Compounds from African Medicinal Plants with Activities Against Selected Parasitic Diseases: Schistosomiasis, Trypanosomiasis and Leishmaniasis
Abstract
Parasitic diseases continue to represent a threat on a global scale, particularly among the poorest countries in the world. This is particularly because of the absence of vaccines, and in some cases, resistance against available drugs, currently being used for their treatment. In this review emphasis is laid on natural products and scaffolds from African medicinal plants (AMPs) for lead drug discovery and possible further development of drugs for the treatment of parasitic diseases. In the discussion, emphasis has been laid on alkaloids, terpenoids, quinones, flavonoids and narrower compound classes of compounds with micromolar range activities against Schistosoma, Trypanosoma and Leishmania species. In each subparagraph, emphasis is laid on the compound subclasses with most promising in vitro and/or in vivo activities of plant extracts and isolated compounds. Suggestions for future drug development from African medicinal plants have also been provided. This review covering 167 references, including 82 compounds, provides information published within two decades (1997-2017).Graphical Abstract
Keywords
African medicinal plants Leishmaniasis Natural products Parasitic diseases Schistosomiasis Trypanosomiasis1 Introduction
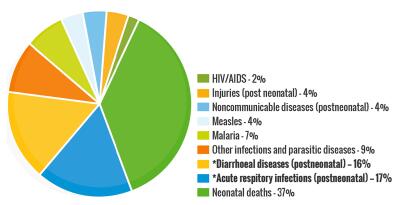
Parasites are considered as organisms that obtain their food by eating other organisms or their products in nature. These diseases continue to be a cause of considerable morbidity and mortality globally [1-4], including Trypanosomiasis (African sleeping sickness and Chagas disease) [5-7], Leishmaniasis [8] and Schistosomiasis [9, 10]. They threaten almost one-third of the world's population, the most numerous incidents being recorded in over 100 tropical and developing countries and territories, Fig. 1 [11-13]. The African region recorded the most death-related cases, especially amongst infants below the age of 5 and pregnant women. Schistosomiasis, caused by parasites of the Schistosoma genus are responsible for about 200 million sickness cases and about 280, 000 death-related incidents annually worldwide [9, 10, 14]. Only one drug (praziquantel) has been proven to be effective in the treatment of human Schistosomiasis, with no vaccine available or in development so far [15-21]. Serious concerns about drug selectivity and resistance were raised in 2013 when over 30 million people were treated in Sub-Saharan Africa [20]. Moreover, observed resistance and reduced efficiency of praziquantel in laboratory strains have prompted the search for alternative therapeutic strategies [20-27].
Global statistics for disease burdens in 2017
Trypanosomiasis, which represents several diseases caused by parasites of the genus Trypanosoma, is also of interest [5, 27-29]. This disease, which is much arguably the most important disease of man and domesticated animals, accounts for over 8 million reported annual cases globally, especially in the tropical regions of Latin America and Africa [30, 31]. Besides, great socioeconomic effects on the endemic areas by this disease are forecast if inadequate attention (both at the communal, national, and international levels) is not given [7, 29, 32-34]. Leishmaniasis is caused by parasites of the Leishmania type, which is also transmitted by certain types of sandflies [35, 36]. The diseases are reported by the WHO to be responsible for about 1 million new cases leading to approximately 30, 000 deaths annually on a global scale. The major cause is linked to environmental changes and affects mainly the very poor populations [37, 38]. These three diseases represent a real burden to the lives of millions of persons and their domesticated animals. The trio is capable of inflicting long-term disability and social stigmatisation, which can ultimately lead to a highly unproductive population and eventually result in economic loss and the slowdown of a country's development.
With the absence of any vaccine targeting any parasites and resistance against the already existing anti-parasitic drugs, research efforts have been employed and encouraged towards the search for new, cheaper, potent and effective drugs to treat these diseases. Medicinal plants represent a potential source of new drugs. This is because natural products (NPs) from organisms such as animals, fungi and the higher plants have been known to be good sources of pharmacologically active compounds against several ailments, including parasitic infections. Moreover, NPs are believed to have significant advantages as lead molecules over synthetic molecules [39-49]. The criteria for choosing a particular natural product for studies are either based on the pre-existing knowledge on the traditional use of the source species in therapy (ethnobotanical knowledge) or the search for structurally related molecules with known pharmacologically active agents from chemical databases [49-54]. The African continent is highly diverse ethnobotanically. This might explain why about 80% of the population tends to rely on medicinal plants as a primary source of healthcare [55-67]. It is our goal to provide evidence of the efficacy and potency of plants used in traditional medicine against parasitic infections. The systematic documentation of the plant-based chemical constituents of African traditional medicine and attempting to using in silico procedures to investigate their modes of action are ongoing efforts [44-46, 52, 53], particularly on the isolated compounds from African medicinal plants (AMPs) with evaluated in vitro and/or in vivo activities against Trypanosomiasis [68-74], Schistosomiasis, Leishmaniasis [72-74] and other parasitic diseases [4]. However, the most recent review dates about 3 years back and was focused only on plants collected from Nigeria. Thus, an updated review that covers the entire continent for these three parasitic diseases is required now. The information presented herein was retrieved by searching literature from major international journals on natural products and medicinal chemistry, alongside available M.Sc. and Ph.D. theses and online databases [54, 75]. The information gathered is discussed under the main compound classes, as presented below and summarised in Tables 1, 2, and 3.
Bioactive alkaloids from African flora with potential for antitrypanosomal and antileischmanial drug discovery
Bioactive terpenoids from African flora with potential for antitrypanosomal anti-Schistosoma and antileischmanial drug discovery
Other bioactive compounds from African flora with potential for antitrypanosomal and antileischmanial drug discovery
2 Alkaloids
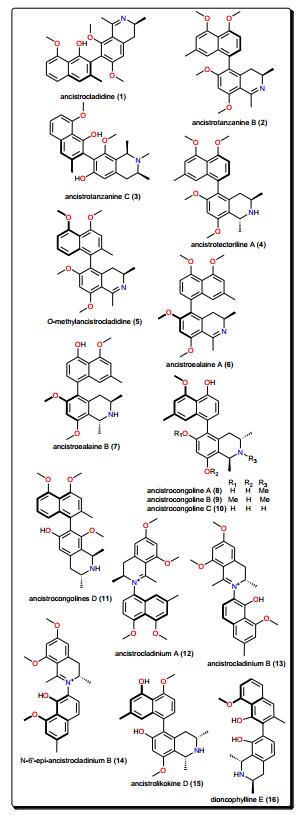
This class is characterized by nitrogen-containing compounds that are naturally occurring. Diverse species (fungi, plants, animals) have yielded several bioactive alkaloids against a broad range of diseases [76-84]. Table 1 summarises the alkaloids (compounds 1–33) isolated from AMPs and evaluated against these parasitic diseases, while Figs. 2, 3, 4 and 5 show a selection of some promising alkaloidal compounds, based on their evaluated activity (< 12.41 μM).
Antiprotozoal naphthylisoquinoline alkaloids
Aporphine alkaloids with trypanosidal potencies
Quinoline, indoles and other alkaloids showing activities against Trypanosoma species
SAR for W. indica compounds inhibiting T. cruzi, T. b. brucei and T. b. rhodesiense
2.1 Naphthylisoquinolines
The leaves, stem bark and roots of Ancistrocladus sp. (Ancistrocladaceae) are known to be rich sources of naphthylisoquinoline alkaloids (Fig. 2) [85-91]. Ancistrocladidine (1), ancistrotanzanines B (2), and C (3), ancistrotectoriline A (4), O-methylancistrocladidine (5), ancistroealaines A (6) and B (7), ancistrocongolines A-D (8–11), ancistrocladiniums A (12) and B (13), N-6′-epi-ancistrocladinium B (14), ancistrolikokine D (15) and dioncophylline E (16) are few examples of naphthylisoquinoline antiparasitic alkaloids from Ancistrocladus sp. and Dioncophyllum thollonii (Dioncophyllaceae).
The evaluation of the biological activities of these compounds showed them to be a rare set and promising class of antiprotozoal and antiviral agents, which are only found in plants of the Ancistrocladaceae and Dioncophyllaceae, mostly found in Africa. Their anti-Trypanosoma activities are evident (e.g., with IC50 values ranging from 0.17 to 12.41 μM against Trypanosoma brucei rhodesiense), alongside good to moderate activities against Trypanosoma cruzi and Leishmania donovani. It might be worth mentioning that the isoquinoline scaffold has also been explored synthetically for the discovery of novel antiprotozoals and antimicrobials [85, 86, 95-97].
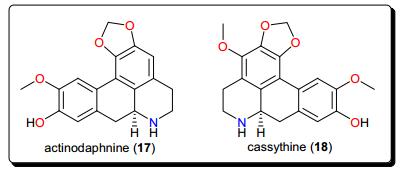
2.2 Aporphines
Other bioactive alkaloids include the aporphines (Fig. 3); actinodaphnine (17) and cassythine (18) from Cassytha filiformis (Lauraceae), a plant whose alkaloidal extract showed activity against T. b. brucei (with an IC50 value of 2.2 μg/mL). This confirmed the use of this plant in African folkloric medicine to treat African Trypanosomiasis and other diseases [92, 98, 99]. The isolated compounds displayed antitrypanosomal activities, with IC50 values of 10.29 and 17.60 μM for compounds 17 and 18, respectively. Although, the compounds showed low selectivity indices to HeLa cells (e.g., for actinodaphnine, IC50 (HeLa)/IC50 (T. b. brucei) < 5), when compared with the alkaloidal fraction (selectivity index = 16), they represent good starting scaffolds that could be optimised in order to improve the efficacy and selectivity in the search for new bioactive molecules with trypanocidal effects.
