New polychlorinated bibenzyls from Rhododendron minutiflorum
Abstract
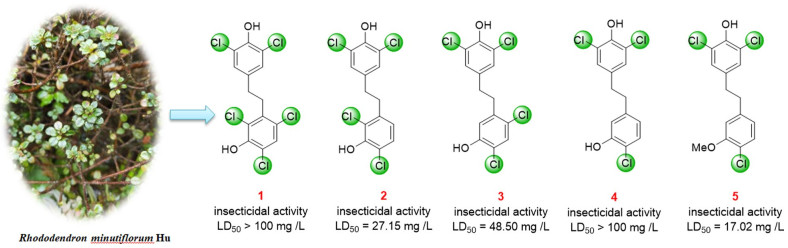
Five new polychlorinated bibenzyls (1–5) along with 3 known compounds (6–8) were isolated from the stems and leaves of Rhododendron minutiflorum. The chemical structures of all the isolates were determined by spectroscopic methods, and compounds 1 and 2 were further verified by single-crystal X-ray diffraction analyses. Compounds 1–5 were halogenated compounds which bear three to five chlorine atoms in their chemical structures. Biologically, compounds 2, 5 and 6 showed varying degrees of toxicity toward the Asian citrus psyllid (Diaphorina citri) with LD50 values 27.15, 17.02 and 16.20 mg/L, respectively. These values were comparable to the positive control matrine (LD50 = 11.86 mg/L), which were calculated using observations on day 6. Meanwhile, compound 4 had α-glucosidase inhibitory activity with IC50 value of 17.87 ± 0.74 μM.Graphical Abstract
Keywords
Rhododendron minutiflorum Polychlorinated bibenzyls Matrine Insecticidal activity Diaphorina citri α-Glucosidase inhibitory activity Acarbose1 Introduction
Bibenzyl compounds are categorized as a class of secondary metabolite with parent nucleus structure that consists of two benzyl units which are linked by a C–C single bond at the benzylic position. Substituents on the benzene ring usually occur at the para- and meta-positions of the benzene ring and common substituents include methyl and hydroxyl groups [1]. Bibenzyl compounds and their derivatives which possessed a variety of biological activities including insecticidal [2], antifungal [3, 4], anti-inflammatory [5], anticancer [6, 7], neuroprotective [8] and other biological activities [9, 10] were discovered from a wide range of plants. Although a large number of halogenated compounds have been found in nature [11], natural halogenated bibenzyls are rarely isolated from higher plants.
Rhododendron, which belongs to the family of Ericaceae, is one of the largest genera of vascular plants that is widely distributed all over the world, including the 542 species which grow in the mainland of China [12, 13]. A variety of compounds, such as terpenoids, lignans, and flavonoids [14-16] that have been identified from plants of this genus were reported with different biological activities [12, 13]. Rhododendron minutiflorum Hu, belonged to evergreen erect shrub, mainly distributed in the southern area of China. Previously, 2 omphalane-type sesquiterpenoids, 30 triterpenoids and their derivatives with potent α-glucosidase inhibitory activity have been isolated from R. minutiflorum by our group [13]. Moreover, partial species of Rhododendron exhibited insecticidal properties [12, 17]. In order to further study the phytochemical components and to analyze the biological activity, this work isolated five new polychlorobenzyls (1–5) and three known bibenzyls (6–8) (Fig. 1) from the leaves and stems of R. minutiflorum. The chemical structures of these compounds were determined by spectroscopic methods, whereas compounds 1 and 2 were further verified by single-crystal X-ray diffraction analyses. Subsequently, the α-glucosidase inhibitory activities and the insecticidal properties of these compounds were evaluated.
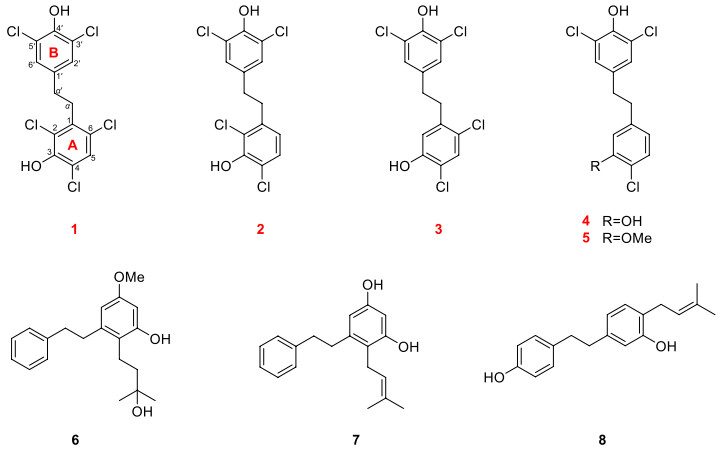
Chemical structures of compounds 1–8
2 Results and discussion
The air-dried stems and leaves of R. minutiflorum were extracted with 95% EtOH-H2O and then the organic solvent was removed under vacuum to obtain EtOH extract. Subsequently, the EtOH extract was suspended with an appropriate amount of distilled water and extracted with EtOAc for 3 times. The organic layer was concentrated to obtain the EtOAc part (900 g). The EtOAc part was further applied to various chromatographic columns (CC) such as Diaion HP20 macroporous resin, silica gel, Sephadex LH-20, reversed-phase C18 and semi-preparative RP-HPLC to obtain 8 different compounds. Among these, compounds 1–5 were undescribed halogenated compounds with multiple chlorine atoms. Based on spectroscopic analysis and comparison with previous literature data, 3 known compounds were determined as: 3-hydroxyl-5-methoxy-2-(3′-hydroxyl-3′-methylbutyl)-bibenzyl (6) [18], 3, 5-dihydroxyl-2-(3′-methyl-2′-butenyl)-bibenzyl (7) [18], 3, 4″-dihydroxyl-4-(3′-methyl-2′-butenyl)-bibenzyl (8) [19], respectively. All these compounds were isolated from R. minutiflorum for the first time.
