The bio-interactions between plants, insecticides and fertilizers: an innovative approach for the research of xenobiotic substances
Abstract
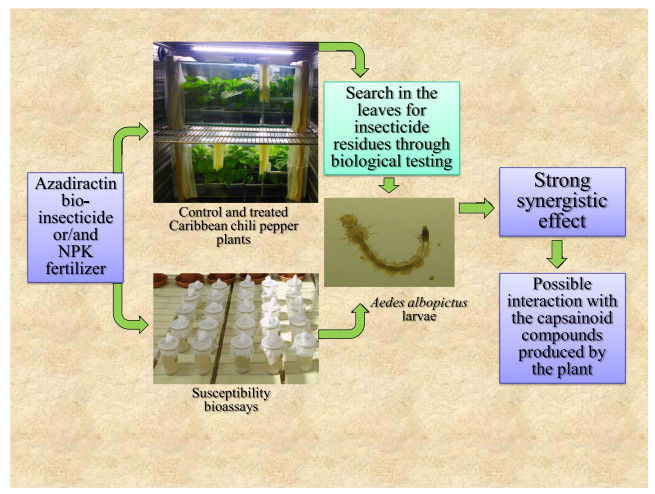
In this experiment carried out on Caribbean chili pepper plants (Capsicum chinensis), the bio-insecticide azadirachtin in combination with an NPK fertilizer proved to have a greater lethal impact on the larvae of Aedes albopictus than each substance on its own. This synergistic effect is noticeably important when both inputs are sprayed directly on the leaves of the plant (foliar application). While the plants treated with azadirachtin or NPK alone cause a 33.6% and 36.4% mortality respectively of the Ae. albopictus larvae, the combination of the two inputs induces a 74.4% mortality on the mosquito larvae. To account for this synergistic effect phenomenon inside the plant, the azadirachtin + NPK combination most likely interacts with the capsaicinoid compounds naturally produced by the plant. Not only does this study carried out on azadirachtin reveal major results but the methodology itself offers a most interesting approach on how to boost the agricultural inputs within the plants. As a matter of fact, this research axis demands developing since the control of pests harmful to men has been dramatically lacking insecticide molecules acting on new targets over the past three decades.Graphical Abstract
Keywords
Chili pepper plant Azadirachtin bio-insecticide NPK fertilizer Capsaicinoid compounds Xenobiotic substance Aedes albopictus Innovative research1 Introduction
Since the mid twentieth century, intensive farming has been using more and more chemical fertilizers and pesticides to increase crop yields and protect plants from pests. 180 millions tons of fertilizers and 2.4 million tons of pesticides are spread every year worldwide [1]. Modern fertilizers such as NPK are associations of minerals (N = nitrogen; P = phosphorous; K = potassium) meant to provide the plants with additional nutrients needed for their growth. Most of the time they are added to the soil but can also to borne by irrigated waters or sprayed straight onto the foliage [2]. The major parts of the insecticides used in agriculture are sprayed on the aerial parts of the plants. Irrigated crops such as rice and market gardening, besides their own pests, also host some mosquito species whose females inoculate vector-borne diseases to men, such as malaria, Japanese encephalitis, lymphatic filariasis and West Nile virus. Field observations and laboratory studies have confirmed that fertilizers do attract the mosquitoes looking for breeding places [3-6]. Fertilizers are not directly assimilated by the mosquito larvae, however the three minerals [nitrogen, phosphorous, and potassium] enhance the development of bacteria, algae, and fungi, increasing the food biomass of the breeding sites [7]. Mosquito larvae exploit this additional biomass to proliferate [8, 9]. To limit the proliferation of mosquitoes and destructive insects dependent on irrigated crops, an innovative control strategy was implemented in laboratory, consisting of adding to the fertilizer spread on the fields, chemical or biological insecticides (AGRIAC concept) [10]. During the laboratory trial of this novel concept of control, the chemical insecticides, thiacloprid (neonicotinoids family) and cyromazine (aminotriazins family) and the bio-insecticide azadirachtin (limonoids family) have been tested on Caribbean chili pepper plants (Capsicum chinense) (Solanales: Solanaceae). Thiacloprid and cyromazine have a systemic action, and azadirachtin, a translaminar action. A systemic insecticide is a substance which gets into the plant so as to be sap-borne while a translaminar compound is a molecule which can penetrate only a few layers of the cells of the same plant organ [11]. A search for insecticide residues in chili pepper plants using biological testing revealed that dried samples from untreated plants displayed natural toxicity (45% mortality) towards Aedes albopictus larvae (Diptera: Culicidae). The search for residues also revealed that thiacloprid, cyromazine and azadirachtin show greater insecticide efficacy (susceptibility tests versus insecticide residues bioassays) once absorbed by C. chinensis. These plants, which belong to the Solanaceae family, synthesize capsaicinoid components (capsaicin and dihydrocapsaicin) in their leaves and fruits that are an irritant to mammals, including humans [12]. To estimate the capsaicin content in the matured fruits of C. chinensis (Umorok cultivar), analyses carried out by high performance liquid chromatography (HPLC) revealed an average content of capsaicin of 2.06% (% w/w) [13]. When thiacloprid, cyromazine and azadirachtin insecticides enter C. chinensis plants (systemic or translaminar effects), their own toxicity probably adds up to the natural toxicity of the capsaicinoid compounds they come across [10]. Moreover, the bioassays have shown that the synergistic effect of the insecticides with the capsaicinoid compounds is quite substantial with azadirachtin [10]. In order to better encompass these synergistic effects inside the pepper plants, further evaluation was performed on the azadirachtin bio-insecticide alone and/or in combination with an NPK fertilizer. Extracted from the neem tree seeds (Azadirachta indica) (Sapindales: Meliaceae), this natural insecticide is used in market gardening to eliminate the phytophagous caterpillars and the piercing and sucking insects feeding on the plant sap [14]. The susceptibility tests were carried out with Ae. albopictus larvae so as to measure the activity of the various substances studied (azadirachtin, fertilizer and capsaicin), alone or in combination. The mortalities recorded in these insecticide susceptibility tests were compared with the mortalities induced by the dried plant leaves which had previously received water, NPK fertilizer, azadirachtin or azadirachtin + NPK combination.
2 Results and discussion
2.1 Search in chili pepper plants for insecticide residues through biological testing
2.1.1 Weight of dried plant leaves and search for insecticide residues (Table 1)
Averages of weight of dried plant leaves and search for insecticide residues in chili pepper plants through biological testing
Whatever the control conditions (negative and positive controls) and treated ones (NPK, azadirachtin and azadirachtin + NPK), the average of weight of dried plant leaves showed no significant differences (P = 0.56). Considering the NPK and azadirachtin doses sprayed on the leaves, the average weight of those leaves in each experimental condition, and the amount selected (100 mg) tested in 100 ml osmotic water, the concentrations of the two agricultural inputs can thus be expressed in mg per liter of water (mg/l). The concentrations in active ingredients amount to 2.07 mg/l for NPK fertilizer, 0.25 mg/l for azadirachtin and 0.218 + 2.20 mg/l for azadirachtin + NPK combination. The negative control (water without plant matter) showed 2.4% larval mortality. Dried plant leaves from the water (positive control), NPK fertilizer and azadirachtin insecticide had a moderate level of toxicity on Ae. albopictus larvae with 29.2%, 33.6% and 36.4% mortalities, respectively. If the water, NPK and azadirachtin conditions do not generate significantly different mortalities between them (0.22 < P < 0.55), they nonetheless are statistically different from the negative control (P = 0.008). A sample from chili pepper plants treated previously with azadirachtin + NPK combination showed much higher toxicity with a mortality rate of 74.4% (NPK + azadirachtin versus other experimental situations: P < 0.008).
2.1.2 Observed and expected mortalities of NPK + azadirachtin combination (Table 2)
Activity of NPK+azadirachtin combination (observed and expected mortalities) against susceptible strain IRD Aedes albopictus
Compared with the action of azadirachtin and NPK fertilizer alone, the azadirachtin + NPK combination showed a synergistic insecticidal effect against susceptible Aedes albopictus (P = 0.0001).
