However, no antibacterial activity was found [35].
Chemical structures of emindole DA series
Name, bioactivities and source of emindole DA series
2.4 Aflavinine series
This series difference from the emindole DA series is that the 3-position of the indole ring is directly connected with the 6/6-membered ring (Fig. 4 and Table 4). In 1988, aflavinine (52) and its natural derivative products 20, 25-dihydroxyaflavinine (53), 14-hydroxyflavinine (54), 24, 25-dihydro-10, 11-dihydro-20-hydroxyflavinine (55) and 10, 11-dihydro-11, 12-dihydro-20-hydroxyflavinine (56) were isolated from the fungus Aspergillus flavus, and these metabolites were selectively distributed to the sclerotia, It also showed antifeedant activity to fungus eating insects that usually encounter sclerotia in nature [8]. Compound 52 was non-toxic and non-tremor to 1-day-old chickens at 300 mg/kg. Compounds 54–56 were inactive against C. hemipterus at 100 ppm, but showed significant feeding deterrence when tested at the levels found in the sclerotia (400–1100 ppm). Compounds 53 and 54–56 also showed mild activity against Bacillus subtilis in a standard disk assay of 100 mg/disk, but were not toxic to brine shrimp at 250 mg/ml [36]. In 2019, Han X et al. isolated a new IDT cladosporine A (57) from the extract of the fungal strain Cladosporium sp. JNU17DTH12-9-01, which was the first report of the existence of IDT in Cladosporium spp. The MIC(minimum inhibitory concentration) of this compound to Staphylococcus aureus 209P and Candida albicans FIM 709 was 4 μg/mL and 16 μg/mL, respectively [37].
Chemical structures of aflavinine series
Name, bioactivities and source of aflavinine series
2.5 Tubingensin A series
The structure of this series is characterized by the presence of a benzene ring attached to the indole ring B (Fig. 5 and Table 5). In 1989, tubingensin A (58) and its structural isomer tubingensin B (59) were isolated from the fungus Aspergillus tubingensis by Gloer JB and colleagues, and 58 was found to be resistant to the general crop pest Heliothis zea, and exhibit showed in vitro antiviral activity against herpesvirus type I [38], while 59 showed mild activity against the crop pest H. zea, resulting in a 10% mortality rate when added to a standard diet at 125 ppm. The compound also showed almost identical activity to 58 in assays against herpes simplex virus type I with an IC50 of 9 μg/mL, but was more cytotoxic to HeLa cells (IC50 4 μg/mL) [39]. In 1990, the compound aflavazole (60) was isolated from Aspergillus flavus. When added at 100 ppm to the standard test diet, 60 showed significant feeding-rejecting activity against the fungus-eating beetle Carpophilus hemipterus and was second only to dihydroxyaflavinine in activity against C. hemipterus among the IDT mycorrhizal metabolites of A. flavus [40]. When added to diets at concentrations found in A. flavus sclerotia (200–600 ppm), almost complete feeding deterrence was observed [40, 41]. In 2019, Miles CO and his colleagues isolated the compound shearilicine (61) from the strain Penicillium sp. ZO-R1-1, which had an IC50 value of less than 10 μM against L5178Y or A2780 cells, was tested against the human embryonic kidney cell line HEK-293. The results showed the highest selectivity in tests with SI (selectivity index) values in the range 3.3–8.1 and were also the most active metabolite against L5178Y cells with an IC50 value of 3.6 μM and A2780 cells with an IC50 value of 8.7 μM [42].
Chemical structures of tubingensin A series
Name, bioactivities and source of tubingensin A series
2.6 Other non-paspaline skeleton type compounds
This series contain irregular non-paspaline type compounds (Fig. 6 and Table 6). In 1992, the compound paxinorol (62), isolated from the fungus Penicillium paxilli, which was found to be toxic to mammals, and it reduced the activity behavior of mice, but returned to normal after some time [43]. In the same year, the compound sulpinine C (63) was isolated from Aspergillus sulphureus, which was weakly active against H. zea but inactive against C. hemipterus [44]. In 1992, Gloer JB and his colleagues reported the anti-insect metabolite aspernomine (64) from Aspergillus nomius, which showed moderate activity against H. zea. Incorporating this compound at 100 ppm into the standard test diet resulted in a 35% reduction in body weight gain of the test insects relative to the control. Moreover, it also exhibited cytotoxicity against three human tumor cell lines, with ED50 values of 3.09, 4.93, and 3.08 μg/mL against A-549 lung, MCF-7 breast, and HT-29 colon adenocarcinoma cell lines, respectively [45]. In 1997, petromindole (65) was isolated by Ooike M et al. from the soil fungus Petromyces [46]. In 2002, two anthelmintic IDTs, thiersinine A (66) and B (67), were isolated from an organic extract of P. thiersii NRRL 28, 147, which showed effective activity against S. frugiperda when added to standard test grains at 100 ppm, with growth compared to the control rates were reduced by 83% and 84% respectively. However, they were inactive against both Candida albicans ATCC 90, 029 and Staphylococcus aureus ATCC 29, 213 in the standard assay at 200 μg/plate [47]. In 2010, the natural products asporyzin A (68) and B (69) were isolated from Aspergillus oryzae, where 68 and 69 had lower insecticidal activity than their precursor JBIR-03, and neither of them showed any antifungal activity [25]. In 2010, the IDT JBIR-03 (70) was isolated from the fungus Dichotomyces cejpii var., which showed anti-MRSA (methicillin-resistant Staphylococcus aureus) activity and was tested at 32 and 64 mg/ml, respectively. Inhibits the growth of gram-positive and gram-negative bacteria at a concentration of any cytotoxic activity [48]. In 2013, the compound (6S, 7R, 10E, 14E)-16-(1H-indol-3-yl)-2, 6, 10, 14-tetramethylhexadeca-2, 10, 14-triene-6, 7-diol (71) was isolated from an acid fungal strain Penicillium camemberti OUCMDZ-1492, which showed significant protection against H1N1 virus-induced cytopathic with IC50 values of 34.1 μM, respectively [7]. In 2016, Gao SS et al. discovered the compound rhizovarin D (72) from Rhizomucor Mucor irregularis QEN-189, which represents the most complex member of IDT derivatives [49]. In 2018, Zhao JC et al. isolated a new 1(2), 2(18)-diseco IDT drechmerin H (73) from the fermentation broth of Drechmeria sp. This compound exhibits a significant agonistic effect on the pregnane X receptor (PXR) with an EC50 (concentration for 50% of maximal effect) value of 134.91 ± 2.01 nM [50]. In 2019, the IDT tolypocladin H (74) was isolated from the strain Tolypocladium sp. XL115, the compound is active against the fungus A. fragariae with MIC values of 6.25–50 μg/mL; also active against all bacteria tested, the MIC value is 12.5–25 μg/mL, but no cytotoxicity [51]. In 2020, Nur EAA et al. isolated a new IDT terpendole N (75) from Volutella citrinella BF-0440, but no physiological activity was found [52]. In 2021, the compound penerpene N (76) was identified from the fungus Penicillium sp. KFD28, which represents a second paxilline-type IDT with a 1, 3-dioxane ring, has a low cytotoxic effect on Hela cancer cell lines, and no antimicrobial activity was found [35]. In 2021, the compound ascandinine A (77) was isolated from the Antarctic sponge-derived fungus Aspergillus candidus HDN15-152, which has an unprecedented 2-oxabicyclo [2.2.2]octan-3-ol motif embedded in a pentacyclic system [53].
