Isoprenylated flavonoids and clerodane diterpenoids from Dodonaea viscosa
Abstract
Phytochemical investigation of the aerial parts of Dodonaea viscosa led to the isolation of six new compounds including four isoprenylated flavonoids, dodovisones A-D (1-4), and two clerodane diterpenoids, dodovislactones A and B (5 and 6). Their structures were established by extensive spectroscopic analysis.Keywords
Dodonaea viscosa isoprenylated flavonoid clerodane diterpenoid dodovisone dodovislactoneIntroduction
The genus Dodonaea (Sapindaceae) is composed of approximately 50 species and is mainly distributed throughout Australia and its nearby islands. Only one species exists in China, Dodonaea viscosa; a eurytopic species growing throughout the tropics and sub-tropics.1 This plant has been used as a folk medicine for the treatment of fever, skin diseases, stranguria, toothache, rheumatism, gout, inflammation, and swelling.2, 3 Previous chemical investigations of D. viscosa found flavonoids, diterpenoids, triterpenoid saponins, cyanolipids, and phenylpropanoids, among which isoprenylated flavonoids and clerodane diterpenoids predominated.2-7 Pharmacological studies on the title plant have shown bioactivities such as antibacterial8 and antioxidant8 activities, inhibition against urease9 and enoyl-ACP reductase, 10 and activity against liver fibrosis.11 Hou et al. reported that some isoprenylated flavonoids from this plant enhanced the accumulation of lipid droplets significantly and induced the up-regulation of the expression of the adipocyte-specific genes aP2 and GLUT4.2 Recent pharmacological research on hautriwaic acid and related terpenes derived from this plant also displayed potent inhibitions against edema-associated inflammation which were similar or higher than those of reference compound indomethacin when evaluated in the chronic test.12
As part of our effort to assemble a large-scale natural compound library of thousands of structures as well as to examine opportunities for the development of natural resources, 13 the study described herein was undertaken to determine the chemical constituents in the aerial parts of D. viscosa. The resulting investigation characterized four isoprenylated flavonoids, dodovisones A-D (1-4), as well as two clerodane diterpenoids, dodovislactones A and B (5 and 6), together with 12 known compounds: dodoviscin J, 2 dodoviscin A, 2 dodoviscin I, 2 dodoviscin H, 2 aliarin, 14 5, 7, 4'-trihydroxy-3, 6-dimethoxy-3'-prenylflavone, 15 5'-prenylaliarin, 3 5, 7, 4'-trihydroxy-3, 6-dimethoxyflavone, 16 sakuranetin, 17 15-methoxypatagonic acid, 18 6α-hydroxycleroda-3, 13-dien-16, 15-olid-18-oic acid; 19 and hautriwaic acid.20 Herein, we describe the isolation and structure elucidation of the new compounds.
|
Results and Discussion
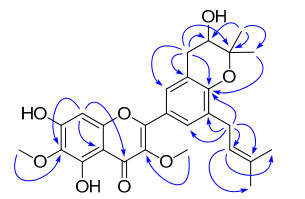
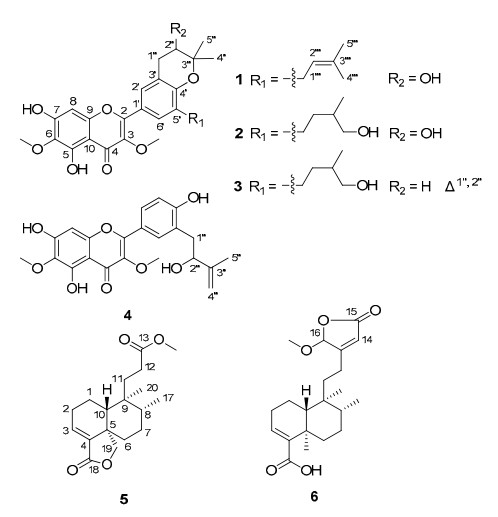
