Phytochemical and pharmacological studies on Solanum lyratum: a review
Abstract
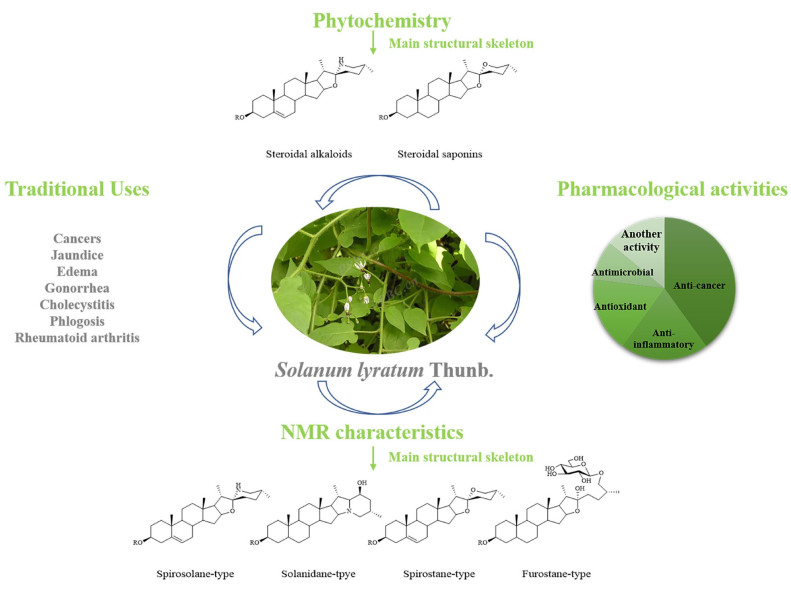
Solanum lyratum is one of the temperate plants, broadly distributed in Korea, China, Japan, India, and South-East Asia and well-documented in those oriental ethnic medicine systems for curing cancers, jaundice, edema, gonorrhea, cholecystitis, phlogosis, rheumatoid arthritis, etc. This review systematically summarized the research progress on S. lyratum respecting the botany, traditional uses, phytochemistry, pharmacology, and toxicology to increase people's in-depth understanding of this plant, by data retrieval in a series of online or off-line electronic databases as far as we can reach. Steroidal saponins and alkaloids, terpenoids, nitrogenous compounds, and flavonoid compounds are the main chemical constituents in S. lyratum. Among them, steroidal alkaloids and saponins are the major active ingredients ever found in S. lyratum, exerting activities of anti-cancer, anti-inflammation, anti-microbial, anti-allergy, and anti-oxidation in vivo or in vitro. As a result, S. lyratum has been frequently prescribed for the abovementioned therapeutic purposes, and there are substantial traditional and modern shreds of evidence of its use.Graphical Abstract
Keywords
Solanum lyratum Thunb. Steroidal saponins Steroidal alkaloids Anti-cancer Toxicity1 Introduction
Solanum lyratum Thunb. is a herbaceous vine of the family Solanaceae, with white villous hairs on violin-shaped leaves and stems, distributed throughout China, Japan, Korea, etc. [1]. S. lyratum prefers a warm and humid environment and is distributed widely in valley grass, roadside and field. S. lyratum is commonly known as "Bai-Mao-Teng" in traditional Chinese medicine and "Back-Mo-Deung" in traditional Korean medicine [2]. In traditional Chinese medicine (TCM), S. lyratum has the functions of clearing heat and removing toxicity ("Qingre Jiedu" in Chinese), dispelling wind and eliminating dampness ("Qufeng Lishi" in Chinese). Therefore, S. lyratum has been traditionally prescribed mainly for healing jaundice, edema, gonorrhea, cholecystitis, phlogosis, and rheumatoid arthritis [3]. Modern phytochemistry and pharmacological studies revealed that S. lyratum consists of a variety of active ingredients, including steroidal saponins, steroidal alkaloids, terpenoids, lignans, and flavonoids [4]. And steroidal saponins and steroidal alkaloids have been used in the modern clinic to treat various cancers, especially lung cancer, cervical cancer, and liver cancer [3].
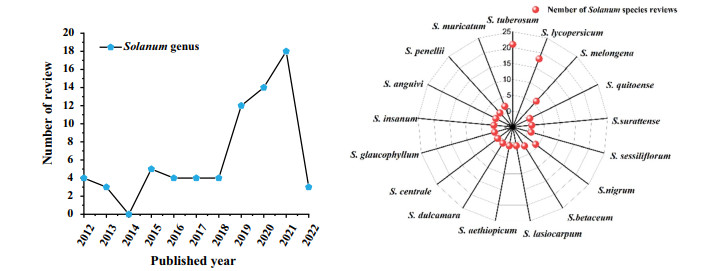
In the last ten years, dozens of reviews on the research progress of Solanum plants have been published, occasionally referring few phytochemistry and pharmacological reports on S. lyratum [5-8] (Fig. 1). However, there is no specialized and systematic research review on the S. lyratum species, especially on its phytochemistry and pharmacological aspects. Thus, this review intends to provide an updated and comprehensive summary on the botanical characterization, phytochemistry, and pharmacological and toxicity studies of S. lyratum to fill a gap in the research review of this plant and provides for a better exploration and application of S. lyratum. The literature for this manuscript was obtained from reports published from 1981 to Mar 2022.
Reviews of Solanum species published in last ten years
2 Botany
S. lyratum is a herbaceous vine of the family Solanaceae, well-known native in China, India, Japan, Korea, North Vietnam, and the Indochina Peninsula [1]. This plant grows in a warm and humid environment, prefers light and fertile organic soil, and is distributed on hillsides, grass, ditch, and roadside at altitudes of 100–850 m. S. lyratum is 0.5–1 m long, and its stems and twigs are densely covered with white villous hairs [2]. The botanical characteristics of S. lyratum were recorded in many classics of TCM, including "Tang Xinxiu Bencao" in the Tang Dynasty [9], "Zhenglei Bencao" in the Song Dynasty [10], and "Compendium of Materia Medica" in Ming Dynasty [11].
S. lyratum is commonly known as "Bai-Mao-Teng" in TCM. It should be noted that there are several adulterants of S. lyratum, including Aristolochia mollissima, Paederia scandens (Lour.) Merr. and Solanumrr dulcamara L., all of which are so called as "Bai-Mao-Teng" that it may easily cause an event of medication confusion [12]. For example, a misuse of A. mollissima instead of S. lyratum, has ever led to a renal failure event in patients of Hong Kong [13]. Those adulterants closely resemble S. lyratum in botanical morphology, it is very important to seek advice from a professional or pharmacist before use. The plant morphological characteristics of S. lyratum are as follows: Root is slender and cylindrical. Leaves are mostly violin-shaped, with 3.5–5.5 cm long and 2.5–4.8 cm wide, and the base is 3–5 cm deep-lobed. Lateral lobes are smaller near the base. Middle lobes are usually larger oval and tend to apex acuminate. Both sides of the leaves were covered with white shiny villous hairs, and the levels own midvein and lateral veins. Flowers are sparsely terminal inflorescence or extra-axillary inflorescence, and the pedicel is approximately 2–2.5 cm long. Corollas are blue-purple or white and corollas are about 1.1 cm in diameter. Fruits are spherical and about 8 cm in diameter, which become reddish-black when it matures. Seeds are nearly disc-shaped and about 1.5 mm in diameter. The flowering period of S. lyratum is between May and June, while the fruiting period is between August and October. Significantly, the suitable harvest time has been recommended to be between October and December (Fig. 2) [14, 17].
Plant morphology of S. lyratum, (a) leaves, (b) flowers (c) fruits (d) the whole plant [data originated from the Plant Photo Bank of China (http://ppbc.iplant.cn/), accessed 10 April 2022]
3 Traditional uses
In TCM, S. lyratum has been considered as one of the "Top-grade" herbs in "Shennong Bencao Jing" (100 BC-200 AD, Han Dynasties) [18]. For centuries, it has been used for the treatments of cold and fever, malaria, jaundice, nephritis, edema, cholecystitis, rheumatoid arthritis, vaginitis, uterine erosion, and several types of cancer including lung cancer, cervical cancer, and gastric cancer [19, 21]. External applications of S. lyratum [22] have been recorded to treat carbuncle, furuncle and swollen poison, etc. In the "Compendium of Materia Medica", S. lyratum is documented [11] to have the effects of clearing heat, detoxification, and expelling rheumatism, for the treatment of rubella, erysipelas, malaria, cancer, etc. The traditional uses of S. lyratum in Korea, Japan, and the Indochina Peninsula focused mainly on the treatments of several types of cancers, warts, herpes, pyretic syndrome, diarrhea, etc., as summarized in Table 1.
Traditional uses of S. lyratum
4 Phytochemistry
So far, hundreds of phytochemicals have been isolated and identified from S. lyratum, including steroidal alkaloids (1–41), steroids and steroidal saponins (42–101), terpenoids (102–153), nitrogenous compounds (154–178), phenylpropanoids (179–227), flavonoids (228–258) and other compounds (259–270). Among them, steroidal alkaloids, steroidal saponins and terpenoids are so often recognized as the main active constituents of S. lyratum [32, 33].
4.1 Steroidal alkaloids
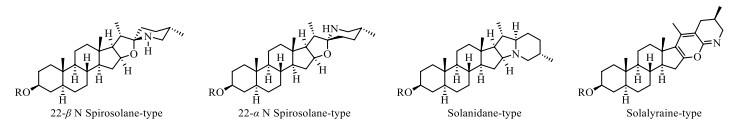
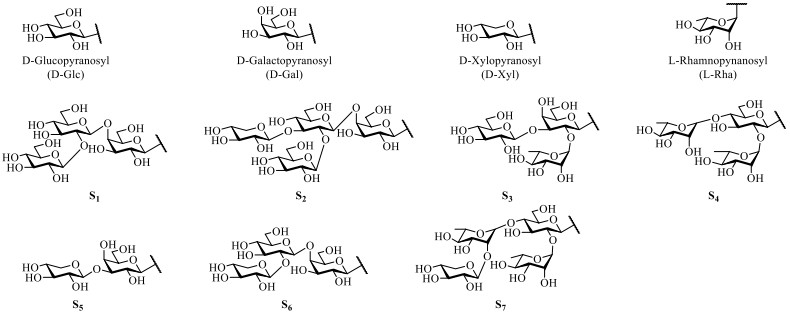
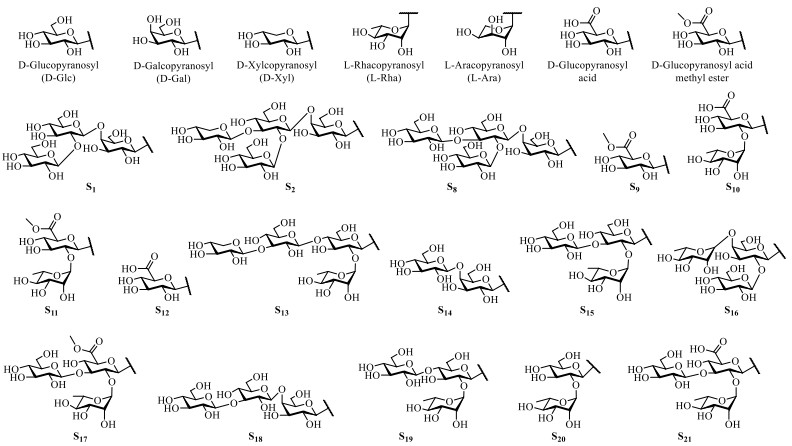
Steroidal alkaloids in S. lyratum include mostly solanidane (27 carbon atoms), spirosolane (27 carbon atoms) and solayraine (27 carbon atoms) (Fig. 3) types of nitrogenous sapogenins. The glycone moieties are most likely to be substituted at C-3 position of the nitrogenous sapogenin aglycone. D-glucose (D-Glc), D-galactose (D-Gal), D-xylose (D-Xyl), and L-rhamnose (L-Rha) are the common components of the glycones, in which one to four monosaccharides linked linearly or with one or more branched chains, as shown in Fig. 4.