Chemical structures of other types of compounds
Name, bioactivities and source of other types of compounds
3 Paspaline skeleton type
3.1 Only paspaline skeleton
There are twenty-five IDT compounds containing only the paspaline skeleton (Fig. 7 and Table 7). In 1966, Arigoni D and his colleagues isolated the compound paspaline (78) from Claviceps paspali, which did not cause BK channel inhibition and tremor, but showed stronger anti-proliferative, anti-migratory, and Wnt/β-catenin inhibition than compound emindole SB [23, 54]. In the same year, Sarah et al. isolated the compound paspaline B (79) from the fungus Penicilium paxilli, which was the first oxidized analog of paspaline to be isolated, and also had tremor-causing activity for animals [55]. Paxilline (80) was first isolated from P. paxilli in 1974, and later Cole et al. reported that administration of 25 mg/kg of this compound caused severe intermittent tremors in roosters and mice [56, 57]. In 1989, Miles CO and colleagues discovered the compounds α-paxitriol (81) and β-paxitriol (82), neither of which caused tremors in mice [43]. In 1989, 1'-O-acetylpaxilline (83) was isolated from Emericella striata. When the injection concentration was 3.125 mg/kg, it could cause tremors in mice, and its tremor intensity was the same as that of paxilline. However, at the same time, it can also cause horn arch in mice [29]. In 1989, the compound 13-desoxypaxilline (84) was isolated from Emericella spp., which was active against human A-549 and HL-60 cancer cell lines, but had no antibacterial activity [35, 58]. In 1990, PC-M6 (85) was isolated from P. crustosum. The compound 85 showed moderate inhibitory activity against Staphylococcus aureus ATCC 6538, and also had activity for human gastric cancer cells [35, 59, 60]. In 1994, two compounds, 10β-hydroxy-13-desoxypaxilline (86) and 7α-hydroxy-13-desoxypaxilline (87), were isolated from the fungus P. paxilli, of which 86 showed significant resistance to human A-549 and HL-60 cancer cell lines, and it is the only paspaline-type IDTs that exhibits activity against both cell lines. 87 has tremor activity [61]. In 1995, Tomoda H et al. isolated and characterized terpendoles E (88), F (89), and G (90) from the culture broth of Albophoma yamanashiensis by using different production media [62]. They have a weak inhibitory effect on cholesterol acyltransferase (ACAT) activity, and 88 can be oxidatively modified to desoxyterpendole I (123) [63, 64]. In 1995, Belofsky et al. isolated the compound 7α-hydroxyl-13-dehydroxypaxilline (91) from Eupenicillium Shearii [65], which showed moderate inhibitory activity against Staphylococcus aureus ATCC 6538 and antibacterial activity against Bacillus subtilis ATCC 6633 (MIC = 16 μg/mL), but showed no inhibitory activity against E. coli ATCC 25, 922 and L. monocytogenes ATCC 1911 [35]. In 2009, the compound penijanthine A (92) was isolated from the fungus Penicillium janthinellum, which had no antifungal activity against Aspergillus fumigatus IFM 41, 362, Aspergillus niger IFM 41, 398, Candida albicans ATCC 90, 028 or Cryptococcus neoformans ATCC 90, 112 [66]. In 2013, 4α-demethylpaspaline-4α-carboxylic-acid (93) and 4α-demethylpaspaline-3, 4, 4α-triol (94) were isolated from an acid fungal strain Penicillium camemberti OUCMDZ-1492, and compound 94 was significant protection against H1N1 virus-induced cytopathic in MDCK cells with an IC50 value of 32.2 μM [7]. In 2013, the IDTs 3-deoxo-4β-deoxypaxilline (95) and 2'-hydroxypaxilline (96) were isolated from an acid fungal strain Penicillium camemberti OUCMDZ-1492, and compound 95 exhibited significant protection against H1N1 virus-induced cytopathic with IC50 value of 28.3 μM [7]. In 2014, the IDT 4β-deoxypenijanthine A (97) was isolated from the soil fungus Penicillium sp. CM-7, which showed no activity against human A-549 and HL-60 cancer cell lines [67]. In 2014, the IDT 4β-deoxy-1'-O-acetylpaxilline (98) was isolated from the soil fungus Penicillium sp. CM-7, which showed no effect on human A-549 and HL-60 cancer cell lines [67]. In 2019, during chemical research on the endophyte Penicillium sp. ZO-R1-1 was isolated from the medicinal plant ginger root, and the compounds 7-hydroxypaxilline-13-ene (99) and 7-methoxypaxilline (100) were discovered. Compound 99 showed cytotoxicity with IC50 values in the range of 5.3–8.1 μM [42]. In 2021, the IDT penerpene K (101) was isolated from a fermentation broth produced by adding L-tryptophan to the medium of the fungus Penicillium sp. KFD28. It has inhibitory activity against PTP1B and TCPTP, but has no antibacterial activity and cytotoxicity [35]. In 2021, the compound epi-paxilline (102) was isolated from the marine-derived fungus Penicillium sp., which has inhibitory activity against PTP1B with IC50 values of 31.5 μM, respectively [26, 35].
Chemical structures of paspaline-type compounds with only a paspaline skeleton
Name, bioactivities and source of compounds with only paspaline skeleton
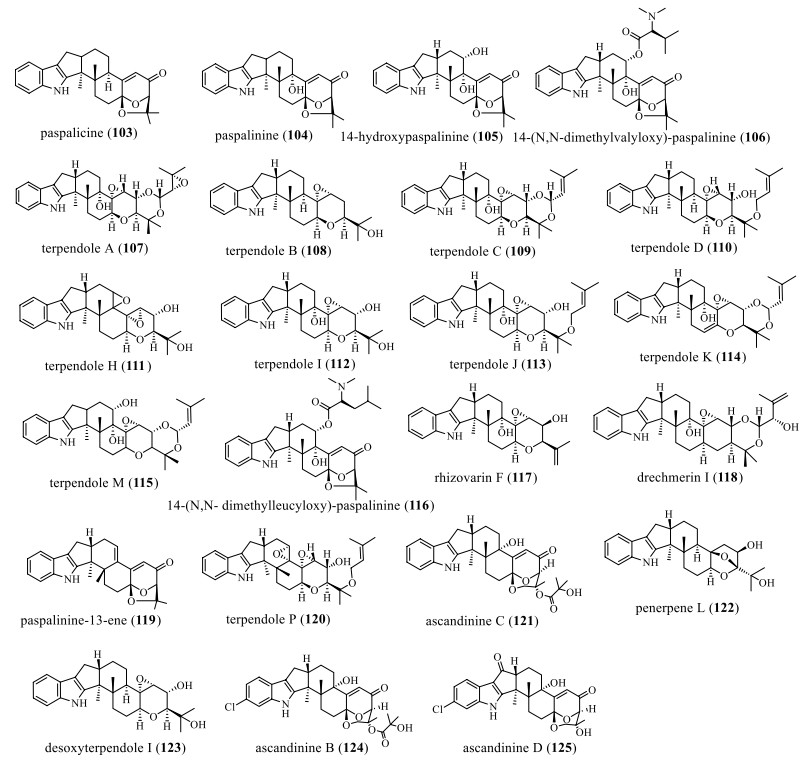
3.2 F-ring modification
Based on the paspaline skeleton, its F-ring was modified by epoxidation (Fig. 8 and Table 8). In 1966, Arigoni D and his colleagues isolated the compound paspalicine (103) from Claviceps paspali, a dehydroxylated analog of paspalinine lacking tremor activity [23]. It can effectively block maxi-K (high-conductance Ca2+-activated K+) channels [24, 54]. In 1980, Gallagher RT et al. discovered the compound paspalinine (104) from Claviceps paspali, a mycotoxin that causes tremors in mice [68]. In 1993, compounds 14-hydroxypaspalinine (105) and 14-(N, N-dimethylvalyloxy)-paspalinine (106) were isolated from the fungus Aspergillus nomius. At 100 ppm levels, the two compounds resulted in a 90% reduction in body weight gain in tests against the corn roundworm H. zea. However, at this concentration, 105 does not have any effect [69]. In 1995, Huang XH et al. isolated and characterized terpendole A (107), B (108), C (109), and D (110) from Albophoma yamanashiensis and found that they showed strong inhibition of ACAT activity [70]. In 1995, Tomoda et al. isolated and characterized terpendoles H–K (111–114) from the culture broth of A. yamanashiensis using different production media [62]. 113 and 114 have moderate inhibitory effects on ACAT activity with IC50 values of 38.8 μm and 38.0 μm in rat liver microsomes, respectively, but 114 has a weaker activity [62-64]. In 1999, terpendole M (115) was isolated from perennial ryegrass (Lolium perenne) infected with the endophytic fungus Neotyphodium lolii. In standard mouse bioassays, this compound was less tremor than 109 [71]. In 2006, Junker et al. isolated and discovered the compound 14-(N, N-dimethylleucyloxy)-paspalinine (116) from Aspergillus alliaceus culture medium by optimizing the culture conditions [72]. In 2016, Gao et al. discovered the compound rhizovarins F (117) from Rhizomucor Mucor irregularis QEN-189 [49]. In 2019, Liang JH and colleagues isolated a new IDT drechmerin I (118) from the fermentation broth of Drechmeria sp., which has antibacterial activity against Bacillus subtilis with a MIC value of 200 μg/mL [73]. In 2019, during chemical research on the endophyte Penicillium sp. ZO-R1-1 isolated from the root of the medicinal plant ginger, paspalinine-13-ene (119), was discovered, which shows cytotoxicity with IC50 values in the range of 5.3–8.1 μM [42]. In 2020, the compound terpendole P (120) was isolated from the culture medium of the fungus Volutella citrinella BF-0440, which has 6 consecutive ring systems and an indole ring and can inhibit sterol O-acyltransferases 1 and 2 (SOAT1 and 2) [52]. In 2021, the compound ascandinine C (121) was isolated from the Antarctic sponge-derived fungus Aspergillus candidus HDN15-152. It is a rare IDT with a 6/5/5/6/6/6/6-fused ring system. The compound 121 has anti-influenza virus A (H1N1) activity with an IC50 value of 26 μM [53]. In 2021, the IDT penerpene L (122) was isolated from a fermentation broth produced by adding L-tryptophan to the medium of the fungus Penicillium sp. KFD28. It has inhibitory activity against PTP1B and TCPTP, but has no antibacterial activity and cytotoxicity [35]. In the same year, the IDTs ascandinine B (124) and D (125) were isolated from the Antarctic sponge-derived fungus Aspergillus candidus HDN15-152. They represent a rare IDT with a 6/5/5/6/6/6/6-fused ring system. Among them, 125 is cytotoxic to HL-60 cells with an IC50 value of 7.8 μM [53].
