Carascynol A, a hybrid of caryophyllane-type terpenoid and a C6 unit degraded by polyprenylated acylphloroglucinols from Hypericum ascyron
Abstract
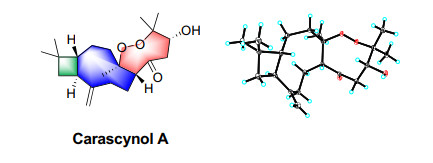
Carascynol A, an unprecedented 4/9/8 ring system hybrid with a peroxide bridge, was characterized from Hypericum ascyron. The architecture contains a caryophyllane-type moiety and a C6 unit derived from polyprenylated acylphloroglucinols. Its structure and absolute configuration were determined by comprehensive spectroscopic and X-ray diffraction data. Biologically, compound 1 inhibited cell proliferation in LoVo, SW480, and HCT116 cell lines (IC50 = 12.30–24.57 µM).Graphical Abstract
Keywords
Hypericum ascyron Caryophyllane Polyprenylated acylphloroglucinols Cytotoxicity Colon cancer1 Introduction
Colorectal cancer (CRC) ranks as the second most lethal cancer and the third most prevalent malignant tumor worldwide [1]. Colon cancer, one of three types of CRC, accounts for the highest percentage of incidence and mortality rate [1, 2]. For cancer patients, surgery and chemotherapy are usually the first choices. Current chemotherapy includes single-agent therapy, mainly fluoropyrimidine (5-FU)-based, and multiple-agent regimens containing one or several drugs [3]. Due to chemical and biological diversity, natural products have always been a major source for pharmacotherapy, especially for cancer diseases [4].
Polycyclic polyprenylated acylphloroglucinols (PPAP), a special class of structurally diverse and biologically broad natural products, are rich in the plants of Hypericum. As one of the most widely distributed Hypericum species in China, Hypericum ascyron is a medicinal herb used in the treatment of abscesses and wounds [5]. Our previous studies on this plant have led to the characterization of some seco- and nor-PPAPs with anti-cancer activities [6, 7]. As a part of our systematic search for novel and bioactive natural PPAPs from Hypericum plants, further investigation on H. ascyron obtained an unprecedented hybrid condensed by a caryophyllane-type sesquiterpenoid and an uncommon C6 unit (Fig. 1). It is proposed that the C6 unit was derived from polyprenylated acylphloroglucinols by a cascade of ring-contracting isomerization, addition, and degradation reactions. Herein, the isolation, structure elucidation, plausible biosynthetic pathways, and biological evaluation of compound 1 were elaborated in this paper.
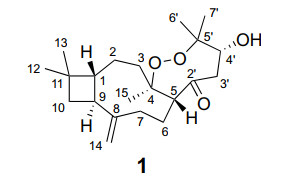
Structure of carascynol A (1)
2 Result and discussion
2.1 Structure elucidation
Carascynol A (1) was obtained as colorless needles crystals. Its molecular formula C21H34O4 was established by its 13C NMR and HRESIMS data (m/z 373.2351, [M + Na]+, calcd for 373.2355), corresponding to 5 indices of hydrogen deficiency. The IR absorptions implied the presence of hydroxy (3430 cm−1), carbonyl (1712 cm−1), and terminal double-bond (3086, 1643, and 908 cm−1) groups. Its 1H NMR spectrum exhibited two olefinic protons (δH 4.82 and 4.96) and five methyl singlets (δH 0.96, 1.00, 1.01, 1.21, and 1.40). The 13C NMR and DEPT data presented a total of 21 carbon signals, including one non-conjugated carbonyl (δC 207.4), four quaternary carbons (including one unsaturated hydrocarbon at δC 151.7 and two oxygenated ones at δC 88.0 and 83.9), four methines (including an oxygenated one at δC 71.1), seven methylenes (including an olefinic one at δC 110.5), and five methylenes (Table 1).
