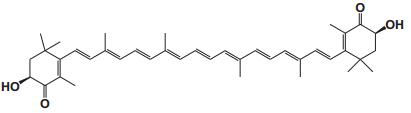
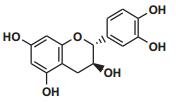
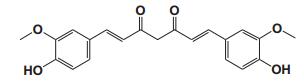
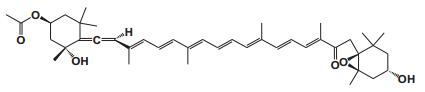
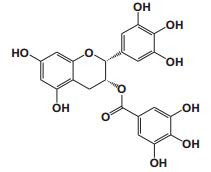
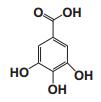
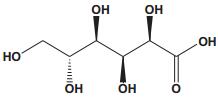
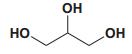
Current Perspective in the Discovery of Anti-aging Agents from Natural Products
Abstract
Aging is a process characterized by accumulating degenerative damages, resulting in the death of an organism ultimately. The main goal of aging research is to develop therapies that delay age-related diseases in human. Since signaling pathways in aging of Caenorhabditis elegans (C. elegans), fruit flies and mice are evolutionarily conserved, compounds extending lifespan of them by intervening pathways of aging may be useful in treating age-related diseases in human. Natural products have special resource advantage and with few side effect. Recently, many compounds or extracts from natural products slowing aging and extending lifespan have been reported. Here we summarized these compounds or extracts and their mechanisms in increasing longevity of C. elegans or other species, and the prospect in developing antiaging medicine from natural products.Keywords
Aging Natural products Anti-aging Drug screening1 Introduction
Since realizing the inevitability of death, the fear of death and pursuit of immortality might have preoccupied with human beings. In the Epic of Gilgamesh, Gilgamesh (the Sumerian king of Uruk) was obsessed in pursuit of immortality herbal. About 200 BC, Qin Shi Huang (the first emperor of a unified China) feared death and desperately sought the fabled elixir of life. A more recent story was the Spanish explorer Ponce de Leon who was looking for the mythical fountain of youth. Unexpectedly, all these human activities of pursuing for immortality were failed. We now know that there is no such elixir of immortality placed in somewhere by god and waited for human to find it.
On the other hand, early medical practice was developed in Babylon, Egypt, Greece, India, and China. Along with the development of biology, chemistry, physics and math, the west medical tradition developed into modern medical science. Great success has been achieved in prevention and treatment of disease. Consequently, the longevity of human has been greatly extended. The aged population is growing rapidly in modern world. Aging is the most risk factor for many age-associated diseases, such as neurodegenerative disease, diabetes, stroke, and cancer. The aged people are often suffering from one or multiple aging associated diseases, which brings enormous social and economic burden. While current medicine is focused on treatment of individual disease, the aging people recovered for one disease would probably suffer from other disease soon later.
Two thousand years ago, a systematic theory and practice to achieve healthy aging with core idea of "preventive treatment of disease" was proposed in Huang Di Nei Jing (one of the most important classical texts of traditional Chinese medicine). Current geroscience research have revealed key molecular processes that underlie biological aging [1], and that delaying aging process could delay the onset and progress of age-associated diseases and the disability of aging people [2]. As the modern version of "the preventative treatment of disease", anti-aging medicine could be the most effective way to combat the age-associated diseases and the disability of aging people. Currently, many compounds with anti-aging activity have been discovered. A large portion of these compounds are natural products. Therefore, we summarized these natural products or extracts that are reported to have anti-aging effects. We also discussed the prospect and challenges of natural products in development of anti-aging medicine.
2 Current Progress in Aging Research
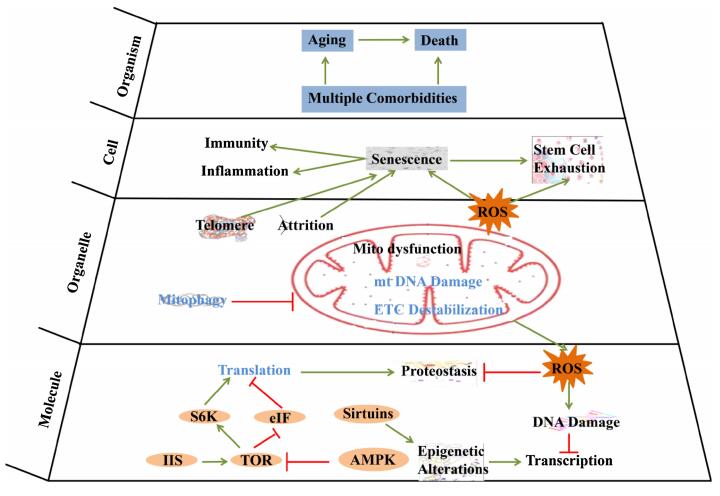
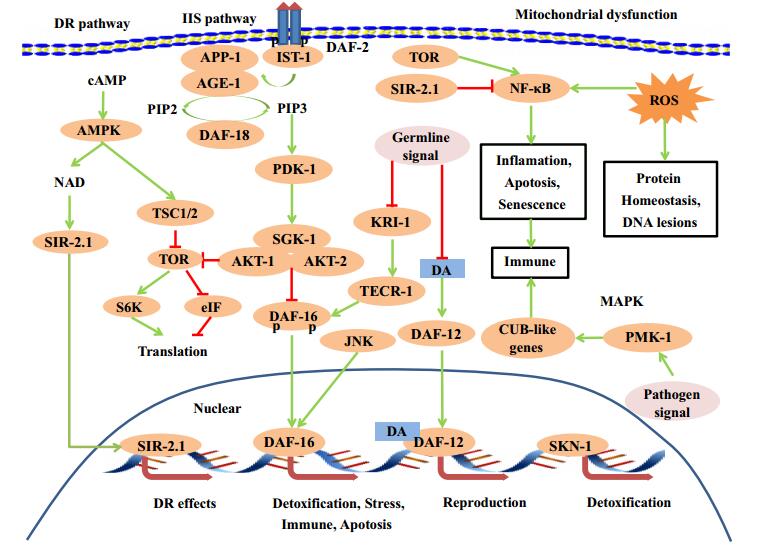
Biological process was relying on the delicate interaction of biomolecules. These building blocks of organism were selected during the origin of life, and were imperfect and intrinsic to generation of damage in every biological process, such as in DNA replication, epigenetic modification, transcription and translation, protein post-translational modification, protein fold, and metabolic process. For some of the damages were endangering species survival, their correction mechanisms were evolved by natural selection, such as DNA repair, protein unfolding response, antioxidant mechanism, detoxification, autophagy, and proteasome. The failure of these protection processes would cause the occurrence of aging and pathological phenotypes, while enhancing these protection processes would delay aging and related phenotypes. Here we summarized how the dynamic interactions between various damages and errors occurred in biological process and their evoked response of correction mechanisms contribute to genome stability, proteostasis and metabolic homeostasis, to cellular homeostasis and finally to aging process (Figs. 1, 2). For the detailed mechanisms of aging, we refer to the reviews elsewhere [1, 3-8].
Aging mechanisms in different hierarchies
Signaling networks in aging. Dietary restriction (DR), insulin/IGF-1-like signaling (IIS), germline, MAPK and mitochondrial dysfunction pathway networks in aging
2.1 Genome Stability and Aging
Accumulation of genome damage is one of the major causes of aging [9]. The intrinsic threats to DNA integrity, including DNA replication errors, spontaneous hydrolytic reactions, and reactive oxygen species (ROS), together with exogenous physical (e.g. UV/IR radiation), chemical and biological agents (e.g. virus) cause various genetic lesions, such as point mutations, translocations, chromosomal gains and losses, telomere shortening, and gene disruption. About 70, 000 lesions per day were estimated to happen in each normal human cell [10]. Accordingly, a complex repair mechanisms, such as base excision repair (BER), nucleotide excision repair (NER), transcription-coupled repair (TCR), homologous recombination, nonhomologous end-joining (NHEJ), and telomere elongation have been evolved in the organism. The deletion of genes for BER were lethal in mice [11], while mutations affecting NER and TCR were associated with numerous disorders and accelerated aging [12-14]. Mice with defected in NHEJ were subjected to early onset of aging [15]. The discovery of the causality between telomere shortening and cell replication limits, has led to the generation of telomere theory of aging [16]. Patients with inherited telomere syndrome presents greater overall telomere attrition and premature aging [16]. Compounds with improving telomerase activity or suppressing telomere shortening play distinct roles in anti-aging [17].
The epigenetic changes are one of the hallmarks of aging, including alterations in transcription factor binding, histone marks, DNA methylation, and nucleosome positioning [18]. These epigenetic changes can either happen spontaneously or modulated by environmental stimuli, nutrient signaling, and metabolic state, via multiple enzymatic systems including DNA methyltransferases, histone acetylases, deacetylases, methylases, demethylases, and other protein complex. These epigenetic changes can cause aberrant transcription and noncoding RNA expression and impair DNA integrity, affect cellular function and stress resistance, heavily influence the progression of aging. Diet or environment and genetic influencing epigenetic information could alter aging process [19]. Humans and mice with genetic defects in genome maintenance present accelerated aging symptoms, while enhancing DNA maintenance could delay aging [20].
2.2 Proteostasis and Aging
Errors happen on proteins including abnormally synthesized proteins, protein unfolding, abnormal cleavage, undesirable posttranslational modifications, can cause protein self-assembling into toxic oligomeric structures or aggregation into cytosolic inclusions. These damaged proteins can be recognized by chaperones or heat shock proteins and delivered to degradation by the ubiquitin/proteasome system or the lysosomes/autophagy. Increased protein damages would compromise endo-reticulum (ER) homeostasis, lead to increased synthesis of ER chaperones and reduced protein translation to maintain proteostasis, this response is called the unfolding protein response (UPR) [21]. The ability to maintain the protein homeostasis decline with age, many age-related diseases, such as Alzheimer's disease, Parkinson's disease, and ALS are associated with intracellular accumulation of abnormal proteins in the form of protein inclusions and aggregates [22]. Chaperone defective could lead to accelerated aging [23], while activation of the master regulator of the heat-shock response, the transcription factor HSF-1, could upregulate heat-shock proteins and increase longevity in C. elegans and mice [24, 25].
2.3 Metabolic Homeostasis and Aging
Metabolism provides energy for cell activity, molecules attending signaling transmission, and building block of cell components. Genome instability, proteostasis failure, and environmental influence could lead to abnormal energy supply and metabolite production, such as excessive free oxygen radicals and toxic molecules. Free oxygen radicals including reactive oxygen species (ROS) and diffusible hydrogen peroxide (H2O2), could lead to accumulated oxidative damages, such as carbonylation, oxidized methionine, glycation, aggregation of proteins and DNA damage, and contribute to aging and age-related diseases [26]. This process was proposed by the famous free radical theory of aging. Many compounds increase longevity or improve age-related diseases via scavenging free radicals, such as resveratrol, astaxanthin and gallic acid [27-29].
JNK, a MAP kinase family member, activated by oxidative stress increases longevity in fruit flies and worms [30, 31]. Reduced function of electron transport chain (ETC) could dramatic extend the lifespan of C. elegans and Drosophila [32, 33]. Recently research shows that mitophagy modulates bioenergetics and survival in the neurodegenerative disease by reducing redox and damage [34].
The regulation of metabolism is closely coupled with nutrient sensing pathways, including insulin-like growth factor (IGF) signaling (IIS) pathway [35], target of rapamycin (TOR) signaling [36], adenosine monophosphate activated protein kinase (AMPK) pathway [37], and sirtuins [38]. These signaling pathways sense nutrient or metabolites to regulate the level of glucose, amino acid, cAMP and nicotinamide adenine dinucleotide (NAD+). These pathways regulate growth, metabolic and aging process. Genetic or pharmacological intervention of their components can extend lifespan and delay age-associated dysregulation [8].
2.4 Cellular Homeostasis and Aging
Failure to maintain genome stability, proteostasis and metabolic homeostasis will lead to imbalance of cellular homeostasis and cellular senescence. Genome instability could lead to abnormality of nuclear structure, while excessive protein aggregation could cause ER malfunction. Genome damage, defective proteins, and excessive production of ROS could impair mitochondria. Mitochondria damage could induce rescue mechanisms: mitochondrial biogenesis, mitochondria specific unfolded protein response and mitophagy (macroautophagy that targets deficient mitochondria for proteolytic degradation) [39]. Recently research shows that mitophagy modulates bioenergetics and survival in the neurodegenerative disease by reducing redox and damage [34]. The increased damage and reduced repair response are important to aging process.
Senescent cells secret signaling molecules enriched in proinflammatory cytokines and matrix metalloproteinases, which could attract mast cells to clear the senescent cells through macrophage. But deficient clearance of senescent cells will induce inflammation, impair adjacent cells and tissue function, and lead to stem cell exhaustion, and finally contribute to aging [40]. Either genetic or pharmacological elimination of senescent cells could delay age-related pathologies [41, 42].