Three types of steroidal alkaloid aglycones reported in S. lyratum
Glycones of steroidal alkaloids reported in S. lyratum
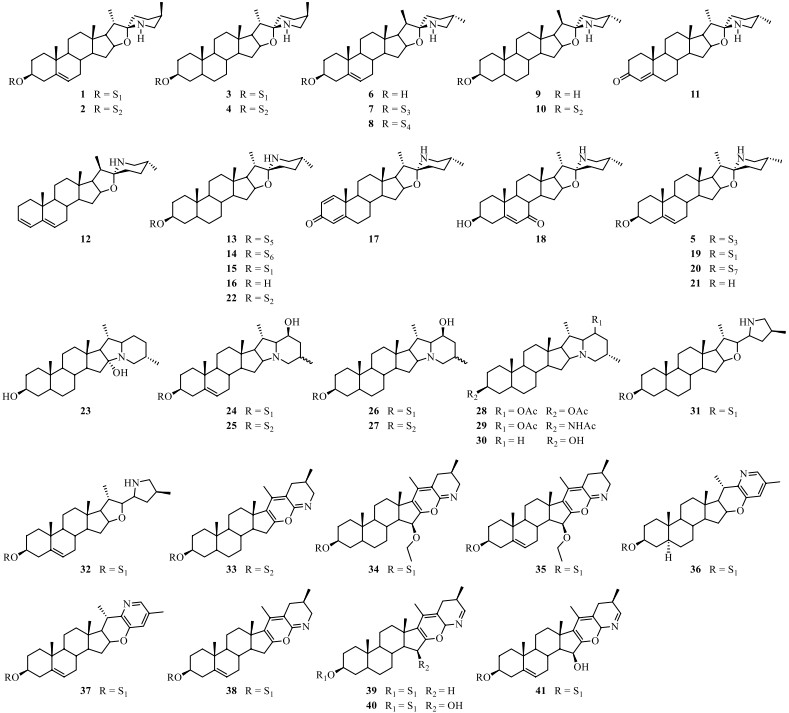
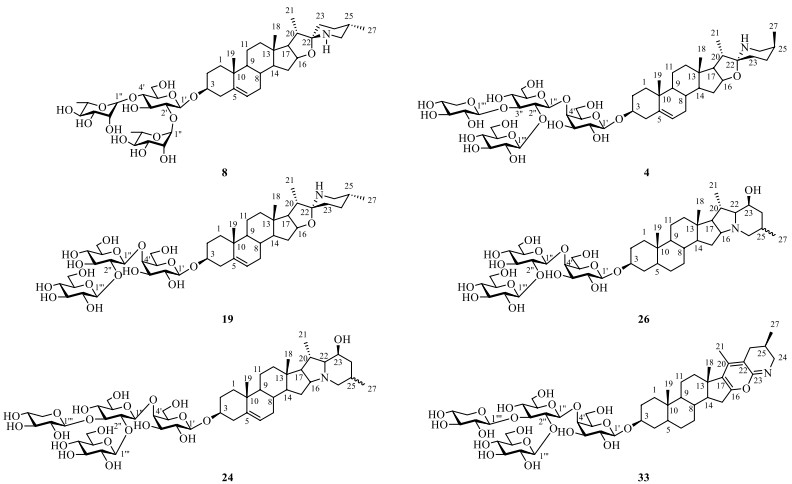
Steroidal alkaloid is one of the characteristic ingredients of Solanum plants [34]. Until now, a total of forty-one steroidal alkaloids (1–41) have been identified from S. lyratum (Table 2). It is noteworthy that there were two epimers for the most abundant spirosolane-type steroidal alkaloids in Solanum plants, one is 22-β N type (1–4, 6–11) [33, 35-39] and the other is 22-α N type (5, 13–22) [24, 35-40] in clue of the existence of an oxa-azaspirodecane system. In addition, solanidane-type steroidal alkaloids (23–30) [35, 36, 41, 42], with a unique octahydroindolizine complex cholestane skeleton, have also been found to be existed in this plant. Further, other unusual spirosolane-type glycoalkaloids with a deformed E and F rings (piperidine, pyridine or other derived F rings) have been also occasionally discovered from S. lyratum, exemplified by compounds 31–41 [39, 43], as shown in Fig. 5.
Steroidal alkaloids isolated from S. lyratum
Chemical structures of steroidal alkaloids from S. lyratum
4.2 NMR characteristics of steroidal alkaloids
Representative NMR data of the common steroidal alkaloids and saponins from S. lyratum were summarized in Tables 3, 4, 5 and 6. 13C NMR spectra of the spirosolane-type, spirostanol-type, and furostanol-type steroidal alkaloids or saponins, with intrinsic twenty-seven steroid skeleton, normally exhibit characteristic carbon signals for C-22 around δC 98.0 [35], 109.0 [22], and 112.0 [40] ppm, respectively. In addition, in the high field of the 1H NMR spectra of steroidal alkaloids and saponins, the resonance signals of methylene and methine protons are generally around δH 1.1–3.0, while the four methyl groups show proton resonances at δH 0.6–1.4, among which there are two singlets for the methyl groups at C-18 and C-19 [44], and two doublets for those methyls at C-21 and C-27 [45].
Representative 13C NMR data of six common steroid alkaloids
Steroids isolated from S. lyratum
Steroidal saponins isolated from S. lyratum
Representative 13C NMR data of six common steroidal saponins (in C5H5N-d5)
In the 13C NMR spectra, spirosolane-type of steroidal alkaloids, characterizing a A/B/C/D ring system of C27 steroid scaffold (Table 3 and Fig. 6), show twenty-seven carbon signals generally containing four methyl carbon signals around at δC 16.0 (C-18), 19.0 (C-19), 15.0 (C-21), and 19.0 (C-27). Steroidal alkaloid can be simply recognized to be a steroidal saponin with a replacement of the oxygen atom in F ring by a nitrogen atom, resulting in the high-field shifting of the carbon chemical shifts of C-22 and C-26, from δC 109.0 and 67.0 to 98.0 and 47.5, respectively [30, 39, 40, 46]. In the 13C NMR spectra of the solanidane-type steroidal alkaloids, there were four methyl carbon signals around at δC 16.0 (C-18), 12.0 (C-19), 19.0 (C-21), and 22.0 (C-27) [30, 38, 39, 46] besides those characteristic carbon signals around at δC 69.0 (C-16), 78.0 (C-22) and 58.0 (C-26) [35, 41]. And, chemical shifts of the glycosyl anomeric carbon are at δC 100.0–108.0, especially when the glycosidation taking place at OH-3 of the steroidal alkaloids [47-49].
Representative six common steroidal alkaloids
4.3 Steroids and steroidal saponins
4.3.1 Steroids
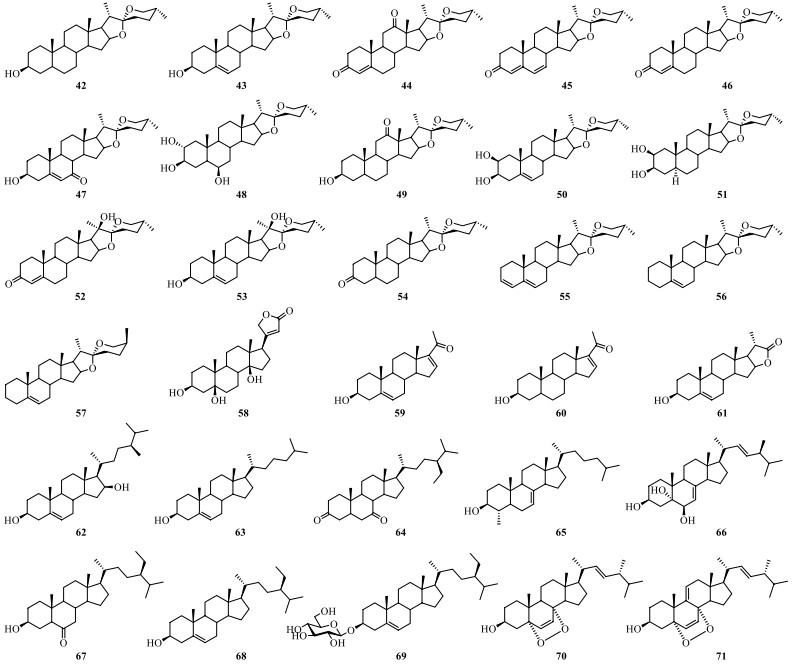
Cholestanol, containing a perhydrocyclopentenophenanthrene moiety (rings A, B, C and D) with a acyclic side-chain, has been considered as the precursor of furostanol and spirostanol (Fig. 7). At present, thirty steroids have been isolated from S. lyratum (42–71) (Table 4) [35, 36, 39, 47, 48, 50, 52].
Chemical structures of steroids from S. lyratum
4.3.2 Steroidal saponins
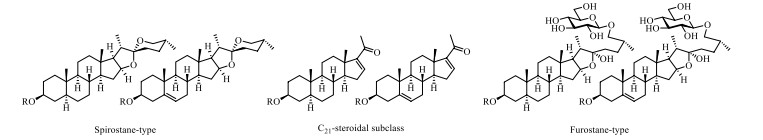
Steroidal saponins reported in S. lyratum are well-known characterized by possessing the steroid-derived aglycones normally consisting of a hydrophobic C27-skeleton of cholestane with an oxygen fused into the F ring, exemplified by the spirostanol (27 carbon atoms), furostanol (27 carbon atoms) and cholestanol (27 carbon atoms) as the most common scaffolds. Nevertheless, C21- steroidal saponins have also been reported to be existed in S. lyratum (Fig. 8). As is known, the remaining hydrophilic glycone unit of a steroidal saponin has been frequently reported to be substituted at the C-3 position of the sapogenin. D-Glc, D-Gal, D-Xyl, L-Rha, and L-arabinose (L-Ara) are the common members of the glycones, in which one to five monosaccharides linked linearly or with one or more branched chains, as shown in Fig. 9.
