Pterocarpadiols A-D, Rare 6a, 11b-Dihydroxypterocarpans from Derris robusta
Abstract
Four hitherto unknown 6a, 11b-dihydroxypterocarpans, namely pterocarpadiols A-D(1-4), were isolated from the ethanol extract of the twigs and leaves of Derris robusta. Their structures were elucidated on the basis of extensive spectroscopic analysis. Pterocarpadiols A-D are a kind of very rare 6a, 11b-dihydroxypterocarpans, and their presence as markers may be helpful in chemotaxonomical classification.Graphical Abstract
Keywords
Derris robusta 6a, 11b-Dihydroxypterocarpan Pterocarpadiol1 Introduction
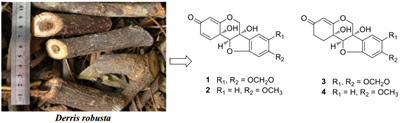
The genus Derris (Leguminosae) contains about 800 species, widely distributed in tropical and subtropical parts of the world [1]. The bark and leaves of Derris species are commonly utilized as folk medicine to treat human diseases such as arthritis and eczema [2]. The extracts and reported chemical constituents exhibit antioxidant, antibacterial and pesticidal activities [2]. Previous studies show the genus is a rich source of flavonoids, isoflavonoids, pterocarpans and rotenoids [2]. As part of a BioBioPha [http://www.chemlib.cn] objective to assemble a large-scale natural product library very valuable in the discovery of new drug leads from nature [3-7], the phytochemical investigation on the twigs and leaves of Derris robusta led to the isolation of four new 6a, 11b-dihydroxypterocarpans, namely pterocarpadiols A-D (1-4), together with 10 known pterocarpans: 1, 11b-dihydro-11b-hydroxymaackiain (5) [8], 1, 11b-dihydro-11b-hydroxymedicarpin (6) [8], pisatin (7) [9], variabilin (8) [10], 6a-hydroxymaackiain (9) [11], 6a-hydroxymedicarpin (10) [12], maackiain (11) [13], medicarpin (12) [14], 3-hydroxy-8, 9-methylenedioxypterocarpene (13) [15], and anhydroglycinol (14) [16]. Among the known pterocarpans, compounds 5-10 and 14 were isolated from the genus for the first time. This paper reports the isolation and structure elucidation of pterocarpadiols A-D.
2 Results and Discussion
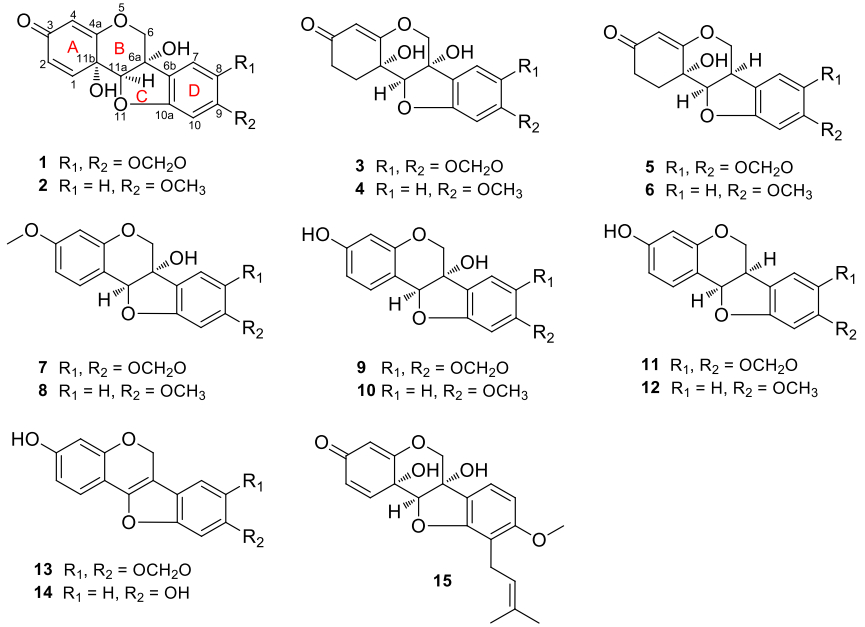
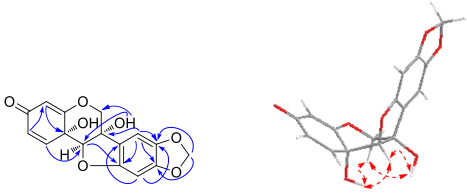
Compound 1, obtained as amorphous powder, had a molecular formula of C16H12O7 as deduced from its positive-ion HRESIMS at m/z 339.0462 [M+Na]+ (calcd for C16H12O7Na, 339.0475), requiring 11 degrees of unsaturation. The 1H NMR spectrum (Table 1) showed three characteristic aliphatic protons of 6a-hydroxypterocarpan skeleton at δH 4.99, 4.37 (each 1H, d, J = 10.5 Hz, H-6), and 4.73 (s, H-11a) [17]. The 13C NMR spectrum (Table 3) displayed a total of 16 carbon resonances, including three typical oxygen-bearing carbons of 6a-hydroxypterocarpan at δc 70.2 (t, C-6), 78.7 (s, C-6a) and 