13C (150 MHz) and 1H (600 MHz) NMR spectroscopic data of compound 1 in CDCl3
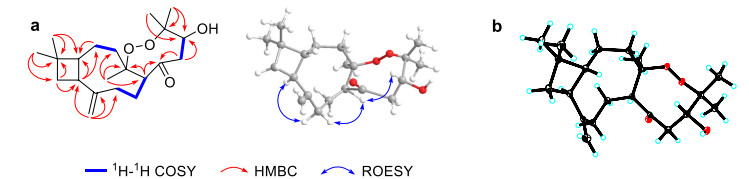
The correlations from a gem-dimethyl at δH 1.00 (Me-12) and 0.96 (Me-13) to C-1 (δC 57.9)/C-10 (δC 36.3)/C-11 (δC 34.6), from H2-10 (δH 1.75 and 1.60) to C-9 (δC 41.3)/C-11, and from H-9 (δH 2.42) to C-1/C-10 in the HMBC spectrum, combined with the correlations of H-1 (δH 1.66)/H-9/H2-10 in the 1H-1H COSY spectrum, suggested the presence of a cyclobutane, which is characteristic for caryophyllene with 4/9-fused ring nucleus. Another nine-membered ring was established by the 1H-1H COSY correlations of H2-2 (δH 1.43 and 1.64)/H2-3 (δH 1.44 and 1.88) and H-5 (δH 2.79)/H2-6 (δH 1.59 and 1.99)/H2-7 (δH 1.93 and 2.10), together with the HMBC correlations from H2-2 to C-1, from H2-14 (δH 4.82 and 4.96) to C-1/C-7 (δC 36.2)/C-8 (δC 151.7)/C-9 (δC 41.3), and from Me-15 (δH 1.21) to C-3 (δC 39.6)/C-4 (δC 88.0)/C-5 (δC 52.3) (Fig. 2a).
Key 2D NMR correlations (a) and ORTEP drawing (b) of 1
Besides the existence of a caryophyllane-type sesquiterpenoid monomer, the remaining 6 carbons were connected by the correlations of H-5 to C-2′ (δC 207.4), H2-3′ (δH 2.51 and 2.55) to C-2′/C-4′ (δC 71.1), and Me-6′/7′ (δH 1.40 and 1.01) to C-4′/C-5′ (δC 83.9) in the HMBC spectrum. Additionally, a changeable proton and three additional oxygen atoms were unassigned in the molecular formula of 1. Except for three unsaturated degrees attributed to the caryophyllane unit and one deficiency due to the carbonyl group, there should be one unsaturated degree left, which indicated that the C6 unit should be involved in further cyclization. We assumed that a peroxide bridge should lie between C-4 and C-5′ according to their apparently downfield chemical shifts at δC 88.0 and 83.9, respectively, while a hydroxyl group was located at C-4′ (δC 71.1). The single crystals of 1 [Flack parameter = − 0.09(10), CCDC 2212335] were obtained in methanol and subjected to an X-ray diffraction experiment with Cu Kα radiation. The XRD result confirmed the planar structure (Fig. 2b).
In the ROESY spectrum (Fig. 2a), the cross-peaks of H-7b (δH 1.93)/H-5, H-5/H-4′ (δH 4.64), and H-7a (δH 2.10)/H-9 suggested that H-5 and H-4′ placed in the same orientation, while H-9 adopted the opposite orientation and was assigned as α. This deduction was consistent with the XRD result (Fig. 2b). Consequently, the absolute configuration of 1 was determined as 1R, 4R, 5R, 9S, 4′R.