Chemical structures of paspaline-type compounds with F-ring modification
Name, bioactivities and source of compounds with F-ring modification
3.3 A-ring prenylation
Based on the paspaline skeleton, the modification of isopentenyl was added to the 20, 21 or (and) 22 positions of the A ring of its indole ring (Fig. 9 and Table 9). In 1964, Wilson BJ isolated the compounds ɑ-aflatrem (126) and β-aflatrem (127) from Aspergillus flavus, which are fibrillating mycotoxins with acute neurotoxic effects [74, 75]. In 1977, Cole RJ et al. discovered the compound paspalitrem A (128) from Claviceps paspali, a toxin that can vibrate muscles [76, 77]. In 1992, compounds sulpinine A (129) and B (130) were isolated from Aspergillus sulphureus, both of which were active against H. zea but not against C. hemipterus [44]. Among them, 129 have the most potent activity. When this compound was added to the standard test diet at 100 ppm, a 96.0% reduction in body weight gain compared to the control was noted after one week, and a 10% mortality rate was also observed in this assay. 130 brought a similar weight gain reduction of 87.2%. Moreover, 129 was also cytotoxic to human lung cancer A549, breast cancer MCF7, and colon adenocarcinoma HT-29 cells with ED50 values of 25.7, 58.1, and 3.7 µg/ mL [44, 78]. In 1995, Tomoda H et al. isolated and characterized terpendole L (131) from the culture broth of Albophoma yamanashiensis by using different production media [62]. This compound has a moderate inhibitory effect on ACAT activity with an IC50 value of 32.4 μM in rat liver microsomes [62-64]. In 1996, the first systematic study of the effect of paspalitrem C (132) on the spontaneous contractile activity of a variety of mammalian smooth muscles [79], increased the spontaneous contractility of the bladder and duodenum in guinea pigs and rats, and caused tracheal tension in guinea pigs. These effects are attributed to blocking high conductance, Ca2+-activated K+ channels [77, 79]. In 2007, shearinine K (133) and J (134) were isolated and characterized from the endophytic fungus Penicillium sp. [80]. In 2013, the IDT 21, 22-diprenylpaxilline (135) was isolated from an acid fungal strain Penicillium camemberti OUCMDZ-1492, which exhibits significant protection against H1N1 virus-induced cytopathic in MDCK cells with an IC50 value of 73.3 μM [7]. In 2014, when studying JanD and AmyD protein function, the compound 20, 21-diprenylpaxilline (136) were discovered [13, 81, 82]. In 2019, the isoprene IDT tolypocladin A (137) was isolated from the fungus Tolypocladium sp., which showed no inhibitory activity against three pathogenic fungi (F. oxysporum, A. solani, and R. solani). However, it showed significant inhibitory activity against seven pathogenic fungi (A. fragariae, C. cassiicola, A. alternata, B. cinereal, C. personata, V. dahliae Kleb, and S. sclerotiorum), with MIC values of 6.25–25 μg /mL. It is also active against Bacillus cereus and Staphylococcus aureus, with MIC values of 25 and 12.5 μg/mL, respectively [51]. In 2019, two new prenylated IDTs, namely tolypocladin K (138) and L (139), were isolated from the fungus Tolypocladium sp. XL115. The compound 138 exhibits moderate antifungal activity against S. sclerotiorun, H. maydis, B. cinereal, and C. acutatum with a MIC value of 50 μg/mL [64].
Chemical structures of paspaline-type compounds with A-ring prenylation
Name, bioactivities and source of compounds with A-ring prenylation
3.4 The isopentenyl group on the A ring is modified
The difference from the previous classification is that the isopentenyl group on the A ring is further modified by oxidation, halogenation, or epoxidation (Fig. 10 and Table 10). In 1977, Cole et al. discovered the compound paspalitrem B (140) from Claviceps paspali [76]. Cattle are affected by tremors (also known as "staggering") as they graze on toxic pastures; the compound identified at the highest concentration was the compound 140 (~ 150 mg/kg) in Claviceps cynodontis-infected Cynodon dactylon collected from pastures causing staggered syndrome in South African cattle herds [76, 77]. In 1990, PC-M5 (141) was isolated from Penicillium crustosum, which is toxic to PC12 cells [35, 59, 60]. In 2002, Tsuchiya et al. found the isolated and characterized compound NK12838 (142), which inhibits the activities of SOAT1 and SOAT2 with a SI value (log (IC50 for SOAT1)/(IC50 for SOAT2)) of + 0.27, but has no cytotoxicity [83, 84]. In 2016, while studying the biosynthesis of shearinine, the compound protoshearinine (143) was characterized [85]. In 2018, the compound sespelline (144) was reported while studying the biosynthesis of sespendole [86]. In 2019, new isoprenindole diterpenes tolypocladins B–G (145–150), I (151), and J (152) were isolated from the fungus Tolypocladium sp., they showed no inhibitory activity against three pathogenic fungi (F. oxysporum, A. solani and R. solani). All of them are active against A. fragariae with MIC values of 6.25–50 μg/mL, and compound 145 has weak activity against Staphylococcus aureus [51]. In 2020, the compound terpendole O (153) was isolated from the culture medium of the fungus Volutella citrinella BF-0440, which has 7 consecutive ring systems and an indole ring. It can inhibit sterol SOAT1 and 2 [52]. In 2020, Ohshiro T and colleagues isolated new compounds, termed voluhemins A (154) and B (155), from the culture broth of the fungal strain Volutella citrinella BF 0440. They have a common IDT core and two additional isoprenyl moieties, and 155 are O-methylated 154. 154 can inhibit the activities of SOAT1 and SOAT2 with a SI value of + 0.45, and 155 can selectively inhibit the SOAT2 isoenzyme. However, none of which is cytotoxic [83].