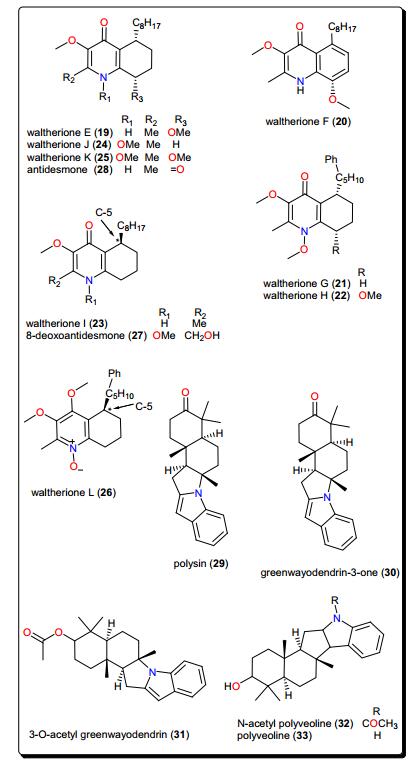
2.3 Quinolines
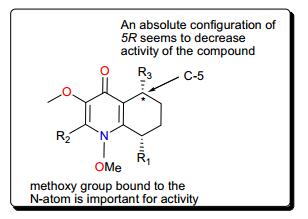
Other trypanocidal alkaloids include the quinolines (Fig. 4); waltheriones E–L (19–26), 8-deoxoantidesmone (27) and antidesmone (28) from Waltheria indica (Malvaceae) [93]. This plant is used in traditional medicine for the treatment of several ailments, including malaria [63, 100-104]. The dichloromethane root extract showed activities against T. cruzi (IC50 = 0.74 μg/mL), T. b. brucei (2.3% survival at 20 μg/mL) and T. b. rhodesiense (IC50 = 17.4 μg/mL) [93]. With the exception of waltherione L (26), with a slightly higher IC50(3.1 μM), the isolated compounds all displayed potent growth inhibition toward the amastigote form of T. cruzi (the Tulahuen C4 strain), with IC50 values lower than that of the reference drug benznidazole (IC50 = 2.9 μM). Structure–activity relationships (SARs) provide suggestions that, a methoxy group, bound to the nitrogen atom is important for activity (e.g., as in compounds 22, 24 and 25). This group at this position increased the lethality of T. cruzi. Furthermore, the absolute configuration (5R) (as in compounds 23, 26, 27) seems to result in a decrease of activity, while the presence of an N-oxide function (as in compound 26) is detrimental for T. cruzi inhibitory activity (Fig. 5). Finally, a comparison of the IC50 values of the isolated compounds against T. brucei sp. and T. cruzi highlighted selective toxicity towards the latter. This suggests that these molecules (or the waltherione scaffold) is a potential starting point for new safe antitrypanocidal drug development, although antidesmone (28) has already been patented for its potential as an antiprotozoal drug since 2003 [93, 105, 106].
2.4 Indoles and Other Alkaloids
Polysin (29), an indolosesquiterpene alkaloid from Polyalthia suaveolens (Annonaceae), was isolated together with the known alkaloids (Fig. 4); greenwayodendrin-3-one (30), 3-O-acetyl greenwayodendrin (31), N-acetyl polyveoline (32) and polyveoline (33). These alkaloids have demonstrated interesting activities on selected glycolytic enzymes, e.g., phosphofructo kinase (PFK), glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and aldolase [94]. Of particular interest are polysin (29) and 3-O-acetyl greenwayodendrin (31). Compound 29 acted as a competitive reversible inhibitor against T. brucei PFK (Ki = 10 μM), while compound 31 acted as a selective inhibitor of T. brucei aldolase (with IC50 ~ 0.5 μM). Meanwhile, polyveoline (33) acted as a selective inhibitor of T. brucei PFK and is a mixed reversible inhibitor of T. brucei GAPDH. These compounds, therefore, represent a good starting point for the design of new selective and potent trypanosomal drugs.
3 Terpenoids
Terpenoids constitute a large and diverse class of naturally occurring secondary metabolites, with interesting physiological and pharmacological functions [44, 107-110]. Their main scaffolds occur as multicyclic structures, e.g., hemi-terpenoids (5 carbon atoms), monoterpenoids (10 carbon atoms), sesquiterpenoids (15 carbon atoms), diterpenoids (20 carbon atoms), sesterterpenoids (25 carbon atoms), triterpenoids (30 carbon atoms), tetraterpenoids (40 carbon atoms), and polyterpenoids (more than 40 carbon atoms), which are all primarily derived from the five-carbon isoprene units [45, 107]. Terpenoids have been proven to possess interesting pharmacological activities as seen in the summary presented in Table 2 (compounds 34–61) and their corresponding structures shown in Figs. 6, 7, 8, 9 and 10[44, 111-114].
Sesquiterpenoids which have demonstrated anti-Trypanosoma activities
Carvotacetones with potent antileishmanial activities
Diterpenoids and a triterpenoid with selective inhibitory activity against T. brucei GAPDH
Potent compound with selective activity for L. donovani, when compared with the activity against T. b. brucei
Triterpenoids with antiparasitic activities
3.1 Sesquiterpenoids
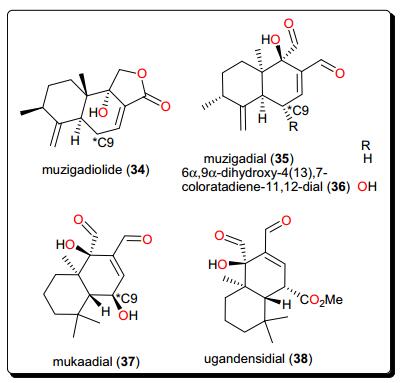
The sesquiterpenoids (Fig. 6), muzigadiolide (34), muzigadial (35), 6α, 9α-dihydroxy-4(13), 7-coloratadiene-11, 12-dial (36), mukaadial (37) and ugandensidial (38), from the East African medicinal plant Warburgia ugandensis (Canellaceae) have demonstrated anti-Trypanosoma activities [115]. The compounds displayed in vitro activities (with IC50 values ranging from 0.64 to 6.4 μM) against T. b. rhodesiense, the parasite responsible for African sleeping sickness. Compound 37 had previously been isolated from the same plant, also showing antitrypanocidal activity [116]. This plant (now regarded as an endangered species) has attracted many researchers because of its traditional use for the treatment of a variety of ailments, including malaria and diverse fevers [115-117]. SAR studies suggested that an additional dialdehyde functional group to the sesquiterpene lactone backbone, together with a hydroxyl group attached to C-9 contribute to the activity of the compounds.
3.2 Carvotacetone Derivatives
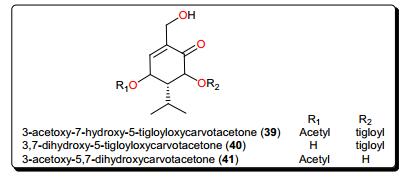
The native tropical East African medicinal plant Sphaeranthus bullatus (synonym: S. gallensis Sacleux, Family: Asteraceae) has been the origin of several compounds (Fig. 7) [118-120], including the carvotacetone derivatives; 3-acetoxy-7-hydroxy-5-tigloyloxycarvotacetone (39), 3, 7-dihydroxy-5-tigloyloxycarvotacetone (40) and 3-acetoxy-5, 7-dihydroxycarvotacetone (41). Compounds 39–41 demonstrated antileishmanial activities, with IC50 values of 2.16, 10.64 and 2.89 μM, respectively, against the parasite L. donovanii promastigotes.
3.3 Diterpenoids
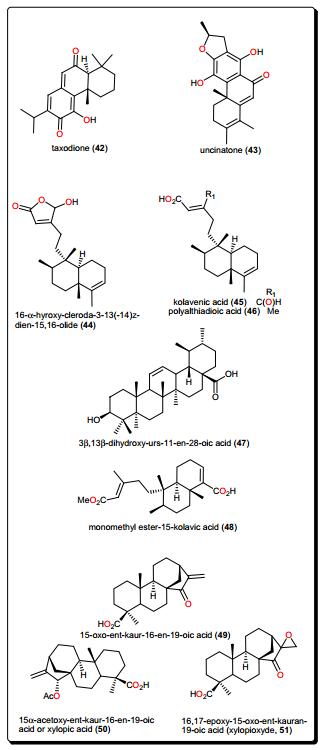
Other terpenoids include the abietane diterpenoids, taxodione (42) and uncinatone (43), Fig. 8, from the roots of Clerodendrum eriophyllum (Verbenaceae) [121], which displayed potent antileishmanial activities (with IC50 values of 0.25 and 0.61 μM, respectively) against L. donovanii. The activities of the crude extracts, e.g., the ethyl acetate extracts of Newbouldia laevis (Bignoniaceae) (EC50 4.2 μg/mL) and Eucalyptus maculata (Myrtaceae) (EC5012.3 μg/mL) and the hexane extract of Polyalthia longifolia (Annonaceae) (EC50 2.4 μg/mL) as well as their isolated active compounds (Fig. 8); 16-α-hydroxy-cleroda-3-13(-14)z-dien-15, 16-olide (44), kolavenic acid (45), polyalthiadioic acid (46) and the triterpenoid 3β, 13β-dihydroxy-urs-11-en-28-oic acid (47) were observed against different trypanosomes strains (s427 WT, B48 and AQP2/3KO) [122]. While these pure compounds exhibited activities against the tested strains, with EC50 values ranging from 1.16 to 40.46 μM, it was remarkable that no toxicity towards Human Embryonic Kidney cells was observed even at concentrations up to 400 μg/mL (1.31 μM), thus suggesting new scaffolds to be further developed for the treatment of the wild-type and multi-drug resistant T. brucei [122, 123]. Also interesting is the kolavic acid derivative; monomethyl ester-15-kolavic acid (48) isolated from Entada abyssinica (Fabaceae) [124], which demonstrated interesting selective inhibitory activity (IC50 value of 12 μM) against T. brucei GAPDH [125].
