The Genus Solanum: An Ethnopharmacological, Phytochemical and Biological Properties Review
Abstract
Over the past 30 years, the genus Solanum has received considerable attention in chemical and biological studies.Solanum is the largest genus in the family Solanaceae, comprising of about 2000 species distributed in the subtropical and tropical regions of Africa, Australia, and parts of Asia, e.g., China, India and Japan.Many of them are economically significant species.Previous phytochemical investigations on Solanum species led to the identification of steroidal saponins, steroidal alkaloids, terpenes, flavonoids, lignans, sterols, phenolic comopunds, coumarins, amongst other compounds.Many species belonging to this genus present huge range of pharmacological activities such as cytotoxicity to different tumors as breast cancer (4T1 and EMT), colorectal cancer (HCT116, HT29, and SW480), and prostate cancer (DU145) cell lines.The biological activities have been attributed to a number of steroidal saponins, steroidal alkaloids and phenols.This review features 65 phytochemically studied species of Solanum between 1990 and 2018, fetched from SciFinder, Pubmed, ScienceDirect, Wikipedia and Baidu, using "Solanum" and the species'names as search terms ("all fields").Keywords
Solanum Solanaceae Phytochemistry Steroidal saponins and alkaloids EthnopharmacologyAbbreviations
1 Introduction
The genus Solanum is considered to be one of the largest and most complex genera among the Angiosperms [1], and the most representative and largest genus of the family Solanaceae [1-4]. It is comprised of about 2000 species distributed across subtropical and tropical regions of Asia [3-9], tropical Africa [10-29], non-arid Africa [30-43], Americas [44-87], Australia [71-74, 81-84] and India [71]. The genus is well represented in Brazil with about 350 species widely distributed from north to south in diverse phytogeographic regions [70, 80]. In Brazil (Ceará, Bahia, Mato Grosso do Sul, Paraná and north-central coast of Santa Catarina State), many Solanum species, usually known as 'yubeba', the word that refers to the prickles found on the stems of several of the species, are widely used in traditional medicine [66, 80, 87]. In the northeast of Brazil, 80 Solanum species are distributed throughout the region and used in folk medicine. One of such species is S. capsicoides, commonly known as "Gogoia" [87]. In East Africa, several Solanum species such as S. arundo and S. incanum are known to be poisonous and are reportedly used to induce miscarriages [64].
Solanum genus is rich in economically significant species; the food crops include S. aethiopicum [20, 21], S. anguivi [30, 31] S. lycopersicum, S. melongena, S. muricatum, S. torvum and S. tuberosum [1]. Ornamental species include S. aviculare, S. capsicastrum, S. crispum, S. laciniatum, S. laxum, S. pseudocapsicum, S. rantonnetii, S. seaforthianum and S. wendlandii [1].
A series of pharmacological studies have been carried out to verify and validate the traditional medicinal applications of many plants in this genus. The studied pharmacological activities include analgesic, anthelminthic, antiallergic, anti-anemic, anti-asthmatic, antibacterial, anti- cancer, anti-convulsant, anti-depressant, anti-diabetic, anti-fungal, antihistaminic, antihyperten- sive, anti-inflammatory, anti-leishmanial, antimelanogenetic, anti-molluscicidal, anti-nociceptive, anti-psoriatic, antiplasmodial, antiprotozoa, anti-trypanosomal, antiurolithiatic, antiviral, cardio- vascular, diuretic, hepatoprotective, hypolipidemic, mosquito larvicidal, nephrotoxic, spasmolytic, schistosomicidal and vasorelaxant activities.
In the past, several reviews on Solanum genus have been documented [88-101], however, mostly with singular focus on particular species. The present review is multi faceted, and features 66 medicinal species of Solanum in their geographical distribution, traditional uses, and 670 isolated chemical constituents, including 134 steroidal saponins, 63 steroidal alkaloids, 13 pregnane glycosides, 128 terpenes, 75 flavonoids, 31 lignans, 31 other types of alkaloids, 66 sterols, 52 phenolic compounds, 20 coumarins and coumestans, 4 coumarinolignoids, 23 fatty acids and esters and 30 other compounds. Where applicable, the biological activities of compounds isolated from various species are noted.
2 Distribution and Ethnopharmacological Uses
Sixty-six species commonly used as important folk medicine, ornamental plants, or wild food sources were selected in this review, and their local names, distribution and ethnopharmacologi- cal uses were summarized in Table 1. Local names are given in different languages with which the inhabitants of a particular region use to identify a specific species. Each species' natural habitat and/or places of cultivation are mentioned. Traditional as well as modern day applications are presented.
Distribution and ethnopharmalogical uses of Solanum species
3 Chemical Constituents and Their Biological Properties
At least 670 compounds, including 134 steroidal saponins (1-134), 63 steroidal alkaloids (135-197), 13 pregnane glycosides (198-210), 128 terpenes (211-338), 72 flavonoids (339-413), 31 lignans (414-444), 31 other types of alkaloids (445-475), 66 sterols (476-541), 52 phenols (542-593), 20 coumarins and coumestans (594-613), 4 coumarinolignoids (614-617), 23 fatty acids and esters (618-640) and 30 other compounds (641-670) were reported from the genus Solanum. Most of them were investigated for various biological activities. The chemical constituents and their biological properties are presented in Table 2, together with their plant sources and parts, alongside the classification of structures.
Phytochemistry, biological properties and classification of Solanum compounds
3.1 Steroidal Saponins
Steroidal saponins are prominent characteristic components in Solanum species, from which 134 compounds, 1-134, have been obtained (Fig. 1). Among all the studied species, S. torvum was the one studied mostly, resulting in the isolation of 32 saponins including chlorogenone (1), (5α, 25S)-spirostan-3, 6-dione (2), diosgenone (13), 56-72, neochlorogenin (73), solanolactosides A-C (91-93), torvosides J-L (95-97) and 98-102 from the leaves, fruits, aerial parts and the whole plant [323, 325, 430, 435, 436, 448, 449, 451, 452, 463].
Steroidal saponins 1-134 from Solanum
Included herein are spirostane saponins, SC1-SC6 (35-40), isolated from the leaves of S. chrysotrichum [113, 114, 115, 117], and lyconosides Ⅰa (46), Ⅰb (47), Ⅱ (48), Ⅲ (49), and Ⅳ (50) reported from the fruits of S. lycocarpum. Indiosides G (82) and H (83) with an iso-type F ring were isolated from the methanolic extract of the whole plant of S. violaceum, together with indioside Ⅰ (86), and two unusual furostanol saponins with a deformed F ring, indiosides J (87) and K (88) [391, 392]. In addition, four steroidal sapogenins, indiosides L-O (78-81) were also obtained from this plant [391]. Indioside L (78) is a rare spirostanoid possessing a 1, 4-dien-3-one moiety in ring A. Compounds 80 and 81 represent rare examples of spirostane with the 3β, 7α-diol-5, 6-ene moiety compared to the normal 3β, 7β-diol-5, 6-ene derivatives [391].
Two C-22 steroidal lactone saponins, namely solanolactosides A, B (91, 92) and two spirostanol glycosides, torvosides M, N (23, 8) were isolated from ethanol extract of aerial parts of S. torvum. Compounds 91 and 92 possess the aglycon of solanolide (94), while 23 and 8 have the aglycons of yamogenin (76) and neochlorogenin (73), resp. The aglycon of 94 is an unusual C-22 steroidal lactone sapogenin [316].
An avenacoside-type saponin (51) was isolated from aerial parts of S. surattense [305]. Two 23-keto-spirostanol glycosides, torvoside Q (18) and paniculonin B (126) were obtained from aerial parts of S. torvum [323, 331]. Torvosides A (64), B (65), F (67) and G (112) displayed a positive reaction with Ehrlich reagent, suggesting these to be furostanol glycosides [449]. Abutilosides L (106), M (107) and N (108), a 22S, 25S-epoxy-furost-5-ene type glycosides, and abutiloside O, being a 20, 22-seco-type steroidal glycoside, were isolated from the fresh fruits of S. abutiloides [4].
Anguiviosides Ⅲ (118) and Ⅺ (119) are hydroxylated at C-23 and C-26 on the spirostanol and furostanol skeletons, resp. Anguiviosides XV (120) and XVI (121) are based on a 16, 22-dicarbonyl aglycon, with 121 hydroxylated at C-23 and C-26 followed by ring closure. The biogenetic pathway of 16, 22-dicarbonyl compounds such as 120 and 121 might be considered via a 17R-hydroxy spirostanol such as pennogenin, 11 or via a 3β, 16β, 22, 26-tetrahydroxycholesterol glycoside such as anguivioside A (114) [43].
Solanum saponins were reported to have various bioactivies, e.g. cytotoxic [257], anticancer [316, 317, 392], hepatoprotective [242, 247], antihypertensive [289, 291], antimelanogenesis [211], antifungal [113, 114, 117], anti-inflammatory [331, 392, 448] anticonvulsant [305] and antiviral [257].
Nuatigenosido (15) from the roots of S. sisymbriifolium presented anti-hypertensive effect in experimental hypertensive rats [291]. Dioscin (19) showed antimelanogenesis effect on α-melanocyte stimulating hormone (α-MSH)induced melanogenesis in B16 murine melanoma cells. It significantly downregulated the expression of tyrosinase, TRP-1, and TRP-2, which led to the reduction of α-MSH-induced melanogenesis in B16 cells [211]. Degraded diosgenone (13) from S. nudum exhibited hepatoprotective effect on the liver of mice infected with Plasmodium berghei; necrosis of hepatocytes in mice infected with malaria decreased 47-65 [249].
Spirostanic saponins SC2-SC6 (36-40) from the leaves of S. chrysotrichum displayed activity against dermatophytes and yeasts. 36 was the most active in indicating fungicidal effect against Candida albicans and non-albicans strains [113, 114, 117].
Indioside H (83), borassoside E (85), indioside Ⅰ (86) and yamoscin (89) demonstrated cytotoxic activity against six human cancer cell lines (HepG2, Hep3B, A549, Ca9-22, MDA-MB-231, and MCF-7) (IC50=1.83-8.04 μg/mL) [392]. Seperately, 85 and 86 presented inflammation inhibitory effects on SAG (IC50=0.62±0.03 and 2.84±0.18 μg/mL, resp.). Compound 85 also inhibited elastase release with IC50 values of 111.05±7.37 μg/mL [392], while 89 showed anti-neutrophilic inflammatory activity against SAG with an IC50 value of 3.59 μM [331].
Torvosides N (8) and M (23) revealed significant cytotoxicity against MGC-803, HepG2, A549 and MCF-7 as compared to the positive control, CDDP [316]. Torvosides J-L (95-97), isolated from the leaves of S. torvum, exhibited substantial anticonvulsant activity in zebrafish seizure assays [323], while 96 also showed considerable antifungal activity against Aspergillus flavus and Fusarium verticillioides with MIC ranging from 31.25 to 250 μg/mL [318]. Compounds 99 and 100 inhibited both inflammatory mediators SAG (IC50=3.49 and 2.87 μM) and elastase release (IC50=2.69 and 0.66 μM) [331], while 123-125 convinced cytotoxicities against melanoma A375 [317].
3.2 Steroidal Alkaloids
Sixty-three steroidal alkaloids (135-197), as other principal components in Solanum were reported from this genus (Fig. 2). Compounds 139-156 are derivatives of solasodine (145), one of the main glycoalkaloid constituents in Solanum spp., even as indicated by several numbers of species from which it has been isolated. Solamargine (139) is the major steroidal alkaloid constituent of Solanum plants and literature data showed that it has been revealed in 18 species.
Steroidal alkaloids 135-197 from Solanum
Compounds such as 139, solasonine (142), β1-solasonine (143) and solanigroside P (156) with three sugar units and α-L-rhamnose at C-2 or a hydroxyl group on the steroidal backbone may be potential candidates for the treatment of gastric cancer [228].
Featured here are steroidal pseudoalkaloid oligoglycosides, robeneosides A (153) and B (154) and lobofrutoside (155) from the fruits of S. lycocarpum [182, 447], and a rare 16β-H steroidal alkaloid (157) from aerial parts of S. surattense [305]. Also included are leptinine Ⅰ (171) and Ⅱ (172), the solanidane alkaloid glycosides, isolated from aerial parts of S. orbignianum [46].
Two rare C-3 amino steroidal alkaloids, 188 and 189, were isolated from aerial parts of S. triste [362, 471]. Three C-27 steroidal glycoalkaloids, spiralosides A (194), B (193), C (192), were obtained from the fruits of S. spirale [474]. Esculeoside A (197), a tomato saponin, is a significant component of ripened tomatoes isolated by Toshihiro et al. [475].
Various bioactivities e.g. antibacterial [80, 384, 403, 406, 407], anticancer [13, 305, 458], antidiabetic [182, 183], antifungal [279], anti-inflammatory [303], CNS depressant [294], leishmanicidal [182, 183], molluscicidal [384, 403, 406, 407], neurotoxicity [106], schistosomicidal [185, 186, 447, 457], spasmolytic [70] and trypanocidal [185, 186, 447, 457] were highlighted as have been exhibited by steroidal alkaloids of Solanum.
Antioxidant activity of 145 and tomatidine (167) from the berries of S. aculeastrum was investigated using DPPH, ABTS and reducing power assays, and the highest inhibition was observed when the two compounds were combined, followed by 145 and 167 [13]. Furthermore, 145 exhibited significant anti-inflammatory activity at doses of 30 mg/kg, with a maximum inhibition of 77.75% in carrageenan-induced rat paw edema, comparing to indomethacin (81.69%). It also showed stronger (46.79effect in xylene induced ear edema in mice [303]. Intraperitoneal injection of 145 (25 mgkg) significantly delayed latency of hind limb tonic extensor phase in the picrotoxin-induced convulsions, and it also potentiated thiopental-provoked sleep in a dose-dependent manner [294]. Moreover, 145 exhibited not only the antibacterial activity against Klebsiella and Staphylococcus spp. at concentration of 1 mg, together with 139 and 141 [403], but also a potent stemness and invasion inhibitory effect on human colorectal cancer HCT116 cells [155]. Colony Spheroid formation assay showed that solasodine dose-dependently prohibited HCT116 cell stemness. CD133, CD44, Nanog, Oct-4 and Sox-2 were inhibited by 145 to reverse stemness and similar mechanism was stimulated in vivo. Transwell and scratch wound assays revealed that 145 impeded HCT116 cell invasion and migration potential strengthened by TGF-β1. Moreover, solasodine attenuated TGF-β1-induced EMT and decreased MMPs while in vivo study showed the same trend. The results of this study implied that 145 may be a novel therapeutic drug for CRC treatment [155].
Burger et al. documented that the crude extract and aqueous fraction containing 139 displayed potent non-selective cytotoxicity (IC50 15.62 μgmL) and noteworthy 9.1-fold P-glycoprotein inhibition at 100 μgmL [15]. Zhang et al. assessed the molecular mechanism underlying the anti-cancer effect of 139 in human cholangiocarcinoma QBC939 cells. The results revealed that 139 inhibited the viability of QBC939 cells in a dose-dependent manner. Furthermore, it significantly induced the apoptosis of QBC939 cells and altered the mitochondrial membrane potential of cells. Quantitative polymerase chain reaction analysis revealed that 139 decreased the mRNA level of B cell lymphoma-2 (Bcl-2) Bcl-extra-large and X-linked inhibitor of apoptosis protein but increased the mRNA level of Bcl-2-associated X protein (Bax) In addition, western blot analysis demonstrated that 139 inhibited the protein expression of Bcl-2 and poly ADP ribose polymerase (PARP) and promoted the protein expression of Bax, cleaved PARP, caspase 3, cleaved caspase 3 and caspase [97].
Compounds 139, 141 and 157 demonstrated cytotoxicity against A549, whereas 139 and 156 showed cytotoxicity against HepG2 cell lines [305]. Compounds 139 and 141 were confirmed as the effective components for Oncomelania snail control. The death rate of Oncomelania snails was 94.2 at a concentration of 2.50 mg/L (139) [406], while 141 exhibited a lethality of 100against O. hupensis [407]. Moreover, 139 and solasonine (142) displayed not only leishmanicidal activity against promastigote forms of Leishmania amazonensis [185], but also antidiabetic activity by inhibiting the serum glucose increase in oral sucrose-loaded rats and suppressing gastric emptying in mice [182]. A synergistic effect was observed for a mixture of the compounds [183]. Compound 139 also expressed stronger trypanocidal activity (IC50=15.3 μg/mL), when compared to benznidazol (IC50=9.0 μg/mL), the only drug used to treat Chagas' disease [186].
Tomatine (168) was illustrated to exert significant neuroprotective effect on H2O2-induced SH-SY5Y cells, by enhancing intracellular anti-oxidant enzyme activity and brain-derived neurotrophic factor expression and restraining H2O2-induced oxidative stress [106]. Isojuripidine (190) displayed spasmolytic activity by hindering phasic contractions induced by both histamine and acetylcholinein guinea-pig ileum [69].
