Drimane sesquiterpenoids from a wetland soil-derived fungus Aspergillus calidoustus TJ403-EL05
Abstract
Soil-derived fungi represent an insufficiently tapped reservoir for discovering new and bioactive natural products (NPs), and despite an ever-increasing number of unknown NPs have been discovered over the past few decades, much of the hidden biosynthetic potential is still in an urgent need to be disclosed. In this research, a chemical investigation was performed on a wetland soil-derived fungus Aspergillus calidoustus TJ403-EL05, leading to the isolation of a total of fourteen drimane sesquiterpenoids (1-14), incorporating three new ones, namely ustusols F-H (1-3). Their structures, comprising absolute configurations, were completely authenticated by widespread spectroscopic data, quantum chemical 13C NMR and ECD calculations, and X-ray crystallography experiments. Compound 14 exhibited moderate anti-inflammatory activity by inhibiting the LPS-induced NO release (IC50=25.6 μM).Graphical Abstract

Keywords
Aspergillus calidoustus Drimane sesquiterpenoids Structure elucidation Anti-inflammatory activity1 Introduction
Over the past few decades, a large proportion of medicines originate from various natural resources, especially from the field of microbiology [1]. Terrestrial microorganisms have a huge biosynthetic capacity to produce structurally diverse and pharmacologically active NPs, which have become important chemical entities in drug discovery [2]. For example, cyclosporine, isolated from the soil-derived Tolypocladium inflatum, was the first immunosuppressive agent to enable selective immune regulation of T cells, without excessive toxicity [3]. The discovery of cyclosporine in 1971 initiated a new era in the immunopharmacology field, and is still widely used in clinical practice. Therefore, soil-derived fungi have attracted, and will attract increasing attention in the NPs-related research fields.
Aimed at searching for structurally unique and pharmacologically attractive NPs from the soil-derived fungi [4, 5, 6], strain A. calidoustus TJ403-EL05 that was separated from a wetland soil collected from East Lake in Wuhan City, caught our attention and was thus chemically investigated, which afforded three new drimane sesquiterpenoids, namely ustusols F–H (1–3), and eleven known congeners (4–14). In this paper, the isolation, structural characterization, and anti-inflammatory activity of these drimane sesquiterpenoids (Fig. 1) were elaborated.
Chemical structures of compounds 1–14
2 Results and discussion
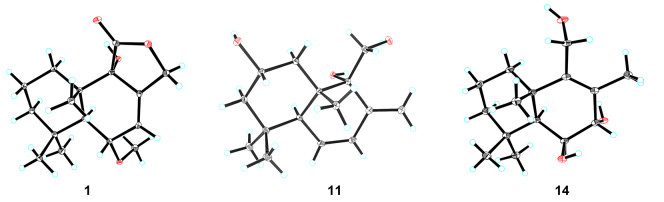
Compound 1 was purified as a colorless crystal. According to the HRESIMS analysis showing a sodium adduct ion at m/z 303.1567 (calcd for 303.1567), its molecular formula was determined as C16H24O4, implying 5 degrees of unsaturation. The 1H NMR data (Table 1) of 1 showed obvious signals as three methyl protons (δH 1.08, 1.04, and 0.90), four methylene protons (δH 4.97/4.70, 1.62/2.08, 1.42/1.33, and 1.58), three methine protons (δH 6.05, 3.97, and 1.98) and one methoxy proton (δH 3.31). With the help of DEPT and HSQC spectroscopic analyses, the 13C NMR data (Table 1) of 1 demonstrated the existence of 16 carbon signals that were attributable to three methyls (δC 35.0, 23.1 and 18.5), one methoxy (δC 54.4), four methylenes (δC 69.0, 43.1, 30.5, and 18.4), three methines (δC 125.2, 76.3, and 46.1), four quaternary carbons (δC 135.6, 74.6, 41.7 and 33.8) and one ester carboxyl carbon (δC 175.2). The 1D and 2D NMR data of 1 were highly similar to those of the known 