2.1 Structural identification of compounds
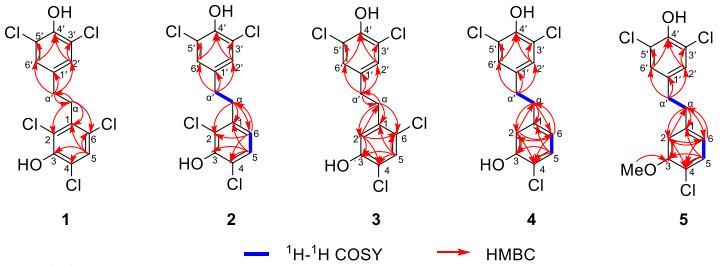
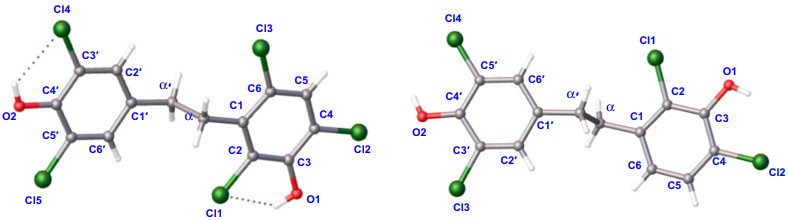
Compound 1, colorless crystals, has a molecular formula of C14H9Cl5O2 with eight degrees of unsaturation based on its 1H and 13C NMR data and the (–) HRESIMS [M−H]− ion at m/z 382.8992 (calcd 382.8972). IR absorption bands confirmed the hydroxy (3417 cm−1) and aromatic ring (1453, 1395 cm−1) functionalities in compound 1. The NMR data (Tables 1 and 2) showed characteristic signals for two phenyl rings [δH 7.35 (1H, s), 7.14 (2H, s); δC 119.3–147.2] and two sp3 methylene groups [δH 3.11 (2H, m), 2.71 (2H, m); δC 33.8, 33.0]. According to the key HMBC correlations from H-2′/H-6′ to C-4′ and C-3′/C-5′, H-α′ to C-α, C-1′ and C-2′/C-6′ (Fig. 2), a 3′, 4′, 5′ trisubstituted benzyl skeleton moiety was expounded. Moreover, the 2, 3, 4, 6-tetrasubstituted benzyl skeleton moiety of 1 was further deduced by the HMBC correlations from H-5 to C-1, C-3, C-4 and C-6, H-α to C-α′, C-1, C-2 and C-6, indicating that 1 belonged to the bibenzyl compounds. Based on the molecular formula, the positions of the five chlorine atoms and the two hydroxyl groups could not determine. By analyzing the NMR data of bibenzyl compounds previously reported in the literature, the chemical shift of aromatic carbon with hydroxyl group was above 145 ppm [18]. Therefore, it was inferred to that aromatic quaternary carbons with chemical shifts of δC 147.2 and δC 146.4 were linked to hydroxyl groups, while the other aromatic quaternary carbons were attached to the chlorine atoms. Finally, the single-crystal X-ray crystallographic study (Fig. 3) confirmed the positions for hydroxyl groups at C-3 and C-4′ and chlorine atoms at C-2, C-4, C-6, C-3′ and C-5′. Consequently, compound 1 was assigned as 2, 4, 6, 3′, 5′-pentachloro-3, 4′-dihydroxybibenzyl.
