Three new pyrrole alkaloids from the endophytic fungus Albifimbria viridis
Abstract
Three new pyrrole alkaloids albifipyrrols A-C (1-3), were isolated from the endophytic fungus Albifimbria viridis collected from the Chinese medicinal plant. Their structures were elucidated by extensive NMR and HRESIMS spectrometric analyses. All compounds were evaluated for immunosuppressive activity. Fortunately, compound 2 exhibits certain inhibition specifically against the LPS-induced proliferation of B lymphocyte cells with IC50 value 16.16 μM.Graphical Abstract
Keywords
Pyrrole alkaloids Coptis chinensis Endophytic fungus Albifimbria viridis Immunosuppressive activity1 Introduction
The human immune system is a complex network of defensing against foreign invaders. Autoimmune diseases arise when the immune system fails to distinguish between self and non-self [1, 2]. Immunosuppressants are often used to prevent and treat the immune rejection of organs and tissues of transplant patients and play an important role in the treatment of various autoimmune diseases [3-8]. Nevertheless, some common immunomodulatory drugs such as mycophenolate mofetil (MMF) and cyclosporin A (CsA) have low efficacy, toxicity, and serious side effects in transplant patients [9-12]. Therefore, it is necessary to find more efficient, safe and novel immunosuppressants to improve rejection.
In recent years, endophytic fungi from plants have been widely regarded as a significant source of drugs [13]. A great quantity of compounds with novel structures and multiple bioactivities are constantly isolated [14-16]. For instance, the well-known anticancer drug paclitaxel can be produced from Pacific yew by the endophytic fungus Taxomyces andreance [17]. Coptis chinensis Franch is a famous Chinese medicine in China. Modern pharmacological and clinical studies have indicated that it has anti-tumor, anti-inflammatory, antibacterial, hypoglycemic and other pharmacological activities [18-20]. However, there are few reports on endophytic fungus of C. chinensis Franch.
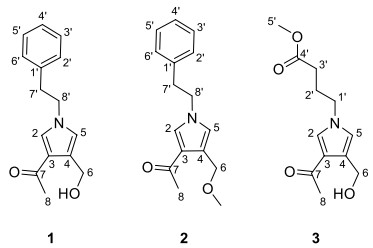
During the past few years, we had the aim of finding new potential immunosuppressive agents from endophytic fungus of C.chinensis Franch. Fortunately, we obtained a pyrrole alkaloid with immunosuppressive activity from Albifimbria viridis. Herein, we report the details of the isolation, structure elucidation, and bioactivities of three pyrrole alkaloids albifipyrrols A–C (1–3) (Fig. 1).
The chemical structures of compounds 1–3
2 Results and discussion
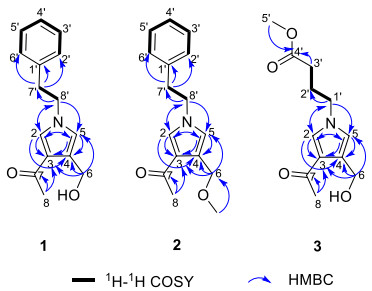
Compound 1 was obtained as yellow oil. The molecular ion peak of HR-ESI–MS was at m/z 266.1150 [M + Na]+ (calcd for 266.1157), which indicated that the molecular formula of compound 1 is C15H17NO2, with eight degrees of unsaturation. In the 1H-NMR spectrum (Table 1), a monosubstituted benzene moiety at δH 7.13 (2H, d, J = 7.8 Hz, H-2′, H-6′), 7.24–7.28 (2H, m, H-3′, H-5′) and 7.18–7.22 (1H, m, H-4′), two mutually coupling aromatic protons at δH 6.72 (1H, d, J = 2.2 Hz, H-5) and 7.40 (1H, d, J = 2.2 Hz, H-2), two heteroatom-bearing methylenes at δH 4.56 (2H, s, H-6) and 4.16 (2H, t, J = 7.1 Hz, H-8′), one conventional methylene at δH 3.06 (2H, t, J = 7.1 Hz, H-7′) and one methyl group at δH 2.32 (3H, s, H-8) were clearly shown. The 13C-NMR and DEPT spectrum (Table 1) of 1 showed the presence of fifteen carbons, including one methyl, three methylenes [including two heteroatom-bearing methylenes at δC 58.5 (C-6), 52.5 (C-8′)], seven aromatic or olefinic methines and four nonprotonated carbons [including one ketone carbonyl at δC 197.4 (C-7)]. Among them, one benzene ring, an acetyl group and four olefinic carbons occupied seven degrees of unsaturation. Hence, the remaining one degree of unsaturation can only be due to the presence of one ring. The key HMBC correlations (Fig. 2) from H-2 to C-3/C-4/C-5 and from H-5 to C-2/C-3/C-4 and from H-8′ to C-2/C-5 demonstrated the existence of a pyrrole nucleus. The 1H-1H COSY correlations (Fig. 2) between H2-7′ and H2-8′ and the key HMBC correlations from H-7′ to C-1′/C-2′/C-6′, H-8′ to C-2/C-5/C-7′ showed the phenylethyl was attached to the nitrogen atom. In addition, the acetyl can be confirmed by the key HMBC correlation from H-8 to C-7. Finally, the locations of the two substituents (an acetyl group and an ethoxy group) on the pyrrole nucleus were also confirmed at C-3, C-4 based on the HMBC correlations from H-8 to C-3 and from H-6 to C-3/C-4/C-5. Compound 1 was, therefore, established as albifipyrrol A, as depicted.