Other bioactive diterpenoids include 15-oxo-ent-kaur-16-en-19-oic acid (49), 15α-acetoxy-ent-kaur-16-en-19-oic acid or xylopic acid, (50) and 16, 17-epoxy-15-oxo-ent-kauran-19-oic acid or xylopioxyde (51), from the fruits of Xylopia aethiopica (Annonaceae) [126]. These compounds and their synthetic epoxide analogues were screened on antitrypanosomal and cytotoxicity assays, showing that only the naturally-occurring compounds (49–51) displayed cytotoxic effects on the mammalian fibroblast cell line MRC-5 (with ED50 values ranging from 22 to 121 μM), as well as inhibitory effects on the growth of the bloodstream forms of T. b. brucei cells (strain 241) (ED50 ranging from 27 to 205 μM).
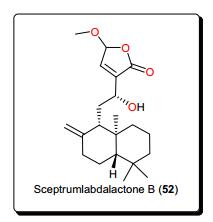
The genus Aframomum (Zingiberaceae), has been the source of the antitryposonomals. Sceptrumlabdalactone B (52, Fig. 9) was identified, from the rhizomes of A. sceptrum, a plant locally used for the treatment of infectious diseases including human African Trypanosomiasis (sleeping sickness), together with sceptrumlabdalactone A [127, 134-136]. The activity of compound 52 (with IC50 value of 5.7 μM) against L. donovani was comparable to that of reference drugs (IC50 of 2.5 and 3.0 μM for pentamidine and miltefosine respectively). Additionally, this molecule demonstrated selective activity for L. donovani, when compared with the activity against T. b. brucei.
3.4 Triterpenoids
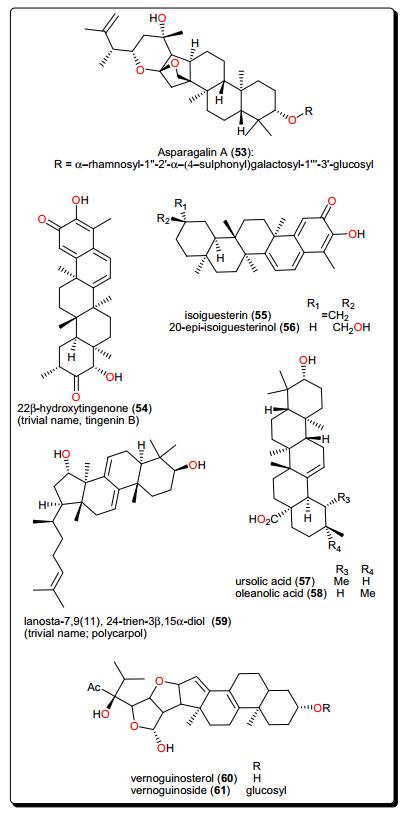
The anti-schistosomal activity of Asparagalin A (53, Fig. 10), from the Egyptian medicinal plant Asparagus stipularis (Asparagaceae) has been evaluated [128]. It was found that this compound was able to significantly reduce the ability of adult female worms to lay eggs. It was further shown that the compound had some suppressive effect on egg-laying capacity in a dose-dependence manner [137]. Elaeodendron schlechteranum (Celastraceae) is the source of tingenin B or 22β-hydroxytingenone (54) [129]. This compound has displayed a broad range of activities, e.g., against T. cruzi (IC50 < 0.57 μM), T. brucei (< 0.57 μM), L. infantum (1.67 μM), and P. falciparum (0.83 μM), confirming the claim of the applicability of the plant in traditional medicine to treat various non-infectious diseases [63, 138]. Albeit, being highly cytotoxic to MRC-5 cells (CC50 0.45 μg/mL), compound 54 indicates a poor selectivity to normal cells. Further studies on this compound could be considered in order to suggest less toxic and more selective analogues for the development of novel antiparasitics. The bisnortriterpenes from Salacia madagascariensis (Celastraceae); isoiguesterin (55) and 20-epi-isoiguesterinol (56) showed potent activities against Leishmania sp. [130]. Meanwhile, isoiguesterin (55) and 20-epi-isoiguesterinol (56) displayed comparable activities with chloroquine and artemisinin against the D6 clone, being more potent and selective against L. donovani (a species known to cause visceral Leishmaniasis). When compared with amphotericin B, used currently in the treatment of Leishmaniasis, compounds 55 and 56 show great potential for future selective drug development against Leishmania.
Keetia leucantha (synonym: Plectronia leucantha Krause) is a West African tree of the Rubiaceae, used to treat a variety of infections, including parasitic infections [139, 140]. Ursolic acid (57) and oleanolic acid (58), along with other constituents were isolated from the leaves of this plant. An investigation of the antitrypanosomal activities of essential oil, the dichloromethane extract and isolated compounds on T. b. brucei bloodstream forms (Tbb BSF) and procyclic forms (Tbb PF) [131] showed that ursolic acid (57) and oleanolic acid (58) were the most bioactive tested compounds [131]. Ursolic acid displayed IC50 values of 5.48 and 14.25 μM, respectively, on Tbb BSF and Tbb PF, while oleanolic acid displayed an IC50 value of 16.00 μM on Tbb BSF. This could explain why the plant is effective in the traditional treatment of related parasitic ailments. Another identified triterpenoid was polycarpol or lanosta-7, 9(11), 24-trien-3β, 15α-diol (59) from Piptostigma preussi (Annonaceae) [132]. The compound showed antitrypanosomal activity with an ED50 value of 5.11 μM on T. brucei cells. An investigation of its mode of action showed that the compound acted by inhibiting T. brucei glycolytic enzymes GAPDH and PFK (glycolytic pathway enzymes validated by WHO as good targets for the development of drugs against trypanosomiasis), with IC50 values of 650 and 180 μM, respectively. The glycolytic enzymes GAPDH are responsible for ATP production and have been reported to be vital for the survival of Trypanosomatids [141, 142]. From the stem bark of Vernonia guineensis (Asteraceae), vernoguinosterol (60) and vernoguinoside (61), exhibited interesting trypanocidal activity with IC50 values in the range 4.60–7.67 μM [133].
4 Other Compound Classes
Other compound classes from AMP with reported activities on Leishmaniasis and Trypanosomiasis are shown in Figs. 11, 12, 13, 14, and 15, while a summary of the reported molecules is given in Table 3 (compounds 62–82).
An antitrypansomal amide and two diarylhepanoids
Acylphloroglucinols and a xanthone with very potent in vitro antileishmanial activities in the nanomolar range
Taccalonolides, a rare class of antiprotozoals
Quiniones and klaivanolide, which showed lower micromolar activities against against several Leishmania species
Flavonoids and phytosterols with trypanocidal activities
4.1 Amides
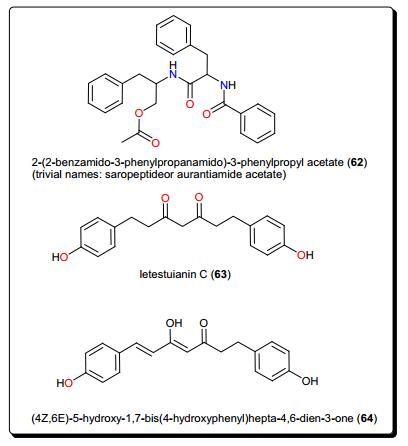
Plants from the genus Zapoteca (Fabaceae) have been the origin of diverse compounds with antiprotozoal activities. These include the ester 2-(2-benzamido-3-phenylpropanamido)-3-phenylpropyl acetate (trivial names; saropeptide or aurantiamide acetate) (62) from Z. portoricensis (Fig. 11) [143]. The IC50 values of compound 62 were 3.63, 41.65 and 92.05 μM against T. b. rhodesiense, T. cruzi and against rat skeletal myoblast cell line (L6 cells), respectively. The compound had been previously reported to possess anti-inflammatory as well as antiplatelet aggregation activities, which are complementary to the observed trypanocidal property [144-148]. Since inflammation poses major problems in the advanced stages of Trypanosomiasis, compound 62 represents a promising natural hit with a reasonable selectivity for T. b. rhodesiense.
4.2 Diarylhepanoids
Other potent antitrypanosomal compounds are the diarylheptanoid; letestuianin C (63) and (4Z, 6E)-5-hydroxy-1, 7-bis(4-hydroxyphenyl)hepta-4, 6-dien-3-one (64) from the species Aframomum letestuianum, Fig. 11 [136]. The activities of compounds 63 (4.49 μM) and 64 (8.39 μM) validate the use of the Aframomum sp. in treating parasitic ailments amongst others.