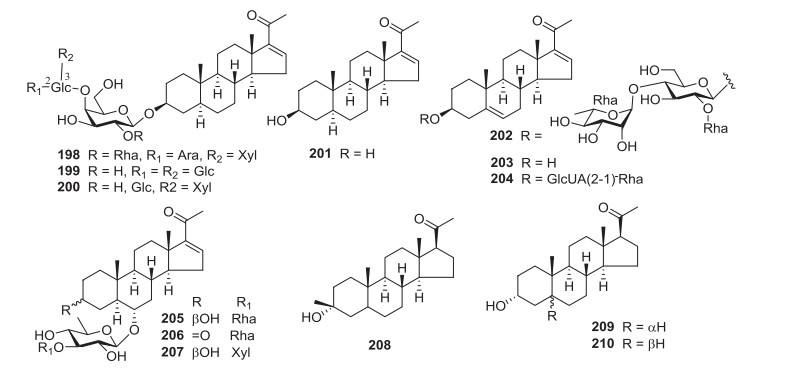
3.3 Pregnane Glycosides
Compounds 198-210 from Solanum comprise pregnane glycosides (Fig. 3). These compounds coexist in small amounts and could be biosynthesised from steroidal glycosides [194]. Solanigrosides A (198), B (199), 200 and hypoglaucin H (202) were isolated from S. nigrum [476]. Aerial parts of S. torvum gave the highest number of pregnane glycosides, torvpregnanosides A (205) and B (207), ganaxolone (208), allopregnanolone (209) and pregnanolone (210). The whole plant of S. lyratum afforded compounds 203 and 204 [194].
Pregnane glycosides 198-210 from Solanum
Pregnane glycosides have reportedly demonstrated anticancer properties [194, 317]. Compound 203 exhibited substantial cytotoxic activity against A375-S2, HeLa, SGC-7901, and Bel-7402 cell lines, with IC50 values of 13.1 to 49.8 μg/mL [194]. Compound 206 indicated cytotoxicity against human melanoma A375 (IC50=39.66 μM) [317].
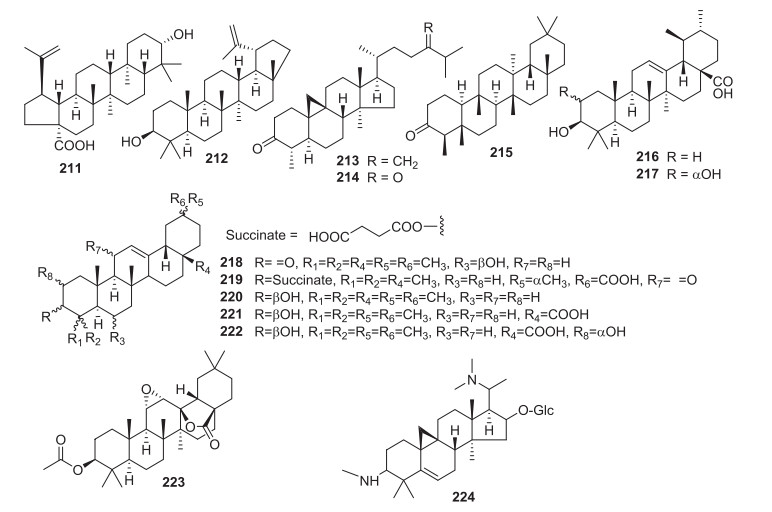
3.4 Triterpenes
Fourteen triterpenes (211-224) were identified in Solanum spp. (Figure 4), with lupeol (212) from S. cathayanum [472, 473, 477], S. schimperianum [278], S. spirale [297] and ursolic acid (216) from S. lyratum [197], S. torvum [463] and S. xanthocarpum [427], as the major ones. Six triterpenes 216-217 and 221-224 were reported from the aerial parts of S. torvum [314, 463]. Two cycloartane triterpenoids, cycloeucalenone (213) and 24-oxo-31-norcycloartanone (214) are the main constituents of S. cernuum leaves [107]. Daturaolone (218) was isolated for the first time from S. arundo [65].
Triterpenoids 211-224 from Solanum
Solanum triterpenes have indicated to possess anticancer properties. For instance, 213 presented significant activity against KB-Oral cavity cancer (IC50=26.73 μgmL) [297], while 213 exhibited selective activity against lung tumor cell line (NCIH460). The anti-nociceptive activity observed for 213 and 214 was found to be related to the inhibition of different mediators involved in inflammation and nociceptive process. Both compounds decreased cyclooxygenase 2 (COX-2) protein expression, although only 214 reached a significant response (P < 0.05 vs control) [107].
3.5 Diterpenes
Four diterpenes, e.g., phytol (225) from S. pseudocapsicum [263], kaur-16-ene (226) from S. aculeastrum [11], solanerioside A (227) from S. erianthum [138], and tricalysioside U (228) from S. violaceum [392] were reported from Solanum spp. (Figure 5). Solanerioside A (227) was the first example of a diterpenoid glucoside featuring a 14, 15-dinor-cyclophytane scaffold [138].
Diterpenes 225-228 from Solanum
3.6 Sesquiterpenes
Sesquiterpenes, 229-310, have been characterized from Solanum spp. (Figure 6). Majority of these compounds, 260-282, were from S. lyratum [196, 197, 199, 200, 484, 485, 486] and S. septemlobum [281, 482, 483]. Likewise, 283-285 and 298-303 were reported from S. septemlobum [281, 482, 483]. Compounds 229-231 and 245-255 were isolated from the leaves and fruits of S. erianthum [138, 481], while 286-293 were from the roots of S. torvum [487]. Compounds 236-239 were isolated from the roots of S. aethiopicum [29], while 240-242 were obtained from the leaves of S. aculeastrum [11]. The fruits of S. betaceum yielded compounds 306-310 [77].
Sesquiterpenes 229-310 from Solanum
The bioactivities notedly displayed by sesquiterpenes include anticancer [197, 198, 199, 200, 281, 484] and antifungal [3]. 3-β-Hydroxysolavetivone (232), solavetivone (233) and lubimin (235) from the roots of S. abutiloides exhibited anti-fungal activities against Fusarium oxysporum f. sp. Melongenae [3]. The eudesmane-type, solajiangxin D (276), and vetispirane-type, solajiangxin E (277) from S. lyratum demonstrated crucial cytotoxicities (ED50=2.1-3.7 μg/mL) against three human cancer lines (P-388, HONE-1, and HT-29) [200]. Solajiangxin B (258), A (274) and C (275) from the whole plant of S. lyratum [198] and Septemlobin D (259), and 11, 12-O-isopropylidene solajiangxin F (298) [483] also showed significant cytotoxicities (ED50=1.9-3.7, and 3.0-7.3 μM, resp.) against these three cancer cell lines. Lyratol D (257), blumenol A (260), dehydrovomifoliol (262) and lyratol C (272) from the whole plant of S. lyratum displayed critical cytotoxic activities against HONE-1 nasopharyngeal, KB oral epidermoid carcinoma, and HT29 colorectal carcinoma cells (IC50=3.7-8.1 μM) [199].
Eudesmane-related sesquiterpenes, septemlobins A (301) and B (302) and vetispirane-type, septemlobin C (303) exhibited significant cytotoxicities against three cancer cell lines (P-388, HONE-1, and HT-29) (IC50=3.8-7.5 mΜ) [281].
3.7 Monoterpenes
Twenty-eight monoterpenes (311-338) have been characterized from Solanum spp. (Fig. 7), with β-Ionone (320) reported from S. aculeastrum [11], S. pseudocapsicum [263] and S. betaceum [77], and loliolide (323) obtained from S. erianthum [137], S. americanum [49] and S. pseudocapsicum [263], as dominant monoterpenes. Majority of the compounds, 316-318 and 324-333 [468, 489, 490, 491, 492], were obtained from the fruits of S. vestissimum. Hotrienol (324), with very sweet and flowery flavor is a well-known constituent of the leaf oil of Cinnamomum camphora. It has also been found in a large number of other natural tissues, such as tea, grapes, wines passion fruit, elderberry flowers, Achillea ligustica and papaya fruit [468]. Seven monoterpenes, 311-313 and 319-322 were reported from the leaves of S. aculeastrum [11], and glycosides 329-332 were the aroma precursors in S. vestissimum fruit peelings [468, 492].
Monoterpenes 311-338 from Solanum
3.8 Flavonoids
Seventy-two flavonoids 339-413 have been identified in the genus Solanum (Fig. 8), with quercetin (340) and kaempferol (351) as the primary flavonoids. Several glycosylated flavonoids, e.g., afzelin (344), astragalin (346), kaempferol 3-O-[apiofuranosyl-(1→2)]- α-rhamnoside (347) and -β-galactoside (348) from S. cernuum [501], and camelliaside C (352) from S. erianthum [137] were obtained. Five kaempferol derivatives 373-377 were reported from S. elaeagnifolium [502]. Moreover, three anthocyanins 361-363 were isolated from the red and purple tubers of S. tuberosum [508], while five anthocyanin rutinosides 364-368 were reported from the fruits of S. betaceum [75, 76]. Anthocyanins are the largest group of water-soluble pigments in the plant kingdom. They are responsible for most red and blue colours in fruits, vegetables, and have been used in the food industry as pigments, owing to their bright attractive colours, high water solubility and associated health benefits [76]. In addition, diverse flavonoids, such as 388-397 from S. jabrense [167] and S. palodusum [513] and 399-403 from S. lyratum [514] were reported.
Flavonoids 339-413 from Solanum
Flavonoids of Solanum have displayed various biactivities e.g., anticancer [31, 75, 76, 503], anti-depressant and antiviral [322, 332] and hepatoprotective [502] characteristics. Compound 373 exhibited significant hepatoprotective and curative effects against histopathological and histochemical damage induced by paracetamol in liver [502], while 349 and 371 displayed cytotoxicity against breast MCF7 and liver HPG2 cancer cell lines [503].
Compound 340 and rutin (342) indicated potent and concentration-dependent free radical-scavenging activity [45]. They also inhibited peroxidation of cerebral and hepatic lipids subjected to iron oxidative assault. Compound 340 induced in vitro antiproliferative and apoptotic activities on Jurkat cells (IC50=11.77±2.4 mg/mL) [23], while 364-367 showed antioxidant activities [75]. Torvanol A (409) from the roots of S. torvum exhibited antidepressant, anxiolytic and adaptogenic effects [316], as well as anti-HSV-1 activity (IC50=9.6 μgmL) [322].
3.9 Lignans
Lignans, widely distributed in the plant kingdom, are a family of secondary metabolites produced by oxidative dimerization of two phenylpropanoid units. Although their molecular scaffold consists only of two phenylpropane (C6-C3) units, lignans exhibit an enormous structural diversity originating from various linkage patterns of these phenylpropane units. As the C-8-C-3′/C-7-O-C-4′ linked lignans containing two chiral centers (C-7 and C-8) comprise the core of 2, 3-dihydrobenzo[b]furan [480].
Lignans are rare in the genus Solanum [79], with only 31 compounds (414-444) having been isolated (Fig. 9). Compounds 414-419 were obtained from the stems of S. buddleifolium [79], while 424-432, 434 and 442 were isolated from the roots of S. melongena [208, 209, 210]. Several neo-lignans, sisymbrifolin (433) from the fruits of S. sisimbriifolium [519], ficusal (442) from the roots of S. melongena [209], glycosmisic acid (439), simulanol (440) and balanophonin (443) from the whole plant of S. surattense [518] were identified. A pair of new C-8-C-3′/C-7-O-C-4′ linked neolignan enantiomers, 420 and 421, were isolated from the stems of S. erianthum [480]. Lignanamides 424-432 and 434 were obtained from the roots of S. melongena [210].
Lignans 414-444 from Solanum
Among lignans from the genus Solanum, only lignanamides (425-432) were reported with bioactivities. They displayed anti-inflammatory activities by inhibition of nitric oxide production in lipopoly-saccharide-induced RAW 264.7 macrophages (IC50=16.2 to 58.5 μM) [210].
3.10 Other Alkaloids
The alkaloids have a natural (2-aminopyrrolidin-1-yl) carboxamidine alkaloidal base acylated with isoferulic (3-hydroxy-4-methoxycinnamic) acid with Z and E configurations, resp. [111]. Thirty-one alkaloids 445-475 have been isolated from Solanum spp. (Fig. 10), comprising types of cyclic guanidine alkaloids, e.g., cernumidine (446) and isocernumidine (447) from the leaves of S. cernuum [109, 111, 112]. Bioactive long chain amides, 454-456, exhibiting antimicrobial activity against Escherichia coli and Candida albicans were isolated from aerial parts of S. schimperianum [277]. Compounds 472-474 were obtained from S. sessiliflorum [525].
Other alkaloids 445-475 from Solanum
Antidiabetic activity was illustrated by Solanum alkaloids [49, 209]. Four amides, N-trans-p-coumaroyl -octopamine (464) and -tyramine (466), and N-trans-p-feruloyl -octopamine (465) and -tyramine (467) exhibited antidiabetic properties by enhancing α-glucosidase inhibitory activity in a study involving dual high-resolution α-glucosidaseradical scavenging inhibition profiling [35]. Moreover, 459, 466 and 468 demonstrated possession of inhibitory activity against α-glucosidase (IC50=500.6, 5.3 and 46.3 μM, resp.) [209].
3.11 Sterols
Sixty-six sterols (476-541) were obtained from the genus Solanum (Fig. 11), with β-sitosterol (483), daucosterol (484) and stigmasterol (485) as the main sterol constituents. Clistol G (476) and capsisteroids A-F (477-482) were obtained from the leaves of S. capsicoides [85], tumacones A (507) and B (508) and tumacosides A (509) and B (510) were from the leaves of S. nudum [242-247], carpesterol (517) was isolated from the seeds of S. capsicoides [86], and its derivatives (518-521) were reported from the fruits of S. xanthocarpum [401]. From the seeds of S. elaeagnifolium, 491, 495, 496 and 498 were yielded [134]. Additionally, two 26-aminochole- stane-type glycosides, abutilosides A (528) and B (529), and five 26-hydroxycholestane-type glycosides, abutilosides C-G (534-538), were isolated from the fresh roots of S. abutiloides [5-9]. These compounds are important intermediates in the biogenesis of steroidal alkaloids [5].
Sterols 476-541 from Solanum
Sterols in Solanum have indicated possession of anticancer [86], antifungal [401], and antiplasmodial [242, 245, 247] features. For instance, 509 and 510 displayed in vitro antimalarial activity against P. falciparum chloroquine-resistant FCB-1 strain (IC50=27 and 16 μM) [247]. Compounds 511-515 from aerial parts of S. nudum demonstrated antiplasmodial activity on hepatic trophozoites of P. vivax. All the steroids reduced the number of hepatic P. vivax trophozoites. Among them, 506 and 512 reduced the number of hepatic trophozoites by 47and 39resp. [245]. Compound 517 produced antiproliferative activity in glioma (U251), breast (MCF-7), kidney (786-0), ovary (OVCAR-03), and K562 cell lineages [86]. In addition, 505-509 displayed antifungal activity by inhibiting radial growth of A. niger and T. viride [401].
3.12 Phenolic Compounds
Fifty-two phenolic compounds (542-593) were recorded from Solanum (Fig. 12). The fruits of S. crinitum have yielded 552, 561-564 [122]. Aerial parts of S. torvum indicated a great wealth of phenolic compunds, e.g. 558-559, 576, 591-593 [315, 320, 335-337, 521, 524, 533]. The highest numbers of phenols, 542-546, 549-540, 552, 555 and 589 were reported from stems of S. melongena [205] while 574-575 and 577-584 were mentioned from the fruits S. sessiliflorum [525].
Phenolic compounds 542-593 from Solanum
Phenolic compounds in Solanum have displayed antibacterial [297, 320, 335-337, 524], anticancer [31], anti- diabetic [297, 320, 335-337, 524] and antihypertensive [521] activities. Chlorogenic acid (546) (21.90±0.02 mgg), gallic acid (551) (17.54±0.04 mgg) and caffeic acid (555) (16.64±0.01 mgg) have indicated potent and concentration-dependent DPPH radical-scavenging activity (IC50=275.03±7.8 μg/mL) [31], and 551 and 555 reportedly have great potentials as natural source of antidiabetic and antioxidant drug [336]. trans-Cinnamic acid (590) showed antibacterial activities (MIC=250 μg/mL) against Staphylococcus aureus [297], and antimycobacterial activities (inhibition zone=0-22 mm) against Proteus vulgaris, Klebsiella pneumoniae (ESBL-), M. tuberculosis (H37Rv) and M. tuberculosis (Rifampin) [320]. Methyl caffeate (591) not only significantly reduced the cell proliferation, but also increased formation of fragmented DNA and apoptotic body in MCF-7 cells. In this study, Bcl-2, Bax, Bid, p53, caspase-3, PARP and cytochrome c release were detected by western blot analyses [474]. The effects of oral administration of 591 (10, 20 and 40 mgkg) in streptozotocin induced diabetic rats, including body weight, fasting blood glucose, plasma insulin, hemoglobin, glycated hemoglobin, total protein, hepatic glycogen and carbohydrate metabolism enzymes have been studied for 28 days. At 40 mgkg, the compound significantly prevented the increase in blood glucose level after glucose administration at 60 min in comparison to the hyperglycemic control group. It also produced remarkable reductions in blood glucose and increased body weight in streptozotocin induced diabetic rats [335]. Takahashi et al. further established that 591 has a most favorable structure for both sucrase and maltase inhibition against sucrose and that its moderate inhibitory action against alpha-glucosidase provides a prospect for antidiabetic usage of S. torvum fruit [337].
