Sesquiterpenoids from the Rhizomes of Homalomena occulta
Abstract
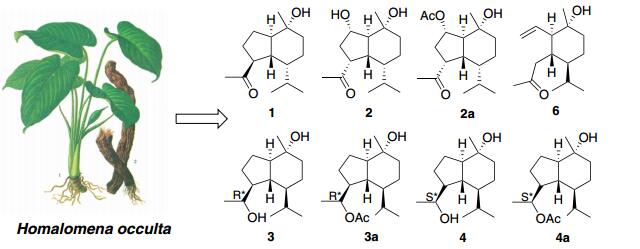
Naturally occurring oplopanane sesquiterpenoids are rarely reported. A phytochemical investigation on the rhizomes of Homalomena occulta (Lours) has resulted in the discovery of six oplopanane sesquiterpenoids (1-6), including four new (1-4) and one 3, 5-seco-oplopanane (6), together with three previously reported sesquiterpenoids (7-9). In addition three new oplopananes (2a-4a) were also obtained by chemical transformation. All structures of these sesquiterpenoids were established based on the comprehensive spectroscopic analyses, including NMR, MS, and IR, and comparing with the literatures.Graphical Abstract
Keywords
Homalomena occulta Sesquiterpenoids Oplopananes1 Introduction
With its rich cultural heritage and biodiversity, traditional Chinese medicine (TCM) has potential as a source for the discovery of structurally novel bioactive compounds. During the last twenty years, considerable efforts have been dedicated towards the exploration of the natural product chemistry of TCM. Homalomena occulta (Lour.) was officially listed in the Chinese Pharmacopoeia (named Qian-nian-jian) [1], and was found to occur in the tropical and sub-tropical areas of Asia and America. The plant has been used for the treatment of rheumatoid arthritis, trengthening tendons and bones, and invigorating the kidney and liver [2, 3]. Recent reports of H. occulta have also shown that the species is one of the most prolific sources of compounds with new structures [2-6].
Our research group has considerably focused on phytochemical investigations of TCM [7-10]. As part of our continuing efforts to obtain novel compounds with exquisite structural architectures, we initiated a chemical investigation of H. occulta, which has thus far led to the isolation and structural elucidation of six oplopanane sesquiterpenoids (1-6), including four new (1-4) and one 3, 5-seco-oplopanane (6), together with three previously reported sesquiterpenoids (7-9).
2 Results and Discussion
By means of diverse chromatographic methods, including silica gel and LH-20, four new oplopanane sesquiterpenoids (1-4) (Fig. 1) have been purified from an 88 % ethanol/water extract of the rhizomes of H. occulta.
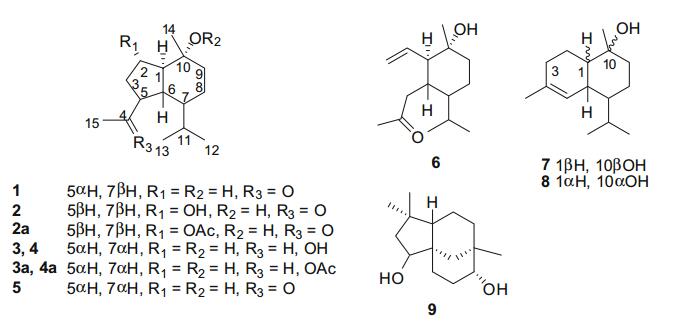
Molecular structures of sesquiterpenoids 1-9
Compound 1 was isolated as an optically active colorless oil with
NMR spectroscopic data for compound 1 in CDCl3
Compound 2, obtained as an optically active colorless oil with
NMR spectroscopic data for compounds 2, 2a, and 3 in CDCl3
Compounds 3 and 4, both isolated as optically active white floc with
NMR spectroscopic data for compounds 3a, 4, and 4a in CDCl3
On the basis of NMR, MS, optical rotation data and comparison with literature values, the known sesquiterpenoids were elucidated as oplopanone (5) [11], taiwaninone A (6) [12], T-muurolol (7) [13], α-cadinol (8) [14], and clovane-2β, 9α-diol (9) [15]. Naturally occurring oplopanane sesquiterpenoids are rarely reported [16, 17]. Literature searching showed that there were no more than 20 such type sesquiterpenoids reported up to now, distributed among the families of Alismataceae [16, 17], Araliaceae [18], Araceae [19], Asteraceae [20], Chloranthaceae [21], Cyperaceae [22], Magnoliaceae [23], Meliaceae [24], Schisandraceae [25], Salicaceae [26], and Zingiberaceae [27]. In this study, six oplopananes (1-6), including four new (1-4) and one 3, 5-seco-oplopanane (6), were discovered from the rhizomes of H. occulta. In addition three new oplopananes (2a-4a) were also obtained by chemical transformation. These results indicated that H. occulta was a rich source of novel natural products.