4.3 Acylphloroglucinols and Xanthones
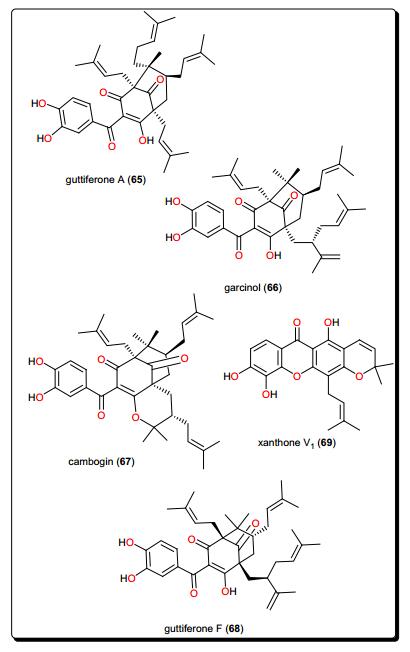
Guttiferone A (65), garcinol (66), cambogin (67) and guttiferone F (68) from Allanblackia monticola (Guttiferae or Clusiaceae) fruits and xanthone V1 (69) from Symphonia globulifera leaves have shown antileishmanial activities (Fig. 12) [149]. The compounds exhibited very potent in vitro antileishmanial activities, particularly compounds 65–67, with IC50 values of 0.16, 0.33 and 0.2 μM, for compounds 65, 66 and 67, respectively. These were lower than that of the reference drug, miltefosine (0.46 μM). SAR studies could further improve the activities of these compounds in order to enhance their selectivity indices against human cancer cell lines.
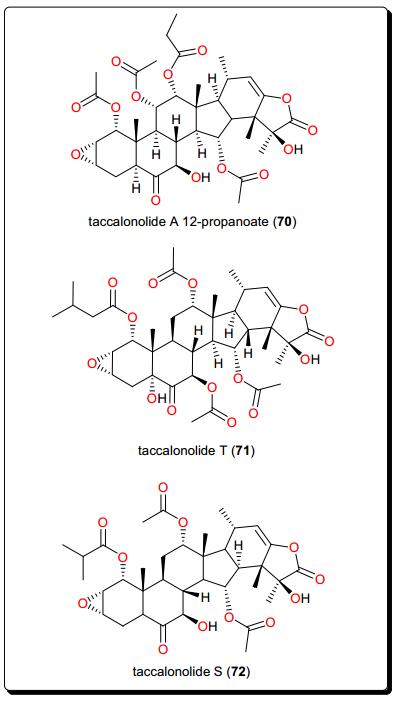
4.4 Taccalonolides
Beside their proven anticancer potential, these represent a quite potent class of antitrypanosomal compounds identified from Tacca leontopetaloides (Taccaceae) [157-162]. These include taccalonolide A 12-propanoate (70), taccalonolide T (71) and taccalonolide S (72) from the tubers of T. leontopetaloide (Fig. 13). They have shown activities against the T. b. brucei s427 lister strain [150]. These compounds and crude fractions yielded EC50 values as low as 0.79 μg/mL.
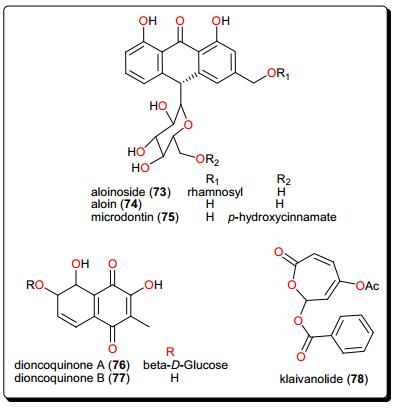
4.5 Quiniones and Klaivanolide
Quinones from Aloe species have also shown antileishmanial activities. These include aloinoside (73), aloin (74) and microdontin (75) from the leaf latex of A. calidophila (Fig. 14) [151]. It is noteworthy that, the activities of the most potent compounds, with IC50 values ranging from 3.12 to 10.92 μM against Leishmania aethiopica and from 3.70 to 15.26 μM against Leishmania major, were comparable to the control drug amphotericin B (IC50 = 0.12 and 0.07 μM against L. aethiopica and L. major respectively). The selectivity indices of aloinoside (73) (813.35 and 694.90, respectively, against L. aethiopica and L. major) were much better than those of the control, amphotericin B (423.49 and 688.96, respectively). This suggests that the isolated compounds could serve as potential scaffolds for the development of safe, specific and cost-effective antileishmanial agents [151]. Additionally, the dioncoquinones A (76) and B (77) isolated from Triphyophyllum peltatum (Dioncophyllaceae) showed good and specific activity against L. major by inhibiting the growth of the parasite at very low concentrations [152]. Klaivanolide (78), from the stems of Uvaria klaineana (Annonaceae), was also reported as a potent molecule (in vitro IC50 values of 1.75 and 3.12 μM, respectively) against sensitive and amphotericin B-resistant promastigote forms of L. donovani [153].
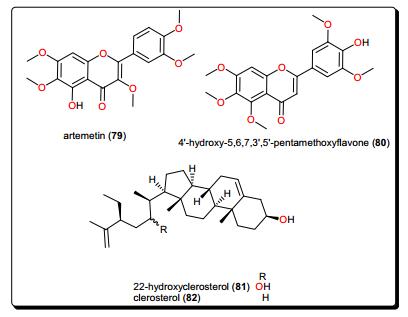
4.6 Flavonoids
Artemetin (79, Fig. 15), from Vitex simplicifolia (Verbenaceae) leaves, exhibited promising trypanocidal activity with an IC50 value of 4.7 μg/mL and a selectivity index of 9.8 against L6 cells [154]. While this activity confirms the use of this plant in the traditional treatment of ailments including parasitic diseases [163-165], phytochemical evaluation of trypanocidal activities were not reported before. Hence, the plant could further be investigated for the unidentified compounds. An investigation of Ageratum conyzoides (Asteraceae), a plant known for its importance in the treatment of sleeping sickness patients traditionally [155, 166, 167], led to the isolation of several flavonoids; 5, 6, 7, 8, 5′-pentamethoxy-3′, 4′-methylenedioxyflavone (trivial name: eupalestin), 5, 6, 7, 5′-tetramethoxy-3′, 4′-methylenedioxyflavone, 5, 6, 7, 8, 3′, 4′, 5′-heptamethoxy-flavone (trivial name: 5′-methoxynobiletine), 5, 6, 7, 3′, 4′, 5′-hexamethoxy-flavone and 4′-hydroxy-5, 6, 7, 3′, 5′-pentamethoxyflavone (trivial name: ageconyflavone C, 80) which displayed antiprotozoal activities, some in the lower micromolar range [155]. Among the tested NPs, compound 80 showed the highest activity against T. b. rhodesiense and L. donovani with IC50 values of 7.8 and 9.2 μM respectively. However, all the isolated compounds showed an activity weaker than that of the crude extract, implying that the activities of the compounds in the mixture could be synergistic.
4.7 Phytosterols
22-Hydroxyclerosterol (81) and clerosterol (82), Fig. 15, were isolated from the stem bark of Allexis cauliflora (Violaceae) [155]. These compounds were evaluated for trypanocidal activities, and the activity of compound 81 (ED50 = 1.12 μM) was far better than that of compound 82 (ED50 = 134.34 μM). These results prompted an investigation of their cytotoxic activities. It was observed that compound 81 inhibited mammalian cells at quite a similar concentration (ED50 = 1.56 μM), while compound 82 had no effect. This difference in activity could be attributed to the presence of the hydroxyl group at C-22 in the side chain of compound 81 which is absent in compound 82. Additionally, it was observed that compound 81 was more active and selective on the parasite enzyme glycolytic enzymes (PGI and GAPDH), when compared with compound 82.
5 Conclusions
Parasitic diseases continue to represent a menace on a global scale and require attention due to lack of vaccines and reported resistance against available drugs for their treatment. This review focuses on different natural compounds and scaffolds that could lead drug discovery research groups into reasonable starting points for further development of fast, effective and affordable novel molecules for the treatment of parasitic diseases. Drug discovery and development now place efforts on the search for new moieties or chemical scaffolds of natural/semisynthetic origin and in the development of phytomedicines. As a means to facilitate accessibility of information, our research team has as one of its goals, to develop free online natural products libraries from African flora (http://african-compounds.org/). In this paper, an attempt has been made to draw together original research works on natural products from AMP with micromolar range activities against Schistosoma, Trypanosoma and Leishmania species. The compounds presented herein have demonstrated a diverse range of activities against different forms of Trypanosomiasis, Schistosomiasis and Leishmaniasis, with some scaffolds and molecules showing great potential as starting points for further development into drugs. We recently collected a dataset of several hundred bioactive plant based metabolites from AMPs with activities against Trypanosoma sp. (Afrotryp) [68]. It becomes interesting to perform in silico prediction of binding modes and binding free energy calculations of some of the compounds against some selected targets.
Notes
Acknowledgements
CVS is currently a Ph.D. student funded by the German Academic Exchange Services (DAAD). FNK currently holds a Georg Forster postdoctoral fellowship from the Alexander von Humboldt Foundation, Germany.
Compliance with Ethical Standards
Conflict of interest
The authors declare no conflict of interest.