Sapogenin scaffolds of saponins reported in S. lyratum
Glycones of saponins reported in S. lyratum
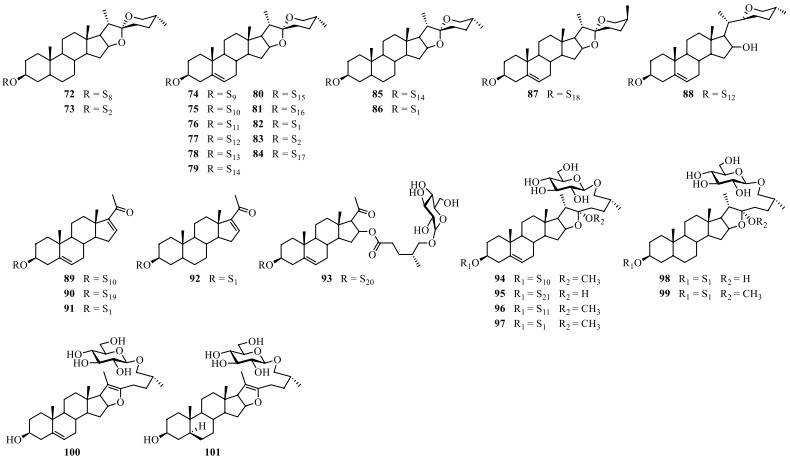
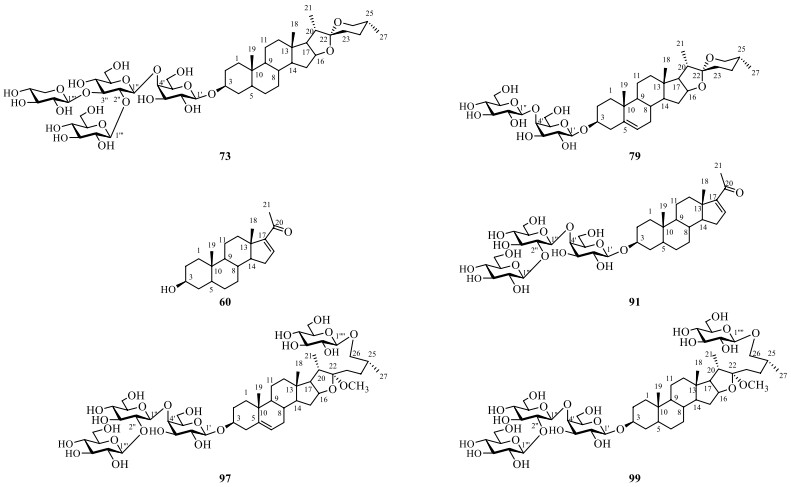
Till now, a total of thirty steroidal saponins (72–101) have been identified from S. lyratum (Table 5 and Fig. 10). Most of the isolated steroidal saponins of S. lyratum belong to the spirostane-type (72–87) [4, 22, 46, 47, 49], C21-steroidal subclass (89–93) [40, 46, 51] and furostanol-type (94–101) [22, 23, 40, 46]. Notably, when F-ring of a spirostanol is ring-opened, a new sapogenin skeleton of a furostanol is then afforded. As far as we know, the furostanol and its derivatives are the only reported ring-opened steroidal saponins ever isolated from S. lyratum up to now (94–101) [22, 23, 40, 46].
Chemical structures of steroidal saponins from S. lyratum
4.4 NMR characteristics of steroidal saponins
In short, in the 13C NMR spectra, spirostanol and furostanol-types of steroidal saponins, characterizing a A/B/C/D ring system of C27-steroidal scaffold, show twenty-seven carbon signals generally containing four methyl carbon signals at δC 16.0 (C-18), 19.0 (C-19), 14.0 (C-21), and 17.0 (C-27) or two olefinic carbon signals at δC 140.0 (C-5) and 120.0 (C-6) [47, 49] (Table 6 and Fig. 11). The carbon chemical shift of CH3-19 will down-field shifted from δC 12.0 to δC 19.0 [47, 49], when the two methylenes at C-5, 6 being dehydrogenated (-H2) to form a double bond [53]. The carbon resonances of C-16, 17, 22 (spiroketal carbon) and C-26 in a spirostanol-type steroidal saponin, were at about δC 81.0, 62.0, 109.0 and 67.0, respectively [47, 49], while those in a furostanol-type steroidal saponin were at about δC 81.0, 64.0, 112.0 and 75.0, respectively [22, 48]. It is worth noting that the proton chemical shift (δH) difference (∆ab = δa-δb) of the two geminal protons (Ha and Hb) of CH2-26 has been recognized for ascertaining 25R or 25S orientation of the CH3-27 [∆ab ≤0.48 for 25R, ∆ab≥ 0.57 for 25S] in the spirostanol and furostanol-types of steroidal saponins [45, 54, 55]. Similarly, the abovementioned empirical law for configuration assignment of C-25 applies equally well to spirosolane-type of steroidal alkaloids (Table 3).
Representative six common steroidal saponins
4.5 Terpenoids
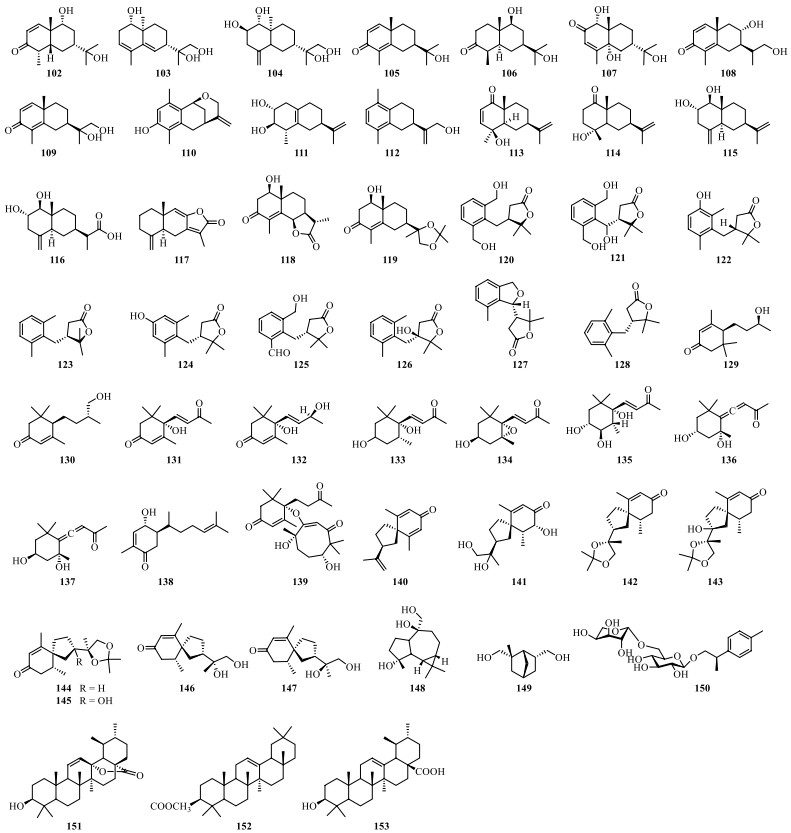
So far, fifty-one terpenoids, including sesquiterpenoids, monoterpenoids and triterpenoids (Table 7 and Fig. 12), have been isolated from S. lyratum. Among them, sesquiterpenoids are the most common terpenoids in S. lyratum, including eudesmane-type sesquiterpenoids (102–119) [52, 56-64] and the related derivatives (120–128) [39, 47, 58, 60, 62], monocyclic sesquiterpenoids (129–139) [25, 47, 56, 58, 62], vetispirane-type sesquiterpenoids (140–147) [1, 56, 62, 64], and guaiane-type sesquiterpenoids (148) [47]. In addition to the abovementioned constituents, two monoterpenoids (149–150) and three pentacyclic triterpenoids (151–153) have also been found in S. lyratum.
Terpenoids isolated from S. lyratum
Chemical structures of terpenoids from S. lyratum
4.6 Nitrogenous compounds
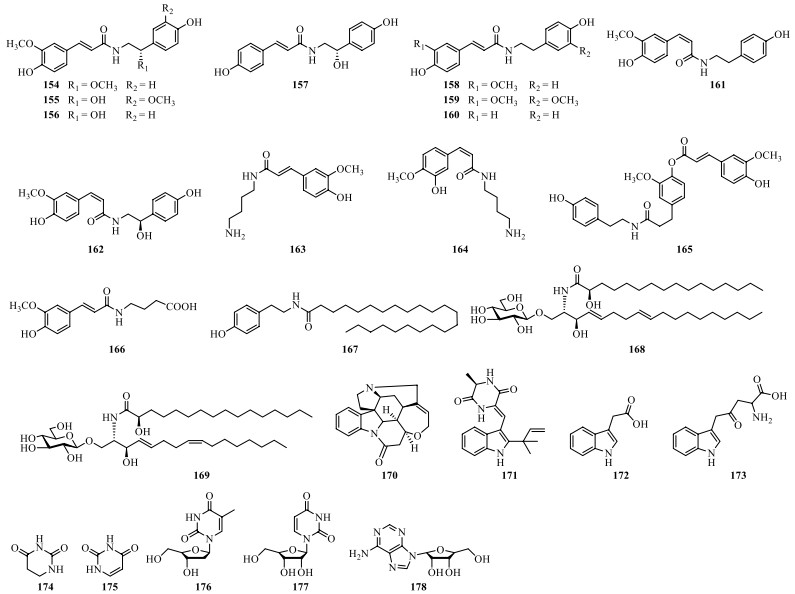
Nitrogenous compounds found in S. lyratum include arylamides (154–167) [36, 47, 65-68], aliphatic amides (168–169) [47], and other nitrogenous compounds (170–178) (Table 8 and Fig. 13) [47, 65].
Nitrogenous compounds isolated from S. lyratum
Chemical structures of nitrogenous compounds from S. lyratum
4.7 Phenylpropanoids
4.7.1 Lignans
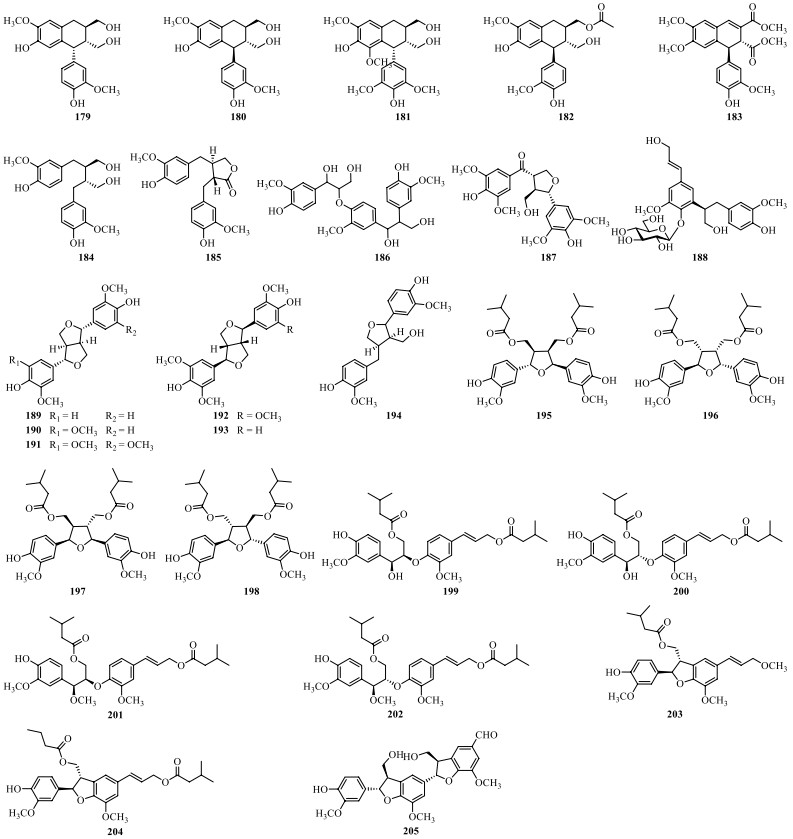
Till now, a total of twenty-eight lignans (179–205) have been isolated from S. lyratum (Table 9, Fig. 14), including simple lignans (184, 188) [32, 47, 68], lignanolides (185) [47], cyclolignans (179–183) [32, 47], monoepoxyligans (187), bisepoxylignans (189–193) [32, 47, 66], and norlignans (203–205) [20, 32, 47]. Lignans with one or more isovaleroyloxyl substitution, as exemplified by compounds 195–204 [20], has been frequently uncovered from S. lyratum in recent years. Notably, neolignans including compounds 186–187 [47], and 195–202, exhibited neuroprotective effects against human neuroblastoma SH-SY5Y cell injury induced by H2O2 [20].