Compound 1 was isolated as a yellowish powder with a molecular formula of C27H30O8, as evidenced by HREIMS at m/z 482.1960 (calcd 482.1941) and NMR spectroscopic data, with 13 degrees of unsaturation. The IR spectrum suggested the presence of hydroxy (3417 cm-1) and conjugated carbonyl (1656 cm-1) groups in 1. The UV spectrum (MeOH) of 1 showed maxima at 251, 271, and 352 nm suggestive of a flavone nucleus and also closely reflecting the UV absorbances of related flavones isolated from the same species2. The 1H NMR spectrum (Table 1) displayed a chelated phenolic hydroxy group at δH 12.94 (1H, br, s), an aromatic proton at δH 6.55 (1H, s), two meta-coupled aromatic protons at δH 7.66 and 7.76 (each 1H, d, J = 1.7 Hz), and two methoxy groups at δH 3.83 and 4.03 (each 3H, s). The 1H NMR data also exhibited two isoprenoid units: a 2, 2-dimethyl-3-hydroxydihydropyran ring21 [δH 1.41 and 1.36 (each 3H, s), 3.88 (1H, dd, J = 5.5, 4.8 Hz), 2.87 (1H, dd, J = 16.7, 5.5 Hz), and 3.16 (1H, dd, J = 16.7, 4.8 Hz)]; and a 3-methyl-2-butenyl group2 [δH 1.74 and 1.76 (each 3H, s), 5.31 (1H, t, J = 7.5 Hz), and 3.36 and 3.32 (each 1H, dd, J = 15.5, 7.5 Hz)]. The 13C NMR (Table 1) spectrum showed 27 carbon signals corresponding to a flavonol derivative with two isoprenoid moieties and two methoxy groups. The presence of the dihydropyran ring was further supported by the HMBC correlations (Figure 1) from H-1" to C-2" and C-3", and from H-5" to C-2", C-3", and C-4". Given that no correlation was observed for H-2" to C-4′, weak correlation from H3-4" to C-4a (4J) strongly suggested that an oxygen atom was to bridge C-4′ (δC 153.2) and C-3" (δC 77.2) and not C-4′ and C-2". This unit was fused to ring B as shown via C-3′ and C-4′ by the HMBC correlations from H-1" to C-2′, C-3′, and C-4′. Another isoprenoid unit was confirmed by HMBC correlations from H-2''' to C-1''', C-4''', and C-5''', and from H-1''' to C-2''' and C-3''' and was attached to C-5′ by correlations from H2-1''' to C-4′, C-5′, and C-6′. During our work to elucidate hundreds of structures of aromatic methoxycontaining compounds, we found that the particular low field chemical shifts of the aromatic methoxy carbon signals at about 60 ppm (aromatic methoxy carbon signals were commonly at about 56 ppm), were without exception accompanied by o-substitutions on both sides of the methoxy groups. 22 This observation may be explained by the steric hindrance of neighboring groups within the same plane. According to this regulation, the two methoxy groups (δH 3.83; δC 60.1/δH 4.03; δC 60.9) were assigned at C-3/C-6, since a chelated phenolic hydroxy group at δH 12.94 (1H, br. s) must be connected to C-5 and the two low field meta-coupled aromatic protons at δH 7.66 and 7.76 (each 1H, d, J = 1.7 Hz) can easily be assigned as H-2′/H-6′. This deduction was further supported by the HMBC correlations from δH 3.83 to C-3 (δC 138.2) and from δH 4.03 to C-6 (δC 129.9). The HMBC correlations from the aromatic singlet at δH 6.55 to five quaternary carbons C-6, C-7, C-9, C-10, and C-4 (4J, ω-coupled) indicated that the singlet was assigned to H-8. Accordingly, the hydroxy group leftover must be attached to C-7 (δC 154.8). On the basis of the above discussion, the structure of 1 was finally assigned as 2-[3, 4-dihydro-3-hydroxy-8-(3-methylbut-2-en-1-yl)-2, 2-dimethyl-2H-1-benzopyran-6-yl]-5, 7-dihydroxy-3, 6-dimethoxy-4H-1-benzopyran-4-one, and it was given the trivial name dodovisone A.