References
-
1.P. Mansueto, A. Seidita, G. Vitale, A. Cascio, Travel Med. Infect. Dis. 12, 563-581 (2014) CrossRef PubMed Google Scholar
-
2.B.Y. Lee, K.M. Bacon, M.E. Bottazzi, P.J. Hotez, Lancet Infect. Dis. 13, 342-348 (2013) CrossRef PubMed Google Scholar
-
3.T.N. Wells, P.L. Alonso, W.E. Gutteridge, Nat. Rev. Drug Discov. 8, 879-891 (2009) CrossRef PubMed Google Scholar
-
4.F. Ntie-Kang, P.A. Onguene, L.L. Lifongo, J.C. Ndom, W. Sippl, L.M. Mbaze, Malar. J. 13, 81 (2014) CrossRef PubMed Google Scholar
-
5.M.C. Nunes, W. Dones, C.A. Morillo, J.J. Encina, A.L. Ribeiro, Council on Chagas Disease of the Interamerican Society of Cardiology, J. Am. Coll. Cardiol. 62, 767-776 (2013) PubMed Google Scholar
-
6.P.G. Kennedy, Lancet Neurol. 12, 186-194 (2013) CrossRef PubMed Google Scholar
-
7.H. de Vries, A.P. Wagelmans, E. Hasker, C. Lumbala, P. Lutumba, S.J. de Vlas, J.V. Klundert, PLoS Comput. Biol. 12, e1005103 (2016) CrossRef PubMed Google Scholar
-
8.N.K. Copeland, N.E. Aronson, Curr. Opin. Infect. Dis. 28, 426-437 (2015) CrossRef PubMed Google Scholar
-
9.D.G. Colley, A.L. Bustinduy, W.E. Secor, C.H. King, Lancet 383, 2253-2264 (2014) CrossRef PubMed Google Scholar
-
10.D. Rollinson, S. Knopp, S. Levitz, J.R. Stothard, L.A. Tchuem Tchuente, A. Garba, K.A. Mohammed, N. Schur, B. Person, D.G. Colley, J. Utzinger, Acta Trop. 128, 423-440 (2013) CrossRef PubMed Google Scholar
-
11.M. Noma, H.G. Zoure, A.H. Tekle, P.A. Enyong, B.E. Nwoke, J.H. Remme, Parasit. Vector 7, 325 (2014) CrossRef PubMed Google Scholar
-
12.H.G. Zoure, M. Noma, A.H. Tekle, U.V. Amazigo, P.J. Diggle, E. Giorgi, J.H. Remme, Parasit Vectors 7, 326 (2017) PubMed Google Scholar
-
13.World Health Organization. Accessed 10 January 2018 (2017) PubMed Google Scholar
-
14.D.J. Gray, A.G. Ross, Y.S. Li, D.P. McManus, Br. Med. J. 342, d2651 (2011) CrossRef PubMed Google Scholar
-
15.A. Olotu, G. Fegan, J. Wambua, G. Nyangweso, A. Leach, M. Lievens, D.C. Kaslow, P. Njuguna, K. Marsh, P. Bejon, N Engl. J. Med. 374, 2519-2529 (2016) CrossRef PubMed Google Scholar
-
16.E.H. Ntege, N. Arisue, D. Ito, T. Hasegawa, N.M.Q. Palacpac, T.G. Egwang, T. Horii, E. Takashima, T. Tsuboi, Vaccine 34, 5612-5622 (2016) CrossRef PubMed Google Scholar
-
17.K.E. Lyke, A.S. Ishizuka, A.A. Berry, S. Chakravarty, A. DeZure, M.E. Enama, E.R. James, P.F. Billingsley, A. Gunasekera, A. Manoj, M. Li, A.J. Ruben, T. Li, A.G. Eappen, R.E. Stafford, K.C. Natasha, T. Murshedkar, F.H. Mendoza, I.J. Gordon, K.L. Zephir, L.A. Holman, S.H. Plummer, C.S. Hendel, L. Novik, P.J.M. Costner, J.G. Saunders, N.M. Berkowitz, B.J. Flynn, M.C. Nason, L.S. Garver, M.B. Laurens, C.V. Plowe, T.L. Richie, B.S. Graham, M. Roederer, B.K.L. Sim, J.E. Ledgerwood, S.L. Hoffman, R.A. Seder, Proc. Natl. Acad. Sci. USA 114, 2711-2716 (2017) CrossRef PubMed Google Scholar
-
18.B. Hanboonkunupakarn, N.J. White, Trop. Dis. Travel Med. Vaccines 2, 10 (2016) CrossRef PubMed Google Scholar
-
19.B. Blasco, D. Leroy, D.A. Fidock, Nat. Med. 23, 917-928 (2017) CrossRef PubMed Google Scholar
-
20.J.P. Webster, D.H. Molyneux, P.J. Hotez, A. Fenwick, Philos. Trans. R. Soc. Lond. B 369, 20130434 (2014) CrossRef PubMed Google Scholar
-
21.M.J. Doenhoff, J.R. Kusel, G.C. Coles, D. Cioli, Trans. R. Soc. Trop. Med. Hyg. 96, 465-469 (2002) CrossRef PubMed Google Scholar
-
22.T. Heimburg, A. Chakrabarti, J. Lancelot, M. Marek, J. Melesina, A.T. Hauser, T.B. Shaik, S. Duclaud, D. Robaa, F. Erdmann, M. Schmidt, C. Romier, R.J. Pierce, M. Jung, W. Sippl, J. Med. Chem. 59, 2423-2435 (2016) CrossRef PubMed Google Scholar
-
23.M. Ismail, S. Botros, A. Metwally, S. William, A. Farghally, L.F. Tao, T.A. Day, J.L. Bennett, Am. J. Trop. Med. Hyg. 60, 932-935 (1999) CrossRef PubMed Google Scholar
-
24.M.J. Doenhoff, D. Cioli, J. Utzinger, Curr. Opin. Infect. Dis. 21, 659-667 (2008) CrossRef PubMed Google Scholar
-
25.S.G. Sparg, J. Ethnopharmacol. 73, 209-214 (2000) CrossRef PubMed Google Scholar
-
26.D.A. Stolfa, M. Marek, J. Lancelot, A.T. Hauser, A. Walter, E. Leproult, J. Melesina, T. Rumpf, J.M. Wurtz, J. Cavarelli, W. Sippi, R.J. Pierce, C. Romier, M. Jung, J. Mol. Biol. 426, 3442-3453 (2014) CrossRef PubMed Google Scholar
-
27.K.T. Andrews, A. Haque, M.K. Jones, Immunol. Cell Biol. 90, 66-77 (2012) CrossRef PubMed Google Scholar
-
28.P.J. Hotez, A. Kamath, .PLoS Negl. Trop. D 3, e412 (2009) CrossRef PubMed Google Scholar
-
29.R. Brun, J. Blum, F. Chappuis, C. Burri, Lancet 375, 148-159 (2010) CrossRef PubMed Google Scholar
-
30.World Health Organization (WHO). Chagas disease (American trypanosomiasis). http://www.who.int/mediacentre/factsheets/fs340/en/. Accessed 9 November 2017 (2017) PubMed Google Scholar
-
31.World Health Organization (WHO). Trypanosomiasis, human African (sleeping sickness). http://www.who.int/mediacentre/factsheets/fs259/en/. Accessed 9 November 2017 (2017) PubMed Google Scholar
-
32.P.P. Simarro, J. Jannin, P. Cattand, PLoS Med. 5, 174-180 (2008) CrossRef PubMed Google Scholar
-
33.S. Aksoy, P. Buscher, M. Lehane, P. Solano, J. Van Den Abbeele, PLoS Negl. Trop. Dis. 11, e0005454 (2017) CrossRef PubMed Google Scholar
-
34.J.R. Franco, P.P. Simarro, A. Diarra, J.A. Ruiz-Postigo, J.G. Jannin, Parasitology 141, 748-760 (2014) CrossRef PubMed Google Scholar
-
35.P.D. Ready, Clin. Epidemiol. 6, 147-154 (2014) PubMed Google Scholar
-
36.S. Aluru, M. Hide, G. Michel, A.L. Banuls, P. Marty, C. Pomares, Parasite 22(16) (2015) PubMed Google Scholar
-
37.World Health Organization (WHO). Leishmaniasis. http://www.who.int/mediacentre/factsheets/fs375/en/. Accessed 9 November 2017 (2017) PubMed Google Scholar
-
38.F.R. Martins-Melo, M.D. Lima, A.N. Ramos, C.H. Alencar, J. Heukelbach, PLoS ONE 9, e93770 (2014) CrossRef PubMed Google Scholar
-
39.A.I. Einzig, H. Hochster, P.H. Wiernik, D.L. Trump, J.P. Dutcher, E. Garowski, J. Sasloff, T.J. Smith, Invest. New Drugs 9, 59-64 (1991) PubMed Google Scholar
-
40.M.C. Wani, H.L. Taylor, M.E. Wall, P. Coggon, A.T. McPhail, J. Am. Chem. Soc. 93, 2325-2327 (1971) CrossRef PubMed Google Scholar
-
41.G.M. Rishton, Am. J. Cardiol. 101, 43d-49d (2008) PubMed Google Scholar
-
42.J.D. Phillipson, Phytochemistry 56, 237-243 (2001) CrossRef PubMed Google Scholar
-