Chemical structures of paspaline-type compounds in which the isopentenyl group on the A-ring is modified
Name, bioactivities and source of compounds with the isopentenyl group on the A-ring is modified
3.5 A-ring with 6/5 member ring
The 21 and 22 positions of the A-ring of the indole ring are modified with diprenyl groups, and then further oxidatively cyclized into a 6/5-membered ring (Fig. 11 and Table 11). In 1984, Jesus et al. isolated and identified the tremor toxin janthitrems E–G (156–158) from the fungus P. janthinellum [87]. In 1992, Wilkins et al. isolated janthitrem B (159) [88]. In 1993, Penn and colleagues isolated and identified the compound janthitrem C (160) [89]. In 1995, compounds shearinines A (161) and B (162) were discovered from the fungus Eupenicillium shearii, both of which showed potent activity against H. zea and Carpophilus hemipterus [65]. 161 also induces apoptosis in human leukemia HL-60 cells, while 162 causes significant mortality in leaf disc assays against Spodoptera frugiperda [65, 90]. In 1995, Belofsky et al. discovered the compound shearinine C (163) from Eupenicillium Shearii, which can be formed from 160 through an autoxidative process, which has anti-insect activity [65]. In 2007, Smetanina OF and colleagues isolated shearinines D(164), E(165), and F(166) from marine-derived strains of the fungus Penicillium janthinellum [90]. 166 inhibits EGF-induced malignant transformation of JB6 P+ Cl 41 cells in soft agar with INCC50 (inhibition of colony number 50) equal to 13 μM concentration. It may be a strongly effective cancer preventive agent in humans or animals. 164 and 165 induce apoptosis in human leukemia HL-60 cells at a concentration of 100 μM. Moreover, the apoptosis rates of apoptotic cells are 39% and 34%, respectively, compared with control cells [80, 90]. In 2007, shearinines D–G was isolated and characterized from the endophytic fungus Penicillium sp., in which shearinine G (167) had inhibitory effects on BK channels [80]. Shearinines H (168) and I (169) were isolated and characterized from the endophytic fungus Penicillium sp. in 2007 [80]. In 2010, the compound epoxy-janthitrems I–IV (170–173) was isolated and identified from the endophyte Epichloë endophytes, and the compound epoxy-Janthitrems produced by the endophyte had strong inhibitory activity against insect larvae. However, when ryegrass plants are grown at a constant low temperatures for a long time, the concentration of the compounds in the plants is significantly reduced, and the insect resistance is less effective [91, 92]. In 2014, the compound pyrapaxilline (174) was isolated from Eupenicillium shearii. Lipopolysaccharide (LPS) increases NO production by approximately 2.5-fold over basal levels. When the mouse macrophage cell line RAW264.7 was pretreated with this compound for 2 h before LPS stimulation, it inhibited NO production by 40% at 10–30 μg/ml with no toxicity [93]. In 2018, new compounds 11, 12-epoxyjanthitrem B and 11, 12-epoxyjanthitrem C were isolated from the fungus Penicillium janthinellum, and named janthitrem A (175) and janthitrem D (176), respectively. Injecting mice with 175 at a concentration of 4 mg/kg can achieve high-intensity tremor effects in 15 min [94]. In 2019, Ariantari NP and colleagues isolated compounds shearinine P (177), 7-methoxyshearinine P (178), and shearinine Q (179) from strain Penicillium sp. ZO-R1-1. Among them, the IC50 value of 179 on L5178Y or A2780 cells is 10 μM [42]. In 2019, during chemical research on the endophyte Penicillium sp. ZO-R1-1 isolated from the medicinal plant ginger root, the compounds 7-methoxypyrapaxilline (180) and pyrapaxilline-6-ene (181) were discovered. Among them, the compound 181 showed cytotoxicity with IC50 values in the range of 5.3–8.1 μM; and also showed significant cytotoxic activity against the A2780 human ovarian cancer cell line, with IC50 values of 5.3–8.7 μM [42].
Chemical structures of paspaline-type compounds with A-ring 6/5 member ring
Name, bioactivities and source of compounds with A-ring 6/5 member ring
3.6 A-ring with 5/6 member ring
The difference from the previous type is that this type is further oxidatively cyclized into a 5/6-membered ring based on the diprenyl modification at the 20 and 21 positions of the A ring of the indole ring (Fig. 12 and Table 12). In 1981, two strong neurotoxins, lolitrems A (182) and B (183), were isolated from herbs that developed a livestock disease known as "ryegrass staggered disease." They can poison livestock with tremors that do not directly impair spatial learning and memory, but reduce voluntary movements in poisoned animals; later, perennial ryegrass toxicosis (PRGT) was prevented by limiting the concentration of 183 [95]. In 1992, lolitriol (184) was found in extracts of endophyte-infected ryegrass leaves and cultures of A. lolii [43]. Moreover, 183 is quickly degraded to compound 184, which does not cause tremors even at 20 mg/kg, so its activity is at least 20-fold lower than 183 [96]. In 1994, Christopher et al. obtained the abundant secondary compound lolitrem E (185) when 183 was purified from ryegrass staggers (RGS), which has intense BK channel activity but no tremor effect in animals [97, 98]. In 1996, Sarah et al. isolated lolitrem F (186), a stereoisomer of the vibratory mycotoxin 183, from ryegrass infected with Acremonium Lolii. The compound 186 was found to have similar potency and duration of action as 183 in standard mouse bioassays, but was slightly less active than 183 [99]. In 1997, the compound lolitrem H (187) was discovered [71]. In 1997, Sarah et al. isolated lolilline (188) from an extract of ryegrass seeds infected with the endophytic fungus Acremonium lolii, which does not have tremor effects [100]. In 1998, lolitrem N (189), lolicine A (190), and B (191) were identified in an extract of perennial ryegrass (Lolium perenne) seeds infected with the endophytic fungus Neotyphodium lolii, and they are lolitrem-like compounds [101].
Chemical structures of paspaline-type compounds with A-ring 5/6 member ring
Name, bioactivities and source of compounds with A-ring 5/6 member ring
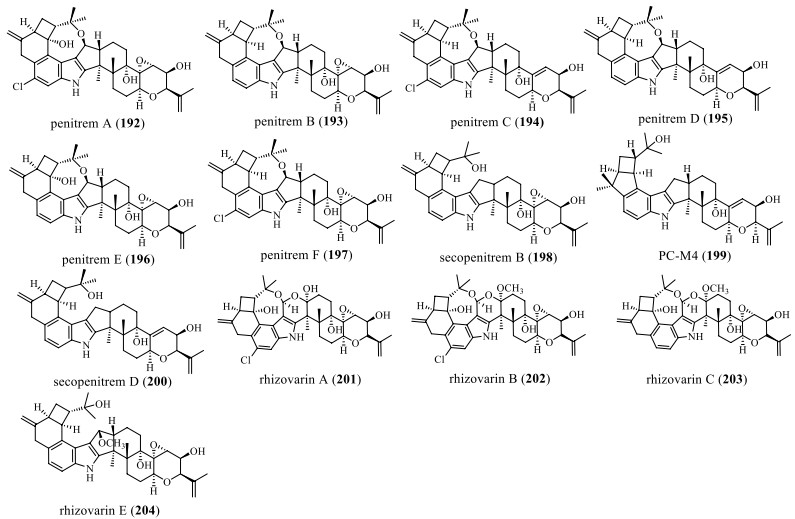
3.7 A-ring 4/5 or 6 membered ring
The difference between this type and the last type is that the oxidative cyclization is modified into a 4/5 or 4/6-membered ring, and even further forms an oxygen-containing 8-membered ring with the 17th position of the C ring (Fig. 13 and Table 13). In 1983, Amelia et al. isolated 6 IDTs penitrems A–F (192–197) from Penicillium crustosum, wherein the compounds 192, 194, and 197 showed the anti-cancer effect on human A-549 and HL-60 cancer cell lines [49, 102]. It was also found that all chlorinated compounds (192, 194, and 197) exhibited more vigorous activity than their chlorine-free analogs, 193, 195, and 196 [24, 49]. In 1992, the compound secopenitrem B (198) was isolated from Aspergillus sulphureus, which was active against H. zea but inactive against C. hemipterus. It reduced weight gain by 87.0%, while 198 also caused 32.0% larval mortality [44]. In 1993, Yamaguchi et al. isolated PC-M4 (199) from P. crustosum, which could be biosynthesized by PC-M6, and then added isoprenyl to give PC-M5, which had no cancer cell activity [59]. In 2011, the compound secopenitrem D (200) was isolated and characterized from P. crustosum, which caused poisoning in animals [103]. In 2016, Gao et al. discovered the compounds rhizovarins A–C (201–203) and E (204) from Rhizomucor Mucor irregularis QEN-189, which represent the most complex members of the IDT derivatives. Among them, 201 and 202 showed activity against human A-549 and HL-60 cancer cell lines, and compound 204 showed activity against the A-549 cancer cell line, but not the Hela cell line [49].