Lignans isolated from S. lyratum
Chemical structures of lignans from S. lyratum
4.7.2 Coumarins, simple phenylpropanoids and their derivatives
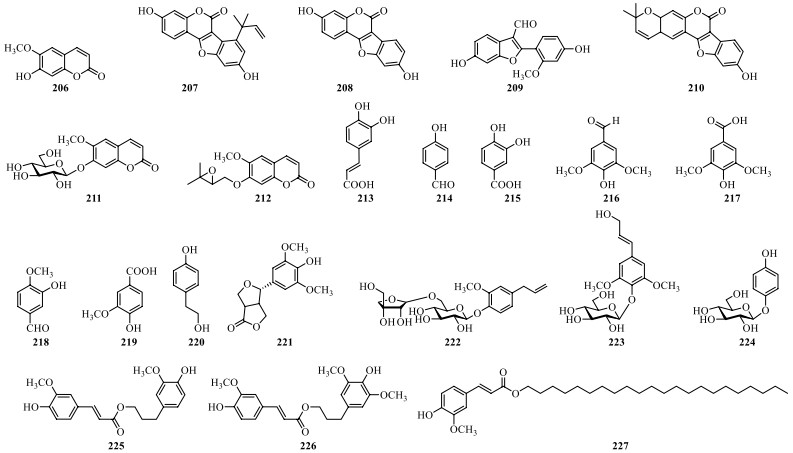
So far, seven coumarins (206–212) and eight simple phenylpropanoids (213–220) and their derivatives (221–227) have been isolated from S. lyratum as shown in Table 10 and Fig. 15.
Coumarins, simple phenylpropanoids and their derivatives isolated from S. lyratum
Chemical structures of coumarins, simple phenylpropanoids and their derivatives from S. lyratum
4.8 Flavonoids
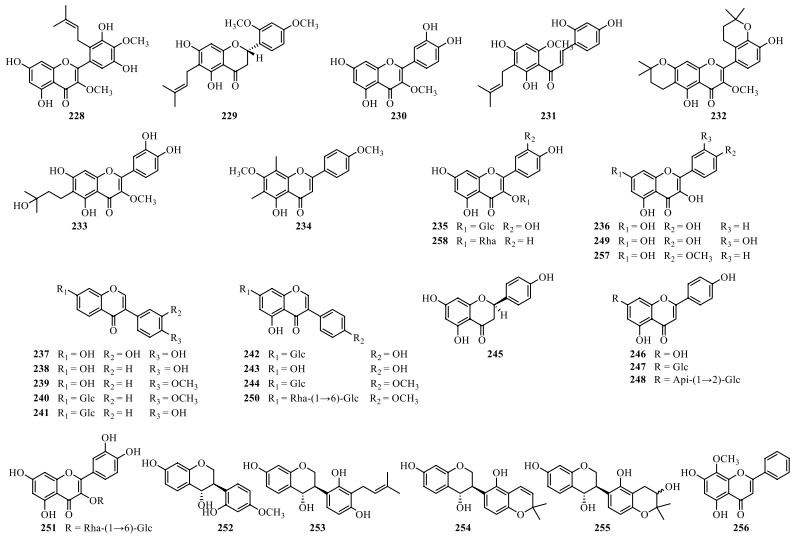
Thirty-one flavonoids (228–258) have been reported from S. lyratum (Table 11, Fig. 16), including flavonols (228, 230, 232–236, 246–249, 251, 256–258) [47, 54, 68, 70-72], flavanones (229, 245) [47, 72], isoflavones (237–244, 250) [47, 54, 69, 71], chalcones (231) [47], and isoflavan-4-ols (252–255) [73], usually in the form of flavonoid glycoside with C-3 or C-7 substitution of the monosaccharides (D-Glc, D-Gal, D-Xyl, L-Rha, and L-Ara) and disaccharides [Rha (1 → 6) Glc, Xyl (1 → 2) Glc, Api (1 → 2) Glc and Xyl (1 → 6) Glc].
Flavonoids isolated from S. lyratum
Chemical structures of flavonoids from S. lyratum
4.9 Other compounds
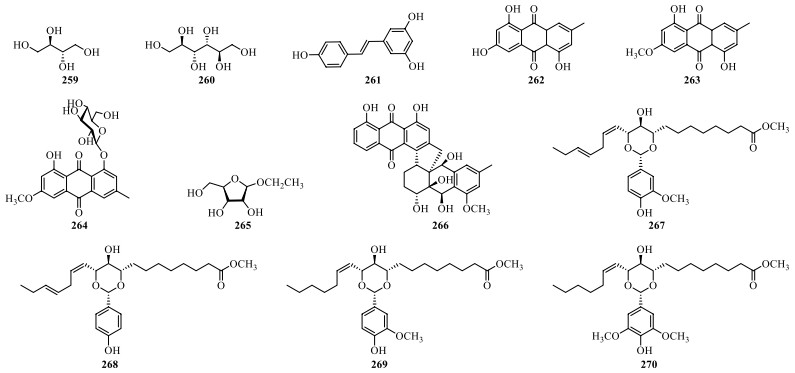
In addition to the abovementioned constituents, other compounds including anthraquinones and fatty acids have also been isolated from S. lyratum (Table 12, Fig. 17).
Other compounds isolated from S. lyratum
Chemical structures of other compounds reported from S. lyratum
5 Pharmacology
Water decoction of the whole herb of S. lyratum was commonly used to treat various diseases, and the fresh whole herb was mashed to remedy herpes and warts for external use. Modern pharmacological evaluations revealed that extracts, fractions or compounds isolated from S. lyratum possessed various therapeutic potentials. Recently, the plant has been most extensively studied for its anti-cancer pharmacological properties. Meanwhile, other pharmacological effects such as anti-inflammatory, anti-oxidant, anti-microbial, anti-allergy, and hepatoprotective activities of S. lyratum have also been assessed, as summarized in Table 13.
Pharmacological activities of S. lyratum
5.1 Anti-cancer
5.1.1 Extracts and fractions
The heat-clearing and detoxicating property of S. lyratum is favorable in the treatment of cancer [17, 19, 21]. It has been reported that S. lyratum treats various cancers by inhibiting the tumor growth [74, 75], enhancing immunity [76, 78] and inducing apoptosis via activating both extrinsic and intrinsic apoptotic pathways [27, 34, 79].
In S180 tumor-bearing mice, both ethanol and aqueous extracts of S. lyratum could improve immune function and exhibited anti-cancer potential with certain tumor inhibitory effect by improving the activities of natural killer (NK) and cluster of differentiation 4 (CD4) cells, and elevating the contents of serum Interleukin-2 (IL-2) and tumor necrosis factor-α (TNF-α) [77, 78, 80]. Total alkaloids from S. lyratum (SLTA, 24 mL/kg) could inhibit the tumor growth in mice with Lewis lung cancer, and when combined with cisplatin, a synergistic effect had been shown to down-regulate the mRNA expression of Notch1, Notch3 and Jagged1 in Notch signaling pathway [81]. In addition, the hexane fraction of the methanol extract (50 mg/kg) of S. lyratum showed similar inhibitory activity on tumor growth in mice with Lewis lung carcinoma tumor, potentially acting through up-regulating Fas, caspase-8, caspase-3, and p53, and down-regulating FasL and B-cell lymphoma-2 (Bcl-2) in the mitochondrial pathway [27, 79].
Further studies revealed that the 70% ethanol extract of S. lyratum (SLE) could suppress tumor angiogenesis in vitro by repressing migration, invasion, and tube formation of tumor-derived vascular endothelial cells (Td-ECs). The mechanism of the anti-angiogenic effect of SLE may be related to the inhibitory activity of vascular endothelial growth factor (VEGF) via reducing the number of lipid rafts in the cell membrane [43] and interfering with the lipid rafts by agglutinating cell membrane cholesterol [75]. These changes led to the inhibition of VEGFR2 phosphorylation and activation of its downstream signaling molecules, thereby inhibiting tumor angiogenesis [43]. In addition, SLTA could induce apoptosis of lung carcinoma A549 cells by inhibiting the nuclear factor-kappa B (NF-κB) signaling pathway [82], while glycoalkaloids of S. lyratum (SLGS) significantly inhibited the activity of A549-derived exosomes with IC50 = 99.59 μg/mL [43].
5.1.2 Compounds
The cytotoxic tests involved in most in vitro studies of S. lyratum have shown that the compounds isolated from S. lyratum possess a good cytotoxic potential for several cancer cells.
In the process of cytotoxic investigation by MTT assay and flow cytometry, the characteristic ompounds 5, 8, 21 from the methanolic extract of S. lyratum showed significant cytotoxicities against huh-7 and HepG2 cell lines with IC50 values of 9.6 ± 0.5 and 10.8 ± 0.1 μM, 11.7 ± 0.3 and 19.4 ± 0.4 μM, and 10.3 ± 1.5 and 91.8 ± 9.4 μM, respectively. The mechanism was attributed to cell cycle arrest at S-phase [83]. while sesquiterpenoids 104, 106, 108, 109, 116, 118, 124, 126, 127, 132, 135, 142, and 143 were evaluated for their cytotoxicity activities with IC50 1.9–8.6 μg/mL against HONE-1 cells [1, 57, 58, 60, 61]. Among them, compounds 126–127 showed potent cytotoxicity activity with IC50 2.1 and 1.9 μg/mL, slightly weaker than the positive controls etoposide and cisplatin (IC50 1.6 and 1.7 μg/mL) [1, 57, 58, 60, 61]. Notably, the IC50 differences of the positive controls (etoposide and cisplatin) may have been caused by the operation of the author, so the experimental cytotoxicity results need to be further verified.