43.G.A. Cordell, Phytochem. Lett. 10, 28-40 (2014) CrossRef PubMed Google Scholar
-
44.F. Ntie-Kang, L.L. Lifongo, C.V. Simoben, S.B. Babiaka, W. Sippl, L.M. Mbaze, RSC Adv. 4, 35348-35370 (2014) CrossRef PubMed Google Scholar
-
45.F. Ntie-Kang, L.L. Lifongo, C.V. Simoben, S.B. Babiaka, W. Sippl, L.M. Mbaze, RSC Adv. 4, 28728-28755 (2014) CrossRef PubMed Google Scholar
-
46.C.V. Simoben, F. Ntie-Kang, L.L. Lifongo, S.B. Babiaka, W. Sippl, L.M. Mbaze, RSC Adv. 4, 40095-40110 (2014) CrossRef PubMed Google Scholar
-
47.I. Tietjen, F. Ntie-Kang, P. Mwimanzi, P.A. Onguene, M.A. Scull, T.O. Idowu, A.O. Ogundaini, L.M. Meva'a, B.M. Abegaz, C.M. Rice, K. Andrae-Marobela, M.A. Brockman, Z.L. Brumme, D. Fedida, PLoS ONE 10, e0121099 (2015) CrossRef PubMed Google Scholar
-
48.D.J. Newman, G.M. Cragg, K.M. Snader, Nat. Prod. Rep. 17, 215-234 (2000) CrossRef PubMed Google Scholar
-
49.B.E. van Wyk, J. Ethnopharmacol. 119, 342-355 (2008) CrossRef PubMed Google Scholar
-
50.A.L. Harvey, R. Edrada-Ebel, R.J. Quinn, Nat. Rev. Drug Discov. 14, 111-129 (2015) CrossRef PubMed Google Scholar
-
51.M.A. Ibrahim, A. Mohammed, M.B. Isah, A.B. Aliyu, J. Ethnopharmacol. 154, 26-54 (2014) CrossRef PubMed Google Scholar
-
52.F. Ntie-Kang, L.L. Lifongo, L.M. Mbaze, N. Ekwelle, L.C. Owono Owono, E. Megnassan, P.N. Judson, W. Sippl, S.M. Efange, BMC Complement. Altern. Med. 13, 147 (2013) CrossRef PubMed Google Scholar
-
53.L.L. Lifongo, C.V. Simoben, F. Ntie-Kang, S.B. Babiaka, P.N. Judson, Nat. Prod. Bioprospect 4, 1-19 (2014) CrossRef PubMed Google Scholar
-
54.F. Ntie-Kang, D. Zofou, S.B. Babiaka, R. Meudom, M. Scharfe, L.L. Lifongo, J.A. Mbah, L.M. Mbaze, W. Sippl, S.M. Efange, PLoS ONE 8, e78085 (2013) CrossRef PubMed Google Scholar
-
55.H.M. Adamu, O.J. Abayeh, M.O. Agho, A.L. Abdullahi, A. Uba, H.U. Dukku, B.M. Wufem, J. Ethnopharmacol. 99, 1-4 (2005) CrossRef PubMed Google Scholar
-
56.F.B. Magassouba, A. Diallo, M. Kouyate, F. Mara, O. Mara, O. Bangoura, A. Camara, S. Traore, A.K. Diallo, M. Zaoro, K. Lamah, S. Diallo, G. Camara, S. Traore, A. Kaita, M.K. Camara, R. Barry, S. Keita, K. Oulare, M.S. Barry, M. Donzo, K. Camara, K. Tote, D. Vanden Berghe, J. Totte, L. Pieters, A.J. Vlietinck, A.M. Balde, J. Ethnopharmacol. 114, 44-53 (2007) CrossRef PubMed Google Scholar
-
57.T. Brendler, B.E. van Wyk, J. Ethnopharmacol. 119, 420-433 (2008) CrossRef PubMed Google Scholar
-
58.I.P. Dike, O.O. Obembe, F.E. Adebiyi, J. Ethnopharmacol. 144, 618-626 (2012) CrossRef PubMed Google Scholar
-
59.L. Kambizi, A.J. Afolayan, J. Ethnopharmacol. 77, 5-9 (2001) CrossRef PubMed Google Scholar
-
60.K.A. Abo, A.A. Fred-Jaiyesimi, A.E.A. Jaiyesimi, J. Ethnopharmacol. 115, 67-71 (2008) CrossRef PubMed Google Scholar
-
61.J.S. Ashidi, P.J. Houghton, P.J. Hylands, T. Efferth, J. Ethnopharmacol. 128, 501-512 (2010) CrossRef PubMed Google Scholar
-
62.S. Semenya, M. Potgieter, L. Erasmus, J. Ethnopharmacol. 141, 440-445 (2012) CrossRef PubMed Google Scholar
-
63.S.M. Maregesi, O.D. Ngassapa, L. Pieters, A.J. Vlietinck, J. Ethnopharmacol. 113, 457-470 (2007) CrossRef PubMed Google Scholar
-
64.World Health Organization. Accessed 7 January 2017. (2004) PubMed Google Scholar
-
65.C. Vonthron-Senecheau, B. Weniger, M. Ouattara, F.T. Bi, A. Kamenan, A. Lobstein, R. Brun, R. Anton, J. Ethnopharmacol. 87, 221-225 (2003) CrossRef PubMed Google Scholar
-
66.O. Jansen, L. Angenot, M. Tits, J.P. Nicolas, P. De Mol, P.Y. Sacre, M.C. Jonville, M. Frederich, Planta Med. 74, 1142 (2008) PubMed Google Scholar
-
67.A.W. Mbaya, U.I. Ibrahim, Int. J. Pharmacol. 7, 1-11 (2011) CrossRef PubMed Google Scholar
-
68.A. Ibezim, B. Debnath, F. Ntie-Kang, C.J. Mbah, N.J. Nwodo, Med. Chem. Res. 26, 562-579 (2017) CrossRef PubMed Google Scholar
-
69.N.J. Nwodo, A. Ibezim, F. Ntie-Kang, M.U. Adikwu, C.J. Mbah, Molecules 20, 7750-7771 (2015) CrossRef PubMed Google Scholar
-
70.S. Hoet, F. Opperdoes, R. Brun, J. Quetin-Leclereq, Nat. Prod. Rep. 21, 353-364 (2004) CrossRef PubMed Google Scholar
-
71.I.V. Ogungbe, W.N. Setzer, Molecules 14, 1513-1536 (2009) CrossRef PubMed Google Scholar
-
72.T.J. Schmidt, S.A. Khalid, A.J. Romanha, T.M. Alves, M.W. Biavatti, R. Brun, F.B. Da Costa, S.L. de Castro, V.F. Ferreira, M.V.G. de Lacerda, J.H.G. Lago, L.L. Leon, N.P. Lopes, R.C.D. Amorim, M. Niehues, I.V. Ogungbe, A.M. Pohlit, M.T. Scotti, W.N. Setzer, M.D.C. Soeiro, M. Steindel, A.G. Tempone, Curr. Med. Chem. 19, 2128-2175 (2012) CrossRef PubMed Google Scholar
-
73.T.J. Schmidt, S.A. Khalid, A.J. Romanha, T.M.A. Alves, M.W. Biavatti, R. Brun, F.B. Da Costa, S.L. de Castro, V.F. Ferreira, M.V.G. de Lacerda, J.H.G. Lago, L.L. Leon, N.P. Lopes, R.C.D. Amorim, M. Niehues, I.V. Ogungbe, A.M. Pohlit, M.T. Scotti, W.N. Setzer, M.D.C. Soeiro, M. Steindel, A.G. Tempone, Curr. Med. Chem. 19, 2176-2228 (2012) CrossRef PubMed Google Scholar
-
74.F. Ntie-Kang, K.K. Telukunta, K. Doring, C.V. Sirnoben, A.F.A. Moumbock, Y.I. Malange, L.E. Njurne, J.N. Yong, W. Sippl, S. Gunther, J. Nat. Prod. 80, 2067-2076 (2017) CrossRef PubMed Google Scholar
-
75.F. Ntie-Kang, Springerplus 2, 353 (2013) CrossRef PubMed Google Scholar
-
76.A. Inada, R. Ogasawara, I. Koga, N. Nakatani, Y. Inatomi, H. Murata, M. Nishi, T. Nakanishi, Chem. Pharm. Bull. 56, 727-729 (2008) CrossRef PubMed Google Scholar
-
77.K.P. Devkota, J.D. Wansi, B.N. Lenta, S. Khan, M.I. Choudhary, N. Sewald, Planta Med. 76, 1022-1025 (2010) CrossRef PubMed Google Scholar
-
78.L. Cheesman, J.J. Nair, J. van Staden, J. Ethnopharmacol. 140, 405-408 (2012) CrossRef PubMed Google Scholar
-
79.B.B. Mishra, R.R. Kale, R.K. Singh, V.K. Tiwari, Fitoterapia 80, 81-90 (2009) CrossRef PubMed Google Scholar
-
80.B.V. Tsassi, H. Hussain, A. Geagni, E. Dongo, I. Ahmed, M. Riaz, K. Krohn, Helv. Chim. Acta 94, 1035-1040 (2011) CrossRef PubMed Google Scholar
-
81.A. Ndagijimana, X.M. Wang, G.X. Pan, F. Zhang, H. Feng, O. Olaleye, Fitoterapia 86, 35-47 (2013) CrossRef PubMed Google Scholar
-
82.C.O. Nnadi, N.J. Nwodo, M. Kaiser, R. Brun, T.J. Schmidt, Molecules 22, 1129 (2017) CrossRef PubMed Google Scholar
-
83.P.F. Uzor, W. Ebrahim, P.O. Osadebe, J.N. Nwodo, F.B. Okoye, W.E.G. Muller, W.H. Lin, Z. Liu, P. Proksch, Fitoterapia 105, 147-150 (2015) CrossRef PubMed Google Scholar
-