2.2 Results of the susceptibility tests
2.2.1 Susceptibility tests of capsaicin compound and NPK fertilizer (Table 3)
Comparative toxicity of capsaicin compound and NPK fertilizer against susceptible strain IRD Aedes albopictus tested at the larval stage
Capsaicin showed little toxicity on Ae. albopictus, the 1 mg/l to 10 mg/l concentrations causing a mere 19% to 38.5% mortality among the larvae. However the 1 mg/l concentration of capsaicin inducing 19% larval mortality was retained to conduct all of the other tests. As a matter of fact, at the concentration of 5.43 mg/l, the NPK fertilizer induces a very limited lethal response with only 20.7% of dead larvae.
2.2.2 Susceptibility tests of azadirachtin alone, azadirachtin + capsaicin and azadirachtin + capsaicin + NPK combinations (Table 4)
Comparative toxicity of azadirachtin alone, azadirachtin+capsaicin and azadirachtin+capsaicin+NPK fertilizer combinations against susceptible strain IRD Aedes albopictus tested at the larval stage
For the azadirachtin alone, the IE50 value was 1.31 mg/l. The IE50 value of azadirachtin + capsaicin was 0.89 mg/l, indicating that combination activity was 1.5 times as high as azadirachtin alone. Azadirachtin + capsaicin + NPK mixture issues a 0.54 mg/l IE50. The latter combination proves 2.4 times as high a larvicidal action as azadirachtin alone.
2.3 Comparison of mortalities recorded in dried plant leaves versus susceptibility tests (Tables 1 and 4)
In 100 mg of dried chili pepper leaves brewed in 100 ml of osmotic water, the concentration of azadirachtin is estimated at 0.25 mg/l. This residual load in plants kills 36.4% of Ae albopictus larvae (Table 1). To get similar mortality rates (IE40) in the susceptibility tests, the concentrations of azadirachtin alone or combined with capsaicin have to be 1.17 and 0.8 mg/l (Table 4). The capsaicin naturally produced by the chili pepper plants thus increases 4.7 fold the toxic action of azadirachtin (1.17 mg/l versus 0.25 mg/l).
The azadirachtin + NPK combination recorded in 100 mg of dried chili pepper leaves (0.218 + 2.20 mg/l) induced a 74.4% larval mortality (Table 1). To reach the same levels of mortality in the susceptibility tests (IE70), the concentration of azadirachtin has to equal 0.79 mg/l (for the azadirachtin + capsaicin + NPK combination) (Table 4). The azadirachtin + NPK treatment directly applied onto the chili pepper leaves has demonstrated an insecticidal action 3.6 times as important as the same combination evaluated during the susceptibility tests (0.218 mg/l versus 0.79 mg/l).
2.4 An innovative approach for the research of xenobiotic substances
The results obtained during the susceptibility tests reveal that an NPK fertilizer added to azadirachtin + capsaicin combination increases the toxicity of the latter on the Ae. albopictus larvae, the more so when both the azadirachtin and NPK are absorbed by Caribbean chili pepper leaves. This synergistic phenomenon inside the plant may be accounted for by fact the azadirachtin + NPK combination interacts with the capsaicinoid compounds naturally synthesized by the plant. A similar action had been observed with the thiacloprid and cyromazine insecticides (neonicotinoids and aminotriazins families respectively) also combined with an NPK fertilizer [10]. In this study the content of capsaicin as such in the leaves of C. chinensis could not be assessed. However the bioassays carried out on the larvae of Ae. albopictus indirectly, yet undoubtedly, testify that once the azadirachtin + NPK combination is sprayed then absorbed by the plant leaves, the latter proves a lot more toxic than each substance separately. If the results of this study carried out with azadirachtin raises interest, even more so the novel approach which discloses the potential to be expected from the interaction with the agricultural inputs within the plants. The chili peppers synthesize capsaicinoid compounds (alkaloids family) but plenty of the other plants worldwide also produce many various similar compounds [15, 16]. Knowing organic molecules from plants can interact with agricultural fertilizer and pesticides to produce xenobiotic compounds even more toxic than the original substances reveals interesting perspectives of research on the isolation and characterization of new substances applicable to agriculture and/or public health. As resistance phenomenons among insects harmful to humans are spreading and intensifying, to find substances with novel modes of action has become urgent, all the more so as the chemical industry has a harder and harder time finding new pesticides families.