3 Natural Products with Anti-aging Activity
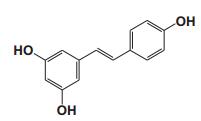
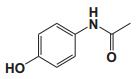
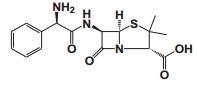
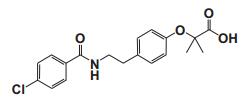
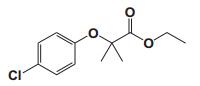
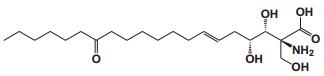
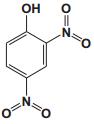
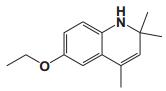
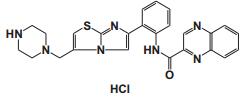
To date, there are about 5, 400 scientific research/review articles published under the terms of "anti-aging" and "anti-ageing" terms (obtained from Web of Science, May 2017; keywords restricted to the topics: anti-aging and anti-ageing, at the search domain of Science & Technology). These reports revealed more than 300 compounds with anti-aging activity. Here we summarized the compounds or natural product extracts with explicit anti-aging activity, including 185 compounds from natural products (Table 1), 55 complex or extracts from natural products (Table 2), 62 from clinical drugs (of which more than 50% are also from natural products or natural products analogues, Table 3), 35 from synthesized chemicals (Table 4). Some of them received popular interest and under vigorous investigation, present anti-aging activities in multiple aging models, such as resveratrol [28, 43-53], α-lipoic acid [54-56], astaxanthin [29, 57-59], catechin [60-62], curcumin [63-65], fucoxanthin [66, 67], spermidine [68, 69], metformin [70-72], caffeine [73-75], and rapamycin [76-84], all show anti-aging activity in both D. melanogaster and C. elegans, as well as in other aging models (Table 1). There are 39 compounds present anti-aging activity in two aging models, 32 of them with anti-aging activity in C. elegans. 19 of the 39 compounds are antioxidant (including acacetin, antcin M, agmatine, baicalein, caffeic acid, carnosine, chlorogenic acid, coenzyme Q10, dimethyl sulfide, gallic acid, gluconate, glycerol, hesperidin, icariin, lactate, oleanolic acid, minocycline, vitamin E, and vitexin). Compound betaine, catalpol, (-)-epicatechin, huperzine A and polydatin regulate inflammation. 11 compounds act through energy sensing pathway, including acetic acid, α-ketoglutarate, D-glucosamine, epigallocatechin gallate, nordihydroguaiaretic acid, oligonol, polydatin, rosmarinic acid, sesamin, aspirin, and tetrahydrocurcumin. There are 14, 9, and 109 natural products with anti-aging activity reported only in mice or rat, fruit fly, and C. elegans, respectively, while 14 compounds present anti-aging activities in other aging models, such as mammalian cells and S. cerevisiae. Among the 109 compounds with anti-aging activity in C. elegans, 18 with antioxidative activity, five regulating IIS pathway, four regulating AMPK, four regulating mTOR signaling, 10 regulating SIR-2.1, six regulating SKN-1/Nrf2 pathway, seven regulating JNK-1, 16 with unknown mechanisms, and about half of 109 compounds revealed to regulate multiple signaling pathways.
Compounds from natural products with anti-aging activities
Natural product extracts with anti-aging activities
Clinical medicine with anti-aging activities
Synthetic compounds with anti-aging activities
Among the 55 complex or extracts from natural products, 8, 14 and 29 of them were tested in mice, fruit fly and C. elegans, respectively. A majority of these extracts present antioxidative activity.
Among the 62 clinical medicine with anti-aging activity, three (rapamycin, metformin, caffeine) present anti-aging activities in three aging models, six (aspirin, berberine, huperzine A, minocycline, phenformin, and vitamin E) in two aging models, two (buformin and melatonin) in rats, four (ivabradine, acarbose, metoprolol, and nebivolol) in mice, 8 in D. melanogaster, 37 in C. elegans, cortisone in Asplanchna brightwelli and myriocin in S. cerevisiae, respectively. Interestingly, the anti-aging mechanisms of the most drugs are different from their clinical applications.
We also summarized 35 synthetic compounds with explicit anti-aging activity (Table 4). 2, 4-Dinitrophenol presents anti-aging activities in mice and fruit fly, ethoxyquin and SRT1720 in mice. Seven and 24 compounds present anti-aging activity in fruit fly and C. elegans, respectively. 3, 3′-thiodipropionic acid with anti-aging activity in Asplanchna brightwelli. Twenty-one of the 35 compounds present antioxidative activity.
In total, there are 212 and 46 compounds present anti-aging activity in C. elegans and fruit fly, respectively, indicating C. elegans and fruit fly are the most popular aging models for anti-aging screening. Those compounds present anti-aging activity in both C. elegans and fruit fly are worth to be further investigated in mammalian models.
4 Prospects of Discovering Anti-aging Molecules from Natural Products
Many clinical medicines are derived from natural products. But in the past two decades, pharmaceutical companies have been enthusing the drug development strategy of high-throughput screening (HTS) and combinatorial synthesis of enormous synthetic libraries of small molecules. Natural products were largely neglected for unsuitable for HTS of targeted protein assay and difficult in compound isolation and synthesis. But the achievement of new lead discovery and new drug approval was disappointing [85]. Compared with synthetic compounds, natural products are secondary metabolite, evolutionarily optimized with biologically relevant chemical space and preferred ligand binding motif, are not only biologically active, but with a high degree of bioavailability, suitable for functional and phenotypic assays [86]. Recent innovation in techniques for structural elucidation, metabolomics for profiling and isolation, and metagenomics or gene manipulation for synthetic pathways has facilitated to explore the enormous biodiversity on earth, including plant, microorganism and marine organism [87]. Engineered production of natural products from uncultivated species could extremely expand the chemical space of natural products by synthetic biology [88]. Moreover, modern computer-assisted drug design could utilize natural-product-derived fragments to computationally infer the biomolecular targets and activities of natural products and fragment-based de novo design. As summarized in above, currently discovered agents with anti-aging activity, majority of them are natural products. Therefore, natural products are invaluable sources and provide great promise for developing anti-aging medicine.
Notes
Acknowledgements
This work was supported by the Natural Science Foundation of China (81671405 and 81370453), Natural Science Foundation of Yunnan province (2013FA045 and 2015FB172), and Open Funds of Guangdong Key Laboratory of Marine Materia Medica.
Compliance with Ethical Standards
Conflict of interest
The authors declare no conflict of interest.
References
-
1.L. Fontana, L. Partridge, V.D. Longo, Science 328, 321-326 (2010) CrossRef PubMed Google Scholar
-
2.M. Kaeberlein, F1000Prime Rep. 5, 5 (2013) PubMed Google Scholar
-
3.C.E. Riera, C. Merkwirth, C.D. De Magalhaes Filho, A. Dillin, Annu. Rev. Biochem. 85, 35-64 (2016) CrossRef PubMed Google Scholar
-
4.J. Campisi, Annu. Rev. Physiol. 75, 685-705 (2013) CrossRef PubMed Google Scholar
-
5.C. Lopez-Otin, M.A. Blasco, L. Partridge, M. Serrano, G. Kroemer, Cell 153, 1194-1217 (2013) CrossRef PubMed Google Scholar
-
6.C. Lopez-Otin, L. Galluzzi, J.M. Freije, F. Madeo, G. Kroemer, Cell 166, 802-821 (2016) CrossRef PubMed Google Scholar
-
7.P. Sen, P.P. Shah, R. Nativio, S.L. Berger, Cell 166, 822-839 (2016) CrossRef PubMed Google Scholar
-
8.C.J. Kenyon, Nature 464, 504-512 (2010) CrossRef PubMed Google Scholar
-
9.A.A. Moskalev, M.V. Shaposhnikov, E.N. Plyusnina, A. Zhavoronkov, A. Budovsky, H. Yanai, V.E. Fraifeld, Ageing Res. Rev. 12, 661-684 (2013) CrossRef PubMed Google Scholar
-
10.T. Lindahl, D.E. Barnes, Cold Spring Harb. Symp. Quant. Biol. 65, 127-133 (2000) CrossRef PubMed Google Scholar
-
11.R.S. Tebbs, M.L. Flannery, J.J. Meneses, A. Hartmann, J.D. Tucker, L.H. Thompson, J.E. Cleaver, R.A. Pedersen, Dev. Biol. 208, 513-529 (1999) CrossRef PubMed Google Scholar
-
12.A. de Vries, C.T. van Oostrom, F.M. Hofhuis, P.M. Dortant, R.J. Berg, F.R. de Gruijl, P.W. Wester, C.F. van Kreijl, P.J. Capel, H. van Steeg, S.J. Verbeek, Nature 377, 169-173 (1995) CrossRef PubMed Google Scholar
-
13.J. de Boer, J.O. Andressoo, J. de Wit, J. Huijmans, R.B. Beems, H. van Steeg, G. Weeda, G.T. van der Horst, W. van Leeuwen, A.P. Themmen, M. Meradji, J.H. Hoeijmakers, Science 296, 1276-1279 (2002) CrossRef PubMed Google Scholar
-