13C NMR data of compounds 1–5
1H NMR data of compounds 1–5
The key 1H–1H COSY and HMBC correlations for compounds 1–5
X-ray ORTEP drawing of 1 and 2
Compound 2 was obtained as colorless crystals and its molecular formula was C14H10Cl4O2 with eight degrees of unsaturation according to its (−) HRESIMS [M−H]− ion at m/z 348.9391 (calcd 348.9362) and the NMR data. From the IR absorption bands, compound 2 contains hydroxy (3316 cm−1) and aromatic ring (1419 cm−1) functionalities. The NMR data (Tables 1 and 2) indicated the characteristic signals for two methylene groups [δH 2.94 (2H, m), 2.78 (2H, m); δC 35.7, 34.6] and two benzene rings [δH 7.17 (1H, d, J = 8.4 Hz), 7.07 (2H, s), 6.66 (1H, d, J = 8.4 Hz); δC 119.0–148.1]. The 1H-1H COSY correlations (Fig. 2) of 2 showed two spin systems of H-5/H-6 and H-α/H-α′. The key HMBC correlations (Fig. 2) from H-α to C-1, C-2 and C-6, H-5 to C-3, C-4 and C-1, H-6 to C-α, C-2 and C-4 and the chemical shifts further verified the presence of a 2, 3, 4-trisubstituted benzyl skeleton and the positions for hydroxyl group at C-3 and chlorine atoms at C-2 and C-4. The other HMBC correlations from δH 2.78 (H-α′) to δC 128.3 (C-2′, 6′) and δC 134.5 (C-1′), δH 7.07 (H-2′, 6′) to δC 146.3 (C-4′) and δC 120.9 (C-3′, 5′) supported that the positions of C-3′, C-5′ and C-4′ were substituted by two chlorine atoms and hydroxyl group, respectively. Furthermore, detailed analysis of the NMR data and molecular formula revealed that the absence of a chlorine atom at C-6 in 2 than 1. Finally, the structure of 2 was further verified by single-crystal X-ray diffraction analysis with Flack parameter of − 0.008 (14) (Fig. 3), and the structure of compound 2 was deduced as 2, 4, 3′, 5′-tetrachloro-3, 4′-dihydroxybibenzyl, as shown in Fig. 1.
Compound 3, a white amorphous powder, had a molecular formula of C14H10Cl4O2, with eight degrees of unsaturation, deduced from its NMR data and the (−) HRESIMS [M−H]− ion at m/z 348.9390 (calcd 348.9362). The detailed NMR data (Tables 1 and 2) analysis revealed that the structure of 3 was similar to 2, with only a change in position of the chlorine atom at C-2 in 2 to C-6 in 3. In the HMBC spectrum (Fig. 2), the correlations from δH 2.89 (H-α) to δC 34.7 (C-α′), 117.7 (C-2), 139.1 (C-1), 134.5 (C-1′) and 125.3 (C-6), δH 6.82 (H-2) to δC 35.4 (C-α), 150.3 (C-3), 118.4 (C-4) and 125.3 (C-6), δH 7.34 (H-5) to δC 139.1 (C-1), 150.3 (C-3), 118.4 (C-4) and 125.3 (C-6) can confirm the above conclusion. From the perspective of biosynthesis, the locations for hydroxyl group at C-3 and chlorine atom at C-4 could be presumed. Therefore, the structure of 3 was defined as shown in Fig. 1 and named as 4, 6, 3′, 5′-tetrachloro-3, 4′-dihydroxybibenzyl.
Compound 4 was obtained as a white amorphous powder and had a molecular formula of C14H11Cl3O2 based on the (−) HRESIMS [M−H]− ion at m/z 314.9781 (calcd 314.9752) and NMR data. The NMR data (Tables 1 and 2) indicated characteristic signals for two phenyl rings [δH 7.21 (1H, d, J = 8.4 Hz), 7.04 (2H, s), 6.82 (1H, d, J = 2.0 Hz), 6.64 (1H, dd, J = 8.4, 2.0 Hz); δC 151.3–116.2] and two methylene groups [δH 2.79 (4H, m); δC 37.2, 36.4]. The NMR data suggested that 4 was a bibenzyl compound. The 1H–1H COSY correlations (Fig. 2) of 4 showed two spin systems of H-5/H-6 and H2-α/H2-α′. Moreover, the key HMBC correlations (Fig. 2) supported that the positions of C-3, C-4, C-3′, C-4′ and C-5′ in 4 were substituted by two hydroxyl groups and three chlorine atoms. The aforementioned analyses suggested that the structure of 4 closely resembled 2, except for the absence of a chlorine atom at C-2 in 4. Thus, 4 was characterized as a polychlorinated bibenzyl and assigned as 4, 3′, 5′-trichloro-3, 4′-dihydroxybibenzyl.
Compound 5, a white amorphous powder, had a molecular formula of C15H13Cl3O2 with eight degrees of unsaturation deduced from its NMR data and the (−) HRESIMS [M−H]− ion at m/z 328.9931 (calcd 328.9908). The detailed analyses of 1D NMR data (Tables 1 and 2) and 2D NMR data (Fig. 2) showed that the structure of 5 was similar to 4, differing only by the hydroxy at C-3 in 4 being replaced by a methoxy in 5, which was confirmed by the HMBC correlation (Fig. 2) from δH 3.86 (–OMe) to δC 155.0 (C-3). Thus, the structure of 5 was elucidated as shown in Fig. 1 and named as 4, 3′, 5′-trichloro-4′-hydroxyl-3-methoxybibenzyl.