3.13 Coumarins and Coumestans
Seventeen coumarins 594-610 and three coumastans 611-613 were isolated from Solanum spp. (Fig. 13). The seeds of S. indicum yielded the highest number of coumarins 597-598 and 600-604 [535, 536], while coumestans 611-613 were from the whole plant of S. lyratum [88]. Scopolin (594), scopoletin (595) and coumarin (596) are the main coumarins in Solanum. Compounds 611-613 showed in vitro anti-inflammatory activities with IC50 values in the range of 6.3-9.1 μM [88].
Coumarins and coumestans 594-613 from Solanum
3.14 Coumarinolignoids
Four coumarinolignoids known as indicumines A-D (614-617) were obtained from the seeds of S. indicum [535] (Fig. 14). Coumarinolignoids, including cleomiscosins, aquillochins and malloapelins, are unique and rare in nature. Coumarinolignoids of the cleomiscosins type bearing cleomiscosins A-D, 8-epi-cleomiscosin A, and malloapeli A functionalities have been identified in a few genera, including Cleome viscosa, Mallotus apelta, and Rhododendron collettianum. The compounds with such functionalities, especially cleomiscosins A-C and 8-epi-cleomiscosin A, which contributed to biological activities, have been reported with hepatoprotective and tyrosinase inhibition activities [535].
Coumarinolignoids 614-617 from Solanum
3.15 Fatty Acids and Esters
Nine saturated (618-619, 621, 627-628, 631, 634, 638-639) and 13 unsaturated (620, 622-626, 629, 630, 632, 633, 635-637, 640) fatty acids were reported from Solanum (Fig. 15). The whole plant of S. glabratum has yielded the highest number of fatty acid and esters (627-635) in Solanum spp. [140]. Hexadecanoic acid (618), notably the major fatty acid component in Solanum, was isolated from aerial parts of S. aculeastrum [11] S. vestissimum [489] and S. villosum [434, 479].
Fatty acids and esters 618-640 from Solanum
3.16 Others
Thirty other kinds of compounds (641-670) were also obtained from Solanum spp. (Fig. 16). Most of them, 642-653, were from the leaves of S. aculeastrum [11] and 654-659 were yielded from the fruits of S. betaceum [78]. An aldehyde puerariafuran (641) and a cyclic eight-membered α, β-unsataturated ketone, solalyratin B (661) were isolated from the whole plant of S. lyratum [88]. Compounds 641 and 661 showed in vitro anti-inflammatory activities, with IC50 values in the range 6.3-9.1 μM [88]. Also presented here are two furans, ethyl-α-D-arabinofuranoside (660) from the whole plant of S. lyratum and 5-hydroxymethyl furfural (663) from the stems of S. torvum [533]. Five aromatic glycosides (666-670) were also isolated from the aerial part of S. incanum [494] and the fruit of S. lycopersicum [511].
Other compounds 641-670 from Solanum
4 Conclusion and Future Prospects
From 1990 to 2017, phytochemical studies on the 65 Solanum species have yielded at least 670 compounds (134 steroidal saponins, 63 steroidal alkaloids, 13 pregnane glycosides, 128 terpenes, 75 flavonoids, 31 lignans, 31 alkaloids, 66 steroids, 52 phenolic compounds, 20 coumarins and coumestans, 4 coumarinolignoids, 23 fatty acids and esters, and 30 other types of compounds).
Pharmacological studies on Solanum genus have focused on antioxidants and anticancer activities. A total of 17 species (fruits of S. aculeastrum, S. americanum, S. muricatum, S. sessiliflorum and S. spirale, seeds of S. capsicoides, the stems of S. cathayanum and S. tuberosum, the roots of S. diphyllum, aerial parts of S. surattense and S. torvum and the whole plant parts of S. aethiopicum, S. nigrum, S. anguivi, S. septemlobum, S. violaceum and S. xanthocarpum) have been explored for anticancer activities and have exhibited significant results.
S. xanthocarpum has outstandingly demonstrated the most diverse pharmacological activities e.g. antioxidants and antitumor, anti-fungal, anti-bacterial, antileishmanial, mosquito larvicidal, molluscicidal, antidiabetic, asthmatic, hepatoprotective, diuretic, nephrotoxicity, antinociceptive, anti-psoriatic, and antiurolithiatic.
Steroidal alkaloids have been presented as being largely responsible for various pharmacological activities of Solanum species, e.g. antibacterial (139, 141 and 145), anticonvulsant and CNS depressant (145), antidiabetic (139, 142 and 144), anti-fungal (145 and 174), anti-inflammatory (145), antileishmanial (139 and 142), molluscicidal (139 and 141), nephrotoxicity (168), antioxidants and antitumor (139, 141, 145, 158, 168 and 180), antiprotozoa (139 and 142), schistosomicidal (139 and 142), spasmolytic (190) and anti-trypanosomal (139).
The genus Solanum seems to possess great potential, yet majority of the species remain unknown or scantily studied for the chemical constituents. It would be very necessary for the phytochemistry researchers to explore and investigate more of its species. The vast pharmacological activities envinced by many compounds from Solanum genus should attract the attention of the pharmacological community to determine their exact target sites, structure-activity relationships and other medicinal applications.
Notes
Compliance with Ethical Standards
Conflict of interest
The authors declare no conflict of interest.
References
-
1.https://en.wikipedia.org/wiki/Solanum. Accessed 22 Aug 2017 PubMed Google Scholar
-
2.https://en.wikipedia.org/wiki/Solanum_abutiloides. Accessed 22 Aug 2017 PubMed Google Scholar
-
3.T. Yokose, K. Katamoto, S. Park, H. Matsuura, T. Yoshihara, Biosci. Biotechnol. Biochem. 68, 2640-2642 (2004) CrossRef PubMed Google Scholar
-
4.H. Yoshimitsu, M. Nishida, T. Nohara, Phytochemistry 64, 1361-1366 (2003) CrossRef PubMed Google Scholar
-
5.H. Yoshimitsu, M. Nishida, M. Yoshida, T. Nohara, Chem. Pharm. Bull. 50, 284-286 (2002) CrossRef PubMed Google Scholar
-
6.H. Yoshimitsu, M. Nishida, T. Nohara, Chem. Pharm. Bull. 48, 556-558 (2000) CrossRef PubMed Google Scholar
-
7.R.H. Tian, E. Ohmura, M. Matsui, T. Nohara, Phytochemistry 44, 723-726 (1997) CrossRef PubMed Google Scholar
-
8.R.H. Tian, E. Ohmura, H. Yoshimitsu, T. Nohara, M. Matsui, Chem. Pharm. Bull. 44, 1119-1121 (1996) CrossRef PubMed Google Scholar
-
9.E. Ohmura, T. Nakamura, R.H. Tian, S. Yahara, H. Yoshimitsu, T. Nohara, Tetrahedron Lett. 36, 8443-8444 (1995) CrossRef PubMed Google Scholar
-
10.http://pza.sanbi.org/solanum-aculeastrum. Accessed 22 Aug 2017 PubMed Google Scholar
-
11.S. Koduru, O.T. Asekun, D.S. Grierson, A.J. Afolayan, J. Ess, Oil-Bear. Plants 9, 65-69 (2006) CrossRef PubMed Google Scholar
-
12.A.W. Wanyonyi, S.C. Chhabra, G. Mkoji, W. Njue, P.K. Tarus, Fitoterapia 74, 298-301 (2003) CrossRef PubMed Google Scholar
-
13.S. Koduru, F.O. Jimoh, D.S. Grierson, A.J. Afolayan, J. Pharm. Toxicol. 2, 160-167 (2007) CrossRef PubMed Google Scholar
-
14.S. Koduru, D.S. Grierson, J. O'Callaghan, A.J. Afolayan, Pharm. Biol 45, 613-618 (2007) CrossRef PubMed Google Scholar
-
15.T. Burger, V. Steenkamp, W. Cordier, T. Mokoka, G. Fouche, P. Steenkamp, BMC Comp. Alt. Med. 18, 137 (2018) CrossRef PubMed Google Scholar
-
16.L.M. Njoki, S.A. Okoth, P.M. Wachira, Int. J. Microbiol. 2017, 5273893 (2017) PubMed Google Scholar
-
17.K.K. Francisca, M. Jacob, L.K. Omosa, J. Nganga, N. Maina, J. Ethnopharmacol. 192, 524-534 (2016) CrossRef PubMed Google Scholar
-
18.L.T. Laban, J.M. Mutiso, S.G. Kiige, M.M. Ngedzo, Iran. J. Basic. Med. Sci. 18, 64-71 (2015) PubMed Google Scholar
-
19.A.W. Wanyonyi, S.C. Chhabra, G. Mkoji, U. Eilert, W.M. Njue, Phytochemistry 59, 79-84 (2002) CrossRef PubMed Google Scholar
-
20.https://en.wikipedia.org/wiki/Solanum_aethiopicum. Accessed 22 Aug 2017 PubMed Google Scholar
-
21.http://www.pfaf.org/user/Plant.aspx?LatinName=Solanum+aethiopicum. Accessed 22 Aug 2017 PubMed Google Scholar
-
22.A. Chioma, A. Obiora, U. Chukwuemeka, J. Trop. Med. 4, 163-166 (2011) PubMed Google Scholar
-
23.K.C. Kouassi, B.J.A. Mamyrbekova, Res. J. Rec. Sci. 4, 81-87 (2015) PubMed Google Scholar
-
24.C.A. Anosike, N.E. Ogbodo, A.L. Ezugwu, R.I. Uroko, C.C. Ani, O. Abonyi, Am.-Eur J. Toxicol. Sci. 7, 104-109 (2015) PubMed Google Scholar
-
25.A. Adetutu, O.S. Olorunnisola, E.B. Oyewo, Can. J. Pure Appl. Sci. 7, 2357-2362 (2013) PubMed Google Scholar
-
26.E.E. Nwanna, E.O. Ibukun, G. Oboh, Adv. Food Sci. 35, 30-36 (2013) PubMed Google Scholar
-
27.C.A. Anosike, O. Obidoa, L.U.S. Ezeanyika, Asian Pac. J. Trop. Med. 5, 62-66 (2012) CrossRef PubMed Google Scholar
-
28.C. Tagawa, M. Okawa, T. Ikeda, T. Yoshida, T. Nohara, Tetrahedron Lett. 44, 4839-4841 (2003) CrossRef PubMed Google Scholar
-
29.T. Nagaoka, K. Goto, A. Watanabe, Y. Sakata, T. Yoshihara, J. Biosci. 56, 707-713 (2001) PubMed Google Scholar
-
30.https://en.wikipedia.org/wiki/Solanum_anguivi. Accessed 22 Aug 2017 PubMed Google Scholar
-
31.O.O. Elekofehinti, J.P. Kamdem, A.A. Bolingon, M.L. Athayde, S.R. Lopes, Asian Pac. J. Trop. Biomed. 3, 757-766 (2013) CrossRef PubMed Google Scholar
-
32.C. Dalavi, S. Patil, Int. J. Pharm. Sci. Rev. Res. 30, 112-120 (2016) PubMed Google Scholar
-
33.O.O. Elekofehinti, J.P. Kamdem, I.J. Kade, J.B.T. Rocha, S. Afr. J. Bot. 88, 56-61 (2013) CrossRef PubMed Google Scholar
-
34.O.O. Elekofehinti, I.G. Adanlawo, J.A. Saliu, S.A. Sodehinde, Pharm. Lett. 4, 811-814 (2012) PubMed Google Scholar
-
35.O.O. Elekofehinti, I.G. Adanlawo, A. Fakoya, Asian J. Pharm. Health Sci. 2, 416-419 (2012) PubMed Google Scholar
-
36.O.O. Elekofehinti, J.P. Kamdem, D.F. Meinerz, I.J. Kade, I. Joseph, Arch. Pharmacal Res (2015) PubMed Google Scholar
-
37.O.O. Elekofehinti, J.P. Kamdem, I.J. Kade, I.G. Adanlawo, Asian J. Pharm. Clin. Res. 6, 252-257 (2013) PubMed Google Scholar
-
38.J. Gandhiappan, R. Rengasamy, Pharm. Lett. 4, 875-880 (2012) PubMed Google Scholar
-
39.O.O. Elekofehinti, I.G. Adanlawo, J.A. Saliu, S.A. Sodehinde, Curr. Res. J. Biol. Sci. 4, 530-533 (2012) PubMed Google Scholar
-
40.I.G. Adanlawo, M.A. Akanji, Rec. Prog. Med. Plants 19, 1-7 (2008) PubMed Google Scholar
-
41.X.H. Zhu, T. Ikeda, T. Nohara, Chem. Pharm. Bull. 48, 568-570 (2000) CrossRef PubMed Google Scholar
-
42.H. Ripperger, U. Himmelreich, Phytochemistry 37, 1725-1727 (1994) CrossRef PubMed Google Scholar
-
43.T. Honbu, T. Ikeda, X.H. Zhu, O. Yoshihara, M. Okawa, A.M. Nafady, T. Nohara, J. Nat. Prod. 65, 1918-1920 (2002) CrossRef PubMed Google Scholar
-
44.http://eol.org/pages/5695130/data. Accessed 22 Aug 2017 PubMed Google Scholar
-
45.A.C. Correia, C.L. Macedo, F.S. Monteiro, G. Alves de Oliveira, J. Med. Plants Res. 7, 2293-2299 (2013) CrossRef PubMed Google Scholar
-
46.
-
47.https://en.wikipedia.org/wiki/Solanum_americanum. Accessed 22 Aug 2017 PubMed Google Scholar
-
48.J.N. Kadima, F.M. Kasali, B. Bavhure, A.O. Mahano, F.M. Bwironde, Int. J. Pharmacol. Pharm. 5, 196-206 (2016) PubMed Google Scholar
-
49.E.L. Silva, L.R.C. Almeida, R.M. Borges, D. Staerk, Fitoterapia 118, 42-48 (2017) CrossRef PubMed Google Scholar
-
50.J.M. Vagula, J. Bertozzi, J.C. Castro, O.C. Celestino, Nat. Prod. Res. 30, 2230-2234 (2016) CrossRef PubMed Google Scholar
-
51.I. Fidrianny, A. Rizkiya, K. Ruslan, J. Chem. Pharm. Res. 7, 666-672 (2015) PubMed Google Scholar
-
52.L.U. Colmenares, J. Lai, Determination of Phenolic Content, Antioxidant Activity and Mineral Content of Popolo (Solanum americanum) Leaves. Abstracts, 64th Northwest Regional Meeting of the American Chemical Society, (Tacoma, WA, United States, 2009) PubMed Google Scholar
-
53.B.E. Hope, D.G. Massey, M.G. Fournier, Hawaii Med. J. 52, 160-166 (1993) PubMed Google Scholar
-
54.V.A. Maritza, N.L. Clara, Afinidad 56, 393-396 (1999) PubMed Google Scholar
-
55.L. Al Chami, R. Mendez, B. Chataing, J. O'Callaghan, A. Usubillaga, Phytother. Res 56, 254-258 (2003) PubMed Google Scholar
-
56.A. Vazquez, F. Ferreira, P. Moyna, L. Kenne, Phytochem. Anal. 10, 194-197 (1999) CrossRef PubMed Google Scholar
-
57.F. Ferreira, A. Vazquez, P. Moyna, L. Kenne, Phytochemistry 36, 1473-1478 (1994) CrossRef PubMed Google Scholar
-
58.http://www.boldsystems.org/index.php/Taxbrowser_Taxonpage?taxid=148709. Accessed 22 Aug 2017 PubMed Google Scholar
-
59.A. Maxwell, R. Pingal, W.F. Reynolds, S. McLean, Phytochemistry 43, 913-915 (1996) CrossRef PubMed Google Scholar
-
60.F. Londono, W. Cardona, F. Alzate, F. Cardona, I.D. Velez, J. Med. Plants Res. 10, 100-107 (2016) CrossRef PubMed Google Scholar
-
61.C.M. Chinchilla, C.I. Valerio, P.R. Sanchez, M.V. Bagnarello, C.D. Rodriguez, Rev. Biol. Trop. 62, 1229-1240 (2014) CrossRef PubMed Google Scholar
-
62.M. Chinchilla, I. Valerio, R. Sanchez, V. Mora, V. Bagnarello, L. Martinez, Rev. Biol. Trop. 60, 881-891 (2012) PubMed Google Scholar
-
63.A. Maxwell, R. Pingal, W.F. Reynolds, M.S. McLean, Stewart Phytochem. 42, 543-545 (1996) CrossRef PubMed Google Scholar
-
64.K. Fukuhara, K. Shimizu, I. Kubo, Arudonine Phytochem. 65, 1283-1286 (2004) CrossRef PubMed Google Scholar
-
65.M.H. Grace, M.M. Saleh, Pharm 51, 593-595 (1996) PubMed Google Scholar
-
66.T.M.S. Silva, C.A. Camara, K.R.L. Freire, T.G. da Silva, J. Braz. Chem. Soc. 19, 1048-1052 (2008) CrossRef PubMed Google Scholar
-
67.F.C.L. Pinto, D.E.A. Uchoa, E.R. Silveira, O.D.L. Pessoa, L.S. Espindola, Quim. Nova 34, 284-288 (2011) CrossRef PubMed Google Scholar
-
68.P.C.B. Silva, N.J. Clementino, A.D.S. da Silva, S. da Melo, S. Karoline, M.S. Tania, Rev. Bras. Farmacogn. 22, 131-136 (2012) CrossRef PubMed Google Scholar
-
69.R.C.M. Oliveira, J.T. Lima, L.A.A. Ribeiro, J.L.V. Silva, F.S. Monteiro, J. Biosci. 61, 799-805 (2006) PubMed Google Scholar
-
70.T.M.S. Silva, R.A. Costa, E.J. Oliveira, F.J.M. Barbosa, J. Braz. Chem. Soc. 16, 1467-1471 (2005) CrossRef PubMed Google Scholar
-
71.http://www.hear.org/pier/species/solanum_betaceum.htm. Accessed 23 Aug 2017 PubMed Google Scholar
-
72.https://en.wikipedia.org/wiki/Tamarillo. Accessed 23 Aug 2017 PubMed Google Scholar
-
73.http://eol.org/pages/486416/overview. Accessed 23 August 2017 PubMed Google Scholar
-
74.http://www.iucnredlist.org/details/34636/0. Accessed 23 Aug 2017 PubMed Google Scholar
-
75.N.H. Hurtado, A.L. Morales, M.L. Gonzalez-Miret, M.L. Escudero-Gilete, F. Heredia, Food Chem. 117, 88-93 (2009) CrossRef PubMed Google Scholar
-
76.C. Osorio, N. Hurtado, C. Dawid, T. Hofmann, M. Heredia, J. Francisco, A.L. Morales, Food Chem. 132, 1915-1921 (2012) CrossRef PubMed Google Scholar
-
77.A.A. Durant, C. Rodriguez, A.I. Santana, C. Herrero, J.C. Rodriguez, Rec. Nat. Prod. 7, 15-26 (2013) PubMed Google Scholar
-
78.G.J. Maria, P.L. Juliana, O. Coralia, G. Alirio, M. Diana, Mol. 21 (2016) PubMed Google Scholar
-
79.F.D.C.L. Pinto, M.D.C.M. Torres, E.R. Silveira, Quim. Nova 36, 1111-1115 (2013) CrossRef PubMed Google Scholar
-
80.N.P. Vaz, E.V. Costa, E.L. Santos, S.B. Mikich, F.A. Marques, R.A. Braga, J. Braz. Chem. Soc. 23, 361-366 (2012) CrossRef PubMed Google Scholar
-
81.https://en.wikipedia.org/wiki/Solanum_capsicoides. Accessed 24 Aug 2017 PubMed Google Scholar
-
82.http://florida.plantatlas.usf.edu/Plant.aspx?id=609. Accessed 24 Aug 2017 PubMed Google Scholar
-
83.