Further, the cytotoxic potentials of nine steroids saponins and alkaloids (36, 72, 73, 81–83, 86) against ASGC7901 and BEL-7402 cancer cell lines were tested, and compounds 72, 73, and 83 showed attractive antiproliferative activities with respective IC50 values of 6.39–9.11 μM, 3.19–8.86 μM and 0.39–1.16 μM, as compared with IC50 values of 0.17–5.34 μM and 8.15–23.06 μM of positive control adriamycin and 5-fluorouracil, respectively [22]. Another, a glycoalkaloid (10) exhibited significant cytotoxicity against mouse colon cancer CT-26 cells with IC50 3.5 μM, as compared to IC50 values of 1.8 μM of positive control etoposide, in clue of the inhibition on the expressions of survivin and NF-κB/p65 and the induction of the AIF nuclear translocation [74]. Besides those characteristic constituents of S. lyratum, four other compounds 267–270 have been evaluated their cytotoxicities against hepatocellular carcinoma cell lines, and 267 and 269 showed significant inhibitory activities against HepG2 cell lines with IC50 values of 46.07 μM and 45.39 μM, respectively [26].
5.2 Anti-inflammatory
5.2.1 Extracts and fractions
Inflammation is closely related to cancer disease [84]. The detoxication and detumescence effect of S. lyratum can be used as a supplement to modern anti-inflammatory agents.
Total alkaloid fraction from the 70% ethanol extract of S. lyratum significantly relieved the inflammatory effect of the lipopolysaccharide-stimulated RAW264.7 macrophages for 48 h. Further evaluation revealed that this total alkaloid fraction could inhibit the release of Cyclooxygenase-2 (COX-2), and Prostaglandin E2 (PGE2) from lipopolysaccharide-stimulated RAW264.7 macrophages [85].
5.2.2 Compounds
In vitro, diosgenin-3-O-α-L-rhamnosyl-(1 → 2)-O-β-D-glucopyranosiduronic acid (75) could inhibit the lipopolysaccharide-induced expression of intercellular cell adhesion molecule-1 (ICAM-1) protein at 16 μg/mL, and exhibited anti-inflammatory activities [86]. In addition, in the anti-inflammatory experiments with polymorphonuclear leukocytes of rats (rat PMNs) with ginkgolide B as the positive control, compounds 139, 207–210 showed significant β-glucuronidase inhibitory activities with IC50 values range of 6.3–9.1 μM [25], while four 4-hydroxyisoflavans 252–255 afforded anti-inflammatory activities with inhibitory ratios release of β-glucuronidase in the range of 30.3–38.6% at 10 μM [73].
5.3 Antioxidant activity
5.3.1 Extracts
Modern pharmacological studies have revealed that cancer or other diseases are primarily associated with the production and accumulation of excessive free radicals [87], which are commonly produced by the continuous contact between our body and the outside world. Thus, antioxidants can effectively relieve the harmful effects of free radicals. It has been confirmed that S. lyratum extracts and compounds possess significant antioxidant activities.
50% Ethanol extract of S. lyratum (10 μg/mL) could protect against oxidized low-density lipoprotein (Ox-LDL)-induced injury in cultured human umbilical vein endothelial cells (HUVECs) by direct antioxidative action [88]. In the DPPH radical-scavenging tests in vitro using the spectrophotometric method with vitamin C as the positive control, the ethanol and ethyl acetate extracts from S. lyratum showed antioxidative potential with IC50 of 0.230 mg/mL and 1.010 mg/mL, respectively [89].
5.3.2 Compounds
Five flavones 236, 248, 249, 251, and 258 from the ethanol extracts of S. lyratum possessed the capability of scavenging DPPH free radicals with IC50 values of 2.56–21.33 μg/mL [70]. It seems that the glycosidation of the flavone C-3 and C-5 is essential for the scavenging of DPPH free radicals.
5.4 Antimicrobial
5.4.1 Extracts and fractions
There were reports verified the antibacterial potential of S. lyratum extracts. For example, the water-soluble polysaccharide of S. lyratum exerted a significant antibacterial activity against Staphylococcus aureus, Salmonella, Pasteurella, Escherichia coli, Candida albicans, and Pseudomonas aeruginosa. The inhibition zone diameters were > 13 mm at a concentration of 120 mg/mL [90].
5.4.2 Compounds
Several gram-positive bacteria (S. aureus and Enterococcus faecalis) were used to assess the antimicrobial activity of a new compound 266 from S. lyratum, with minimum inhibitory concentration (MIC) values of 2.0 μM (1.08 μg/mL) and 10.0 μM (5.44 μg/mL), respectively [21].
5.5 Other activities
The extracts of S. lyratum showed a therapeutic potential on the tetrachloride-induced liver damage in rats, by decreasing significantly the activity of transaminase in rat serum [91, 92]. Further, there was an in vivo report revealed that the aqueous extract of S. lyratum possessed strong antiallergy activity [93] by inhibiting dose-dependently the histamine release from the rat peritoneal mast cells and decreasing the mRNA expression of L-histamine decarboxylase [94]. Lastly, the ethanol extract of S. lyratum possesses molluscicidal activities with IC50 values of 30–50 mg/mL [95].
Notably, several Chinese patent prescriptions with S. lyratum as the major component herb, showed significant anticancer efficacies in reported clinic trials. For example, 'Baiyingtang' (composed of S. lyratum, Herba Patriniae, Houttuynia cordata, Lilium brownii var., Asparaguscochinchinensis(Lour.)Merr.) retention enema could reduce plasma transforming growth factor-β1 (TGF-β1), interleukin-6 (IL-6) levels and increase plasma IL-4 levels in patients with pelvic tumors receiving radiotherapy [96]. 'Baiying decoction' (composed of S. lyratum, Ophiocordyceps sinensis, Houttuynia cordata, Lilium brownii var., Asparaguscochinchinensis(Lour.)Merr.) treatment could ameliorate the marrow suppression and the quality of life in patients with advance non-small cell lung cancer, with high safety [97].
6 Toxicology
Up to now, the toxicity studies of the isolated compounds and extracts of S. lyratum may have been overlooked by researchers, while few studies have found the toxic potential of Solanum glycoalkaloid [125]. The toxic properties of Glycoalkaloids including solamargine (8) have been reviewed by Sinani AI S.S.S. et al. are due to (1) their ability to disrupt cell-membrane function by complexation with membrane 3β-hydroxysterols to form aggregates and damage the membrane integrity [126], (2) their anti-acetylcholinesterase activity on the central nervous system [126, 127], and (3) changes caused by them in active transport of ions through membranes, resulting in disorders in general body metabolism [126]. Additionally, in the acute toxicity experiment with rats, neither mortality nor clinical alterations were shown, except for the mild transient diarrhea with 70% ethanol extract of S. lyratum at 5000 mg/kg [31]. In future, more pharmacological evidences should be sought on the possible adverse effects and the toxicities of S. lyratum extracts and their bioactive constituents when used in treatments of acute, subchronic, or chronic diseases. Further, more clinical trials must be conducted to evaluate the safety and clinical efficacy of S. lyratum in humans.
7 Conclusion
This review summarized the latest advancements of S. lyratum in botany, traditional uses, phytochemistry, pharmacology, and toxicology. Phytochemical and pharmacological studies have validated many modern usages of this plant. A total of 270 chemical constituents have been isolated from S. lyratum, including steroidal alkaloids, steroidal saponins, terpenoids, nitrogenous compounds, phenylpropanoids, flavonoids, etc. It has been popular in traditional practices due to its potential efficacy on cancer and inflammation, and showed important biological properties in scientific investigations. In the phytochemical analysis of S. lyratum extracts, aqueous and ethanol extracts were commonly acquired from S. lyratum, whose main components included total alkaloids and total saponins. In modern pharmacological studies, compounds and extracts from S. lyratum were evaluated in vivo and in vitro, and their anticancer and cytotoxic, anti-inflammatory, antioxidant, antimicrobial, anti-allergy, and hepatoprotective activities have been demonstrated. However, many aspects of S. lyratum have not been studied yet and some relative studies on S. lyratum should be further explored in the following aspects in the future.
Firstly, the pharmacological activities are mostly proven from the aqueous and ethanol extracts from S. lyratum, while insufficient pharmacological studies have been conducted on pure compounds. In addition, some activities are lacking comparisons to standards or positive and negative controls. Other studies especially on anticancer and anti-inflammatory activities have shown that the IC50 values of the test extracts/compounds of S. lyratum are above 200 μg/mL, which can be considered that such extracts/compounds are actually poorly active.
Secondly, pharmacological studies were mostly performed in cell models and animals while investigations in humans have been seldomly performed. Hence, the future investigation should be focused on the bioactivity of S. lyratum in various clinical studies with humans. In addition, the DPPH radical scavenging test and antimicrobial activities also should be guaranteed in vivo, instead of solely relying on method models in vitro.
Thirdly, global quality control standards of S. lyratum are needed urgently and should be improved. Simultaneous qualitative and quantitative measures are recommended to be used for those major active constituents of S. lyratum.
Finally, in toxicological studies of S. lyratum, no unequivocal proof of the toxicological activities in human exists. Further, relationship studies between systematic toxicity and safety evaluation are still needed to assure safety for clinical application in the future. Pharmacological effects of S. lyratum have been demonstrated by ethanol and aqueous extracts of high doses, the effectiveness of high doses extracts in treating diseases provides the possibility of finding active compounds. Therefore, it is important to study the therapeutic window (the range between the doses that produce the desired therapeutic effect and doses that produce toxicity) and the long-term in vivo toxicity for further research on S. lyratum.
In summary, S. lyratum can be considered as an important and valuable resources for human's health. Further research is needed in terms of quality control, toxicity and pharmacological mechanism to provide a theoretical basis for exploitation of the medicinal functions of S. lyratum.
Notes
Acknowledgements
The authors are grateful to the staff of researchers at the State Key Laboratory of Component-based Chinese Medicine, Institute of Traditional Chinese Medicine, Tianjin University of Traditional Chinese Medicine. The authors acknowledge the support of the Tianjin Committee of Science and Technology of China, the National Key Research and Development Project of China, and the Important Drug Development Fund, Ministry of Science and Technology of China.
Author contributions
The manuscript was prepared by Y. Zhao. Y. Zhao, W.-K. Gao, and X.-D. Wang completed the writing of this review. The research work was supported by the projects of H.-H. Wu. All the authors reviewed the final version of the manuscript and approve it for publication. To the best of our knowledge and belief, this manuscript has not been published in whole or in part nor is it being considered for publication elsewhere. All authors have seen the manuscript and approved to submit to your journal. All authors read and approved the final manuscript.
Funding
This study was funded by a grant (No. 21ZYJDJC00080) from the Tianjin Com- mittee of Science and Technology of China, the National Key Research and Development Project of China (No. 2018YFC1707904, 2018YFC1707905, and 2018YFC1707403) and the Important Drug Development Fund, Ministry of Science and Technology of China (No. 2018ZX09735-002).
Declarations
Competing interests
The authors declare no conflict of interest.
