A Systemic Review on Topical Marketed Formulations, Natural Products, and Oral Supplements to Prevent Androgenic Alopecia: A Review
Abstract
Androgens have an intense consequence on the human scalp and body hair. Scalp hair sprouts fundamentally in awol of androgens whereas the body hair hike is vulnerable to the activity of androgens. Androgenetic alopecia (AGA) invoked as males emulate Alopecia due to the cause of the dynamic reduction of scalp hair. Androgens are medium of terminus growth of hair although the body. Local and system androgens convert the extensive terminal follicles into lesser vellus like structure. The out start of this type of alopecia is intensely irregular and the reason behind this existence of enough circulating steroidal hormones androgens and due to genetic predisposition. Effective treatments are available in the market as well as under clinical and preclinical testing. Many herbal formulations are also available but not FDA approved. Different conventional and NDDS formulations are already available in the market. To avoid various systemic side effects of both Finasteride and Minoxidil, topical formulations and natural products (nutrients, minerals, vitamins) now a days are being widely used to treat Androgenic alopecia. CAM (complementary and alternative medicine) provides the option to elect favorable, low-risk, adjuvant and alternative therapies. Herein, we offer a widespread review of topical marketed formulations, natural products, and CAM treatment options for AGA.Graphical Abstract
Keywords
Androgenetic alopecia FDA approved drugs Natural products Herbal and novel topical marketed formulations Brief descriptions about formulations Formulation under clinical trialsAbbreviations
1 Introduction
Two efficacious natural androgens in our body are sex steroidal hormone (5-alpha-dihydrotestosterone and testosterone). 5Alpha-reductase remains in 2 forms type 1 and type 2 and both of them mainly present in scalp follicles [1, 2]. AGA, also recognized as male or female pattern baldness affecting up to 50% of the adult male and 40% of the adult female population. Androgens bind with human androgen receptor (AR) which composed of 2 domain one is ligand bind and another is DNA bind domain. When both the steroidal hormone binds with bind site then DNA binding site activates. After the activation, the androgenic sensitive genes are exposed [3]. Androgenic receptors are normally needed in the male body for their male characterization such as testes, muscles, male reproductive systems, immune systems [4]. Studies informed that in the case of balding scalp follicles, the concentration of DHT is more intense rather than non-balding scalp follicles [5]. Experts researched and suggested that hair loss occurs due to increased concentration of both 5alpha reductase and AR, they are not surely assured about the mechanism but in hair loss, the probable reason is the gene which controls the hair follicles growth cycle is remoted by androgens [6]. Although there are only two FDA drugs (Topical Minoxidil and Oral Finasteride) are allowed for treating this AGA (Androgenic alopecia) but there are various side effects related to Minoxidil include facial hypertrichosis in 3-5% of women and contact dermatitis in 6.5% of patients and systemic finasteride also showed a large side effects including sexual dysfunction, mood disturbance and post-finasteride syndrome with related depression. At present accessible regular treatments of going bald utilizing synthetic drugs are as yet defective and have various restrictions. Their adequacy just as the security of their utilization is regularly addressed. It has prompted an expanded enthusiasm for alternative medicines with less reactions, for example, herbal plants having therapeutic potential constituents. For this herbal products now a days become crucial to treat androgenic alopecia [7]. Natural products envelop an assortment of subgroups including nutrients and minerals, botanicals, and probiotics, which are all internationally showcased as dietary enhancements and don't need Food and Drug Administration (FDA) endorsement. The 2012 National Health Interview Survey reports concluded that CAM (complementary and alternative medicine) approach for dermatological conditions were utilized by 17.7% of Americans [8]. The reports showed the enhanced hair growth by using amino acids, caffeine, capsaicin, curcumin, garlic gel, marine proteins, melatonin, onion juice, procyanidin, pumpkin seed oil, rosemary oil, saw palmetto, vitamin B7 (biotin), vitamin D, vitamin E, and zinc.
2 Management of Androgenic Alopecia
Beyond treatment, the androgenic alopecia increasing day by day. Researchers found that approximately the rate is near about 5% per year. There are many disguises and surgical management procedures are available but, in this paper, we have discussed the therapeutic management procedures. Figuring out the functional sequence alternate in or around the AR gene will lead to the dedication of the exact variation in AR proteins between bald and non-bald people. By this proficiency, treatments can be arranged that the point and reverse these inequalities, through that impeding exact hair loss mechanism. Now recent pharmaceutical treatments for androgenic alopecia do not mark the particular cellular mechanisms in this procedure. Rather they impede the activity of the enzyme which boosts the AR in the balding scalp, thus they are abolishable than curative, with different success rates.
2.1 FDA Approved Drug for Alopecia
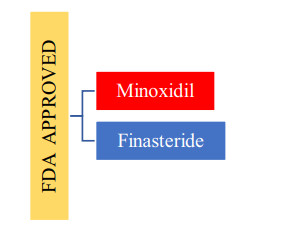
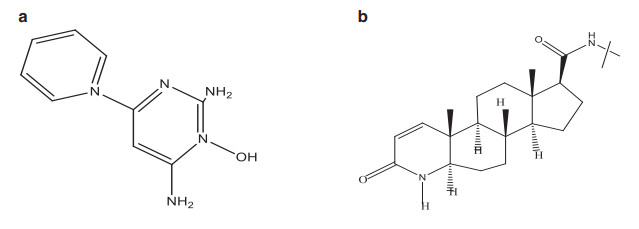
Drugs that are approved by the FDA for the treatment of alopecia are shown in Fig. 1 and their respective chemical structures are in Fig. 2a and b.
FDA approved drugs
a Chemical structure of Minoxidil. b Chemical structure of Finasteride
Only two approved drugs by the FDA still are there for Alopecia are Finasteride and Minoxidil. The patent of Finasteride was filed in 1984 and accepted for medical purposes in 1982 and was available in generic form. Whereas Minoxidil was developed in the 1950s by the Upjohn Company (now as Pfizer). Then the company had synthesized many derivatives and in 1963 named Minoxidil [9]. In 1979 it was authorized by FDA for the treatment of high BP in tablet form with Loniten trade name [10]. In 1988 FDA approved it for treating male pattern baldness in men with the trade name of 'Rogaine' [11]. In 1998, 5% of Minoxidil formulation was allowed by the FDA [10]. In the year 1998 minoxidil came for sale nonprescription ally by FDA and in 2014 it was the only topical choice by FDA approved for treating androgenic alopecia [12]. The drug is available in the topical formulation in the UK, US, Sweden, and Germany.
2.1.1 Minoxidil
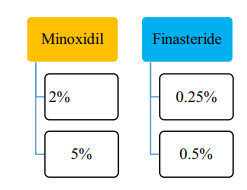
At first, Minoxidil was used to treat high BP due to its systemic side effects. The formulation changed to the topical formulation used to treat baldness [13]. Minoxidil is present as a 2%, 5% topical solution approved by USFDA. In 1998 it was approved first for male pattern baldness and then for female in 2001 as 2% minoxidil solution and 5% minoxidil was approved in 2007 for male androgenic alopecia. 5% Foam minoxidil also approved by FDA in 2006 but only in men, not in case of women's hair loss purpose, it was off-label treatment formulation. 2% and 5% Topical solution indicated twice a day as 1 mL of the solution.