-
84.http://davesgarden.com/guides/pf/go/38381/. Accessed 24 Aug 2017 PubMed Google Scholar
-
85.B.W. Chen, Y.Y. Chen, Y.C. Lin, RSC Adv. 5, 88841-88847 (2015) CrossRef PubMed Google Scholar
-
86.M. Petreanu, A.A.A. Guimaraes, M.F. Broering, Nau.-Sch Arch. Pharm. 389, 1123-1131 (2016) CrossRef PubMed Google Scholar
-
87.L.O. Simoes, F.G. Conceicao, T.S. Ribeiro, A.M. Jesus, D.F. Silva, Phytomedicine 23, 498-508 (2016) CrossRef PubMed Google Scholar
-
88.J. He, B.Z. Ma, X.F. Tian, F.L. Wei, T. Zhao, Chin. Pharm. Mag. 25, 3713-3718 (2014) PubMed Google Scholar
-
89.S. Xu, L. Wang, H. Liu, Shizhen Chin. Med. J. 17, 523-524 (2006) PubMed Google Scholar
-
90.G.M. Nino, O.V. Urias, M.D.R. Muy, J.B. Heredia, S. Afr. J. Bot. 111, 161-169 (2017) CrossRef PubMed Google Scholar
-
91.J. Sun, Y.F. Gu, M.M. Li, X.Q. Su, H. Li, J. Li, P.F. Tu, Chin. Herb. Med. 44, 2615-2622 (2013) PubMed Google Scholar
-
92.T.F. Cao, L. Zhou, X.H. Huang, L. Wang, D.K. Wang, Q. Wu, W.Y. Hou, Chin. Vet. Med. Mag. 33, 31-33 (2014) PubMed Google Scholar
-
93.J. He, C.D. Zhou, B.Z. Ma, F. Liu, X. Liu, T. Zhao, Chin. Pharm. 26, 4433-4436 (2015) PubMed Google Scholar
-
94.H. Huang, J. Zhou, Food Ind. Tech. 30, 315-318 (2009) PubMed Google Scholar
-
95.L. An, J.T. Tang, X.M. Liu, N.N. Gao, Chin. J. of Chin. Mat. Med. 31(1225-1226), 1260 (2006) PubMed Google Scholar
-
96.G. Butt, M.A. Romero, F. Tahir, A.A. Farooqi, J. of Cell. Biochem. 119, 9640-9644 (2018) CrossRef PubMed Google Scholar
-
97.X. Zhang, Z. Yan, T. Xu, Z. An, W. Chen, F. Zhu, Onc. Lett. 15, 6329-6335 (2018) PubMed Google Scholar
-
98.S.E. Potawale, S.D. Sinha, K.K. Shroff, H.J. Dhalawat, S.S. Boraste, S.P. Gandhi, A.D. Tondare, Pharmacol. Onl. 3, 140-163 (2008) PubMed Google Scholar
-
99.K. Aftab, R. Noreen, S.A. Bukhari, A. Malik, A. Sahar, Oxid. Commun. 39, 3079-3089 (2016) PubMed Google Scholar
-
100.Z. Yousaf, Y. Wang, E. Baydoun, J. App. Pharm. Sci. 3, 152-160 (2013) PubMed Google Scholar
-
101.J. Lachman, K. Hamouz, M. Orsak, V. Pivec, Rost. Vyr. 47, 181-191 (2001) PubMed Google Scholar
-
102.Y. Zhou, Z.S. Deng, F. Cheng, W.J. Dong, K. Zou, Chem. Nat. Comp. 52, 920-921 (2016) CrossRef PubMed Google Scholar
-
103.F. Cheng, X. Li, J.Z. Wang, Chin. Chem. Lett. 19, 68-70 (2008) CrossRef PubMed Google Scholar
-
104.K. Yushan, S. Huang, M. Xie, Y. Xue, J. Zou, Med. Plant 6, 1-3 (2015) CrossRef PubMed Google Scholar
-
105.Y.S. Kong, S.L. Huang, M.X. Xie, Y.H. Xue, K. Zou, S.P. Liu, Nat. Prod. Res. Dev. 26, 943-946 (2014) PubMed Google Scholar
-
106.S.L. Huang, H.B. He, K. Zou, C.H. Bai, Y.H. Xue, J.Z. Wang, J.F. Chen, J. Pharm. Pharmacol. 66, 844-854 (2014) PubMed Google Scholar
-
107.L.C. Lopes, J.E. de Carvalho, M. Kakimore, C.D.B. Vendramini, Inflammopharmacology 22, 179-185 (2014) PubMed Google Scholar
-
108.R. Grando, M.A. Antonio, C.E.P. Araujo, C. Soares, M.A. Medeiros, J. Biosci. 63, 507-514 (2008) PubMed Google Scholar
-
109.A.M. Mariza, M. Lemos, A.C. Kamila, D. Rodenburg, J.D. McChesney, J. Ethnopharmacol. 172, 421-429 (2015) CrossRef PubMed Google Scholar
-
110.J.L. Damasceno, P.F. Oliveira, M.A. Miranda, L.F. Leandro, Biomed. Pharmacother. 83, 1111-1115 (2016) CrossRef PubMed Google Scholar
-
111.L.C. Lopes, B. Roman, M.A. Medeiros, A. Mukhopadhyay, Tetrahedron Lett. 52, 6392-6395 (2011) CrossRef PubMed Google Scholar
-
112.J.L. Damasceno, P.F. de Oliveira, M.A. Miranda, M. Lima, C Denise, Biol. Pharm. Bull 39, 920-926 (2016) CrossRef PubMed Google Scholar
-
113.A. Herrera, F.E. Jimenez, A. Zamilpa, R.M.A. Martinez, Planta Med. 75, 466-471 (2009) CrossRef PubMed Google Scholar
-
114.H.A. Armando, J.F. Enrique, V.P.A. Maria, Planta Med. 70, 483-488 (2004) CrossRef PubMed Google Scholar
-
115.H.A. Armando, V.E.O. Lopez, T.A.V. Rodriguez, A. Zamilpa, R.M.A. Martinez, Afr. J. Trad. Comp. Altern. Med. 10, 410-417 (2013) PubMed Google Scholar
-
116.H.A. Armando, R.S. Artemio, M.R. Maria, M.C. Eugenia, J. Tortoriello, Planta Med. 69, 390-395 (2003) CrossRef PubMed Google Scholar
-
117.A. Zamilpa, J. Tortoriello, V. Navarro, G. Delgado, L. Alvarez, J. Nat. Prod. 65, 1815-1819 (2002) CrossRef PubMed Google Scholar
-
118.L. Alvarez, M.D.C. Perez, J.L. Gonzalez, V. Navarro, M.L. Villarreal, Planta Med. 67, 372-374 (2001) CrossRef PubMed Google Scholar
-
119.X. Lozoya, V. Navarro, M. Garcia, M. Zurita, J. Ethnopharmacol. 36, 127-132 (1992) CrossRef PubMed Google Scholar
-
120.L. Alvarez, H.A. Armando, S. Marquina, J. Tortoriello, A. Zamilpa, V. Navarro, Curr. Top. Steroid Res. 6, 89-104 (2009) PubMed Google Scholar
-
121.I. Gaitaan, A.M. Paz, S.A. Zacchino, G. Tamayo, A. Gimeenez, R. Pinzoon, Pharm. Biol. 49, 907-919 (2011) CrossRef PubMed Google Scholar
-
122.M.T.F. Cornelius, M.G. de Carvalho, T.M.S. da Silva, C.C.F. Alves, J. Braz. Chem. Soc 21, 2211-2219 (2010) CrossRef PubMed Google Scholar
-
123.E.S. Andressa, T.M.S. da Silva, C.C.F. Alves, M.G. de Carvalho, J. Braz. Chem. Soc 13, 838-842 (2002) CrossRef PubMed Google Scholar
-
124.https://en.wikipedia.org/wiki/Solanum_diphyllum. Accessed 10 Sept 2017 PubMed Google Scholar
-
125.https://toptropicals.com/catalog/uid/Solanum_diphyllum.htm. Accessed 10 Sept 2017 PubMed Google Scholar
-
126.M.A. El-Sayed, A.E.H. Mohamed, M.K. Hassan, M.E.F. Hegazy, J. Biosci. 64, 644-649 (2009) PubMed Google Scholar
-
127.
-
128.https://en.wikipedia.org/wiki/Solanum_dulcamara. Accessed 10 Sept 2017 PubMed Google Scholar
-
129.T. Yamashita, T. Matsumoto, S. Yahara, N. Yoshida, T. Nohara, Chem. Pharm. Bull. 39, 1626-1628 (1991) CrossRef PubMed Google Scholar
-
130.T. Sabudak, O. Kaya, E. Cukurova, Nat. Prod. Res. 29, 308-314 (2015) CrossRef PubMed Google Scholar
-
131.N. Mimica-Dukic, L. Krstic, P. Boza, Oxid. Commun. 28, 536-546 (2005) PubMed Google Scholar
-
132.H. Tunon, H.C. Olavsdotter, L. Bohlin, J. Ethnopharmacol. 48, 61-76 (1995) CrossRef PubMed Google Scholar
-
133.https://en.wikipedia.org/wiki/Solanum_elaeagnifolium. Accessed 13 Sept 2017 PubMed Google Scholar
-
134.H. Feki, I. Koubaa, H. Jaber, J. Makni, M. Damak, J. Eng. Appl. Sci. 8, 708-712 (2013) PubMed Google Scholar
-
135.http://www.cabi.org/isc/datasheet/120139. Accessed 14 Sept 2017 PubMed Google Scholar
-
136.https://en.wikipedia.org/wiki/Solanum_erianthum. Accessed 14 Sept 2017 PubMed Google Scholar
-
137.S.C. Chou, T.J. Huang, E.H. Lin, C.H. Huang, C.H. Chou, Nat. Prod. Commun. 7, 153-156 (2012) PubMed Google Scholar
-
138.S.Y. Peng, H. Li, D. Yang, B. Bai, L.P. Zhu, Q. Liu, Nat. Prod. Res. 31, 810-816 (2017) CrossRef PubMed Google Scholar
-
139.S.D. Priyadharshini, V. Sujatha, Int. J. Pharm. Pharm. Sci. 5, 652-658 (2013) PubMed Google Scholar
-
140.M.A. Monem, R. Azza, Bull. Fac. Pharm. 47, 59-66 (2009) PubMed Google Scholar
-
141.A.S. Essam, M.A. Farag, E.A. Mahrous, Rec. Nat. Prod. 9, 94-104/1 (2015) PubMed Google Scholar
-
142.N.A. Siddiqui, M.A. Parvez, A.J. Al-Rehaily, M.S. Al Dosari, Saudi Pharm. J. 25, 184-195 (2017) PubMed Google Scholar
-
143.R.A. Mothana, N.M. Al-Musayeib, M.F. Al-Ajmi, P. Cos, L. Maes, Evidence-based Comp. Altern. Med. 2014, 905639 (2014) PubMed Google Scholar
-
144.R.A.A. Mothana, R. Gruenert, P.J. Bednarski, U. Lindequist, Pharmazie 64, 260-268 (2009) PubMed Google Scholar
-
145.https://en.wikipedia.org/wiki/Solanum_glaucophyllum. Accessed 14 Sept 2017 PubMed Google Scholar
-
146.C.N. Zanuzzi, F. Nishida, E.L. Portiansky, P.A. Fontana, E.J. Gimeno, C.G. Barbeito, Res. Vet. Sci. 93, 336-342 (2012) CrossRef PubMed Google Scholar
-
147.M. Zadra, M. Piana, T. de Brum, F. Thiele, A.A. Boligon, Molecules 17, 2560-12574 (2012) PubMed Google Scholar
-
148.G. Bonfanti, K.S.D. Bona, L.D. Lucca, L. Jantsch, M.B. Moretto, Red. Rep. 19, 206-213 (2014) CrossRef PubMed Google Scholar
-
149.G. Bonfanti, P.R. Bitencourt, S.B. Karine, S.S. Priscila, Molecules 18, 9179-9194 (2013) CrossRef PubMed Google Scholar
-
150.https://en.wikipedia.org/wiki/Solanum_incanum. Accessed 14 Sept 2017 PubMed Google Scholar
-
151.
-
152.S.A. Alamri, M.F. Moustafa, Saudi Med. J. 33, 272-277 (2012) PubMed Google Scholar
-
153.B. Taye, M. Giday, A. Animut, J. Seid, Asian Pac. J. Trop. Biomed. 1, 370-375 (2011) CrossRef PubMed Google Scholar
-
154.S.S. Al-Sokari, N.A.A. Ali, L. Monzote, M. Al-Fatimi, Biomed. Res. Int. 2015, 938747 (2015) PubMed Google Scholar
-
155.M.J. Manase, O.A.C. Mitaine, D. Pertuit, T. Miyamoto, C. Tanaka, S. Delemasure, Fitoterapia 83, 1115-1119 (2012) CrossRef PubMed Google Scholar
-
156.S. Sundar, Y.J.K. Pillai, Asian J. Pharm. Clin. Res. 8, 179-188 (2015) PubMed Google Scholar
-
157.