Key HMBC correlations of 1
NMR spectroscopic data for 1–4
Compound 2 was isolated as a yellowish powder with the molecular formula C27H32O9, as determined by HREIMS at m/z 500.2034 [M]+ (calcd 500.2046). The 1H and 13C NMR data (Table 1) of 2 indicated this compound to be an isoprenylated flavonoid, similar to 1. The only difference between them was the 3-methyl-2-butenyl group in 1 changing into a 4-hydroxy-3-methylbutyl group2 [δH 1.37, 1.60, 1.73, 2.58, and 2.69 (each 1H, m), 3.37 (1H, dd, J = 10.6, 6.6 Hz), 3.46 (1H, dd, J = 10.6, 5.9 Hz), and 0.99 (3H, d, J = 6.7 Hz); δC 28.8 (CH2), 34.6 (CH2), 36.2 (CH), 68.4 (CH2), and 17.1 (CH3)] in 2. This group was confirmed by HMBC correlations from H-5''' to C-2''', C-3''', and C-4''', and from H-1''' to C-2''' and C-3'''. The methylene protons (δH 2.69 and 2.58) of the isoprenoid side chain showed HMBC correlations to C-4′, C-5′, and C-6′, positioning this group at C-5′ as in 1. Hence, compound 2 was elucidated as 2-[3, 4-dihydro-3-hydroxy-8-(4-hydroxy-3-methylbutyl)-2, 2-dimethyl-2H-1-benzopyran-6-yl]-5, 7-dihydroxy-3, 6-dimethoxy-4H-1-benzopyran-4-one, and it was given the name dodovisone B.
Compound 3 gave a molecular formula of C27H30O8 by HREIMS. Comparison of its 13C NMR data (Table 1) with those of 2 revealed similarities, except for the evident methine signals of a double bond (δC 122.1 and 130.8) in 3 instead of a methylene (δC 32.3, C-1") and an oxygenated methane (δC 70.1, C-2") in 2, which was supported by HMBC correlations from H-1" (δH 6.39) to C-2′, C-3′, and C-4′ and from H-2" (δH 5.69) to C-3′, C-1", C-3", C-4", and C-5". Consequently, compound 3 was elucidated as 2-[8-(4-hydroxy-3-methylbutyl)-2, 2-dimethyl-2H-1-benzopyran-6-yl]-5, 7-dihydroxy-3, 6-dimethoxy-4H-1-benzopyran-4-one and was named dodovisone C.
Compound 4 was assigned the molecular formula C22H22O8 by HREIMS. It was found to be an isoprenylated flavonol with the same ring A and C moieties as 1-3. The ABX spin system observed for aromatic protons in the 1H NMR (Table 1) at δH 7.88 (1H, d, J = 2.2 Hz), 6.88 (1H, d, J = 8.5 Hz), and 7.82 (1H, dd, J = 8.5, 2.2 Hz) suggested a 3′, 4′-disubstitution pattern in ring B. A 2-hydroxy-3-methyl-3-butenyl unit2 was indicated by 1H NMR signals at δH 2.83 (1H, dd, J = 13.7, 7.8 Hz), 2.96 (1H, dd, J = 13.7, 5.2 Hz), 4.39 (1H, dd, J = 7.8, 5.2 Hz), 4.77 (1H, s), 4.87 (1H, s), and 1.81 (3H, s), and another hydroxy group was implied by the presence of an sp2 quarternary carbon at δC 160.1. This butenyl unit was confirmed by HMBC correlations from H-5''' to C-2''', C-3''', and C-4''', and from H-1''' to C-2''' and C-3'''. The above two groups were fixed at C-3′ and C-4′, respectively, by strong HMBC correlations, from a meta-coupled aromatic proton at δH 7.88 (1H, d, J = 2.2 Hz) to the methylene of the isoprenoid group at δC 38.1 and the oxygenated carbon at δC 160.1. Therefore, compound 4 was elucidated as 2-[3-(2-hydroxy-3-methylbut-3-en-1-yl)-4-hydroxyphenyl]-5, 7-dihydroxy-3, 6-dimethoxy-4H-1-benzopyran-4-one and was named dodovisone D.