1H and 13C NMR data (δ in ppm and J in Hz) of compounds 1–3
Key HMBC and 1H-1H COSY correlations of compounds 1–3
Compound 2 was obtained as yellow oil. The molecular ion peak of HR-ESI–MS was at m/z 280.1306 [M + Na]+ (calcd for 280.1313), which deduced that the molecular formula of compound 2 was C16H19NO2, with eight degrees of unsaturation. The 1H-NMR and 13C-NMR data (Table 1) suggested 2 was similar to 1 and the only observed difference was that the hydroxy group in 1 was replaced by a methoxy group in 2. This change can be confirmed by the key HMBC correlations (Fig. 2) from H3-OMe to C-6. Compound 2 was, therefore, established as albifipyrrol B, as depicted.
Compound 3 was obtained as yellow oil. The molecular ion peak of HR-ESI–MS is at m/z 262.1046 [M + Na]+ (calcd for 262.1055), which indicated that the molecular formula of compound 3 is C12H17NO4, with five degrees of unsaturation. The 1H-NMR (Table 1) and HSQC spectrum of 3 revealed 3 has the same pyrrole ring as 1 and the major difference was the substituents on nitrogen. The 1H-NMR showed the signals of one methoxy [δH 3.65 (3H, s, H-5′); δC 52.2 (C-5′)], one carboxyl group [δC 174.8 (C-4′)], three methylenes [δH 2.32 (2H, t, J = 7.3 Hz, H-3′), 2.08 (2H, m, H-2′), 3.99 (2H, t, J = 7.0 Hz, H-1′); δC 31.4 (C-3′), 27.4 (C-2′), 49.9 (C-1′)]. The methyl butyrate unit was established by the 1H-1H COSY correlations between H2-1′, H2-2′ and H2-3′ and the key HMBC correlations (Fig. 2) from H-5′ to C-4′ and from H-3′ to C-4′. Finally, the attachment position of the methyl butyrate residue to the pyrrole ring was defined on the basis of HMBC correlations between H-1′and C-2/C-5. Compound 3 was, therefore, established as albifipyrrol C, as depicted.
All new compounds were evaluated for their in vitro inhibition activities on concanavalin A (Con A) induced T cell proliferation and lipopolysaccharide (LPS) induced B cell proliferation. Compound 2 exhibited certain inhibition specifically against the LPS-induced proliferation of B lymphocyte cells with IC50 value 16.6 μM (Table 2).
Immunosuppressive tests of compounds 1–3
3 Experimental section
3.1 General experimental procedures
1D and 2D NMR spectra were recorded on Bruker DXR-600 instrument (600 and 150 MHz) and Bruker DXR-500 instrument (500 and 126 MHz). The UV data were detected by Hitachi UH5300 spectrophotometer (Hitachi, Kyoto, Japan). IR spectra were conducted on IRT racer-100 (SHIMADZU, Kyoto, Japan) with KBr pellets. HR-ESI–MS data were obtained on a UPLC-Q Exactive MS system (Thermo Fisher, Santa Clara, CA, USA). The packing for column chromatography (CC) is silica gel (200–300 mesh, Qingdao Haiyang Chemical Co., Ltd., Qingdao, China) or Sephadex LH-20 (Amersham Biosciences, Upssala, Sweden). The semi-prepared HPLC was carried out on an Agilent Technologies 1260 Infinity II system with a diode array detector. And the chromatographic column was C18 reversed phase column (5 μm, 10 × 250 mm) (Agela, Tianjin, China).