-
158.http://www.geniusherbs.com/solanum-indicum.html. Accessed 25 Sept 2017 PubMed Google Scholar
-
159.Y.W. Zhuang, C.E. Wu, J.Y. Zhou, Z.M. Zhao, C.L. Liu, S.L. Liu, Biochem. Biophy. Res. Commun. 505, 485-491 (2018) CrossRef PubMed Google Scholar
-
160.H.C. Chiang, T.H. Tseng, C.J. Wang, C.F. Chen, W.S. Kan, Anticancer Res. 11, 1911-1917 (1991) PubMed Google Scholar
-
161.A. Aberoumand, S.S. Deokule, J. Food Technol. 8, 131-133 (2010) CrossRef PubMed Google Scholar
-
162.P. Ma, T.T. Cao, G.F. Gu, X. Zhao, Y.G. Du, Y. Zhang, Chin. J. Cancer 25, 438-442 (2006) PubMed Google Scholar
-
163.W.J. Syu, M.J. Don, G.H. Lee, C.M. Sun, J. Nat. Prod. 64, 1232-1233 (2001) CrossRef PubMed Google Scholar
-
164.W.H. Huang, C.W. Hsu, J.T. Fang, Clin. Toxicol. 46, 293-296 (2008) CrossRef PubMed Google Scholar
-
165.https://link.springer.com/article/10.2307/2807835. Accessed 26 Sept 2017 PubMed Google Scholar
-
166.T.M.S. Silva, R. Braz-Filho, M.G. de Carvalho, M.F Agra, Biochem. Syst. Ecol 30, 1083-1085 (2002) CrossRef PubMed Google Scholar
-
167.T.M.S. Silva, G.M. de Carvalho, B.F Raimundo, Quim. Nova 32, 1119-1128 (2009) PubMed Google Scholar
-
168.E.S. Andressa, T.M.S.S. da Silva, C.C.F. Alves, M.G. de Carvalho, A Echevarria, Aurea J. Braz. Chem. Soc. 13, 838-842 (2002) CrossRef PubMed Google Scholar
-
169.T.M.S. Silva, C.A. Camara, M.F. Agra, M.G. de Carvalho, B.F. Raimundo, Fitoterapia 77, 449-452 (2006) CrossRef PubMed Google Scholar
-
170.E.E. Jarald, S. Edwin, V. Saini, L. Deb, V.B. Gupta, S.P. Wate, K.P. Busari, Nat. Prod. Res. 22, 267-274 (2008) PubMed Google Scholar
-
171.R. Chand, Indian Drugs 30, 650 (1993) PubMed Google Scholar
-
172.G. Rosangkima, G.C. Jagetia, J. Pharm. Res. 4, 98-103 (2015) PubMed Google Scholar
-
173.http://www.plantoftheweek.org/week351.shtml. Accessed 27 Sept 2017 PubMed Google Scholar
-
174.http://www.anbg.gov.au/gnp/gnp12/solanum-laciniatum.html. Accessed 27 Sept 2017 PubMed Google Scholar
-
175.https://en.wikipedia.org/wiki/Solanum_laxum. Accessed 27 Sept 2017 PubMed Google Scholar
-
176.https://plantsam.com/solanum-laxum/. Accessed 27 Sept 2017 PubMed Google Scholar
-
177.S. Soule, C. Guntner, A. Vazquez, V. Argandona, P. Moyna, F. Ferreira, Phytochemistry 55, 217-222 (2000) CrossRef PubMed Google Scholar
-
178.F. Ferreira, S. Soule, A. Vazquez, P. Moyna, L. Kenne, Phytochemistry 42 (1409) PubMed Google Scholar
-
179.C. Delporte, N. Backhouse, R. Negrete, P. Salinas, P. Rivas, B.K. Cassels, Phytother. Res. 12, 118-122 (1998) CrossRef PubMed Google Scholar
-
180.C.C. Munari, P.F. Oliveira, J.C.L. Campos, S.P.L. Martins, J. Nat. Med. 68, 236-241 (2014) CrossRef PubMed Google Scholar
-
181.C.C. Munari, P.F. de Oliveira, L.F. Leandro, L.M. Pimenta, N.H Ferreira, PLoS ONE 9, 111999 (2014) CrossRef PubMed Google Scholar
-
182.M. Yoshikawa, S. Nakamura, K. Ozaki, A. Kumahara, T. Morikawa, H. Matsuda, J. Nat. Prod. 70, 210-214 (2007) CrossRef PubMed Google Scholar
-
183.M.A. Miranda, L.G.M. Magalhaes, R.F.J. Tiossi, C.C. Kuehn, Parasit. Res. 111, 257-262 (2012) CrossRef PubMed Google Scholar
-
184.A.M. Mariza, C.C. Kuehn, J.F.R. Cardoso, L.G.R. Oliveira, L.G. Magalhaes, Exp. Parasit 133, 396-402 (2013) CrossRef PubMed Google Scholar
-
185.A.M. Mariza, R.F.J. Tiossi, M.R. da Silva, K.C. Rodrigues, C.C Kuehn, Chem. Biodiversity 10, 642-648 (2013) CrossRef PubMed Google Scholar
-
186.R.R.D. Moreira, G.Z. Martins, N.O. Magalhaes, A.E. Almeida, R.C.L.R. Pietro, Anais Acad. Bras. Cienc. 85, 903-907 (2013) CrossRef PubMed Google Scholar
-
187.G.Z. Martins, R.R.D. Moreira, C.S. Planeta, A.E. Almeida, J.K. Bastos, Pharmacogn. Mag. 11, 161-165 (2015) CrossRef PubMed Google Scholar
-
188.https://en.wikipedia.org/wiki/Tomato. Accessed 03 Oct 2017 PubMed Google Scholar
-
189.M. Kralova, M. Sanda, M. Mackova, T. Macek, Collec. Symp. Series 13, 73-76 (2011) PubMed Google Scholar
-
190.S. Paoli, A.P.M. Dias, P.V.S.Z. Capriles, T.E.M.M. Costa, Rev. Bras. Farmacogn. 18, 190-196 (2008) CrossRef PubMed Google Scholar
-
191.E. Fuentes, R. Castro, L. Astudillo, G. Carrasco, Evidence-based Comp. Altern. Med. 2012, 147031 (2012) PubMed Google Scholar
-
192.M. Friedman, C.E. Levin, S.U. Lee, H.J. Kim, I.S. Lee, J. Agric. Food Chem. 57, 5727-5733 (2009) CrossRef PubMed Google Scholar
-
193.http://www.fpcn.net/a/guanmu/20131109/Solanum_lyratum.html. Accessed 07 Oct 2017 PubMed Google Scholar
-
194.L.X. Sun, W.W. Fu, J. Ren, L. Xu, K.S. Bi, M.W. Wang, Arch. Pharm. Res. 29, 135-139 (2006) CrossRef PubMed Google Scholar
-
195.S.H. Liu, X.H. Shen, X.F. Wei, X.H. Mao, T. Huang, Immunopharmcol. Immunotoxicol. 33, 100-106 (2011) CrossRef PubMed Google Scholar
-
196.X.P. Nie, F. Yao, X.D. Yue, G.S. Li, S.J. Dai, Nat. Prod. Res. 28, 641-645 (2014) CrossRef PubMed Google Scholar
-
197.G.S. Li, F. Yao, L. Zhang, X.D. Yue, S.J. Dai, Chin. Chem. Lett. 24, 1030-1032 (2013) CrossRef PubMed Google Scholar
-
198.F. Yao, Q.L. Song, L. Zhang, G.S. Li, S.J. Dai, Fitoterapia 89, 200-204 (2013) CrossRef PubMed Google Scholar
-
199.Y. Ren, L. Shen, D.W. Zhang, S.J. Dai, Chem. Pharm. Bull. 57, 408-410 (2009) CrossRef PubMed Google Scholar
-
200.F. Yao, Q.L. Song, L. Zhang, G.S. Li, S.J. Dai, Phytochem. Lett. 6, 453-456 (2013) CrossRef PubMed Google Scholar
-
201.D.W. Zhang, Y. Yang, F. Yao, Q.Y. Yu, S.J. Dai, J. Nat. Med. 66, 362-366 (2012) CrossRef PubMed Google Scholar
-
202.https://en.wikipedia.org/wiki/Eggplant. Accesed 02 Nov 2017 PubMed Google Scholar
-
203.https://www.thoughtco.com/eggplant-history-solanum-melongena-170820. Accessed 02 Nov 2017 PubMed Google Scholar
-
204.M.A.S. Atta, M.T.K. Shahid, Oxid. Commun. 39, 2249-2259 (2016) PubMed Google Scholar
-
205.A.P. Singh, D. Luthria, T. Wilson, N. Vorsa, V. Singh, Food Chem. 114, 955-961 (2009) CrossRef PubMed Google Scholar
-
206.A. Zhao, Y. Sakurai, K. Shibata, F. Kikkawa, Y. Tomoda, H. Mizukami, Nippon Shokuhin Kagaku Gakkaish 21, 42-47 (2014) PubMed Google Scholar
-
207.M.M. Shabana, M.M. Salama, S.M. Ezzat, L.R. Ismail, J. Carcinog. Mutagen. 4, 1000149/1-1000149/6 (2013) PubMed Google Scholar
-
208.K. Yoshikawa, K. Inagaki, T. Terashita, J. Shishiyama, S. Kuo, D.M. Shankel, Mutagen. Res. Gen. Toxicol. 371, 65-71 (1996) CrossRef PubMed Google Scholar
-
209.X. Liu, J. Luo, L. Kong, Nat. Prod. Commun. 6, 851-853 (2011) PubMed Google Scholar
-
210.J. Sun, Y.F. Gu, X.Q. Su, M.M. Li, H.X. Huo, J. Zhang, Fitoterapia 98, 110-116 (2014) CrossRef PubMed Google Scholar
-
211.A. Nishina, K. Ebina, M. Ukiya, M. Fukatsu, M. Koketsu, M. Ninomiya, J. Food Sci. 80, H2354-H2359 (2015) CrossRef PubMed Google Scholar
-
212.W. Ren, D.G. Tang, Antican. Res. 19, 403-408 (1999) PubMed Google Scholar
-
213.F.J. Herraiz, M. Plazas, S. Vilanova, J. Prohens, D. Villano, F. Ferreres, Int. J. Mol. Sci. 17, 394 (2016) CrossRef PubMed Google Scholar
-
214.V.H.C. Chang, T.H. Chiu, S.C. Fu, J. Sci. Food Agric. 96, 192-198 (2016) CrossRef PubMed Google Scholar
-
215.C.C. Hsu, Y.R. Guo, Z.H. Wang, M.C. Yin, J. Sci. Food Agric. 91, 1517-1522 (2011) CrossRef PubMed Google Scholar
-
216.http://www.efloras.org/florataxon.aspx?flora_id=601&taxon_id=200020596. Accessed 07 Nov 2017 PubMed Google Scholar
-
217.
-
218.https://www.wikidata.org/wiki/Q15239976. Accessed 07 Nov 2017 PubMed Google Scholar
-
219.https://en.wikipedia.org/wiki/Solanum_nigrum. Accessed 08 Nov 2017 PubMed Google Scholar
-
220.http://naturalhomeremedies.co/Snigrum.html. Accessed 08 Nov 2017 PubMed Google Scholar
-
221.
-
222.http://www.pfaf.org/user/Plant.aspx?LatinName=Solanum+nigrum. Accessed 08 Nov 2017 PubMed Google Scholar
-
223.B.K. Sharma, D. Iyer, U.K. Patil, J. Herb. Spic. Med. Plants 18, 257-267 (2012) CrossRef PubMed Google Scholar
-
224.T.M. Sridhar, P. Josthna, C.V. Naidu, J. Exp. Sci. 2, 24-29 (2011) PubMed Google Scholar
-
225.L.G. Matasyoh, H.M. Murigi, J.C. Matasyoh, Afr. J. Microbiol. Res. 8, 3923-3930 (2014) PubMed Google Scholar
-
226.F.Z. Khan, M.A. Saeed, M. Alam, A.R. Chaudhry, M. Ismail, J. Fac. Pharm. Gazi Uni. 10, 105-116 (1993) PubMed Google Scholar
-
227.H.L. Yuan, X.L. Liu, Y.J. Liu, Asian Pac. J. Cancer Prev. 15, 10469-10473 (2014) PubMed Google Scholar
-
228.X. Ding, F. Zhu, Y. Yang, M. Li, Food Chem. 141, 1181-1186 (2013) CrossRef PubMed Google Scholar
-
229.K.V. Prashanth, S. Shashidhara, M.M. Kumar, B.Y. Sridhara, .Fitoterapia 72, 481-486 (2001) CrossRef PubMed Google Scholar
-
230.H.C. Huang, K.Y. Syu, J.K. Lin, J. Agric. Food Chem. 58, 8699-8708 (2010) CrossRef PubMed Google Scholar
-
231.G.T. El-Sherbini, R.A. Zayed, E.T. El-Sherbini, J. Parasit. Res. 2009, 474360 (2009) PubMed Google Scholar
-
232.H. Hammami, A. Ayadi, J. Helminth. 82, 235-239 (2008) CrossRef PubMed Google Scholar
-
233.A. Rawani, A.S. Ray, G. Chandra, A. Ghosh, M. Sakar, BMC Res. Notes 10, 135 (2017) CrossRef PubMed Google Scholar
-
234.Z.A. Zakaria, H.K. Gopalan, H. Zainal, P.N.H. Mohd, Yakugaku Zasshi 126, 1171-1178 (2006) CrossRef PubMed Google Scholar
-
235.M. Jainu, C.S.S. Devi, J. Ethnopharmacol. 104, 156-163 (2006) CrossRef PubMed Google Scholar
-
236.H.M. Lin, H.C. Tseng, C.J. Wang, J.J. Lin, C.W. Lo, Chem.-Biol. Int. 171, 283-293 (2008) CrossRef PubMed Google Scholar
-
237.C.C.H.L. Fang, W.C. Lina, J. Ethnopharmacol. 119, 117-121 (2008) CrossRef PubMed Google Scholar
-
238.R.M. Perez, J.A. Perez, L.M. Garcia, J. Ethnopharmacol. 62, 43-48 (1998) CrossRef PubMed Google Scholar
-
239.http://eol.org/pages/5695318/overview. Accessed 10 Nov 2017 PubMed Google Scholar
-
240.A. Pabon, S. Blair, J. Carmona, M. Zuleta, J. Saez, Pharm. 58, 263-267 (2003) PubMed Google Scholar
-
241.G. Alvarez, A. Pabon, J. Carmona, S. Blair, Phytother. Res. 18, 845-848 (2004) CrossRef PubMed Google Scholar
-
242.M.L. Lopez, R. Vommaro, M. Zalis, W. Souza, S. Blair, C. Segura, Parasit. Int. 59, 217-225 (2010) CrossRef PubMed Google Scholar
-
243.M.L. Lopez, S. Blair, J. Saez, C. Segura, Memo. Inst. Oswaldo Cruz 104, 683-688 (2009) CrossRef PubMed Google Scholar
-
244.A. Pabon, E. Deharo, L. Zuluaga, J.D. Maya, J. Saez, S. Blair, Exp. Parasit. 122, 273-279 (2009) CrossRef PubMed Google Scholar
-
245.B. Londono, E. Arango, C. Zapata, S. Herrera, J. Saez, S. Blair, F.J. Carmona, Phytother. Res. 20, 267-273 (2006) CrossRef PubMed Google Scholar
-
246.O.M.S. Cardoso, E.J.N. Gomez, L.A. Garces, S.B. Trujillo, Rev. Colomb. Biotechnol. 13, 186-192 (2011) PubMed Google Scholar
-
247.J. Saez, W. Cardona, D. Espinal, S. Blair, J. Mesa, M. Bocar, A. Jossang, Tetrahedron 54, 10771-10778 (1998) CrossRef PubMed Google Scholar
-
248.G.H. Paola, A. Pabon, C. Arias, S. Blair, Biomed. 33, 78-87 (2013) PubMed Google Scholar
-
249.M. Echeverri, S. Blair, J. Carmona, P. Perez, Am. J. Chin. Med. 29, 477-484 (2001) CrossRef PubMed Google Scholar
-
250.R.M. Coelho, M.C. Souza, M.H. Sarragiotto, Phytochemistry 49, 893-897 (1998) CrossRef PubMed Google Scholar
-
251.https://commons.wikimedia.org/wiki/Category:Solanum_paludosum. Accessed 14 Nov 2017 PubMed Google Scholar
-
252.F.S. Monteiro, A.C.L. Silva, I.R.R. Martins, A.C.C. Correia, J. Ethnopharmacol. 141, 895-900 (2012) CrossRef PubMed Google Scholar
-
253.M.L.C. Valverde, J. Boustie, H.E. Badaoui, B. Muguet, M. Henry, Planta Med. 59, 483-484 (1993) PubMed Google Scholar
-
254.https://en.wikipedia.org/wiki/Solanum_paniculatum. Accessed 14 Nov 2017 PubMed Google Scholar
-
255.http://rain-tree.com/jurubeba.htm#.WgqWOrVx3IU. Accessed 14 Nov 2017 PubMed Google Scholar
-
256.http://www.pfaf.org/user/Plant.aspx?LatinName=Solanum+paniculatum. Accessed 14 Nov 2017 PubMed Google Scholar
-
257.Y.M. Valadares, G.C. Brandao'a, E.G. Kroon, J.D.S. Filho, A.B. Oliveira, F.C. Braga, J. Biosci 64, 813-818 (2009) PubMed Google Scholar
-
258.G.M.J. Vieira, Q.C. Rocha, R.T. Souza, C.L.H. Lima, W. Vilegas, Food Chem. 186, 160-167 (2015) CrossRef PubMed Google Scholar
-
259.V.S. Mesia, M.T. Santos, C. Souccar, L.M.T.R. Lima, A.J. Lapa, Phytomed. Int. J. Phytother. Phytopharm. 9, 508-514 (2002) PubMed Google Scholar
-
260.A.B. Valerino-Diaz, G.T. Daylin, A.C. Zanatta, W. Vilegas, L. dos Santos, J. Agric. Food Chem. 66, 8703-8713 (2018) CrossRef PubMed Google Scholar
-
261.https://en.wikipedia.org/wiki/Solanum_pseudocapsicum. Accessed 14 Nov 2017 PubMed Google Scholar
-
262.https://davesgarden.com/guides/pf/go/54393/. Accessed 14 Nov 2017 PubMed Google Scholar
-
263.A.A. Aliero, O.T. Asekun, D.S. Grierson, A.J. Afolayan, Asian J. Plant Sci. 5, 1054-1056 (2006) CrossRef PubMed Google Scholar
-
264.P. Vijayan, H.C. Prashanth, P. Vijayaraj, S.A. Dhanaraj, S. Badami, B. Suresh, Pharm. Biol. 41, 443-448 (2003) CrossRef PubMed Google Scholar
-
265.https://en.wikipedia.org/wiki/Solanum_rostratum. Accessed 15 Nov 2018 PubMed Google Scholar
-
266.