2.1.2 Finasteride
The 5α-reductase inhibitor finasteride blocks the conversion of testosterone to dihydrotestosterone (DHT), the androgen responsible for male pattern hair loss (androgenic alopecia) in genetically predisposed men. Finasteride, another FDA approved drug has been reported effective in 0.25% and 0.5% topical solution compared to an oral 1 mg/dose. Oral Finasteride tablet have different systemic side effects, to overcome these problems topical formulation of Finasteride now-a-days successfully applied on male pattern alopecia.
% Drug effectiveness [14, 15] for the FDA are elaborated in Fig. 3.
% drug effectiveness of FDA approved drugs
2.2 Adverse Effects of Both FDA Approved Drug Formulation
Common side effects or adverse effects for both the drugs i.e. Minoxidil and Finasteride are illustrated in Table 1.
Side effects of minoxidil and finasteride
3 Role of Various Oral Supplements Like Vitamins and Minerals to Prevent Androgenic Alopecia
Dietary micronutrients such as vitamins, minerals are nowadays becoming another option for treating androgenic alopecia. These minerals play a key role in the normal hair follicle cycle. The deficit of micronutrients represents an adjustable risk factor associated with the development, prevention, and treatment of alopecia. Vitamins and minerals are vital for normal cell growth and function and may cause hair loss due to deficiency of them [19]. Where supplementation is relatively inexpensive and easily available, now it will be discussed which vitamins and minerals help treat hair loss. In total hair follicles, about 90% of them are present in the anagen phase where there is no chance of hair loss. Some essential elements, such as proteins, vitamins, and minerals are very much needed to produce healthy hair [20]. So, micronutrients, including vitamins and trace minerals, are therefore vital components of our diet [21] (Table 2).
Brief elaboration of different vitamins to treat androgenic alopecia
3.1 Role of Essential Trace Elements in Hair Loss (Table 3)
Trace elements for androgenic alopecia
4 Role of Dandruff in Hair Fall
Dandruff is a common scalp disorder, characterized by the presence of corneocytes that form clusters due to their high cohesive power, in the form of flaky white to yellowish scales, accompanied by itching and mainly occurs when sebaceous glands are most active.
Dandruff directly not responsible for hair loss, but the two may be linked indirectly. Due to some infections and medical conditions are responsible for dandruff and hair loss. The harshness of dandruff plays a role in severe conditions among the subjects; the scales may be trapped in a crowded terminal. The presence of dandruff may lead to telogen effluvium [44] and may also cause Androgenic alopecia. However due to dandruff itchiness occurs which leads to scratching and injure the hair follicles, leading to hair loss to some extent. One common dandruff-related health problems that may cause hair loss is seborrheic dermatitis. Seborrheic dermatitis is a flaky, scaly rash that forms on the scalp and face. When it develops on the scalp, it causes dandruff and creates temporary hair loss. New York dermatologist Michele Green, MD says FDA approved drug Minoxidil can cause dandruff-like flaking as a side effect. The presence of alcohol in minoxidil can dry out the scalp, and after a few months of treatment, dandruff may develop, and on the other hand, Finasteride, another medication for hair loss, does not cause flaking.
5 Role of PRP (Platelet-Rich Plasma) in Hair Regrowth
Platelet-rich plasma therapy has become a new popular treatment for hair regrowth in male pattern baldness. Activated platelets release numerous growth factors and cytokines from their alpha granules as part of the wound healing process. Platelets in PRP become activated when injected into the scalp and release multiple growth factors, which promote hair growth. Khatu et al. [45] reported 11 patients who were suffering from Androgenic Alopecia taking Finasteride and Minoxidil for 6 months but there was no improvement in hair growth. A total volume of 2-3 cm3 PRP was injected in the scalp with an insulin syringe. The treatment was done for every 2 weeks, for a total of four times. The result was assessed after 3 months through clinical examination, macroscopic photos, hair pull test, and patient's overall satisfaction. The result showed that hair count improved from an average number of 71 hair follicular units to 93 hair follicular units. That means the average mean gain was 22.09 follicular units/cm2. After the fourth session, the pull test was negative in 9 patients among 11. A crucial decrease in hair loss was observed between the first and fourth injection. Gkini et al. [46] did a nonrandomized trial to check the efficacy of PRP injection in 22 patients affected by androgenic alopecia. This study based on 3 treatment sessions with an interval of 3 weeks. At 6 months from the starting of the treatment, a booster assembly was also performed and hair density significantly increased at 6 weeks. Kang et al. [47] reported the clinical efficacy of injection of CD34+ cell-containing PRP preparations for male and female pattern hair loss. In this study, 3 months after the first treatment, the results showed clinical improvement in the mean number of hairs (20.5%±17.0%), mean hair thickness (31.3%±30.1%). At 6 months, the results were increased in mean hair count (29.2%±17.8%), mean hair thickness (46.4%±37.5%). Trink et al. [48] reported the effect and safety of PRP on alopecia areata in a randomized, double-blind, placebo and active-controlled, half-head, parallel-group study. 45 Patients having alopecia areata were given intralesional injections of PRP and triamcinolone acetonide (TrA). Gentile and Garcovich [49] described a systematic review of Platelet-Rich Plasma Use in Androgenetic Alopecia Compared with Minoxidil, Finasteride, and Adult Stem Cell-Based Therapy. The results showed patients who were treated with PRP had significantly increased hair regrowth compared with those treated with TrA. Platelet-rich plasma (PRP) has appeared as a new treatment modality in reformative plastic surgery, and the preliminary result suggested that it might have a valuable role in hair regrowth. The results of a randomized, placebo-controlled, half-head group study to compare the hair regrowth with PRP versus placebo are reported and it was seen that in every aspect of view PRP showed better results than other options. PRP tends to progress hair caliber and hair growth for about 4 to 6 weeks which needed repeated treatments once a month for 3 months. On average most patients must require repetitive PRP treatment after 6-12 months to keep the hair growth effects. In the case of hair loss, the injected platelets prompt inactive or newly implanted hair follicles to enter an active growth phase, causing the hair to start growing again.
6 Role of Low-Level-Laser Therapy Hair Comb in Hair Loss Prevention
Nowadays laser comb has been approved by the FDA for both safety and effectiveness in case of the treatment of the hair loss. It is a non-chemical, non-invasive option to help people grow fuller, thicker, healthier hair [50]. LLLT stimulated hair growth in mice when they have induced chemotherapy for producing alopecia and also in alopecia areata. Various controlled clinical trials confirmed that LLLT stimulated hair growth in both men and women. There are many mechanisms are established but among them, the key mechanism is the stimulation of epidermal stem cells in the hair follicle bulge and shifting the follicles into anagen phase which promotes hair growth as well as prevents hair loss [51]. In the late 1960s, Endre Mester, a Hungarian physician experimented on the carcinogenic potential of lasers by using a low-power ruby laser (694 nm) on mice. It was seen that the laser did not cause cancer and unexpectedly improved hair growth around the shaved region on the animal's back [52]. Laser phototherapy is supposed to stimulate anagen phase re-entry in telogen phase hair follicles that extend the duration of the anagen phase, enhances rates of proliferation in active anagen hair follicles, and to avoid premature catagen development [53]. LLLT has been demonstrated to modulate inflammatory processes and immunological responses, that result in an effect in hair regrowth [54]. Wikramanayake et al. described the C3H/HeJ mouse model which was treated with laser comb and resulted in an enhanced number of hair follicles with the majority in the anagen phase were observed with reduced inflammatory infiltrates. Shukla et al. [55] studied the outcome of helium-neon (He-Ne) laser on the hair follicle growth cycle of testosterone-treated and un-treated Swiss albino mice skin and the result showed testosterone treatment led to the inhibition of hair growth that was characterized by substantial growth in catagen follicles. The results showed that testosterone-treated mice to the He-Ne laser led more hair follicles in the anagen phase when compared to the other groups. Satino and Markou [56] tested the efficacy of LLLT on hair growth and tensile strength on 28 male and 7 female androgenic alopecia patients who were given a HairMax LaserComb® 655 nm, to use at home for 6 months for 5-10 min every other day. Results show in the case of hair tensile strength it was seen that better improvement in the vertex area for males and temporal areas for females but both sexes got benefited in all areas ominously. LLLT reported very few side effects over the past 50 years. LLLT has only one adverse effect in humans was the temporary onset of TE developing in the first 1-2 months after instigation LaserComb treatment. However, more studies are required to optimize treatment parameters and determine long-term efficacy as well as the safety of emerging LLLT technologies.