3.2 Fungal material
The strain were isolated from the roots of C. chinensis collected from Enshi, Hubei province, and was identified as Albifimbria viridis via 18S rDNA sequences and deposited at South-Central University for Nationalities, China. The sequence data for this strain had been submitted to the DDBJ/EMBL/Genbank with accession No. MT110686.1.
3.3 Extraction and isolation
The fungus Albifimbria viridis was fermented on solid rice medium (100 g of rice, 100 mL of water, in each 500 mL culture flask) and was cultured at 30 ℃ for one month. The fermented material was soaked in absolute methanol (20 L × 4). The combined extracts were evaporated under reduced pressure to afford an crude extract, which was further dissolved in water and extracted three times with EtOAc (10 L × 4) to yield 110 g of the extract. The crude extract was subjected to silica gel column chromatography (petroleum ether: ethyl acetate, 15:1 to 0:1; ethyl acetate: methyl alcohol, 15:1 to 0:1) to yield six fractions (A‒F). Fraction B (6 g) was separated into eight sub-fractions (B1‒B8) by ODS MPLC. The eluent is composed of methyl alcohol: H2O (from 10:90 to 100:0, v/v). Fraction B3 was purified by semi-preparative HPLC (CH3CN/H2O = 55:45, v/v) to give compound 1 (4.7 mg, tR = 18.7 min). Fraction C (5 g) was isolated from Sephadex LH-20 eluting with MeOH and purified by semi-preparative HPLC (CH3CN/H2O = 40:60, v/v) to obtain compound 2 (1.3 mg, tR = 25 min). Fraction D (7.5 g) was isolated by Sephadex LH-20 column chromatography (MeOH) to obtain six sub-fractions (D1‒D6). Fraction D5 was purified by semi-preparative HPLC (CH3CN/H2O from 25:75 to 45:55 in 20 min, v/v) to yield compound 3 (2.4 mg, tR = 13.2 min).
3.4 Spectroscopic data of compounds
3.4.1 Albifipyrrol A (1)
Yellow oil. UV (MeOH) λmax (log ε): 210 (1.97). HR-ESI–MS m/z found 266.1150 [M + Na]+ (Calcd for C15H17NO2Na, 266.1157). IR (KBr) νmax (cm–1): 3401, 2949, 2837, 1655, 1450, 1117, 1024. 1H and 13C-NMR see (Table 1).
3.4.2 Albifipyrrol B (2)
Yellow oil. UV (MeOH) λmax (log ε): 210 (1.82). HR-ESI–MS m/z found 280.1306 [M + Na]+ (Calcd for C16H19NO2Na, 280.1313). IR (KBr) νmax (cm–1): 3364, 2945, 2833, 1670, 1452, 1119, 1032. 1H and 13C-NMR see (Table 1).
3.4.3 Albifipyrrol C (3)
Yellow oil. UV (MeOH) λmax (log ε): 255 (2.01). HR-ESI–MS m/z found 262.1046 [M + Na]+ (Calcd for C12H17NO4Na, 262.1055). IR (KBr) νmax (cm–1): 3400, 2950, 1734, 1632, 1526, 1157. 1H and 13C-NMR see (Table 1).
3.5 Immunosuppressive activities assay
Fresh spleen cells were obtained from female BALB/c mice (6–8 weeks old). Spleen cells (1 × 106 cells) were cultured in triplicate on a 96-well plate for 48 h at 37 ℃ in a humidified incubator containing 5% CO2 (with or without different concentrations of compounds). During the last 8 h of culture, a certain amount of CCK-8 was added to each well. At the end of culture, the OD values at 450 nm was measured by a bio-RAD 650 microplate reader. Cells with viability above 85% were further screened for their inhibitory activity against T and B lymphocytes. The 5 × 105 spleen cells were cultured at the same conditions as those mentioned above. T cell or B cell proliferation was induced with 10 µg ml−1 of LPS or 5 µg ml−1 of ConA, respectively. Proliferation was assessed in terms of uptake of [3H]-thymidine during 8 h of pulsing with 25 µL/well of [3H]-thymidine, and then cells will be harvested onto glass fiber filters. The incorporated radioactivity was counted using a Beta scintillation counter (MicroBeta Trilux, PerkinElmer Life Sciences). Cells treated without any stimuli were used as negative control. The immunosuppressive activity of each compound was expressed as the concentration of compound that inhibited ConA induced T cell proliferation or LPS-induced B cell proliferation to 50% (IC50) of the control value. Both the cytotoxicity and proliferation assessment repeated twice. Cyclosporin A (CsA) an immunosuppressive agent, was used as a positive control (Table 2; Additional file 1: Figs. S1–S24).