14.M. Murai, Y. Enokido, N. Inamura, M. Yoshino, Y. Nakatsu, G.T. van der Horst, J.H. Hoeijmakers, K. Tanaka, H. Hatanaka, Proc. Natl. Acad. Sci. USA 98, 13379-13384 (2001) CrossRef PubMed Google Scholar
-
15.H. Vogel, D.S. Lim, G. Karsenty, M. Finegold, P. Hasty, Proc. Natl. Acad. Sci. USA 96, 10770-10775 (1999) CrossRef PubMed Google Scholar
-
16.E.H. Blackburn, E.S. Epel, J. Lin. Sci. 350, 1193-1198 (2015) PubMed Google Scholar
-
17.X.L. Guan, P.F. Wu, S. Wang, J.J. Zhang, Z.C. Shen, H. Luo, H. Chen, L.H. Long, J.G. Chen, F. Wang, Aging Cell (2016) PubMed Google Scholar
-
18.P. Oberdoerffer, D.A. Sinclair, Nat. Rev. Mol. Cell Biol. 8, 692-702 (2007) CrossRef PubMed Google Scholar
-
19.S. Pal, J.K. Tyler, Sci. Adv. 2, e1600584 (2016) CrossRef PubMed Google Scholar
-
20.D.J. Baker, M.M. Dawlaty, T. Wijshake, K.B. Jeganathan, L. Malureanu, J.H. van Ree, R. Crespo-Diaz, S. Reyes, L. Seaburg, V. Shapiro, A. Behfar, A. Terzic, B. van de Sluis, J.M. van Deursen, Nat. Cell Biol. 15, 96-102 (2013) PubMed Google Scholar
-
21.D. Ron, P. Walter, Nat. Rev. Mol. Cell Biol. 8, 519-529 (2007) CrossRef PubMed Google Scholar
-
22.E.T. Powers, R.I. Morimoto, A. Dillin, J.W. Kelly, W.E. Balch, Annu. Rev. Biochem. 78, 959-991 (2009) CrossRef PubMed Google Scholar
-
23.C. Marques, W. Guo, P. Pereira, A. Taylor, C. Patterson, P.C. Evans, F. Shang, Faseb J. 20, 741-743 (2006) PubMed Google Scholar
-
24.W.C. Chiang, T.T. Ching, H.C. Lee, C. Mousigian, A.L. Hsu, Cell 148, 322-334 (2012) CrossRef PubMed Google Scholar
-
25.W.R. Swindell, M.M. Masternak, J.J. Kopchick, C.A. Conover, A. Bartke, R.A. Miller, Mech. Ageing Dev. 130, 393-400 (2009) CrossRef PubMed Google Scholar
-
26.D.K. Woo, G.S. Shadel, Cell 144, 11-12 (2011) CrossRef PubMed Google Scholar
-
27.N. Saul, K. Pietsch, S.R. Sturzenbaum, R. Menzel, C.E. Steinberg, J. Nat. Prod. 74, 1713-1720 (2011) CrossRef PubMed Google Scholar
-
28.J.G. Wood, B. Rogina, S. Lavu, K. Howitz, S.L. Helfand, M. Tatar, D. Sinclair, Nature 430, 686-689 (2004) CrossRef PubMed Google Scholar
-
29.K. Yazaki, C. Yoshikoshi, S. Oshiro, S. Yanase, Oxid. Med. Cell Longev. 2011, 596240 (2011) PubMed Google Scholar
-
30.S.W. Oh, A. Mukhopadhyay, N. Svrzikapa, F. Jiang, R.J. Davis, H.A. Tissenbaum, Proc. Natl. Acad. Sci. USA 102, 4494-4499 (2005) CrossRef PubMed Google Scholar
-
31.M.C. Wang, D. Bohmann, H. Jasper, Dev. Cell 5, 811-816 (2003) CrossRef PubMed Google Scholar
-
32.A. Dillin, A.L. Hsu, N. Arantes-Oliveira, J. Lehrer-Graiwer, H. Hsin, A.G. Fraser, R.S. Kamath, J. Ahringer, C. Kenyon, Science 298, 2398-2401 (2002) CrossRef PubMed Google Scholar
-
33.B. Rogina, R.A. Reenan, S.P. Nilsen, S.L. Helfand, Science 290, 2137-2140 (2000) CrossRef PubMed Google Scholar
-
34.A. Shaik, A. Schiavi, Shaik A., Schiavi A. (2016) PubMed Google Scholar
-
35.C. Kenyon, Philos. Trans. R. Soc. Lond. B 366, 9-16 (2011) CrossRef PubMed Google Scholar
-
36.M.A. McCormick, S.Y. Tsai, B.K. Kennedy, Philos. Trans. R. Soc. Lond. B 366, 17-27 (2011) CrossRef PubMed Google Scholar
-
37.A. Salminen, K. Kaarniranta, A. Kauppinen, Ageing Res. Rev. 28, 15-26 (2016) CrossRef PubMed Google Scholar
-
38.Y. Kida, M.S. Goligorsky, Can. J. Cardiol. 32, 634-641 (2016) CrossRef PubMed Google Scholar
-
39.K. Wang, D.J. Klionsky, Autophagy 7, 297-300 (2011) CrossRef PubMed Google Scholar
-
40.F. Rodier, J. Campisi, J. Cell Biol. 192, 547-556 (2011) CrossRef PubMed Google Scholar
-
41.D.J. Baker, T. Wijshake, T. Tchkonia, N.K. LeBrasseur, B.G. Childs, B. van de Sluis, J.L. Kirkland, J.M. van Deursen, Nature 479, 232-236 (2011) CrossRef PubMed Google Scholar
-
42.J. Chang, Y. Wang, L. Shao, R.M. Laberge, M. Demaria, J. Campisi, K. Janakiraman, N.E. Sharpless, S. Ding, W. Feng, Y. Luo, X. Wang, N. Aykin-Burns, K. Krager, U. Ponnappan, M. Hauer-Jensen, A. Meng, D. Zhou, Nat. Med. 22, 78-83 (2016) PubMed Google Scholar
-
43.T.M. Bass, D. Weinkove, K. Houthoofd, D. Gems, L. Partridge, Mech. Ageing Dev. 128, 546-552 (2007) CrossRef PubMed Google Scholar
-
44.R. Strong, R.A. Miller, C.M. Astle, J.A. Baur, R. de Cabo, E. Fernandez, W. Guo, M. Javors, J.L. Kirkland, J.F. Nelson, D.A. Sinclair, B. Teter, D. Williams, N. Zaveri, N.L. Nadon, D.E. Harrison, J. Gerontol. A Biol. Sci. Med. Sci. 68, 6-16 (2013) CrossRef PubMed Google Scholar
-
45.C. Wang, C.T. Wheeler, T. Alberico, X. Sun, J. Seeberger, M. Laslo, E. Spangler, B. Kern, R. de Cabo, S. Zou, Age (Dordr.) 35, 69-81 (2013) CrossRef PubMed Google Scholar
-
46.J.A. Baur, K.J. Pearson, N.L. Price, H.A. Jamieson, C. Lerin, A. Kalra, V.V. Prabhu, J.S. Allard, G. Lopez-Lluch, K. Lewis, P.J. Pistell, S. Poosala, K.G. Becker, O. Boss, D. Gwinn, M. Wang, S. Ramaswamy, K.W. Fishbein, R.G. Spencer, E.G. Lakatta, D. Le Couteur, R.J. Shaw, P. Navas, P. Puigserver, D.K. Ingram, R. de Cabo, D.A. Sinclair, Nature 444, 337-342 (2006) CrossRef PubMed Google Scholar
-
47.B.T. Tung, E. Rodriguez-Bies, H.N. Thanh, H. Le-Thi-Thu, P. Navas, V.M. Sanchez, G. Lopez-Lluch, Aging Clin. Exp. Res. 27, 775-783 (2015) CrossRef PubMed Google Scholar
-
48.S. Ringholm, J. Olesen, J.T. Pedersen, C.T. Brandt, J.F. Halling, Y. Hellsten, C. Prats, H. Pilegaard, Exp. Gerontol. 48, 1311-1318 (2013) CrossRef PubMed Google Scholar
-
49.P. Toth, S. Tarantini, Z. Springo, Z. Tucsek, T. Gautam, C.B. Giles, J.D. Wren, A. Koller, W.E. Sonntag, A. Csiszar, Z. Ungvari, Aging Cell 14, 400-408 (2015) CrossRef PubMed Google Scholar
-
50.M. Liu, Y. Yin, X. Ye, M. Zeng, Q. Zhao, D.L. Keefe, L. Liu, Hum. Reprod. 28, 707-717 (2013) CrossRef PubMed Google Scholar
-
51.Y. Cui, B. Zhang, R. Zhang, C. Li, Y. Zhao, Y. Ren, J. Yang, Wei Sheng Yan Jiu 42(995-998), 1003 (2013) PubMed Google Scholar
-
52.J.J. Collins, K. Evason, K. Kornfeld, Exp. Gerontol. 41, 1032-1039 (2006) CrossRef PubMed Google Scholar
-
53.C. Regitz, E. Fitzenberger, F.L. Mahn, L.M. Dussling, U. Wenzel, Eur. J. Nutr. 55, 741-747 (2016) CrossRef PubMed Google Scholar
-
54.J.H. Bauer, S. Goupil, G.B. Garber, S.L. Helfand, Proc. Natl. Acad. Sci. USA 101, 12980-12985 (2004) CrossRef PubMed Google Scholar
-
55.M.K. Brown, J.L. Evans, Y. Luo, Pharmacol. Biochem. Behav. 85, 620-628 (2006) CrossRef PubMed Google Scholar
-
56.S.A. Farr, T.O. Price, W.A. Banks, N. Ercal, J.E. Morley, J Alzheimers Dis. 32, 447-455 (2012) PubMed Google Scholar
-
57.J. Huangfu, J. Liu, Z. Sun, M. Wang, Y. Jiang, Z.Y. Chen, F. Chen, J. Agric. Food Chem. 61, 7800-7804 (2013) CrossRef PubMed Google Scholar
-
58.M. Kuraji, T. Matsuno, T. Satoh, J. Clin. Biochem. Nutr. 59, 79-85 (2016) CrossRef PubMed Google Scholar
-
59.W. Wu, X. Wang, Q. Xiang, X. Meng, Y. Peng, N. Du, Z. Liu, Q. Sun, C. Wang, X. Liu, Food Funct. 5, 158-166 (2014) CrossRef PubMed Google Scholar
-
60.N. Saul, K. Pietsch, R. Menzel, S.R. Sturzenbaum, C.E. Steinberg, Mech. Ageing Dev. 130, 477-486 (2009) CrossRef PubMed Google Scholar
-
61.H. Si, Z. Fu, P.V. Babu, W. Zhen, T. Leroith, M.P. Meaney, K.A. Voelker, Z. Jia, R.W. Grange, D. Liu, J. Nutr. 141, 1095-1100 (2011) CrossRef PubMed Google Scholar
-
62.K. Unno, F. Takabayashi, H. Yoshida, D. Choba, R. Fukutomi, N. Kikunaga, T. Kishido, N. Oku, M. Hoshino, Biogerontology 8, 89-95 (2007) CrossRef PubMed Google Scholar
-
63.K. Cuanalo-Contreras, K.W. Park, A. Mukherjee, L. MillanPerez Pena, C. Soto. Biochem. Biophys. Res. Commun. (2016) PubMed Google Scholar
-
64.B.S. Fleenor, A.L. Sindler, N.K. Marvi, K.L. Howell, M.L. Zigler, M. Yoshizawa, D.R. Seals, Exp. Gerontol. 48, 269-276 (2013) CrossRef PubMed Google Scholar
-
65.L.R. Shen, F. Xiao, P. Yuan, Y. Chen, Q.K. Gao, L.D. Parnell, M. Meydani, J.M. Ordovas, D. Li, C.Q. Lai, Age (Dordr.) 35, 1133-1142 (2013) CrossRef PubMed Google Scholar
-
66.E. Lashmanova, E. Proshkina, S. Zhikrivetskaya, O. Shevchenko, E. Marusich, S. Leonov, A. Melerzanov, A. Zhavoronkov, A. Moskalev, Pharmacol. Res. 100, 228-241 (2015) CrossRef PubMed Google Scholar
-
67.I. Urikura, T. Sugawara, T. Hirata, Biosci. Biotechnol. Biochem. 75, 757-760 (2011) CrossRef PubMed Google Scholar
-
68.T. Eisenberg, H. Knauer, A. Schauer, S. Buttner, C. Ruckenstuhl, D. Carmona-Gutierrez, J. Ring, S. Schroeder, C. Magnes, L. Antonacci, H. Fussi, L. Deszcz, R. Hartl, E. Schraml, A. Criollo, E. Megalou, D. Weiskopf, P. Laun, G. Heeren, M. Breitenbach, B. Grubeck-Loebenstein, E. Herker, B. Fahrenkrog, K.U. Frohlich, F. Sinner, N. Tavernarakis, N. Minois, G. Kroemer, F. Madeo, Nat. Cell Biol. 11, 1305-1314 (2009) CrossRef PubMed Google Scholar
-
69.A. Beach, V.I. Titorenko, Subcell. Biochem. 69, 153-167 (2013) CrossRef PubMed Google Scholar
-
70.G. Garg, S. Singh, A.K. Singh, S.I. Rizvi, Rejuvenation Res. 20, 15-24 (2017) CrossRef PubMed Google Scholar
-
71.B. Onken, M. Driscoll, PLoS ONE 5, e8758 (2010) CrossRef PubMed Google Scholar
-
72.C. Slack, A. Foley, L. Partridge, PLoS ONE 7, e47699 (2012) CrossRef PubMed Google Scholar