91.4 (d, C-11a). Three olefinic protons at δH 6.80 (d, J = 10.0 Hz, H-1), 6.09 (dd, J = 10.0, 1.8 Hz, H-2), and 5.41 (d, J = 1.8 Hz, H-4) were assignable to A-ring by comparison with hydroxycristacarpone (15) (Fig. 1) [18], and their spectral difference was almost completely rooted in the D ring. Two aromatic singlets at δH 6.81 (s, H-7), 6.26 (s, H-10) and a methylenedioxy signal at δH 5.90, 5.89 (each 1H, d, J = 1.0 Hz) were newly detected, while the prenyl and methoxy signals disappeared, which suggested that the methylenedioxy group should be connected to C-8 and C-9. The inference was confirmed by the HMBC correlations from the proton at δH 6.81 (s, H-7) to the carbons at δc 78.7 (s, C-6a), 144.2 (s, C-8), and 151.6 (s, C-9), and from the methylenedioxy protons to the carbons at δc 144.2 (s, C-8), and 151.6 (s, C-9). Regrettably, it was inconclusive to establish relative configurations at C-6a, C-11a and C-11b by ROESY analysis, since the pivotal hydroxy signals were undetectable in CD3OD. As we know, hydroxy proton signals were observable and often appeared as sharp peaks in DMSO-d6, and their HMBC and ROESY correlations often played an important role in structure elucidation, especially the determination of relative configuration [19]. The clear ROESY correlation (DMSO-d6, Fig. 2) of 6a-OH ↔ H-11a revealed a cis fusion of the B/C ring junction, while the correlations of 11b-OH ↔ H-11a and H-6α indicated α-orientation of the hydroxy group at C-11b. Accordingly, the structure of 1 was established and named as pterocarpadiol A.
1H NMR spectroscopic data for pterocarpadiols A-D (1-4) in CD3OD (δH 3.30 ppm)
13C NMR spectroscopic data for pterocarpadiols A-D (1-4)
Pterocarpans from Derris robusta (1-14) and hydroxycristacarpone (15)
Key HMBC (

Compound 2, white amorphous powder, had a molecular formula of C16H14O6 based on the positive-ion HRESIMS at m/z 325.0674 [M+Na]+ (calcd for C16H14O6Na, 325.0683). The NMR spectroscopic data (Tables 1, 3) were similar to those of pterocarpadiol A (1), and the major difference was that its NMR spectra newly displayed a methoxy group (δH 3.72; δc 56.0) instead of the methylenedioxy. And then three aromatic protons at δH 7.25 (d, J = 8.5 Hz, H-7), 6.55 (dd, J = 8.5, 2.4 Hz, H-8), and 6.29 (d, J = 2.4 Hz, H-10) were assignable to an ABX-type aromatic D-ring. The methoxy group was linked to C-9 on the basis of the HMBC correlations from the proton at δH 7.25 (d, J = 8.5 Hz, H-7) to the carbons at δc 78.0 (s, C-6a), 164.2 (s, C-9), and 162.9 (s, C-10a), and from the methoxy protons at δH 3.72 (s) to the carbon at δc 164.2 (s, C-9). Therefore, the structure of 2 was established and named as pterocarpadiol B.