7 CAM Therapy to Treat AGA
Though there are a variability in CAM treatment choices on the market for both androgenic alopecia and alopecia areata, only a few completed multiple randomized controlled clinical trials. So, there is a requirement for additional studies about CAM for alopecia with more robust, clinical design and standardized, quantitative results.
7.1 Acupuncture
The concepts behind this treatment are it can enhance blood circulation, stimulate the hair follicles and diminish inflammatory infiltrates [57]. It was reported that 78 patients were treated with plum-blossom acupuncture and with 2% topical MXD. Results showed total regrowth was 58.1% in acupuncture therapy whereas 34.3% in MXD treated patients [58]. A case study reported improvement in AGA by combining therapy of pharmacopuncture, acupuncture and needle [59].
7.2 Aromatherapy
This therapy uses massaging of essential oils with jojoba, grape seed carrier oils on scalp. Reports said that eighty four patients were treated for 7 months and result showed that 44% patients having increased hair growth whereas only 15% who were treated with carrier oil only [60].
7.3 Psychotherapy
This treatment directly works on the state of mind. A report said that treatment with additional relaxation and prednisolone 5-10 mg/day for 30 min, 2 months and further prednisolone for another 4-5 months improved hair growth in 83% of treated people.
8 Natural Sources for Medication
Androgenetic Alopecia can be treated with the help of some natural marketed preparations. These natural preparations commonly employed due to their less or no toxic effects means these all preparations are safe to use. Some of the medications whose natural sources are elaborated in Table 4 where their Composition, Action, and Dose are well described.
Different natural sources medication related to androgenetic alopecia
8.1 Different Natural Products for Preventing Hair Loss
According to the National Center for Complementary and Integrative Health (NCCIH), a branch of the National Institutes of Health (NIH, Bethesda, MD, USA) has approached for natural products. CAM (complementary and alternative medicine) offers to select promising, low-risk, adjuvant, and alternative therapies. Here a comprehensive update of CAM treatment options for alopecia, with most evidence in androgenetic alopecia (AGA) has discussed. Natural products are categorized in various subgroups including vitamins and minerals, herbs/botanicals, and probiotics, all of which are globally marketed as dietary supplements and do not require the Food and Drug Administration (FDA) approval (Table 5).
Natural methods for preventing hair loss
8.2 Herbal Medication for Treating Alopecia and Their Marketed Herbal Formulations
Herbal formulations are nowadays has become more eye-catching due to some specific advantages over a novel drug delivery system and dosage form. Origin of herbal formulations is mainly natural occurring, therefore; it provides fewer side effects and toxicity than the synthetic carrier for alopecia. Some of the Herbal Formulations which are available in the market are described in detail.
8.2.1 Saw Palmetto
The market herbal formulation for Saw Palmetto [89] is given in Table 6 which describes the source, type of dosage form, the dose that can be given, route of administration, name of a brand for which formulation is available in the market, usage about the formulation and cautions that one kept in mind before using the formulation. Not only this their storage (as is the important part of any formulation whether that formulation is prepared from natural or synthetic forms), mode of action, possible side effects, and their interaction also mentioned.
Market formulation for Saw Palmetto
8.2.2 Green Tea
Green tea has beneficial effects such as anti-cancer and anti-oxidant properties mediated by epigallocatechin-3-gallate (EGCG), a major constituent of polyphenols. Recently, reported that EGCG can be useful in the treatment of androgenetic alopecia by selectively inhibiting 5alpha-reductase activity [90]. The Market formulation for green tea its dosage form, Route for application and how to use given in Table 7.
Market formulation for green tea
8.2.3 Rosemary
Rosemary is a sweet-smelling evergreen herb that has medicinal properties. Conventionally it is used to improve memory, reduces muscle pain, speeds up the immune and circulatory system, and help in hair growth. Rosemary has potential health benefits which include antioxidant and anti-inflammatory compounds, helps in improving digestion, memory enhancement, increasing concentration, helps in neurological protection, helps in preventing brain aging, reduces the formation of cancer-causing agents that acts as an anti-tumor agent. Several side effects are generated due to an increase in the dose. Side effects include coma, muscle spasms, vomiting, and an increase in dose lead to miscarriage; therefore, pregnant women should avoid the use of any rosemary products [91].
A brief about the rosemary market formulation [92] given in Table 8 which includes its dosage form, brand name, the dose that can be taken, route, and how to apply.
Market formulation for rosemary
8.2.4 Grapeseed
Grapeseed, a byproduct of the wine production from grapes. Other names of grape seed are Grape-seed oil, Grape seed extract, and many more. Grapefruit juice or Grapefruit products are different from grape seeds. As researchers had said that grape seed is not effective in the treatment of allergies related to seasonal changes. Several side effects of grape seed include vomiting, nausea, dry mouth, cough, headache, muscle pain, and upset stomach. There are some drugs with which grape seed cannot be administered includes; some antidepressants; asthma medicines; medicines related to heart or blood pressure; medicines for the treatment of mental illness or anxiety [93].
Description of the market formulation of grape seed [94] given in Table 9 which has dosage form, name of brand available in market, route, and direction for use.
Market formulation for grape seed
8.2.5 Licorice
Licorice an herb that belongs to the Mediterranean, southern, and central Russia. Some of the species nowadays grown over Europe, Asia, and the Middle East. Licorice contains the acid i.e. Glycyrrhizic acid that can cause problems when consumed in larger quantities. Licorice can be administered in a single form or can be combined with other herbs like problems related to the digestive system; ulcers, heartburn, chronic gastritis. In several conditions like itchy skin, inflamed skin, psoriasis, or brown spots licorice applied to the skin. Licorice also used in foods for flavoring, in beverages, and tobacco products [95].
Brief about the Licorice available market preparation [96] given in Table 10.
Market formulation for Licorice
9 Marketed Topical Formulation for Androgenic Alopecia
9.1 Minoxidil Topical Solution USP 5% (w/v)
Mintop Forte 5% Solution is a medicinal drug used in the cure of male pattern baldness. It is the highest quality in treating familial hair loss or thinning at the top of the scalp, no longer in front. Mintop Forte 5% Solution helps to stimulate hair increase with the aid of growing the blood circulation to the hair follicles. However, the amount of hair growth is one-of-a-kind for every person. During the first 2 weeks of application, your hair fall may extend temporarily. This is every day and is a sign that the remedy is working. This medicine must strictly be taken following the doctor's advice in Table 11.