Chemical structures of paspaline-type compounds with A-ring 4/5 or 6 membered ring
Name, bioactivities and source of compounds with A-ring 4/5 or 6 membered ring
4 Conclusion
This paper reviews the chemical structures of IDTs and their derivatives discovered in the past 50 years. Based on previous classifications, we divided 77 non-paspaline compounds into 6 categories according to their structural characteristics, and 127 paspaline-type compounds are divided into 7 categories according to oxidative modification. This provides convenient data for the future discovery of new compounds with similar structures or different oxidative modifications. At the same time, we also summarize the biophysiological activities of these compounds and their strong applications in pharmaceutical and agricultural markets. This also shows more compounds and provides more potent options for the development summary of future market applications.
Notes
Acknowledgements
This work was funded by the National Natural Science Foundation of China (Project No. 22077102 and 21877089), and the Shaanxi Key Laboratory of Natural Product & Chemical Biology Open Foundation (Project No. SXNPCB 2021001).
Author contributions
All authors read and approved the final manuscript.
Declarations
Competing interests
The author declares that there are no conflicts of interest associated with this work.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
-
1.Saikia S, Nicholson MJ, Young C, Parker EJ, Scott B, The genetic basis for indole-diterpene chemical diversity in filamentous fungi[J]. Mycol Res 112, 184-99 (2008) CrossRef PubMed Google Scholar
-
2.Andersen B, Frisvad JC, Natural occurrence of fungi and fungal metabolites in moldy tomatoes[J]. J Agric Food Chem 52, 7507-13 (2004) CrossRef PubMed Google Scholar
-
3.Evans TJ, Gupta RC. Tremorgenic mycotoxins. In: Gupta RC, editor. Veterinary toxicology. Academic; 2018. p. 1033–41. PubMed Google Scholar
-
4.Roll DM, Barbieri LR, Bigelis R, McDonald LA, Arias DA, Li PC, et al, The lecanindoles, nonsteroidal progestins from the terrestrial fungus Verticillium lecanii 6144[J]. J Nat Prod 72, 1944-8 (2009) CrossRef PubMed Google Scholar
-
5.Reddy P, Guthridge K, Vassiliadis S, Hemsworth J, Hettiarachchige I, Spangenberg G, et al, Tremorgenic mycotoxins: structure diversity and biological activity[J]. Toxins (Basel) 11, 302 (2019) CrossRef PubMed Google Scholar
-
6.Garcia ML, Goetz MA, Kaczorowski GJ, Mcmanus OB, Monaghan RL, Strohl WR, et al. U.S. Patent WO-03105724-A2, 13 Jun 2003. PubMed Google Scholar
-
7.Fan Y, Wang Y, Liu P, Fu P, Zhu T, Wang W, et al, Indole-diterpenoids with anti-H1N1 activity from the aciduric fungus Penicillium camemberti OUCMDZ-1492[J]. J Nat Prod 76, 1328-36 (2013) CrossRef PubMed Google Scholar
-
8.Gloer JB, TePaske MR, Sima JS, Wicklow DT, Dowd PF, Antiinsectan aflavinine derivatives from the sclerotia of Aspergillus flavus[J]. J Org Chem 53, 5457-60 (1988) CrossRef PubMed Google Scholar
-
9.Jesus AE, Gorst-Allman CP, Steyn PS, Heerden FR, Vleggaar R, Wessels PL, et al, Tremorgenic mycotoxins from Penicillium crustosum[J]. Biosynthesis of penitrem A. J Chem Soc, Perkin Trans 1(1), 1863-8 (1983) PubMed Google Scholar
-
10.Tagami K, Liu C, Minami A, Noike M, Isaka T, Fueki S, et al, Reconstitution of biosynthetic machinery for indole-diterpene paxilline in Aspergillus oryzae[J]. J Am Chem Soc 135, 1260-3 (2013) CrossRef PubMed Google Scholar
-
11.Kozák L, Szilágyi Z, Tóth L, Pócsi I, Molnár I, Tremorgenic and neurotoxic paspaline-derived indole-diterpenes: biosynthetic diversity, threats and applications[J]. Appl Microbiol Biot 103, 1599-616 (2019) CrossRef PubMed Google Scholar
-
12.Schatz DJ, Kuenstner EJ, George DT, Pronin SV, Synthesis of rearranged indole diterpenes of the paxilline type[J]. Nat Prod Rep 39, 946-68 (2022) CrossRef PubMed Google Scholar
-
13.Liu CW, Minami A, Ozaki T, Oikawa H, Biosynthesis of indole diterpenes. Comprehensive natural products III: chemistry and biology[J]. Elsevier, 446-66 (2022) PubMed Google Scholar
-
14.Minami A, Liu C, Oikawa H, Total biosynthesis of fungal indole diterpenes using cell factories[J]. HETEROCYCLES 92, 397-421 (2016) CrossRef PubMed Google Scholar
-
15.Ozaki T, Minami A, Oikawa H. Biosynthesis of indole diterpenes: a reconstitution approach in a heterologous host. Nat Prod Rep. 2022. https://doi.org/10.1002/chin.201617243. PubMed Google Scholar
-
16.Ondeyka JG, Helms GL, Hensens OD, Singh SB, Etal EA, Nodulisporic acid A, a novel and potent insecticide from a Nodulisporium sp. isolation, structure determination, and chemical transformations[J]. J Am Chem Soc 119, 8809-16 (1997) PubMed Google Scholar
-
17.Meinke PT, Smith MM, Shoop WL, Nodulisporic acid: its chemistry and biology[J]. Curr Top Med Chem 2, 655-74 (2002) CrossRef PubMed Google Scholar
-
18.Singh SB, Ondeyka JG, Jayasuriya H, Zink DL, Ha SN, Dahl-Roshak A, et al, Nodulisporic acids D-F: structure, biological activities, and biogenetic relationships[J]. J Nat Prod 67, 1496-506 (2004) CrossRef PubMed Google Scholar
-
19.Hensens OD, Ondeyka JG, Dombrowski AW, Ostlind DA, Zink DL, Isolation and structure of nodulisporic acid A1 and A2, novel insecticides from a Nodulisporium sp[J]. Tetrahedron Lett 40, 5455-8 (1999) CrossRef PubMed Google Scholar
-
20.Ondeyka JG, Dahl-Roshak AM, Tkacz JS, Zink DL, Zakson-Aiken M, Shoop WL, et al, Nodulisporic acid B, B1, and B2: a series of 1'-deoxy-nodulisporic acids from Nodulisporium sp[J]. Bioorg Med Chem Lett 12, 2941-4 (2002) CrossRef PubMed Google Scholar
-
21.Ondeyka JG, Byrne K, Vesey D, Zink DL, Shoop WL, Goetz MA, et al, Nodulisporic acids C, C1, and C2: a series of D-ring-opened nodulisporic acids from the fungus Nodulisporium sp[J]. J Nat Prod 66, 121-4 (2003) CrossRef PubMed Google Scholar
-
22.Zhang YH, Li L, Li YQ, Luo JH, Li W, Li LF, et al, Oxalierpenes A and B, unusual indole-diterpenoid derivatives with antiviral activity from a marine-derived strain of the fungus Penicillium oxalicum[J]. J Nat Prod 85, 1880-5 (2022) CrossRef PubMed Google Scholar
-