Compound 3, white amorphous powder, possessed a molecular formula of C16H14O7 according to its positive-ion HRESIMS at m/z 341.0620 [M+Na]+ (calcd for C16H14O7Na, 341.0632), which was 2 m.u. higher than that of 1. Signals of an oxymethylene at δH 4.49, 4.28 (each 1H, d, J = 10.0 Hz, H-6), an oxymethine at δH 4.44 (s, H-11a), an olefinic proton at δH 5.22 (s, H-4), two aromatic singlets at 6.93 (s, H-7), 6.49 (s, H-10) and a methylenedioxy at δH 5.96, 5.94 (each 1H, s) were observed in the 1H NMR spectrum (Table 2). By comparison of the NMR spectra (Tables 2, 3) of 3 and 1, two ortho-coupled doublets and the corresponding olefinic carbons were absent whereas two sp3 carbons at δc 31.2 (t) and δc 31.8 (t) were newly detected, which hinted that 3 should be 1, 2-dihydropterocarpadiol A. The inference was confirmed by the HMBC correlations from the proton at δH 2.43 (td, 14.1, 4.5, H-1β) to the carbon at δc 89.9 (d, C-11a) and from the proton at δH 2.16 (ddd, 16.0, 4.5, 2.8, H-2β) to the carbon at δc 107.5 (d, C-4). The clear ROESY correlations (DMSO-d6) of 6a-OH/11b-OH ↔ H-11a indicated that 3 possessed the same stereochemistry as 1. Thus, the structure of 3 was established and named as pterocarpadiol C.
1H NMR spectroscopic data for pterocarpadiol A (1) and pterocarpadiol C (3) in DMSO-d6 (δH 2.49 ppm)
Compound 4, white amorphous powder, had a molecular formula of C16H16O6 determined by the positive-ion HRESIMS at m/z 327.0819 [M+Na]+ (calcd for C16H16O6Na, 327.0839). The NMR spectroscopic data (Tables 1, 3) were similar to those of pterocarpadiol C (3), and the obvious difference was that a methoxy signal (δH 3.74; δc 56.0) replaced the methylenedioxy in 3. According to the HMBC correlations from the proton at δH 7.25 (d, J = 8.0 Hz, H-7) to the carbons at δc 78.1 (s, C-6a), 164.1 (s, C-9), and 162.2 (s, C-10a), and from the methoxy protons at δH 3.74 (s) to the carbon at δc 164.1 (s, C-9), the methoxy group was positioned at C-9 as with the previous structure. Thus, the structure of 4 was established and named as pterocarpadiol D.
Pterocarpans isolated in our current research such as pisatin (7), variabilin (8), and maackiain (11) exhibited without exception negative optical rotation values (-286°, -304°, and -260°, respectively), and their absolute configurations had been established as 6aS, 11aS (7), 6aS, 11aS (8), and 6aR, 11aR (11) [20]. As their related co-constituents, pterocarpadiols A-D (1-4) also gave large negative optical rotation values (-484.0°, -397.0°, -507.0° and -476.0°, respectively), thereupon we assumed that the absolute configurations of 1-4 could be assigned as 6aS, 11aR, 11bS depicted in Fig. 1. Nonetheless, this issue deserved further studies in the future. Until now, only two 6a, 11b-dihydroxypterocarpans: hydroxytuberosone [21] and hydroxycristacarpone [18], were reported and only from the family Leguminosae, therefore pterocarpadiols A-D as chemical markers in Derris species may be helpful in chemotaxonomical classification.