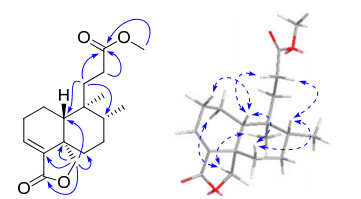
Compound 5 was isolated as colorless oil. Its molecular formula was determined as C18H26O4 from HREIMS at m/z 306.1828 [M]+ (calcd 306.1831), requiring six degrees of unsaturation. The IR spectrum showed absorptions at 1773 and 1737, which were attributed to two carbonyl groups. Analysis of the 13C NMR data (Table 2) revealed, in addition to a methoxy group at δC 51.6, a total of 17 signals consisting of two methyls, seven methylenes (one oxygenated), three methines (one olefinic), and five quarternary carbons (one olefinic and two carbonylic). The 1H and 13C NMR data (Table 2) were in part close to those of mkapwanin, 23 a butenolidecontaining clerodane diterpenoid from D. angustifolia. However, a methoxy signal and the molecular formula (C18H26O4) required a tri-norclerodane diterpenoid. The absence of signals for butenolide moiety and HMBC correlations (Figure 2) from H2-11, H2-12, and the methoxy protons to C-13 indicated that instead of a butenolidecontaining side chain, a methyl propionate one was linked at C-9; therefore, the planar structure of 5 was defined.
Key HMBC and ROESY correlations of 5
NMR spectroscopic dataa for 5 and 6
The relative configuration of 5 was established by ROESY correlations and proton coupling constants based on computergenerated 3D drawing with minimized energy by MM2 calculation (Figure 2). The pro-19S diastereotopic proton, which was ω-coupled (4J = 2.0 Hz) with H-6β, 23 showed correlations with H-1α and H3-20, indicating α-and axial orientation for both C-20 and C-19. In addition, the axially oriented proton H-6β at δH 1.13 (1H, dddd, 13.1, 13.1, 3.7, 2.0) showed correlations with H-8 and H-10, revealing β-and axial orientation for H-8 and H-10. Thus, compound 5 was finally established as shown and was named dodovislactone A.
Compound 6 was isolated as an inseparable C-16 epimeric mixture (1:1) where some of the signals appeared as duplicate in the NMR spectra (Table 2). HREIMS gave a molecular formula of C21H30O5. Its NMR data indicated a clerodane diterpenoid similar to 16-hydroxycleroda-3, 13-dien-16, 15-olide-18-oic acid, 24 except that a methoxy group was evident in 6. HMBC correlation in 6 from the methoxy protons (δH 3.57) to C-16 verified that the methoxy group was at C-16. In the ROESY spectrum, correlations of H3-19/H3-20 and H-10/H-12 revealed the orientation of C-19, C-20, and H-10 as α, α, and β, respectively. However, no useful information about the stereochemistry at C-8 was obtained. In consideration of the co-occurrence of 5, 15-methoxypatagonic acid, 6α-hydroxycleroda-3, 13-dien-16, 15-olid-18-oic acid, and hautriwaic acid, C-8 should have the R* configuration of these analogues. This deduction was also supported by referring to the 1H and 13C NMR data of the diterpenoid hardwickiic acid25 recorded in the same solvent. The diterpenoid was therefore elucidated as shown and was named dodovislactone B.
Experimental Section
General Experimental Procedures. Optical rotations were measured on a Jasco P-1020 automatic digital polarimeter. UV spectra were obtained in an HPLC (Agilent 1200, DAD). IRspectra were obtained using a Bruker Tensor 27 FT-IR spectrometer with KBr pellets. The NMR spectra were acquired with an Avance Ⅲ 600 or Bruker DRX-500 instrument at room temperature. EIMS (including HREIMS) were measured on a VG-Auto-Spec-3000 spectrometer. ESIMS were measured on API QSTAR Pulsar i mass spectrometers. Silica gel (200-300 mesh, Qingdao Marine Chemical Inc., China) and Sephadex LH-20 (Amersham Biosciences, Sweden) were used for column chromatography. Fractions were monitored by TLC (Qingdao Marine Chemical Inc., China) in combination with reversed-phase HPLC (Agilent 1200, Extend-C18 column, 5 μm, 4.6 × 150 mm). Prep. HPLC was performed using an Agilent 1100 series (ZORBAX SB-C18 column, 5 μm, 9.4 × 150 mm for 10 mL/min). Silica gel for prep. TLC was obtained from Qingdao Marine Chemical Inc., China.
Plant Material. The aerial parts of D. viscosa were collected from Yuanyang County in Yunnan Province, China, on May 2011 and were identified by Prof. Yu Chen of Kunming Institute of Botany, Chinese Academy of Sciences. The voucher specimen was deposited at BioBioPha Co., Ltd.
