Traditional Herbal Medicines Against CNS Disorders from Bangladesh
Abstract
The majority of the population in Bangladesh uses traditional plant-based medicines to manage various ailments, including central nervous system (CNS) disorders. This review presents ethnobotanical information and relevant scientific studies on plants used in traditional healthcare for the management of various CNS disorders in Bangladesh. The information on the medicinal plants of Bangladesh effective against CNS disorders published in scientific journals, books, and reports was compiled from different electronic databases using specific key words. The present article provides comprehensive information on a total of 224 medicinal plant species belonging to 81 families used for the treatment of CNS disorders by the various peoples of Bangladesh. In total, we reviewed more than 290 relevant papers. In this study, leaves were found as the most often used plant organ, followed by roots, fruits, whole plants, barks, seeds, stems, rhizomes, and flowers. The Fabaceae family contributes the highest number of used species, followed by Rubiaceae, Lamiaceae, Cucurbitaceae, Vitaceae, Euphorbiaceae, Malvaceae, and Zingiberaceae. The most frequently used species (in decreasing order) are Asparagus racemosus, Centella asiatica, Stephania japonica, Aegle marmelos, Coccinia grandis, Tabernaemontana divaricata, Bacopa monnieri, Abroma augusta, and Scoparia dulcis. This review may serve as a starting point for a rational search for neuroactive natural products against CNS disorders within the Flora of Bangladesh.Graphical Abstract
Keywords
CNS disorder Medicinal plants Traditional plants Ethnopharmacology ReviewAbbreviations
1 Introduction
The central nervous system (CNS), as an integral part of the nervous system, is associated with a number of important functions and mainly consists of the brain and the spinal cord. A CNS disorder refers to a disease that affects the structure or function of brain (encephalopathy) or spinal cord (myelopathy) causing neurological or psychiatric or neurodegenerative complications. Neuroprotection denotes strategies to defend the central nervous system (CNS) against a number of factors such as structural defects, infections, neuronal injury, autoimmune disorders, tumors, neurodegeneration, and others, which may lead to CNS disorders [1]. In recent years, these disorders are rising due to the increase of life expectancy, and thus place a tremendous burden on families and social economies. A new report from the World Health Organization (WHO) shows that neurological disorders affect up to one billion people worldwide, among them 6.8 million people die every year. In addition, the prevalence of CNS disorders is around two times higher in developing countries than in the developed world [2].
Herbal supplements have long played important roles to treat various neuronal and pathological disorders without or with limited side effects. During recent years, complementary and alternative medicine (CAM) has become more popular worldwide. Many plant species have emerged as herbal medicines, and their active components have been subjected to extensive scientific research around the world [3-5]. CAM or traditional medicines are considered safe and effective in sensitive and complicated diseases like CNS disorder, while having less side effects than synthetic compounds [6]. Newman and Cragg reported that more than two thirds of the active agents recently introduced into the market have some relationship to natural sources and only 30% of new chemical entities used as medicines are of purely synthetic origin [7]. The knowledge of ethnobotany therefore continues to provide a valuable starting point for many successful drug-screening projects [8].
Also in western societies, there has been an increasing interest in herbal medicines, which are often perceived as more 'natural' and 'softer' treatments compared to synthetic drugs [9]. Drug discovery based on traditional knowledge has been termed 'reverse pharmacology'; in this approach, drug candidates are first identified based on large-scale usage in the population before initiating clinical trials. This approach can cut the time span, needed for drug discovery, from on average twelve years (classical approach) to five years or even less (reverse pharmacology); the latter has the additional advantage of far lower development costs [10].
Traditional knowledge of medicinal plants as a complementary and alternative therapy has additionally the great significance for conserving cultural traditions and identities. Moreover, community healthcare is fostered and interesting leads for future drug development projects can be found. From this perspective, ethnopharmacological data of medicinal plants on CNS disorders will ease the identification of important species utilized in traditional medicine. In this review, we summarize ethnopharmacological knowledge of all currently known popular CNS active herbal remedies in Bangladesh. Additionally, we provided more details on six selected species: Bacopa monnieri, Centella asiatica, Curcuma longa, Cyperus rotundus, Morinda citrifolia, and Withania somnifera (author citations for these and all other scientific species names mentioned in this text have been consistently omitted from the main body of the text, but are provided in Table 1). This review on species from Bangladesh is intended to stimulate the interest in a deeper evaluation of the mentioned species as potential sources for structurally and functionally novel CNS active drug leads or hits.
Plant species along with their experimental records used for care of CNS disorders in Bangladesh
The CNS is a complex and sophisticated system, and today, CNS disorders are categorized and treated considering critical single or multiple targets. The traditional healers, particularly herbal medicine practitioners, focus on a typical category of disease commensurate with their knowledge and experience rather than employing a specific single biomarker targeted therapy. However, this review highlights ethnobotanical together with the respective experimental records focused on broadly categorized CNS disorders. The reviewed plant species, as a group, have been recommended against almost all classical types of CNS disorders.
2 Materials and Methods
2.1 Search Strategy
A comprehensive literature study published in journals, books, and reports was performed to get a systematic overview about the medicinal plants used against CNS disorders in Bangladesh. Various electronic databases were searched, including Web of Science, SciFinder, PubMed, Science Direct, Scopus, Springer, Taylor & Francis online, Wiley online library, and Google Scholar. The following keywords were employed in combination with Bangladesh: brain, memory, CNS, neurological disorder, neurodegenerative disease, psychological disorder, medicinal plants, traditional plants, survey of medicinal plants, ethnobotanical survey, ethnomedicinal survey, and survey of plants acting on CNS.
2.2 Study Selection and Data Extraction
All publications dealing with plant species effective against CNS disorder have been identified from all of the possible sources published until the end of July 2020. The search was limited to literature published in English. The name of the plant species responsible in the treatment of CNS disorders has only been extracted among all other uses and species. For the pharmacological evidence, articles presenting first-hand research information including clinical, pre-clinical, ex-vivo, and in-vitro studies were also part of the inclusion criteria.
3 History and Present Status of Traditional Bangladeshi Medicine (TBM)
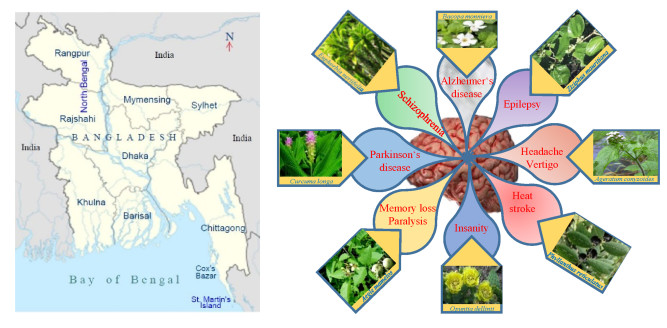
Bangladesh, a tropical South Asian country, harbors a huge range of biodiversity including numerous medicinal plant species due to its diverse landscape and pronounced seasonal diversity [11]. Large parts of Bangladesh are covered by tropical forests featuring heterogeneous ecologic conditions such as fertile alluvial lands, warm and humid climates. Bangladesh is home to a rich plant diversity with more than 5300 species of higher plants [12]. Around 80% of the population of Bangladesh use herbal medicines for their primary healthcare where plants used in traditional ethnomedicine constitute a major component [13]. Bangladesh is also home to 35 indigenous communities living in various, mostly hilly, remote areas of Bangladesh; these communities contribute about 2% to the total population of the country. Each of these communities has a diverse cultural background and practices their own traditional ethnomedicine for primary healthcare [14].
4 Distribution of Plant Species and Their Taxonomy
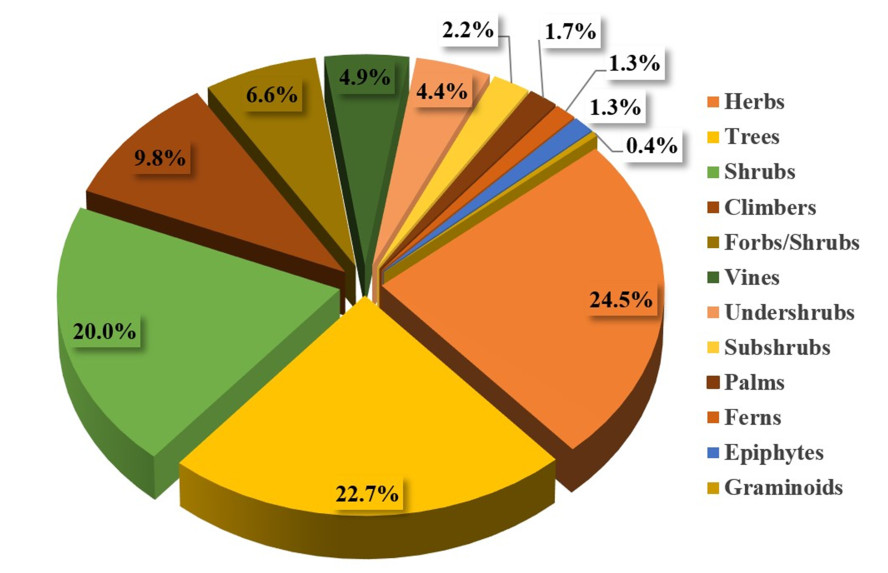
A total of 224 plant species from 182 genera and from 81 different families were reported to be used against CNS disorders. All recorded plant species are presented in Table 1, detailing their family, local name(s), life-form, plant part(s) used, traditional uses, and the available pharmacological data supporting their traditional use. The life forms of the documented species were (in decreasing order) herbs (24.5%), trees (22.7%), shrubs (20.0%), climbers (9.8%), forbs/herbs (6.6%), vines (4.9%), undershrubs (4.4%), subshrubs (2.2%), palms (1.7%), ferns (1.3%), and epiphytes (1.3%) (Fig. 1). Analogous studies from other areas in tropical Asia yielded similar results regarding the life form of the medicinally used species [15-17].
Growth habits of the covered species
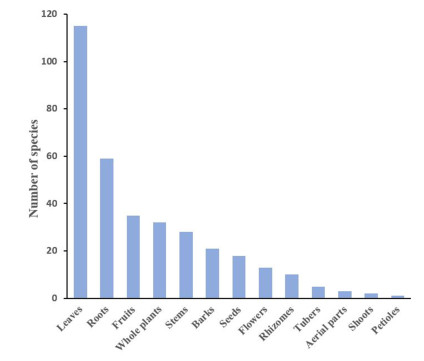
The most often utilized plant parts were leaves (51.3%), followed by roots (26.3%), fruits (15.6%), whole plants (14.2%), stems (12.5%), barks (9.3%), seeds, flowers, and rhizomes; while other parts were only rarely utilized (Fig. 2). Leaves are very often used in herbal medicine, because they often contain high amounts of active compounds and are easy to collect and prepare, and consequently, a larger number of plant natural product studies are available for leaves compared to studies concerning other plant organs. In our survey, roots were the second most frequently used plant organs, possibly due to their high concentration of bioactive compounds [18]. Regarding botanical systematics, the families with the highest number of species used against CNS disorders were the Fabaceae (syn.: Leguminosae; seventeen), Rubiaceae (twelve), Lamiaceae (ten), Apocynaceae, Cucurbitaceae and Vitaceae (each eight species), Euphorbiaceae, Malvaceae, and Zingiberaceae (each seven species), Araceae, Compositae, Fabaceae, Piperaceae, and Urticaceae (each five species), Amaranthaceae, Asteraceae, Moraceae, and Solanaceae (each four species). The remainder of the medicinally used plant families contributed only one to three species (Table 1).
List of the most frequently used plant parts along with the number of corresponding species used in ethnomedicinal preparations
According to the fundamental book on the Bangladeshi Flora [12], the largest five families in Bangladesh are the Poaceae, Fabaceae, Orchidaceae, Rubiaceae, and Asteraceae, respectively. The dominance of Fabaceae and Rubiaceae species in treating CNS disorder might amongst other factors, be explained by the presence of bioactive alkaloids, flavonoids, and terpenoids in many members of these families [19].
5 CNS-Active Natural Products
Numerous plant natural products have been reported to have beneficial effects on the human CNS. Table 2 presents some of these natural products and their mechanism of actions. Two general postulates try to explain why natural products elicit effects on the human CNS: firstly, due to the connection of the numerous molecular signaling pathways that are conserved between the taxa and the systematic actions in natural product synthesis within plants [20]. The second hypothesis is that plant natural products exhibit similar effects on the nervous systems of humans and the most prevalent natural herbivores, via the same mechanisms [21].
Bioactive compounds against CNS disorders from native species of Bangladesh
Alkaloids are one of the largest groups of plant natural products. These compounds usually act as agonists and antagonists to a variety of neurotransmitter through direct binding to neuro-receptors and/or by interference with neurotransmitter metabolism. Plant-derived alkaloids possess potential therapeutic effects against several neurodegenerative disorders (Alzheimer's disease, Huntington's disease, and Parkinson's disease), epilepsy, schizophrenia, and stroke [22].