References
-
1.Yao F, Song QL, Zhang L, Li GS, Dai SJ. Three new cytotoxic sesquiterpenoids from Solanum lyratum[J]. Phytochem Lett 6, 453-6 (2013) CrossRef PubMed Google Scholar
-
2.Kang B, Lee E, Hong I, Lee J, Kim H. Abolition of anaphylactic shock by Solanum lyratum Thunb[J]. Int J Immunopharmacol 19, 729-34 (1997) PubMed Google Scholar
-
3.Ren J, Feng G, Wang M, Sun L. The primary study on the anti-tumor effect of total saponin of Solanum lyratum Thunb[J]. Cancer Res Prevent Treat 33, 262-4 (2006) PubMed Google Scholar
-
4.Shoji Y, Murakami N, Yamasaki M. A furostanol glucuronide from Solanum lyratum[J]. Phytochemistry 24, 2748-50 (1985) CrossRef PubMed Google Scholar
-
5.Chen FF, Zhang YW, Huang XF. Steroidal saponins and their pharmacological activities in Solanum plants[J]. Zhongguo Zhong Yao Za Zhi 41, 976-88 (2016) PubMed Google Scholar
-
6.Bártová V, Bárta J, Jarošová M. Antifungal and antimicrobial proteins and peptides of potato (Solanum tuberosum L.) tubers and their applications[J]. Appl Microbiol Biotechnol 103, 5533-47 (2019) CrossRef PubMed Google Scholar
-
7.Kaunda JS, Zhang YJ. The genus solanum: an ethnopharmacological, phytochemical and biological properties review[J]. Nat Prod Bioprospect 9, 77-137 (2019) CrossRef PubMed Google Scholar
-
8.Elizalde-Romero CA, Montoya-Inzunza LA, Contreras-Angulo LA, Heredia JB, Gutiérrez-Grijalva EP. Solanum fruits: phytochemicals, bioaccessibility and bioavailability, and their relationship with their health-promoting effects[J]. Front Nutr 8, 790582 (2021) CrossRef PubMed Google Scholar
-
9.Su J. Xinxiu Bencao. Shanghai: Shanghai Scientific & Technical Publishers; 1957. p. 174. PubMed Google Scholar
-
10.Tang SW, Shang ZJ. Zhenglei Bencao. Shanghai: Shanghai Classics Publishing House; 1991. p. 176. PubMed Google Scholar
-
11.Wu P, Sun XY, Sun FY. Compendium of Materia Medica. (Beijing: Scientific and technical documentation press, 1996), p. 20. PubMed Google Scholar
-
12.Du X. Study on the material basis mechanism and preclinical safety of total alkaloids from Solanum lyratum against NSCLC, Doctoral thesis. China Academy of Chinese Medical Sciences. 2020. PubMed Google Scholar
-
13.Jiang XW, Wu QN. Comparison and identification of Solanum lyratum Thunb and Aristolochia mollissima Hance[J]. J Jiangxi Univ Tradit Chin Med 18, 35-6 (2006) PubMed Google Scholar
-
14.Chen XM, Chen QH. Solanum lyratum and its mixed varieties[J]. Zhong Yao Cai 28, 462-3 (2005) PubMed Google Scholar
-
15.Liu XB, Wu J. Study on pharmacognostic identification of Solanum lyratum[J]. J Sci Teachers' Univ 41, 72-6 (2021) PubMed Google Scholar
-
16.Knapp S. A revision of the Dulcamaroid Clade of Solanum L. (Solanaceae)[J]. PhytoKeys 22, 1-432 (2013) CrossRef PubMed Google Scholar
-
17.Editorial Board of Flora of China. Flora of China. Beijing: China Science Publishing; 1978. p. 86–7. PubMed Google Scholar
-
18.Ming HY, Wang E, Ma XP. Study on the ethnic medicine Solanum lyratum Thunb. and patent literature analysis[J]. Asia-Pacific Tradit Med 13, 59-61 (2017) PubMed Google Scholar
-
19.Xiao ZM, Wang AM, Wang XY, Shen SR. A study on the inhibitory effect of Solanum lyratum Thunb extract on Lewis lung carcinoma lines[J]. Afr J Tradit Complement Altern Med 10, 444-8 (2013) CrossRef PubMed Google Scholar
-
20.Li SS, Hou ZL, Yao GD, Guo R, Wang YX, Lin B, Huang XX, Song SJ. Lignans and neolignans with isovaleroyloxy moiety from Solanum lyratum Thunb.: chiral resolution, configurational assignment and neuroprotective effects[J]. Phytochemistry 178, 112461 (2020) CrossRef PubMed Google Scholar
-
21.Chen H, Du K, Sun Y, Hao Z, Zhang Y, Bai J, Wang Q, Hu H, Feng W, Solanrubiellin A. a hydroanthraquinone dimer with antibacterial and cytotoxic activity from Solanum lyratum[J]. Nat Prod Res 34, 3176-81 (2020) CrossRef PubMed Google Scholar
-
22.Xu YL, Lv J, Wang WF, Liu Y, Xu YJ, Xu TH. New steroidal alkaloid and furostanol glycosides isolated from Solanum lyratum with cytotoxicity[J]. Chin J Nat Med 16, 499-504 (2018) PubMed Google Scholar
-
23.Shoji Y, Mitsuyuki M, Murakami N. Two new steroidal glucuronides from Solanum lyratum, Ⅱ1[J]. Planta med 6, 496-8 (1986) PubMed Google Scholar
-
24.Lee Y, Hsu FL, Nohara T. Two new soladulcidine glycosides from Solanum lyratum[J]. Chem Pharm Bull 45, 1381-2 (1997) CrossRef PubMed Google Scholar
-
25.Zhang DW, Yang Y, Yao F, Yu QY, Dai SJ. Solalyratins A and B, new anti-inflammatory metabolites from Solanum lyratum[J]. J Nat Med-Tokyo 66, 362-6 (2012) CrossRef PubMed Google Scholar
-
26.Li SS, Liu QB, Zhang YY, Shi SC, Guo R, Yao GD, Lin B, Huang XX, Song SJ. Oxylipin vanillyl acetals from Solanum lyratum[J]. Fitoterapia 143, 104559 (2020) CrossRef PubMed Google Scholar
-
27.Lee JH, Lee YH, Lee HJ, Lee HJ, Lee EO, Ahn KS, Shim BS, Bae H, Choi SH, Ahn KS, Baek NI, Kim DK, Kim SH. Caspase and mitogen activated protein kinase pathways are involved in Solanum lyratum herba induced apoptosis[J]. J Ethnopharmacol 123, 121-7 (2009) CrossRef PubMed Google Scholar
-
28.Ikeda T, Tsumagari H, Honbu T, Nohara T. Cytotoxic activity of steroidal glycosides from Solanum plants[J]. Biol Pharm Bull 26, 1198-201 (2003) CrossRef PubMed Google Scholar
-
29.Yang JS, Wu CC, Kuo CL, Lan YH, Yeh CC, Yu CC, Lien JC, Hsu YM, Kuo WW, Wood WG, Tsuzuki M, Chung JG. Solanum lyratum extracts induce extrinsic and intrinsic pathways of apoptosis in WEHI-3 murine leukemia cells and inhibit allograft tumor[J]. Evid Based Complement Alternat Med 2012, 254960 (2012) PubMed Google Scholar
-
30.Lee MH, Cheng JJ, Lin CY, Chen YJ, Lu MK. Precursor-feeding strategy for the production of solanine, solanidine and solasodine by a cell culture of Solanum lyratum[J]. Process Biochem 42, 899-903 (2007) CrossRef PubMed Google Scholar
-
31.Guo XH, Weng LJ, Yi LT, Geng D. Toxicological safety evaluation in acute and 21-day studies of ethanol extract from Solanum lyratum Thunb[J]. Evid Based Complement Alternat Med 31, 8518324 (2022) PubMed Google Scholar
-
32.Xu YL, Xu YJ, Shi HX, Liu Y, Xu TH. Lignanoids from Solanum lyratum[J]. J Chin Pharm Sci 27, 289-94 (2018) PubMed Google Scholar
-
33.Han L, Wang JN, Sun CX, Cao XQ, Du X. Anti-angiogenic activities of glycoalkaloids isolated from Solanum lyratum in tumor-derived vascular endothelial cells[J]. Phytochem Lett 29, 212-9 (2019) CrossRef PubMed Google Scholar
-
34.Zhao DK, Zhao Y, Chen SY, Kennelly EJ. Solanum steroidal glycoalkaloids: structural diversity, biological activities, and biosynthesis[J]. Nat Prod Rep 38, 1423-44 (2021) CrossRef PubMed Google Scholar
-
35.Zang YL. Study on antitumor active components of Solanum lyratum Thunb, Master's thesis. China Academy of Chinese Medical Sciences. 2008. PubMed Google Scholar
-
36.Yang YD. Studies on the chemical constitutents and bioactivities of three plants, Doctoral thesis. Huazhong University of Science and Technology. 2014. PubMed Google Scholar
-
37.Jia YR, Tian XL, Liu K, Chen C, Wang XL, Zhang CC, Sun LX. Simultaneous determination of four alkaloids in Solanum lyratum Thunb by UPLC-MS/MS method[J]. Pharmazie 67, 111-5 (2012) PubMed Google Scholar
-
38.Ye WC, Wang H, Zhao SX, Che CT. Steroidal glycoside and glycoalkaloid from Solanum lyratum[J]. Biochem Syst Ecol 29, 421-3 (2001) CrossRef PubMed Google Scholar
-
39.Wu T, Du X, Wang JN, Liu LY, Yang YK. Two new glycoalkaloids from Solanum lyratum Thunb[J]. J Chin Pharm Sci 30, 518-23 (2021) CrossRef PubMed Google Scholar
-
40.Yin HL. Study on the active constituents from Solanum indicum and Solanum lyratum for ANTI-HSN1 influenza virus, Doctoral thesis. Beijing University of Technology. 2013. PubMed Google Scholar
-
41.MurakamI K, Ezima H, Takaishi Y, Takeda Y, Fujita T, Sato A, Tomimatsu T. Studies on the constituents of Solanum plants. V. The constituents of S. lyratum Thunb Ⅱ[J]. Chem Pharm Bull 33, 67-73 (1985) CrossRef PubMed Google Scholar
-
42.Qi W. Study on chemical constituents and pharmacokinetics of anti-tumor traditional Chinese medicine Solanum lyratum, master's thesis. Shenyang Pharmaceutical University. 2009. PubMed Google Scholar
-
43.Du X, Wang JN, Sun J, Wu T, Cao XQ, Liu LY, Yang YK. Steroidal glycoalkaloids from Solanum lyratum inhibit the pro-angiogenic activity of A549-derived exosomes[J]. Fitoterapia 141, 104481 (2020) CrossRef PubMed Google Scholar
-
44.Sun LX, Li F, Wang C, Li W, Wang M, Bi K. Isolation and identification of chemical constituents of Solanum lyratum Thunb[J]. J Shenyang Pharm Univ 25, 36-46 (2008) PubMed Google Scholar
-
45.Simonet AM, Durán AG, Pérez AJ, Macías FA. Features in the NMR spectra of the aglycones of Agave spp. saponins. HMBC method for aglycone identification (HMAI)[J]. Phytochem Anal 32, 38-61 (2021) CrossRef PubMed Google Scholar