3 Experimental Section
3.1 General Experimental Procedures
Optical rotations were measured on a Jasco P-1020 automatic digital polarimeter. UV data were obtained from HPLC online analysis. NMR spectra were carried out on a Bruker AV-400, Bruker DRX-500 or Bruker AV-800 spectrometer with deuterated solvent signals used as internal standards. ESI and HRESIMS were performed with a Shimadzu LC-IT-TOF mass spectrometer equipped with an ESI interface (Shimadzu, Kyoto, Japan). Silica gel 200-300 mesh (Qingdao Marine Chemical Inc., Qingdao, China), Chromatorex C-18 (40-75 μm, Fuji Silysia Chemical Ltd., Japan) and Sephadex LH-20 (Amersham Biosciences, Uppsala, Sweden) were used for normal pressure column chromatography (CC). Fractions were monitored and analyzed by TLC, in combination with Agilent 1200 series HPLC system equipped by Extend-C18 column (5 μm, 4.6 × 150 mm).
3.2 Plant Material
The twigs and leaves of D. robusta were collected from the Pu'er region of Yunnan Province, China, in May 2011, and identified by Mr. Yu Chen of Kunming Institute of Botany. A voucher specimen (BBP0350021DR) was deposited at BioBioPha Co., Ltd.
3.3 Extraction and Isolation
The air-dried and powdered twigs and leaves (12.0 kg) of D. robusta were extracted with 95 % EtOH at room temperature, and the solvent was removed under reduced pressure to give crude extract (ca. 870 g), which was fractionated by silica gel CC successively eluted with petroleum ether (PE)/acetone gradient and then MeOH to yield nine fractions A-I. Fraction C (PE/acetone, 6:1) was subjected to silica gel CC (CHCl3/MeOH, 100:0 → 100:2) and Sephadex LH-20 (CHCl3/MeOH, 1:1; or MeOH) to give 5 (51 mg), 6 (185 mg), 7 (138 mg), 8 (262 mg), 11 (665 mg), and 12 (608 mg), and the remainder was separated by RP-18 (40 % MeOH/H2O) and Sephadex LH-20 (MeOH) to yielded 9 (661 mg) and 10 (349 mg). The fraction E (PE/acetone, 4:1) was repeatedly applied to silica gel CC (CHCl3/MeOH, 30:1 → 15:1) and Sephadex LH-20 (MeOH) to yiele 13 (23 mg) and 14 (114 mg). The fraction F (PE/acetone, 3:1) was repeatedly separated on silica gel CC (CHCl3/MeOH, 10:1), Sephadex LH-20 (CHCl3/MeOH, 1:1) and RP-18 (40 % MeOH/H2O) to yield 1 (233 mg) and 2 (33 mg), and the remainder was further isolated on silica gel (CHCl3/MeOH, 10:1), Sephadex LH-20 (MeOH) and RP-18 (45 % MeOH/H2O) to yield 3 (47 mg) and 4 (28 mg). The retention times (tR) of 1-4 on an analytical HPLC Extend-C18 column (20 % → 100 % MeOH in H2O over 8.0 min followed by 100 % MeOH to 13.0 min, 1.0 ml/min, 25 ℃) were 6.03, 6.16, 6.50, and 6.60 min, respectively.
3.4 Pterocarpadiol A (1)
White amorphous powder; UV (MeOH) λmax: 235, 306 nm;
3.5 Pterocarpadiol B (2)
White amorphous powder; UV (MeOH) λmax: 230, 285, 305 nm;
3.6 Pterocarpadiol C (3)
White amorphous powder, UV (MeOH) λmax: 260, 308 nm;
3.7 Pterocarpadiol D (4)
White amorphous powder, UV (MeOH) λmax: 232, 261 nm;
Notes
Acknowledgments
This work was financially supported by the "Large-scale Compound Library" project of National Development and Reform Commission of China.
Compliance with Ethical Standards
Conflict of Interest
The authors declare no conflict of interest.