-
267.https://plants.usda.gov/core/profile?symbol=soro. Accessed 15 Nov 2018 PubMed Google Scholar
-
268.https://www.cabi.org/isc/datasheet/50544. Accessed 15 Nov 2018 PubMed Google Scholar
-
269.https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/solanum-rostratum. Accessed 15 November 2018 PubMed Google Scholar
-
270.http://ucjeps.berkeley.edu/eflora/eflora_display.php?tid=44902. Accessed 15 Nov 2018 PubMed Google Scholar
-
271.https://www.illinoiswildflowers.info/weeds/plants/buffalo_bur.html. Accessed 15 Nov 2018 PubMed Google Scholar
-
272.https://www.invasiveplantatlas.org/subject.html?sub=6463. Accessed 15 Nov 2018 PubMed Google Scholar
-
273.C.A. Ibarra, A. Rojas, S. Mendoza, M. Bah, D.M. Gutierrez, S.L. Hernandez, M. Martinez, Pharmaceu. Bio. 48, 732-739 (2010) CrossRef PubMed Google Scholar
-
274.
-
275.
-
276.http://plants.jstor.org/compilation/solanum.schimperianum. Accessed 15 Nov 2017 PubMed Google Scholar
-
277.W.H.B. Hassan, M. Al-Oqail, M.S. Ahmad, A.J. Al-Rehaily, Biosci. Biotechnol. Res Asia 9, 593-599 (2012) CrossRef PubMed Google Scholar
-
278.W.H.B. Hassan, M. Al-Oqail, M.S. Ahmad, A.J. Al-Rehaily, Saudi Pharm. J. 20, 371-379 (2012) CrossRef PubMed Google Scholar
-
279.A.J. Al-Rehaily, J. Adnan, M.S. Ahmad, J. Mustafa, M.M. Al-Oqail, I.A. Khan, J. Saudi Chem. Soc. 17, 67-76 (2013) CrossRef PubMed Google Scholar
-
280.http://plants.for9.net/edible-and-medicinal-plants/solanum-septemlobum/. Accessed 15 Nov 2017 PubMed Google Scholar
-
281.L. Zhang, G.S. Li, F. Yao, X.D. Yue, S.J. Dai, Phytochem. Lett. 11, 173-176 (2015) CrossRef PubMed Google Scholar
-
282.https://en.wikipedia.org/wiki/Solanum_sessiliflorum. Accessed 15 Nov 2017 PubMed Google Scholar
-
283.https://www.hort.purdue.edu/newcrop/morton/cocona.html. Accessed 15 Nov 2017 PubMed Google Scholar
-
284.http://www.tradewindsfruit.com/content/cocona.htm. Accessed 15 Nov 2017 PubMed Google Scholar
-
285.J.R.P. Maia, M.C. Schwertz, R.F.S. Sousa, J.P.L. Rev, Bras. Plantas Med. 17, 112-119 (2015) CrossRef PubMed Google Scholar
-
286.https://en.wikipedia.org/wiki/Solanum_sisymbriifolium. Accessed 15 Nov 2017 PubMed Google Scholar
-
287.
-
288.http://www.pfaf.org/user/Plant.aspx?LatinName=Solanum+sisymbriifolium. Accessed 15 Nov 2017 PubMed Google Scholar
-
289.D.A. Ibarrola, M.D.C. Hellion-Ibarrola, N.L. Alvarenga, Pharm. Biol. 44, 378-381 (2006) CrossRef PubMed Google Scholar
-
290.A.S. Apu, S.H. Bhuyan, M. Matin, F. Hossain, F. Khatun, A. Taiab Abu, M Matin, Avicenna J. Phytomed 3, 302-312 (2013) PubMed Google Scholar
-
291.D.A. Ibarrola, M.C.H. Ibarrola, Y. Montalbetti, O. Heinichen, M.A. Campuzano, Phytomedicine 18, 634-640 (2011) CrossRef PubMed Google Scholar
-
292.D.A. Ibarrola, M.C.I. Hellion, Y. Montalbetti, O. Heinichen, N. Alvarenga, J. Ethnopharmacol. 70, 301-307 (2000) CrossRef PubMed Google Scholar
-
293.V.K. Gupta, A. Simlai, M. Tiwari, K. Bhattacharya, A. Roy, J. Appl. Pharm. Sci. 4, 75-80 (2014) PubMed Google Scholar
-
294.K. Chauhan, N. Sheth, V. Ranpariya, S. Parmar, Pharm. Biol. 49, 194-199 (2011) CrossRef PubMed Google Scholar
-
295.J.J.M. Bagalwa, V.N. Laurence, C. Sayagh, A.S. Bashwira, Fitoterapia 81, 767-771 (2010) CrossRef PubMed Google Scholar
-
296.T.O. Siddiqi, J. Ahmad, S.U. Khan, K. Javed, M.S.Y. Khan, Philipp. J. Sci. 119, 41-47 (1990) PubMed Google Scholar
-
297.S. Keawsa-ard, S. Natakankitkul, S. Liawruangrath, A. Teerawutgulrag, Chiang Mai J. Sci. 39, 445-454 (2012) PubMed Google Scholar
-
298.K.A. Sukanya, B. Liawruangrath, S. Liawruangrath, A. Teerawutgulrag, Nat. Prod. Commun. 7, 955-958 (2012) PubMed Google Scholar
-
299.T. Payum, A.K. Das, R. Shankar, C. Tamuly, M. Hazarika, Am. J. PharmTech Res. 5, 307-314 (2015) PubMed Google Scholar
-
300.http://www.zhiwutong.com/latin/Solanaceae/Solanum-surattense-Burm-F.htm. Accessed 29 Nov 2017 PubMed Google Scholar
-
301.http://frps.eflora.cn/frps/Solanum%20surattense. Accessed 29 Nov 2017 PubMed Google Scholar
-
302.
-
303.S.A. Patil, S.N. Sambrekar, Int. J. Res. Pharma. Biomed. Sci. 3, 1559-1566 (2012) PubMed Google Scholar
-
304.T. Ahmed, R. Kanwal, N. Ayub, M. Hassan, Hum. Ecol. Risk Assess. 15, 624-635 (2009) CrossRef PubMed Google Scholar
-
305.Y. Lu, J. Luo, L. Kong, Phytochemistry 72, 668-673 (2011) CrossRef PubMed Google Scholar
-
306.M. Qasim, Z. Abideen, M.Y. Adnan, S. Gulzar, B. Gul, M. Rasheed, S. Afr. J. Bot. 110, 240-250 (2017) CrossRef PubMed Google Scholar
-
307.A. Yadav, R. Bhardwaj, R.A. Sharma, Int. J. Pharm. Pharm. Sci. 5, 489-493 (2013) PubMed Google Scholar
-
308.M.M. Ahmed, S. Andleeb, F. Saqib, B.A. Ch, M. Hussain, M.N. Khatun, H. Rahman, B.M.C. Comp, Altern. Med. 16, 166 (2016) PubMed Google Scholar
-
309.A. Ramazani, S. Zakeri, S. Sardari, N. Khodakarim, N.D. Djadidt, Malaria J. 9, 124 (2010) CrossRef PubMed Google Scholar
-
310.https://en.wikipedia.org/wiki/Solanum_torvum. Accessed 6 Dec 2017 PubMed Google Scholar
-
311.http://fleppc.org/ID_book/solanum%20torvum.pdf. Accessed 6 Dec 2017 PubMed Google Scholar
-
312.http://tropical.theferns.info/viewtropical.php?id=Solanum+torvum. Accessed 6 Dec 2017 PubMed Google Scholar
-
313.W.H. Maser, N.D. Yuliana, N. Andarwulan, J. Liq. Chrom. Rel. Technol. 38, 1230-1235 (2015) CrossRef PubMed Google Scholar
-
314.S.B. Paul, M.D. Choudhury, R. Choudhury, Asian J. Chem. 21, 581-588 (2009) PubMed Google Scholar
-
315.C. Balachandran, N. Emi, Y. Arun, Y. Yamamoto, B. Ahilan, B. Sangeetha, Chem.-Biol. Inter. 242, 81-90 (2015) CrossRef PubMed Google Scholar
-
316.Y. Lu, J. Luo, X. Huang, L. Kong, Steroids 74, 95-101 (2009) CrossRef PubMed Google Scholar
-
317.J. Li, L. Zhang, C. Huang, F. Guo, Y. Li, Fitoterapia 93, 209-215 (2014) CrossRef PubMed Google Scholar
-
318.R.U. Abhishek, S. Thippeswamy, K. Manjunath, D.C. Mohana, J. Appl. Microbiol. 119, 1624-1636 (2015) CrossRef PubMed Google Scholar
-
319.K.F. Chah, K.N. Muko, S.I. Oboegbulem, Fitoterapia 71, 187-189 (2000) CrossRef PubMed Google Scholar
-
320.C. Balachandran, V. Duraipandiyan, N.A. Al-Dhabi, K. Balakrishna, Indian J. Microbiol. 52, 676-681 (2012) CrossRef PubMed Google Scholar
-
321.T.B. Nguelefack, C.B. Feumebo, G. Ateufack, P. Watcho, S. Tatsimo, J. Ethnopharmacol. 119, 135-140 (2008) CrossRef PubMed Google Scholar
-
322.D. Arthan, J. Svasti, P. Kittakoop, D. Pittayakhachonwut, M. Tanticharoen, Phytochemistry 59, 459-463 (2002) CrossRef PubMed Google Scholar
-
323.S. Challal, O.E.M. Buenafe, E.F. Queiroz, S. Maljevic, L. Marcourt, M. Bock, A.C.S. Chem, .Neuroscience 5, 993-1004 (2014) PubMed Google Scholar
-
324.T.B. Nguelefack, H. Mekhfi, T. Dimo, S. Afkir, M. Nguelefack, P. Elvine, A. Legssyer, A. Ziyyat, J. Comp. Integ. Med. 5 (2008) PubMed Google Scholar
-
325.M. Mohan, B.S. Jaiswal, S. Kasture, J. Ethnopharmacol. 126, 86-89 (2009) CrossRef PubMed Google Scholar
-
326.M. Mohan, S. Kamble, P. Gadhi, S. Kasture, F. Chem, Toxicology 48, 436-440 (2010) PubMed Google Scholar
-
327.C.H. Ramamurthy, A. Subastri, A. Suyavaran, C. Thirunavukkarasu, Environ. Sci. Pollut. Res. Int. 23, 7919-7929 (2016) CrossRef PubMed Google Scholar
-
328.C. Lalmuanthanga, C. Lalchhandama, M.C. Lallianchhunga, M.A. Ali, World J. Pharm. Res. 4, 1752-1759 (2015) PubMed Google Scholar
-
329.W. Kusirisin, C. Jaikang, C. Chaiyasut, P. Narongchai, Med. Chem. 5, 583-588 (2009) CrossRef PubMed Google Scholar
-
330.C.H. Ramamurthy, M.S. Kumar, V.S.A. Suyavaran, J. Food Sci. 77, 907-913 (2012) CrossRef PubMed Google Scholar
-
331.C.L. Lee, T.L. Hwang, W.J. He, Y.H. Tsai, C.T. Yen, H.F. Yen, C.J. Chen, Phytochemistry 95, 315-321 (2013) CrossRef PubMed Google Scholar
-
332.M. Mohan, D. Attarde, R. Momin, S. Kasture, Nat. Prod. Res. 27, 2140-2143 (2013) CrossRef PubMed Google Scholar
-
333.R. Momin, M. Mohan, Nat. Prod. Res. 26, 416-422 (2012) CrossRef PubMed Google Scholar
-
334.C. Kamaraj, N.K. Kaushik, D. Mohanakrishnan, G. Elango, A. Bagavan, Parasit. Res. 111, 703-715 (2012) CrossRef PubMed Google Scholar
-
335.G.R. Gandhi, S. Ignacimuthu, M.G. Paulraj, P. Sasikumar, Eur. J. Pharmacol. 670, 623-631 (2011) CrossRef PubMed Google Scholar
-
336.G.R. Gandhi, S. Ignacimuthu, M.G. Paulraj, Food Chem. Toxicol. 49, 2725-2733 (2011) CrossRef PubMed Google Scholar
-
337.K. Takahashi, Y. Yoshioka, E. Kato, S. Katsuki, K. Hosokawa, J. Kawabata, Biosci. Biotechnol. Biochem. 74, 741-745 (2010) CrossRef PubMed Google Scholar
-
338.C. Kamaraj, A.A. Rahuman, G. Elango, A. Bagavan, A.A. Zahir, Parasit. Res. 109, 37-45 (2011) PubMed Google Scholar
-
339.http://swbiodiversity.org/seinet/taxa/index.php?taxon=28414. Accessed 19 Dec 2017 PubMed Google Scholar
-
340.
-
341.L. Brito, F. Wendy, S. Mejia, M. Gaspar, C.M. Francisco, Afinidad 52, 49-52 (1995) PubMed Google Scholar
-
342.http://tropical.theferns.info/viewtropical.php?id=Solanum+trilobatum. Accessed 21 Dec 2017 PubMed Google Scholar
-
343.http://naturalhomeremedies.co/Strilobatum.html. Accessed 21 Dec 2017 PubMed Google Scholar
-
344.A. Kanchana, C. Panneerselavam, Int. J. Curr. Res. Rev. 3, 37-51 (2011) PubMed Google Scholar
-
345.R.R. Thanigaiarassu, K. Kannabiran, V.G. Khanna, J. Pharm. Res. 2, 273-276 (2009) PubMed Google Scholar
-
346.K. Kannabiran, R.R. Thanigaiarassu, G.K. Venkatesan, J. Appl. Biol. Sci. 2, 109-112 (2008) PubMed Google Scholar
-
347.M. Vanaja, K. Paulkumar, G. Gnanajobitha, S. Rajeshkumar, C. Malarkodi, G. Annadurai, International Journal of Metals, pp. 1-9 (2014) PubMed Google Scholar
-
348.A.K. Zameer, S.S.Z. Ahmed, P. Ponnusamy, K.B. Senthil, Pak. J. Pharm. Sci. 29, 1578 (2016) PubMed Google Scholar
-
349.M.B. Kanchana, Int. J. Pharm. Pharm. Sci. 3, 356-364 (2011) PubMed Google Scholar
-
350.P.V. Mohanan, K.S. Devi, Cancer Lett. 110, 71-76 (1996) CrossRef PubMed Google Scholar
-
351.P.V. Mohanan, K.S. Devi, Cancer Lett. 112, 219-223 (1997) CrossRef PubMed Google Scholar
-
352.M. Shahjahan, G. Vani, C.S. Shyamaladevi, Chem.-Biol. Int. 156, 113-123 (2005) CrossRef PubMed Google Scholar
-
353.P. Jagadeesan, D.A. Prasad, P. Pandikumar, S. Ignacimuthu, Indian J. Nat. Prod. Res. 2, 156-163 (2011) PubMed Google Scholar
-
354.P.V. Mohanan, K.S. Devi, J. Exp. Clin. Cancer Res. 17, 159-164 (1998) PubMed Google Scholar
-
355.P. Govindarajan, C. Chinnachamy, Pak. J. Pharm. Sci. 27, 2101-2107 (2014) PubMed Google Scholar
-
356.P.N. Venkatesan, P. Rajendran, G. Ekambaram, D. Sakthisekaran, Nat. Prod. Res. 22, 1094-1106 (2008) CrossRef PubMed Google Scholar
-
357.S. Rajkumar, A. Jebanesan, J. Ins. Sci. 5, 15 (2005) PubMed Google Scholar
-
358.A. Pandurangan, R.L. Khosa, S. Hemalatha, Nat. Prod. Res. 25, 1132-1141 (2011) CrossRef PubMed Google Scholar
-
359.A. Pandurangan, R.L. Khosa, S. Hemalatha, J. Asian Nat. Prod. Res. 12, 691-695 (2010) CrossRef PubMed Google Scholar
-
360.M. Shahjahan, K.E. Sabitha, M. Jainu, D.C.S. Shyamala, Indian J. Med. Res. 120, 194-198 (2004) PubMed Google Scholar
-
361.http://solanaceaesource.org/taxonomy/term/110074/descriptions. Accessed 21 Dec 2017 PubMed Google Scholar
-
362.A. Maxwell, M. Seepersaud, R. Pingal, D.R. Mootoo, W.F. Reynolds, J. Nat. Prod. 59, 200-201 (1996) CrossRef PubMed Google Scholar
-
363.https://en.wikipedia.org/wiki/Potato. Accessed 22 Dec 2017 PubMed Google Scholar
-
364.https://www.hort.purdue.edu/newcrop/duke_energy/Solanum_tuberosum.html. Accessed 22 Dec 2017 PubMed Google Scholar
-
365.K.D. Chandrashekara, S.M. Dharmesh, J. Pharm. Res. 8, 1148-1157 (2014) PubMed Google Scholar
-
366.A.A.A. Mohdaly, M.A. Sarhan, I. Smetanska, A. Mahmoud, J. Sci. Food Agric. 90, 218-226 (2010) CrossRef PubMed Google Scholar
-
367.D. Paik, P. Das, T.P. De, T. Chakraborti, Exp. Parasit. 146, 11-19 (2014) CrossRef PubMed Google Scholar
-
368.D. Paik, P. Das, P.K. Pramanik, K. Naskar, T. Chakraborti, Biomed. Pharmacother. 83, 1295-1302 (2016) CrossRef PubMed Google Scholar
-
369.T. Zuber, D. Holm, P. Byrne, L. Ducreux, M. Taylor, M. Kaiser, C. Stushnoff, Food Funct. 6, 72-83 (2015) PubMed Google Scholar
-
370.E. Langner, F.M. Nunes, P. Pozarowski, M. Kandefer-Szerszen, Food Chem. Toxicol. 57, 246-255 (2013) CrossRef PubMed Google Scholar
-
371.L. Reddivari, J. Vanamala, S. Chintharlapalli, S.H. Safe, J.C. Miller Jr., Carcinogenesis 28, 2227-2235 (2007) PubMed Google Scholar
-
372.M. Friedman, K.R. Lee, H.J. Kim, I.S. Lee, N. Kozukue, J. Agric. Food Chem. 53, 8420 (2005) CrossRef PubMed Google Scholar
-
373.J.A.O. Ojewole, D.R. Kamadyaapa, C.T. Musabayane, Cardiovasc. J. S. Afr. 17, 166-171 (2006) PubMed Google Scholar
-
374.