-
73.G.L. Sutphin, E. Bishop, M.E. Yanos, R.M. Moller, M. Kaeberlein, Healthspan 1, 9 (2012) PubMed Google Scholar
-
74.F.F. Cruz, C.E. Leite, L.W. Kist, G.M. de Oliveira, M.R. Bogo, C.D. Bonan, M.M. Campos, F.B. Morrone, Comp. Biochem. Physiol. C Toxicol. Pharmacol. 194, 28-36 (2017) CrossRef PubMed Google Scholar
-
75.F. Ullah, T. Ali, N. Ullah, M.O. Kim, Neurochem. Int. 90, 114-124 (2015) CrossRef PubMed Google Scholar
-
76.D.E. Harrison, R. Strong, Z.D. Sharp, J.F. Nelson, C.M. Astle, K. Flurkey, N.L. Nadon, J.E. Wilkinson, K. Frenkel, C.S. Carter, M. Pahor, M.A. Javors, E. Fernandez, R.A. Miller, Nature 460, 392-395 (2009) PubMed Google Scholar
-
77.J.E. Wilkinson, L. Burmeister, S.V. Brooks, C.C. Chan, S. Friedline, D.E. Harrison, J.F. Hejtmancik, N. Nadon, R. Strong, L.K. Wood, M.A. Woodward, R.A. Miller, Aging Cell 11, 675-682 (2012) CrossRef PubMed Google Scholar
-
78.R.A. Miller, D.E. Harrison, C.M. Astle, E. Fernandez, K. Flurkey, M. Han, M.A. Javors, X. Li, N.L. Nadon, J.F. Nelson, S. Pletcher, A.B. Salmon, Z.D. Sharp, S. Van Roekel, L. Winkleman, R. Strong, Aging Cell 13, 468-477 (2014) CrossRef PubMed Google Scholar
-
79.Y. Zhang, A. Bokov, J. Gelfond, V. Soto, Y. Ikeno, G. Hubbard, V. Diaz, L. Sloane, K. Maslin, S. Treaster, S. Rendon, H. van Remmen, W. Ward, M. Javors, A. Richardson, S.N. Austad, K. Fischer, J. Gerontol. A Biol. Sci. Med. Sci. 69, 119-130 (2014) PubMed Google Scholar
-
80.W.C. Fok, Y. Chen, A. Bokov, Y. Zhang, A.B. Salmon, V. Diaz, M. Javors, W.H. Wood 3rd, Y. Zhang, K.G. Becker, V.I. Perez, A. Richardson, PLoS ONE 9, e83988 (2014) CrossRef PubMed Google Scholar
-
81.N.G. Kolosova, A.O. Vitovtov, N.A. Muraleva, A.E. Akulov, N.A. Stefanova, M.V. Blagosklonny, Aging (Albany.. NY) 5, 474-484 (2013) PubMed Google Scholar
-
82.R.A. Miller, D.E. Harrison, C.M. Astle, J.A. Baur, A.R. Boyd, R. de Cabo, E. Fernandez, K. Flurkey, M.A. Javors, J.F. Nelson, C.J. Orihuela, S. Pletcher, Z.D. Sharp, D. Sinclair, J.W. Starnes, J.E. Wilkinson, N.L. Nadon, R. Strong, J. Gerontol. A Biol. Sci. Med. Sci. 66, 191-201 (2011) PubMed Google Scholar
-
83.I. Bjedov, J.M. Toivonen, F. Kerr, C. Slack, J. Jacobson, A. Foley, L. Partridge, Cell Metab. 11, 35-46 (2010) CrossRef PubMed Google Scholar
-
84.S. Robida-Stubbs, K. Glover-Cutter, D.W. Lamming, M. Mizunuma, S.D. Narasimhan, E. Neumann-Haefelin, D.M. Sabatini, T.K. Blackwell, Cell Metab. 15, 713-724 (2012) CrossRef PubMed Google Scholar
-
85.J.W. Li, J.C. Vederas, Science 325, 161-165 (2009) CrossRef PubMed Google Scholar
-
86.D.J. Newman, G.M. Cragg, J. Nat. Prod. 75, 311-335 (2012) CrossRef PubMed Google Scholar
-
87.A. Bouslimani, L.M. Sanchez, N. Garg, P.C. Dorrestein, Nat. Prod. Rep. 31, 718-729 (2014) CrossRef PubMed Google Scholar
-
88.C.J. Paddon, P.J. Westfall, D.J. Pitera, K. Benjamin, K. Fisher, D. McPhee, M.D. Leavell, A. Tai, A. Main, D. Eng, D.R. Polichuk, K.H. Teoh, D.W. Reed, T. Treynor, J. Lenihan, M. Fleck, S. Bajad, G. Dang, D. Dengrove, D. Diola, G. Dorin, K.W. Ellens, S. Fickes, J. Galazzo, S.P. Gaucher, T. Geistlinger, R. Henry, M. Hepp, T. Horning, T. Iqbal, H. Jiang, L. Kizer, B. Lieu, D. Melis, N. Moss, R. Regentin, S. Secrest, H. Tsuruta, R. Vazquez, L.F. Westblade, L. Xu, M. Yu, Y. Zhang, L. Zhao, J. Lievense, P.S. Covello, J.D. Keasling, K.K. Reiling, N.S. Renninger, J.D. Newman, Nature 496, 528-532 (2013) CrossRef PubMed Google Scholar
-
89.S.J. Park, F. Ahmad, A. Philp, K. Baar, T. Williams, H. Luo, H. Ke, H. Rehmann, R. Taussig, A.L. Brown, M.K. Kim, M.A. Beaven, A.B. Burgin, V. Manganiello, J.H. Chung, Cell 148, 421-433 (2012) CrossRef PubMed Google Scholar
-
90.C. Liu, R. Zhang, C. Sun, H. Zhang, C. Xu, W. Liu, W. Gao, S. Huang, L. Chen, J. Neurochem. 135, 466-478 (2015) CrossRef PubMed Google Scholar
-
91.S. Chen, N. Zhou, Z. Zhang, W. Li, W. Zhu, Biochem. Biophys. Res. Commun. 457, 608-613 (2015) CrossRef PubMed Google Scholar
-
92.L. Quoc Trung, J.L. Espinoza, A. Takami, S. Nakao, PLoS ONE 8, e55183 (2013) CrossRef PubMed Google Scholar
-
93.L.A. Madrigal-Perez, G.M. Nava, J.C. Gonzalez-Hernandez, M. Ramos-Gomez, J. Bioenerg. Biomembr. 47, 331-336 (2015) CrossRef PubMed Google Scholar
-
94.X. Escote, M. Miranda, S. Menoyo, B. Rodriguez-Porrata, D. Carmona-Gutierrez, H. Jungwirth, F. Madeo, R.R. Cordero, A. Mas, F. Tinahones, J. Clotet, J. Vendrell, Yeast 29, 251-263 (2012) CrossRef PubMed Google Scholar
-
95.T. Liu, H. Qi, L. Ma, Z. Liu, H. Fu, W. Zhu, T. Song, B. Yang, G. Li, Rejuvenation Res. 18, 225-233 (2015) CrossRef PubMed Google Scholar
-
96.Y.M. Li, H.Y. Chan, Y. Huang, Z.Y. Chen, Mol. Nutr. Food Res. 51, 546-554 (2007) CrossRef PubMed Google Scholar
-
97.X. Wang, H. Perumalsamy, H.W. Kwon, Y.E. Na, Y.J. Ahn, Sci. Rep. 5, 16127 (2015) CrossRef PubMed Google Scholar
-
98.J. Asthana, B.N. Mishra, R. Pandey, Free Radic. Res. 50, 861-874 (2016) CrossRef PubMed Google Scholar
-
99.M.H. Chuang, S.H. Chiou, C.H. Huang, W.B. Yang, C.H. Wong, Bioorg. Med. Chem. 17, 7831-7840 (2009) CrossRef PubMed Google Scholar
-
100.W.C. Burhans, M. Weinberger, Cell Cycle 8, 2300-2302 (2009) CrossRef PubMed Google Scholar
-
101.K.J. Senthil Kumar, M. Gokila Vani, J.L. Mau, C.C. Lin, F.H. Chu, C.C. Wei, V.H. Liao, Wang. S.Y., Oncotarget (2016) PubMed Google Scholar
-
102.M. Rushaidhi, H. Zhang, P. Liu, Neuroscience 234, 116-124 (2013) CrossRef PubMed Google Scholar
-
103.C. Edwards, J. Canfield, N. Copes, A. Brito, M. Rehan, D. Lipps, J. Brunquell, S.D. Westerheide, P.C. Bradshaw, BMC Genet. 16, 8 (2015) PubMed Google Scholar
-
104.T. Niemiec, J. Sikorska, A. Harrison, M. Szmidt, E. Sawosz, E. Wirth-Dzieciolowska, J. Wilczak, S. Pierzynowski, J. Physiol. Pharmacol. 62, 37-43 (2011) PubMed Google Scholar
-
105.R.M. Chin, X. Fu, M.Y. Pai, L. Vergnes, H. Hwang, G. Deng, S. Diep, B. Lomenick, V.S. Meli, G.C. Monsalve, E. Hu, S.A. Whelan, J.X. Wang, G. Jung, G.M. Solis, F. Fazlollahi, C. Kaweeteerawat, A. Quach, M. Nili, A.S. Krall, H.A. Godwin, H.R. Chang, K.F. Faull, F. Guo, M. Jiang, S.A. Trauger, A. Saghatelian, D. Braas, H.R. Christofk, C.F. Clarke, M.A. Teitell, M. Petrascheck, K. Reue, M.E. Jung, A.R. Frand, J. Huang, Nature 510, 397-401 (2014) PubMed Google Scholar
-
106.S. Havermann, H.U. Humpf, W. Watjen, Fitoterapia 113, 123-127 (2016) CrossRef PubMed Google Scholar
-
107.S. Zhang, J. Ye, G. Dong, J. Mol. Neurosci. 40, 311-320 (2010) CrossRef PubMed Google Scholar
-
108.E.K. Lee, E.J. Jang, K.J. Jung, D.H. Kim, B.P. Yu, H.Y. Chung, Exp. Gerontol. 48, 517-524 (2013) CrossRef PubMed Google Scholar
-
109.J. Coban, I. Bingul, K. Yesil-Mizrak, S. Dogru-Abbasoglu, S. Oztezcan, M. Uysal, Curr. Aging Sci. 6, 199-205 (2013) CrossRef PubMed Google Scholar
-
110.M. Esrefoglu, M. Iraz, B. Ates, M. Gul, Ultrastruct. Pathol. 36, 244-251 (2012) CrossRef PubMed Google Scholar
-
111.R. Deshmukh, M. Kaundal, V. Bansal, Samardeep. Biomed. Pharmacother. 81, 56-62 (2016) CrossRef PubMed Google Scholar
-
112.K. Pietsch, N. Saul, S. Chakrabarti, S.R. Sturzenbaum, R. Menzel, C.E. Steinberg, Biogerontology 12, 329-347 (2011) CrossRef PubMed Google Scholar
-
113.A.F. Aydin, J. Coban, I. Dogan-Ekici, E. Betul-Kalaz, S. DogruAbbasoglu, M. Uysal, Metab. Brain Dis. 31, 337-345 (2016) CrossRef PubMed Google Scholar
-
114.A.F. Aydin, J. Coban, I. Dogan-Ekici, S. Dogru-Abbasoglu, M. Uysal, N. Kocak-Toker, Andrologia 47, 1131-1138 (2015) CrossRef PubMed Google Scholar
-
115.A.O. Yuneva, G.G. Kramarenko, T.V. Vetreshchak, S. Gallant, A.A. Boldyrev, Bull. Exp. Biol. Med. 133, 559-561 (2002) CrossRef PubMed Google Scholar
-
116.S. Stvolinsky, M. Antipin, K. Meguro, T. Sato, H. Abe, A. Boldyrev, Rejuvenation Res. 13, 453-457 (2010) CrossRef PubMed Google Scholar
-
117.X. Zhang, C. Jin, Y. Li, S. Guan, F. Han, S. Zhang, Food Chem. Toxicol. 58, 50-55 (2013) CrossRef PubMed Google Scholar
-
118.M. Wei, Y. Lu, D. Liu, W. Ru, Biol. Pharm. Bull. 37, 1444-1449 (2014) CrossRef PubMed Google Scholar
-
119.H.W. Seo, S.M. Cheon, M.H. Lee, H.J. Kim, H. Jeon, D.S. Cha, Evid. Based Complement Altern. Med. 2015, 524878 (2015) PubMed Google Scholar
-
120.Y. Feng, Y.H. Yu, S.T. Wang, J. Ren, D. Camer, Y.Z. Hua, Q. Zhang, J. Huang, D.L. Xue, X.F. Zhang, X.F. Huang, Y. Liu, Pharm. Biol. 54, 1027-1034 (2016) CrossRef PubMed Google Scholar
-
121.S.Q. Zheng, X.B. Huang, T.K. Xing, A.J. Ding, G.S. Wu, Luo. H.R., J. Gerontol. A (2016) PubMed Google Scholar
-
122.R.A. Shetty, U.S. Ikonne, M.J. Forster, N. Sumien, Exp. Gerontol. 58, 208-218 (2014) CrossRef PubMed Google Scholar
-
123.N. Ishii, N. Senoo-Matsuda, K. Miyake, K. Yasuda, T. Ishii, P.S. Hartman, S. Furukawa, Mech. Ageing Dev. 125, 41-46 (2004) CrossRef PubMed Google Scholar
-