3 Material and methods
3.1 The biological material
The plant used was the Caribbean chili pepper Capsicum chinensis (seeds produced by TECHNISEM). This chili pepper originated from South America is very hot. On the Scoville scale, it scores from 100, 000 to 350, 000 depending on the variety. For capsaicin [8-méthyl-N-vanillyl-6-nonenamide (alkaloids family)] it scores zero on the same scale, which means no burning sensation is detected, even without dilution. For the C. chinensis, the Scoville scale score of 100, 000 to 350, 000 means that extracts have to be diluted 100, 000 to 350, 000 times before capsaicin becomes undetectable [17]. By way of an example, the Umorok cultivar of C. chinensis has shown that the capsaicin content in the fruits reaches 2.06% (% w/w) with a score of 320, 100 on the Scoville scale [13]. All bioassays were conducted with the insecticide-susceptible (SS) tiger mosquito Aedes Albopictus IRD strain, raised at the insectarium of the Institute of Research for Development (IRD) (Vectopole) in Montpellier, France.
3.2 The NPK fertilizer, azadirachtin insecticide and capsaicin compound
The 0.058-0.034-0.058 composition of NPK fertilizer (liquid formulation) (Masso Garden) contains 0.058% total nitrogen (N) with 0.0135% ureic nitrogen, 0.0275% ammonium nitrogen (NH4 +), and 0.017% nitric nitrogen (NO3 −); 0.034% phosphorous (P) in the form of phosphoric anhydride (P2O5) and 0.058% potassium (K) in the form of potassium oxide (K2O). The bio-insecticide azadirachtin (Neemazal®), is presented as a suspension concentrate [EC] at the dosage of 9.8 g/l (Andermatt). The capsaicin compound, analytical standard, (Pestanal™), titrates 98.5% of technical grade (Sigma-Aldrich).
3.3 The experimental design
NPK fertilizer or/and the bio-insecticide azadirachtin were evaluated in plastic containers [L: 0.115 m; l: 0.08 m; H: 0.055 m; S: 0.0092 m2] two thirds of their surface representing the soil space (0.00616 m2). The remaining third of the surface (0.00307 m2) was allotted to the water supply needed for the normal development of the plant (water space). The soil and water spaces were separated by a plexiglass sheet with three holes for the water space to communicate with the soil space (Fig. 1). The Caribbean chili peppers seedlings were transplanted in the soil space once they would number about fifteen fully-formed leaves.
Experimental device used to search for xenobiotics substances in plants (©Frédéric Darriet)
3.4 The treatments of Caribbean chili pepper plants
The experiment consisted in evaluating azadirachtin and NPK fertilizer alone and in combination. The treatments were made starting from the dosages applicable for agriculture use of fertilizer and insecticide. The recommended dosage of azadirachtin on market gardening is 30 g/ha, to be reiterated 3 times every 7 days [18]. In foliar spraying, the optimal amount of NPK solution to be sprayed is 200 l/ha [19]. The treated conditions were evaluated in parallel with the water situation in five replicates. The fertilizer and insecticide doses were calculated in relation to the surface of the soil space (0.00616 m2) (Table 5). Water, NPK, azadirachtin or azadirachtin + NPK were sprayed three times at weekly intervals. The sprayings were performed on the plant leaves with a small pressure sprayer (https://www.massogarden.com/fr/produits/produit/engrais-foliaire-plantes-d-interieur-spray-pret-a-l-emploi-250ml).
Quantities of azadirachtin insecticide or/and NPK fertilizer poured in a foliar treatment
3.5 Search in chili pepper plants for insecticide residues through biological testing
One week after the first treatment, all the leaves of the chili pepper plants of each experimental condition (water – NPK – azadirachtin – azadirachtin + NPK) were slipped between two sheets of paper and left to dry for 15 days at room temperature. The dried-up leaves of each plant were weighed, following which a precise quantity of 100 mg of dried leaves was put into 100 ml of osmotic water (macerate titrated to 1 g/l). A negative control condition with no dried plant was assessed following the same experimental conditions. After 24 h and in each condition, 50 Ae. albopictus 2nd instar larvae were introduced, fed with aquarium shrimp food and monitored until adult emergence. The temperature was maintained at 27 ℃ throughout the test.