Phenols are the most widespread and ubiquitous class of natural products. Besides free radical and reactive oxygen species scavenging, and metal chelating abilities, phenolic compounds demonstrate a significant role in various CNS disorders by direct interaction with neurotransmitter systems including sedative, anxiolytic, antipsychotic, cognitive enhancement, cholinergic upregulation, and antidepressant effects [23].
Saponins are a structurally diverse group of glycosidic compounds, featuring either pentacyclic triterpenoids or steroids as aglycones. Saponins have significant neuroprotective effects on the attenuation of CNS disorders, such as stroke, Alzheimer's disease, Parkinson's disease, and Huntington's disease [24]. In this review, Table 2 displays a selection of saponins (Fig. 9), which are potentially effective on brain disorders. Terpenes are a large class of natural products exhibiting a wide range of effects within the CNS. Many natural terpenoids have been reported to interact with the octopaminergic and noradrenergic systems, to inhibit cholinesterase, and to directly or allosterically bind to the GABAergic system; all with a relation to disorders like anxiety, insomnia, convulsion, pain, and cognitive deficits [25].
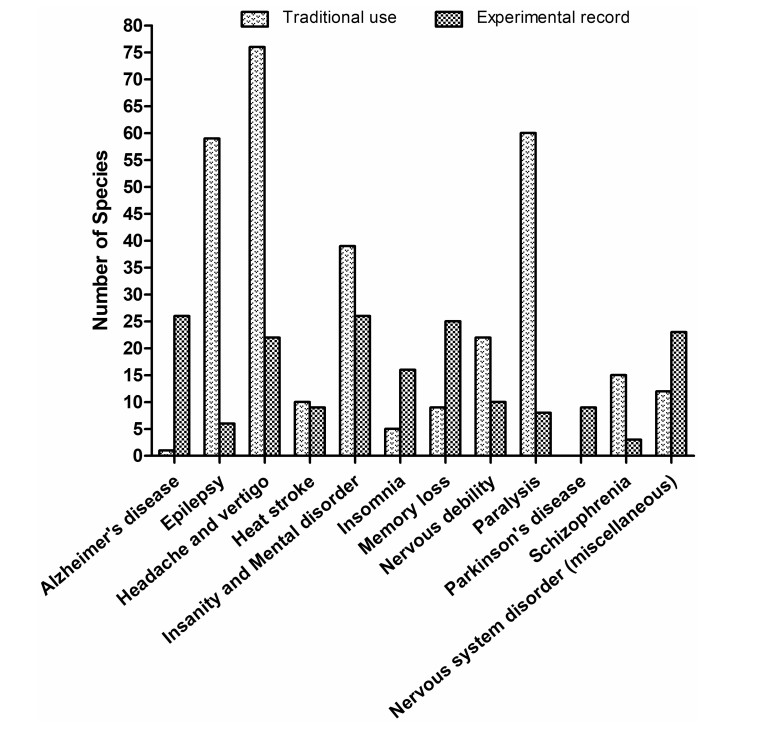
Comparison of the documented plant species with traditional use and experimental evidence over categorized CNS disorders
Chemical structure of some commonly used natural products for the treatment of nervous system disorders
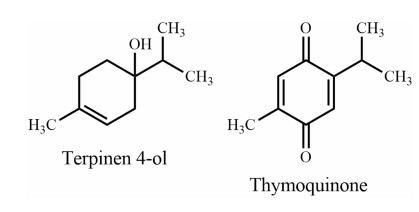
Monoterpenes
Sesquiterpenes
Diterpene
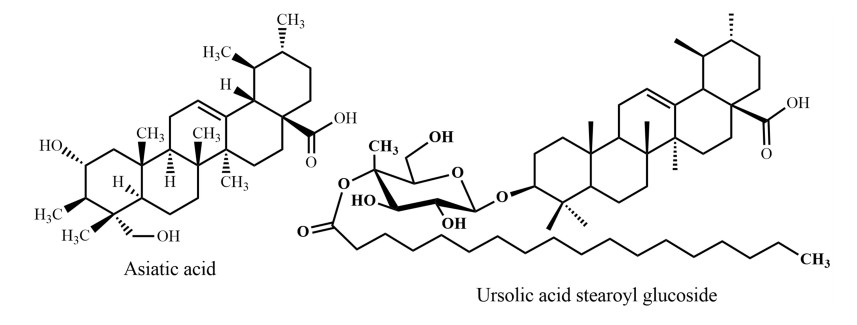
Triterpenes 1: ursane derivatives
Triterpenes 2: steroidal saponins
6 Plants, Traditional Medicines, and CNS Disorder: Globally
Approximately one out of nine human deaths is related to a nervous system disorder worldwide, and more than 28% have to live with disability caused by nervous system disorder at some stage of their lives [26]. Depression is the major cause of disability and is globally more frequent than all other nervous system disorders. The top twenty leading causes for disability also include anxiety disorders, schizophrenia, autism and Asperger syndrome, Alzheimer's disease and other dementias, and illicit drug use [27].
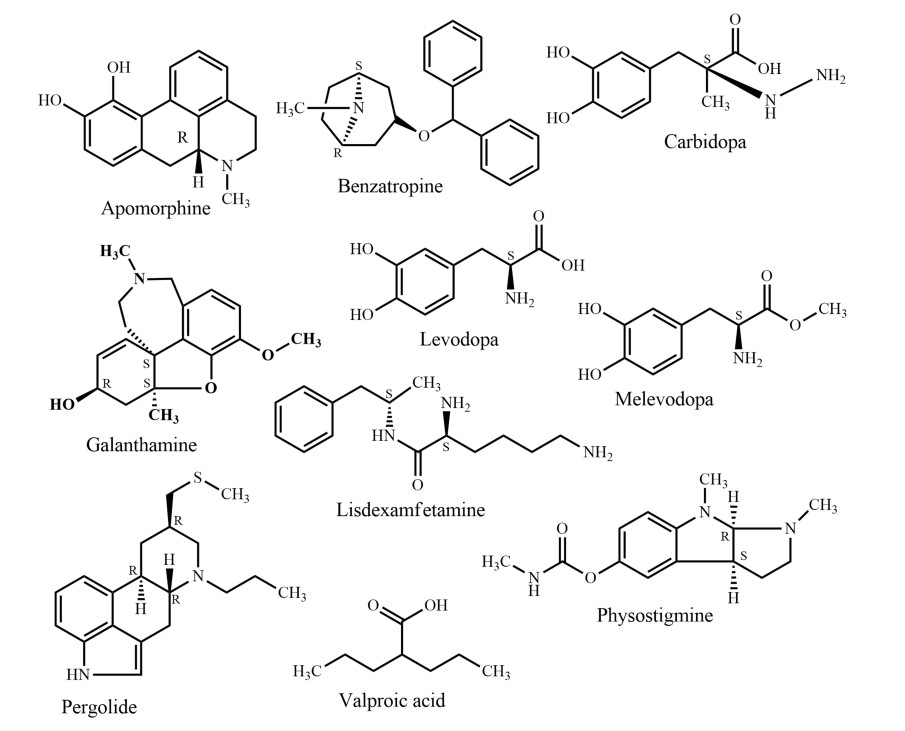
In traditional systems of medicine, plants have been used to treat a huge number of disorders including nervous disorder for centuries, because they are easily available and affordable. The latest global survey of traditional and complementary medicine (T & CM) shows that significant momentum has been achieved over the past decade (WHO, 2013). Over 100 million Europeans are currently using T & CM. Thus, in Europe one fifth of the population regularly use T & CM and the same share is preferring healthcare, which includes T & CM [28]. It is evident that there are many more T & CM users in Africa, Asia, Australia, and North America [29]. Traditional medicines could be a potential source of novel compounds or phytomedicines/supplements in the management of nervous disorders. Apomorphine, galanthamine, lisdexamfetamine, and valproic acid (Fig. 4) are the first line drugs currently used to treat Parkinson's disease, Alzheimer's disease, attention-deficit/hyperactivity disorder, and epilepsy, respectively. The active compounds were originally derived from Papaver somniferum L, Galanthus nivalis L., Ephedra sinica Stapf., and Valeriana officinalis L., respectively. Since the 1950s, the FDA approved six plant derived drugs (Fig. 4), namely benzatropine (1954) (derived from atropine from e.g. Atropa belladonna L.), levodopa (1970) [from Mucuna pruriens (L.) DC.], carbidopa (1975) (from levodopa, e.g. from Mucuna pruriens), pergolide (1988) [from ergot alkaloids from, e.g. Claviceps purpurea (Fr.) Tul.], melevodopa (1993) (from levodopa from, e.g. Mucuna pruriens), and apomorphine (2004) (from morphine from e.g. Papaver somniferum) to treat Parkinson's disease. A report showed that by the end of 2013, the FDA had approved 307 natural products and natural product derivatives from plants, bacteria, fungi, and marine organisms, respectively. These comprise 21% of all approved new chemical entities [30].
7 Plants, Traditional Medicines, and CNS Disorder: in Bangladesh
In a global study, Bangladesh has been ranked 133rd among 195 countries regarding personal healthcare access and quality [31]. In Bangladesh, over six million people experience depressive disorders and almost seven million people are suffering from anxiety disorders [32]. It is estimated that more than ten thousand people are dying every year by suicide in the country [33]. Most of the nervous system disorders are chronic and polygenic in nature. The development of more effective treatments, for example in schizophrenia and depression, based on selective drugs for single molecular targets has been largely unsuccessful [34]. Hence, multi-targeted therapeutic approach of nervous system disorders employing traditional medicine is often advantageous, easier, cheaper, and more cost effective. A handful of ethnomedicinal surveys on medicinal plants over different divisions, districts, villages, and even hill tract and tribal areas of the country revealed that medicinal plants are used to treat various disorders including nervous system disorders. Among the medicinal plants used in nervous system disorders Sotamuli (Asparagus racemosus), Thankuni (Centella asiatica), Akanadi (Stephania japonica), Bel (Aegle marmelos), Telakucha (Coccinia grandis), Tagar (Tabernaemontana divaricate), Misridana (Scoparia dulcis), Brammishak (Bacopa monnieri), and Aswagandha (Withania somnifera) are the most popular herbal medications for nervous system disorders in Bangladesh (Table 1). Table 1 contains all local names of the plant species used against CNS disorders in Bangladesh.
Ulothkombal (Abroma augusta), Apang (Achyranthes aspera), Halud (Curcuma longa), Noni (Morinda citrifolia), Sajina (Moringa oleifera), and Mutha (Cyperus rotundus) are also widely used in the management of CNS disorders. All of the above-mentioned species have demonstrated their notable pharmacological activity against nervous system disorders in different experimental models. The experimental evidence available for Achyranthes aspera, Aegle marmelos, Asparagus racemosus, Bacopa monnieri, Bryophyllum pinnatum, Centella asiatica, Clitoria ternatea, Coccinia grandis, Convolvulus pluricaulis, Curcuma aromatica, Curcuma longa, Datura metel, Euphorbia neriifolia, Hemidesmus indicus, and Musa sapientum also support the claims of traditional users (Table 1). In addition, for some of the species traditionally used in various nervous disorders, no pharmacological investigations have been performed yet, including Ghetkaachu (Typhonium trilobatum), Kundri (Solena amplexicaulis), Lal Chita (Plumbago rosea), Dheki shak (Lygodium altum), and Kanchan (Bauhinia acuminata) (Table 1). To cure paralysis, epilepsy, insanity and mental disorder, and nervous debility are the most often mentioned indications among all covered CNS disorders. In contrast, most experimental evidence so far has been provided for activity against insanity and mental disorder, memory loss, and Alzheimer's disease (Fig. 3).
Traditional medicine and conventional healthcare systems are offered in separate facilities at secondary and tertiary levels in nine countries in South-Eastern Asia (Bangladesh, Bhutan, India, Indonesia, Maldives, Myanmar, Nepal, Sri Lanka, and Thailand), while all three levels of care are available in the same health care facilities in South Korea. In Bangladesh, there are 469 small factories (268 Unani and 201 Ayurvedic) producing traditional drugs worth approximately US$ 100 million every year [35] (Fig. 4).
8 Evidence-Linked Plants and Active Metabolites of TBM Effective on CNS Disorder
Many plant-derived natural products are claimed to have beneficial effects against CNS disorders. Some pure natural products derived from the plant species mentioned in this review, have already been tested as efficacious candidates against CNS disorders. Table 2 displays these metabolites with the corresponding disorder, where they were found to be active. Name and structures of all mentioned plant natural products from different source species have been summarized in Table 2 and in Figs. 5, 6, 7, 8, 9, 10, 11, 12, 13, and 14 (based on chemical compound classes). From all of the mentioned species, Bacopa monnieri, Centella asiatica, Curcuma longa, Cyperus rotundus, Morinda citrifolia, and Withania somnifera have been selected and discussed in some detail below. The focus of the discussion is on their impact on nervous system disorders. The species have been selected based on their widespread use, a large body of experimental records, and commercial availability. The main point of giving in-depth records on some selected species is to show the large potential of such traditional medicinal plants both from a medicinal and from a commercial perspective.