3 Experimental Section
3.1 General
Optical rotations were recorded on a 241 polarimeter (Perkin-Elmer). Infrared (IR) spectra were obtained with a FTS 165-IR instrument (Bio-Rad, USA). NMR spectra were acquired on a Varian INOVA-400 FT-NMR spectrometer (USA). HRESIMS were measured on a Bruker APEX II spectrometer. Sephadex LH-20 (Amersham Biosciences) and silica gel (200-300 mesh, Qingdao Haiyang Chemical Co., Ltd) were used for column chromatography (CC), whereas TLC analyses were carried out with glass plates pre-coated with silica gel and the spots were visualized by spraying with 98 % H2SO4/EtOH in (5/95, v/v) followed by heating. All solvents used were analytical grade.
3.2 Plant Materials
The rhizomes of H. occulta Lours (Araceae), collected from Guangxi in China, were purchased from Lanzhou Fuxinghou Herbal Medicines Ltd. Co. in February 2007. The materials were identified by Dr. Huan-Yang Qi at Lanzhou Institute of Chemical Physics (LICP), and a voucher specimen (ZY2007H001) was deposited at the herbarium of LICP.
3.3 Extraction and Isolation
The air-dried rhizomes (13.0 kg) of H. occulta were powdered and extracted with 88 % ethanol/water (v/v) at 60 ℃ (12 h × 3). After dried in vacuum, the residue (410 g) was suspended in water (1.5 L) and applied to a liquid-liquid partitioning against petroleum ether (PE), EtOAc, and n-BuOH (each 1.0 L × 3) continuously. The dried PE part (257 g) was chromatographed over silica gel (1.5 kg), using gradient PE/acetone (v/v, from 80:1 to 1:1, each about 8.0 L) to yield eleven fractions (A1-A11). Fraction A2 (55 g) was subjected to silica gel CC eluting with CHCl3/PE gradient system to afford compound 8 (18.2 mg). Fraction A3 (21 g) was purified over silica gel with PE/CHCl3 (v/v, 1:1) to afford 7 (4.5 mg). Fraction A5 (34 g) was fractionated consecutively over silica gel and Sephadex LH-20 (CHCl3/MeOH, 1:1, v/v) to yield compound 5 (4.5 mg). Fraction A6 (12 g) was chromatographed on a silica gel column eluting with PE/acetone (v/v, 10:1, 8:1, 5:1, 3:1, and 1:1) to afford compound 1 (5.1 mg). Fraction A7 (15 g) was fractionated consecutively over silica gel with PE/acetone (8:1) and Sephadex LH-20 (CHCl3/MeOH, 1:1, v/v) to yield 2 (7.8 mg), 3 (23.1 mg), 4 (17.7 mg), 6 (17.8 mg), and 9 (2.1 mg).
3.3.1 7-Epi-oplopanone (1)
Colorless oil;
3.3.2 5, 7-Diepi-2α-hydroxyoplopanone (2)
Colorless oil;
3.3.3 Oplopananol (3)
White floc;
3.3.4 4-Epi-oplopananol (4)
White floc;
3.4 Acetylation of 2 to 4
A solution of compounds in a mixture of acetic anhydride-pyridine (1:1) were stirred fully and settled at 25 ℃ for 12 h. After concentration and storage in vacuo compounds 2a, 3a, and 4a were obtained and identified.
3.4.1 5, 7-Diepi-2α-acetoxyoplopanone (2a)
Colorless oil;
3.4.2 4-Acetoxyoplopananol (3a)
Colorless oil;
3.4.3 4-Epi-acetoxyoplopananol (4a)
White floc;
Notes
Acknowledgement
The work was financially supported by the National Nature Science Foundation of China (Nos. 21375136 and 21575150), and the scientific research project of Central Asia Drug Discovery and Development Centre of Chinese Academy of Sciences (No. CAM201404) and the CAS Pioneer Hundred Talents Program.
Compliance with Ethical Standards
Conflict of Interest
The authors declare no competing financial interest.