3.6 The susceptibility tests
Susceptibility tests on 2nd instar larvae of Ae. albopictus were carried out with azadirachtin, capsaicin, azadirachtin + capsaicin and azadirachtin + capsaicin + NPK fertilizer. Groups of 50 larvae were placed in 99 ml of osmotic water completed by 0.1 to 1 ml of azadirachtin insecticide or/and capsaicin and NPK fertilizer (100 ml per plastic cup). Larval bioassays were carried out with technical material (capsaicin) or formulations (azadirachtin and fertilizer). The technical grade of capsaicin was diluted in absolute alcohol and the formulation of azadirachtin and fertilizer in osmotic water. The concentration of capsaicin dropped in each cup was determined according to preliminary bioassays determining the sub lethal concentrations [20% of inhibition of mosquito adult emergence (IE)]. The concentration of NPK put into each cup containing 100 ml of water was 0.543 mg (N: 0.21 mg + P: 0.123 mg + K: 0.21 mg). It corresponds to the among of fertilizer administered in foliar spraying on each chili pepper plant. Translated in mg/l of water, the NPK concentrations respectively amount to 2.1 mg/l, 1.23 mg/l and 2.1 mg/l (5.43 mg/l). The capsaicin compound and the NPK fertilizer were put, separately, in the azadirachtin susceptibility tests. Two plastic cups per concentration and a minimum of 4 concentrations per replicate, providing mortality within a range of 01–99%, were used for each replicate. Each bioassay was done in four to six replicates. The larvae were fed with aquarium shrimp food and monitored until adult emergence. The temperature was maintained at 27 ℃ throughout the test.
3.7 Statistical analyses
The statistical comparisons were performed with Statistica V10 software [20]. When the experimental situations respond to a Gaussian distribution (averages of weight of dried plants leaves), the statistical comparisons were analyzed using the one-way ANOVA test. When the experimental situations didn't show a Gaussian distribution (% inhibition of mosquito adult emergence), the Kruskal Wallis test was used. The expected mortality [21] for the azadirachtin + NPK fertilizer mixture was calculated by 1 – (frequency of adults surviving on azadirachtin) × (frequency of adults surviving on NPK fertilizer). It is considered to be synergy (positive interaction) when the observed mortality is significantly higher (Fisher's exact test) than the expected mortality. The insecticide susceptibility tests were analyzed according to the method of Finney [22] by using Probit® software [23]. This software uses an iterative method of maximum likelihood to fit a regression line between the logarithm of concentration and the probit inhibition of mosquito adult emergence (IE). This software then provided an estimation of IE1-99 with their 95% confidence intervals.
Notes
Acknowledgements
Special thanks to Hélène Darriet for kindly translating this paper from French into English.
Author contributions
FD conceived the study, analyzed the results and wrote the article. The author read and approved the final manuscript.
Declarations
Competing interests
The author declares that he has no competing interests.