Triterpenes 3: steroidal lactones
Flavonoids
Phenylpropanoids
Diphenylheptanoids
Miscellaneous (cannabinoid, lignan, oleamide, and alkaloid)
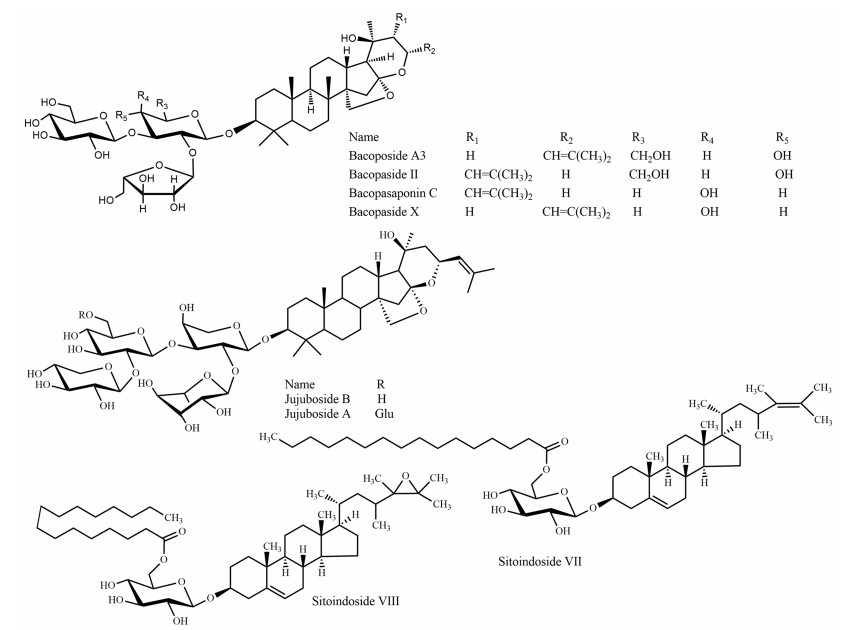
Bacopa monnieri, "Brammishak", a small herb from the Plantaginaceae family, is distributed mainly in the coastal area of Bangladesh such as Chittagong, Cox's Bazar, and Saint Martin's island. Brammishak is named after the word 'Brama', the mythical 'creator' in the Hindu pantheon. 'Brahmi', which also means 'bringing knowledge of the Supreme Reality' [36]. The herb was used by ancient Vedic scholars to sharpen the cognitive functions and is mentioned as part of many Ayurvedic preparations. Brammishak is also traditionally used as a green leafy vegetable (shak) due to its well-known health benefits [37]. The experimental evidence has proven potent activity of Brammishak on the regulation of reactive oxygen species, neuroprotection, acetylcholinesterase (AChE) inhibition, choline acetyltransferase activation, β-amyloid reduction, increased cerebral blood flow, and monoamine potentiation and modulation [38]. Brammishak contains triterpenoid saponins called bacosides. Among the twelve analogs of bacosides, bacoside A is the best studied and most potent constituent of Brammishak, which additionally includes bacoside A3, bacopaside Ⅱ, bacopasaponin C, and bacopaside X (a jujubogenin isomer of bacosaponin C) (Fig. 9) [39]. Bacoside A significantly inhibit β-amyloid toxicity, fibrillation, improve memory and cognitive functions, decreased GABA receptors associated with epilepsy as well as increased the activities of superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase [40, 41]. In a review study on human trials, Neale et al. [42] compared the nootropic effects of two neutraceuticals Brammishak and Panax ginseng with modafinil (a synthetic eugeroic drug); in this comparison, Brammishak displayed the most consistent and largest effect of the three tested preparations.
Centella asiatica, "Thankuni", a perennial herbaceous creeper with kidney shaped leaves belonging to the Apiaceae family, is distributed throughout Bangladesh in fallow lands. Thankuni leaf is an ancient Ayurvedic, Unani, and has been used as a folk medicine in Bangladesh and South Asian countries for many centuries. The species is used as a revitalizing herb that supposedly strengthens nervous function and memory. An aqueous extract of C. asiatica leaves contributes to improved learning and memory processes by modulating dopamine, 5-hydroxytryptamine (5-HT), and noradrenaline systems in rat brains in vivo [43]. This result suggested that the polar compounds, for example asiatic acid present in C. asiatica leaves, may enhance cognitive functions by influencing neurotransmitter systems in the CNS. Further research proved that asiatic acid (triterpenoid) (Fig. 8) from C. asiatica down-regulates β-secretase (BACE1) as well as up-regulates ADAM10 in primary rat cortical neurons [44], inhibits induced neurotoxicity of aged rats [45], attenuates glutamate-induced cognitive deficiencies of mice, and protects SH-SY5Y cells against glutamate-induced apoptosis [46], which are all related to potential routes in Alzheimer's disease treatment. Asiatic acid from C. asiatica effectively offered neuro-protection in chronic Parkinson's disease by activation of dopaminergic neurons [47]. Orhan et al. [48] showed that butyrylcholinesterase inhibitory activity of South Asian C. asiatica is stronger than from Chinese sources.
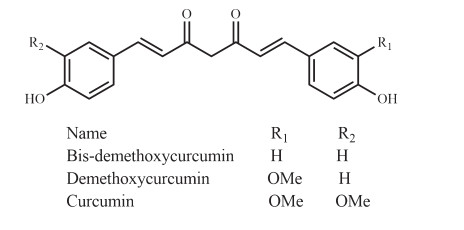
Curcuma longa, "Halud", is a perennial rhizomatous herb from the Zingiberaceae family and is cultivated all over Bangladesh and used as one of the main spice. Along with the protection of memory loss, it contributes to a wide range of potential medicinal applications because of the presence of curcuminoids (Fig. 13). Curcumin, an extensively studied plant natural product isolated from the rhizome of Curcuma longa has displayed neuro-pharmacological activity against neuro-inflammation, memory impairment, and different biomarkers of Alzheimer's disease and Parkinson's disease in vitro and in vivo [49-51]. More importantly, curcumin has already been clinically evaluated against a few central nervous system disordersseases. Initially, Rainey-Smith et al. [52] reported a low efficacy of curcumin against dementia symptoms. However, recently developed novel curcumin formulations (Longvida® and Theracurmin) ensure a higher bioavailability, combined with good acute and chronic activities for both products, even at low doses (80-180 mg/day) [53]. The study carried out by Burns et al. [54] showed a marked improvement in a patient trial of Déjérine-Sottas disease, where curcumin was administered for twelve months in two escalating doses (1500 and 2500 mg/day). In the curcumin-treated group, it was observed that curcumin decreased IL-1β, TNFα, salivary cortisol levels, and increased plasma BDNF [55]. Lopresti et al. [56] identified a significant increase in urinary molecular markers thromboxane B2, substance P, baseline plasma endothelin-1, and leptin that can all be related to the antidepressant mechanism of action of curcumin.
Cyperus rotundus, "Mutha", a perennial herb as well as an obnoxious weed, is widely distributed in tropical and subtropical regions, including Bangladesh. This species traditionally used in the management of paralysis in Bangladesh, and epilepsy in India [57]. Additionally, experimental evidence showed a potential role in improving memory and cognition. Rhizomes of C. rotundus possess anti-AChE activity [58], anticonvulsant properties [59], inhibits memory loss [60] and pyramidal cell loss. Nóbrega et al. [61] reported that terpinen-4-ol (Fig. 5) (contained in the essential oil of C. rotundus) is effective against convulsion in behavioral and electrophysiological studies. Azimi et al. [62] identified α-cyperone from C. rotundus as capable of interactions with tubulin and as a destabilizing agent of microtubule polymerization. This interaction results in reduction of inflammation, which could be beneficial for the treatment of inflammatory diseases such as Alzheimer's disease.
Morinda citrifolia, "Noni", a small tropical tree of the Rubiaceae family, is native to South Asia and cultivated all over Bangladesh [63]. All parts of the plant are claimed to have various pharmacological properties, in particular, the fruit has a long history of dietary use in tropical regions [64]. In 2002, Noni fruit juice has been recognized as a novel food the European Union [65]. Evidence showed that Noni fruit juice had a preventive effect against cerebral ischemic neuronal damage in a mice model [66]. Muto et al. [67] also reported that Noni juice protected mice brains from stress induced cognitive dysfunction, predominantly reducing the blood vessel density caused by stress. The administration of an ethyl acetate extract of noni fruit increased serotonin, dopamine, and antioxidant-enzyme serum levels in mice model with beta-amyloid induced cognitive dysfunction [68]. The ethanol extract of Noni fruit also improved memory, brain blood flow, and attenuated oxidative stress, acetylcholinesterase activity in a mice model [69]. A behavioral test revealed that the administration of the methanolic extract of Noni fruits decreased the negative effects of heroin and alcohol dependence [70, 71]. Despite a number of experimental evidence related to nervous system disorders, no specific natural product from this species has so far been identified and evaluated against nervous system disorders.
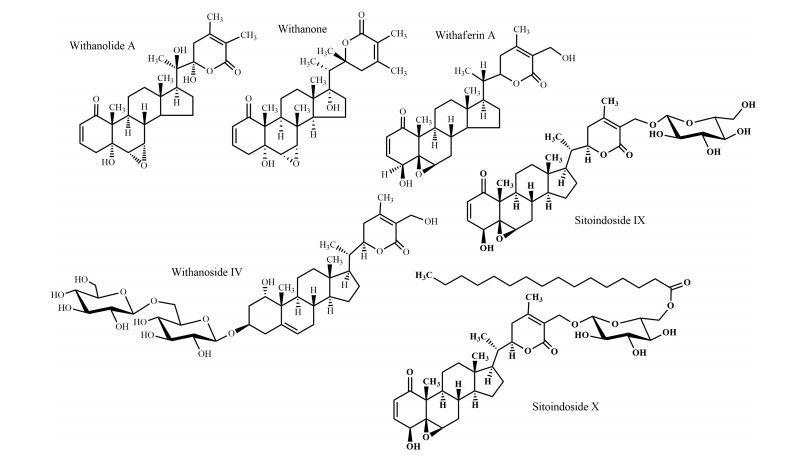
Withania somnifera, "Ashwagandha", is an undershrub commonly used in the traditional medicine of Bangladesh, naturally occurring in the North Bengal region. Among the 23 species of genus Withania, Ashwagandha is the most highly valued medicinal plant in traditional medicine and has been used since more than 3000 years. Various uses of this species including nervous system disorders (tonic, senile debility, nervous tension, loss of memory) reflect the ethno-pharmacological importance. Recent studies also demonstrated its multiple activities on nervous system disorders, particularly neuritic regeneration activity [72], neuroprotective activity [73], anti-anxiety and anti-depression activity [74], anti-Parkinson's activity [75], nootropic and anti-Alzheimer's activity [76], and anti-convulsant effects [77]. Roots are the most frequently used parts and the compounds isolated from these roots are effective against nervous system disorders. For example, withanolide A and withanoside Ⅳ (steroidal lactones) (Fig. 10) attenuated the β-amyloid (25-35) protein with the hope of enabling Alzheimer's disease management [78, 79]. In an in vivo experimental report, it has been demonstrated that bioactive glyco-withanolides (Fig. 10) enhanced the activity levels of various antioxidant enzymes in the frontal cortex and striatum of rats, which may also be relevant for Alzheimer's disease therapy [80].
9 Plants Used Against CNS Disorder: Economical and Botanical Context
Apart from the medicinal benefits, many of the mentioned species are economically important and cultivated or collected as part of Bangladeshi tradition. Many medicinal plant species have also other uses such as foodstuff, in cosmetics and hygiene, as additives in different preparations, as part of rituals, and as medicines for ailments not related to the CNS.
The fruits of many medicinal plant species, including Aegle marmelos, Citrullus lanatus, Citrus grandis, Phoenix sylvestris, Phyllanthus embelica, Solanum torvum, and Terminalia chebula, are predominantly used as foods. The same holds true for various green leaves commonly consumed as vegetables namely, Alpinia nigra, Amaranthus viridis, Bacopa monnieri, Centella asiatica, Coccinia grandis, Ipomoea aquatica, Moringa oleifera, and Nelumbo nucifera. Aloe vera, Curcuma longa, Curcuma aromatica, and Santalum album are natural cosmetics used in Bangladesh since centuries. Spices are substances with pungent and aromatic properties used to flavor foods or beverages. Cissus repens, Curcuma longa, Dillenia indica, Kaempferia galanga, Ocimum americanum, and Ocimum gratissimum are common spices used in different curries and beverages. Species used as ornament (Tabennaemontana divaricata), masticatory substances (Achyranthes aspera, Areca catechu, and Piper betel), aquatic plants (Nelumbo nucifera), and incense plants (Santalum album) are sometimes included in the management of nervous system disorder [37].