23.Fehr T, Acklin W, Die isolierung zweier neuartiger indol-derivate aus dem mycel von Claviceps paspali STEVENSet HALL[J]. Helv Chim Acta 49, 1907-10 (1966) CrossRef PubMed Google Scholar
-
24.Sallam AA, Ayoub NM, Foudah AI, Gissendanner CR, Meyer SA, El-Sayed KA, Indole diterpene alkaloids as novel inhibitors of the Wnt/β-catenin pathway in breast cancer cells[J]. Eur J Med Chem 70, 594-606 (2013) CrossRef PubMed Google Scholar
-
25.Qiao MF, Ji NY, Liu XH, Li K, Zhu QM, Xue QZ, Indoloditerpenes from an algicolous isolate of Aspergillus oryzae[J]. Bioorg Med Chem Lett 20, 5677-80 (2010) CrossRef PubMed Google Scholar
-
26.Chen MY, Xie QY, Kong FD, Ma QY, Zhou LM, Yuan JZ, et al, Two new indole-diterpenoids from the marine-derived fungus Penicillium sp[J]. KFD28. J Asian Nat Prod Res 23, 1030-6 (2021) CrossRef PubMed Google Scholar
-
27.Chaiyosang B, Kanokmedhakul K, Yodsing N, Boonlue S, Yang JX, Wang YA, et al, Three new indole diterpenoids from Aspergillus aculeatus KKU-CT2[J]. Nat Prod Res 36, 4973-81 (2022) CrossRef PubMed Google Scholar
-
28.Nozawa K, Nakajima S, Kawai K, Udagawa S, Studies on fungal products. Part 17. Isolation and structures of novel indoloditerpenes, emindoles DA and DB, from Emericella desertorum: X-ray molecular structure of emindole DA acetate[J]. J Chem Soc 7, 1689-94 (1988) PubMed Google Scholar
-
29.Nozawa K, Horie Y, Udagawa S, Kawai K, Yamazaki M, Isolation of a new tremorgenic indoloditerpene, 1'-O-acetylpaxilline, from Emericella striata and distribution of paxilline in Emericella spp[J]. Chem Pharm Bull (Tokyo) 37, 1387-9 (1989) CrossRef PubMed Google Scholar
-
30.Gloer JB, Rinderknecht BL, Wicklow DT, Dowd PF, Nominine: a new insecticidal indole diterpene from the sclerotia of Aspergillus nomius[J]. J Org Chem 54, 2530-2 (1989) CrossRef PubMed Google Scholar
-
31.Laakso JA, Gloer JB, Wicklow DT, Dowd PF, Radarins A-D: new antiinsectan and cytotoxic indole diterpenoids from the sclerotia of Aspergillus sulphureus[J]. J Org Chem 57, 138-41 (1992) CrossRef PubMed Google Scholar
-
32.Kimura Y, Nishibe M, Nakajima H, Hamasaki T, Shigemitsu N, Sugawara F, et al, Emeniveol: a new pollen growth inhibitor from the fungus, Emericella nivea[J]. Tetrahedron Lett 33, 6987-90 (1992) CrossRef PubMed Google Scholar
-
33.Hosoe T, Itabashi T, Kobayashi N, Udagawa S, Kawai K, Three new types of indoloditerpenes, emindole PA—PC, from Emericella purpurea. Revision of the structure of emindole PA[J]. Chem Pharm Bull (Tokyo) 54, 185-7 (2006) CrossRef PubMed Google Scholar
-
34.Liu L, Xu W, Li S, Chen MY, Cheng YJ, Yuan WJ, et al, Penicindopene A, a new indole diterpene from the deep-sea fungus Penicillium sp[J]. YPCMAC1. Nat Prod Res 33, 2988-94 (2019) CrossRef PubMed Google Scholar
-
35.Dai LT, Yang L, Kong FD, Ma QY, Xie QY, Dai HF, et al, Cytotoxic indole-diterpenoids from the marine-derived fungus Penicillium sp[J]. KFD28. Mar Drugs 19, 613 (2021) CrossRef PubMed Google Scholar
-
36.Tepaske MR, Gloer JB, Wicklow DT, Dowd PF, Three new aflavinines from the sclerotia of Aspergillus tubingensis[J]. Tetrahedron 45, 4961-8 (1989) CrossRef PubMed Google Scholar
-
37.Han X, Bao XF, Wang CX, Xie J, Song XJ, Dai P, et al, Cladosporine A, a new indole diterpenoid alkaloid with antimicrobial activities from Cladosporium sp[J]. Nat Prod Res 35, 1115-21 (2021) CrossRef PubMed Google Scholar
-
38.TePaske MR, Gloer JB, Wicklow DT, Dowd PF, Tubingensin A: an antiviral carbazole alkaloid from the sclerotia of Aspergillus tubingensis[J]. J Org Chem 54, 4743-6 (1989) CrossRef PubMed Google Scholar
-
39.Tepaske MR, Gloer JB, Wicklow DT, Dowd PF, The structure of tubingensin B: a cytotoxic carbazole alkaloid from the sclerotia of Aspergillus tubingensis[J]. Tetrahedron Lett 30, 5965-8 (1989) CrossRef PubMed Google Scholar
-
40.Tepaske MR, Gloer JB, Wicklow DT, Dowd PF, Aflavazole: a new antiinsectan carbazole metabolite from the sclerotia of Aspergillus flavus[J]. J Org Chem 55, 5299-301 (1990) CrossRef PubMed Google Scholar
-
41.Li H, Chen Q, Lu Z, Li A, Total syntheses of aflavazole and 14-hydroxyaflavinine[J]. J Am Chem Soc 138, 15555-8 (2016) CrossRef PubMed Google Scholar
-
42.Ariantari NP, Ancheeva E, Wang C, Mándi A, Knedel TO, Kurtán T, Indole diterpenoids from an endophytic Penicillium sp[J]. J Nat Prod 82, 1412-23 (2019) CrossRef PubMed Google Scholar
-
43.Miles CO, Wilkins AL, Gallagher RT, Hawkes AD, Munday SC, Towers NR, Synthesis and tremorgenicity of paxitriols and lolitriol: possible biosynthetic precursors of lolitrem B[J]. J Agric Food Chem 40, 234-8 (1992) CrossRef PubMed Google Scholar
-
44.Laakso JA, Gloer JB, Wicklow DT, Dowd PF, Sulpinines A-C and secopenitrem B: new antiinsectan metabolites from the sclerotia of Aspergillus sulphureus[J]. J Org Chem 57, 2066-71 (1992) CrossRef PubMed Google Scholar
-
45.Staub GM, Gloer J, Wicklow DT, Dowd PF, Aspernomine: a cytotoxic antiinsectan metabolite with a novel ring system from the sclerotia of Aspergillus nomius[J]. J Am Chem Soc 114, 1015-7 (1992) CrossRef PubMed Google Scholar
-
46.Ooike M, Nozawa K, Udagawa S, Kawai K, Structures of a new type of indoloditerpene, petromindole, and a new asterriquinone derivative, PM-53, from the ascostromata of Petromyces muricatus[J]. Chem Pharm Bull (Tokyo) 45, 1694-6 (1997) CrossRef PubMed Google Scholar
-
47.Chen L, Gloer JB, Wicklow DT, Dowd PF, Thiersinines A and B: novel antiinsectan indole diterpenoids from a new fungicolous Penicillium species (NRRL 28147)[J]. Org Lett 4, 3095-8 (2002) CrossRef PubMed Google Scholar
-
48.Ogata M, Ueda JY, Hoshi M, Hashimoto J, Nakashima T, Anzai K, et al, A novel indole-diterpenoid, JBIR-03 with anti-MRSA activity from Dichotomomyces cejpii var. cejpii NBRC 103559. cejpii NBRC 103559[J]. J Antibiot (Tokyo) 60, 645-8 (2007) CrossRef PubMed Google Scholar
-
49.Gao SS, Li XM, Williams K, Proksch P, Ji NY, Wang BG, Rhizovarins A-F, indole-diterpenes from the mangrove-derived endophytic fungus Mucor irregularis QEN-189[J]. J Nat Prod 79, 2066-74 (2016) CrossRef PubMed Google Scholar
-
50.Zhao JC, Luan ZL, Liang JH, Cheng ZB, Sun CP, Wang YL, et al, Drechmerin H, a novel 1(2), 2(18)-diseco indole diterpenoid from the fungus Drechmeria sp. as a natural agonist of human pregnane X receptor[J]. Bioorg Chem 79, 250-6 (2018) PubMed Google Scholar