Minoxidil topical solution USP 5% (w/v)
9.1.1 Mechanism of Action
Minoxidil is a vasodilator it widens the blood vessels which improves blood flow. Using Mintop solution on the top of scalp increase the blood flow which provides more the oxygen and nutrient to the hair follicle that lower the death of hair follicle cell. This medication triggered the anagen chemical messenger.
9.1.2 Expert Comments
Should be applied directly to the scalp area. If it comes in contact with eyes and mouth immediately wash with cool water properly. Do not use a hairdryer as it reduces effectiveness. Do not apply shampoo for 4 h after applying the Mintop Forte solution. Use carefully because in contact with face it will cause unwanted facial hair growth.
Some of the alternative brands for Mintop Forte Solution have been described in Table 12 with their; brand name, the composition of salt, type of dosage form, name of the manufacturer, and the volume of the dosage form.
Alternative brands for Mintop Forte solution
9.2 Men's Rogaine 2%, 5% Aerosol (Easy to Use Foam)
Aerosol helps in hair growth when applied to the vertex part. Outline of this aerosol is described in Table 13 which includes ingredients that are used in this aerosol, type of dosage form, use of aerosol, what are storage conditions, and inactive ingredients used. Some of the drawbacks of this aerosol include; chest pain, rapid heartbeat, dizziness, increase in body weight, irritation on the scalp, undesired facial hair growth, no improvement after applying for 4 months.
Men's Rogaine 2%, 5% aerosol (easy to use foam)
Precautions that one should be kept in mind include; only for external use; away for children; as it is in aerosol so it is extremely flammable.
9.3 EquateTM
EquateTM which is Minoxidil 2%, 5% topical solution which contains alcohol, propylene glycol, purified water as inactive ingredients. It should be used only for the vertex portion; only for male baldness and applied two times a day directly to the scalp. It is contraindicated in the case of women's baldness. As it contains alcohol so chances of fire are more; keep away for the flame as well as fire.
Some of the information about Minoxidil 2%, a 5% solution is given in CIMS. Information is elaborated in Table 14 that covers male and female adults, safety measures, contraindication as well as interaction and adverse drug reaction.
Minoxidil 2%, 5% solution (CIMS data)
9.4 Inoxi 10% Solution
Inoxi 10% solution is commonly used for the treatment of hair growth and only male pattern baldness and effective treatment for hereditary hair loss; thinning of hair at the top of the scalp, not the front. This solution is manufactured by Psycoremedies which has 10% Minoxidil.
9.4.1 Mechanism of Action
Increasing blood circulation to hair follicles. That to leads more oxygen supply to hair follicles and leads to less death of hair scalp cells. During 1st 2 weeks of application, hair fall may increase temporarily this is a normal sign of medicine effectiveness.
Other available brands include; Morr 10% solution manufactured by Intas Pharmaceuticals; Grewit 10% solution manufactured by Kivi Labs Ltd.; Trichoton M 10% solution manufactured by Med Manor Organics Pvt. Ltd.
This solution is unsafe for women and children.
The Topical solution is available in 20 mg/60 mL which costs about Rs. 153.00 and 100 mg/60 mL which costs about Rs. 550.00.
9.5 Inoxi Forte
Inoxi Forte which is 5% Minoxidil lotion manufactured by Psycoremedies and its mechanism of action includes widening blood vessels and opening potassium channel.
9.5.1 Uses
This lotion is used for the; Hair growth stimulation; Androgenic alopecia; Hereditary hair loss problems.
9.5.2 Supportive Measures
5% Minoxidil lotion has interaction with other drugs those include; Clonidine, Nadolol, Nicorandil, Propranolol, Tretinoin.
Side effects of this lotion comprise of increase in heart rate; chest pain; tenderness in the breast; rare palpitations.
The lotion is opposed in cases like hypersensitivity; angina pectrosis; pregnancy.
It must be stored at room temperature. Don't freeze this lotion.
Apply only to scalp; avoid contact with eyes and mucous membrane.
9.6 Minch
Minch containing about Minoxidil 3% and Minoxidil 12.5% (w/v) manufactured by Aamorb (Sioux) having volume 60 mL.
It is used to treat male pattern baldness only on the top of the scalp, not in front. It works by increasing the blood circulation, oxygen supply, and nutrition to the hair follicles. During the first 2 weeks of hair fall may enhance for a time that means medicine has shown effective results.
9.6.1 Expert Advice
Apply directly to the scalp, do not shampoo for 4 h after applying the medication, during first 2 weeks hair fall may increase.
9.6.2 Supportive Measures
Side effects include; irritation at the applied site; itching; headache.
9.6.3 Unsafe During Pregnancy
Other brands available for this include; more 3% solution by Intas Pharmaceuticals Ltd.; MNX-3 topical solution by Salve Pharmaceuticals Ltd.
If you have missed the dose take as soon as possible if it is too late then following the next day schedule but not double the dose.
9.7 Radixil
Radixil is a topical solution of Minoxidil 2%, 5% and 10% manufactured by Signova Pharma Pvt. Ltd. Several side effects include; irregularity in a heartbeat; an increase in weight; shortness of breath; skin redness; irritation in the eye.
Different drug interactions with Radixil; Alprazolam; Corticosteroids; Guanethidine.
A substitute available includes Exidil 5%, Morr 5%, Rootz 5%, Tugain 5%.
9.8 Pilogro
Pilogro is a topical 2%, 5% Minoxidil manufactured by Fulford. Its onset of action can be observed within 4-5 h after application and may cause sleepiness.
Side effects of this include; shortness of breath; chest pain; weight gain; an irregular heartbeat.
Contraindicated in patients with congestive heart failure, unsafe for pregnant and breastfeeding women.
9.9 Regero
Regero, Minoxidil 2% topical solution manufactured by Shinto Organics Ltd. Having a volume of 60 mL. It is a type of vasodilator used in the treatment of male pattern baldness. Applied on the scalp directly not used for the front portion.
Side effects of this solution include; itching; headache; irritation; chest pain; unwanted hair growth to the undesired site; swollen hands and feet.
Other than these alternative brands are also available in Table 15.
Alternative brand available for Regero
9.10 Retreat
Retreat is Minoxidil 5% (w/w) topical gel manufactured by Segment Care whose volume is 60 g.
9.10.1 Mechanism of Action
Vasodilator expands blood vessels which promotes blood supply and provides nutrition and oxygen to hair follicles that prevent hair cell death and result in an improvement in hair growth by prolonging the action of chemical messenger anagen.
How to use: use it in the dose and duration as advised by the Doctor. Check the level for direction before using it. Clean and dry the affected area and then apply the gel. Wash hands after applying.
Some of the alternative brands include; Tugain 5% gel (Cipla Ltd.), Curlz pep gel 5% (w/v) (Canixa Life Science Pvt.), Regrowee 5% gel (w/w) (West Coast Pharmaceutical Works Ltd.), Menexil 5% gel (Dabur India Ltd.)
9.11 Stonark
Minoxidil 5% (w/v) solution manufactured by Cutis Derma. Use it in the dose as prescribed by the physician. Alternative brands available for Stonark include; Tugain 5%-Cipla Ltd. Regaine 5%-Janssen Pharma. MX-5-Hedge and Hedge Pharmaceuticals, Mintop Forte 5%-Dr. Reddy's Laboratories. Minoqilib 5%-Galderma India Pvt. Ltd.