-
375.http://calscape.org/Solanum-umbelliferum. Accessed 23 Dec 2017 PubMed Google Scholar
-
376.https://en.wikipedia.org/wiki/Solanum_umbelliferum. Accessed 23 Dec 2017 PubMed Google Scholar
-
377.https://www.wildflower.org/plants/result.php?id_plant=soum. Accessed 23 Dec 2017 PubMed Google Scholar
-
378.http://www.watershednursery.com/nursery/plant-finder/solanum-umbelliferum/. Accessed 23 Dec 2017 PubMed Google Scholar
-
379.http://www.theodorepayne.org/mediawiki/index.php?title=Solanum_umbelliferum. Accessed 23 Dec 2017 PubMed Google Scholar
-
380.Y.C. Kim, Q.M. Che, A.A.L. Gunatilaka, D.G.I. Kingston, J. Nat. Prod. 59, 283-285 (1996) CrossRef PubMed Google Scholar
-
381.
-
382.http://www.asklepios-seeds.de/gb/solanum-uporo-seeds.html. Accessed 25 Dec 2017 PubMed Google Scholar
-
383.http://www.tradewindsfruit.com/content/cannibal-tomato.htm. Accessed 25 Dec 2017 PubMed Google Scholar
-
384.H. Ripperger, Phytochemistry 44, 731-734 (1997) CrossRef PubMed Google Scholar
-
385.L.E.C. Suarez, D.R.M. Cendales, I.O.P. Clara, Rev. Colomb. Quimica 35, 59-65 (2006) PubMed Google Scholar
-
386.https://en.wikipedia.org/wiki/Solanum_vestissimum. Accessed 25 Dec 2017 PubMed Google Scholar
-
387.http://tropical.theferns.info/viewtropical.php?id=Solanum+vestissimum. Accessed 25 Dec 2017 PubMed Google Scholar
-
388.https://en.wikipedia.org/wiki/Solanum_villosum. Accessed 25 Dec 2017 PubMed Google Scholar
-
389.http://florida.plantatlas.usf.edu/Plant.aspx?id=56. Accessed 25 Dec 2017 PubMed Google Scholar
-
390.http://tropical.theferns.info/viewtropical.php?id=Solanum+violaceum. Accessed 25 Dec 2017 PubMed Google Scholar
-
391.F.R. Chang, C.T. Yen, M. El-Shazly, C.Y. Yu, Bioorg. Med. Chem. Lett. 23, 2738-2742 (2013) CrossRef PubMed Google Scholar
-
392.C.T. Yen, C.L. Lee, F.R. Chang, T.L. Hwang, H.F. Yen, C.J. Chen, S.L. Chen, J. Nat. Prod. 75, 636-643 (2012) CrossRef PubMed Google Scholar
-
393.G.S. Raju, M.R. Moghal, S.M.R. Dewan, M.N. Amin, M. Billah, Avic. J. Phytomed. 3, 313-320 (2013) PubMed Google Scholar
-
394.
-
395.http://www.himalayawellness.com/herbfinder/solanum-xanthocarpum.htm. Accessed 28 Dec 2017 PubMed Google Scholar
-
396.https://herbpathy.com/Uses-and-Benefits-of-Solanum-Xanthocarpum-Cid1087. Accessed 28 Dec 2017 PubMed Google Scholar
-
397.D. Rani, P.K. Dantu, Nat. Acad. Sci. Lett. 38, 275-279 (2015) CrossRef PubMed Google Scholar
-
398.S. Khanam, R. Sultana, Int. J. Pharm. Sci. Res. 3, 1057-1060 (2012) PubMed Google Scholar
-
399.G.P. Vadnere, R.S. Gaud, A.K. Singhai, Pharmacol. 1, 513-522 (2008) PubMed Google Scholar
-
400.S. Govindan, S. Viswanathan, V. Vijayasekaran, R. Alagappan, J. Ethnopharmacol. 66, 205-210 (1999) CrossRef PubMed Google Scholar
-
401.O.M. Singh, K. Subharani, N.I. Singh, N.B. Devi, L. Nevidita, Nat. Prod. Res. 21, 585-590 (2007) CrossRef PubMed Google Scholar
-
402.G. Shiv, S. Gaherwal, C.S. Shrivastava, N. Wast, Eur. J. Exp. Biol. 5, 81-89 (2015) PubMed Google Scholar
-
403.L.N.C.J. Packia, B.M.S. Bharath, M.A. Anita, B.J. Raja, S. Jeeva, Int. J. Inv. Pharm. Sci. 1, 433-437 (2013) PubMed Google Scholar
-
404.K. Abbas, U. Niaz, T. Hussain, M.A. Saeed, Z. Javaid, A. Idrees, S. Rasool, Acta Poloniae Pharm. 71, 415-421 (2014) PubMed Google Scholar
-
405.D.K. Kajaria, M. Gangwar, D. Kumar, S.A. Kumar, R. Tilak, G. Nath, Asian Pac. J Trop. Biomed. 2, 905-909 (2012) CrossRef PubMed Google Scholar
-
406.Z. Li, X. Cheng, C.J. Wang, G.L. Li, S.Z. Xia, F.H. Wei, Chin. J. Parasitol. Parasit. Diseases 23, 206-208 (2005) PubMed Google Scholar
-
407.W. Li, X. Huang, C. Qi, S. Liu, O. Kodama, K. Utaka, China Pest. 46, 591-593 (2007) PubMed Google Scholar
-
408.T. Changbunjong, W. Wongwit, S. Leemingsawat, Y. Tongtokit, V. Deesin, S. Asian J. Trop. Med. Pubic Health 41, 320-325 (2010) PubMed Google Scholar
-
409.T. Hussain, R.K. Gupta, K. Sweety, M.S. Khan, M.D. Hussain, A.M.D. Sarfaraj, Asian Pac. J Trop. Biomed. 2, 454-460 (2012) CrossRef PubMed Google Scholar
-
410.G.B. Jalali, H. Ghaffari, H.S. Prakash, K.R. Kini, Pharm. Biol. 52, 1060-1068 (2014) CrossRef PubMed Google Scholar
-
411.R.K. Gupta, T. Hussain, G. Panigrahi, A. Das, G.N. Singh, K. Sweety, Asian Pac. J Trop. Med. 4, 964-968 (2011) CrossRef PubMed Google Scholar
-
412.K. Poongothai, P. Ponmurugan, K.S.Z. Ahmed, B.S. Kumar, Asian Pac. J Trop. Med. 4, 778-785 (2011) CrossRef PubMed Google Scholar
-
413.D.M. Kar, L. Maharana, S. Pattnaik, G.K. Dash, J. Ethnopharmacol. 108, 251-256 (2006) CrossRef PubMed Google Scholar
-
414.C. Archana, J. Jacob, Asian J. Phytomed Clin. Res. 3, 32-36 (2015) PubMed Google Scholar
-
415.P. Velu, P. Iyappan, A. Vijayalakshmi, D. Indumathi, Biomed. Pharmacother. 84, 430-437 (2016) CrossRef PubMed Google Scholar
-
416.S. Kumar, A.K. Pandey, BMC Comp. Altern. Med. 14, 112 (2014) CrossRef PubMed Google Scholar
-
417.S. Kumar, U.K. Sharma, A.K. Sharma, A.K. Pandey, Cell. Mol. Biol. 58, 174-181 (2012) PubMed Google Scholar
-
418.K.K. Bhutani, A.T. Paul, W. Fayad, S. Linder, Phytomedicine, 789-793 (2010) PubMed Google Scholar
-
419.M.T. Rahman, M. Ahmed, M. Alimuzzaman, J.A. Shilpi, Fitoterapia 74, 119-121 (2003) CrossRef PubMed Google Scholar
-
420.T. Hussain, R.K. Gupta, K. Sweety, B. Eswaran, M. Vijayakumar, C.V. Rao, Asian Pac. J. Trop. Med. 5, 686-691 (2012) CrossRef PubMed Google Scholar
-
421.K.P. Mahesh, K. Murugan, K. Kovendan, J. Subramaniam, D. Amaresan, Parasit. Res. 110, 2541-2550 (2012) CrossRef PubMed Google Scholar
-
422.K.P. Mahesh, K. Murugan, K. Kovendan, C. Panneerselvam, Parasit. Res. 111, 609-618 (2012) CrossRef PubMed Google Scholar
-
423.S.K. Bansal, K.V. Singh, S. Kumar, J. Environ. Biol. 30, 221-226 (2009) PubMed Google Scholar
-
424.L. Mohan, P. Sharma, C.N. Srivastava, J. Environ. Biol. 26, 366-401 (2005) PubMed Google Scholar
-
425.K.M. Parmar, P.R. Itankar, A. Joshi, S.K. Prasad, J. Ethnopharmacol. 198, 158-166 (2017) CrossRef PubMed Google Scholar
-
426.D. Ranka, M. Aswar, U. Aswar, B.S. Bodhankar, Indian J. Exp. Biol. 51, 833-839 (2013) PubMed Google Scholar
-
427.B. Bhatt, J. Chem. Pharm. Res. 3, 176-181 (2011) PubMed Google Scholar
-
428.P.K. Patel, M.A. Patel, B.A. Vyas, D.R. Shah, T.R. Gandhi, J. Ethnopharmacol. 144, 160-170 (2012) CrossRef PubMed Google Scholar
-
429.O.M. Singh, T.P. Singh, J. Sci. Ind. Res. 69, 732-740 (2010) PubMed Google Scholar
-
430.A.C. Cuervo, B. Gerald, A.V. Patel, Phytochemistry 30, 1339-1341 (1991) CrossRef PubMed Google Scholar
-
431.W. Putalun, L.J. Xuan, H. Tanaka, Y. Shoyama, J. Nat. Prod. 62, 181-183 (1999) CrossRef PubMed Google Scholar
-
432.X.M. Zuo, L.Z. Guo, F. Cheng, Z.Y. Guo, K. Zou, Nat. Prod. Res. Dev. 25, 1205-1208 (2013) PubMed Google Scholar
-
433.Y. Lee Y., F. Hashimoto, S. Yahara, T. Nohara, N. Yoshida, Chem. Pharm. Bull. 42, 707-709 (1994) CrossRef PubMed Google Scholar
-
434.A. Mohy-ud-din, Z. Khan, M. Ahmad, M.A. Kashmiri, Asian J. Chem. 22, 2919-2927 (2010) PubMed Google Scholar
-
435.W. Shu, Y. Zhang, W. Ye, G. Zhou, J. Jinnan Univ. Nat. Sci. Med. 32, 493-497 (2011) PubMed Google Scholar
-
436.W. Shu, C. Wu, Y. Zhang, W.C. Ye, G. Zhou, Nat. Prod. Res. 27, 1982-1986 (2013) CrossRef PubMed Google Scholar
-
437.L.J. Hao, S. Wang, J.J. Zhu, Z.M. Wang, S.H. Wei, China J. Chin. Mat. Med. 39, 2034-2038 (2014) PubMed Google Scholar
-
438.R.H. Al-Sofany, O.A. Rashwan, Bull. Fac. Pharm. 39, 99-101 (2001) PubMed Google Scholar
-
439.K.M. Ahmed, Egyptian J. Pharm. Sci. 37, 37-44 (1996) PubMed Google Scholar
-
440.A.C. Suthar, R.M. Mulani, Pharmacogn. Mag. 4, 112-115 (2008) PubMed Google Scholar
-
441.K.E. Hellenas, A. Nyman, P. Slanina, L. Loof, J. Gabrielsson, J. Chromatogr. 573, 69-78 (1992) CrossRef PubMed Google Scholar
-
442.R.F. Keeler, D.C. Baker, W. Gaffield, Offic. J. Int. Soc Toxicon. 28, 873-884 (1990) CrossRef PubMed Google Scholar
-
443.E.A. Mona, H. Miyashita, T. Ikeda, J.H. Lee, H. Yoshimitsu, T. Nohara, K. Murakami, Chem. Pharm. Bull. 57, 747-748 (2009) CrossRef PubMed Google Scholar
-
444.X. Teng, Y.J. Zhang, C. Yang, Acta Bot. Yunnan. 30, 239-242 (2008) PubMed Google Scholar
-
445.Y. Lu, J. Luo, L. Kong, Mag. Reson. Chem. 47, 808-812 (2009) CrossRef PubMed Google Scholar
-
446.E.A. Ferro, N.L. Alvarenga, D.A. Ibarrola, I.M.C. Hellion, A.G. Ravelo, Fitoterapia 76, 577-579 (2005) CrossRef PubMed Google Scholar
-
447.S. Nakamura, M. Hongo, S. Sugimoto, H. Matsuda, M. Yoshikawa, Phytochemistry 69, 1565-1572 (2008) CrossRef PubMed Google Scholar
-
448.Y.Y. Lu, J.G. Luo, L.Y. Kong, Chin. J. Nat. Med. 9, 30-32 (2011) PubMed Google Scholar
-
449.S. Yahara, T. Yamashita, N. Nozawa, T. Nohara, Phytochemistry 43, 1069-1074 (1996) CrossRef PubMed Google Scholar
-
450.W. Shu, G. Zhou, W. Ye, Chin. Trad. Herb. Drugs 42, 424-427 (2011) PubMed Google Scholar
-
451.A.P. Colmenares, L.B. Rojas, O.A.C. Mitaine, L. Pouysegu, S. Quideau, T. Miyamoto, C. Tanaka, T. Paululat, Phytochemistry 86, 137-143 (2013) CrossRef PubMed Google Scholar
-
452.Y. Iida, Y. Yanai, M. Ono, T. Ikeda, T. Nohara, Chem. Pharm. Bull. 53, 1122-1125 (2005) CrossRef PubMed Google Scholar
-
453.M. Ohno, K. Murakami, M. El-Aasr, J.R. Zhou, K. Yokomizo, M. Ono, T. Nohara, J. Nat. Med. 66, 658-663 (2012) CrossRef PubMed Google Scholar
-
454.H. Nawaz, E. Ahmed, A. Sharif, M. Arshad, N. Batool, M.A. Rasool, U.H. Mukhtar, Chem. Nat. Comp. 49, 1091-1094 (2014) CrossRef PubMed Google Scholar
-
455.H.T. Nguyen, V.T. Nguyen, D.T. Nguyen, V.M. Chau, Tap Chi Duoc Hoc 48, 31-36 (2008) PubMed Google Scholar
-
456.W. Putalun, N. Fukuda, H. Tanaka, Y. Shoyama, J. Liq. Chrom. Rel. Technol. 25, 2387-2398 (2002) CrossRef PubMed Google Scholar
-
457.A. Schwarz, E. Pinto, M. Haraguchi, Phytother. Res. 21, 1025-1028 (2007) CrossRef PubMed Google Scholar
-
458.J.J. Jared, L.K. Murungi, B. Torto, Pest Manag. Sci. 72, 828-836 (2016) CrossRef PubMed Google Scholar
-
459.M.T.F. Cornelius, C.C.F. Alves, T.M.S. da Silva, K.Z. Alves, M.G. de Carvalho, Rev. Bras. Farmacia 85, 57-59 (2004) PubMed Google Scholar
-
460.F.J.M. Barbosa, M.F. Agra, R.A. Oliveira, M.Q. Paulo, G. Trolin, Memo. Inst. Oswaldo Cruz 86, 189-191 (1991) CrossRef PubMed Google Scholar
-
461.J. Manosroi, A. Manosroi, P. Sripalakit, Acta Hort. 679, 105-111 (2005) PubMed Google Scholar
-
462.T. Purwanti, Majalah Farmasi Indon. 8, 115-122 (1997) PubMed Google Scholar
-
463.H. Zhou, F. Wang, Z. Fang, China J. Chin. Mat. Med. 36, 2096-2098 (2011) PubMed Google Scholar
-
464.J. Bhattacharyya, I.J.L.D. Basilio, L.C.S.L. Morais, M.F. Agra, Biochem. Syst. Ecol. 37, 228-229 (2009) CrossRef PubMed Google Scholar
-
465.F. Njeh, H. Feki, I. Koubaa, N. Hamed, M. Damak, Pharm. Biol. 54, 726-731 (2016) CrossRef PubMed Google Scholar
-