84.T.C. Okoye, P.A. Akah, A.C. Ezike, P.F. Uzor, U.E. Odoh, S.O. Igboeme, U.B. Onwuka, S.N. Okafor, BMC Complement. Altern. Med. 14, 376 (2014) CrossRef PubMed Google Scholar
-
85.G. Bringmann, M. Dreyer, J.H. Faber, P.W. Dalsgaard, D. Staerk, J.W. Jaroszewski, H. Ndangalasi, F. Mbago, R. Brun, S.B. Christensen, J. Nat. Prod. 67, 743-748 (2004) CrossRef PubMed Google Scholar
-
86.G. Bringmann, M. Dreyer, J.H. Faber, P.W. Dalsgaard, D. Staerk, J.W. Jaroszewski, H. Ndangalasi, F. Mbago, R. Brun, M. Reichert, K. Maksimenka, S.B. Christensen, J. Nat. Prod. 66, 1159-1165 (2003) CrossRef PubMed Google Scholar
-
87.G. Bringmann, A. Hamm, C. Gunther, M. Michel, R. Brun, V. Mudogo, J. Nat. Prod. 63, 1465-1470 (2000) CrossRef PubMed Google Scholar
-
88.G. Bringmann, K. Messer, R. Brun, V. Mudogo, J. Nat. Prod. 65, 1096-1101 (2002) CrossRef PubMed Google Scholar
-
89.G. Bringmann, I. Kajahn, M. Reichert, S.E.H. Pedersen, J.H. Faber, T. Gulder, R. Brun, S.B. Christensen, A. Ponte-Sucre, H. Moll, G. Heubl, V. Mudogo, J. Org. Chem. 71, 9348-9356 (2006) CrossRef PubMed Google Scholar
-
90.G. Bringmann, W. Saeb, M. Ruckert, J. Mies, M. Michel, V. Mudogo, R. Brun, Phytochemistry 62, 631-636 (2003) CrossRef PubMed Google Scholar
-
91.G. Bringmann, K. Messer, K. Wolf, J. Muhlbacher, M. Grune, R. Brun, A.M. Louis, Phytochemistry 60, 389-397 (2002) CrossRef PubMed Google Scholar
-
92.S. Hoet, C. Stevigny, S. Block, F. Opperdoes, P. Colson, B. Baldeyrou, A. Lansiaux, C. Bailly, J. Quetin-Leclercq, Planta Med. 70, 407-413 (2004) CrossRef PubMed Google Scholar
-
93.S. Cretton, L. Breant, L. Pourrez, C. Ambuehl, L. Marcourt, S.N. Ebrahimi, M. Hamburger, R. Perozzo, S. Karimou, M. Kaiser, M. Cuendet, P. Christen, J. Nat. Prod. 77, 2304-2311 (2014) CrossRef PubMed Google Scholar
-
94.I. Ngantchou, B. Nyasse, C. Denier, C. Blonski, V. Hannaert, B. Schneider, Bioorg. Med. Chem. Lett. 20, 3495-3498 (2010) CrossRef PubMed Google Scholar
-
95.G. Bringmann, M. Dreyer, M. Michel, F.S.K. Tayman, R. Brun, Phytochemistry 65, 2903-2907 (2004) CrossRef PubMed Google Scholar
-
96.M.N. Ngemenya, J.N. Hanna, J.A. Komtchou, S.M.N. Efange, Asian Pac. J. Trop. Biomed. 5, 472-477 (2015) CrossRef PubMed Google Scholar
-
97.J.N. Hanna, F. Ntie-Kang, M. Kaiser, R. Brun, S.M.N. Efange, RSC Adv. 4, 22856-22865 (2014) CrossRef PubMed Google Scholar
-
98.H. Montenegro, M. Gutierrez, L.I. Romero, E. Ortega-Barria, T.L. Capson, L.C. Rios, Planta Med. 69, 677-679 (2003) CrossRef PubMed Google Scholar
-
99.S.E. Atawodi, D.A. Ameh, S. Ibrahim, J.N. Andrew, H.C. Nzelibe, E.O. Onyike, K.M. Anigo, E.A. Abu, D.B. James, G.C. Njoku, A.B. Sallau, J. Ethnopharmacol. 79, 279-282 (2002) CrossRef PubMed Google Scholar
-
100.M.S. Abubakar, A.M. Musa, A. Ahmed, I.M. Hussaini, J. Ethnopharmacol. 111, 625-629 (2007) CrossRef PubMed Google Scholar
-
101.J.D. Olowokudejo, A.B. Kadiri, V.A. Travih, Ethnobot. Leafl. 12, 851-865 (2008) PubMed Google Scholar
-
102.M.O. Soladoye, M.O. Adetayo, E.C. Chukwuma, A.N. Adetunji, Ann. Biol. Res. 1, 1-15 (2010) PubMed Google Scholar
-
103.H. de Wet, M.N. Nkwanyana, S.F. van Vuuren, J. Ethnopharmacol. 130, 284-289 (2010) CrossRef PubMed Google Scholar
-
104.H.A. Garro, M. Juri Ayub, M. Nieto, Estrada C. Lucero, C.R. Pungitore, C.E. Tonn, Cell Mol. Biol. 56(24), 1318-1323 (2010) PubMed Google Scholar
-
105.A.H. Tekle, H.G. Zoure, M. Noma, M. Boussinesq, L.E. Coffeng, W.A. Stolk, J.H. Remme, Am. J. Trop. Med. Hyg. 95, 1 (2017) PubMed Google Scholar
-
106.A. Buske, A. S. Kekule, A. Haring, G. Adam, patent WO2003000272 A1. https://www.google.com/patents/WO2003000272A2003000271?cl=en (2003) PubMed Google Scholar
-
107.L.M. Leandro, F.D. Vargas, P.C.S. Barbosa, J.K.O. Neves, J.A. da Silva, V.F. da Veiga, Molecules 17, 3866-3889 (2012) CrossRef PubMed Google Scholar
-
108.R.J. Thoppil, A. Bishayee, World J. Hepatol. 3, 228-249 (2011) CrossRef PubMed Google Scholar
-
109.H. Achenbach, A. Schwinn, Phytochemistry 38, 1037-1048 (1995) CrossRef PubMed Google Scholar
-
110.M.H.H. Nkunya, Pure Appl. Chem. 77, 1943-1955 (2005) CrossRef PubMed Google Scholar
-
111.K. Nakano, C. Yoshida, W. Furukawa, Y. Takaishi, K. Shishido, Phytochemistry 49, 1821-1824 (1998) CrossRef PubMed Google Scholar
-
112.P.A. Onguene, F. Ntie-Kang, L.L. Lifongo, J.C. Ndom, W. Sippl, L.M. Mbaze, Malar. J. 12, 449 (2013) CrossRef PubMed Google Scholar
-
113.L. Faiella, A. Temraz, T. Siciliano, N. De Tommasi, A. Braca, Phytochem. Lett. 5, 297-300 (2012) CrossRef PubMed Google Scholar
-
114.J.N. Nwodo, A. Ibezim, C.V. Simoben, F. Ntie-Kang, AntiCancer Agent Med. Chem. 16, 108-127 (2016) PubMed Google Scholar
-
115.A.A. Wube, F. Bucar, S. Gibbons, K. Asres, L. Rattray, S.L. Croft, Phytother. Res. 24, 1468-1472 (2010) CrossRef PubMed Google Scholar
-
116.D. Olila, J. Opuda-Asibo, O. Olwa, Afr. Health Sci. 1, 12-15 (2001) PubMed Google Scholar
-
117.J. O. Kokwaro, in Medicinal plants of East Africa, 3rd edn., ed. by J. O. Kokwaro (University of Nairobi Press, Kampala, 2009), p. 215 PubMed Google Scholar
-
118.F. Machumi, A. Yenesew, J.O. Midiwo, M. Heydenreich, E. Kleinpeter, B.L. Tekwani, S.I. Khan, L.A. Walker, I. Muhammad, Nat. Prod. Commun. 7, 1123-1126 (2012) PubMed Google Scholar
-
119.C.Y. Ragasa, P.W. Tsai, C.T. Galvez, C.C. Shen, Planta Med. 76, 146-151 (2010) CrossRef PubMed Google Scholar
-
120.J. Jakupovic, M. Grenz, F. Bohlmann, G.M. Mungai, Phytochemistry 29, 1213-1217 (1990) CrossRef PubMed Google Scholar
-
121.F. Machumi, V. Samoylenko, A. Yenesew, S. Derese, J.O. Midiwo, F.T. Wiggers, M.R. Jacob, B.L. Tekwani, S.I. Khan, L.A. Walker, I. Muhammad, Nat. Prod. Commun. 5, 853-858 (2010) PubMed Google Scholar
-
122.G.U. Ebiloma, J.O. Igoli, E. Katsoulis, A.M. Donachie, A. Eze, A.I. Gray, H.P. de Koning, J. Ethnopharmacol. 202, 256-264 (2017) CrossRef PubMed Google Scholar
-
123.A.B. Aliyu, A.M. Musa, M.S. Abdullahi, A.O. Oyewale, U.S. Gwarzo, Niger. J. Pharm. Res. 7, 1-8 (2008) PubMed Google Scholar
-
124.B. Nyasse, I. Ngantchou, E.M. Tchana, B. Sonke, C. Denier, C. Fontaine, Pharmazie 59, 873-875 (2004) PubMed Google Scholar
-
125.F. Freiburghaus, E.N. Ogwal, M.H.H. Nkunya, R. Kaminsky, R. Brun, Trop. Med. Int. Health 1, 765-771 (1996) PubMed Google Scholar
-