9.12 Ximinox
2%, 5% Minoxidil lotion manufactured by Rowan Biopharmaceuticals Pvt Ltd. Side effects of this lotion-irritation, itching, headache. Different alternative brands are available for lotion-Arodil lotion-Dr. Johns Laboratories Pvt. Ltd., AGA 1% lotion-Nidus Pharma Pvt. Ltd., Mxd 10 lotion-L Med India, Minfin 10% lotion-HBC Life Science Pvt. Ltd.
9.13 Gaine Hair
Gaine hair is a 2% topical solution manufactured by Wockhardt Ltd. composed of Aminexil and Minoxidil. Other alternative brands available Tugain 2% Solution-Cipla Ltd., MX 2 Solution.
Hegde and Hegde Pharmaceuticals and Black Crown 2% Solution-Derma joint India.
Side effects of this solution headache, allergic reaction, rashes on the skin, and chest pain.
9.14 Hair 4U
Hair 4U is lotion (60 mL) manufactured by Glenmark Pharmaceuticals which is composed of Min 2% w/v + diaminopyridine oxide 1.5% w/v, Min 5% w/diamino pyridine oxide 1.5% w/v, Min 10% w/diamino pyridine 1.5% w/v.
9.15 Hair 4U 2% Lotion and Hair 4U Spray
Hair 4U 2% lotion manufactured by Glenmark Pharmaceuticals composed of Aminoxil and Minoxidil. Side effects of headache, skin rashes, edema, and dermatitis. It cannot be used in case of allergy.
Hair 4U Spray 60 mL manufactured by Glenmark Pharmaceutical Ltd. (Grace well) and composed of Minoxidil 2%, 5%, 10% + diamino pyridine oxide 1.5% Generic-Diamino pyridine oxide.
9.16 Radixil A and Radixil F
Both the solutions are manufactured by Signova Pharma Pvt. Ltd. Composition of Radixil A includes Minoxidil 5% + diamino pyridine 1.5 and Radixil F includes Minoxidil 5% + 0.1% Finasteride. Side effects of this topical solution redness and dry skin change in hair color and texture. Keep away from children and must be stored at 15-25 ℃.
9.17 Stonark-AX and Stonark-2AX Solution
Both are topical solution manufactured by Cutis Derma Care. Composition of Stonark-AX Minoxidil 2% w/w + diaminopyridine oxide 1.5% w/w and Stonark-2AX Minoxidil 2% w/v + Aminexil 1.5% w/v and alternative available brands Hairjoy 2%-solution-Torrent Pharmaceuticals.
9.18 Hairslim-F Topical Solution
Solution is lipid-based composed of Minoxidil 5% w/v + Finasteride 0.1% w/v. Adverse effects are dry skin, tingling, headache, dermatitis, erythema, dizziness. After applying to allow to dry for completely 2-4 h; hairdryer not to be used.
9.19 Keshgaine-5%
Minoxidil 5% solution manufactured by Connote Health Care. It should be applied once or twice daily and the solution must contact the scalp for at least 4 h before washing.
9.20 Regenepure Precision Minoxidil 5% Spray
Spray composed of deionized water, aloe vera gel, sodium-cocoyl isethionate, carbomer, niacin, PEG-8, hydrolyzed wheat protein, allantoin, ascorbic acid, Vit-B6, linolenic acid, ketoconazole, menthol, salicylic acid, PEG-25, lemon oil, ZnO, Polysorbate-80, Phenoxyethanol, FD, and C blue. For external use only.
9.21 Formula 82F/M
Composed of 0.25% Finasteride + 5% Minoxidil, retinoic acid, oleanolic-acid, algae extract with propylene glycol. Must be used twice a day i.e. 30 drops. The common area that includes the crown, top of the scalp, and around hair transplants are the area that can be treated with this.
9.22 Andro Block F
Formulation composed of Min 5% + Azelaic acid 12.5% + Finasteride 0.1% + Ketoconazole 2% which is manufactured by Empower Pharmacy. It should be stored at temperature 68-77°F and away from heat, moisture, and light.
9.23 Minoxidil Store Plus
Formulation composed of Min 10% + Azelaic acid 5% + Finasteride 0.1%, ethyl alcohol, propylene glycol, applied to the scalp and avoided in patients having high blood pressure. Side effects are itching, difficulty in breathing, increase in heart rate.
9.24 Regre Topical Spray
Spray composed of only 5% Minoxidil (60 mL), which is used twice a day.
9.25 MX-2 Solution
The solution is composed of Minoxidil 2% manufactured by Hedge and Hedge Pharmaceuticals. This solution used only for male patterns. Other alternative available brands includes Mintop 2%, Tugain 2%, Black crown 2%, Regaine 2%.
9.26 Coverit 5%
Minoxidil 5% belongs to Schedule H, Manufactured by Micro Labs Ltd. Side effects are skin redness, weight gain, headache, and irregular heart rate.
Warnings include during pregnancy, congestive heart failure, and breastfeeding and scalp irritation.
Drug-drug interaction includes Alprazolam-moderate, Corticosteroids-moderate, and Guanethidine-severe.
9.27 Exidil 5% Solution
The solution comprises of Minoxidil 5% which is manufactured by Ranbaxy Laboratories Ltd.
9.28 Rootz 5% Solution
A solution composed of Minoxidil 5% manufactured by Cipla Ltd.
9.29 Tinfal
Composed of Minoxidil 5% (w/v), ethanol 95% and volume is 60 mL.
9.30 Tinfal Plus
Composed of Minoxidil 5% + Aminexil 1.5%, volume 60 mL, and used on dry hair once at night time.
9.31 Lipogaine (Men)
Serum composed of Minoxidil 5% with biotinyl-tripeptide, niacin, apple polyphenol. Not use more than 1 mL of serum each time, no massage required to apply directly to the scalp in the hair loss area. Stored at temperature 10-35 ℃, away from children.
9.32 Lipogaine (Women)
Composed of only Minoxidil 2% and apply 1 mL to the scalp in hair loss area, apply twice a day but at least 8 h after first applied.
Keep away from children as well as from fire.
9.33 Imxia
Composed of Minoxidil 5% which is manufactured by KLM Laboratories Pvt. Ltd.
9.34 Minokem
The spray contains Minoxidil 2%, 5% (60 mL) manufactured by Alkem Laboratories.
Severe interactions with Guanethidine and moderate with Alprazolam and Corticosteroids.
Associated side effects are weight gain, irritation in the eye, chest pain, and redness of the skin.
9.35 Minotreat
Lotion that contains Minoxidil 5% manufactured by Ikon Remedies Pvt. Ltd.
For external use only and do not show interaction with any formulation.
9.35.1 Available Alternative Brands
Biodens hair lotion, Minotress lotion-Prism Life Science Ltd.., Arodil Forte lotion-Dr. Johns Laboratories Pvt. Ltd., TM 5 lotion-New trimed.
9.36 Proanagen Solution
Composed of diaminopyrimidine oxide topical solution, diamino pyridine oxide 1.5% (w/v) in green apple skin extract base and Kopexil (1NCI name diaminopyrimidine oxide, trade name Aminexil) manufactured by Curatio Healthcare Pvt. Ltd.
Stored at a cool place, away from light, must be applied as instructions given by dermatologists.