Notes
Supplementary Information
The online version contains supplementary material available at https://doi.org/10.1007/s13659-022-00327-2.
Additional file 1: Figure S1. 1H NMR (600 MHz, CD3OD) spectrum of compound 1. Figure S2. 13C NMR (150 MHz, CD3OD) spectrum of compound 1. Figure S3. HSQC spectrum of compound 1. Figure S4. COSY spectrum of compound 1. Figure S5. HMBC spectrum of compound 1. Figure S6. HRESIMS of compound 1. Figure S7. UV spectrum of compound 1. Figure S8. IR spectrum of compound 1. Figure S9. 1H NMR (600 MHz, CD3OD) spectrum of compound 2. Figure S10. 13C NMR (150 MHz, CD3OD) spectrum of compound 2. Figure S11. HSQC spectrum of compound 2. Figure S12. COSY spectrum of compound 2. Figure S13. HMBC spectrum of compound 2. Figure S14. HRESIMS of compound 2. Figure S15. UV spectrum of compound 2. Figure S16. IR spectrum of compound 2. Figure S17. 1H NMR (500 MHz, CD3OD) spectrum of compound 3. Figure S18. 13C NMR (126 MHz, CD3OD) spectrum of compound 3. Figure S19. HSQC spectrum of compound 3. Figure S20. COSY spectrum of compound 3. Figure S21. HMBC spectrum of compound 3. Figure S22. HRESIMS of compound 3. Figure S23. UV spectrum of compound 3. Figure S24. IR spectrum of compound 3.
Acknowledgements
This work was supported by National Natural Science Foundation of China (grant No. 31870513, 32000011). We thank the Analytical & Measuring Center, School of Pharmaceutical Sciences, SCUN for their help with NMR measurements.
Author contributions
All authors read and approved the final manuscript.
Funding
National Natural Science Foundation of China (31870513), Zheng-Hui Li, National Aerospace Science Foundation of China (32000011), Hong-Lian Ai.
Declarations
Competing interests
The authors declare no conflict of interest.
References
-
1.HP Chen, ZZ Zhao, GG Cheng, K Zhao, KY Han, L Zhou, T Feng, ZH Li, JK Liu, Immunosuppressive nor-isopimarane diterpenes from cultures of the fungicolous fungus Xylaria longipes HFG1018[J]. J Nat Prod 83, 401-12 (2020) CrossRef PubMed Google Scholar
-
2.A Lleo, P Invernizzi, B Gao, M Podda, ME Gershwin, Definition of human autoimmunity-autoantibodies versus autoimmune disease[J]. Autoimmun Rev 9, A259-66 (2010) CrossRef PubMed Google Scholar
-
3.Y Gao, FF Duan, L Liu, XG Peng, XG Meng, HL Ruan, Hypothemycin-type resorcylic acid lactones with immunosuppressive activities from a Podospora sp[J]. J Nat Prod 84, 483-94 (2021) CrossRef PubMed Google Scholar
-
4.T Feng, KT Duan, SJ He, B Wu, YS Zheng, HL Ai, ZH Li, J He, JP Zuo, JK Liu, Ophiorrhines A and B, two immunosuppressive monoterpenoid indole alkaloids from Ophiorrhiza japonica[J]. Org Lett 20, 7926-8 (2018) CrossRef PubMed Google Scholar
-
5.QY Shou, RZ Fu, Q Tan, ZW Shen, Geranylated flavonoids from the roots of Campylotropis hirtella and their immunosuppressive activities[J]. J Agric Food Chem 57, 6712-9 (2009) CrossRef PubMed Google Scholar
-
6.M Kiuchi, K Adachi, T Kohara, M Minoguchi, T Hanano, Y Aoki, T Mishina, M Arita, N Nakao, M Ohtsuki, Y Hoshino, K Teshima, K Chiba, S Sasaki, T Fujita, Synthesis and immunosuppressive activity of 2-substituted 2-aminopropane-1, 3-diols and 2-aminoethanols[J]. J Med Chem 43, 2946-61 (2000) CrossRef PubMed Google Scholar
-