References
-
1.Chinese Pharmacopoeia Commission, Chinese Pharmacopoeia (Part I, 2015 Edition), p33, China Medical Science Press (2015) PubMed Google Scholar
-
2.M. Elbandy, H. Lerche, H. Wagner, M.A. Lacaille-Dubois, Biochem. Syst. Ecol. 32, 1209-1213 (2004) CrossRef PubMed Google Scholar
-
3.Y.M. Hu, C. Liu, K.W. Cheng, H.H.Y. Sung, L.D. Williams, Z.L. Yang, W.C. Ye, Phytochemistry 69, 2367-2373 (2008) CrossRef PubMed Google Scholar
-
4.Y.M. Hu, Z.L. Yang, H. Wang, W.C. Ye, Nat. Prod. Res. 23, 1279-1283 (2009) CrossRef PubMed Google Scholar
-
5.X.Y. Tian, Y. Zhao, S.S. Yu, W.S. Fang, Chem. Biodivers. 7, 984-992 (2010) CrossRef PubMed Google Scholar
-
6.Y.F. Wang, X.Y. Wang, G.F. Lai, C.H. Lu, S.D. Luo, Chem. Biodivers. 4, 925-931 (2007) CrossRef PubMed Google Scholar
-
7.X.H. Li, W.J. Zhang, H.Y. Qi, Y.P. Shi, Can. J. Chem. 87, 1218-1221 (2009) CrossRef PubMed Google Scholar
-
8.R. Wang, W.H. Chen, Y.P. Shi, J. Nat. Prod. 73, 17-21 (2010) CrossRef PubMed Google Scholar
-
9.R. Wang, L.L. Liu, Y.P. Shi, Helv. Chim. Acta 93, 2081-2085 (2010) CrossRef PubMed Google Scholar
-
10.J.L. Yang, L.L. Liu, Y.P. Shi, Tetrahedron Lett. 50, 6315-6317 (2009) CrossRef PubMed Google Scholar
-
11.G.Y. Zhu, G.P. Peng, Nat. Prod. Res. Dev. 14, 85-88 (2002) PubMed Google Scholar
-
12.Y.H. Kuo, C.F. Chyu, Tetrahedron Lett. 44, 7221-7223 (2003) CrossRef PubMed Google Scholar
-
13.T.V. Sung, L. Kutschabsky, A. Porzel, W. Steglich, G. Adam, Phytochemistry 31, 1659-1661 (1992) CrossRef PubMed Google Scholar
-
14.W. Herz, K. Watanabe, Phytochemistry 22, 1457-1459 (1983) CrossRef PubMed Google Scholar
-
15.H. Heymann, Y. Tezuka, T. Kikichi, S. Supriyatna, Chem. Pharm. Bull. 42, 138-146 (1994) CrossRef PubMed Google Scholar
-
16.G.P. Peng, F.C. Lou, Nat. Prod. Res. Dev. 13, 9-11 (2001) PubMed Google Scholar
-
17.G.Y. Zhu, G.P. Peng, Nat. Prod. Res. Dev. 14, 85-88 (2002) PubMed Google Scholar
-
18.Z.F. Li, Z.H. Wu, G. Chen, Q.H. Zhang, Y.H. Pei, J. Asian Nat. Prod. Res. 11, 715-718 (2009) PubMed Google Scholar
-
19.K.C. Wong, A. Hamid, I.M.S. Eldeen, M.Z. Asmawi, S. Baharuddin, H.S. Abdillahi, J. van Staden, Nat. Prod. Res. 26, 850-858 (2012) CrossRef PubMed Google Scholar
-
20.L.S. Gan, Z.J. Zhan, S.P. Yang, J.M. Yue, J. Asian Nat. Prod. Res. 8, 589-594 (2006) CrossRef PubMed Google Scholar
-
21.S. Yang, H. Chen, J. Yue, Chin. J. Chem. 30, 1243-1248 (2012) CrossRef PubMed Google Scholar
-
22.H.B. Xu, Y.B. Ma, X.Y. Huang, C.A. Geng, H. Wang, Y. Zhao, T.H. Yang, X.L. Chen, C.Y. Yang, X.M. Zhang, J.J. Chen, J. Ethnopharmacol. 171, 131-140 (2015) CrossRef PubMed Google Scholar
-
23.G.J. Zhang, Y.H. Li, J.D. Jiang, S.S. Yu, X.J. Wang, P.Y. Zhuang, Y. Zhang, J. Qu, S.G. Ma, Y. Li, Y.B. Liu, D.Q. Yu, Tetrahedron 70, 4494-4499 (2014) CrossRef PubMed Google Scholar
-
24.L. Zhang, J.H. Zhang, S.M. Yang, C.H. Tan, H.F. Luo, D.Y. Zhu, J. Asian Nat. Prod. Res. 12, 215-219 (2010) CrossRef PubMed Google Scholar
-
25.Y. Narukawa, C. Komatsu, R. Yamauchi, S. Shibayama, M. Hachisuka, F. Kiuchi, J. Nat. Med., p 1-7 (2016) PubMed Google Scholar
-
26.W. Wang, Z. Ali, X.C. Li, T.A. Smillie, D.A. Guo, I.A. Khan, Fitoterapia 80, 404-407 (2009) CrossRef PubMed Google Scholar
-
27.N. Liu, X. Yu, H. Zhao, Y. Zhao, Zhongcaoyao 40, 29-32 (2009) PubMed Google Scholar
Copyright information
© The Author(s) 2016
Open Access
This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.