9.36.1 Mechanism of Action of Aminexil
The mechanism of action of Aminexil and Minoxidil is only structurally related by conjecture. It prolongs the hair development phase through non-hormonal mechanisms. Furthermore, hair follicles get hardened by DHT are softened by Aminexil. It expands the blood vessels and provides better blood flow to the hair follicles. Commonly used in hair cosmetic products to fight against hair loss due to premature exhaustion of the hair root. The drug acts by stimulating or inhibiting a receptor or an enzyme or a protein most of the time. Medications are produced in such a way that the ingredients target the specific site and bring about chemical changes in the body that can stop or reverse the chemical reaction which is causing the disease.
9.36.2 Mechanism of Action of Diaminopyridine (3, 4-Diaminopyridine)
Blocks calcium-dependent potassium channels and prolongs the duration of the action potential at motor nerve terminals; this enhances calcium influx into nerve endings and causes the release of acetylcholine [97]. It selectively blocks presynaptic fast voltage-gated potassium channels thus prolonging cell membrane depolarization and action potential, and increasing calcium transport into the nerve endings.
9.37 Keraglo Eva
Keraglo Eva is Manufactured by Ipca Laboratories Ltd., generic uses include Biotin, Folic Acid, Selenium. Composed of Biotin (10 Mg), Folic Acid (300 Mcg), Selenium (40 Mcg) Gamma Linolenic Acid (found mostly in plant-based oils such as borrage seed oil), multivitamin, multimineral. Gamma-linolenic acid is an essential fatty acid that helps to stop hair loss, as well as the growth of hair. No side effects, have been reported at appropriate doses. Daily dosage is 1 tablet daily with a glass of water, before or after a meal.
9.37.1 Drug Interaction of Biotin
May Interact With Clozapine, Fluvoxamine, Haloperidol, Mexiletine, Imipramine, Olanzapine, Pentazocine, Propranolol, Tacrine, Theophylline, Zileuton, and Zolmitriptan. Carbamazepine, Phenytoin, Phenobarbital, Valproic Acid.
9.37.1.1 Drug Interaction of Folic acid
Fluorouracil, Sulphonamide, Phenytoin, Methotrexate, Sulfasalazine, Cholestyramine.
9.37.2 Side effects of Folic Acid
The common side effects are urge to vomit, bloating of stomach, excessive passage of wind, loss of appetite.
9.37.3 Drug Interaction of Selenium
Seborrheic dermatitis.
9.37.4 Side Effects of Selenium
Nausea, stomach upset, skin rash, acute toxicity.
9.38 Biodens Hair Lotion
Hair lotion which is manufactured by Adonis Phytoceuticals Pvt. Ltd. and having a salt composition of Minoxidil 5% (w/v).
9.38.1 Side Effects of Biodens Lotion
Excessive hair growth on the face, Rash, Edema (swelling), Skin irritation.
9.38.2 Mechanism of Action
It works by increasing blood flow to the hair follicles on the scalp, which prevents hair cell death and also enhances new hair growth. It is effective for baldness or thinning at the top of the scalp but less effective at the front or for receding hairline. Biodens hair lotion is not appropriate for sudden or inexplicable hair loss. It is considered as a safe medicine to promote hair growth after hereditary hair loss (male pattern baldness).
Applied directly to the scalp area in the amount. Clean and dry your scalp before using it.
Alternate Brands of lotion includes Hairex Lotion-Swiss Pharma Pvt. Ltd.: Minotress Lotion-Prism Life Sciences Ltd.: TM 5 Scalp Lotion-Newtrimed.
10 Formulations Under Clinical Trials [98]
Some the formulations are under clinical trials i.e. under the different phases of clinical trials that are elaborated in Table 16.
Formulation under clinical trials
11 Conclusion
Topical minoxidil and finasteride can be useful adjuncts to hair transplant surgery for AGA. Topical use of this therapeutics leads less side effects than oral use. The oral finasteride causes different systemic side effects including liver metabolism, decreased sex drive, ejaculation disorder and so on. By using the topical formulations and other supplements we can easily reduce the side effects. Different natural supplements also helpful for preventing hair loss. According to patients medical history, it is always significant to be detailed and include over-the-counter vitamins, minerals, and supplements. It is very important to go for allergy history as some allergies may impede the use of certain CAMs. In this article we reviewed different topical marketed formulations of different brands, natural products, supplements which are reported to treat against androgenic alopecia.
Notes
Acknowledgements
We are highly thankful to the management of ISF College of Pharmacy for continuous support and guidance.
Compliance with Ethical Standards
Conflict of interest
No conflict of interest.
References
-
1.E.P. Jenkins, S. Andersson, J. Imperato-McGinley, J.D. Wilson, D.W. Russell, J. Clin. Investig. 89, 293-300 (1992) CrossRef PubMed Google Scholar
-
2.W. Eicheler, M. Dreher, R. Hoffmann, R. Happle, G. Aumüller, Br. J. Dermatol. 133, 371-376 (1995) CrossRef PubMed Google Scholar
-
3.O.A. Jänne, J.J. Palvimo, P. Kallio, M. Mehto, Ann. Med. 25, 83-89 (1993) CrossRef PubMed Google Scholar
-
4.M.J. McPhaul, M. Young, J. Am. Acad. Dermatol. 45, S87-S94 (2001) PubMed Google Scholar
-
5.H.U. Schweikert, J.D. Wilson, J. Clin. Endocrinol. Metab. 38, 811-819 (1974) CrossRef PubMed Google Scholar
-
6.A.L. Dallob, N.S. Sadick, W. Unger, S. Lipert, L.A. Geissler, S.L. Gregoire, H.H. Nguyen, E.C. Moore, W.K. Tanaka, J. Clin. Endocrinol. Metab. 79, 703-706 (1994) CrossRef PubMed Google Scholar
-
7.A. Herman, A.P. Herman, Arch. Dermatol. Res. 309, 595-610 (2017) CrossRef PubMed Google Scholar
-
8.N. Smith, D.B. Shin, J.A. Brauer, J. Mao, J.M. Gelfand, J. Am. Acad. Dermatol. 60, 419-425 (2009) CrossRef PubMed Google Scholar
-
9.