9α-hydroxy-5α-drim-7-ene-6-one-11, 12-olide (6) [7], uncovering 1 and 6 to possess the same drimane sesquiterpenoid core skeleton. The significant difference of 1 and 6 was the existence of one methoxy group linked at C-6 in 1 instead of a conjugated ketone carbonyl (C-6) in 6, as further supported based on the key HMBC correlations (Fig. 2) of 6-OMe (δH 3.31) with C-6 (δC 76.3) and of H-6 (δH 3.97) with C-5 (δC 46.1), C-7 (δC 125.2), and C-8 (δC 135.6). In the NOESY experiment (Fig. 3), the key NOE correlations of H-6 with Me-14 (δH 1.04)/Me-13 (δH 0.90) and of H-5 (δH 1.98) with Me-15 (δH 1.08) suggested that H-6, Me-13 and Me-14 should all be β-oriented, while H-5 and Me-15 were all α-oriented. However, no useful NOE signals could be applied to verify the configuration of C-9. Fortunately, a suitable crystal of 1 was acquired by recrystallization and then furnished for X-ray crystallographic experiment (Fig. 4). According to a Flack parameter of 0.01(3), the absolute configuration of 1 was unequivocally confirmed as 5S, 6S, 9S, and 10S. Accordingly, the absolute structure of 1, named as ustusol F, was defined.
NMR data of 1–3 (δ in ppm, J in Hz)
Key 1H–1H COSY and HMBC correlations of compounds 1–3
Key NOESY correlations (dashed black arrows) of compounds 1–3
X-ray crystallographic structures of 1, 11, and 14
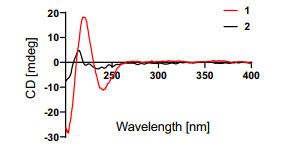
Compound 2, obtained as a white powder, was determined to possess a molecular formula of C15H24O3, as evidenced by its positive HRESIMS data at m/z 275.1618 (calcd for C15H24O3Na+, 275.1618). By comparing its 1H, 13C, and DEPT NMR data (Table 1) with those of the known 6-epi-pereniporin A (4) [8], we could speculate that both compounds were structural analogues, with the only distinction being that one hydroxy group linked at C-11 was absent in 2, as fully supported by the HMBC correlations (Fig. 2) of H2-11 (δH 3.45) with C-8 (δC 138.2), C-9 (δC 74.6), and C-12 (δC 61.5). Similar NOESY data (Fig. 3) and ECD curves (Fig. 5) between 1 and 2 proved that these two compounds possessed the identical absolute configuration. Accordingly, the absolute structure of 2, named as ustusol G, was defined.
Experimental ECD curves of compounds 1 and 2 in MeOH
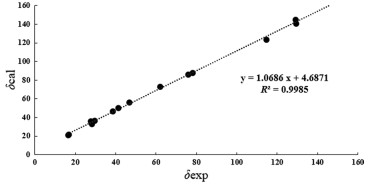
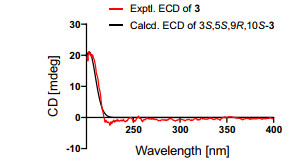
Compound 3 was deduced to have a molecular formula of C15H24O3, as evidenced via its HRESIMS data. By comparing the 1D NMR data (Table 1) of 3 to those of the known ustusol D (11) [9] (Fig. 1) whose absolute structure was verified via crystallography experiment (Fig. 4), it revealed that both compounds possessed the identical drimane sesquiterpenoid core skeleton, with the only exception that a hydroxy group was linked at C-2 in 11 by C-3 in 3. This conclusion was further corroborated via the key 1H–1H COSY cross-peaks of H2-1/H2-2/H-3, as well as the HMBC correlations of both Me-14 and Me-15 with C-3, C-4, and C-5 (Fig. 2). The NOE cross-peaks (Fig. 3) of Me-15α/H-3/H-5 and H2-11/Me-13β demonstrated that OH-3 was β-oriented in 3. To validate this speculation, the 13C NMR chemical shifts of 3 were predicted at the B972/pcSseg-2 level showing the correlation coefficient (R2) value of 0.9985 (Fig. 6), which completely supported our proposed relative structure. Lastly, the quantum chemical electronic circular dichroism (ECD) calculation was employed for 3. To our expectation, the calculated ECD plot was closely similar to the experimental one (Fig. 7), proclaiming its absolute configuration as 3S, 5S, 9R, and 10S, and this compound was named as ustusol H.