2.2 Insecticidal activity and α-glucosidase inhibitory activity
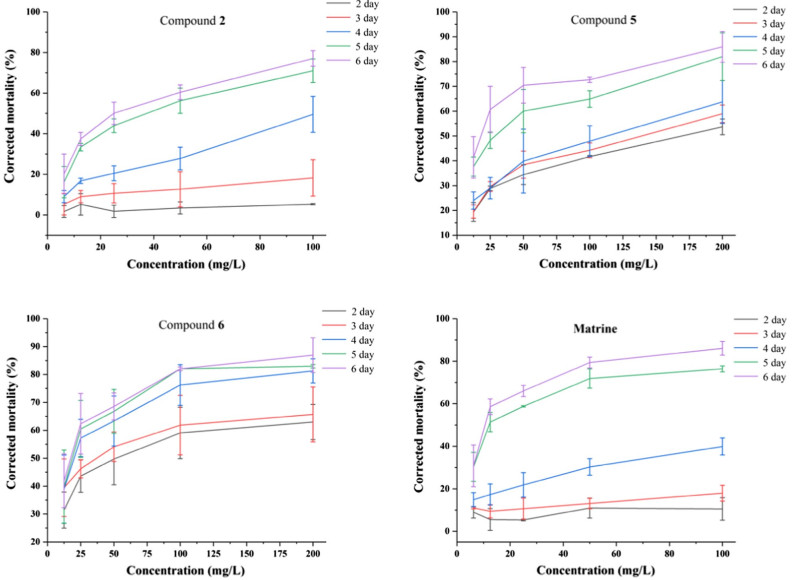
In order to evaluate the insecticidal activities of all the isolated compounds, their toxicities on D. citri Kuwayama were tested. With compound 4 as the only exception, compounds 1–3 and 5–8 exhibited different toxicities to D. citri compared to the 10% methanol or DMSO controls. Compounds 2, 5 and 6 showed remarkable insecticidal activities, with LD50 values of 27.15, 17.02 and 16.20 mg/L, respectively. While the LD50 of matrine was 11.86 mg/L (Table 3). Interestingly, on the 4th day after the test started, the corrected mortality rate of citrus psyllid is higher than 50% when the compound concentration is higher than 50 mg/L, indicating that compounds 2, 5 and 6 had insecticidal activity comparable to positive control matrine (Fig. 4). These data could provide reference and scientific basis for the further development and utilization of new pesticides against the Asian citrus psyllid D. citri. Besides, the α-glucosidase inhibitory activity of compounds 1–8 were detected by PNPG method, with acarbose served as a positive control (Table 4). The results showed that compounds 1, 3–8 gave different degrees of inhibitory activity with IC50 values from 17.87 to 82.84 μM (the IC50 of acarbose was 3.07 × 10–3 μM).
Toxicity of compounds 2, 3, 5–7 and matrine against D. citri during 6 days after treatment
Toxic activity anainst D. citri of compounds 2, 5, 6 and matrine
Inhibitory effects of compounds 1 and 3–8 on α-glucosidase (mean ± SE)
Furthermore, the preliminary structure–activity relationships (SAR) analyses showed that compounds with two chlorine atoms at C-2 and C-4 or C-4 and C-6 in ring A possessed insecticidal activity while compounds with three chlorine atoms at C-2, C-4 and C-6 in ring A had no more insecticidal activity, such as the insecticidal activity of 2, 3 > 1. Besides, if there was only one chlorine atom in ring A, the insecticidal activity was decreased (2, 3 > 4); however, methoxylation of C-3 can significantly increase the insecticidal activity (5 > 4).
3 Experimental
3.1 General experimental procedures
The instruments, materials and reagents used in this study were consistent with those reported in the literature [13, 20].
3.2 Plant materials
Dr. Shao-Qing Tang of Guangxi Normal University, Guangxi Province, People's Republic of China identified the stems and leaves of Rhododendron minutiflorum that were collected from Wuming County, Guangxi Province, in May 2019. The voucher specimen (No. TS-20190505) was deposited at the State Key Laboratory of Chemistry and Molecular Engineering of Medicinal Resources, Guangxi Normal University.
3.3 Extraction and isolation
The air-dried stems and leaves of R. minutiflorum (13.4 kg) were extracted with 95% EtOH-H2O (4 × 70 L, each 3 h) heating reflux to obtain the EtOH extract. The extract was concentrated under reduced pressure and then suspended with an appropriate amount of distilled water. Subsequently, the suspension liquid was extracted by EtOAc for 3 times and concentrated to generate the EtOAc part (900 g). The EtOAc part was subjected to a Diaion HP20 macroporous resin column eluting with EtOH-H2O (from 30:70 to 95:5) and acetone to yield five fractions (A–E).