-
51.Xu LL, Hai P, Zhang SB, Xiao JF, Gao Y, Ma BJ, et al, Prenylated indole diterpene alkaloids from a mine-soil-derived Tolypocladium sp[J]. J Nat Prod 82, 221-31 (2019) CrossRef PubMed Google Scholar
-
52.Nur EAA, Kobayashi K, Amagai A, Ohshiro T, Tomoda H, New terpendole congeners, inhibitors of sterol O-acyltransferase, produced by Volutella citrinella BF-0440[J]. Molecules 25, 3079 (2020) CrossRef PubMed Google Scholar
-
53.Zhou G, Sun C, Hou X, Che Q, Zhang G, Gu Q, et al, Ascandinines A-D, indole diterpenoids, from the sponge-derived fungus Aspergillus candidus HDN15-152[J]. J Org Chem 86, 2431-6 (2021) CrossRef PubMed Google Scholar
-
54.Springer JP, Clardy J, Paspaline and paspalicine, two indole-mevalonate metabolites from Claviceps paspali[J]. Tetrahedron Lett 21, 231-4 (1980) CrossRef PubMed Google Scholar
-
55.Munday-Finch SC, Wilkins AL, Miles CO, Isolation of paspaline B, an indole-diterpenoid from Penicilium paxilli[J]. Phytochemistry 41, 327-32 (1996) CrossRef PubMed Google Scholar
-
56.Cole RJ, Kirksey JW, Wells JM, A new tremorgenic metabolite from Penicillium paxilli[J]. Can J Microbiol 20, 1159-62 (1974) CrossRef PubMed Google Scholar
-
57.Springer JP, Clardy J, Wells JM, Cole RJ, Kirksey JW, The structure of paxilline, a tremorgenic metabolite of bainier[J]. Tetrahedron Lett 16, 2531-4 (1975) CrossRef PubMed Google Scholar
-
58.Nozawa K, Horie Y, Udagawa S, Kawai K, Yamazaki M, Isolation of a new tremorgenic indologiterpene, 1'-O-acetylpaxilline, from Emericella striata and distribution of paxilline in Emericella spp[J]. Chem Pharm Bull (Tokyo) 37, 1387-9 (1989) CrossRef PubMed Google Scholar
-
59.Yamaguchi T, Nozawa K, Hosoe T, Nakajima S, Kawai K, Indoloditerpenes related to tremorgenic mycotoxins, penitrems, from Penicillium crustosum[J]. Phytochemistry 32, 1177-81 (1993) CrossRef PubMed Google Scholar
-
60.Zhao LL. Search for new antineoplastic substances from marine-derived fungi. Dissertation, Fudan University, 2008. PubMed Google Scholar
-
61.Mantle PG, Weedon CM, Biosynthesis and transformation of tremorgenic indolediterpenoids by Penicillium paxilli and Acremonium lolii[J]. Phytochemistry 36, 1209-17 (1994) CrossRef PubMed Google Scholar
-
62.Tomoda H, Tabata N, Yang DJ, Takayanagi H, Omura S, Terpendoles, novel ACAT inhibitors produced by Albophoma yamanashiensis. III. Production, isolation and structure elucidation of new components[J]. J Antibiot 48, 1-4 (1995) PubMed Google Scholar
-
63.Tarui Y, Chinen T, Nagumo Y, Motoyama T, Hayashi T, Hirota H, et al, Terpendole E and its derivative inhibit STLC- and GSK-1-resistant Eg5[J]. ChemBioChem 15, 934-8 (2014) CrossRef PubMed Google Scholar
-
64.Xu LL, Pang XJ, Shi Q, Xian PJ, Tao YD, Yang XL, Two new prenylated indole diterpenoids from Tolypocladium sp. and their antimicrobial activities. and their antimicrobial activities[J]. Chem Biodivers 16, e1900116 (2019) PubMed Google Scholar
-
65.Belofsky GN, Gloer JB, Wicklow DT, Dowd PF, Antiinsectan alkaloids: shearinines A-C and a new paxilline derivative from the ascostromata of Eupenicillium shearii[J]. Tetrahedron 51, 3959-68 (1995) CrossRef PubMed Google Scholar
-
66.Itabashi T, Hosoe T, Wakana D, Fukushima K, Takizawa K, Yaguchi T, et al, A new indoloditerpene derivative, penijanthine A, isolated from Penicillium janthinellum[J]. J Nat Med 63, 96-9 (2009) CrossRef PubMed Google Scholar
-
67.Zhang YH, Huang SD, Pan HQ, Bian XQ, Wang ZY, Han AH, et al, Structure determination of two new indole-diterpenoids from Penicillium sp[J]. CM-7 by NMR spectroscopy. Magn Reson Chem 52, 306-9 (2014) PubMed Google Scholar
-
68.Gallagher RT, Finer J, Clardy J, Leutwiler A, Weibel FR, Acklin W, et al, Paspalinine, a tremorgenic metabolite from Claviceps paspali Stevens et Hall[J]. Tetrahedron Lett 21, 235-8 (1980) CrossRef PubMed Google Scholar
-
69.Staub GM, Gloer KB, Gloer JB, Wicklow DT, Dowd PF, New paspalinine derivatives with antiinsectan activity from the sclerotia of Aspergillus nomius[J]. Tetrahedron Lett 34, 2569-72 (1993) CrossRef PubMed Google Scholar
-
70.Huang XH, Nishida H, Tomoda H, Tabata N, Shiomi K, Yang DJ, Terpendoles, novel ACAT inhibitors produced by Albophoma yamanashiensis. II. Structure elucidation of terpendoles A, B, C and D[J]. J Antibiot (Tokyo) 48, 5-11 (1995) PubMed Google Scholar
-
71.Gatenby WA, Munday-Finch SC, Wilkins AL, Miles CO, Terpendole M, a novel indole-diterpenoid isolated from Lolium perenne infected with the endophytic fungus Neotyphodium lolii[J]. J Agric Food Chem 47, 1092-7 (1999) CrossRef PubMed Google Scholar
-
72.Junker B, Walker A, Connors N, Seeley A, Masurekar P, Hesse M, Production of indole diterpenes by Aspergillus alliaceus[J]. Biotechnol Bioeng 95, 919-37 (2006) CrossRef PubMed Google Scholar
-
73.Liang JH, Huo XK, Cheng ZB, Sun CP, Zhao JC, Kang XH, et al, An indole diterpenoid isolated from the fungus Drechmeria sp. and its antimicrobial activity[J]. Nat Prod Res 33, 2770-6 (2019) PubMed Google Scholar
-
74.Valdes JJ, Cameron JE, Cole RJ, Aflatrem: a tremorgenic mycotoxin with acute neurotoxic effects[J]. Environ Health Persp 62, 459-63 (1985) CrossRef PubMed Google Scholar
-
75.Gallagher RT, Wilson BJ, Aflatrem, the tremorgenic mycotoxin from Aspergillus flavus[J]. Mycopathologia 66, 183-5 (1979) CrossRef PubMed Google Scholar
-
76.Cole RJ, Dorner JW, Lansden JA, Cox RH, Pape C, Cunfer B, et al, Paspalum staggers: isolation and identification of tremorgenic metabolites from sclerotia of Claviceps paspali[J]. J Agric Food Chem 25, 1197-201 (1977) CrossRef PubMed Google Scholar
-
77.Uhlig S, Botha CJ, Vrålstad T, Rolén E, Miles CO, Indole-diterpenes and ergot alkaloids in Cynodon dactylon (bermuda grass) infected with Claviceps cynodontis from an outbreak of tremors in cattle[J]. J Agric Food Chem 57, 11112-9 (2009) CrossRef PubMed Google Scholar
-
78.Alley MC, Scudiero DA, Monks A, Hursey ML, Czerwinski MJ, Fine DL, et al, Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay[J]. Cancer Res 48, 589-601 (1988) PubMed Google Scholar
-