124.S. Weimer, J. Priebs, D. Kuhlow, M. Groth, S. Priebe, J. Mansfeld, T.L. Merry, S. Dubuis, B. Laube, A.F. Pfeiffer, T.J. Schulz, R. Guthke, M. Platzer, N. Zamboni, K. Zarse, M. Ristow, Nat. Commun. 5, 3563 (2014) PubMed Google Scholar
-
125.J. Chen, Y. Li, Q. Zhu, T. Li, H. Lu, N. Wei, Y. Huang, R. Shi, X. Ma, X. Wang, J. Sheng, Mech. Ageing Dev. 164, 1-7 (2017) CrossRef PubMed Google Scholar
-
126.S. Abbas, M. Wink, Planta Med. 75, 216-221 (2009) CrossRef PubMed Google Scholar
-
127.L. Li, T.B. Ng, W. Gao, W. Li, M. Fu, S.M. Niu, L. Zhao, R.R. Chen, F. Liu, Life Sci. 77, 230-240 (2005) CrossRef PubMed Google Scholar
-
128.H.R. Massie, T.R. Williams, Exp. Gerontol. 14, 109-115 (1979) CrossRef PubMed Google Scholar
-
129.T.W. Snell, R.K. Johnston, Exp. Gerontol. 57, 47-56 (2014) CrossRef PubMed Google Scholar
-
130.S.O. Rotimi, G.E. Bankole, I.B. Adelani, O.A. Rotimi, Immunopharmacol. Immunotoxicol. 38, 364-371 (2016) CrossRef PubMed Google Scholar
-
131.K. Sun, L. Xiang, S. Ishihara, A. Matsuura, Y. Sakagami, J. Qi, Biosci. Biotechnol. Biochem. 76, 640-645 (2012) CrossRef PubMed Google Scholar
-
132.S.Q. Zhang, W.J. Cai, J.H. Huang, B. Wu, S.J. Xia, X.L. Chen, X.M. Zhang, Z.Y. Shen, Exp. Gerontol. 69, 226-235 (2015) CrossRef PubMed Google Scholar
-
133.W.J. Cai, J.H. Huang, S.Q. Zhang, B. Wu, P. Kapahi, X.M. Zhang, Z.Y. Shen, PLoS ONE 6, e28835 (2011) CrossRef PubMed Google Scholar
-
134.H. Ma, Y. Ma, Z. Zhang, Z. Zhao, R. Lin, J. Zhu, Y. Guo, L. Xu, Int. J. Environ. Res. Public Health, 13 (2016) PubMed Google Scholar
-
135.R. Strong, R.A. Miller, C.M. Astle, R.A. Floyd, K. Flurkey, K.L. Hensley, M.A. Javors, C. Leeuwenburgh, J.F. Nelson, E. Ongini, N.L. Nadon, H.R. Warner, D.E. Harrison, Aging Cell 7, 641-650 (2008) CrossRef PubMed Google Scholar
-
136.N.P. Buu-Hoi, A.R. Ratsimamanga, C. R. Seances, Soc. Biol. Fil. 153, 1180-1182 (1959) PubMed Google Scholar
-
137.Jr.J.P. Richie, B.J. Mills, C.A. Lang, Proc. Soc. Exp. Biol. Med. 183, 81-85 (1986) CrossRef PubMed Google Scholar
-
138.S.R. Spindler, P.L. Mote, A.L. Lublin, J.M. Flegal, J.M. Dhahbi, R. Li, J. Gerontol. A 70, 1479-1489 (2015) CrossRef PubMed Google Scholar
-
139.S.J. Tsai, M.C. Yin, Eur. J. Pharmacol. 689, 81-88 (2012) CrossRef PubMed Google Scholar
-
140.J. Zhang, L. Lu, L. Zhou, Biochem. Biophys. Res. Commun. 468, 843-849 (2015) CrossRef PubMed Google Scholar
-
141.S.K. Park, R.K. Seong, J.A. Kim, S.J. Son, Y. Kim, T. Yokozawa, O.S. Shin, Nutr. Res. Pract. 10, 3-10 (2016) CrossRef PubMed Google Scholar
-
142.Y.Y. Choi, T. Maeda, H. Fujii, T. Yokozawa, H.Y. Kim, E.J. Cho, T. Shibamoto, Nutr Res. 34, 595-603 (2014) CrossRef PubMed Google Scholar
-
143.L.Q. Xu, Y.L. Xie, S.H. Gui, X. Zhang, Z.Z. Mo, C.Y. Sun, C.L. Li, D.D. Luo, Z.B. Zhang, Z.R. Su, J.H. Xie, Food Funct. 7, 4545-4555 (2016) CrossRef PubMed Google Scholar
-
144.H. Wen, X. Gao, J. Qin, Integr. Biol. (Camp) 6, 35-43 (2014) CrossRef PubMed Google Scholar
-
145.Y. Zhang, X. Chen, L. Yang, Y. Zu, Q. Lu, Food Funct. 6, 927-931 (2015) CrossRef PubMed Google Scholar
-
146.Y. Zuo, C. Peng, Y. Liang, K.Y. Ma, H.Y. Chan, Y. Huang, Z.Y. Chen, Biogerontology 14, 107-119 (2013) CrossRef PubMed Google Scholar
-
147.Y. Yaguchi, T. Komura, N. Kashima, M. Tamura, E. KageNakadai, S. Saeki, K. Terao, Y. Nishikawa, Eur. J. Nutr. 53, 1659-1668 (2014) CrossRef PubMed Google Scholar
-
148.K. Kitani, T. Yokozawa, T. Osawa, Ann. N. Y. Acad. Sci. 1019, 424-426 (2004) CrossRef PubMed Google Scholar
-
149.W. Sangartit, P. Pakdeechote, V. Kukongviriyapan, W. Donpunha, S. Shibahara, U. Kukongviriyapan, Vascul. Pharmacol. 87, 199-208 (2016) CrossRef PubMed Google Scholar
-
150.K. Okada, C. Wangpoengtrakul, T. Tanaka, S. Toyokuni, K. Uchida, T. Osawa, J. Nutr. 131, 2090-2095 (2001) PubMed Google Scholar
-
151.K. Kitani, T. Osawa, T. Yokozawa, Biogerontology 8, 567-573 (2007) CrossRef PubMed Google Scholar
-
152.L. Xiang, Y. Nakamura, Y.M. Lim, Y. Yamasaki, Y. KurokawaNose, W. Maruyama, T. Osawa, A. Matsuura, N. Motoyama, L. Tsuda, Aging (Albany.. NY) 3, 1098-1109 (2011) PubMed Google Scholar
-
153.D. Ryu, L. Mouchiroud, P.A. Andreux, E. Katsyuba, N. Moullan, A.A. Nicolet-Dit-Felix, E.G. Williams, P. Jha, G. Lo Sasso, D. Huzard, P. Aebischer, C. Sandi, C. Rinsch, J. Auwerx. Nat. Med. 22, 879-888 (2016) CrossRef PubMed Google Scholar
-
154.F. An, G. Yang, J. Tian, S. Wang, Neural Regen. Res. 7, 2565-2575 (2012) PubMed Google Scholar
-
155.E.B. Lee, J.H. Kim, Y.S. Cha, M. Kim, S.B. Song, D.S. Cha, H. Jeon, J.S. Eun, S. Han, D.K. Kim, Biomol. Ther. (Seoul) 23, 582-589 (2015) CrossRef PubMed Google Scholar
-
156.D.G. Souza, B. Bellaver, L.D. Bobermin, D.O. Souza, A. Quincozes-Santos. Purinergic Signal (2016) PubMed Google Scholar
-
157.F. de la Coba, J. Aguilera, M.V. de Galvez, M. Alvarez, E. Gallego, F.L. Figueroa, E. Herrera, J. Dermatol. Sci. 55, 161-169 (2009) CrossRef PubMed Google Scholar
-
158.S. Hur, H. Lee, Y. Kim, B.H. Lee, J. Shin, T.Y. Kim, Eur. J. Pharmacol. 582, 1-11 (2008) CrossRef PubMed Google Scholar
-
159.T. Kawamura, N. Mori, K. Shibata, J. Nutr. Sci. Vitaminol. (Tokyo) 62, 272-276 (2016) CrossRef PubMed Google Scholar
-
160.B. Bernardes de Jesus, K. Schneeberger, E. Vera, A. Tejera, C.B. Harley, M.A. Blasco, Aging Cell 10, 604-621 (2011) CrossRef PubMed Google Scholar
-
161.M. Kiaei, K. Kipiani, S. Petri, J. Chen, N.Y. Calingasan, M.F. Beal, Neurodegener. Dis. 2, 246-254 (2005) CrossRef PubMed Google Scholar
-
162.A. Bender, J. Beckers, I. Schneider, S.M. Holter, T. Haack, T. Ruthsatz, D.M. Vogt-Weisenhorn, L. Becker, J. Genius, D. Rujescu, M. Irmler, T. Mijalski, M. Mader, L. QuintanillaMartinez, H. Fuchs, V. Gailus-Durner, M.H. de Angelis, W. Wurst, J. Schmidt, T. Klopstock, Neurobiol. Aging 29, 1404-1411 (2008) CrossRef PubMed Google Scholar
-
163.H.A. Bakshi, S. Sam, A. Feroz, Z. Ravesh, G.A. Shah, M. Sharma, Asian Pac. J. Cancer Prev. 10, 887-890 (2009) PubMed Google Scholar
-
164.X.M. Peng, L. Gao, S.X. Huo, X.M. Liu, M. Yan, Phytother. Res. 29, 1137-1144 (2015) CrossRef PubMed Google Scholar
-
165.H. Wu, J. Zhao, M. Chen, H. Wang, Q. Yao, J. Fan, M. Zhang, J. Mol. Neurosci. 61, 449-458 (2017) CrossRef PubMed Google Scholar
-
166.Y. Li, Z. Zhang, Int. J. Clin. Exp. Pathol. 8, 14099-14109 (2015) PubMed Google Scholar
-
167.J. Zhu, X. Mu, J. Zeng, C. Xu, J. Liu, M. Zhang, C. Li, J. Chen, T. Li, Y. Wang, PLoS ONE 9, e101291 (2014) CrossRef PubMed Google Scholar
-
168.J. Li, D. Cai, X. Yao, Y. Zhang, L. Chen, P. Jing, L. Wang, Y. Wang, Int. J. Mol. Sci., 17 (2016) PubMed Google Scholar
-
169.B. Hada, M.R. Yoo, K.M. Seong, Y.W. Jin, H.K. Myeong, K.J. Min, J. Gerontol. A 68, 226-234 (2013) CrossRef PubMed Google Scholar
-
170.W. Pan, S. Jiang, P. Luo, J. Wu, P. Gao, Nat. Prod. Res. 22, 719-725 (2008) CrossRef PubMed Google Scholar
-
171.Z. Zhang, S. Han, H. Wang, T. Wang, Arch. Gerontol. Geriatr. 58, 153-159 (2014) CrossRef PubMed Google Scholar
-
172.C. Peng, H.Y. Chan, Y.M. Li, Y. Huang, Z.Y. Chen, Exp. Gerontol. 44, 773-783 (2009) CrossRef PubMed Google Scholar
-
173.S. Yang, L.H. Long, D. Li, J.K. Zhang, S. Jin, F. Wang, J.G. Chen, Aging Cell 14, 1024-1033 (2015) CrossRef PubMed Google Scholar
-
174.A. Danilov, M. Shaposhnikov, E. Plyusnina, V. Kogan, P. Fedichev, A. Moskalev, Oncotarget 4, 1507-1526 (2013) CrossRef PubMed Google Scholar
-
175.Y. Nakagawa-Yagi, Y. Sato, E. Matsumoto, S. Nakatsuka, T. Sakaki, Y. Muramatsu, T. Hara, T. Aigaki, BMC Complement Altern. Med. 12, 101 (2012) CrossRef PubMed Google Scholar
-
176.X. Ye, J.M. Linton, N.J. Schork, L.B. Buck, M. Petrascheck, Aging Cell 13, 206-215 (2014) CrossRef PubMed Google Scholar
-
177.E. Wang, M. Wink, PeerJ 4, e1879 (2016) CrossRef PubMed Google Scholar
-
178.Z. Zhang, Y. Zhao, X. Wang, R. Lin, Y. Zhang, H. Ma, Y. Guo, L. Xu, B. Zhao, Food Funct. 7, 1975-1984 (2016) CrossRef PubMed Google Scholar
-
179.A.A. Sayed, J. Pharm. Pharmacol. 63, 423-428 (2011) CrossRef PubMed Google Scholar
-
180.E.B. Lee, D. Ahn, B.J. Kim, S.Y. Lee, H.W. Seo, Y.S. Cha, H. Jeon, J.S. Eun, D.S. Cha, D.K. Kim, Biomol. Ther. (Seoul) 23, 77-83 (2015) CrossRef PubMed Google Scholar
-
181.M. Fischer, C. Regitz, M. Kahl, M. Werthebach, M. Boll, U. Wenzel, Mol. Nutr. Food Res. 56, 957-965 (2012) CrossRef PubMed Google Scholar
-
182.Y.L. Xue, T. Ahiko, T. Miyakawa, H. Amino, F. Hu, K. Furihata, K. Kita, T. Shirasawa, Y. Sawano, M. Tanokura, J. Agric. Food Chem. 59, 5927-5934 (2011) CrossRef PubMed Google Scholar
-
183.S. Ayyadevara, P. Bharill, A. Dandapat, C. Hu, M. Khaidakov, S. Mitra, R.J. Shmookler Reis, J.L. Mehta, Antioxid. Redox Signal. 18, 481-490 (2013) CrossRef PubMed Google Scholar
-
184.J. Asthana, A.K. Yadav, A. Pant, S. Pandey, M.M. Gupta, R. Pandey, Comp. Biochem. Physiol. C 169, 25-34 (2015) PubMed Google Scholar
-