Linear correlation between the experimental and calculated 13C NMR chemical shifts for 3
Experimental and calculated ECD spectra of compound 3
Apart from new compounds 1–3, eleven known congeners were also isolated from A. calidoustus TJ403-EL05 and identified as 6-epi-pereniporin A (4) [8], 6-epi-O-methyl-pereniporin A (5) [8], 9a-hydroxy-5a-drim-7-ene-6-one-11, 12-olide (6) [7], 6-dehydroxy-6-oxopereniporin A (7) [8], strobilactone A (8) [10], pereniporin B (9) [11], dendocarbin C (10) [12], ustusol D (11) [9], 9a, 11, 12-trihydroxydrim-7-en-6-one (12) [13], 12-hydroxyalbrassitriol (13) [14] and drim-8-en-6β, 7a, 11-triol (14) [15], by comparison of their HRESIMS and NMR data with those reported in the literature.
In the bioactivity assay, due to the limited amounts of 3, other compounds (1–2 and 4–14) were tested for anti-inflammatory activity by using LPS-induced murine macrophages RAW264.7 cells. As a result, only compound 14 was found to show an inhibitory effect against the NO release (IC50=25.6 μM), and the remaining compounds did not exhibit significant activity with IC50 values of > 40 µM (positive control MG132: IC50=0.32 µM).
Notes
Acknowledgements
Acknowledgements This project was supported financially by the National Natural Science Foundation for Distinguished Young Scholars (No. 81725021), the National Natural Science Foundation of China (Nos. 81573316 and 31870326), the Innovative Research Groups of the National Natural Science Foundation of China (No. 81721005), the Fundamental Research Funds for the Central Universities (No. 2020kfyXJJS083), the National Key R & D Program of China (No. 2021YFA0910500), the Research and Development Program of Hubei Province (No. 2020BCA058), and the Chinese Medicine Research Foundation of Health Commission of Hubei Province (No. ZY2021Z019).
Author contributions
All authors read and approved the final manuscript.
Funding
The National Natural Science Foundation for Distinguished Young Scholars, 81725021, Yonghui Zhang, the National Natural Science Foundation of China, Nos. 81573316, 31870326, the Innovative Research Groups of the National Natural Science Foundation of China, No. 81721005, Yonghui Zhang, the Fundamental Research Funds for the Central Universities, No. 2020kfyXJJS083, Zhengxi Hu, the National Key R & D Program of China, No. 2021YFA0910500, Yonghui Zhang, the Research and Development Program of Hubei Province, No. 2020BCA058, Jianping Wang, the Chinese Medicine Research Foundation of Health Commission of Hubei Province, No. ZY2021Z019, Jianping Wang.
Declarations
Competing interests
The authors declare no conflict of interest.