126.D. Soh, E. Nkwengoua, I. Ngantchou, B. Nyasse, C. Denier, V. Hannaert, K.H. Shaker, B. Schneider, J Appl Pharm Sci 3, 13-19 (2013) PubMed Google Scholar
-
127.Z. Cheikh-Ali, T. Okpekon, F. Roblot, C. Bories, M. Cardao, J.C. Jullian, E. Poupon, P. Champy, Phytochem. Lett. 4, 240-244 (2011) CrossRef PubMed Google Scholar
-
128.H.R. El-Seedi, R. El-Shabasy, H. Sakr, M. Zayed, A.M.A. ElSaid, K.M.H. Helmy, A.H.M. Gaara, Z. Turki, M. Azeem, A.M. Ahmed, L. Boulos, A.K. Borg-Karlson, U. Goransson, Rev. Bras. Farmacogn. 22, 314-318 (2012) CrossRef PubMed Google Scholar
-
129.S.M. Maregesi, N. Hermans, L. Dhooghe, K. Cimanga, D. Ferreira, C. Pannecouque, D.A. Vanden Berghe, P. Cos, L. Maes, A.J. Vlietinck, S. Apers, L. Pieters, J. Ethnopharmacol. 129, 319-326 (2010) CrossRef PubMed Google Scholar
-
130.D.A. Thiem, A.T. Sneden, S.I. Khan, B.L. Tekwani, J. Nat. Prod. 68, 251-254 (2005) CrossRef PubMed Google Scholar
-
131.J. Bero, C. Beaufay, V. Hannaert, M.F. Herent, P.A. Michels, J. Quetin-Leclercq, Phytomedicine 20, 270-274 (2013) CrossRef PubMed Google Scholar
-
132.I. Ngantchou, E. Nkwengoua, Y. Nganso, B. Nyasse, C. Denier, V. Hannaert, B. Schneider, Fitoterapia 80, 188-191 (2009) CrossRef PubMed Google Scholar
-
133.A.T. Tchinda, A. Tsopmo, P. Tane, J.F. Ayafor, J.D. Connolly, O. Sterner, Phytochemistry 59, 371-374 (2002) CrossRef PubMed Google Scholar
-
134.P. Tane, S.D. Tatsimo, G.A. Ayimele, J.D. Connolly, Bioactive metabolites from Aframomum species. In:11th NAPRECA Symposium Book of Proceedings, Antananarivo, Madagascar, pp., 214-223 (2005) PubMed Google Scholar
-
135.T. Okpekon, S. Yolou, C. Gleye, F. Roblot, P. Loiseau, C. Bories, P. Grellier, F. Frappier, A. Laurens, R. Hocquemiller, J. Ethnopharmacol. 90, 91-97 (2004) CrossRef PubMed Google Scholar
-
136.P. Kamnaing, A. Tsopmo, E.A. Tanifum, M.H. Tchuendem, P. Tane, J.F. Ayafor, O. Sterner, D. Rattendi, M.M. Iwu, B. Schuster, C. Bacchi, J. Nat. Prod. 66, 364-367 (2003) CrossRef PubMed Google Scholar
-
137.L. Boulos, Medicinal plants of North Africa (Reference Publications, Inc., Algonac, 1983), p. 130 PubMed Google Scholar
-
138.S.M. Maregesi, L. Pieters, O.D. Ngassapa, S. Apers, R. Vingerhoets, P. Cos, D.A.V. Vanden Berghe, A.J. Vlietinck, J. Ethnopharmacol. 119, 58-66 (2008) CrossRef PubMed Google Scholar
-
139.J. Bero, H. Ganfon, M.C. Jonville, M. Frederich, F. Gbaguidi, P. Demol, M. Moudachirou, J. Quetin-Leclercq, J. Ethnopharmacol. 122, 439-444 (2009) CrossRef PubMed Google Scholar
-
140.J. Bero, V. Hannaert, G. Chataigne, M.F. Herent, J. QuetinLeclercq, J. Ethnopharmacol. 137, 998-1002 (2011) CrossRef PubMed Google Scholar
-
141.V. Hannaert, F.R. Opperdoes, P.A.M. Michels, Protein Express Purif. 6, 244-250 (1995) CrossRef PubMed Google Scholar
-
142.B. Nyasse, J.J. Nono, Y. Nganso, I. Ngantchou, B. Schneider, Fitoterapia 80, 32-34 (2009) CrossRef PubMed Google Scholar
-
143.N.J. Nwodo, F.B.C. Okoye, D.W. Lai, A. Debbab, R. Brun, P. Proksch, Molecules 19, 5470-5477 (2014) CrossRef PubMed Google Scholar
-
144.P.C. Kuo, T.L. Hwang, Y.T. Lin, Y.C. Kuo, Y.L. Leu, Arch. Pharm. Res. 34, 715-722 (2011) CrossRef PubMed Google Scholar
-
145.C.A.N. Catalan, C.S. de Heluani, C. Kotowicz, T.E. Gedris, W. Herz, Phytochemistry 64, 625-629 (2003) CrossRef PubMed Google Scholar
-
146.M. Nwagwu, A.L. Inyang, R.I. Molokwu, E.M. Essien, Afr. J. Med. Med. Sci. 18, 283-287 (1989) PubMed Google Scholar
-
147.G.B.S. Lundkvist, M.T. Sellix, M. Nygard, E. Davis, M. Straume, K. Kristensson, G.D. Block, J. Biol. Rhythm. 25, 92-102 (2010) CrossRef PubMed Google Scholar
-
148.V. Kuete, T. Efferth, J. Ethnopharmacol. 137, 752-766 (2011) CrossRef PubMed Google Scholar
-
149.B.N. Lenta, C. Vonthron-Senecheau, B. Weniger, K.P. Devkota, J. Ngoupayo, M. Kaiser, Q. Naz, M.I. Choudhary, E. Tsamo, N. Sewald, Molecules 12, 1548-1557 (2007) CrossRef PubMed Google Scholar
-
150.V.T. Dike, B. Vihiior, J.A. Bosha, T.M. Yin, G.U. Ebiloma, H.P. de Koning, J.O. Igoli, A.I. Gray, Phytochem. Anal. 27, 217-221 (2016) CrossRef PubMed Google Scholar
-
151.F. Abeje, D. Bisrat, A. Hailu, K. Asres, Phytother. Res. 28, 1801-1805 (2014) CrossRef PubMed Google Scholar
-
152.G. Bringmann, S. Rudenauer, A. Irmer, T. Bruhn, R. Brun, T. Heimberger, T. Stuhmer, R. Bargou, M. Chatterjee, Phytochemistry 69, 2501-2509 (2008) CrossRef PubMed Google Scholar
-
153.B. Akendengue, F. Roblot, P.M. Loiseau, C. Bories, E. NgouMilama, A. Laurens, R. Hocquemiller, Phytochemistry 59, 885-888 (2002) CrossRef PubMed Google Scholar
-
154.N. Nwodo, F. Okoye, D. Lai, A. Debbab, M. Kaiser, R. Brun, P. Proksch, BMC Complement. Altern. Med. 15, 82-86 (2015) CrossRef PubMed Google Scholar
-
155.A.M.M. Nour, S.A. Khalid, M. Kaiser, R. Brun, W.E. Abdalla, T.J. Schmidt, J. Ethnopharmacol. 129, 127-130 (2010) CrossRef PubMed Google Scholar
-
156.Y.O. Nganso, I.E. Ngantchou, E. Nkwenoua, B. Nyasse, C. Denier, V. Hannert, B. Schneider, Sci. Pharm. 79, 137-144 (2011) CrossRef PubMed Google Scholar
-
157.J.H. Jiang, H.M. Yang, Y.L. Wang, Y.G. Chen, Trop. J. Pharm. Res. 13, 635-648 (2014) CrossRef PubMed Google Scholar
-
158.G. Ni, H.Z. Yang, N.J. Fu, L.L. Zhang, M.C. Wang, J. Chen, C.L. Zhang, Y. Li, X.G. Chen, R.Y. Chen, D.Q. Yu, Planta Med. 81, 247-256 (2015) CrossRef PubMed Google Scholar
-
159.A. Yokosuka, Y. Mimaki, H. Sakagami, Y. Sashida, J. Nat. Prod. 65, 283-289 (2002) CrossRef PubMed Google Scholar
-
160.Z.L. Chen, B.D. Wang, M.Q. Chen, Acta Chim. Sin. 46, 1201-1206 (1988) PubMed Google Scholar
-
161.J. Peng, A.L. Risinger, G.A. Fest, E.M. Jackson, G. Helms, L.A. Polin, S.L. Mooberry, J. Med. Chem. 54, 6117-6124 (2011) CrossRef PubMed Google Scholar
-
162.T.I. Borokini, A.E. Ayodele, Int. J. Mod. Bot. 2, 97-102 (2012) PubMed Google Scholar
-
163.F. Liang, Z. XiangZhen, C. Lan, Med. Plants 3, 16-22 (2012) PubMed Google Scholar
-
164.A.A. Munir, J. Adel Bot. Gard. 10, 31-97 (1987) PubMed Google Scholar
-
165.R. W. J. Keay, Trees of Nigeria (Clarendon Press, Wotton-UnderEdge, 1989), pp. 340-345 PubMed Google Scholar
-
166.A. Kamboj, A.K. Saluja, Int. J. Green Pharm. 2, 59-68 (2008) CrossRef PubMed Google Scholar
-
167.A.L. Okunade, Fitoterapia 73, 1-16 (2002) CrossRef PubMed Google Scholar
Copyright information
© The Author(s) 2018
Open Access
This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.