-
10.P. Conrad, Sociol. Health Illn. 31, 147-148 (2009) CrossRef PubMed Google Scholar
-
11.W. Lester, Daily Gaz. (2015) PubMed Google Scholar
-
12.W.S. Pray, Am. J. Pharm. Educ. 70, 94-97 (2006) CrossRef PubMed Google Scholar
-
13.S. Varothai, W.F. Bergfeld, Am. J. Clin. Dermatol. 15, 217-230 (2014) CrossRef PubMed Google Scholar
-
14.M.C. Jones, US Pharm. 43, 12-16 (2018) PubMed Google Scholar
-
15.V. Mysore, Indian Dermatol. Online J. 3, 62 (2012) CrossRef PubMed Google Scholar
-
16.K. Padois, C. Cantiéni, V. Bertholle, C. Bardel, F. Pirot, F. Falson, Int. J. Pharm. 416, 300-304 (2011) PubMed Google Scholar
-
17.S. Sheikh, A. Ateeq, M.A. Shoukath, U.A. Moghis, P. Mahesh, D. Saptarshi, P. Kale, K. Maheshwari, H.V. Barkate, H.L. Pathak, M. Mushtaq, J. Clin. Exp. Dermatol. Res. 6, 253-258 (2015) PubMed Google Scholar
-
18.L.L. Levy, J.J. Emer, Int. J. Women's Health 5, 541-556 (2013) PubMed Google Scholar
-
19.H.M. Almohanna, A.A. Ahmed, J.P. Tsatalis, A. Tosti, Dermatol. Ther. 9, 51-70 (2019) CrossRef PubMed Google Scholar
-
20.G.G. Aksoy, Turkderm 48, 45 (2014) CrossRef PubMed Google Scholar
-
21.J.B. Mason, L. Goldman, D. Ausiello, Cecil Textb. Med. 23, 1626-1639 (2007) PubMed Google Scholar
-
22.H.B. Everts, Biochim. Biophys. Acta. 1821, 222-229 (2012) CrossRef PubMed Google Scholar
-
23.K. Yamamoto, K. Sadahito, M. Yoshikawa, O. Nobuyuki, O. Mikami, M. Yamada, K. Nakamura, N. Yasuyuki, Vet. Hum. Toxicol. 45, 85-87 (2003) CrossRef PubMed Google Scholar
-
24.C. Girard, O. Dereure, V. Blatiere, B. Guillot, D. Bessis. Pediatr. Dermatol. 23, 346-349 (2006) CrossRef PubMed Google Scholar
-
25.W. Fujimoto, M. Inaoki, T. Fukui, Y. Inoue, T. Kuhara, J. Dermatol. 32, 256-261 (2005) CrossRef PubMed Google Scholar
-
26.P.M. Coates, M.C. Paul, M. Blackman, M.R. Blackman, G.M. Cragg, M. Levine, J.D. White, J. Moss, Encyclopedia of Dietary Supplements (Online) (CRC Press, Boca Raton, 2004), pp. 421-428 PubMed Google Scholar
-
27.T.H. Harvard, Three of the B vitamins: folate, vitamin B6, and vitamin B12 (Harvard TH Chan School of Public Health, Boston, MA, 2018) PubMed Google Scholar
-
28.J.M. Olmedo, J.A. Yiannias, E.B. Windgassen, M.K. Gornet, Int. J. Dermatol. 45, 909-913 (2006) CrossRef PubMed Google Scholar
-
29.M.B. Demay, Arch. Biochem. Biophys. 523, 19-21 (2012) CrossRef PubMed Google Scholar
-
30.R. Bouillon, G. Carmeliet, L. Verlinden, E. van Etten, A. Verstuyf, H.F. Luderer, L. Lieben, C. Mathieu, M. Demay, Endocr. Rev. 29, 726-776 (2008) CrossRef PubMed Google Scholar
-
31.V. Vegesna, J. O'Kelly, M. Uskokovic, J. Said, N. Lemp, T. Saitoh, T. Ikezoe, L. Binderup, H.P. Koeffler, Endocrinology 143, 4389-4396 (2002) CrossRef PubMed Google Scholar
-
32.L.A. Beoy, W.J. Woei, Y.K. Hay, Trop. Life Sci. Res. 21, 91-99 (2010) PubMed Google Scholar
-
33.M. Moeinvaziri, P. Mansoori, K. Holakooee, Z. Safaee Naraghi, A. Abbasi, Acta Dermatovenerol. Croat. 17, 279-284 (2009) CrossRef PubMed Google Scholar
-
34.D.H. Rushton, Clin. Exp. Dermatol. 27, 396-404 (2002) CrossRef PubMed Google Scholar
-
35.L.B. Trost, W.F. Bergfeld, E.J. Calogeras, Am. Acad. Dermatol. 54, 824-844 (2006) CrossRef PubMed Google Scholar
-
36.S.A. Pierre, G.M. Vercellotti, J.C. Donovan, M.K. Hordinsky, J. Am. Acad. Dermatol. 63, 1070-1076 (2010) CrossRef PubMed Google Scholar
-
37.H. Rushton, I.D. Ramsay, Clin. Endocrinol. 36, 421-427 (1992) CrossRef PubMed Google Scholar
-
38.E. Alhaj, N. Alhaj, N.E. Alhaj, SKINmed 6, 199-200 (2007) CrossRef PubMed Google Scholar
-
39.A.E. Slonim, N. Sadick, M. Pugliese, C.H. Meyers-Seifer, J. Pediatr. 121, 890-895 (1992) CrossRef PubMed Google Scholar
-
40.M.S. Kil, C.W. Kim, S.S. Kim, Ann. Dermatol. 25, 405-409 (2013) CrossRef PubMed Google Scholar
-
41.P.M. Plonka, B. Handjiski, M. Popik, D. Michalczyk, R. Paus, Exp. Dermatol. 14, 844-853 (2005) CrossRef PubMed Google Scholar
-
42.J.M. Bates, V.L. Spate, J.S. Morris, D.L. Germain, V.A. Galton, Endocrinology 141, 2490-2500 (2000) CrossRef PubMed Google Scholar
-
43.J.H. Upton, R.F. Hannen, A.W. Bahta, N. Farjo, B. Farjo, M.P. Philpott, J. Investig. Dermatol. 135, 1244-1252 (2015) CrossRef PubMed Google Scholar
-
44.C. Pierard-Franchimont, G.E. Pierard, Dermatology 203, 115-117 (2001) CrossRef PubMed Google Scholar
-
45.S.S. Khatu, Y.E. More, N.R. Gokhale, D.C. Chavhan, N.J. Bendsure, Cutan. Aesthet. Surg. 7, 107 (2014) CrossRef PubMed Google Scholar
-
46.M.A. Gkini, A.E. Kouskoukis, G. Tripsianis, D. Rigopoulos, K. Kouskoukis, J. Cutan. Aesthet. Surg. 7, 213 (2014) CrossRef PubMed Google Scholar
-
47.J.S. Kang, Z. Zheng, M.J. Choi, S.H. Lee, D.Y. Kim, S.B. Cho, J. Eur. Acad Dermatol. Venereol. 28, 72-79 (2014) CrossRef PubMed Google Scholar
-
48.A. Trink, E. Sorbellini, P. Bezzola, L. Rodella, R. Rezzani, Y. Ramot, F. Rinaldi, Br. J. Dermatol. 169, 690-694 (2013) CrossRef PubMed Google Scholar
-
49.P. Gentile, S. Garcovich, Int. J. Mol. Sci. 21, 2702 (2020) CrossRef PubMed Google Scholar
-
50.T.C. Wikramanayake, R. Rodriguez, S. Choudhary, L.M. Mauro, K. Nouri, L.A. Schachner, J.J. Jimenez, Lasers Med. Sci. 27, 431-436 (2012) CrossRef PubMed Google Scholar
-
51.P. Avci, A. Gupta, M. Sadasivam, D. Vecchio, Z. Pam, N. Pam, M.R. Hamblin, Semin. Cutan. Med. Surg. NIH Public Access 32, 41 (2013) PubMed Google Scholar
-