References
-
1.Newman DJ, Cragg GM, Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019[J]. J Nat Prod 83, 770-803 (2020) CrossRef PubMed Google Scholar
-
2.Hamed A, Ismail M, El-Metwally MM, Frese M, Ibrahim TMA, El-Haddad AF, Sewald N, Shaaban M, Diverse polyketides and alkaloids from Penicillium sp. KHMM: structural elucidation, biological and molecular docking studies[J]. Z Naturforsch C 74, 131-7 (2019) PubMed Google Scholar
-
3.Stähelin HF, The history of cyclosporin A (Sandimmune®) revisited: another point of view[J]. Experientia 52, 5-13 (1996) CrossRef PubMed Google Scholar
-
4.Liu M, Zhang X, Shen L, Sun W, Lin S, Liu J, Cao F, Qi C, Wang J, Hu Z, Zhang Y, Bioactive polyketide-terpenoid hybrids from a soil-derived fungus Bipolaris zeicola[J]. J Org Chem 86, 10962-74 (2021) CrossRef PubMed Google Scholar
-
5.Li H, Xu D, Sun W, Yang B, Li F, Liu M, Wang J, Xue Y, Hu Z, Zhang Y, HPLC-DAD-directed isolation of linearly fused prenylated indole alkaloids from a soil-derived Aspergillus versicolor[J]. J Nat Prod 82, 2181-8 (2019) CrossRef PubMed Google Scholar
-
6.Mo S, Yin J, Ye Z, Li F, Lin S, Zhang S, Yang B, Yao J, Wang J, Hu Z, Zhang Y, Asperanstinoids A-E: undescribed 3, 5-dimethylorsellinic acid-based meroterpenoids from Aspergillus calidoustus[J]. Phytochemistry 190, 112892 (2021) CrossRef PubMed Google Scholar
-
7.Zhuravleva OI, Afiyatullov SS, Denisenko VA, Ermakova SP, Slinkina NN, Dmitrenok PS, Kim NY, Secondary metabolites from a marine-derived fungus Aspergillus carneus Blochwitz[J]. Phytochemistry 80, 123-31 (2012) CrossRef PubMed Google Scholar
-
8.Kwon J, Lee H, Seo YH, Yun J, Lee J, Kwon HC, Guo Y, Kang JS, Kim JJ, Lee D, Cytotoxic drimane sesquiterpenoids isolated from Perenniporia maackiae[J]. J Nat Prod 81, 1444-50 (2018) CrossRef PubMed Google Scholar
-
9.Xu K, Zhou Q, Li XQ, Luo T, Yuan XL, Zhang ZF, Zhang P, Cadinane-and drimane-type sesquiterpenoids produced by Paecilomyces sp. TE-540, an endophyte from Nicotiana tabacum L., are acetylcholinesterase inhibitors[J]. Bioorg Chem 104, 104252 (2020) CrossRef PubMed Google Scholar
-
10.Shiono Y, Hiramatsu F, Murayama T, Koseki T, Funakoshi T, Ueda K, Yasuda H, Two drimane-type sesquiterpenes, Strobilactones A and B, from the liquid culture of the edible mushroom Strobilurus ohshimae[J]. Z Naturforsch B 62, 1585-9 (2007) CrossRef PubMed Google Scholar
-
11.Rajab MS, Ndegwa JM, 11α-Hydroxy muzigadiolide, a novel drimane sesquiterpene from the stem bark of Warburgia ugandensis[J]. Bull Chem Soc Ethiop 14, 45-9 (2000) PubMed Google Scholar
-
12.Sakio Y, Hirano YJ, Hayashi M, Komiyama K, Ishibashi M, Dendocarbins A-N, new drimane sesquiterpenes from the Nudibranch Dendrodoris carbunculosa[J]. J Nat Prod 64, 726-31 (2001) PubMed Google Scholar
-
13.Urones JG, Díez D, Gómez PM, Marcos IS, Basabe P, Moro RF, Chemistry of zamoranic acid. Part 10 Homochiral hemisynthesis of pereniporin A[J]. J Chem Soc Perkin Trans 1, 1815-8 (1997) PubMed Google Scholar
-
14.Ding JH, Ding ZG, Chunyu WX, Zhao JY, Wang HB, Liu SW, Wang F, Three new drimane sesquiterpenoids from cultures of the fungus Penicillium sp[J]. J Asian Nat Prod Res 19, 780-5 (2017) PubMed Google Scholar
-
15.Zhou H, Zhu T, Cai S, Gu Q, Li D, Drimane sesquiterpenoids from the mangrove-derived fungus Aspergillus ustus[J]. Chem Pharm Bull 59, 762-6 (2011) PubMed Google Scholar
Copyright information
© The Author(s) 2022
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.