185.M.G. Benedetti, A.L. Foster, M.C. Vantipalli, M.P. White, J.N. Sampayo, M.S. Gill, A. Olsen, G.J. Lithgow, Exp. Gerontol. 43, 882-891 (2008) CrossRef PubMed Google Scholar
-
186.K. Zarse, S. Jabin, M. Ristow, Eur. J. Nutr. 51, 765-768 (2012) CrossRef PubMed Google Scholar
-
187.H. Adachi, N. Ishii, J. Gerontol. A 55, B280-B285 (2000) CrossRef PubMed Google Scholar
-
188.A. Kampkotter, Gombitang Nkwonkam C., R.F. Zurawski, C. Timpel, Y. Chovolou, W. Watjen, R. Kahl, Arch. Toxicol. 81, 849-858 (2007) CrossRef PubMed Google Scholar
-
189.V. Shukla, D. Yadav, S.C. Phulara, M.M. Gupta, S.K. Saikia, R. Pandey, Free Radic. Biol. Med. 53, 1848-1856 (2012) CrossRef PubMed Google Scholar
-
190.C. Buchter, S. Havermann, K. Koch, W. Watjen, Eur. J. Nutr. 55, 257-265 (2016) CrossRef PubMed Google Scholar
-
191.A. Kampkotter, C. Timpel, R.F. Zurawski, S. Ruhl, Y. Chovolou, P. Proksch, W. Watjen, Comp. Biochem. Physiol. B 149, 314-323 (2008) PubMed Google Scholar
-
192.A. Pant, J. Asthana, A.K. Yadav, L. Rathor, S. Srivastava, M.M. Gupta, R. Pandey, Free Radic. Res. 49, 1384-1392 (2015) CrossRef PubMed Google Scholar
-
193.Y. Honda, M. Tanaka, S. Honda, Aging Cell 9, 558-569 (2010) CrossRef PubMed Google Scholar
-
194.B.A. Akhoon, S. Pandey, S. Tiwari, R. Pandey, Exp. Gerontol. 78, 47-56 (2016) CrossRef PubMed Google Scholar
-
195.A. Canuelo, F.J. Esteban, J. Peragon, Eur. J. Nutr. 55, 639-650 (2016) CrossRef PubMed Google Scholar
-
196.A. Canuelo, J. Peragon, Proteomics 13, 3064-3075 (2013) PubMed Google Scholar
-
197.A. Canuelo, B. Gilbert-Lopez, P. Pacheco-Linan, E. MartinezLara, E. Siles, A. Miranda-Vizuete, Mech. Ageing Dev. 133, 563-574 (2012) CrossRef PubMed Google Scholar
-
198.P. Shen, Y. Yue, Q. Sun, N. Kasireddy, K.H. Kim, Y. Park. Biofactors (2017) PubMed Google Scholar
-
199.A. Schlernitzauer, C. Oiry, R. Hamad, S. Galas, F. Cortade, B. Chabi, F. Casas, L. Pessemesse, G. Fouret, C. Feillet-Coudray, G. Cros, G. Cabello, R. Magous, C. Wrutniak-Cabello, PLoS ONE 8, E78788 (2013) CrossRef PubMed Google Scholar
-
200.D.S. Williams, A. Cash, L. Hamadani, T. Diemer, Aging Cell 8, 765-768 (2009) CrossRef PubMed Google Scholar
-
201.J. Lee, G. Kwon, J. Park, J.K. Kim, Y.H. Lim, Exp. Biol. Med. (Maywood) 241, 1757-1763 (2016) CrossRef PubMed Google Scholar
-
202.L. Gao, R. Zhang, J. Lan, R. Ning, D. Wu, D. Chen, W. Zhao, J. Nat. Prod. 79, 3039-3046 (2016) CrossRef PubMed Google Scholar
-
203.X. Fu, R.M. Chin, L. Vergnes, H. Hwang, G. Deng, Y. Xing, M.Y. Pai, S. Li, L. Ta, F. Fazlollahi, C. Chen, R.M. Prins, M.A. Teitell, D.A. Nathanson, A. Lai, K.F. Faull, M. Jiang, S.G. Clarke, T.F. Cloughesy, T.G. Graeber, D. Braas, H.R. Christofk, M.E. Jung, K. Reue, J. Huang, Cell Metab. 22, 508-515 (2015) CrossRef PubMed Google Scholar
-
204.Y. Honda, Y. Araki, T. Hata, K. Ichihara, M. Ito, M. Tanaka, S. Honda, J. Aging Res. 2015, 425261 (2015) PubMed Google Scholar
-
205.A.H. Ludewig, Y. Izrayelit, D. Park, R.U. Malik, A. Zimmermann, P. Mahanti, B.W. Fox, A. Bethke, F. Doering, D.L. Riddle, F.C. Schroeder, Proc. Natl. Acad. Sci. USA 110, 5522-5527 (2013) CrossRef PubMed Google Scholar
-
206.J. Kim, Y.G. Kang, J.Y. Lee, D.H. Choi, Y.U. Cho, J.M. Shin, J.S. Park, J.H. Lee, W.G. Kim, D.B. Seo, T.R. Lee, Y. Miyamoto, K.T. No, Mol. Cell. Endocrinol. 412, 216-225 (2015) CrossRef PubMed Google Scholar
-
207.D.L. Miller, M.B. Roth, Proc. Natl. Acad. Sci. USA 104, 20618-20622 (2007) CrossRef PubMed Google Scholar
-
208.B. Qabazard, L. Li, J. Gruber, M.T. Peh, L.F. Ng, S.D. Kumar, P. Rose, C.H. Tan, B.W. Dymock, F. Wei, S.C. Swain, B. Halliwell, S.R. Sturzenbaum, P.K. Moore, Antioxid. Redox Signal. 20, 2621-2630 (2014) CrossRef PubMed Google Scholar
-
209.T.T. Nguyen, S.W. Caito, W.E. Zackert, J.D. West, S. Zhu, M. Aschner, J.P. Fessel, L.J. Roberts, Aging (Albany.. NY) 8, 1759-1780 (2016) PubMed Google Scholar
-
210.T. Heidler, K. Hartwig, H. Daniel, U. Wenzel, Biogerontology 11, 183-195 (2010) CrossRef PubMed Google Scholar
-
211.L. Huang, P. Li, G. Wang, S. Guan, X. Sun, L. Wang, Free Radic. Res. 47, 316-324 (2013) CrossRef PubMed Google Scholar
-
212.T. Hashimoto, M. Horikawa, T. Nomura, K. Sakamoto, Biogerontology 11, 31-43 (2010) CrossRef PubMed Google Scholar
-
213.C.B. Edwards, N. Copes, A.G. Brito, J. Canfield, P.C. Bradshaw, PLoS ONE 8, e58345 (2013) CrossRef PubMed Google Scholar
-
214.A.A. Powolny, S.V. Singh, S. Melov, A. Hubbard, A.L. Fisher, Exp. Gerontol. 46, 441-452 (2011) CrossRef PubMed Google Scholar
-
215.P.R. Hunt, T.G. Son, M.A. Wilson, Q.S. Yu, W.H. Wood, Y. Zhang, K.G. Becker, N.H. Greig, M.P. Mattson, S. Camandola, C.A. Wolkow, PLoS ONE 6, e21922 (2011) CrossRef PubMed Google Scholar
-
216.E.F. Fang, T.B. Waltz, H. Kassahun, Q. Lu, J.S. Kerr, M. Morevati, E.M. Fivenson, B.N. Wollman, K. Marosi, M.A. Wilson, W.B. Iser, D.M. Eckley, Y. Zhang, E. Lehrmann, I.G. Goldberg, M. Scheibye-Knudsen, M.P. Mattson, H. Nilsen, V.A. Bohr, K.G. Becker, Sci. Rep. 7, 46208 (2017) CrossRef PubMed Google Scholar
-
217.T. Ogawa, Y. Kodera, D. Hirata, T.K. Blackwell, M. Mizunuma, Sci. Rep. 6, 21611 (2016) CrossRef PubMed Google Scholar
-
218.S. Su, M. Wink, Phytochemistry 117, 340-350 (2015) CrossRef PubMed Google Scholar
-
219.A. Pant, S.K. Saikia, V. Shukla, J. Asthana, B.A. Akhoon, R. Pandey, Exp. Gerontol. 57, 81-95 (2014) CrossRef PubMed Google Scholar
-
220.J. Asthana, D. Yadav, A. Pant, A.K. Yadav, M.M. Gupta, R. Pandey, J. Gerontol. A 71, 1160-1168 (2016) CrossRef PubMed Google Scholar
-
221.S. Schmeisser, K. Schmeisser, S. Weimer, M. Groth, S. Priebe, E. Fazius, D. Kuhlow, D. Pick, J.W. Einax, R. Guthke, M. Platzer, K. Zarse, M. Ristow, Aging Cell 12, 508-517 (2013) CrossRef PubMed Google Scholar
-
222.C.W. Yu, C.M. How, V.H. Liao, Chemosphere 150, 632-638 (2016) CrossRef PubMed Google Scholar
-
223.C. Edwards, J. Canfield, N. Copes, M. Rehan, D. Lipps, P.C. Bradshaw, Aging (Albany.. NY) 6, 621-644 (2014) PubMed Google Scholar
-
224.A. Lublin, F. Isoda, H. Patel, K. Yen, L. Nguyen, D. Hajje, M. Schwartz, C. Mobbs, PLoS ONE 6, e27762 (2011) CrossRef PubMed Google Scholar
-
225.N. Baberschke, C.E. Steinberg, N. Saul, Chemosphere 124, 122-128 (2015) CrossRef PubMed Google Scholar
-
226.K. Filippopoulou, N. Papaevgeniou, M. Lefaki, A. Paraskevopoulou, D. Biedermann, V. Kren, N. Chondrogianni, Free Radic. Biol. Med. 103, 256-267 (2017) CrossRef PubMed Google Scholar
-
227.K. Zarse, A. Bossecker, L. Muller-Kuhrt, K. Siems, M.A. Hernandez, W.G. Berendsohn, M. Birringer, M. Ristow, Horm. Metab. Res. 43, 241-243 (2011) CrossRef PubMed Google Scholar
-
228.C. Buchter, D. Ackermann, S. Havermann, S. Honnen, Y. Chovolou, G. Fritz, A. Kampkotter, W. Watjen, Int. J. Mol. Sci. 14, 11895-11914 (2013) CrossRef PubMed Google Scholar
-
229.J. Yang, Q.L. Wan, Q.Z. Mu, C.F. Wu, A.J. Ding, Z.L. Yang, M.H. Qiu, H.R. Luo, Nat. Prod. Bioprospect. 5, 177-183 (2015) CrossRef PubMed Google Scholar
-
230.Y. Chen, B. Onken, H. Chen, S. Xiao, X. Liu, M. Driscoll, Y. Cao, Q. Huang, J. Agric. Food Chem. 62, 3422-3431 (2014) CrossRef PubMed Google Scholar
-
231.M.S. Denzel, N.J. Storm, A. Gutschmidt, R. Baddi, Y. Hinze, E. Jarosch, T. Sommer, T. Hoppe, A. Antebi, Cell 156, 1167-1178 (2014) CrossRef PubMed Google Scholar
-
232.D. Chandler-Brown, H. Choi, S. Park, B.R. Ocampo, S. Chen, A. Le, G.L. Sutphin, L.S. Shamieh, E.D. Smith, M. Kaeberlein, Front. Genet. 6, 316 (2015) PubMed Google Scholar
-
233.K. Pietsch, N. Saul, S.C. Swain, R. Menzel, C.E. Steinberg, S.R. Sturzenbaum, Front. Genet. 3, 48 (2012) PubMed Google Scholar
-
234.L.M. Katiki, J.F. Ferreira, J.M. Gonzalez, A.M. Zajac, D.S. Lindsay, A.C. Chagas, A.F. Amarante, Vet. Parasitol. 192, 218-227 (2013) CrossRef PubMed Google Scholar
-
235.S.J. Kim, S.M. Beak, S.K. Park, J. Food Sci. (2017) PubMed Google Scholar
-
236.K.A. Mark, K.J. Dumas, D. Bhaumik, B. Schilling, S. Davis, T.R. Oron, D.J. Sorensen, M. Lucanic, R.B. Brem, S. Melov, A. Ramanathan, B.W. Gibson, G.J. Lithgow, Cell Rep. 17, 1227-1237 (2016) CrossRef PubMed Google Scholar
-
237.A. Ihara, M. Uno, K. Miyatake, S. Honjoh, E. Nishida, Exp. Gerontol. 87, 40-47 (2016) PubMed Google Scholar
-
238.B. Gerisch, V. Rottiers, D. Li, D.L. Motola, C.L. Cummins, H. Lehrach, D.J. Mangelsdorf, A. Antebi, Proc. Natl. Acad. Sci. USA 104, 5014-5019 (2007) CrossRef PubMed Google Scholar
-
239.F. Broue, P. Liere, C. Kenyon, E.E. Baulieu, Aging Cell 6, 87-94 (2007) CrossRef PubMed Google Scholar
-
240.G. Detienne, W. De Haes, U.R. Ernst, L. Schoofs, L. Temmerman, Exp. Gerontol. 60, 129-135 (2014) CrossRef PubMed Google Scholar
-
241.S. Havermann, Y. Chovolou, H.U. Humpf, W. Watjen, PLoS ONE 9, e100256 (2014) CrossRef PubMed Google Scholar
-