Fraction D (12.0 g) was separated by silica gel column chromatography and eluted with CH2Cl2–MeOH (from 100:1 to 1:1) with increasing polarity to yield ten fractions (D1–D10). Fraction D1 (1.9 g) was chromatographed on a reversed-phase C18 (RP-C18) column eluting with MeOH–H2O (from 60:40 to 100:0), yielding fourteen subfractions (D1f1–D1f14). The subfraction D1f3 (159.0 mg) was separated by silica gel (200–300 mesh) column chromatography (CC) and eluted with PE/EtOAc (from 10:1 to 1:1) with increasing polarity and then purified by semi-preparative RP-HPLC (MeOH–H2O, 65:35) to generate compound 2 (72.2 mg, tR = 156.8 min). The subfraction D1f2 (118.0 mg) was separated by silica gel CC, and followed by purification by semi-preparative RP-HPLC (MeOH–H2O, 65:35) to generate 4 (5.6 mg, tR = 67.3 min). The subfraction D1f4 (142.0 mg) was separated by silica gel CC and eluted with PE/EtOAc (from 10:1 to 1:1) and purified by semi-preparative RP-HPLC (MeOH–H2O, 68:32) to generate 3 (5.1 mg, tR = 128.0 min).
Fraction E (75.3 g) was separated by silica gel CC and eluted with CH2Cl2–MeOH (from 100:1 to 1:1) with increasing polarity to yield nine fractions (E1–E9). Fraction E1 (16.1 g) was chromatographed on a RP-C18 column eluting with MeOH–H2O (from 55:45 to 100:0), yielding twenty-one subfractions (E1k1–E1k21). The subfractions E1k7 (98.0 mg) and E1k6 (259.8 mg) were respectively separated by Sephadex LH-20 column (MeOH) and purified by semi-preparative RP-HPLC (MeCN–H2O, 55:45, 50:50, respectively) to generate 1 (7.6 mg, tR = 81.8 min) and 5 (5.1 mg, tR = 180.0 min).
Fraction C (100 g) was separated by silica gel CC and eluted with CH2Cl2–MeOH (from 100:1 to 1:1) with increasing polarity to yield fifteen fractions (C1–C15). The fraction C5 (2.2 g) was chromatographed on a RP-C18 column eluting with MeOH–H2O (from 50:50 to 100:0), yielding thirteen subfractions (C5h1–C5h13). The subfraction C5h5 (61.0 mg) was purified by semi-preparative RP-HPLC (MeCN–H2O, 45:55) to generate 6 (15.7 mg, tR = 67.8 min) and 7 (28.2 mg, tR = 127.7 min). The subfraction C5h6 (152.0 mg) was separated by Sephadex LH-20 and purified by semi-preparative RP-HPLC (MeCN–H2O, 45:55) to generate 8 (5.0 mg, tR = 182.8 min).
3.3.1 2, 4, 6, 3′, 5′-Pentachloro-3, 4′-dihydroxybibenzyl (1)
Colorless crystals; m.p. 151–152 ℃; UV (MeOH) λmax (log ε) 222 (3.99), 290 (3.28) nm; IR (KBr) νmax 3672, 3417, 2975, 1453, 1395, 1232, 1503, 879 cm−1; 1H (400 MHz) and 13C (100 MHz) NMR (in CDCl3) data, see Tables 1 and 2; (−) HRESIMS m/z 382.8992 [M−H]−, (calcd C14H8Cl5O2: 382.8972).
3.3.2 2, 4, 3′, 5′-Tetrachloro-3, 4′-dihydroxybibenzyl (2)
Colorless crystals; m.p. 114–115 ℃; UV (MeOH) λmax (log ε) 205 (5.19), 225 (4.78), 285 (4.17) nm; IR (KBr) νmax 3316, 2976, 1419, 1049, 881, 799 cm−1; 1H (400 MHz) and 13C (100 MHz) NMR (in CDCl3) data, see Tables 1 and 2; (−) HRESIMS m/z 348.9391 [M−H]−, (calcd C14H9Cl4O2: 348.9362).
3.3.3 4, 6, 3′, 5′-Tetrachloro-3, 4′-dihydroxybibenzyl (3)
White powder; UV (MeOH) λmax (log ε) 227 (3.30), 284 (2.61) nm; IR (KBr) νmax 3664, 3303, 2975, 1407, 1252, 1053, 983 cm−1; 1H (400 MHz) and 13C (100 MHz) NMR (in CDCl3) data, see Tables 1 and 2; (−) HRESIMS m/z 348.9390 [M−H]−, (calcd C14H9Cl4O2: 348.9362).
3.3.4 4, 3′, 5′-Trichloro-3, 4′-dihydroxybibenzyl (4)
White powder; UV (MeOH) λmax (log ε) 226 (3.41), 275 (2.77) nm; IR (KBr) νmax 3391, 2923, 1668, 1575, 1486, 1150, 1048, 874, 800 cm−1; 1H (400 MHz) and 13C (100 MHz) NMR (in CDCl3) data, see Tables 1 and 2; (−) HRESIMS m/z 314.9781 [M−H]−, (calcd C14H10Cl3O2: 314.9752).