10 Future Prospects
Traditional plant-derived medicines are used throughout the world for a range of nervous disorders and may offer leads for drug development. In the past, native people around the world have helped to introduce many plant-derived products currently used to treat nervous disorders. Galanthamine (Fig. 4), a drug used against Alzheimer's disease, is a natural alkaloid and was first isolated from Galanthus nivalis. Evidence-based and safe use of non-expensive plant-derived medications against nervous disorders may offer an enormous public health benefit, particularly for low-income countries. Research showed that fruit juice of noni (Morinda citrifolia, a traditional medicine of Bangladesh) has more inhibitory effects on hydrocephalus-induced degenerative disorders than memantine, a synthetic drug used against Alzheimer's disease [81]. However, most of the pharmacological investigations carried out on the properties of the above-mentioned plants are only on a preliminary level. In addition, plant natural product as well as pharmacological potentials of many species mentioned in this review have not been scientifically examined at all yet.
It is therefore of pronounced interest to perform in-depth phyto-pharmacological assessments of traditionally used species to reveal potential new applications. This will additionally lead to a better understanding of traditional knowledge and clinical observations. For example, acteoside (Fig. 12) previously isolated from Clerodendrum infortunatum [82] and recently has been proved as an efficacious natural product against neurocytotoxicity, cognitive deficit, and neurochemical disturbances [83]. On the other hand, semisynthetic modifications of old and new natural compounds may yield substances for therapy, which are more effective than the genuine natural products they are derived from. One notable example is rivastigmine, which is more active than physostigmine (Fig. 4) (originally isolated from Physostigma venenosum Balf.) in the treatment of Alzheimer's and Parkinson's disease. Moreover, the multifactorial nature of Alzheimer's disease suggests that a multi-targeted therapeutic approach might be more advantageous than single target drugs and combination therapies. This review shows that Bacopa monnieri, Citrus grandis, Piper betel, and Withania somnifera have an interesting activity against different biomarkers of Alzheimer's disease and have distinct mechanism of action (Table 2). A combined therapy of these species or their bioactive natural products may contribute to an all-encompassing treatment strategy for Alzheimer's disease. At the same time, combinatory herbal therapy could be more beneficial for those who are suffering from multiple nervous disorders.
11 Conclusion
In many fields, traditional medicinal knowledge offers interesting leads for pharmacological research. Bangladesh is abundant in medicinal plants with various ethno-medicinal uses. In this review, we have compiled data on a large number of plant species, used as traditional medicine against neurological problems in Bangladesh. Many of these species have also displayed activity in bioassays matching their traditional uses. Based on these observations, future extensive investigations on those particular species can be targeted to identify the compounds responsible for the observed bioactivities as well as to unravel their mechanisms of action. Up to date, only a few of those active natural products and their respective modes of action have been identified (Table 2). We hope that the findings compiled in this review will contribute to the successful usage of ethno-medicinal knowledge of medicinal plants and their bioactive natural products in the treatment of CNS disorders.
Notes
Author contributions
MJU and CZ conceived and designed the review. MJU studied literatures and compiled data. MJU and CZ wrote the manuscript. All authors revised and approved the final version of the manuscript.
Funding
This research did not receive any specific grants.
Compliance with Ethical Standards
Competing interests
The authors declare that they have no competing interest.
References
-
1.R.K. Upadhyay, Biomed. Res. Int. 1, 37 (2014) PubMed Google Scholar
-
2.U.P. Kundap, S. Bhuvanendran, Y. Kumari, I. Othman, M.F. Shaikh, Front. Pharmacol. 8, 76 (2017) CrossRef PubMed Google Scholar
-
3.S. Lakea, UBCMJ. 7, 40-41 (2015) PubMed Google Scholar
-
4.M.N. Ahmed, K. Azam, M. Nur, Schizophr. Res. Treat. 2014, 679810 (2014) CrossRef PubMed Google Scholar
-
5.Y.T. Kantati, K.M. Kodjo, K.S. Dogbeavou, D. Vaudry, J. Leprince, M. Gbeassor, J. Ethnopharmacol. 181, 214-220 (2016) CrossRef PubMed Google Scholar
-
6.H. Kumar, S.V. More, S.D. Han, J.Y. Choi, D.K. Choi, Molecules 17, 10503-10539 (2012) CrossRef PubMed Google Scholar
-
7.D.J. Newman, G.M. Cragg, J. Nat. Prod. 75, 311-335 (2012) CrossRef PubMed Google Scholar
-
8.D.S. Fabricant, N.R. Farnsworth, Environ. Health Perspect. 109, 69-75 (2001) CrossRef PubMed Google Scholar
-
9.M. Adams, F. Gmünder, M. Hamburger, J. Ethnopharmacol. 113, 363-381 (2007) CrossRef PubMed Google Scholar
-
10.V. Kumar, Phytother. Res. 20, 1023-1035 (2006) CrossRef PubMed Google Scholar
-
11.M.H. Rahman, Int. Scholarly Res. Not. 2013, 369138 (2013) CrossRef PubMed Google Scholar
-
12.M.K. Pasha, S.B. Uddin, Dictionary of Plant Names of Bangladesh (Vascular Plants) (Janokalyan Prokashani, Chittagong, 2013), p. 434 PubMed Google Scholar
-
13.M. Yusuf, J. Begum, M.N. Hoque, J.U. Chowdhury, Medicinal Plants of Bangladesh (Bangladesh Council of Scientific and Industrial Research Laboratories, Chittagong, 2009) PubMed Google Scholar
-
14.M.A. Khan, M.K. Islam, M.A. Siraj, S. Saha, A.K. Barman, K. Awang, M.M. Rahman, J.A. Shilpi, R. Jahan, E. Islam, M. Rahmatullah, J. Ethnobiol. Ethnomed. 11, 44 (2015) CrossRef PubMed Google Scholar
-
15.A. Ghorbani, G. Langenberger, L. Feng, J. Sauerborn, J. Ethnopharmacol. 134, 651-667 (2011) CrossRef PubMed Google Scholar
-
16.H. Singh, T. Husain, P. Agnihotri, P.C. Pande, S. Khatoon, J. Ethnopharmacol. 154, 98-108 (2014) CrossRef PubMed Google Scholar
-
17.S. Kayani, M. Ahmad, S. Sultana, Z. KhanShinwari, M. Zafar, G. Yaseen, J. Ethnopharmacol. 164, 186-202 (2015) CrossRef PubMed Google Scholar
-
18.I. Basualdo, E.M. Zardini, M. Ortiz, Econ. Bot. 49, 387-394 (1995) CrossRef PubMed Google Scholar
-
19.E. Rodrigues, F.R. Mendes, G. Negri, Cent. Nerv. Syst. Agents Med. Chem. 6, 211-244 (2006) CrossRef PubMed Google Scholar
-
20.J.C. Schultz, Integr. Comp. Biol. 42, 454-462 (2002) CrossRef PubMed Google Scholar
-
21.D.O. Kennedy, E.L. Wightman, Adv. Nutr. 2, 32-50 (2011) CrossRef PubMed Google Scholar
-
22.G. Hussain, A. Rasul, H. Anwar, N. Aziz, A. Razzaq, W. Wei, M. Ali, J. Li, X. Li, Int. J. Biol. Sci. 14, 341-357 (2018) CrossRef PubMed Google Scholar
-
23.A. Ayokun-nun Aja, A.A. Alimi, O.A. Olatunji, F.O. Balogun, S.A. Saheed, Trans. R. Soc. S. Afr. 73, 33-41 (2018) CrossRef PubMed Google Scholar
-
24.A. Sun, X. Xu, J. Lin, X. Cui, R. Xu, Phytother. Res. 29, 187-200 (2015) CrossRef PubMed Google Scholar
-
25.A. Manayi, S.M. Nabavi, M. Daglia, S. Jafari, Pharmacol. Rep. 68, 671-679 (2016) CrossRef PubMed Google Scholar
-
26.D.C. Bergen, D. Silberberg, Arch. Neurol. 59, 1194-1196 (2002) CrossRef PubMed Google Scholar
-
27.World Health Organization, Bangladesh. https://www.searo.who.int/bangladesh/mental-health/en. Accessed August 16 2019. PubMed Google Scholar
-
28.Q. Zhang, Tradit. Med. Mod. Med. 1, 11-13 (2018) CrossRef PubMed Google Scholar
-
29.P.M. Barnes, B. Bloom, R.L. Nahin, Natl. Health Stat. Report. 12, 1-23 (2008) PubMed Google Scholar
-
30.E. Patridge, P. Gareiss, M.S. Kinch, D. Hoyer, Drug Discov. Today. 21, 204-207 (2015) CrossRef PubMed Google Scholar
-
31.N. Fullman, J. Yearwood, S.M. Abay, C. Abbafati, F. Abd-Allah, J. Abdela, A. Abdelalim, Z. Abebe, T.A. Abebo, V. Aboyans, H.N. Abraha, The Lancet. 391, 2236-2271 (2018) CrossRef PubMed Google Scholar
-
32.World Health Organization, 2017. Depression and other common mental disorders: global health estimates (No. WHO/MSD/MER/2017.2). World Health Organization. PubMed Google Scholar
-
33.World Health Organization, 2014. Preventing suicide: a global imperative. https://www.who.int/mental_health/suicide-prevention/world_report_2014/en/. Accessed August 16 2019 PubMed Google Scholar
-
34.B.L. Roth, D.J. Sheffler, W.K. Kroeze, Nat. Rev. Drug Discov. 3, 353-359 (2004) CrossRef PubMed Google Scholar
-
35.World Health Organization, 2013. WHO traditional medicine strategy: 2014-2023. https://apps.who.int/iris/bitstream/10665/92455/1/9789241506090_eng.pdf. Accessed August 19 2019 PubMed Google Scholar
-
36.L. Braun, M. Cohen, Herbs and Natural Supplements: An Evidence-Based Guide, vol. 2 (Churchill Livingstone Australia, London, 2014), p. 1384 PubMed Google Scholar