References
-
1.FAO, Current world fertilizer trends and outlook to 2016. Food and Agriculture Organization of the United Nations, Rome. 2012. PubMed Google Scholar
-
2.Fernández V, Brown PH, From plant surface to plant metabolism: the uncertain fate of foliar-applied nutrients[J]. Front Plant Sci 4, 289 (2013) PubMed Google Scholar
-
3.Victor TJ, Reuben R, Effects of organic and inorganic fertilizers on mosquito populations in rice fields of southern India[J]. Med Vet Entomol 14, 361-8 (2000) CrossRef PubMed Google Scholar
-
4.Mwangangi JM, Muturi EJ, Shililu J, Muriu SM, Jacob B, Kabiru EW, Mbogo CM, Githure J, Novak R, Survival of immature Anopheles arabiensis (Diptera: Culicidae) in aquatic habitats in Mwea rice irrigation scheme, central Kenya[J]. Malar J 5, 114 (2006) CrossRef PubMed Google Scholar
-
5.Darriet F, Corbel V, Aedes aegypti oviposition in response to NPK fertilizers[J]. Parasite 15, 89-92 (2008) CrossRef PubMed Google Scholar
-
6.Darriet F, Zumbo B, Corbel V, Chandre F, Influence of plant matter and NPK fertilizer on the biology of Aedes aegypti (Diptera: Culicidae)[J]. Parasite 17, 149-54 (2010) PubMed Google Scholar
-
7.Hao XH, Hu RG, Wu JS, Tang SR, Luo XQ, Effects of long-term fertilization on paddy soils organic nitrogen, microbial biomass, and microbial functional diversity[J]. J Appl Ecol 21, 1477-84 (2010) PubMed Google Scholar
-
8.Darriet F, Rossignol M, Chandre F, The combination of NPK fertilizer and deltamethrin insecticide favors the proliferation of pyrethroid-resistant Anopheles gambiae (Diptera: Culicidae)[J]. Parasite 19, 159-64 (2012) CrossRef PubMed Google Scholar
-
9.Darriet F, Synergistic effect of fertilizer and plant material combinations on the development of Aedes aegypti (Diptera: Culicidae) and Anopheles gambiae (Diptera: Culicidae) Mosquitoes[J]. J Med Entomol 55, 496-500 (2018) CrossRef PubMed Google Scholar
-
10.Darriet F, Laboratory study of an innovative concept to control aphid pests and mosquito vectors of pathogens to humans[J]. Pest Manag Sci 78, 1071-80 (2022) CrossRef PubMed Google Scholar
-
11.Index phytosanitaire, Association de Coordination Technique Agricole (ACTA), Paris. 2018. PubMed Google Scholar
-
12.Babakkanian RV, Binat GN, Isakov VD, Mukovskii LA, Forensic medical aspects of injuries inflicted with self-defense capsaicin aerosols[J]. Sud Med Ekspert 44, 9-11 (2001) PubMed Google Scholar
-
13.Sanatombi K, Sharma GJ, Capsaicin content and pungency of different Capsicum spp[J]. Cultivars Not Bot Hort Agrobot Cluj 36, 89-90 (2008) PubMed Google Scholar
-
14.Aribi N, Denis B, Kilani-Morakchi S, Joly D, Azadirachtin, a natural pesticide with multiple effects[J]. Med Sci 36, 44-9 (2020) PubMed Google Scholar
-
15.Kries H, O'Connors SE, Biocatalysts from alkaloid producing plants[J]. Curr Opin Chem Biol 31, 22-30 (2016) CrossRef PubMed Google Scholar
-
16.Schläger S, Dräger B, Exploiting plant alkaloids[J]. Curr Opin Biotechnol 37, 155-64 (2016) CrossRef PubMed Google Scholar
-
17.Cornell Botanic Gardens, Some Like it Hot! 2018. http://cornellbotanicgardens.org/wp-content/uploads/2018/11/heat.index_.poster.pdf. Accessed 5 Oct 2022. PubMed Google Scholar
-
18.Andermatt. NEEMAZAL®-T/S - Insecticide d'origine végétale à base d'extrait d'Azadirachta indica. 2019. https://www.andermatt.fr/img/cms/Fiche%20produit/NeemAzal-Maraichage-FP.pdf. Accessed 5 Oct 2022. PubMed Google Scholar
-
19.Leblanc M. Les règles de base pour l'utilisation des fertilisants folaires en culture maraîchère. Agri réseau, Ministère de l'Agriculture, des pêcheries et de l'Alimentation du Québec. 2005. https://www.agrireseau.net/legumeschamp/documents/fertilisant.pdf. Accessed 5 October 2022. PubMed Google Scholar
-
20.Statistica. Windows Statistical Software, Version 10. StatSoft, France, Maisons-Alfort. 2011. PubMed Google Scholar
-
21.Corbel V, Darriet F, Chandre F, Hougard JM, Insecticides mixtures for mosquito net impregnation against malaria[J]. Parasite 9, 255-9 (2002) PubMed Google Scholar
-
22.Finney DJ, Probit analysis. (Cambridge: Cambridge Univ. Press, 1971). PubMed Google Scholar
-
23.Raymond M, Prato G, Ratsira D. Probit and logit analysis program, version 2.0. Praxème, Biometric, Centre National de la Recherche Scientifique, Montpellier. 1997. PubMed Google Scholar
Copyright information
© The Author(s) 2022
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/