3.3.5 4, 3′, 5′-Trichloro-4′-hydroxyl-3-methoxybibenzyl (5)
White powder; UV (MeOH) λmax (log ε) 226 (2.94), 279 (1.09) nm; IR (KBr) νmax 3366, 2978, 1683, 1456, 1205, 1049, 881, 802 cm−1; 1H (400 MHz) and 13C (100 MHz) NMR (in CDCl3) data, see Tables 1 and 2; (−) HRESIMS m/z 328.9931 [M−H]−, (calcd C15H12Cl3O2: 328.9908).
3.3.6 3, 4″-Dihydroxyl-4-(3′-methyl-2′-butenyl)-bibenzyl (8)
Yellow oil; 1H NMR (600 MHz, MeOH-d4): δH 6.96 (2H, m, H-2′′/6′′), 6.89 (1H, d, J = 7.8 Hz, H-5), 6.66 (2H, m, H-3′′/5′′), 6.56 (1H, overlapped, H-2), 6.55 (1H, overlapped, H-6), 5.29 (1H, m, H-2′), 3.23 (2H, d, J = 7.2 Hz, H2-1′), 2.74 (4H, overlapped, H2-α, H2-β), 1.72 (3H, s, H3-4′), 1.70 (3H, s, H3-5′); 13C NMR (150 MHz, MeOH-d4): δC 156.4 (s, C-4′′), 155.8 (s, C-3), 142.0 (s, C-1), 134.2 (s, C-1′′), 132.6 (s, C-3′), 130.4 (d, C-2′′/6′′), 130.2 (d, C-5), 126.5 (s, C-4), 124.3 (d, C-2′), 120.7 (d, C-6), 116.0 (d, C-2/3′′/5′′), 39.2 (t, C-α), 38.4 (t, C-β), 28.9 (t, C-1′), 25.9 (q, C-4′), 17.8 (q, C-5′); (+) HRESIMS m/z 283.1688 [M + H]+, (calcd C19H23O2: 283.1693) (Additional file 1: Fig. S58).
3.3.7 Single-crystal X-ray diffraction data of 1
Colorless crystals of 1: Moiety formula were C14H9Cl5O2 and H2O(2), M = 422.49, monoclinic, space group I2/a, unit cell dimension a = 27.1575(11) Å, b = 4.3186(3) Å, c = 30.0170(12) Å, α = 90.0°, β = 91.182(4)°, γ = 90°, V = 3519.9(3) Å3, Z = 8, T = 293 K, μ(Cu Kα) = 7.655 mm−1, Dc = 1.594 g/cm3, F(000) = 1712.0. A total of 24, 050 reflections were collected in the range 5.89° ≤ 2θ ≤ 134.15° with 3111 independent reflections [Rint = 0.1191, Rsigma = 0.0698]. The final R indexes [I ≥ 2σ (I)], R1 = 0.0710, wR2 = 0.1946, final R indexes [all data], R1 = 0.1132, wR2 = 0.2257. The goodness of fit on F2 was 1.125.
3.3.8 Single-crystal X-ray diffraction data of 2
Colorless crystals of 2: Molecular formula was C14H10Cl4O2, M = 352.02, orthorhombic, space group P212121, unit cell dimension a = 4.7405(13) Å, b = 12.6052(5) Å, c = 24.1973(7) Å, α = β = γ = 90°, V = 1445.91(8) Å3, Z = 4, T = 293 K, μ(Cu Kα) = 7.423 mm−1, Dc = 1.617 g/cm3, F(000) = 721.0. A total of 6362 reflections were collected in the range 7.306° ≤ 2θ ≤ 134.126° with 2567 independent reflections [Rint = 0.0471, Rsigma = 0.0556]. The final R indexes [I ≥ 2σ (I)], R1 = 0.0330, wR2 = 0.0744, final R indexes [all data], R1 = 0.0386, wR2 = 0.0768. The goodness of fit on F2 was 0.991. Flack parameter: − 0.008(14).
Compounds 1 and 2 are colorless crystals that were obtained by vapor diffusion in MeOH solvent at room temperature. The intensity data were acquired in the way described in the previous literatures [21-23]. These data have been deposited in the Cambridge Crystallographic Data Centre as supplementary publications numbers CCDC 2105148 for 1, 2105091 for 2. Free copies of the data can be obtained from CCDC through www.ccdc.cam.ac.uk.
3.4 The biological assay
In November 2021, D. citri were captured from 5-year-old citrus trees (Citrus reticulata Blanco CV' Shatangju) in an orchard in Lingchuan, Guilin, China (25° 41 N, 110° 36 E). To avoid accidental injury, all the D. citri were carefully collected and cultured. The insecticidal activities of eight compounds were evaluated using methods described in the literature [24, 25] with slightly modified. In brief, D. citri were treated with compounds 1–8 and the positive control at concentrations of 100, 50, 25, 12.5 and 6.25 mg/L or 200, 100, 50, 25 and 12.5 mg/L. The blank solution was prepared with 10% methanol or DMSO in distilled water, respectively. Finally, mortality was calculated every 24 h starting on the second day of the trial. The toxicity data were statistically analyzed by Probit analyses, and the value of 50% lethal dose (LD50) were calculated.