-
37.S.B. Uddin, Medicinal plants database of Bangladesh (www.mpbd.info). Accessed February 26 2019 PubMed Google Scholar
-
38.N.P. Sukumaran, A. Amalraj, S. Gopi, Complement. Ther. Med. 44, 68-82 (2019) CrossRef PubMed Google Scholar
-
39.M. Deepak, G.K. Sangli, P.C. Arun, A. Amit, Phytochem. Anal. 16, 24-29 (2005) CrossRef PubMed Google Scholar
-
40.J. Mathew, S. Soman, J. Sadanandan, C.S. Paulose, J. Ethnopharmacol. 130, 255-261 (2010) CrossRef PubMed Google Scholar
-
41.R. Malishev, S. Shaham-Niv, S. Nandi, S. Kolusheva, E. Gazit, R. Jelinek, A.C.S. Chem, Neuroscience 8, 884-891 (2017) PubMed Google Scholar
-
42.C. Neale, D. Camfield, J. Reay, C. Stough, A. Scholey, Br. J. Clin. Pharmacol. 75, 728-737 (2013) CrossRef PubMed Google Scholar
-
43.K. Nalini, A.R. Aroor, A. Rao, K.S. Karanth, Fitoterapia 63, 231-238 (1992) PubMed Google Scholar
-
44.S.P. Patil, S. Maki, S.A. Khedkar, A.C. Rigby, C. Chan, J. Nat. Prod. 73, 1196-1202 (2010) CrossRef PubMed Google Scholar
-
45.N. Haleagrahara, K. Ponnusamy, J. Toxicol. Sci. 35, 41-47 (2010) CrossRef PubMed Google Scholar
-
46.M.F. Xu, Y.Y. Xiong, J.K. Liu, J.J. Qian, L. Zhu, J. Gao, Acta Pharmacol. Sin. 33, 578-587 (2012) CrossRef PubMed Google Scholar
-
47.J. Nataraj, T. Manivasagam, A.J. Thenmozhi, M.M. Essa, Neurochem. Res. 42, 1354-1365 (2017) CrossRef PubMed Google Scholar
-
48.I.E. Orhan, E. Atasu, F.S. Senol, N. Ozturk, B. Demirci, K. Das, N. Sekeroglu, Ind. Crops Prod. 47, 316-322 (2013) CrossRef PubMed Google Scholar
-
49.F.S. Yang, G.P. Lim, A.N. Begum, O.J. Ubeda, M.R. Simmons, S.S. Ambegaokar, P.P. Chen, R. Kayed, C.G. Glabe, S.A. Frautschy, G.M. Cole, J. Biol. Chem. 280, 5892-5901 (2005) CrossRef PubMed Google Scholar
-
50.C. Van der Merwe, H.C. van Dyk, L. Engelbrecht, F.H. van der Westhuizen, C. Kinnear, B. Loos, S. Bardien, Mol. Neurobiol. 54, 2752-2762 (2017) CrossRef PubMed Google Scholar
-
51.V. Sorrenti, G. Contarini, S. Sut, S. DallAcqua, F. Confortin, A. Pagetta, P. Giusti, M. Zusso, Front. Pharmacol. 9, 183 (2018) CrossRef PubMed Google Scholar
-
52.S.R. Rainey-Smith, B.M. Brown, H.R. Sohrabi, T. Shah, K.G. Goozee, V.B. Gupta, R.N. Martins, Br. J. Nutr. 115, 2106-2113 (2016) CrossRef PubMed Google Scholar
-
53.G.W. Small, P. Siddarth, Z. Li, K.J. Miller, L. Ercoli, N.D. Emerson, J. Martinez, K.P. Wong, J. Liu, D.A. Merrill, S.T. Chen, Am. J. Geriatr. Psychiatry. 26, 266-277 (2018) CrossRef PubMed Google Scholar
-
54.J. Burns, P.D. Joseph, K.J. Rose, M.M. Ryan, R.A. Ouvrier, Pediatr. Neurol. 41, 305-308 (2009) CrossRef PubMed Google Scholar
-
55.J.J. Yu, L.B. Pei, Y. Zhang, Z.Y. Wen, J.L. Yang, J. Clin. Psychopharmacol. 35, 406-410 (2015) CrossRef PubMed Google Scholar
-
56.A.L. Lopresti, M. Maes, M.J. Meddens, G.L. Maker, E. Arnoldussen, P.D. Drummond, Eur. Neuropsychopharmacol. 25, 38-50 (2015) CrossRef PubMed Google Scholar
-
57.D. Panda, S. Pradhan, S.K. Palita, J.K. Nayak, Ecol. Environ. Conserv. 20, 35-38 (2014) PubMed Google Scholar
-
58.R. Sharma, R. Gupta, Life Sci. 80, 2389-2392 (2007) CrossRef PubMed Google Scholar
-
59.M.M. Sonwa, W.A. König, Phytochemistry 58, 799-810 (2001) CrossRef PubMed Google Scholar
-
60.M. Rabbani, A. Ghannadi, N. Malekian, Adv. Biomed. Res. 3, 217-221 (2014) PubMed Google Scholar
-
61.F.F. Nóbrega, M.G. Salvadori, C.J. Masson, C.F. Mello, T.S. Nascimento, J.H. Leal-Cardoso, D.P. de Sousa, R.N. Almeida, Oxid. Med. Cell. Longev. 2014, 1-9 (2014) CrossRef PubMed Google Scholar
-
62.A. Azimi, S.M. Ghaffari, G.H. Riazi, S.S. Arab, M.M. Tavakol, S. Pooyan, J. Ethnopharmacol. 194, 219-227 (2016) CrossRef PubMed Google Scholar
-
63.S.C. Das, M.A. Rahman, Bangladesh J. Bot. 40, 113-120 (2011) CrossRef PubMed Google Scholar
-
64.M.Y. Wang, B.J. West, C.J. Jensen, D. Nowicki, C. Su, A.K. Palu, G. Anderson, Acta Pharmacol. Sin. 23, 1127-1141 (2002) PubMed Google Scholar
-
65.E. Dussossoy, P. Brat, E. Bony, F. Boudard, P. Poucheret, C. Mertz, J. Giaimis, A. Michel, J. Ethnopharmacol. 133, 108-115 (2011) CrossRef PubMed Google Scholar
-
66.S. Harada, W. Hamabe, K. Kamiya, T. Satake, S. Tokuyama, J. Pharm. Soc. Jpn. 129, 203-207 (2009) CrossRef PubMed Google Scholar
-
67.J. Muto, L. Hosung, A. Uwaya, F. Isami, M. Ohno, T. Mikami, Physiol. Behav. 101, 211-217 (2010) CrossRef PubMed Google Scholar
-
68.P. Muralidharan, V.R. Kumar, G. Balamurugan, Phytother. Res. 24, 252-258 (2010) CrossRef PubMed Google Scholar
-
69.S.D. Pachauri, S. Tota, K. Khandelwal, P.R.P. Verma, C. Nath, K. Hanif, R. Shukla, J.K. Saxena, A.K. Dwivedi, J. Ethnopharmacol. 139, 34-41 (2012) CrossRef PubMed Google Scholar
-
70.M. Narasingam, V. Pandy, Z. Mohamed, Exp. Anim. 65, 157-164 (2016) CrossRef PubMed Google Scholar
-
71.V. Pandy, Y. Khan, Exp. Anim. 65, 437-445 (2016) CrossRef PubMed Google Scholar
-
72.B. Jayaprakasam, M.G. Nair, Tetrahedron 59, 841-849 (2003) CrossRef PubMed Google Scholar
-
73.A. Konar, N. Shah, R. Singh, N. Saxena, S.C. Kaul, PLoS ONE 6, e27265 (2011) CrossRef PubMed Google Scholar
-
74.S.K. Bhattacharya, A. Bhattacharya, K. Sairam, Phytomedicine 6, 463-469 (2000) PubMed Google Scholar
-
75.S. RajaSankar, T. Manivasagam, S. Surendran, Neurosci. Lett. 454, 11-15 (2009) CrossRef PubMed Google Scholar
-
76.S.K. Bhattacharya, A. Kumar, S. Ghosal, Phytother. Res. 9, 110-113 (1995) CrossRef PubMed Google Scholar
-
77.S.K. Kulkarni, K.K. Akula, A. Dhir, Ind. J. Experim. Biol. 46, 465-469 (2008) PubMed Google Scholar
-
78.T. Kuboyama, C. Tohda, K. Komatsu, Eur. J. Neurosci. 23, 1417-1426 (2006) CrossRef PubMed Google Scholar
-
79.N. Sehgal, A. Gupta, R.K. Valli, S.D. Joshi, J.T. Mills, E. Hamel, P. Khanna, S.C. Jain, S.S. Thakur, V. Ravindranath, Proc. Natl. Acad. Sci. 109, 3510-3515 (2012) CrossRef PubMed Google Scholar
-
80.S.K. Bhattacharya, K.S. Satyan, S. Ghosal, Indian J. Exp. Biol. 35, 236-239 (1997) PubMed Google Scholar
-
81.S. Köktürk, S. Ceylan, V. Etus, N. Yasa, S. Ceylan, Neural. Regen. Res. 8, 773-782 (2013) PubMed Google Scholar
-
82.N.K. Sinha, V.B. Pandey, B. Dasgupta, R. Higuchi, T. Kawasaki, Indian J. Chem. B. 22, 97-98 (1982) PubMed Google Scholar
-
83.Y.J. Shiao, M.H. Su, H.C. Lin, C.R. Wu, Int. J. Mol. Sci. 18, 895 (2017) CrossRef PubMed Google Scholar
-
84.P. Ramaprasasd, C.S. Basha, Santosh, Tirupati, Srikanth. Indian J. Pharmacol. 40, 86-86 (2008) PubMed Google Scholar
-
85.C. Blázquez, A. Chiarlone, O. Sagredo, T. Aguado, M.R. Pazos, E. Resel, J. Palazuelos, B. Julien, M. Salazar, C. Börner, C. Benito, Brain 134, 119-136 (2011) CrossRef PubMed Google Scholar
-
86.R. Bhowmik, M.R. Saha, M.A. Rahman, M.A.U. Islam, Bangladesh Pharm. J. 17, 205-214 (2015) CrossRef PubMed Google Scholar
-
87.A.K. Thakur, G. Rai, S.S. Chatterjee, V. Kumar, Pharm. Biol. 54, 1528-1538 (2016) CrossRef PubMed Google Scholar
-
88.M.H. Kabir, N. Hasan, M.M. Rahman, M.A. Rahman, J.A. Khan, N.T. Hoque, M.R.Q. Bhuiyan, S.M. Mou, R. Jahan, M. Rahmatullah, J. Ethnobiol. Ethnomed. 10, 19 (2014) CrossRef PubMed Google Scholar
-
89.S.N. Uddin, Traditional uses of Ethnomedicinal plants of the Chittagong Hill Tracts (Bangladesh National Herbarium, Dhaka, 2006), p. 992 PubMed Google Scholar
-
90.M. Yusuf, M.A. Wahab, J.U. Chowdhury, J. Begum, Bangladesh J. Plant Taxon 13, 55-61 (2006) CrossRef PubMed Google Scholar
-
91.A.H. Mollik, Alzheimer's Dement. 6, S43 (2010) PubMed Google Scholar
-
92.S. Reddy, G. Rao, B. Shetty, G. Hn, Turk. Neurosurg. 25, 425-431 (2015) PubMed Google Scholar
-
93.M.A.H. Mollik, Epilepsia. 53, 1-245 (2012) PubMed Google Scholar
-
94.O. Dulla, F.I. Jahan, J. Intercult. Ethnopharmacol. 6, 316-325 (2017) PubMed Google Scholar
-
95.G.L. Viswanatha, M.V. Venkataranganna, N.B.L. Prasad, A. Godavarthi, Metab. Brain Dis. 32, 867-879 (2017) CrossRef PubMed Google Scholar
-
96.Y. Dinesh, R. Chand, R. Kumar, J. Ethnopharmacol. 202, 97-102 (2017) CrossRef PubMed Google Scholar
-
97.A.H.M.M. Rahman, Wudpecker J. Med. Plants. 2, 44-52 (2013) PubMed Google Scholar
-
98.R. Walid, K.F.A. Suvro, M. Harun-or-Rashid, M. Mukti, S. Rahman, M. Rahmatullah, Am. Eur. J. Sustain. Agric. 7, 61-74 (2013) PubMed Google Scholar
-
99.M.A. Motaleb, M.K. Hossain, M.K. Alam, M.M.A.A. Mamun, M. Sultana, Commonly used medicinal herbs and shrubs by traditional herbal practitioners: glimpses from Thanchi upazila of Bandarban. (International Union for Conservation of Nature and Natural Resources, Bolipara Nari Kalyan Somity, and Keidanren Nature Conservation Fund, 2013), p. 294. PubMed Google Scholar
-
100.B. Ibrahim, A. Sowemimo, A. van Rooyen, M. Van de Venter, J. Ethnopharmacol. 141, 282-289 (2012) CrossRef PubMed Google Scholar
-
101.S. Rudra, K.N. Islam, M.M. Rahman, S.B. Uddin, J. Herbs Spices Med. Plants. (in press): https://doi.org/10.1080/10496475.2020.1786874 PubMed Google Scholar
-