79.DeFarias FP, Carvalho MF, Lee SH, Kaczorowski GJ, Suarez-Kurtz G, Effects of the K+ channel blockers paspalitrem-C and paxilline on mammalian smooth muscle[J]. Eur J Pharmacol 314, 123-8 (1996) CrossRef PubMed Google Scholar
-
80.Xu MJ, Gessner G, Groth I, Lange C, Christner A, Bruhn T, et al, Shearinines D-K, new indole triterpenoids from an endophytic Penicillium sp. (strain HKI0459) with blocking activity on large-conductance calcium-activated potassium channels[J]. Tetrahedron 63, 435-44 (2007) PubMed Google Scholar
-
81.Liu C, Noike M, Minami A, Oikawa H, Dairi T, Functional analysis of a prenyltransferase gene (paxD) in the paxilline biosynthetic gene cluster[J]. Appl Microbiol Biot 98, 199-206 (2014) CrossRef PubMed Google Scholar
-
82.Liu C, Noike M, Minami A, Oikawa H, Dairi T, A fungal prenyltransferase catalyzes the regular diprenylation at positions 20 and 21 of paxilline[J]. Biosci Biotech Bioch 78, 448-54 (2014) CrossRef PubMed Google Scholar
-
83.Ohshiro T, Morita H, Nur EAA, Hosoda K, Uchida R, Tomoda H, Voluhemins, new inhibitors of sterol O-acyltransferase, produced by Volutella citrinella BF-0440[J]. J Antibiot (Tokyo) 73, 748-55 (2020) CrossRef PubMed Google Scholar
-
84.Junko O, Arihiro T, Yoshiyuki T, Masaichi N, JPN. Patent JP2004168680, 18 Nov 2002. PubMed Google Scholar
-
85.Liu C, Minami A, Dairi T, Gomi K, Scott B, Oikawa H, Biosynthesis of shearinine: diversification of a tandem prenyl moiety of fungal indole diterpenes[J]. Org Lett 18, 5026-9 (2016) CrossRef PubMed Google Scholar
-
86.Kudo K, Liu C, Matsumoto T, Minami A, Ozaki T, Toshima H, et al, Heterologous biosynthesis of fungal indole sesquiterpene sespendole[J]. ChemBioChem 19, 1492-7 (2018) CrossRef PubMed Google Scholar
-
87.Jesus AE, Steyn PS, Heerden FR, Vleggaar R, Structure elucidation of the janthitrems, novel tremorgenic mycotoxins from Penicillium janthinellum[J]. J Chem Soc Perkin Trans 1(15), 697-701 (1984) PubMed Google Scholar
-
88.Wilkins AL, Miles CO, Ede RM, Gallagher RT, Munday SC, Structure elucidation of janthitrem B, a tremorgenic metabolite of Penicillium janthinellum, and relative configuration of the A and B rings of janthitrems B, E, and F[J]. J Agric Food Chem 40, 1307-9 (1992) CrossRef PubMed Google Scholar
-
89.Penn J, Swift R, Wigley LJ, Mantle PG, Bilton JN, Sheppard RN, Janthitrems B and C, two principal indole-diterpenoids produced by Penicillium janthinellum[J]. Phytochemistry 32, 1431-4 (1993) CrossRef PubMed Google Scholar
-
90.Smetanina OF, Kalinovsky AI, Khudyakova YV, Pivkin MV, Dmitrenok PS, Fedorov SN, et al, Indole alkaloids produced by a marine fungus isolate of Penicillium janthinellum Biourge[J]. J Nat Prod 70, 906-9 (2007) CrossRef PubMed Google Scholar
-
91.Hennessy LM, Popay AJ, Finch SC, Clearwater MJ, Cave VM, Temperature and plant genotype alter alkaloid concentrations in ryegrass infected with an Epichloë endophyte and this affects an insect herbivore[J]. Front Plant Sci 7, 1097 (2016) PubMed Google Scholar
-
92.Ludlow EJ, Vassiliadis S, Ekanayake PN, Hettiarachchige IK, Reddy P, Sawbridge TI, et al, Analysis of the indole diterpene gene cluster for biosynthesis of the epoxy-janthitrems in Epichloë endophytes[J]. Microorganisms 7, 560 (2019) CrossRef PubMed Google Scholar
-
93.Matsui C, Ikeda Y, Iinuma H, Kushida N, Kunisada T, Simizu S, et al, Isolation of a novel paxilline analog pyrapaxilline from fungus that inhibits LPS-induced NO production[J]. J Antibiot (Tokyo) 67, 787-90 (2014) CrossRef PubMed Google Scholar
-
94.Babu JV, Popay AJ, Miles CO, Wilkins AL, di Menna ME, Finch SC, Identification and structure elucidation of janthitrems A and D from Penicillium janthinellum and determination of the tremorgenic and anti-insect activity of janthitrems A and B[J]. J Agric Food Chem 66, 13116-25 (2018) CrossRef PubMed Google Scholar
-
95.Gallagher RT, White EP, Mortimer PH, Ryegrass staggers: isolation of potent neurotoxins lolitrem A and lolitrem B from staggers-producing pastures[J]. N Z Vet J 29, 189-90 (1981) CrossRef PubMed Google Scholar
-
96.Reddy P, Deseo MA, Ezernieks V, Guthridge K, Spangenberg G, Rochfort S, Toxic indole diterpenes from endophyte-infected perennial ryegrass Lolium perenne L.: isolation and stability[J]. Toxins (Basel) 11, 16 (2019) CrossRef PubMed Google Scholar
-
97.Miles CO, Munday SC, Wilkins AL, Ede RM, Towers NR, Large-scale isolation of lolitrem B and structure determination of lolitrem E[J]. J Agric Food Chem 42, 1488-92 (1994) CrossRef PubMed Google Scholar
-
98.Imlach WL, Finch SC, Dunlop J, Dalziel JE, Structural determinants of lolitrems for inhibition of BK large conductance Ca2+-activated K+ channels[J]. Eur J Pharmacol 605, 36-45 (2009) CrossRef PubMed Google Scholar
-
99.Munday-Finch SC, Wilkins AL, Miles CO, Ede RM, Thomason RA, Structure elucidation of lolitrem F, a naturally occurring stereoisomer of the tremorgenic mycotoxin lolitrem B, isolated from Lolium perenne infected with Acremonium lolii[J]. J Agric Food Chem 44, 2782-8 (1996) CrossRef PubMed Google Scholar
-
100.Munday-Finch SC, Wilkins AL, Miles CO, Tomoda H, Omura S, Isolation and structure elucidation of lolilline, a possible biosynthetic precursor of the lolitrem family of tremorgenic mycotoxins[J]. J Agric Food Chem 45, 199-204 (1997) CrossRef PubMed Google Scholar
-
101.Munday-Finch SC, Wilkins AL, Miles CO, Isolation of lolicine A, lolicine B, lolitriol, and lolitrem N from Lolium perenne infected with Neotyphodium lolii and evidence for the natural occurrence of 31-epilolitrem N and 31-epilolitrem F[J]. J Agric Food Chem 46, 590-8 (1998) CrossRef PubMed Google Scholar
-
102.Laakso JA, Gloer JB, Wicklow DT, Dowd PF, A new penitrem analog with antiinsectan activity from the sclerotia of Aspergillus sulphureus[J]. J Agric Food Chem 41, 973-5 (1993) CrossRef PubMed Google Scholar
-
103.Moldes-Anaya A, Rundberget T, Uhlig S, Rise F, Wilkins AL, Isolation and structure elucidation of secopenitrem D, an indole alkaloid from Penicillium crustosum Thom[J]. Toxicon 57, 259-65 (2011) CrossRef PubMed Google Scholar
Copyright information
© The Author(s) 2023
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.