466.A. Cristea, M. Tanasescu, P.M. Adelina, Farmacia 40, 25-30 (1992) PubMed Google Scholar
-
467.C. Butnaru, L. Vlase, D. Lazar, L. Agoroaei, .Farmacia 59, 172-178 (2011) PubMed Google Scholar
-
468.H. Wintoch, A. Morales, C. Duque, P. Schreier, Nat. Food Chem. 41, 1311-1314 (1993) CrossRef PubMed Google Scholar
-
469.A. Sammani, E. Shammaa, F. Chehna, Int. J. Pharm. Sci. Rev. Res. 23, 23-27 (2013) PubMed Google Scholar
-
470.H. Ripperger, Phytochemistry 43, 705-707 (1996) CrossRef PubMed Google Scholar
-
471.A. Maxwell, M. Seepersaud, R. Pingal, D.R. Mootoo, W.F. Reynolds, J. Nat. Prod. 58, 625-628 (1995) CrossRef PubMed Google Scholar
-
472.C.A. Coy-Barrera, L.E. Cuca-Suarez, I.O. Clara, Acta Biol. 27, 131-134 (2005) PubMed Google Scholar
-
473.E.C.S. Luis, A.C.B. Carlos, C.I. Orozco, Rev. Colomb. Quimica 33, 7-12 (2004) PubMed Google Scholar
-
474.D. Li, Y.L. Zhao, X.J. Qin, L. Liu, X.W. Yang, Y.Y. Chen, X.D. Luo, Nat. Prod. Bioprospect. 6, 225-231 (2016) CrossRef PubMed Google Scholar
-
475.J.L. Liu, S.C. Chen, F.L. Lu, D.P. Li, Guangxi Plant Catal. 32, 415-418 (2012) PubMed Google Scholar
-
476.X.L. Zhou, X.J. He, G.X. Zhou, W.C. Ye, X.S. Yao, J. Asian Nat. Prod. Res. 9, 517-523 (2007) CrossRef PubMed Google Scholar
-
477.M. Xie, Y. Zhou, K. Zou, F. Cheng, R. Liu, J. Chin. Med. Mat. 31, 1332-1334 (2008) PubMed Google Scholar
-
478.J. Luo, B. Zhang, D. Deng, J. Chin. Cereals Oils 30, 125-128 (2015) PubMed Google Scholar
-
479.R. Venkatesh, R. Vidya, K. Kalaivani, Int. J. Pharm. Sci. Res. 5, 5283-5287 (2014) PubMed Google Scholar
-
480.H. Li, S.Y. Peng, D.P. Yang, B. Bai, L.P. Zhu, Z.M. Zhao, Chirality 28, 259-263 (2016) CrossRef PubMed Google Scholar
-
481.R. Mahadev, H. Ramakrishnaiah, V. Krishna, N.N. Kumar, Don. J. Ess. Oil-Bear. Plants 15, 387-391 (2012) CrossRef PubMed Google Scholar
-
482.X. Nie, L. Zhang, F. Yao, K. Xiao, S. Dai, China J. Chin. Mat. Med. 40, 1514-1517 (2015) PubMed Google Scholar
-
483.L. Zhang, H. Lei, H.Q. Lin, G.S. Li, X.D. Yue, S.J. Dai, Nat. Prod. Res. 29, 1889-1893 (2015) CrossRef PubMed Google Scholar
-
484.X.D. Yue, F. Yao, L. Zhang, G.S. Li, S.J. Dai, China J. Chin. Mat. Med. 39, 453-456 (2014) PubMed Google Scholar
-
485.S.J. Dai, L. Shen, Y. Ren, Nat. Prod. Res. 23, 1196-1200 (2009) CrossRef PubMed Google Scholar
-
486.S.M. Yu, H.J. Kim, E.R. Woo, H. Park, Arch. Pharm. Res. 17, 1-4 (1994) CrossRef PubMed Google Scholar
-
487.P. Yuan, F. Guo, K. Zheng, K. Chen, Q. Jia, Y. Li, Nat. Prod. Res. 30, 1682-1689 (2016) CrossRef PubMed Google Scholar
-
488.F. Marx, E.H.A. Andrade, J.G. Maia, Food Res. Technol. 206, 364-366 (1998) PubMed Google Scholar
-
489.M. Suarez, C. Duque, H. Wintoch, P. Schreier, J. Agric. Food Chem. 39, 1643-1645 (1991) CrossRef PubMed Google Scholar
-
490.M. Suarez, C. Duque, J. Agric. Food Chem. 39, 1498-1500 (1991) CrossRef PubMed Google Scholar
-
491.M. Suarez, C. Duque, C. Bicchi, H. Wintoch, G. Full, P. Schreier, Flavour Frag. J. 8, 215-220 (1993) CrossRef PubMed Google Scholar
-
492.C. Duque, H. Wintoch, M. Suarez, P. Schreier, Linalool glycosides as aroma precursors in lulo (Solanum vestissimum D.) fruit peelings (Prog. Flavour Precursor. Stud. Proc. Int. Conf. 1993), pp. 279-281 PubMed Google Scholar
-
493.H. Feki, I. Koubaa, M. Damak, Mediter. J. Chem. 2, 639-647 (2014) CrossRef PubMed Google Scholar
-
494.Y.L. Lin, W.Y. Wang, Y.H. Kuo, C.F. Chen, J. Chin. Chem. Soc. 47, 247-251 (2000) CrossRef PubMed Google Scholar
-
495.S.A. Nirmal, A.P. Patel, S.B. Bhawar, S.R. Pattan, J. Ethnopharmacol. 142, 91-97 (2012) CrossRef PubMed Google Scholar
-
496.Y. Zhao, F. Liu, H. Lou, Chin. Trad. Herb. Drugs 33, 555-556 (2010) PubMed Google Scholar
-
497.A. Mohy-Ud-Din, Z. Khan, M. Ahmad, M.A. Kashmiri, S. Yasmin, H. Mazhar, J. Chilean Chem. Soc. 54, 486-490 (2009) PubMed Google Scholar
-
498.W.W. Tang, X.L. Liu, D.Q. Zeng, J. Jiangxi Agric. Univ. 34, 483-486 (2012) PubMed Google Scholar
-
499.Y. Ji, Y. Sun, C. Hui, Lishizen Med. Mat. Med. Res 20, 2431-2432 (2009) PubMed Google Scholar
-
500.D. Lin, Y. Sun, L. Zhang, X. Zeng, shizen Med. Li, Mat. Med. Res 20, C3-C4 (2009) PubMed Google Scholar
-
501.A.C. Ramos, R.O. Rodrigo, Nat. Prod. Res. 31, 2405-2412 (2017) CrossRef PubMed Google Scholar
-
502.U.W. Hawas, G.M. Soliman, L.T.E. Abou, A.R.H. Farrag, J. Biosci. 68, 19-28 (2013) PubMed Google Scholar
-
503.M.M. Radwan, A. Badawy, R. Zayed, H. Hassanin, M.A. ElSohly, Med. Chem. Res. 24, 1326-1330 (2015) CrossRef PubMed Google Scholar
-
504.A. Badawy, R. Zayed, S. Ahmed, H. Hassanean, J. Nat. Prod. 6, 156-167 (2013) PubMed Google Scholar
-
505.Y. Wang, Z. Li, J. Yang, J. Yunnan Univ. 20, 396-398 (1998) PubMed Google Scholar
-
506.http://www.motherherbs.com/solanum-indicum.html. Accessed 25 Sept 2017 PubMed Google Scholar
-
507.Y. Ren, L. Shen, S. Dai, China J. Chin. Mat. Med. 34, 721-723 (2009) PubMed Google Scholar
-
508.G. Ishii, M. Mori, Y. Umemura, S. Takigawa, S. Tahara, Nippon Shokuhin Kagaku Kogaku Kaishi 43, 887-895 (1996) CrossRef PubMed Google Scholar
-
509.W. Dan, G.W. Chen, C.R. Han, W.J. Liu, H.Z. Yang, Chin. Trad. Herb. Drugs 43, 1068-1070 (2012) PubMed Google Scholar
-
510.J. Cardona, E.C. Juliana, S. Cuca, E. Luis, G. Barrera, A. Jaime, Rev. Colomb. Quimica 40, 185-200 (2011) PubMed Google Scholar
-
511.M. Ono, Y. Shiono, T. Tanaka, C. Masuoka, S. Yasuda, T. Ikeda, T. Nohara, J. Nat. Med. 64, 500-505 (2010) CrossRef PubMed Google Scholar
-
512.S. Siqueira, F.S. Vivyanne, M.F. Agra, C. Dariva, S.J.P. Siqueira, J. Sup. Flu. 58, 391-397 (2011) CrossRef PubMed Google Scholar
-
513.S.T.M.S. Silva, R. Braz-Filho, M.G. Carvalho, M.F. Agra, Biochem. Syst. Ecol. 30, 479-481 (2002) CrossRef PubMed Google Scholar
-
514.H.L. Yin, J. Li, Q.S. Li, J.X. Dong, Bull. Acad. Milit. Med. Sci. 34, 65-67 (2010) PubMed Google Scholar
-
515.H.J. Huang, H.M. Wang, A.C. Cao, C.X. Zhang, S.H. Wei, T.J. Ling, Nat. Prod. Res. 31, 1831-1835 (2014) PubMed Google Scholar
-
516.Q. Shao, L. Chang, Y. Wei, Z. Wei, J. Chromatogr. Sci. 56, 695-701 (2018) CrossRef PubMed Google Scholar
-
517.J. He, X.J. Zhang, B.Z. Ma, F. Liu, Chin. Pharm. J. 50, 2035-2038 (2015) PubMed Google Scholar
-
518.J.H. Li, H.L. Yin, J.X. Dong, Acad. Milit. Med. Sci. 37, 130-134 (2013) PubMed Google Scholar
-
519.A.K. Chakravarty, S. Mukhopadhyay, S. Saha, S.C. Pakrashi, Phytochemistry 41, 935-939 (1996) CrossRef PubMed Google Scholar
-
520.L. Wang, N. Wang, X. Yao, Chin. Trad. Herb. Drugs 30, 792-794 (2007) PubMed Google Scholar
-
521.A. Simaratanamongkol, K. Umehara, H. Niki, H. Noguchi, P. Panichayupakaranant, J. Funct. Foods 11, 557-562 (2014) CrossRef PubMed Google Scholar
-
522.X. Zuo, Z. Deng, Z. Guo, K. Zou, F. Cheng, J. Cent, China Norm. Univ. 46, 322-324 (2012) PubMed Google Scholar
-
523.G. Xie, W. Duan, B. Tao, C. Li, Nat. Prod. Res. Dev. 20(627-629), 643 (2008) PubMed Google Scholar
-
524.H. Zhou, F. Wang, Y. He, J. Tang, Z. Fang, West China J. Pharm. Sci. 26, 522-524 (2011) PubMed Google Scholar
-
525.E. Rodrigues, L.R.B. Mariutti, A.Z. Mercadante, J. Agric. Food Chem. 61, 3022-3029 (2013) CrossRef PubMed Google Scholar
-
526.D. Wu, Z. Fang, Guangdong Med. Coll. J. 24, 139-140 (2008) PubMed Google Scholar
-
527.A.K. Sharma, M.C. Sharma, M.P. Dobhal, Pharm. Lett. 5, 355-361 (2013) PubMed Google Scholar
-
528.V. Aeri, Rajkumari, M. Mujeeb, M. Ali, Indian J. Nat. Prod 21, 40-42 (2005) PubMed Google Scholar
-
529.G.A. Mathis, A. Toggenburger, R. Pokorny, S. Autzen, R. Ibanez, J. Steroid Biochem. Mol. Biol. 144, 40-43 (2014) CrossRef PubMed Google Scholar
-
530.H. Bachmann, S. Autzen, U. Frey, U. Wehr, Brit. Poultry Sci. 54, 642-652 (2013) CrossRef PubMed Google Scholar
-
531.D.R. Baek, M.J. Lee, N.I. Baek, K.H. Seo, Y.H. Lee, J. Appl. Biol. Chem. 59, 103-106 (2016) PubMed Google Scholar
-
532.M.G. Morais, A.F.C. Guilherme, A.A. Aleixo, L.A.R.S. Lima, Nat. Prod. Res. 29, 480-483 (2015) CrossRef PubMed Google Scholar
-
533.P.L. Yuan, X.P. Wang, K.X. Chen, Y.M. Li, Q. Jia, Chin. Patent Med. 38, 104-107 (2016) PubMed Google Scholar
-
534.M.C.D. Ricardo, C.S.L. Enrique, P. Orozco, I. Clara, Actual. Biol. 27, 49-52 (2005) PubMed Google Scholar
-
535.Y.H. Long, J.H. Li, J. Li, B. Li, L. Chen, J.X. Dong, Fitoterapia 84, 360-365 (2013) CrossRef PubMed Google Scholar
-
536.Y.H. Long, L.J. Hui, B. Li, L. Chen, J.X. Dong, J. Asian Nat. Prod. Res. 16, 153-157 (2014) CrossRef PubMed Google Scholar
-
537.J.M. Antonio, J.S. Gracioso, W. Toma, L.C. Lopez, F. Oliveira, A.R.M.S. Brito, J. Ethnopharmacol. 95, 83-88 (2004) CrossRef PubMed Google Scholar
-
538.T. Chen, J. Zhang, X. Sun, X. Xu, S. Zhang, Fine Chem. 26, 47-50 (2009) PubMed Google Scholar
-
539.https://species.wikimedia.org/wiki/Solanum_elaeagnifolium. Accessed 13 Sept 2017 PubMed Google Scholar
-
540.http://www.cabi.org/isc/datasheet/50516. Accessed 13 Sept 2017 PubMed Google Scholar
-
541.https://garden.org/plants/view/146851/Natri-Solanum-ligustrinum/. Accessed 28 Sept 2017 PubMed Google Scholar
-
542.https://garden.org/plants/photo/357234/. Accessed 28 Sept 2017 PubMed Google Scholar
-
543.https://en.wikipedia.org/wiki/Solanum_lycocarpum. Accessed 29 Sept 2017 PubMed Google Scholar
-
544.https://en.wikipedia.org/wiki/Solanum_muricatum. Accessed 06 Nov 2017 PubMed Google Scholar
-
545.http://www.pfaf.org/user/plant.aspx?latinname=Solanum+muricatum. Accessed 06 Nov 2017 PubMed Google Scholar
-
546.https://www.shootgardening.co.uk/plant/solanum-muricatum. Accesed 06 Nov 2017 PubMed Google Scholar
-
547.https://www.vanmeuwen.com/p/solanum-muricatum-mini-melon/v17830VM. Accessed 06 Nov 2017 PubMed Google Scholar
-
548.P.M. Veira, L.P.M. Marinho, S.C.S. Ferri, C. Lee, Anais Acad. Bras. Cienc. 85, 553-560 (2013) CrossRef PubMed Google Scholar
-
549.http://www.asklepios-seeds.de/gb/solanum-sessiliflorum-seeds.html. Accessed 15 Nov 2017 PubMed Google Scholar
-
550.D.R.L.H. Mascato, M. M. Passarinho, D.M.L. Galeno, R. P. Carvalho, J. Nutr. Met., 364185/1-364185/8 (2015) PubMed Google Scholar
-
551.http://tropical.theferns.info/viewtropical.php?id=Solanum+spirale. Accessed 15 Nov 2017 PubMed Google Scholar
-
552.http://www.hear.org/pier/species/solanum_torvum.htm. Accessed 6 Dec 2017 PubMed Google Scholar
-
553.T.B. Nguelefack, H. Mekhfi, A.B. Dongmo, T. Dimo, P. Watcho, J. Zoheir, J. Ethnopharmacol. 124, 592-599 (2009) CrossRef PubMed Google Scholar
-
554.G.T. El-Sherbini, R.A. Zayed, E.T. El-Sherbini, J. Parasit. Res. 2009 (2009) PubMed Google Scholar
-
555.N. Chowdhury, A. Ghosh, G. Chandra, B.M.C. Comp, Altern. Med. 8, 10 (2008) PubMed Google Scholar
Copyright information
© The Author(s) 2019
Open Access
This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.