102.M. Bhatnagar, S.D. Shukla, R. Bhatnagar, J. Herb. Pharmacother. 5, 21-30 (2005) CrossRef PubMed Google Scholar
-
103.M. Faysal, Ethnobot. Leaflets. 12, 231-1235 (2008) PubMed Google Scholar
-
104.N.A. Khan, A.M. Rashid, Afr. J. Tradit. Complement. Altern. Med. 3, 37-47 (2006) CrossRef PubMed Google Scholar
-
105.M.R. Ahmad, A.T. Justin, T. Manivasagam, J. Nataraj, M.E. Mohamed, S.B. Chidambaram, Front. Biosci. Elite Ed. 10, 287-299 (2018) CrossRef PubMed Google Scholar
-
106.F.M.S. Azam, A. Biswas, A. Mannan, N.A. Afsana, R. Jahan, M. Rahmatullah, J. Evid. Based Complement. Altern. Med. 2014, 1-28 (2014) CrossRef PubMed Google Scholar
-
107.M. Akber, S. Seraj, F. Islam, D. Ferdausi, R. Ahmed, D. Nasrin, N. Nahar, S. Ahsan, F. Jamal, M. Rahmatullah, Am. Eur. J. Sustain. Agric. 5, 177-195 (2011) PubMed Google Scholar
-
108.A.H. Mollik, A.I. Hassan, T.K. Paul, M. Sintaha, H.N. Khaleque, F.A. Noor, A. Nahar, S. Seraj, R. Jahan, M.H. Chowdhury, M. Rahmatullah, Am. Eur. J. Sustain. Agric. 1, 349-357 (2010) PubMed Google Scholar
-
109.H. Joshi, M. Parle, J. Med. Food. 9, 413-417 (2006) CrossRef PubMed Google Scholar
-
110.R. Jash, K.A. Chowdary, Pharmacogn. Res. 6, 46-51 (2014) CrossRef PubMed Google Scholar
-
111.M.N.K. Azam, M.N. Ahmed, M.M. Rahman, M. Rahmatullah, Am. Eur. J. Sustain. Agric. 7, 391-402 (2013) PubMed Google Scholar
-
112.T. Yoneyama, M.A. Arai, R. Akamine, K. Koryudzu, A. Tsuchiya, S.K. Sadhu, F. Ahmed, M. Itoh, R. Okamoto, M. Ishibashi, J. Nat. Prod. 80, 2453-2461 (2017) CrossRef PubMed Google Scholar
-
113.M.A.H. Mollik, M.S. Hossan, A.K. Paul, M. Taufiq-Ur-Rahman, R. Jahan, M. Rahmatullah, Ethnobot. Res. Appl. 8, 195-218 (2010) CrossRef PubMed Google Scholar
-
114.M. Rahmatullah, A. Noman, M.S. Hossan, M.H. Rashid, T. Rahman, M.H. Chowdhury, R. Jahan, Am. Eur. J. Sustain. Agric. 3, 862-876 (2009) PubMed Google Scholar
-
115.K. Hegde, S.P. Thakker, A.B. Joshi, C.S. Shastry, K.S. Chandrashekhar, Trop. J. Pharm. Res. 8, 117-125 (2009) PubMed Google Scholar
-
116.A. Kundu, A. Mitra, Plant Foods Hum. Nutr. 68, 247-253 (2013) CrossRef PubMed Google Scholar
-
117.M. Mathew, S. Subramanian, PLoS ONE 9, e86804 (2014) CrossRef PubMed Google Scholar
-
118.M.F. Kadir, M.S.B. Sayeed, N.I. Setu, A. Mostafa, M.M.K. Mia, J. Ethnopharmacol. 155, 495-508 (2014) CrossRef PubMed Google Scholar
-
119.A. Ghani, Medicinal plants of Bangladesh: chemical constituents and uses, 2nd edn. (Asiatic Society of Bangladesh, Dhaka, 2003), p. 603 PubMed Google Scholar
-
120.A.P. Benthall, Trees of Calcutta and its neighborhood, 1st edn. (Thacker, Spink & Co, Calcutta, 1946), p. 613 PubMed Google Scholar
-
121.W. Nakdook, O. Khongsombat, P. Taepavarapruk, N. Taepavarapruk, K. Ingkaninan, J. Ethnopharmacol. 130, 122-126 (2010) CrossRef PubMed Google Scholar
-
122.S. Chattipakorn, A. Pongpanparadorn, W. Pratchayasakul, A. Pongchaidacha, K. Ingkaninan, N. Chattipakorn, J. Ethnopharmacol. 110, 61-68 (2007) CrossRef PubMed Google Scholar
-
123.D. Singh, A. Singh, Chemosphere 60, 135-140 (2005) CrossRef PubMed Google Scholar
-
124.M.Z. Uddin, M.G. Kibria, M.A. Hassan, J. Asiat. Soc. Bangladesh Sci. 41, 203-223 (2015) CrossRef PubMed Google Scholar
-
125.R. Prajapati, M. Kalariya, R. Umbarkar, S. Parmar, N. Sheth, Int. J. Nutr. Pharmacol. Neurol. Dis. 1, 90-96 (2011) CrossRef PubMed Google Scholar
-
126.A.K. Paul, H. Al Arif, S. Seraj, A. Nahar, D. Nasrin, M.H. Chowdhury, F. Islam, R. Jahan, A.A. Bashar, R. Freedma, M. Rahmatullah, Am. Eur. J. Sustain. Agric. 5, 132-145 (2011) PubMed Google Scholar
-
127.M. Yeasmin, S. Karmaker, M.S. Hossain, S. Ahmed, A. Tabassum, I. Malek, M. Rahmatullah, World J. Pharm. Pharmaceut. Sci. 4, 70-78 (2015) PubMed Google Scholar
-
128.M. Faisal, A.I. Hossain, S. Rahman, R. Jahan, M. Rahmatullah, B.M.C. Complement, Altern. Med. 14, 335 (2014) CrossRef PubMed Google Scholar
-
129.M.A. Rahman, S.B. Uddin, C.C. Wilcock, Indian J. Tradit. Knowl. 6, 508-517 (2007) PubMed Google Scholar
-
130.A. Dar, S. Khatoon, Pharmacol. Biochem. Behav. 65, 1-6 (2000) CrossRef PubMed Google Scholar
-
131.M. Rahmatullah, A.H. Mollik, S. Rahman, N. Hasan, B. Agarwal, R. Jahan, Alter. Complement. Med. 16, 419-425 (2010) CrossRef PubMed Google Scholar
-
132.C.N. Chu, J.W. Kim, S.Y. Chung, J.H. Park, J. Oriental Neuropsychiatry. 21, 43-57 (2010) PubMed Google Scholar
-
133.M.S. Shajib, S. Akter, T. Ahmed, M.Z. Imam, Front Pharmacol. 6, 212 (2015) PubMed Google Scholar
-
134.H. Joshi, M. Parle, Cognition improving and antioxidant effects of Asparagus racemosus willd in mice. Paper presented at 28th CINP world congress of neuropsychopharmacology, Stockholm, Sweden, 3-7 June 2012 PubMed Google Scholar
-
135.R. Ojha, A.N. Sahu, A.V. Muruganandam, G.K. Singh, S. Krishnamurthy, Brain Cogn. 74, 1-9 (2010) CrossRef PubMed Google Scholar
-
136.S. Jabbar, M.T. Khan, M.S. Choudhuri, Pharmazie 56, 506-508 (2001) CrossRef PubMed Google Scholar
-
137.V.D. Thakur, S.A. Mengi, J. Ethnopharmacol. 102, 23-31 (2005) CrossRef PubMed Google Scholar
-
138.S.K. Roy, U.K. Mazumder, A. Islam, Pharmacologyonline. 1, 632-643 (2011) PubMed Google Scholar
-
139.P. Amoateng, S. Adjei, D. Osei-Safo, K.K. Kukuia, E.O. Bekoe, T.K. Karikari, S.B. Kombian, B.M.C. Complement, Altern. Med. 17, 389 (2017) CrossRef PubMed Google Scholar
-
140.S. Roy, S. Dutta, T.K. Chaudhuri, J. Basic Clin. Physiol. 26, 395-401 (2015) PubMed Google Scholar
-
141.A. Khatun, M.A.A. Khan, M.A. Rahman, M.S. Akter, A. Hasan, W. Parvin, R.J. Ripa, M. Moniruzzaman, M.J. Mahal, M. Rahmatullah, Am. Eur. J. Sustain. Agric. 7, 319-339 (2013) PubMed Google Scholar
-
142.A.O. Aderibigbe, T. Olufunmilayo, O.I. Agboola, Planta Med. 79, PE4 (2013) PubMed Google Scholar
-
143.S.H. Sohn, M. Yoon, J. Kim, H.L. Choi, M. Shin, M. Hong, H. Bae, Mol. Cell. Toxicol. 8, 343-348 (2012) CrossRef PubMed Google Scholar
-
144.R.G. Fuentes, M.A. Arai, S.K. Sadhu, F. Ahmed, M. Ishibashi, J. Nat. Med. 69, 589-594 (2015) CrossRef PubMed Google Scholar
-
145.G. Oboh, T.L. Akomolafe, S.A. Adefegha, A.O. Adetuyi, Exp. Toxicol. Pathol. 63, 257-262 (2011) CrossRef PubMed Google Scholar
-
146.R. Afroz, N. Islam, K.R. Biswas, T. Ishika, M. Rahman, A. Swarna, T. Khan, M.N. Monalisa, S. Seraj, M.A. Rahman, S.M. Mou, Am. Eur. J. Sustain. Agric. 5, 226-232 (2011) PubMed Google Scholar
-
147.M. Rahmatullah, M.A.H. Mollik, M.A. Khatun, R. Jahan, A.R. Chowdhury, S. Seraj, M.S. Hossain, D. Nasrin, Z. Khatun, Adv. Nat. Appl. Sci. 4, 39-44 (2010) PubMed Google Scholar
-
148.X. Huang, Q. Li, H. Li, L. Guo, Cell. Mol. Neurobiol. 29, 1211-1221 (2009) CrossRef PubMed Google Scholar
-
149.M. Rahmatullah, M.A. Rahman, M.S. Hossan, M. Taufiq-Ur-Rahman, R. Jahan, M.A.H. Mollik, J. Altern. Complement. Med. 16, 769-785 (2010) CrossRef PubMed Google Scholar
-
150.M.A. Holgado, L. Martín-Banderas, J. Álvarez-Fuentes, M. Fernández-Arévalo, J. Drug Delivery Sci. Technol. 42, 84-93 (2017) CrossRef PubMed Google Scholar
-
151.A. Vallée, Y. Lecarpentier, R. Guillevin, J.N. Vallée, Acta Biochim. Biophys. Sin. 49, 853-866 (2017) CrossRef PubMed Google Scholar
-
152.G. Gnahoué, J.D. N'guessan, E. Koffi, F. Traoré, F. Guédé-Guina, Trop. J. Pharm. Res. 8, 11-17 (2009) PubMed Google Scholar
-
153.L.H. Martini, F. Jung, F.A. Soares, L.N. Rotta, D.A. Vendite, M.E. dos Santos Frizzo, R.A. Yunes, J.B. Calixto, S. Wofchuk, D.O. Souza, Neurochem. Res. 32, 1950-1956 (2007) CrossRef PubMed Google Scholar
-
154.L. Yaidikar, S. Thakur, Pharmacol. Rep. 67, 890-895 (2015) CrossRef PubMed Google Scholar
-
155.S. Mubassara, S.J. Hossain, F. Ahmed, M. Yamamoto, N. Tan, H. Aoshima, Food Sci Technol. Res. 15, 315-324 (2009) CrossRef PubMed Google Scholar
-
156.J.H. Park, H.S. Joo, K.Y. Yoo, B.N. Shin, I.H. Kim, C.H. Lee, J.H. Choi, K. Byun, B. Lee, S.S. Lim, M.H. Won, Neurochem. Res. 36, 2043-2050 (2011) CrossRef PubMed Google Scholar
-
157.J.H. Sampson, J.D. Phillipson, N.G. Bowery, M.J. Oneill, J.G. Houston, J.A. Lewis, Phytother. Res. 14, 24-29 (2000) CrossRef PubMed Google Scholar
-
158.J. Ma, Q. Ren, B. Dong, Z. Shi, J. Zhang, D.Q. Jin, J. Xu, Y. Ohizumi, D. Lee, Y. Guo, Bioorg. Chem. 76, 449-457 (2018) CrossRef PubMed Google Scholar
-
159.A.B.M.A. Hossain, Blumea balsamifera DC, in Encyclopedia of flora and fauna of Bangladesh. Angiosperms: Dicotyledons (Acanthaceae-Asteraceae), vol. 6, ed. by Z.U. Ahmed, Z.N.T. Begum, M.A. Hassan, M. Khondker, S.M.H. Kabir, M. Ahmed, A.T.A. Ahmed, A.K.A. Rahman, E.U. Haque (Asiatic Society of Bangladesh, Dhaka, 2008), pp. 272-273 PubMed Google Scholar
-
160.M. Yusuf, M.A. Wahab, M.D. Yousuf, J.U. Chowdhury, J. Begum, Bangladesh J. Plant Taxon 14, 117-128 (2007) PubMed Google Scholar
-
161.V.M. Couto, F.C. Vilela, D.F. Dias, M.H. dos Santos, R. Soncini, C.G.O. Nascimento, A. Giusti-Paiva, J. Ethnopharmacol. 134, 348-353 (2011) CrossRef PubMed Google Scholar
-