References
-
1.Editorial Committee of Flora Reipublicae Popularis Sinicae, Flora Reipublicae Popularis Sinicae. Academic Press: Beijing 40, 194-195 (1994) PubMed Google Scholar
-
2.X.Y. Yang, R.J. Ma, L.Q. Wang, T. Li, Nat. Prod. Res. Dev. 25, 117-128 (2013) PubMed Google Scholar
-
3.F. Wang, Y. Gao, L. Zhang, J.K. Liu, Org. Lett. 12, 2354-2357 (2010) CrossRef PubMed Google Scholar
-
4.F. Wang, Y.J. Li, F.C. Ren, G.Z. Wei, J.K. Liu, Chem. Pharm. Bull. 59, 484-487 (2011) CrossRef PubMed Google Scholar
-
5.Y. Gao, G.Q. Wang, K. Wei, P. Hai, F. Wang, J.K. Liu, Org. Lett. 14, 5936-5939 (2012) CrossRef PubMed Google Scholar
-
6.F. Wang, X.L. Li, G.Z. Wei, F.C. Ren, J.K. Liu, Nat. Prod. Bioprospect. 3, 238-242 (2013) CrossRef PubMed Google Scholar
-
7.P. Hai, S.Z. Wen, Y. Li, Y. Gao, X.J. Jiang, F. Wang, Nat. Prod. Bioprospect. 4, 47-51 (2014) CrossRef PubMed Google Scholar
-
8.A.F. Barrero, E. Cabrera, I.R. Garcia, Phytochemistry 48, 187-190 (1998) CrossRef PubMed Google Scholar
-
9.M.F. Hegazy, A.E.H. Mohamed, A.M. El-Halawany, P.C. Djemgou, A.A. Shahat, P.W. Paré, J. Nat. Prod. 74, 937-942 (2011) CrossRef PubMed Google Scholar
-
10.T.G. van Aardt, H. van Rensburg, D. Ferreira, Tetrahedron 57, 7113-7126 (2001) CrossRef PubMed Google Scholar
-
11.A. Fuchs, F.W.D. Vries, C.A. Landheer, A.V. Veldhuizen, Phytochemistry 19, 917-919 (1980) CrossRef PubMed Google Scholar
-
12.F.A. Macías, A.M. Simonet, J.C.G. Galindo, D. Castellano, Phytochemistry 50, 35-46 (1999) CrossRef PubMed Google Scholar
-
13.T. Kinoshita, K. Ichinose, C. Takahashi, F.C. Ho, J.B. Wu, U. Sankawa, Chem. Pharm. Bull. 38, 2756-2759 (1990) CrossRef PubMed Google Scholar
-
14.J.F. Zeng, D.Y. Zhu, Acta Bot. Sin. 41, 997-1001 (1999) PubMed Google Scholar
-
15.E. Malan, E. Swinny, Phytochemistry 29, 3307-3309 (1990) CrossRef PubMed Google Scholar
-
16.T. Miyase, A. Ueno, T. Noro, S. Fukushima, Chem. Pharm. Bull. 28, 1172-1177 (1980) CrossRef PubMed Google Scholar
-
17.H. Tanaka, T. Tanaka, H. Etoh, Phytochemistry 45, 205-207 (1997) CrossRef PubMed Google Scholar
-
18.H. Tanaka, T. Tanaka, H. Etoh, Phytochemistry 42, 1473-1475 (1996) CrossRef PubMed Google Scholar
-
19.F. Wang, D.S. Zhou, G.Z. Wei, F.C. Ren, J.K. Liu, Phytochemistry 77, 312-317 (2012) CrossRef PubMed Google Scholar
-
20.J.L. Ingham, K.R. Markham, Phytochemistry 19, 1203-1207 (1980) CrossRef PubMed Google Scholar
-
21.A. V. K. Prasad, A. Singh, R. S. Kapil, S. P. Popli, Indian J. Chem. 23B, 1165-1167 (1984) PubMed Google Scholar
Copyright information
© The Author(s) 2015
Open Access
This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.