-
46.MurakamI K, Saijo R, Nohara T, Tomimatsu T. Studies on the constituents of Solanum plants. L. on the constituents of the stem parts of Solanum lyratum Thunb[J]. Yakugaku Zasshi 101, 275-9 (1981) CrossRef PubMed Google Scholar
-
47.Xu YL. Study on chemical constituents of Solanum lyratum Thunb and its antitumor activity, Doctoral thesis. Beijing University of Chinese Medicine. 2019. PubMed Google Scholar
-
48.Kang SY, Sung SH, Park JH, Cho JH, Kim YC. A phenolic glucoside and steroidal sapogenins of Solanum lyratum[J]. Yakhak Hoeji 44, 534-8 (2000) PubMed Google Scholar
-
49.Hirai Y, Konishi T, Sanada S, Ida Y, Hoji J. Studies on the constituents of Aspidistra elatior Blume. I. on the steroids of the underground part[J]. Chem Pharm Bull 30, 3476-84 (1982) CrossRef PubMed Google Scholar
-
50.Yang L, Feng F, Gao Y. Chemical constituents from herb of Solanum lyratum[J]. Zhongguo Zhong Yao Za Zhi 34, 1805-8 (2009) PubMed Google Scholar
-
51.Sun LX, Fu WW, Ren J, Xu L, Bi KS, Wang MW. Cytotoxic constituents from Solanum lyratum[J]. Arch Pharm Res 29, 135-9 (2006) CrossRef PubMed Google Scholar
-
52.Yu SM, Kim HJ, Woo E, Park H. Some Sesquiterpenoids and 5α, 8α-epidioxysterols from Solanum lyratum[J]. Arch Pharm Res 17, 1-4 (1994) CrossRef PubMed Google Scholar
-
53.Wang Y, Kazuhir O, Roji K, Kazuo Y. Steroidal saponins from fruits of Tribulus Terrestis[J]. Phytochemistry 42, 1417-22 (1996) CrossRef PubMed Google Scholar
-
54.Agrawal PK. Assigning stereodiversity of the 27-Me group of furostane-type steroidal saponins via NMR chemical shifts[J]. Steroids 70, 715-24 (2005) CrossRef PubMed Google Scholar
-
55.Agrawal PK. Dependence of 1H NMR chemical shifts of geminal protons of glycosyloxy methylene (H2-26) on the orientation of the 27-methyl group of furostane-type steroidal saponins[J]. Magn Reson Chem 42, 990-3 (2004) CrossRef PubMed Google Scholar
-
56.Yue XD, Yao F, Zhang L, Li GS, Dai SJ. Sesquiterpenoids from Solanum lyratum[J]. Zhongguo Zhong Yao Za Zhi 39, 453-6 (2014) PubMed Google Scholar
-
57.Nie XP, Zhang L, Yao F, Xiao K, Dai SJ. Study on sesquiterpenes from Solanum septemlobum[J]. Zhongguo Zhong Yao Za Zhi 40, 1514-7 (2015) PubMed Google Scholar
-
58.Ren Y, Shen L, Zhang DW, Dai SJ. Two new sesquiterpenoids from Solanum lyratum with cytotoxic activities[J]. Chem Pharm Bull (Tokyo) 57, 408-10 (2009) CrossRef PubMed Google Scholar
-
59.Nie XP, Yao F, Yue XD, Li GS, Dai SJ. New eudesmane-type sesquiterpenoid from Solanum lyratum with cytotoxic activity[J]. Nat Prod Res 28, 64-5 (2014) PubMed Google Scholar
-
60.Yao F, Song QL, Zhang L, Li GS, Dai SJ. Solajiangxins A-C, three new cytotoxic sesquiterpenoids from Solanum lyratum[J]. Fitoterapia 89, 200-4 (2013) CrossRef PubMed Google Scholar
-
61.Li GT, Yao F, Zhang L, Yue XD, Dai SJ. Two new cytotoxic sesquiterpenoids from Solanum lyratum[J]. Chinese Chem Lett 4, 1030-2 (2013) PubMed Google Scholar
-
62.Li SS, Cheng ZY, Zhang YY, Guo R, Wang XB, Huang XX, Li LZ, Song SJ. Sesquiterpenoids from the herbs of Solanum lyratum and their cytotoxicity on human hepatoma cells[J]. Fitoterapia 139, 104411 (2019) CrossRef PubMed Google Scholar
-
63.Shang SZ, Zhao W, Tang JG, Pu JX, Zhu DL, Yang L, Sun HD, Yang GY, Chen YK. 14-Noreudesmane sesquiterpenes from leaves of Nicotiana tabacum and their antiviral activity[J]. Phytochem Lett 17, 173-6 (2016) CrossRef PubMed Google Scholar
-
64.Li GS, Yao F, Zhang L, Yue XD, Dai SJ. New sesquiterpenoid derivatives from Solanum lyratum and their cytotoxicities[J]. J Asian Nat Prod Res 16, 129-34 (2014) CrossRef PubMed Google Scholar
-
65.Sun LX, Qi W, Yang HY, Jia YR, Tong LJ. Nitrogen-containing compounds from Solanum lyratum Thunb[J]. Biochem Syst Ecol 39, 203-4 (2011) CrossRef PubMed Google Scholar
-
66.Wang XW, Zhao Y, Dong XQ, Wu XL, Yu HY, Zhang LH, Han LF, Zhang Y, Wang T, Wu HH. Amides and lignans from Solanum lyratum[J]. Phytochem Lett 45, 25-9 (2021) CrossRef PubMed Google Scholar
-
67.Yang JZ, Guo GM, Zhou LX, Ning D. Study on the chemical constituents of Solanum lyratum[J]. Zhongguo Zhong Yao Za Zhi 27, 46-7 (2002) PubMed Google Scholar
-
68.Li RL. Extraction, isolation and structural identification of chemical constituents of Solanum lyratum Thunb, Master's thesis. Zhengzhou University. 2006. PubMed Google Scholar
-
69.Yin HL, Li JL, Dong JX. Chemical constituents from Solanum lyratum Thunb[J]. Military Med Sci 34, 65-7 (2010) PubMed Google Scholar
-
70.Ji H, Hou LH, Yuan M. Chemical Constituents Having Antioxidant Activities of Solanum lyratum[J]. Chem Nat Compd+ 50, 179-80 (2014) CrossRef PubMed Google Scholar
-
71.Zhang DW, Li GH, Yu QY, Dai SJ. Studies on flavonoids and amides from herbs of Solanum lyratum[J]. China J Chin Mat med 34, 721-3 (2009) PubMed Google Scholar
-
72.Wang LJ. Study on chemical constituents of Chinese herbal medicine for anti-cancer and anti-malarial, Maeter's thesis. ZhengZhou University. 2004. PubMed Google Scholar
-
73.Zhang DW, Li GH, Yu QY, Dai SJ. New anti-inflammatory 4-hydroxyisoflavans from Solanum lyratum[J]. Chem Pharm Bull (Tokyo) 58, 840-2 (2010) CrossRef PubMed Google Scholar
-
74.Kim SP, Nam SH, Friedman M. The tomato glycoalkaloid α-tomatine induces caspase-independent cell death in mouse colon cancer CT-26 cells and transplanted tumors in mice[J]. J Agr Food Chem 63, 1142-50 (2015) CrossRef PubMed Google Scholar
-
75.Han L, Wang JN, Cao XQ, Sun CX, Du X. An-te-xiao capsule inhibits tumor growth in non-small cell lung cancer by targeting angiogenesis[J]. Biomed Pharmacother 108, 941-51 (2018) CrossRef PubMed Google Scholar
-
76.Xiao ZM, Wang AM, Wang XY, Shen SR. A study on the inhibitory effect of Solanum lyratum thumb extract on lewis lung carcinoma lines[J]. Afr J Tradit Complement Altern Med 10, 444-8 (2014) PubMed Google Scholar
-
77.Guan Y, Zhao H, Yan X, Meng J, Wang WL. Study on anti-tumor effect of Solanum lyratum Thunb. extract in S180 tumor-bearing mice[J]. Afr J Tradit Complement Altern Med 10, 345-51 (2013) PubMed Google Scholar
-
78.Yang JS, Wu CC, Kuo CL, Yeh CC, Chueh FS, Hsu CK, Wang CK, Chang CY, Ip SW, Hsu YM, Kuo WW, Chung JG. Solannm lyratum extract affected immune response in normal and leukemia murine animal in vivo[J]. Hum Exp Toxicol 29, 359-67 (2010) CrossRef PubMed Google Scholar
-
79.Mo XQ, Wei HY, Huang GR, Xu LY, Chen YL, Qi J, Xian W, Qin YC, Wei LD, Zhao LJ, Huang YQ, Xing W, Pu HQ, Wei PY, Li CG, Liang QC. Molecular mechanisms of apoptosis in hepatocellular carcinoma cells induced by ethanol extracts of Solanum lyratum Thumb through the mitochondrial pathway[J]. World J Gastroentero 23, 1010-7 (2017) CrossRef PubMed Google Scholar
-
80.Wu T, Wang J, Du X. Effect of the effective parts of total steroidal alkaloids from Solanum lyratum on tumor inhibition in vivo and immune regulation in vitro[J]. Cent South Pham 18, 1786-90 (2020) PubMed Google Scholar
-
81.Sun XF, Hu SL, Zhou F, Wang Y. Effect of total alkaloids from Solanum lyratum Thunb. combined with cisplatin on tumor growth in mice with Lewis lung cancer[J]. Chin J Clin Pharmacol 35, 1781-3 (2019) PubMed Google Scholar
-
82.Wei X, Li CG, Li T, Nong S, Yan WJ. Solanum lyratum Thunberg alkaloid induces human lung adenoearcinoma A549 cells apoptosis by inhibiting NF-κB signaling pathway[J]. Zhong Yao Cai 36, 787-90 (2013) PubMed Google Scholar
-
83.Fekry MI, Ezzat SM, Salama MM, Alshehri OY, Al-Abd AM. Bioactive glycoalkaloides isolated from Solanum melongena fruit peels with potential anticancer properties against hepatocellular carcinoma cells[J]. Sci Rep-UK 9, 1-11 (2019) CrossRef PubMed Google Scholar
-
84.Cao W, Zhou XQ. Protective effect of notoginsenoside and tanshinone ⅡA on inflammation-related colorectal cancer mice and the inhibition effect on COX-2 expression[J]. Digit Chin Med 4, 54-63 (2021) CrossRef PubMed Google Scholar
-
85.Lin SH, Yi Y, Yu NC. Anti-inflammatory activity of total alkaloids from Solanum lyratum[J]. China Pharm 19, 1263-6 (2016) CrossRef PubMed Google Scholar
-
86.Zhou XX. Studies on the separation of chemical constituents of Solanum lyratum Thunb. and the interaction of pharmacodynamics, Master's thesis. Huazhong Agricultural University. 2020. PubMed Google Scholar
-
87.Taveira M, Sousa C, Valentão P, Ferreres F, Teixeira JP, Andrade PB. Neuroprotective effect of steroidal alkaloids on glutamate-induced toxicity by preserving mitochondrial membrane potential and reducing oxidative stress[J]. J Steroid Biochem 140, 106-15 (2014) CrossRef PubMed Google Scholar
-