242.P.V. Castro, S. Khare, B.D. Young, S.G. Clarke, PLoS ONE 7, e29984 (2012) CrossRef PubMed Google Scholar
-
243.C.H. Chang, C.T. Ho, V.H. Liao, Mol. Nutr. Food Res. (2017) PubMed Google Scholar
-
244.M. Katsiki, N. Chondrogianni, I. Chinou, A.J. Rivett, E.S. Gonos, Rejuvenation Res. 10, 157-172 (2007) CrossRef PubMed Google Scholar
-
245.F.C. Ip, Y.P. Ng, H.J. An, Y. Dai, H.H. Pang, Y.Q. Hu, A.C. Chin, C.B. Harley, Y.H. Wong, N.Y. Ip, Neurosignals 22, 52-63 (2014) CrossRef PubMed Google Scholar
-
246.M.J. Choi, B.K. Kim, K.Y. Park, T. Yokozawa, Y.O. Song, E.J. Cho, Biol. Pharm. Bull. 33, 421-426 (2010) CrossRef PubMed Google Scholar
-
247.S.J. Heo, S.C. Ko, S.H. Cha, D.H. Kang, H.S. Park, Y.U. Choi, D. Kim, W.K. Jung, Y.J. Jeon, Toxicol. In Vitro 23, 1123-1130 (2009) CrossRef PubMed Google Scholar
-
248.P. Wang, Z. Zhang, X. Ma, Y. Huang, X. Liu, P. Tu, T. Tong, Mech. Ageing Dev. 124, 1025-1034 (2003) CrossRef PubMed Google Scholar
-
249.P. Wang, Z. Zhang, Y. Sun, X. Liu, T. Tong, DNA Cell Biol. 29, 33-39 (2010) CrossRef PubMed Google Scholar
-
250.N. Chondrogianni, S. Kapeta, I. Chinou, K. Vassilatou, I. Papassideri, E.S. Gonos, Exp. Gerontol. 45, 763-771 (2010) CrossRef PubMed Google Scholar
-
251.A. Torres, M. Hochberg, I. Pergament, R. Smoum, V. Niddam, V.M. Dembitsky, M. Temina, I. Dor, O. Lev, M. Srebnik, C.D. Enk, Eur. J. Biochem. 271, 780-784 (2004) CrossRef PubMed Google Scholar
-
252.P. Belenky, F.G. Racette, K.L. Bogan, J.M. McClure, J.S. Smith, C. Brenner, Cell 129, 473-484 (2007) CrossRef PubMed Google Scholar
-
253.S.P. Lu, M. Kato, S.J. Lin, J. Biol. Chem. 284, 17110-17119 (2009) CrossRef PubMed Google Scholar
-
254.Y. Liu, Z. Wu, S. Feng, X. Yang, D. Huang, Molecules 19, 568-580 (2014) CrossRef PubMed Google Scholar
-
255.K.T. Howitz, K.J. Bitterman, H.Y. Cohen, D.W. Lamming, S. Lavu, J.G. Wood, R.E. Zipkin, P. Chung, A. Kisielewski, L.L. Zhang, B. Scherer, D.A. Sinclair, Nature 425, 191-196 (2003) CrossRef PubMed Google Scholar
-
256.A.A. Goldberg, V.R. Richard, P. Kyryakov, S.D. Bourque, A. Beach, M.T. Burstein, A. Glebov, O. Koupaki, T. Boukh-Viner, C. Gregg, M. Juneau, A.M. English, D.Y. Thomas, V.I. Titorenko, Aging (Albany.. NY) 2, 393-414 (2010) PubMed Google Scholar
-
257.A. Leonov, A. Arlia-Ciommo, S.D. Bourque, O. Koupaki, P. Kyryakov, P. Dakik, M. McAuley, Y. Medkour, K. Mohammad, T. Di Maulo, V.I. Titorenko, Oncotarget (2017) PubMed Google Scholar
-
258.J.E. Beauvais, H.E. Enesco, Exp. Gerontol. 20, 359-366 (1985) CrossRef PubMed Google Scholar
-
259.S.H. Lee, H.S. An, Y.W. Jung, E.J. Lee, H.Y. Lee, E.S. Choi, S.W. An, H. Son, S.J. Lee, J.B. Kim, K.J. Min, Biogerontology 15, 153-164 (2014) CrossRef PubMed Google Scholar
-
260.J. Jiao, Y. Wei, J. Chen, X. Chen, Y. Zhang, J. Funct. Foods 30, 63-73 (2017) CrossRef PubMed Google Scholar
-
261.X.K. Ma, D.D. Guo, E.C. Peterson, Y. Dun, D.Y. Li, Food Funct. 7, 3468-3479 (2016) CrossRef PubMed Google Scholar
-
262.Y. Xu, Y. Gao, M. Zhong, J. Li, H. Cao, S. Huang, R. Wei, K. Zhang, Int. J. Biol. Macromol. 101, 603-611 (2017) CrossRef PubMed Google Scholar
-
263.B. Jing, C. Lv, S.X. Li, M.L. Fu, Z.Q. Yin, Zhong Yao Cai 38, 2563-2567 (2015) PubMed Google Scholar
-
264.J.F. Bisson, A. Nejdi, P. Rozan, S. Hidalgo, R. Lalonde, M. Messaoudi, Br. J. Nutr. 100, 94-101 (2008) PubMed Google Scholar
-
265.M.J. Shahroudi, S. Mehri, H. Hosseinzadeh, J. Pharmacopuncture 20, 29-35 (2017) CrossRef PubMed Google Scholar
-
266.H. Zhao, J. Li, J. Zhang, X. Wang, L. Hao, L. Jia, Int. J. Biol. Macromol. 102, 351-357 (2017) CrossRef PubMed Google Scholar
-
267.H.T. Ngo, E. Hwang, S.A. Seo, B. Park, Z.W. Sun, M. Zhang, Y.K. Shin, T.H. Yi, J. Photochem. Photobiol. B 169, 161-170 (2017) CrossRef PubMed Google Scholar
-
268.C. Peng, H.Y. Chan, Y. Huang, H. Yu, Z.Y. Chen, J. Agric. Food Chem. 59, 2097-2106 (2011) CrossRef PubMed Google Scholar
-
269.S. Bahadorani, A.J. Hilliker, Nutr. Res. 28, 377-382 (2008) CrossRef PubMed Google Scholar
-
270.Y. Zou, Y. Liu, M. Ruan, X. Feng, J. Wang, Z. Chu, Z. Zhang, Int. J. Mol. Med. 36, 939-946 (2015) CrossRef PubMed Google Scholar
-
271.H.P. Liu, R.F. Chang, Y.S. Wu, W.Y. Lin, F.J. Tsai, Evid. Based Complement Altern. Med. 2012, 735481 (2012) PubMed Google Scholar
-
272.S. Rawal, P. Singh, A. Gupta, S. Mohanty, Biomed. Res. Int. 2014, 910290 (2014) PubMed Google Scholar
-
273.D.V. Gospodaryov, I.S. Yurkevych, M. Jafari, V.I. Lushchak, O.V. Lushchak, Longev. Healthspan 2, 5 (2013) CrossRef PubMed Google Scholar
-
274.S. Zou, J.R. Carey, P. Liedo, D.K. Ingram, B. Yu, Age (Dordr.) 34, 269-279 (2012) CrossRef PubMed Google Scholar
-
275.S.E. Schriner, S. Kuramada, T.E. Lopez, S. Truong, A. Pham, M. Jafari, Exp. Gerontol. 60, 220-230 (2014) CrossRef PubMed Google Scholar
-
276.W.S. Lin, J.Y. Chen, J.C. Wang, L.Y. Chen, C.H. Lin, T.R. Hsieh, M.F. Wang, T.F. Fu, P.Y. Wang, Age (Dordr.) 36, 689-703 (2014) CrossRef PubMed Google Scholar
-
277.S. Ghimire, M.S. Kim, Biogerontology 18, 263-273 (2017) CrossRef PubMed Google Scholar
-
278.H.R. Massie, V.R. Aiello, T.R. Williams, Mech. Ageing Dev. 67, 227-237 (1993) CrossRef PubMed Google Scholar
-
279.L. Wang, Y.M. Li, L. Lei, Y. Liu, X. Wang, K.Y. Ma, Z.Y. Chen, Exp. Gerontol. 69, 189-195 (2015) CrossRef PubMed Google Scholar
-
280.S.E. Schriner, N.S. Katoozi, K.Q. Pham, M. Gazarian, A. Zarban, M. Jafari, Biogerontology 13, 105-117 (2012) CrossRef PubMed Google Scholar
-
281.J.K. Park, C.K. Kim, S.K. Gong, A.R. Yu, M.Y. Lee, S.K. Park, Nutr. Res. Pract. 8, 526-532 (2014) CrossRef PubMed Google Scholar
-
282.Q. Wang, Y. Huang, C. Qin, M. Liang, X. Mao, S. Li, Y. Zou, W. Jia, H. Li, C.W. Ma, Z. Huang, Oxid. Med. Cell Longev. 2016, 8956981 (2016) PubMed Google Scholar
-
283.T. Sunagawa, T. Shimizu, T. Kanda, M. Tagashira, M. Sami, T. Shirasawa, Planta Med. 77, 122-127 (2011) CrossRef PubMed Google Scholar
-
284.M.A. Wilson, B. Shukitt-Hale, W. Kalt, D.K. Ingram, J.A. Joseph, C.A. Wolkow, Aging Cell 5, 59-68 (2006) CrossRef PubMed Google Scholar
-
285.H. Liu, F. Liang, W. Su, N. Wang, M. Lv, P. Li, Z. Pei, Y. Zhang, X.Q. Xie, L. Wang, Y. Wang, J. Ethnopharmacol. 147, 366-372 (2013) CrossRef PubMed Google Scholar
-
286.Z. Wu, J.V. Smith, V. Paramasivam, P. Butko, I. Khan, J.R. Cypser, Y. Luo, Cell Mol. Biol. (Noisy-le-grand) 48, 725-731 (2002) PubMed Google Scholar
-
287.J. Chen, J. Zhang, Y. Xiang, L. Xiang, Y. Liu, X. He, X. Zhou, X. Liu, Z. Huang, Food Funct. 7, 943-952 (2016) CrossRef PubMed Google Scholar
-
288.T. Moriwaki, S. Kato, Y. Kato, A. Hosoki, Q.M. ZhangAkiyama, J. Clin. Biochem. Nutr. 53, 81-88 (2013) CrossRef PubMed Google Scholar
-
289.S. Feng, H. Cheng, Z. Xu, S. Shen, M. Yuan, J. Liu, C. Ding, Int. J. Biol. Macromol. 81, 188-194 (2015) CrossRef PubMed Google Scholar
-
290.S.M. Won, H.U. Cha, S.S. Yi, S.J. Kim, S.K. Park, J. Med. Food (2016) PubMed Google Scholar
-
291.F. Wan, D. Zhi, D. Liu, J. Xian, M. Li, A. AbuLizi, W. Ju, H. Li, Biogerontology 15, 377-387 (2014) CrossRef PubMed Google Scholar
-
292.Y. Zhang, T. Lv, M. Li, T. Xue, H. Liu, W. Zhang, X. Ding, Z. Zhuang, Pharmacogn. Mag. 11, 449-454 (2015) CrossRef PubMed Google Scholar
-
293.H. Jeon, D.S. Cha, Chin J. Nat. Med. 14, 335-342 (2016) PubMed Google Scholar
-
294.Z. Wang, X. Ma, J. Li, X. Cui, Exp. Gerontol. 82, 139-149 (2016) CrossRef PubMed Google Scholar
-
295.H. Zhang, N. Pan, S. Xiong, S. Zou, H. Li, L. Xiao, Z. Cao, A. Tunnacliffe, Z. Huang, Biochem. J. 441, 417-424 (2012) CrossRef PubMed Google Scholar
-
296.C.H. Huang, F.Y. Hsu, Y.H. Wu, L. Zhong, M.Y. Tseng, C.J. Kuo, A.L. Hsu, S.S. Liang, S.H. Chiou, J. Nutr. Biochem. 26, 808-817 (2015) CrossRef PubMed Google Scholar
-
297.Y. Honda, Y. Fujita, H. Maruyama, Y. Araki, K. Ichihara, A. Sato, T. Kojima, M. Tanaka, Y. Nozawa, M. Ito, S. Honda, PLoS ONE 6, e23527 (2011) CrossRef PubMed Google Scholar
-
298.I. Parikh, J. Guo, K.H. Chuang, Y. Zhong, R.G. Rempe, J.D. Hoffman, R. Armstrong, B. Bauer, A.M. Hartz, A.L. Lin, Aging (Albany.. NY) 8, 2814-2826 (2016) PubMed Google Scholar
-
299.L. Rathor, A. Pant, H. Awasthi, D. Mani, R. Pandey, Biogerontology 18, 131-147 (2017) CrossRef PubMed Google Scholar
-
300.X. Yang, P. Zhang, J. Wu, S. Xiong, N. Jin, Z. Huang, J. Ethnopharmacol. 141, 41-47 (2012) CrossRef PubMed Google Scholar
-
301.S. Guan, P. Li, J. Luo, Y. Li, L. Huang, G. Wang, L. Zhu, H. Fan, W. Li, L. Wang, Free Radic. Res. 44, 813-820 (2010) CrossRef PubMed Google Scholar
-
302.F.A. Wiegant, S. Surinova, E. Ytsma, M. Langelaar-Makkinje, G. Wikman, J.A. Post, Biogerontology 10, 27-42 (2009) CrossRef PubMed Google Scholar
-
303.C. Scerbak, E.M. Vayndorf, A. Hernandez, C. McGill, B.E. Taylor, Front. Aging Neurosci. 8, 173 (2016) PubMed Google Scholar
-
304.S. Zheng, S. Liao, Y. Zou, Z. Qu, W. Shen, Y. Shi, Age (Dordr.) 36, 9719 (2014) CrossRef PubMed Google Scholar