Extraction and Isolation. The aerial parts of D. viscosa (12 kg) was powdered and extracted with MeOH at room temperature. After filtration, the methanolic extract was evaporated under reduced pressure to get a residue (ca. 900 g), which was fractionized by silica gel column chromatography using petroleum ether (PE)/acetone gradient and then MeOH to yield six main fractions A-F. Separation of fraction C eluted with PE/acetone (9:1→7:3) by silica gel eluted with CHCl3/MeOH gave four subfractions C1 (100:1), C2 (30:1), C3 (20:1), and C4 (10:1). Fr. C1 was purified by Sephadex LH-20 (CHCl3/MeOH, 1:1) and then preparative TLC (PE/EtOAc, 9:1) to afford 5 (4 mg). Fraction C2 was purified by Sephadex LH-20 (CHCl3/MeOH, 1:1) and then preparative HPLC on a ZORBAX SB-C18 column (20%→100% CH3CN in H2O over 10 min) to afford 3 (4 mg, tR = 7.1 min) and 1 (2 mg, tR = 7.6 min). Fraction C3 was purified by silica gel (CHCl3/MeOH, 20:1) and then Sephadex LH-20 (MeOH) to afford 2 (62 mg) and 4 (2 mg). Fraction D eluted by PE/acetone (6:4→1:1) was further separated by silica gel (CHCl3/MeOH, 50:1) into two subfractions -D1 and D2. After repeated preparative TLC (PE/EtOAc/formic acid, 40:10:0.2), fraction D1 afforded 6 (23 mg).
Dodovisone A (1): yellowish powder; [α]D18 -18.7 (c 0.13, CHCl3); UV (MeOH) λmax: 251, 271, 352 nm; IR (KBr) υmax 3417, 2975, 2925, 2852, 1656, 1612, 1591, 1561, 1468, 1368, 1308, 1209, 1170, 1139, 1093, 1049, 952 cm-1; 1H and 13C NMR data (see Table 1); EIMS: m/z 482 [M]+ (100), 467 (25), 439 (10), 413 (12), 395 (11), 355 (12), 337 (6), 269 (8), 183 (8), 105 (17); HREIMS: m/z 482.1960 [M]+ (calcd for C27H30O8, 482.1941).
Dodovisone B (2): yellowish powder; [α]D19 -3.8 (c 0.21, MeOH); UV (MeOH) λmax: 212, 251, 271, 353 nm; IR (KBr) υmax 3425, 2972, 2932, 2873, 1654, 1612, 1594, 1562, 1468, 1369, 1307, 1266, 1210, 1171, 1139, 1092, 1048, 952 cm-1; 1H and 13C NMR data (see Table 1); ESIMS (pos.): m/z 523 [M + Na]+; HREIMS: m/z 500.2034 [M]+ (calcd for C27H32O9, 500.2046).
Dodovisone C (3): yellowish powder; [α]D18 -53.3 (c 0.10, CHCl3); UV (MeOH) λmax: 216, 243, 272, 357 nm; IR (KBr) υmax 3416, 2960, 2927, 2854, 1654, 1611, 1562, 1467, 1378, 1365, 1307, 1269, 1209, 1170, 1122, 1092, 1050, 954 cm-1; 1H and 13C NMR data (see Table 1); EIMS: m/z 482 [M]+ (71), 467 (100), 449 (7), 409 (8), 395 (8), 234 (7), 197 (8), 175 (8), 169 (5), 118 (4); HREIMS: m/z 482.1941 [M]+ (calcd for C27H30O8, 482.1941).
Dodovisone D (4): yellowish powder; [α]D19 -9.2 (c 0.20, MeOH); UV (MeOH) λmax: 248, 270, 349 nm; IR (KBr) υmax 3423, 2933, 2851, 1654, 1610, 1563, 1470, 1366, 1280, 1208, 1169, 1122, 1092, 1046, 997, 807 cm-1; 1H and 13C NMR data (see Table 1); EIMS: m/z 414 [M]+ (85), 399 (10), 381 (17), 344 (70), 329 (33), 285 (10), 84 (77), 66 (100); HREIMS: m/z 414.1345 [M]+ (calcd for C22H22O8, 414.1315).