According to the previously reported method [13], all the isolated compounds were screened for α-glucosidase inhibitory activity. Acarbose (Med Chem Express, NJ, USA, HY-B0089) was used as a positive control.
4 Conclusion
Five new polychlorinated bibenzyls (1–5) and three known bibenzyls (6–8) were isolated from the stems and leaves of R. minutiflorum. All the chemical structures were determined by spectroscopic methods, and the structures of compounds 1 and 2 were further verified by the single-crystal X-ray diffraction analyses. Compounds 1–5 were rare halogenated compounds which bear three to five chlorine atoms. As we know, it's the first time to find polychlorinated bibenzyls from Rhododendron plants. The insecticidal activity against the Asian citrus psyllid D. citri and the inhibitory activity on α-glucosidase of all the isolated compounds were evaluated. It is concluded that compounds 2, 5 and 6 from R. minutiflorum Hu exhibited potential insecticidal activity against the Asian citrus psyllid D. citri, and compounds 4 showed potent inhibitory activity against α-glucosidase.
Notes
Acknowledgements
This work was financially supported by The Natural Science Foundation of China (32160109, 31800294), the Natural Science Foundation of Guangxi Province of China (2018GXNSFBA138006, 2019AC20231), Educational Depart- ment of Guangxi (2018KY0089), the State Key Laboratory for Chemistry and Molecular Engineering of Medicinal Resources (CMEMR2017-A07, 2020-A09), Joint cultivation base of biology and medicine graduate student between Guangxi Normal University and Guilin Pharma Company.
Author contributions
HB, Liao and LJ, Zhang contributed to the project conception/supervision, funding acquisition. YL, Zhu, Y, Tang and XZ, F carried out the isolation, and chemical structure identification. L, Deng and Y, Han carried out the biological evaluations. YL, Zhu, and L, Deng drafted the manuscript. HB, Liao and M, Pan reviewed the manuscript. All authors read and approved the final manuscript.
Declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
-
1.Xu J, Han QB, Li SL, Chen XJ, Wang XN, Zhao ZZ, Chen HB, Chemistry, bioactivity and quality control of Dendrobium, a commonly used tonic herb in traditional Chinese medicine[J]. Phytochem Rev 12, 341-67 (2013) CrossRef PubMed Google Scholar
-
2.Labbé C, Faini F, Villagrán C, Coll J, Rycroft DS, Bioactive polychlorinated bibenzyls from the liverwort Riccardia polyclada[J]. J Nat Prod 70, 2019-21 (2007) CrossRef PubMed Google Scholar
-
3.Xie CF, Qu JB, Wu XZ, Liu N, Ji M, Lou HX, Antifungal macrocyclic bis(bibenzyls) from the Chinese liverwort Plagiochasma intermedium L[J]. Nat Prod Res 24, 515-20 (2010) CrossRef PubMed Google Scholar
-
4.Scher JM, Speakman JB, Zapp J, Becker H, Bioactivity guided isolation of antifungal compounds from the liverwort Bazzania trilobata (L) S.F. Gray[J]. Phytochemistry 65, 2583-8 (2004) CrossRef PubMed Google Scholar
-
5.Li QM, Jiang H, Zha XQ, Wu DL, Pan LH, Duan J, Liu J, Luo JP, Anti-inflammatory bibenzyls from the stems of Dendrobium huoshanense via bioassay guided isolation[J]. Nat Prod Res 34, 563-6 (2020) CrossRef PubMed Google Scholar
-
6.Putri HE, Nutho B, Rungrotmongkol T, Sritullarak B, Vinayanuwattikun C, Chanvorachote P, Bibenzyl analogue DS-1 inhibits MDM2-mediated p53 degradation and sensitizes apoptosis in lung cancer cells[J]. Phytomedicine 85, 153534 (2021) CrossRef PubMed Google Scholar
-
7.Sheng YW, Chen YW, Zeng ZQ, Wu WB, Wang J, Ma YL, Lin Y, Zhang JC, Huang YL, Li WH, Zhu QY, Wei X, Li SY, Wisanwattana W, Li F, Liu WL, Suksamrarn A, Zhang GL, Jiao W, Wang F, Identification of pyruvate carboxylase as the cellular target of natural bibenzyls with potent anticancer activity against hepatocellular carcinoma via metabolic reprogramming[J]. J Med Chem 65, 460-84 (2022) CrossRef PubMed Google Scholar
-
8.Zhou M, Jiang S, Chen CF, Li JY, Lou HY, Wang MY, Liu GZ, Liu HF, Liu T, Pan WD, Bioactive bibenzyl enantiomers from the tubers of bletilla striata[J]. Front Chem 10, 911201 (2022) CrossRef PubMed Google Scholar