-
305.T. Kawano, N. Kataoka, S. Abe, M. Ohtani, Y. Honda, S. Honda, Y. Kimura, Biosci. Biotechnol. Biochem. 69, 2479-2481 (2005) CrossRef PubMed Google Scholar
-
306.M. Stirpe, V. Palermo, M.M. Bianchi, R. Silvestri, C. Falcone, G. Tenore, E. Novellino, C. Mazzoni, BMC Complement Altern. Med. 17, 200 (2017) CrossRef PubMed Google Scholar
-
307.D.J. Snare, A.M. Fields, T.W. Snell, J. Kubanek, Exp. Gerontol. 48, 1420-1427 (2013) CrossRef PubMed Google Scholar
-
308.Q.L. Wan, S.Q. Zheng, G.S. Wu, H.R. Luo, Exp. Gerontol. 48, 499-506 (2013) CrossRef PubMed Google Scholar
-
309.Z. Zhang, X. Li, F. Li, L. An, Int. Immunopharmacol. 38, 426-433 (2016) CrossRef PubMed Google Scholar
-
310.J. Wang, T. Guo, Q.S. Peng, S.W. Yue, S.X. Wang, J. Cell Mol. Med. 19, 2607-2616 (2015) CrossRef PubMed Google Scholar
-
311.V. Navrotskaya, G. Oxenkrug, L. Vorobyova, P. Summergrad, Am. J. Plant Sci. 5, 275-278 (2014) CrossRef PubMed Google Scholar
-
312.Q. Ruan, F. Liu, Z. Gao, D. Kong, X. Hu, D. Shi, Z. Bao, Z. Yu, Mech. Ageing Dev. 134, 89-97 (2013) CrossRef PubMed Google Scholar
-
313.M. Mora, S.J. Medina-Leendertz, E. Bonilla, R.E. Teran, M.C. Paz, J.L. Arcaya, Invest. Clin. 54, 161-170 (2013) PubMed Google Scholar
-
314.V.N. Anisimov, A.V. Semenchenko, A.I. Yashin, Biogerontology 4, 297-307 (2003) CrossRef PubMed Google Scholar
-
315.F. Cabreiro, C. Au, K.Y. Leung, N. Vergara-Irigaray, H.M. Cocheme, T. Noori, D. Weinkove, E. Schuster, N.D. Greene, D. Gems, Cell 153, 228-239 (2013) CrossRef PubMed Google Scholar
-
316.V. Kucukatay, M. Bor-Kucukatay, G. Gundogdu, G. Erken, T.O. Ozcan, F.D. Miloglu, Y. Kadioglu, Folia Biol. (Praha) 58, 157-165 (2012) PubMed Google Scholar
-
317.M.J. Ryan, H.J. Dudash, M. Docherty, K.B. Geronilla, B.A. Baker, G.G. Haff, R.G. Cutlip, S.E. Alway, Exp. Gerontol. 45, 882-895 (2010) CrossRef PubMed Google Scholar
-
318.L.A. Harrington, C.B. Harley, Mech. Ageing Dev. 43, 71-78 (1988) CrossRef PubMed Google Scholar
-
319.C. Vinod, A. Jagota. Biogerontology (2017) PubMed Google Scholar
-
320.S. Gent, P. Kleinbongard, P. Dammann, M. Neuhauser, G. Heusch, Basic Res. Cardiol. 110, 2 (2015) CrossRef PubMed Google Scholar
-
321.D.E. Harrison, R. Strong, D.B. Allison, B.N. Ames, C.M. Astle, H. Atamna, E. Fernandez, K. Flurkey, M.A. Javors, N.L. Nadon, J.F. Nelson, S. Pletcher, J.W. Simpkins, D. Smith, J.E. Wilkinson, R.A. Miller, Aging Cell 13, 273-282 (2014) CrossRef PubMed Google Scholar
-
322.J.J. Tong, G.H. Chen, F. Wang, X.W. Li, L. Cao, X. Sui, F. Tao, W.W. Yan, Z.J. Wei, Behav. Brain Res. 284, 138-152 (2015) CrossRef PubMed Google Scholar
-
323.S.R. Spindler, P.L. Mote, R. Li, J.M. Dhahbi, A. Yamakawa, J.M. Flegal, D.R. Jeske, R. Li, A.L. Lublin, Age (Dordr.) 35, 2099-2109 (2013) CrossRef PubMed Google Scholar
-
324.Y.X. Zou, M.H. Ruan, J. Luan, X. Feng, S. Chen, Z.Y. Chu, J. Nutr. Health Aging 21, 314-319 (2017) CrossRef PubMed Google Scholar
-
325.A. Avanesian, B. Khodayari, J.S. Felgner, M. Jafari, Biogerontology 11, 45-52 (2010) CrossRef PubMed Google Scholar
-
326.H.L. Kang, S. Benzer, K.T. Min, Proc. Natl. Acad. Sci. USA 99, 838-843 (2002) CrossRef PubMed Google Scholar
-
327.C. Bergwitz, Nephrol. Dial. Transplant. 27, 3399-3406 (2012) CrossRef PubMed Google Scholar
-
328.S.R. Spindler, R. Li, J.M. Dhahbi, A. Yamakawa, P. Mote, R. Bodmer, K. Ocorr, R.T. Williams, Y. Wang, K.P. Ablao, PLoS ONE 7, e39581 (2012) CrossRef PubMed Google Scholar
-
329.C. Slack, N. Alic, A. Foley, M. Cabecinha, M.P. Hoddinott, L. Partridge, Cell 162, 72-83 (2015) CrossRef PubMed Google Scholar
-
330.D. Tao, J. Lu, H. Sun, Y.M. Zhao, Z.G. Yuan, X.X. Li, B.Q. Huang, Acta Biochim. Biophys. Sin. (Shanghai) 36, 618-622 (2004) CrossRef PubMed Google Scholar
-
331.T.A. Dubiley, Y.E. Rushkevich, N.M. Koshel, V.P. Voitenko, A.M. Vaiserman, Biogerontology 12, 179-184 (2011) CrossRef PubMed Google Scholar
-
332.S. Calvert, R. Tacutu, S. Sharifi, R. Teixeira, P. Ghosh, J.P. de Magalhaes, Aging Cell 15, 256-266 (2016) CrossRef PubMed Google Scholar
-
333.T.T. Ching, W.C. Chiang, C.S. Chen, A.L. Hsu, Aging Cell 10, 506-519 (2011) CrossRef PubMed Google Scholar
-
334.K. Evason, J.J. Collins, C. Huang, S. Hughes, K. Kornfeld, Aging Cell 7, 305-317 (2008) CrossRef PubMed Google Scholar
-
335.S. Brandstadt, K. Schmeisser, K. Zarse, M. Ristow, Aging (Albany.. NY) 5, 270-275 (2013) PubMed Google Scholar
-
336.K. Evason, C. Huang, I. Yamben, D.F. Covey, K. Kornfeld, Science 307, 258-262 (2005) CrossRef PubMed Google Scholar
-
337.L. Rathor, B.A. Akhoon, S. Pandey, S. Srivastava, R. Pandey, Age (Dordr.) 37, 113 (2015) CrossRef PubMed Google Scholar
-
338.J.J. Collins, K. Evason, C.L. Pickett, D.L. Schneider, K. Kornfeld, PLoS Genet. 4, e1000230 (2008) CrossRef PubMed Google Scholar
-
339.X. Chen, H.V. McCue, S.Q. Wong, S.S. Kashyap, B.C. Kraemer, J.W. Barclay, R.D. Burgoyne, A. Morgan, Mol. Neurodegener. 10, 51 (2015) CrossRef PubMed Google Scholar
-
340.S. Schmeisser, K. Zarse, M. Ristow, Horm. Metab. Res. 43, 687-692 (2011) CrossRef PubMed Google Scholar
-
341.D. Srivastava, U. Arya, T. SoundaraRajan, H. Dwivedi, S. Kumar, J.R. Subramaniam, Biogerontology 9, 309-316 (2008) CrossRef PubMed Google Scholar
-
342.K. Saharia, U. Arya, R. Kumar, R. Sahu, C.K. Das, K. Gupta, H. Dwivedi, J.R. Subramaniam, Exp. Gerontol. 47, 188-197 (2012) CrossRef PubMed Google Scholar
-
343.S. Golegaonkar, S.S. Tabrez, A. Pandit, S. Sethurathinam, M.G. Jagadeeshaprasad, S. Bansode, S.G. Sampathkumar, M.J. Kulkarni, A. Mukhopadhyay, Aging Cell 14, 463-473 (2015) CrossRef PubMed Google Scholar
-
344.S. Liu, N. Saul, B. Pan, R. Menzel, C.E. Steinberg, Chemosphere 93, 2373-2380 (2013) CrossRef PubMed Google Scholar
-
345.R.H. Houtkooper, L. Mouchiroud, D. Ryu, N. Moullan, E. Katsyuba, G. Knott, R.W. Williams, J. Auwerx, Nature 497, 451-457 (2013) CrossRef PubMed Google Scholar
-
346.V. Bozovic, H.E. Enesco, Arch. Gerontol. Geriatr. 9, 45-51 (1989) CrossRef PubMed Google Scholar
-
347.J. Liu, X. Huang, B.R. Withers, E. Blalock, K. Liu, R.C. Dickson, Aging Cell 12, 833-841 (2013) CrossRef PubMed Google Scholar
-
348.C.C. Caldeira da Silva, F.M. Cerqueira, L.F. Barbosa, M.H. Medeiros, A.J. Kowaltowski, Aging Cell 7, 552-560 (2008) CrossRef PubMed Google Scholar
-
349.V.I. Padalko, Biochemistry (Mosc.) 70, 986-989 (2005) CrossRef PubMed Google Scholar
-
350.A. Comfort, I. Youhotsky-Gore, K. Pathmanathan, Nature 229, 254-255 (1971) CrossRef PubMed Google Scholar
-
351.S.J. Mitchell, A. Martin-Montalvo, E.M. Mercken, H.H. Palacios, T.M. Ward, G. Abulwerdi, R.K. Minor, G.P. Vlasuk, J.L. Ellis, D.A. Sinclair, J. Dawson, D.B. Allison, Y. Zhang, K.G. Becker, M. Bernier, R. de Cabo, Cell Rep. 6, 836-843 (2014) CrossRef PubMed Google Scholar
-
352.V.K. Khavinson, D.M. Izmaylov, L.K. Obukhova, V.V. Malinin, Mech. Ageing Dev. 120, 141-149 (2000) CrossRef PubMed Google Scholar
-
353.J. Miquel, J. Fleming, A.C. Economos, Arch. Gerontol. Geriatr. 1, 159-165 (1982) CrossRef PubMed Google Scholar
-
354.S.R. Spindler, R. Li, J.M. Dhahbi, A. Yamakawa, F. Sauer, PLoS ONE 7, e29782 (2012) CrossRef PubMed Google Scholar
-
355.O. Sagi, M. Wolfson, N. Utko, K. Muradian, V. Fraifeld, Mech. Ageing Dev. 126, 249-254 (2005) CrossRef PubMed Google Scholar
-
356.A. Moskalev, M. Shaposhnikov, Aging (Albany.. NY) 3, 391-394 (2011) PubMed Google Scholar
-
357.J.N. Sampayo, A. Olsen, G.J. Lithgow, Aging Cell 2, 319-326 (2003) CrossRef PubMed Google Scholar
-
358.N. Fischer, C. Buchter, K. Koch, S. Albert, R. Csuk, W. Watjen, J. Pharm. Pharmacol. 69, 73-8 (2016) PubMed Google Scholar
-
359.R.A. Mekheimer, A.A. Sayed, E.A. Ahmed, J. Med. Chem. 55, 4169-4177 (2012) CrossRef PubMed Google Scholar
-
360.S. Alavez, M.C. Vantipalli, D.J. Zucker, I.M. Klang, G.J. Lithgow, Nature 472, 226-229 (2011) CrossRef PubMed Google Scholar
-
361.J. Zhu, C.F. Wu, X. Li, G.S. Wu, S. Xie, Q.N. Hu, Z. Deng, M.X. Zhu, H.R. Luo, X. Hong, Bioorg. Med. Chem. 21, 4218-4224 (2013) CrossRef PubMed Google Scholar
-
362.Y. Zhu, Y. Lu, C. Qu, M. Miller, J. Tian, D.P. Thakur, J. Zhu, Z. Deng, X. Hu, M. Wu, O.B. McManus, M. Li, X. Hong, M.X. Zhu, H.R. Luo, Br. J. Pharmacol. 172, 3495-3509 (2015) CrossRef PubMed Google Scholar
-
363.L.P. Yang, F.J. Jiang, G.S. Wu, K. Deng, M. Wen, X. Zhou, X. Hong, M.X. Zhu, H.R. Luo, PLoS ONE 10, e0136255 (2015) CrossRef PubMed Google Scholar
-
364.A.J. Ding, G.S. Wu, B. Tang, X. Hong, M.X. Zhu, H.R. Luo, Mol. Cell Biochem. 426, 101-109 (2016) PubMed Google Scholar
-
365.M. Sawada, J.C. Carlson, H.E. Enesco, Arch. Gerontol. Geriatr. 10, 27-36 (1990) CrossRef PubMed Google Scholar
Copyright information
© The Author(s) 2017
Open Access
This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.