88.Kuo WW, Huang CY, Chung JG, Yang SF, Tsai KL, Chiu TH, Lee SD, Ou HC. Crude extracts of Solanum lyratum protect endothelial cells against oxidized low-density lipoprotein-induced injury by direct antioxidant action[J]. J Vasc Surg 50, 849-60 (2009) CrossRef PubMed Google Scholar
-
89.Chen CJ, Huang KY, Sun CL. Study on DPPH free radicals caventinct activity of Solanum lyratum Thunb fruit extractives[J]. Food Res Dev 27, 45-8 (2006) PubMed Google Scholar
-
90.Yang HL, Sun ZL, Ding R, Cao G. Extraction and in vitro antibacterial test of polysaccharide from Solanum lyratum Thunb[J]. J Tradit Chin Veterinary Med 4, 24-5 (2005) PubMed Google Scholar
-
91.Yang JH, Choi CU, Kim DK, Lee KR, Zee OP. Effect of Solanum lyratum extract on the hepatotoxicity of carbon tetrachloride in rats[J]. Saengyak Hakhoe Chi 27, 167-72 (1996) PubMed Google Scholar
-
92.Li GT. Protective effect of Solanum lyratum Thunb ethanol extract for carbon tetrachloride-induced acute liver injury[J]. Chin Pract Med 10, 8-9 (2015) CrossRef PubMed Google Scholar
-
93.Kim HM, Lee EJ. Solanum lyratum inhibits anaphylactic reaction and suppresses the expression of L-histidine decarboxylase mRNA[J]. Immunopharm Immunot 20, 135-46 (1998) CrossRef PubMed Google Scholar
-
94.Kang B, Lee E, Hong I, Lee J, Kim H. Abolition of anaphylactic shock by Solanum lyratum Thunb[J]. Int J Immunopharmacol 19, 729-34 (1998) CrossRef PubMed Google Scholar
-
95.Cai SX. Studies on extraction, purification, determination and molluscicidal effect of glycosidal steroid alkaloids in Solanum lyratum Thunb, Master's thesis. Hunan Agricultural University. 2005. PubMed Google Scholar
-
96.Yin LH, Zhang J, Pan XY, Zhao FD, Liu QM. Effect of Baiyingtang on inflammatory cytokines in patients with acute radiolectitis[J]. Jiangxi Med J 55, 1781-3 (2020) PubMed Google Scholar
-
97.Liu YS, Xin XB. Efficacy of the Baiying decoction plus chemotherapy on NSCLC[J]. Clin J Chin Med 10, 27-9 (2018) PubMed Google Scholar
-
98.Han L, Zhuo Y, Sun CX, Wang JN. Inhibitory effect of lung cancer cells total alkaloids from Solanum lyratum Thunb. on the growth of transplanted Lewis in mice[J]. Trad Chin Drug Res Clin Pharm 26, 573-6 (2015) PubMed Google Scholar
-
99.Fu HW. SLT screening of anticancer active site and researching of lung cancer A549 cell proliferation inhibition mechanism, Master's thesis. Nanchang University. 2008. PubMed Google Scholar
-
100.Tu S, Wei X, Zhao XM, Yu LH, Wan FS. The influence of Solanum lyratum Thunb extract on apoptosis and the expression of Fas/Fasl, genes in human lung cancer A549 cells[J]. Lishizhen Med Mater Med Res 19, 603-5 (2008) PubMed Google Scholar
-
101.Han L, Sun CX, Wang JN. Effect of total alkaloids from Solanum lyratum on regulating apoptosis and cell cycle of A549 cells through VEGF-related pathway[J]. Trad Chin Drug Res Clin Pharm 27, 509-13 (2016) PubMed Google Scholar
-
102.Tu S, Wan FS, Wei X, Nong S, Zhu JF, Liu ZQ. Solanum lyratum Thunberg alkaloid induces human lung adenoearcinoma A549 cells apoptosis by activating FAS-pathway[J]. Lishizhen Med Mater Med Res 24, 66-8 (2013) PubMed Google Scholar
-
103.Wang MF, Yu LH, Wan HF, Tu SS. Influence of extracts of Solanum lyratum thunb on apoptosis and Bcl-xl/Bid expression in human lung cancer SPC-A-1 cells[J]. J Nanchang Univ Med Sci 51, 8-12 (2013) PubMed Google Scholar
-
104.Wei X, Tu S, Zhao XS, Wan FM, Yu LH. Experimental study on apoptosis induced by ethanol extracts of Solanum lyratum Thunb in human lung cancer SPC-A-1 cells[J]. China Pharm 19, 256-8 (2008) PubMed Google Scholar
-
105.Huo Y, Wang M, Wang XB, Han ZF, Dong X. Effects of Solanum lyratum thunberg alkaloid on the expression of p38 MAPK and Caspase-3 in hepatoma Huh-7cells[J]. Global Tradit Chin Med 11, 987-90 (2018) PubMed Google Scholar
-
106.Liu SH, Shen XH, Wei XF, Mao XH, Huang T. Immunomodulatory activity of butanol extract from Solanum lyratum in tumor-bearing mice[J]. Immunopharm Immunot 33, 100-6 (2010) PubMed Google Scholar
-
107.Wang WF, Liu Y, Wu GM, Yang T, Zhang D, Xu YJ. Screening the anti-tumor active components of the Solanum lyratum Thunb. extract[J]. J Changchun Univ Tradit Chin Med 34, 8883-6 (2018) PubMed Google Scholar
-
108.Liu HR, Peng XD, He HB, Wang YH, Li Y, He GX, Liu YL, Li YL, Zeng CJ. Antiproliferative activity of the total saponin of Solanum lyratum Thunb in Hela cells by inducing apoptosis[J]. Pharmazie 63, 836-42 (2008) PubMed Google Scholar
-
109.Liu SH, Mao XH, Wei XF. High-efficiency inhibitory effect of steroid saponin on HeLa cells proliferation and its mechanism[J]. Shandong J Tradit Chin Med 28, 724-6 (2009) PubMed Google Scholar
-
110.Zhang C, Li ZM, Wang JL, Jiang XT, Xia MF, Wang J, Lu SY, Li SY, Wang HM. Ethanol extracts of Solanum lyratum Thunb regulate ovarian cancer cell proliferation, apoptosis, and epithelial-to-mesenchymal transition (EMT) via the ROS-mediated p53 Pathway[J]. J Immunol Res 2021, 5569354 (2021) PubMed Google Scholar
-
111.Huang HS, Wei X, Huang YQ, Wei LD. Effects of Solanun lyratum Thunb extracts on growth of HO8910 of ovarian cancer cells[J]. J Mod Oncol 19, 1279-81 (2011) PubMed Google Scholar
-
112.Yan J, Luo J, He KY, Pan R, Hu Q, Peng B, Liu X. Apoptosis induced by Solanum lyratum on ovarian carcinoma cell SKOV3[J]. Moder Tradi Chin Med Mat Med-World Sci Tech 2008, 60-3 (2008) PubMed Google Scholar
-
113.Cao JY, Tan XL. The damage effect of Chinese herb medicine Baimaoteng (Solanum lyratum) on G2-PC Chromosome in CHO cells[J]. Chin J Mod Appl Pharm 5, 1-3 (1988) PubMed Google Scholar
-
114.Yang XD, Zhang J, Zhao RJ. Effect of total glycosides of Solamum lyratum survivin and bax in MCF-7 human breast on expression of cancer cells[J]. Chin J New Drags 20, 1123-6 (2011) PubMed Google Scholar
-
115.Chiu CH, Chou YC, Lin JP, Kuo CL, Lu HF, Huang YP, Yu CC, Lin ML, Chung JG. Chloroform extract of Solanum lyratum induced G0/G1 arrest via p21/p16 and induced apoptosis via reactive oxygen species, caspases and mitochondrial pathways in human oral cancer cell lines[J]. Am J Chinese Med 43, 1453-69 (2015) CrossRef PubMed Google Scholar
-
116.Lv J. Study on chemical constituents of Solanum lyratum Thunb, Master's thesis. Changchun University of Chinese Medicine. 2009. PubMed Google Scholar
-
117.Wan FS, Wu J, Li H, Tu S, Yu LH. Study on apoptosis of human stomach SGC-7901 cells induced by extracts of Solanum lyratum[J]. Zhong Yao Cai 32, 245-9 (2009) PubMed Google Scholar
-
118.Wan CX. Study on apoptosis inducing effect of Solanuml lyratum water extract and its rnechanisrn on human colorectal cancer HT29 cells, Master's thesis. Nanchang Uiversity. 2011. PubMed Google Scholar
-
119.Yue XD, Qu GW, Li BF, Xue CH, Li GS, Dai SJ. Two new C13-norisoprenoids from Solanum lyratum[J]. J Asian Nat Prod Res 14, 486-90 (2012) CrossRef PubMed Google Scholar
-
120.Shi WR, Liu Y. Effects of Solanum lyratum Thunb on multiplication in human acute promyelocytic HL-60 Cells[J]. J Fujian Univ Tradit Chin Med 12, 36-9 (2002) PubMed Google Scholar
-
121.Pan B, Zhong WF, Deng ZH, Lai CY, Chu JY, Jiao GL, Liu JF, Zhou QZ. Inhibition of prostate cancer growth by solanine requires the suppression of cell cycle proteins and the activation of ROS/P38 signaling pathway[J]. Cancer Med 5, 3214-22 (2016) CrossRef PubMed Google Scholar
-
122.Lin YT, Huang AC, Kuo CL, Yang JS, Lan YH, Yu CC, Huang WW, Chung JG. Induction of cell cycle arrest and apoptosis in human osteosarcoma U-2 OS cells by Solanum lyratum extracts[J]. Nutr Cancer 65, 469-79 (2013) CrossRef PubMed Google Scholar
-
123.Huo Y, Wang C, Wang M, Wang XB, Han ZF, Dong X. Study on the mechanisms of Solanum lyratum thunberg alkaloid against tert-butyl hydroperoxide-induced oxidative stress and apoptosis in human neuroma metrocyte cell line SH-SYSY cells[J]. Chin Med 13, 1738-41 (2018) PubMed Google Scholar
-
124.Fei YM, Gong CG. Experimental study of anti-inflamatory and analgesic effects of extracts of Solanum lyratum Thunb[J]. J Pharm Pract 27, 111-4 (2009) PubMed Google Scholar
-
125.Li ZW, Zhou BL, Liu X, Zhang P. The progress of researching into physiological and ecological activities of glycoalkaloid in Solanaceae[J]. Acta Agric Shanghai 27, 129-34 (2011) PubMed Google Scholar
-
126.Al Sinani SSS, Eltayeb EA. The steroidal glycoalkaloids solamargine and solasonine in Solanum plants[J]. S Afr J Bot 112, 253-69 (2017) CrossRef PubMed Google Scholar
-
127.Arkhypova VN, Dzyadevych SV, Soldatkin AP, El'Skaya AV, Martelet C, Jaffrezic-Renault N. Development and optimisation of biosensors based on pH-sensitive field effect transistors and cholinesterases for sensitive detection of solanaceous glycoalkaloids[J]. Biosens Bioelectron 18, 1047-53 (2003) CrossRef PubMed Google Scholar
Copyright information
© The Author(s) 2022
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.