Dodovislactone A (5): colorless oil; [α]D18 -96.3 (c 0.28, CHCl3); UV (MeOH) λmax: 240 (sh) nm; IR (KBr) υmax 2957, 2927, 2874, 1773, 1737, 1452, 1437, 1286, 1198, 1189, 1122, 1038, 1005, 988 cm-1; 1H and 13C NMR data (see Table 2); ESIMS (pos.): m/z 329 [M + Na]+; HREIMS: m/z 306.1828 [M]+ (calcd for C18H26O4, 306.1831).
Dodovislactone B (6): colorless oil; [α]D19 -72.8 (c 0.20, CHCl3); UV (MeOH) λmax: 212 nm; IR (KBr) υmax 3440, 2960, 2923, 2862, 1796, 1765, 1711, 1680, 1648, 1629, 1457, 1420, 1382, 1263, 1204, 1172, 1118, 962, 939 cm-1; 1H and 13C NMR data (see Table 2); ESIMS (pos.): m/z 385 [M + Na]+; HREIMS: m/z 362.2097 [M]+ (calcd for C21H30O5, 362.2093).
Notes
Electronic Supplementary Material
Supplementary material is available in the online version of this article at http://dx.doi.org/10.1007/s13659-013-0053-4 and is accessible for authorized users.
Acknowledgments
This work was financially supported by National Basic Research Program of China (973 Program, 2009CB522300), the "West Light" program of Chinese Academy of Sciences, and the "Large-scale Compound Library" project of National Development Reform Commission.
References
-
1.Editorial Committee of Flora Reipublicae Popularis Sinicae. Flora Reipublicae Popularis Sinicae; Academic Press: Beijing, 1985; Vol. 47, pp 58-59. PubMed Google Scholar
-
2.L. B. Zhang, J. Ji, C. Lei, H. Y. Wang, Q. S. Zhao, A. J. Hou, J. Nat. Prod. 75, 699-706 (2012) CrossRef PubMed Google Scholar
-
3.H. M. Niu, D. Q. Zeng, C. L. Long, Y. H. Peng, Y. H. Wang, J. F. Luo, H. S. Wang, Y. N. Shi, G. H. Tang, F. W. Zhao, J. Asian Nat. Prod. Res. 11, 7-14 (2010) PubMed Google Scholar
-
4.A. Ortega, P. E. García, J. Cárdenas, C. Mancera, S. Marquina, M. L. C. Garduño, E. Maldonado, Tetrahedron 57, 2981-2989 (2001) CrossRef PubMed Google Scholar
-
5.S. G. Cao, P. Brodie, M. Callmander, R. Randrianaivo, J. Razafitsalama, E. Rakotobe, V. E. Rasamison, K. TenDyke, Y. C. Shen, E. M. Suh, D. G. I. Kingston, J. Nat. Prod. 72, 1705-1707 (2009) CrossRef PubMed Google Scholar
-
6.H. Wagner, C. Ludwig, L. Grotjahn, M. S. Y. Khan, Phytochemistry 26, 697-701 (1987) CrossRef PubMed Google Scholar
-
7.R. Mata, J. L. Contreras, D. Crisanto, R. Pereda-Miranda, P. Castañeda, F. D. Rio, J. Nat. Prod. 54, 913-917 (1991) CrossRef PubMed Google Scholar
-
8.L. S. Teffo, M. A. Aderogba, J. N. Eloff, South Afric. J. Bot. 76, 25-29 (2010) CrossRef PubMed Google Scholar
-
9.A. Muhammad, I. Anis, A. Khan, B. P. Marasini, M. I. Choudhary, M. R. Shah, Arch. Pharm. Res. 35, 431-436 (2012) CrossRef PubMed Google Scholar
-
10.A. Muhammada, I. Anis, Z. Ali, S. Awadelkarim, A. Khan, A. Khalid, M. R. Shah, M. Galal, I. A. Khan, M. I. Choudhary, Bioorg. Med. Chem. Lett. 22, 610-612 (2012) CrossRef PubMed Google Scholar
-