-
9.Chen DK, Shao HY, Yang L, Hu JM, The bibenzyl derivatives of Dendrobium ofcinale prevent UV-B irradiation induced photoaging via SIRT3[J]. Nat Prod Bioprosp 12, 1 (2022) CrossRef PubMed Google Scholar
-
10.Wang YH, Traditional uses and pharmacologically active constituents of Dendrobium plants for dermatological disorders: a review[J]. Nat Prod Bioprosp 11, 465-87 (2021) CrossRef PubMed Google Scholar
-
11.Engvild KC, Chlorine-containing natural compounds in higher plants[J]. Phytochemistry 25, 781-91 (1986) CrossRef PubMed Google Scholar
-
12.Popescu R, Kopp B, The genus Rhododendron: an ethnopharmacological and toxicological review[J]. J Ethnopharmacol 147, 42-62 (2013) CrossRef PubMed Google Scholar
-
13.Fan XZ, Zhu YL, Yuan RW, Deng L, Hou C, Li W, Liu T, Kong XQ, Zhang LJ, Liao HB, Terpenoids with α-glucosidase inhibitory activity from Rhododendron minutiflorum Hu[J]. Phytochemistry 196, 113083 (2022) CrossRef PubMed Google Scholar
-
14.Liao HB, Lei C, Gao LX, Li JY, Li J, Hou AJ, Two enantiomeric pairs of meroterpenoids from Rhododendron capitatum[J]. Org Lett 17, 5040-3 (2015) CrossRef PubMed Google Scholar
-
15.Zhang ZX, Yan HM, Zhu YX, Zhang HP, Chai LS, Li L, Wang XJ, Liu YB, Li Y, New lignans, sesquiterpenes and other constituents from twigs and leaves of Rhododendron micranthum[J]. Fitoterapia 135, 15-21 (2019) CrossRef PubMed Google Scholar
-
16.Mok SY, Lee S, Identification of flavonoids and flavonoid rhamnosides from Rhododendron mucronulatum for. albiflorum and their inhibitory activities against aldose reductase[J]. Food Chem 136, 969-74 (2013) CrossRef PubMed Google Scholar
-
17.Liang JY, Yang YY, An Y, Shao YZ, He CY, Zhang J, Jia LY, Insecticidal and acetylcholine esterase inhibition activity of Rhododendron thymifolium essential oil and its main constituent against two stored product insects[J]. J Environ Sci Health B 56, 423-30 (2021) CrossRef PubMed Google Scholar
-
18.Zhang CY, Gao Y, Zhou JC, Xu ZJ, Qiao YN, Zhang JZ, Lou HX, Diverse prenylated bibenzyl enantiomers from the Chinese liverwort Radula apiculata and their cytotoxic activities[J]. J Nat Prod 84, 1459-68 (2021) CrossRef PubMed Google Scholar
-
19.Asakawa Y, Takikawa K, Toyota M, Takemoto T, Novel bibenzyl derivatives and ent-cuparenen-type sesquiterpenoids from radula species[J]. Phytochemistry 21, 2481-90 (1982) CrossRef PubMed Google Scholar
-
20.Zhu YL, Deng L, Song JQ, Zhu Y, Yuan RW, Fan XZ, Zhou H, Huang YS, Zhang LJ, Liao HB. Clerodane diterpenoids with anti-inflammatory and synergistic antibacterial activities from Tinospora crispa. Org Chem Front. 2022. https://doi.org/10.1039/D2QO01437H. PubMed Google Scholar
-
21.Dolomanov OV, Bourhis LJ, Gildea RJ, Howard JAK, Puschmann H, OLEX2: a complete structure solution, refinement and analysis program[J]. J Appl Cryst 42, 339-41 (2009) CrossRef PubMed Google Scholar
-
22.Kratzert D, Holstein JJ, Krossing I, DSR: enhanced modelling and refinement of disordered structures with SHELXL[J]. J Appl Crystallogr 48, 933-8 (2015) CrossRef PubMed Google Scholar
-
23.Zheng YK, Su BJ, Wang YQ, Wang HS, Liao HB, Liang D, New tyramine- and aporphine-type alkamides with NO release inhibitory activities from Piper puberulum[J]. J Nat Prod 84, 1316-25 (2021) CrossRef PubMed Google Scholar
-
24.He C, Wang YQ, Yang TM, Wang HS, Liao HB, Liang D, Quassinoids with insecticidal activity against Diaphorina citri Kuwayama and neuroprotective activities from Picrasma quassioides[J]. J Agri Food Chem 68, 117-27 (2020) CrossRef PubMed Google Scholar
-
25.Rizvi SAH, Ling SQ, Tian FJ, Xie F, Zeng XN, Toxicity and enzyme inhibition activities of the essential oil and dominant constituents derived from Artemisia absinthium L. against adult Asian citrus psyllid Diaphorina citri Kuwayama (Hemiptera: Psyllidae)[J]. Int Crop Prod 121, 468-75 (2018) CrossRef PubMed Google Scholar
Copyright information
© The Author(s) 2023
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.