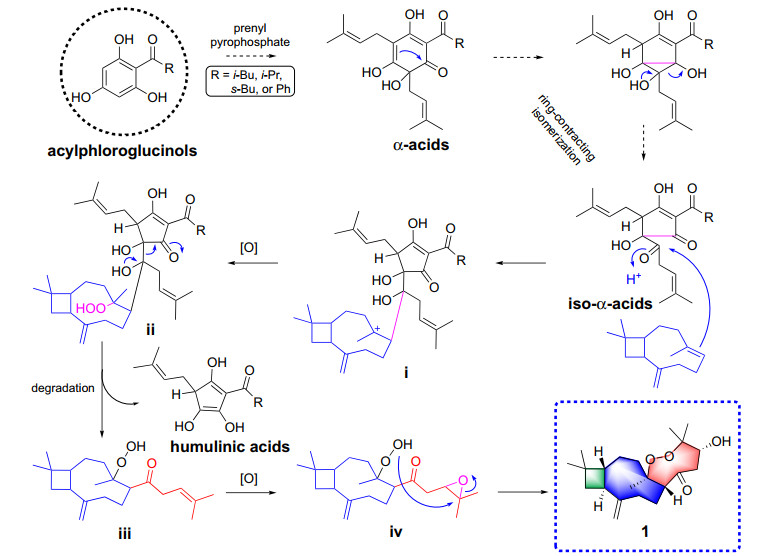
Polycyclic polyprenylated acylphloroglucinols (PPAP) possess highly oxygenated acylphloroglucinol-derived cores decorated with isoprenyl or geranyl side chains. Biosynthetically, prenylation of the acylphloroglucinols core moiety affords monocyclic polyprenylated acylphloroglucinols (MPAPs), which may be further cyclized to PPAP-type metabolites with diverse carbon skeletons [8-13]. In this study, we reckon that prenylation of acyphloroglucinols could also obtain polyprenylated acyphloroglucinols metabolites such as α-acids, which then naturally isomerized as its ring-contracted isomer (iso-α-acids) [14, 15]. Subsequently, iso-α-acids underwent an addition reaction with caryophyllene to form an intermediate i, which was oxidized to create a peroxide ii. Then, ii performed a degradation to produce iii and a humulinic acid [16]. Finally, the epoxidation of iii generated iv, which was cyclized to afford 1 (Scheme 1).
Plausible biosynthetic pathway to compound 1
2.2 Biological activity
The cytotoxicity of compound 1 against three human colon cancer cell lines (HCT116, SW480, and LoVo) was evaluated with fluorouracil (5-FU) as the positive control. As shown in Table 2, compound 1 showed the strongest activity against LoVo with IC50 values 12.30 ± 0.19 µM, while exhibited weaker cytotoxicity to SW480 and HCT116 cell lines (IC50 18.33 ± 1.68 and 24.57 ± 3.09 µM). Compound 1 did not significantly alter the viability of PBMCs, suggesting its selective cytotoxicities on colon cancer cells.
Cytotoxicity of compound 1
3 Conclusion
Actually, the hybridization of acylphloroglucinols core and β-caryophyllene unit has been described previously [9, 17]. However, it is the first time that the condensation of a sesquiterpenoids unit with an unusual C6 polyprenylated acylphloroglucinols degraded moiety in Hypericum species has been reported. Biogenetically, β-caryophyllene and polyprenylated acylphloroglucinols conjugated through a nucleophilic addition to produce a key intermediate, which upon multistep degradation, oxidation, and cyclization afforded 1. Besides, our study revealed that compound 1 exhibited cytotoxicities on LoVo, SW480, and HCT116, with IC50 values in the range of 12.30–24.57 µM. To sum up, the present study may provide a new perspective for the structural and biological explorations of the terpenoid and PPAP hybrids.
Notes
Acknowledgements
This work was financially supported by the NSFC-Joint Foundation of Yunnan Province (Grant No. U1902213) the Second Tibetan Plateau Scientific Expedition and Research (STEP) program (Grant No. 2019QZKK0502-0303), Natural Science Foundation of Yunnan (Grant No. 2019FA003), CAS Interdisciplinary Innovation Team of CAS "Light of West China" Program, and the Fund of State Key Laboratory of Phytochemistry and Plant Resources in West China (Grant No. E0230211Z1).
Author contributions
All authors conceived and designed the study and experiments. HYL conducted the experiments, analyzed the results, and wrote the manuscript. LXR proposed the biosynthetic pathways. XG designed the project and revised the manuscript. All authors read and approved the final manuscript.
Declarations
Competing interests
The authors declare that there are no competing interests associated with this work.