11.N. M. M. Shalaby, H. I. Abd-Alla, M. A. Hamed, S. N. Al-Ghamdi, S. M. S. Jambi, Int. J. Phytomed. 4, 27-39 (2012) PubMed Google Scholar
-
12.D. O. Salinas-Sánchez, M. Herrera-Ruiz, S. Pérez, E. JiménezFerrer, A. Zamilpa, Molecules 17, 4292-4299 (2012) CrossRef PubMed Google Scholar
-
13.(a) Wang, F. ; Gao, Y. ; Zhang, L. ; Bai, B. ; Hu, Y. N. ; Dong, Z. J. ; Zhai, Q. W. ; Zhu, H. J. ; Liu, J. K. Org. Lett. 2010, 12, 3196-3199. (b) Gao, Y. ; Zhou, D. S. ; Kong, L. M. ; Hai, P. ; Li, Y. ; Wang, F. ; Liu, J. K. Nat. Prod. Bioprospect. 2012, 2, 65-69. (c) Gao, Y. ; Wang, G. Q. ; Wei, K. ; Hai, P. ; Wang, F. ; Liu, J. K. Org. Lett. 2012, 14, 5936-5939. (d) Gao, Y. ; Li, G. T. ; Li, Y. ; Hai, P. ; Wang, F. ; Liu, J. K. Nat. Prod. Bioprospect. 2013, 3, 14-19. PubMed Google Scholar
-
14.K. Sachdev, D. K. Kulshreshtha, Phytochemistry 22, 1253-1256 (1983) CrossRef PubMed Google Scholar
-
15.E. Wollenweber, J. N. Roitman, Nat. Prod. Comm. 2, 385-389 (2007) PubMed Google Scholar
-
16.F. R. V. Heerden, A. M. Viljoen, yk W, V. B-E., Fitoterapia 71, 602-604 (2000) CrossRef PubMed Google Scholar
-
17.J. M. J. Vasconcelos, A. M. S. Silva, J. A. S. Cavaleiro, Phytochemistry 49, 1421-1424 (1998) CrossRef PubMed Google Scholar
-
18.P. Singh, S. Jain, J. Jakupovic, Phytochemistry 27, 1537-1539 (1988) CrossRef PubMed Google Scholar
-
19.K. Iqbal, A. Malik, N. Mukhtare, I. Anis, S. N. Khan, M. I. Choudhary, Chem. Pharm. Bull. 52, 785-789 (2004) CrossRef PubMed Google Scholar
-
20.S. D. Jolad, J. J. Hoffmann, K. H. Schram, J. R. Cole, M. S. Tempesta, R. B. Bates, J. Org. Chem. 47, 1356-1358 (1982) CrossRef PubMed Google Scholar
-
21.T. Hatano, M. Takagi, H. Ito, T. Yoshida, Chem. Pharm. Bull. 45, 1485-1492 (1997) CrossRef PubMed Google Scholar
-
22.(a) Ferracin, R. J. ; Silva, M. F. D. G. F. D. ; Fernandes, J. B. ; Vieira, P. C. Phytochemisty 1998, 47, 393-396. (b) Ikeya, Y. ; Taguchi, H. ; Sasaki, H. ; Nakajima, K. ; Yosioka, I. Chem. Pharm. Bull. 1980, 28, 2414-2421. (c) Wu, Y. B. ; Zheng, C. J. ; Qiu, L. P. ; Sun, L. N. ; Han, T. ; Jiao, L. ; Zhang, Q. Y. ; Wu, J. Z. Molecules 2009, 14, 573-583. (d) Sasaki, H. ; Taguchi, H. ; Endo, T. ; Yosioka, I. Chem. Pharm. Bull. 1982, 30, 3555-3562. (e) Sinha, A. ; Taylor, W. H. ; Khan, I. H. ; McDaniel, S. T. ; Esko, J. D. J. Nat. Prod. 1999, 62, 1036-1038. PubMed Google Scholar
-
23.L. K. Omosa, J. O. Midiwo, S. Derese, A. Yenesew, M. G. Peter, M. Heydenreich, Phytochemistry Lett. 3, 217-220 (2010) CrossRef PubMed Google Scholar
-
24.M. Costa, C. M. A. Tanakac, P. M. Imamuraa, A. J. Marsaioli, Phytochemistry 50, 117-122 (1999) CrossRef PubMed Google Scholar
-
25.H. Heymann, Y. Tezuka, T. Kikuchi, S. Supriyatna, Chem. Pharm. Bull. 42, 1204-1207 (1994) PubMed Google Scholar
Copyright information
© The Author(s) 2013
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.