162.A.S. Melo, M.C. Monteiro, J.B. da Silva, F.R. de Oliveira, J.L.F. Vieira, M.A. de Andrade, A.C. Baetas, J.T. Sakai, F.A. Ferreira, P.J. da Cunha Sousa, C.D.S.F. Maia, J. Ethnopharmacol. 147, 293-301 (2013) CrossRef PubMed Google Scholar
-
163.S.W. Bihaqi, M. Sharma, A.P. Singh, M. Tiwari, J. Ethnopharmacol. 124, 409-415 (2009) CrossRef PubMed Google Scholar
-
164.M. Manvar, T. Desai, Indian J. Med. Sci. 67, 49-60 (2013) CrossRef PubMed Google Scholar
-
165.M.K. Islam, S. Saha, I. Mahmud, K. Mohamad, K. Awang, S.J. Uddin, M.M. Rahman, J.A. Shilpi, J. Ethnopharmacol. 151, 921-930 (2014) CrossRef PubMed Google Scholar
-
166.D. Sivaraman, P. Panneerselvam, P. Muralidharan, Int. J. Pharmacol. 12, 52-65 (2016) CrossRef PubMed Google Scholar
-
167.N.A. Rayan, N. Baby, D. Pitchai, F. Indraswari, E.A. Ling, J. Lu, T. Dheen, Front. Biosci. Elite Ed. 3, 1079-1091 (2011) PubMed Google Scholar
-
168.O.K. Yemitan, H.M. Salahdeen, Fitoterapia 76, 187-193 (2005) CrossRef PubMed Google Scholar
-
169.S. Pal, T. Sen, A.N. Chaudhuri, J. Pharm. Pharmacol. 5, 1313-1318 (1999) PubMed Google Scholar
-
170.M.D.D. Sohel, M.D.H. Kawsar, M.D.H.U. Sumon, T. Sultana, Altern. Integr. Med. 5, 1-9 (2016) PubMed Google Scholar
-
171.A.H.M.M. Rahman, Am. J. Life Sci. 1, 77-81 (2010) PubMed Google Scholar
-
172.A.O. Ademosun, G. Oboh, Orient. Pharm. Exp. Med. 17, 269-276 (2017) CrossRef PubMed Google Scholar
-
173.A.H.M.M. Nawaz, M. Hossain, M. Karim, M. Khan, R. Jahan, M. Rahmatullah, Am. Eur. J. Sustain. Agric. 3, 143-150 (2009) PubMed Google Scholar
-
174.R.K. Nitharwal, H. Patel, M.S. Karchuli, R.R. Ugale, Indian J. Pharmacol. 45, 502-507 (2013) CrossRef PubMed Google Scholar
-
175.H.K. Kandikattu, S.N. Deep, S. Razack, N. Amruta, D. Prasad, F. Khanum, Physiol. Behav. 175, 56-65 (2017) CrossRef PubMed Google Scholar
-
176.M.Z. Uddin, M.A. Hassan, M. Sultana, Bangladesh J. Plant Taxon 13, 63-68 (2006) CrossRef PubMed Google Scholar
-
177.M. Rahmatullah, M.A. Khatun, N. Morshed, P.K. Neogi, S.U.A. Khan, M.S. Hossan, M.J. Mahal, R. Jahan, Adv. Nat. Appl. Sci. 4, 52-62 (2010) PubMed Google Scholar
-
178.N. Kaur, L. Kishore, R. Singh, J. Ethnopharmacol. 206, 19-30 (2017) CrossRef PubMed Google Scholar
-
179.A. Biswas, M.A. Bari, M. Roy, S.K. Bhadra, Indian J. Tradit. Know. 9, 77-89 (2010) PubMed Google Scholar
-
180.P. Bigoniya, A.C. Rana, Indian J. Exp. Biol. 43, 859-862 (2005) PubMed Google Scholar
-
181.C.D. Rafael, B.S.S. Kathryn Ana, F.B. Allisson, M. Rodrigo, F.P. Ana, C.M. Flavia, Neuropharmacol. 63, 593-605 (2012) CrossRef PubMed Google Scholar
-
182.P.R. Das, M.T. Islam, M.N. Mostafa, M. Rahmatullah, Anc. Sci. life. 32, 144-149 (2013) CrossRef PubMed Google Scholar
-
183.S. Cabrera, F. Núñez, Rev. Cubana Plant Med. 15, 96-104 (2010) PubMed Google Scholar
-
184.S. Roy, M.Z. Uddin, M.A. Hassan, M.M. Rahman, Bangladesh J. Plant Taxon 15, 67-72 (2008) PubMed Google Scholar
-
185.S.N. Rai, H. Birla, W. Zahra, S.S. Singh, S.P. Singh, J. Chem. Neuroanat. 85, 27-35 (2017) CrossRef PubMed Google Scholar
-
186.P. Shetty, M. Krishnamoorthy, K. Vijayanarayana, D.S. Satyanarayana, Asian J. Chem. 20, 1075-1180 (2008) PubMed Google Scholar
-
187.M. Rahmatullah, D. Ferdausi, A. Mollik, R. Jahan, M.H. Chowdhury, W.M. Haque, Afr. J. Tradit. Complement. Altern. Med. 7, 91-97 (2010) PubMed Google Scholar
-
188.F. Ahmed, I.Z. Shahid, U.K. Biswas, B.A. Roy, A.K. Das, M.S.K. Pharma, Biology 45, 587-593 (2007) CrossRef PubMed Google Scholar
-
189.M.A. Farag, S.M. Ezzat, M.M. Salama, M.G. Tadros, J. Pharm. Biomed. Anal. 125, 292-302 (2016) CrossRef PubMed Google Scholar
-
190.O.I. Olayinka, H.A. Nta, A. Ganiyat, J. Med. Plants Res. 5, 2743-2747 (2011) PubMed Google Scholar
-
191.M. Karthikeyan, K. Deepa, J. Basic Clin. Physiol. Pharmacol. 21, 347-356 (2010) CrossRef PubMed Google Scholar
-
192.N.A. Siddique, M.A. Bari, A.T.M. Naderuzzaman, N. Khatun, M.H. Rahman, R.S. Sultana, M.N. Matin, S. Shahnewaz, M.M. Rahman, J. Biol. Sci. 4, 72-80 (2004) PubMed Google Scholar
-
193.M. Rahmatullah, A.A.B.T. Kabir, M.M. Rahman, M.S. Hossan, Z. Khatun, M.A. Khatun, R. Jahan, Adv. Nat. Appl. Sci. 4, 45-51 (2010) CrossRef PubMed Google Scholar
-
194.M. Umamaheswari, K. Asokkumar, N. Umamageswari, T. Sivashanmugam, V. Subhadradevi, Protective effect of the leaves of Vitex negundo against ethanol-induced cerebral oxidative stress in rats. Tanzan. J. Health Res. 14, 21-28 (2012) CrossRef PubMed Google Scholar
-
195.B.N. Sinha, D. Sasmal, Asian J. Chem. 23, 315-318 (2011) PubMed Google Scholar
-
196.N.N. Biswas, A.K. Acharzo, S. Anamika, S. Khushi, B. Bokshi, Evid. Based Complement. Alternat. Med. 2017, 1-11 (2014) PubMed Google Scholar
-
197.Y.H. Jeong, J.S. Park, D.H. Kim, J.L. Kang, H.S. Kim, Pharmacol. Res. 119, 431-442 (2017) CrossRef PubMed Google Scholar
-
198.L. Leal, R. Silva, T. Araujo, V. Silva, A. Barbosa, J. Medeiros, J. Oliveira, C. Ventura, Rev. Bras. Plant Med. 18, 38-47 (2016) CrossRef PubMed Google Scholar
-
199.M. Rahmatullah, A. Hasan, W. Parvin, M. Moniruzzaman, A. Khatun, Z. Khatun, F.I. Jahan, R. Jahan, Afr. J. Tradit. Complement. Altern. Med. 9, 350-359 (2012) PubMed Google Scholar
-
200.B.N. Ramesh, S.S. Indi, K.S.J. Rao, Neurosci. Lett. 475, 110-114 (2010) CrossRef PubMed Google Scholar
-
201.W.H. Yoon, K.H. Lee, Nat. Prod. Sci. 23, 108-112 (2017) PubMed Google Scholar
-
202.K.S. Rai, K.D. Murthy, K.S. Karantha, M.S. Rao, Indian J. Physiol. Pharmacol. 45, 305-313 (2001) PubMed Google Scholar
-
203.M. Rahmatullah, M.A.H. Mollik, M.K. Islam, M.R. Islam, F.I. Jahan, Z. Khatun, S. Seraj, M.H. Chowdhury, F. Islam, Z.U.M. Miajee, R. Jahan, Am. Eur. J. Sustain. Agric. 4, 363-373 (2010) PubMed Google Scholar
-
204.J. Mehla, M. Pahuja, P. Gupta, S. Dethe, A. Agarwal, Y.K. Gupta, Psychopharmacol. 230, 589-605 (2013) PubMed Google Scholar
-
205.H.H. Ko, J.R. Weng, L.T. Tsao, M.H. Yen, J.P. Wang, C.N. Lin, Bioorg. Med. Chem. Lett. 14, 1011-1014 (2004) PubMed Google Scholar
-
206.M.R. Ayissi, S. Gartside, E. Ngo Bum, N. Njikam, E. Okello, R. McQuade, J. Psychopharmacol. 26, 575-583 (2012) CrossRef PubMed Google Scholar
-
207.G. Patro, S.K. Bhattamisra, B.K. Mohanty, Avicenna J. Phytomed. 6, 696-710 (2016) PubMed Google Scholar
-
208.J.H. Yi, H.J. Park, S. Lee, J.W. Jung, B.C. Kim, Y.C. Lee, J.H. Ryu, D.H. Kim, J. Ethnopharmacol. 178, 50-57 (2016) CrossRef PubMed Google Scholar
-
209.H.A. Jung, M.Y. Ali, H.J. Jung, H.O. Jeong, H.Y. Chung, J.S. Choi, J. Ethnopharmacol. 191, 152-160 (2016) PubMed Google Scholar
-
210.T. Ramesh, C. Sureka, S. Bhuvana, V.H. Begum, Metab. Brain Dis. 30, 573-582 (2015) CrossRef PubMed Google Scholar
-
211.V. Rajesh, T. Riju, S. Venkatesh, G. Babu, Orient. Pharm. Exp. Med. 17, 127-142 (2017) PubMed Google Scholar
-
212.M.S. Hossan, P. Roy, S. Seraj, S.M. Mou, M.N. Monalisa, S. Jahan, T. Khan, A. Swarna, R. Jahan, M. Rahmatullah, Am. Eur. J. Sustain. Agric. 6, 349-359 (2012) PubMed Google Scholar
-
213.A.H.M. Zulfiker, M.A. Hoque, T. Akter, A. Afroz, A.M. Momin, Trop. J. Pharm. Res. 13, 1925-1931 (2014) PubMed Google Scholar
-
214.M.S. Bansod, D.T. Rajgure, U.N. Harle, N.S. Vyawahare, S.R. Yende, Indian J. Pharmacol. 40, 126-127 (2008) PubMed Google Scholar
-
215.A.H.M.M. Rahman, R. Gondha, Open J. Bot. 1, 19-24 (2014) PubMed Google Scholar
-
216.N. Khurana, A. Gajbhiye, Neurotoxicol. 39, 57-64 (2013) PubMed Google Scholar
-
217.S.I. Tumpa, M.I. Hossain, J. Pharmacogn. Phytochem. 3, 23-33 (2014) PubMed Google Scholar
-
218.S.K. Bhattamisra, P.N. Singh, S.K. Singh, Pharm. Biol. 50, 766-772 (2012) CrossRef PubMed Google Scholar
-
219.U. Hossain, M.O. Rahman, Bangladesh J. Plant Taxon 25, 241-255 (2018) PubMed Google Scholar
-
220.M. Moniruzzaman, M.S. Hossain, P.S. Bhattacharjee, J. Ethnopharmacol. 186, 205-208 (2016) PubMed Google Scholar
-
221.N. Izzati, L.E. Fitri, M. Dalhar, Univ. Med. 35, 222-228 (2016) PubMed Google Scholar
-
222.M.S.I. Howlader, M.A. Siraj, S.K. Dey, A. Hira, A. Ahmed, M.H. Hossain, Evid. Based Complement. Alternat. Med. 2017, 7390359 (2017) PubMed Google Scholar
-
223.D. Varija, K.P. Kumar, K.P. Reddy, J. Appl. Anim. Res. 39, 49-52 (2001) PubMed Google Scholar
-
224.D. Singh, A. Mishra, R.K. Goel, Epilepsy Behav. 27, 206-211 (2013) PubMed Google Scholar
-
225.J.O. Bhangale, S.R. Acharya, Adv. Pharmacol. Sci. 2016, 1-9 (2016) PubMed Google Scholar
-
226.C. Sutalangka, J. Wattanathorn, S. Muchimapura, W. Thukham-mee, Oxid. Med. Cell. Longev. 2013, 1-9 (2013) CrossRef PubMed Google Scholar
-
227.M.B. Ekong, M.M. Ekpo, E.O. Akpanyung, D.U. Nwaokonko, Metab. Brain. Dis. 32, 1437-1447 (2017) PubMed Google Scholar
-
228.I.O. Ayoola, B. Gueye, M.A. Sonibare, M.T. Abberton, J. Food Meas. Charact. 11, 488-499 (2017) CrossRef PubMed Google Scholar
-
229.T. Prabsattroo, J. Wattanathorn, P. Somsapt, O. Sritragool, Oxid. Med. Cell Longev. 2016, 1-11 (2016) CrossRef PubMed Google Scholar