References
-
1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA A Cancer J Clin 71, 209-49 (2021) CrossRef PubMed Google Scholar
-
2.Engstrand J, Nilsson H, Stromberg C, Jonas E, Freedman J. Colorectal cancer liver metastases—a population-based study on incidence, management and survival[J]. BMC Cancer 18, 78 (2018) CrossRef PubMed Google Scholar
-
3.Xie YH, Chen YX, Fang JY. Comprehensive review of targeted therapy for colorectal cancer[J]. Signal Transduct Target Therapy 5, 22 (2020) CrossRef PubMed Google Scholar
-
4.Atanasov AG, Zotchev SB, Dirsch VM, the International Natural Product Sciences Taskforce, Supuran CT. Natural products in drug discovery: advances and opportunities[J]. Nat Rev Drug Discov 20, 200-16 (2021) CrossRef PubMed Google Scholar
-
5.Chang SU. Dictionary of Chinese crude drugs. Shanghai: New Medical College Shanghai Scientific Technological Publishers; 1997. p. 1002. PubMed Google Scholar
-
6.Kong LM, Long XW, Yang XW, Xia F, Khan A, Yan H, Deng J, Li X, Xu G. seco-Polycyclic polyprenylated acylphloroglucinols with unusual carbon skeletons from Hypericum ascyron[J]. Tetrahedron Lett 58, 2113-7 (2017) CrossRef PubMed Google Scholar
-
7.Hu YL, Hu K, Kong LM, Xia F, Yang XW, Xu G. Norascyronones A and B, 2, 3, 4-nor-polycyclic polyprenylated acylphloroglucinols from Hypericum ascyron[J]. Org Lett 21, 1007-10 (2019) CrossRef PubMed Google Scholar
-
8.Ciochina R, Grossman RB. Polycyclic polyprenylated acylphloroglucinols Chem[J]. Rev 106, 3963-86 (2006) PubMed Google Scholar
-
9.Yang XW, Grossman RB, Xu G. Research progress of polycyclic polyprenylated acylphloroglucinols[J]. Chem Rev 118, 3508-58 (2018) CrossRef PubMed Google Scholar
-
10.Liao Y, Yang SY, Li XN, Yang XW, Xu G. Polyprenylated acylphloroglucinols from the fruits of Hypericum henryi[J]. Sci China Chem 59, 1216-23 (2016) PubMed Google Scholar
-
11.Yang XW, Yang J, Xu G. Skeleton reassignment of type C polycyclic polyprenylated acylphloroglucinols[J]. J Nat Prod 79, 108-13 (2017) PubMed Google Scholar
-
12.Zhang JJ, Yang XW, Liu X, Ma JZ, Liao Y, Xu G. 1, 9-seco-Bicyclic polyprenylated acylphloroglucinols from Hypericum uralum[J]. J Nat Prod 78, 3075-9 (2015) CrossRef PubMed Google Scholar
-
13.Zhang JJ, Yang XW, Ma JZ, Ye Y, Shen XL, Xu G. Cytotoxic polyprenylated acylphloroglucinol derivatives from Hypericum henryi[J]. Tetrahedron 71, 8315-9 (2015) CrossRef PubMed Google Scholar
-
14.Pouységu L, Marguerit M, Gagnepain J, Lyvinec G, Eatherton AJ, Quideau S. Total synthesis of wasabidienones B1 and B0 via SIBX-mediated hydroxylative phenol dearomatization[J]. Org Lett 10, 5211-4 (2008) CrossRef PubMed Google Scholar
-
15.Huang YR, Tippmann J, Becker T. Kinetic modeling of hop acids during wort boiling[J]. Int J Biosci Biochem Bioinform 3, 47-52 (2013) PubMed Google Scholar
-
16.Rakete S, Berger R, Böhme S, Glomb MA. Oxidation of isohumulones induces the formation of carboxylic acids by hydrolytic cleavage[J]. J Agric Food Chem 62, 7541-9 (2014) CrossRef PubMed Google Scholar
-
17.Yang XW, Li YP, Su J, Ma WG, Xu G. Hyperjapones A–E, terpenoid polymethylated acylphloroglucinols from Hypericum japonicum[J]. Org Lett 18, 1876-9 (2016) CrossRef PubMed Google Scholar