52.E. Mester, G. Ludany, M. Sellyei, B. Szende, G. Gyenes, G. Tota, J. Langenbecks Arch. Chir. 322, 1022-1027 (1968) CrossRef PubMed Google Scholar
-
53.M. Leavitt, G. Charles, E. Heyman, D. Michaels, Clin. Drug Investig. 29, 283-292 (2009) CrossRef PubMed Google Scholar
-
54.R.R. Marinho, R.M. Matos, J.S. Santos, M.A. Ribeiro, R.A. Ribeiro, R.C. Lima, R.L. Albuquerque, S.M. Thomazzi, Lasers Med. Sci. 29, 239-243 (2014) CrossRef PubMed Google Scholar
-
55.S. Shukla, K. Sahu, Y. Verma, K.D. Rao, A. Dube, P.K. Gupta, Skin Pharmacol. Physiol. 23, 79-85 (2010) CrossRef PubMed Google Scholar
-
56.J.L. Satino, M. Markou, Int. J. Cosmet. Surg. Aesthet. Dermatol. 5, 113-117 (2003) CrossRef PubMed Google Scholar
-
57.H.W. Lee, J.H. Jun, J.A. Lee, H.J. Lim, H.S. Lim, M.S. Lee, BMJ Open 5, e008841 (2015) CrossRef PubMed Google Scholar
-
58.Q. Zhu, F. Wu, J. Acupunct. Tuina Sci. 9, 162-164 (2011) CrossRef PubMed Google Scholar
-
59.H.J. Yoon, J. Korean Med. Ophthalmol. Otolaryngol. Dermatol. 27, 162-170 (2014) CrossRef PubMed Google Scholar
-
60.I.C. Hay, M. Jamieson, A.D. Ormerod, Arch. Dermatol. Res. 134, 1349-1352 (1998) PubMed Google Scholar
-
61.S. Murugusundram, J. Cutan. Aesthet. Surg. 2, 31-32 (2009) CrossRef PubMed Google Scholar
-
62.M. Abe, Y. Ito, A. Suzuki, S. Onoue, H. Noguchi, S. Yamada, Anal. Sci. 25, 553-557 (2009) CrossRef PubMed Google Scholar
-
63.P. Pais, A. Villar, S. Rull, Res. Rep. Urol. 8, 41-49 (2016) PubMed Google Scholar
-
64.J.O. Mccoy, C.R. Ziering, Int. J. Life Sci. Pharm. Res. 2, 31-38 (2012) PubMed Google Scholar
-
65.S.M. Chacko, P.T. Thambi, R. Kuttan, I. Nishigaki, Chin. Med. 5, 1-9 (2010) CrossRef PubMed Google Scholar
-
66.A. Jigisha, R. Nishant, K. Navin, G. Pankaj, Int. Res. J. Pharm. 3, 139-148 (2012) PubMed Google Scholar
-
67.S. Farooq, A. Sehgal, Curr. Res. Nutr. Food Sci. J. 6, 35-40 (2018) CrossRef PubMed Google Scholar
-
68.P.K. Jain, D.E. Das, C.H. Das, Innov. J. Med. Sci. 5, 25-33 (2017) PubMed Google Scholar
-
69.B. Lestari, E. Meiyanto, Indones. J. Cancer Chemoprev. 9, 92-101 (2018) CrossRef PubMed Google Scholar
-
70.D. Montesano, F. Blasi, M.S. Simonetti, A. Santini, L. Cossignani, Foods 7, 30 (2018) CrossRef PubMed Google Scholar
-
71.M.R. Al-Sereiti, K.M. Abu-Amer, P. Sena, Indian J. Exp. Biol. 37, 124-131 (1999) PubMed Google Scholar
-
72.Y. Panahi, M. Taghizadeh, E.T. Marzony, A. Sahebkar, Skin Med. 13, 15-21 (2015) PubMed Google Scholar
-
73.J. Garavaglia, M.M. Markoski, A. Oliveira, A. Marcadenti, Nutr. Metab. Insights 9, 59-64 (2016) PubMed Google Scholar
-
74.R. Kaur, H. Kaur, A.S. Dhindsa, Int. J. Pharm. Sci. Res. 4, 2470 (2013) PubMed Google Scholar
-
75.U. Sukirti, S. Vijender, Asian J. Pharm. Clin. Res. 6, 52-55 (2013) PubMed Google Scholar
-
76.K.E. Sharquie, H.K. Al-Obaidi, J. Dermatol. 29, 343-346 (2002) CrossRef PubMed Google Scholar
-
77.Y. Panahi, M. Taghizadeh, E.T. Marzony, A. Sahebkar, SKINmed 13, 15-21 (2015) PubMed Google Scholar
-
78.N. Prager, K. Bickett, N. French, G. Marcovici, J. Altern. Complement. Med. 8, 143-152 (2002) CrossRef PubMed Google Scholar
-
79.V. Wessagowit, C. Tangjaturonrusamee, T. Kootiratrakarn, T. Bunnag, T. Pimonrat, N. Muangdang, P. Pichai, Australas. J. Dermatol. 57, e76-e82 (2016) CrossRef PubMed Google Scholar
-
80.B.E. Carbin, B. Larsson, O. Lindahl, Br. J. Urol. 66, 639-641 (1990) CrossRef PubMed Google Scholar
-
81.A. Kamimura, T. Takahashi, Y. Watanabe, Phytomedicine 7, 529-536 (2000) CrossRef PubMed Google Scholar
-
82.T. Takahashi, A. Kamimura, M. Kagoura, M. Toyoda, M. Morohashi, J. Cosmet. Dermatol. 4, 245-249 (2005) CrossRef PubMed Google Scholar
-
83.Z. Hajheydari, M. Jamshidi, J. Akbari, R. Mohammadpour, Indian J. Dermatol. Venereol. Leprol. 73, 29-32 (2007) CrossRef PubMed Google Scholar
-
84.N. Harada, K. Okajima, M. Arai, H. Kurihara, N. Nakagata, Growth Horm. IGF Res. 17, 408-415 (2007) CrossRef PubMed Google Scholar
-
85.N. Harada, K. Okajima, M. Arai, H. Kurihara, N. Nakagata, Growth Horm. IGF Res. 18, 335-344 (2008) CrossRef PubMed Google Scholar
-
86.T.W. Fischer, U.C. Hipler, P. Elsner, Int. J. Dermatol. 46, 27-35 (2007) PubMed Google Scholar
-
87.H. Hertel, H. Gollnick, C. Matthies, I. Baumann, C.E. Orfanos, Hautarzt 40, 490-495 (1989) PubMed Google Scholar
-
88.P. Morganti, G. Fabrizi, B. James, C. Bruno, J. Appl. Cosmetol. 16, 57-64 (1998) PubMed Google Scholar
-
89.https://www.drugbank.ca/drugs/DB14360. Accessed 4 Mar 2020 PubMed Google Scholar
-
90.P. Ravva, M.R. Gastonguay, H.M. Faessel, T.C. Lee, R. Niaura, Nicotine Tob. Res. 17, 106-113 (2015) CrossRef PubMed Google Scholar
-
91.https://www.medicalnewstoday.com/articles/266370. Accessed 6 May 2020 PubMed Google Scholar
-
92.https://soulflower.biz/products/soulflower-rosemary-essential-oil-15-mL. Accessed 6 May 2020 PubMed Google Scholar
-
93.https://www.drugs.com/mtm/grape-seed.html. Accessed 6 May 2020 PubMed Google Scholar
-
94.
-
95.https://www.webmd.com/vitamins/ai/ingredientmono-881/licorice. Accessed 8 May 2020 PubMed Google Scholar
-
96.SoulTree-Licorice-Shampoo-Hibiscus-Conditioner/dp/B072HWDVDW. Accessed 8 May 2020 PubMed Google Scholar
-
97.S. Lindquist, M. Stangel, Neuropsychiatr. Dis. Treat. 7, 341-349 (2011) CrossRef PubMed Google Scholar
-
98.Clinicaltrials. https://clinicaltrials.gov/ct2/home PubMed Google Scholar
Copyright information
© The Author(s) 2020
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.