7.NT Ujam, DL Ajaghaku, FBC Okoye, CO Esimone, Antioxidant and immunosuppressive activities of extracts of endophytic fungi isolated from Psidium guajava and Newbouldia laevis[J]. Phytomedicine Plus 1, 100028 (2021) CrossRef PubMed Google Scholar
-
8.R D'Alessio, A Bargiotti, O Carlini, F Colotta, M Ferrari, P Gnocchi, A Isetta, N Mongelli, P Motta, A Rossi, M Rossi, M Tibolla, E Vanotti, Synthesis and immunosuppressive activity of novel prodigiosin derivatives[J]. J Med Chem 43, 2557-65 (2000) CrossRef PubMed Google Scholar
-
9.J Liu, H Li, KX Chen, JP Zuo, YW Guo, W Tang, XW Li, Design and synthesis of marine phidianidine derivatives as potential immunosuppressive agents[J]. J Med Chem 61, 11298-308 (2018) CrossRef PubMed Google Scholar
-
10.A Johnston, Equivalence and interchangeability of narrow therapeutic index drugs in organ transplantation[J]. Eur J Hosp Pharm 20, 302-7 (2013) CrossRef PubMed Google Scholar
-
11.JM Smith, TL Nemeth, RA McDonald, Current immunosuppressive agents: efficacy, side effects, and utilization[J]. Pediatr Clin N Am 50, 1283-300 (2003) CrossRef PubMed Google Scholar
-
12.M Gordaliza, GT Faircloth, MA Castro, JM Miguel del Corral, ML López-Vázquez, FA San, Immunosuppressive cyclolignans[J]. J Med Chem 39, 2865-8 (1996) CrossRef PubMed Google Scholar
-
13.G Li, S Kusari, M Lamshoft, A Schuffler, H Laatsch, M Spiteller, Antibacterial secondary metabolites from an endophytic fungus, Eupenicillium sp. LG41[J]. J Nat Prod 77, 2335-41 (2014) CrossRef PubMed Google Scholar
-
14.X Lin, C Lu, Y Huang, Z Zheng, W Su, Y Shen, Endophytic fungi from a pharmaceutical plant, Camptotheca acuminata: isolation, identification and bioactivity[J]. World J Microbiol Biotechnol 23, 1037-40 (2007) CrossRef PubMed Google Scholar
-
15.HJ Chen, T Awakawa, JY Sun, T Wakimoto, I Abe, Epigenetic modifierinduced biosynthesis of novel fusaric acid derivatives in endophytic fungi from Datura stramonium L[J]. Nat Prod Bioprospect 3, 20-3 (2013) CrossRef PubMed Google Scholar
-
16.L Feng, J Wang, S Liu, XJ Zhang, QR Bi, YY Hu, Z Wang, NH Tan, Colletopeptides A-D, anti-inflammatory cyclic tridepsipeptides from the plant endophytic fungus Colletotrichum sp. S8[J]. J Nat Prod 82, 1434-41 (2019) CrossRef PubMed Google Scholar
-
17.C Kuang, SX Jing, Y Liu, SH Luo, SH Li, Drimane sesquiterpenoids and Isochromone derivative from the endophytic fungus Pestalotiopsis sp. M-23[J]. Nat Prod Bioprospect 6, 155-60 (2016) CrossRef PubMed Google Scholar
-
18.D Kim, HL Simborio, AW Reyes, W Min, HJ Lee, J Lee, H Chang, D Kim, Antibacterial effects of Coptis chinensis Franch against Brucella abortus[J]. J Agric Life Sci 48, 107-14 (2014) CrossRef PubMed Google Scholar
-
19.DL Fan, XH Xiao, XJ Ma, Calorimetric study of the effect of protoberberine alkaloids in Coptis chinensis Franch on Staphylococcus aureus growth[J]. Thermochim Acta 480, 49-52 (2008) CrossRef PubMed Google Scholar
-
20.XH Zhang, DJ Zhang, JL Liu, HY Pan, JC Qin, YH Zhang, Antifungal effects of volatile organic compounds from the endophytic fungus Cryptosporiopsis ericae Cc-HG-7 isolated from Coptis chinensis Franch[J]. Biocontrol Sci Technol 28, 496-508 (2018) CrossRef PubMed Google Scholar
Copyright information
© The Author(s) 2022
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.