-
230.Z.U. Ahmed, M.A. Hassan, Z.N.T. Begum, M. Khondker, S.M.H. Kabir, M. Ahmed, A.T.A. Ahmed, A.K.A. Rahman, E.U. Haque, Encyclopedia of flora and fauna of Bangladesh. Angiosperms: Dicotyledons (Fabaceae-Lythraceae), vol. 8 (Asiatic Society of Bangladesh, Dhaka, 2009), pp. 40-41 PubMed Google Scholar
-
231.T. Hongratanaworakit, Nat. Prod. Commun. 5, 157-162 (2010) PubMed Google Scholar
-
232.H.L. Hsieh, S.H. Yang, T.H. Lee, J.Y. Fang, C.F. Lin, Mol. Neurobiol. 53, 5995-6005 (2016) CrossRef PubMed Google Scholar
-
233.M.A. Howlader, M. Alam, K. Ahmed, F. Khatun, A.S. Apu, Pak. J. Biol. Sci. 14, 909-911 (2011) CrossRef PubMed Google Scholar
-
234.M.M. Hossain, Med. Aromat. Plant Sci. Biotechnol. 42, 101-106 (2009) PubMed Google Scholar
-
235.S.M. Khasim, P.R.M. Rao, The Botanica. 49, 86-91 (1999) PubMed Google Scholar
-
236.M.N. Uddin, R. Afrin, M.J. Uddin, M.J. Uddin, A.H.M.K. Alam, A.A. Rahman, G. Sadik, B.M.C. Complement, Altern. Med. 15, 195 (2015) PubMed Google Scholar
-
237.S.J. Uddin, J.A. Shilpi, M.T. Rahman, M. Ferdous, R. Rouf, S.D. Sarker, Die Pharmazi. 61, 362-364 (2006) CrossRef PubMed Google Scholar
-
238.A. Ferdoushi, S. Mahmud, M.M. Rana, M.S. Islam, A.B.A. Salauddin, M.F. Hossain, Eur. J. Med. Plants. 11, 1-22 (2016) PubMed Google Scholar
-
239.B. Shalini, J.D. Sharma, Toxicol. Int. 22, 35-39 (2015) PubMed Google Scholar
-
240.A.J. Thenmozhi, M. Dhivyabharathi, William Raja T.R., T. Manivasagam, M.M. Essa, Nutr. Neurosci. 19, 269-278 (2016) PubMed Google Scholar
-
241.M.R. Khatun, A.M. Rahman, Bangladesh J. Plant Taxon 26, 117-126 (2019) PubMed Google Scholar
-
242.M.S. Uddin, A. Al Mamun, M.A. Iqbal, A. Islam, M.F. Hossain, S. Khanum, M. Rashid, Adv. Alzheimer's Dis. 5, 87-102 (2016) CrossRef PubMed Google Scholar
-
243.A. Pandey, S. Bani, J. Neuroimmunol. 226, 48-58 (2010) PubMed Google Scholar
-
244.G.S. Somani, M.S. Nahire, A.D. Parikh, M.B. Mulik, P.J. Ghumatkar, K.S. Laddha, S. Sathaye, Indian J. Med. Res. 146, 255-159 (2017) PubMed Google Scholar
-
245.U. Subramanian, S. Poongavanam, A.J. Vanisree, Invest. New Drugs. 28, 615-623 (2010) CrossRef PubMed Google Scholar
-
246.Y. Bi, P.C. Qu, Q.S. Wang, L. Zheng, H.L. Liu, R. Luo, X.Q. Chen, Y.Y. Ba, X. Wu, H. Yang, Pharm. Biol. 53, 1516-1524 (2015) CrossRef PubMed Google Scholar
-
247.M. Kubo, R. Ishii, Y. Ishino, K. Harada, N. Matsui, M. Akagi, E. Kato, S. Hosoda, Y. Fukuyama, J. Nat. Prod. 76, 769-773 (2013) CrossRef PubMed Google Scholar
-
248.N. Saini, D. Singh, R. Sandhir, Neurochem. Res. 37, 1928-1937 (2012) PubMed Google Scholar
-
249.C. Promsuban, S. Limsuvan, P. Akarasereenont, K. Tilokskulchai, S. Tapechum, N. Pakaprot, Neuro. Rep. 28, 1031-1035 (2017) CrossRef PubMed Google Scholar
-
250.S. Loganathan, S. Rathinasamy, Pharmacogn. Mag. 12, S7-S13 (2016) PubMed Google Scholar
-
251.V.A. Da-Silva, J.C. De-Freitas, A.P. Mattos, W. Paiva-Gouvea, O.A. Presgrave, F.F. Fingola, M.A. Menezes, F.J. Paumgartten, Braz. J. Med. Biol. Res. 24, 827-831 (1991) PubMed Google Scholar
-
252.S.N. Uddin, M.Z. Uddin, M.A. Hassan, M.M. Rahman, Bangladesh J. Plant Taxon 11, 39-48 (2004) CrossRef PubMed Google Scholar
-
253.G.S. Tayeboon, F. Tavakoli, S. Hassani, M. Khanavi, O. Sabzevari, S.N. Ostad, Vitro Cell. Dev. Biol. Anim. 49, 706-715 (2013) CrossRef PubMed Google Scholar
-
254.Y. Miyazaki, Biosci. Microbiota. Food. Health. 35, 69-75 (2016) PubMed Google Scholar
-
255.G.I. Anuja, P. Latha, S.R. Suja, S. Shyamal, V.J. Shine, S. Sini, S. Pradeep, P. Shikha, S. Rajasekharan, J. Ethnopharmacol. 132, 456-460 (2010) CrossRef PubMed Google Scholar
-
256.N. Ismail, M. Ismail, N.H. Azmi, M.F.A. Bakar, Z. Yida, M.A. Abdullah, H. Basri, Biomed. Pharmacother. 95, 780-788 (2017) CrossRef PubMed Google Scholar
-
257.K. Radad, R. Moldzio, M. Taha, W.D. Rausch, Phytother. Res. 23, 696-700 (2009) PubMed Google Scholar
-
258.A.H.M.M. Rahman, M.C. Biswas, A.K.M.R. Islam, A.T.M.N. Zaman, Wudpecker J. Med. Plants. 2, 99-109 (2013) PubMed Google Scholar
-
259.H.J. Heo, Y.J. Park, Y.M. Suh, S.J. Choi, Biosci. Biotechnol. Biochem. 67, 284-1291 (2003) PubMed Google Scholar
-
260.J.X. Cao, Q.Y. Zhang, S.Y. Cui, X.Y. Cui, J. Ethnopharmacol. 130, 163-166 (2010) CrossRef PubMed Google Scholar
-
261.M.Z. Uddin, M.K. Arefin, M.F. Alam, G. Kibria, S.L. Podder, M.A. Hassan, J. Asiat. Soc. Bangladesh Sci. 43, 101-123 (2017) PubMed Google Scholar
-
262.G. Thamizhoviya, P.P. Kirjayini, A.J. Vanisree, Curr. Sci. 112, 295-303 (2017) PubMed Google Scholar
-
263.M. Asaduzzaman, M.J. Uddin, M.A. Kader, A.H.M.K. Alam, A.A. Rahman, M. Rashid, K. Kato, T. Tanaka, M. Takeda, G. Sadik, Psychogeriatrics. 14, 1-10 (2014) PubMed Google Scholar
-
264.Y. Furukawa, S. Okuyama, Y. Amakura, S. Watanabe, T. Fukata, M. Nakajima, M. Yoshimura, T. Yoshida, Int. J. Mol. Sci. 13, 1832-1845 (2012) PubMed Google Scholar
-
265.T. Satou, Y. Ogawa, K. Koike, Phytother. Res. 29, 1246-1250 (2015) PubMed Google Scholar
-
266.Y. Fu, Z. Si, P. Li, M. Li, H. Zhao, L. Jiang, Y. Xing, W. Hong, L. Ruan, J.S. Wang, Metab. Brain Dis. 32, 1295-1309 (2017) PubMed Google Scholar
-
267.R.L. VanGilder, K.A. Kelly, M.D. Chua, R.L. Ptachcinski, J.D. Huber, Exp. Brain Res. 197, 23-34 (2009) PubMed Google Scholar
-
268.M. Mohan, D. Attarde, R. Momin, S. Kasture, Nat. Prod. Res. 27, 2140-2143 (2013) PubMed Google Scholar
-
269.S. Challal, O.E. Buenafe, E.F. Queiroz, S. Maljevic, L. Marcourt, M. Bock, W. Kloeti, F.M. Dayrit, A.L. Harvey, H. Lerche, C.V. Esguerra, A.C.S. Chem, Neurosci. 5, 993-1004 (2014) PubMed Google Scholar
-
270.De Rose F., R. Marotta, S. Poddighe, G. Talani, T. Catelani, M.D. Setzu, P. Solla, F. Marrosu, E. Sanna, S. Kasture, E. Acquas, PLoS ONE 11, e0146140 (2016) PubMed Google Scholar
-
271.A.A. Reza, M.S. Hossain, S. Akhter, M.R. Rahman, M.S. Nasrin, M.J. Uddin, G. Sadik, A.H.M.K. Alam, B.M.C. Complement, Altern. Med. 18, 123 (2018) PubMed Google Scholar
-
272.I. Kazmi, M. Afzal, B. Ali, Z.A. Damanhouri, A. Ahmaol, F. Anwar, Asian Pac. J Trop. Med. 6, 433-437 (2013) PubMed Google Scholar
-
273.M. Rahmatullah, R. Jahan, F.S. Azam, S. Hossan, M.A.H. Mollik, T. Rahman, Afr. J. Tradit. Complement. Altern. Med. 8, 53-65 (2011) CrossRef PubMed Google Scholar
-
274.M. Shoibe, M.N.U. Chy, M. Alam, M. Adnan, M.Z. Islam, S.W. Nihar, N. Rahman, E. Suez, Biomedicines. 5, 63 (2017) PubMed Google Scholar
-
275.C.W. Chang, W.T. Chang, J.C. Liao, Y.J. Chiu, M.T. Hsieh, W.H. Peng, Y.C. Lin, Evid. Based Complement. Alternat. Med. (2012). https://doi.org/10.1155/2012/135379 PubMed Google Scholar
-
276.M.O. Raihan, M.R. Habib, A. Brishti, M.M. Rahman, M.M. Saleheen, M. Manna, Drug Discov. Ther. 5, 185-189 (2011) PubMed Google Scholar
-
277.L. Kumar, S.A. Ali, Biosci. Biotech. Res. Comm. 8, 197-203 (2015) PubMed Google Scholar
-
278.M.R. Sulaiman, Z.A. Zakaria, A.S. Mohamad, M. Ismail, M.T. Hidayat, D.A. Israf, M. Adilius, Pharm. Biol. 48, 861-868 (2010) PubMed Google Scholar
-
279.F. Sharmen, A. Mannan, M.M. Rahman, M.A.U. Chowdhury, M.E. Uddin, A.A. Ahmed, Asian Pac. J Trop. Biomed. 4, 137-142 (2014) CrossRef PubMed Google Scholar
-
280.Q.Q. Mao, Z. Huang, X.M. Zhong, C.R. Feng, A.J. Pan, Z.Y. Li, S.P. Ip, C.T. Che, J. Ethnopharmacol. 128, 336-341 (2010) PubMed Google Scholar
-
281.M. Chen, Y.Y. Chang, S. Huang, L.H. Xiao, W. Zhou, L.Y. Zhang, C. Li, R.P. Zhou, J. Tang, L. Lin, Z.Y. Du, Mol. Nutr. Food Res. 62, 1700281 (2018) CrossRef PubMed Google Scholar
-
282.X.S. Wang, Z.R. Zhang, M.M. Zhang, M.X. Sun, W.W. Wang, C.L. Xie, B.M.C. Complement, Altern. Med. 17, 412 (2017) PubMed Google Scholar
-
283.M.S. Ali, P.R. Dash, M. Nasrin, BMC Complement. Altern. Med. 15, 158 (2015) CrossRef PubMed Google Scholar
-
284.P.K. Mukherjee, V. Kumar, M. Mac, P.J. Houghton, Planta Med. 73, 283-285 (2007) CrossRef PubMed Google Scholar
-
285.L.M. Eubanks, C.J. Rogers, A.E. Beuscher, G.F. Koob, A.J. Olson, T.J. Dickerson, K.D. Janda, Mol. Pharm. 3, 773-777 (2006) PubMed Google Scholar
-
286.I. Lastres-Becker, F. Molina-Holgado, J.A. Ramos, R. Mechoulam, J. Fernández-Ruiz, Neurobiol. Dis. 19, 96-107 (2005) CrossRef PubMed Google Scholar
-
287.G. Esposito, C. Scuderi, C. Savan, L. Steardo Jr., D. De Filippis, P. Cottone, T. Iuvone, V. Cuomo, L. Steardo, Br. J. Pharmacol. 151, 1272-1279 (2007) PubMed Google Scholar
-
288.A. Alhebshi, M. Gotoh, I. Suzuki, Biochem. Biophys. Res. Commun. 433, 362-367 (2013) CrossRef PubMed Google Scholar
-
289.H. Okugawa, R. Ueda, K. Matsumoto, K. Kawanishi, A. Kato, Phytomedicine 2, 119-126 (1995) PubMed Google Scholar
-
290.T. Kuboyama, C. Tohda, K. Komatsu, Br. J. Pharmacol. 144, 961-971 (2005) CrossRef PubMed Google Scholar
-
291.Y. Zhang, L. Qiao, M. Song, L. Wang, Pharmacogn. Mag. 10, 509-516 (2014) CrossRef PubMed Google Scholar
Copyright information
© The Author(s) 2020
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.