Metabolites and bioactivities of Rhizophoraceae mangroves
Abstract
This review examines the chemical compositions and bioactivities of mangrove plants belonging to the Rhizophoraceae family. The Rhizophoraceae family of true mangrove plants is the most common and is also widely distributed species. It consists of 24 species across four genera. Of the 24 species, 12 species remain unexamined for their phytochemical constituents. There have been 268 metabolites reported from 16 species. The key phytochemical constituents identified across the family are the diterpenoids and triterpenoids. The major diterpenoids include pimaranes, beyeranes, kaurenes, dolabranes and labdanes whereas the significant triterpenoids are lupanes, dammaranes and oleananes. Disulphides, dolabranes and labdanes are considered to be the chemotaxonomic markers of the genera Bruguiera, Ceriops and Rhizophora respectively.Keywords
Rhizophoraceae Bruguiera Rhizophora terpenoids CeriopsIntroduction
Mangrove plants are potential sources of biologically active chemicals that are discernible from their wide spread application in ethnopharmaceutical practices. There habitat exists under stressful conditions and serve as a bridging ecosystem between freshwater and marine systems. These plants have specially adapted their own morphological structures and physiological mechanisms to their harsh natural surroundings. Pneumatophores, stilt roots and buttresses, with salt-excreting glands found in their leaves, and viviparous propagules are some of the several highly specialized adaptations of this group. The path of photosynthesis in mangroves is different from other glycophytes. Furthermore, there are alterations in other physiological processes such as carbohydrate metabolism or polyphenol synthesis. These plants survive under extreme conditions of salinity, temperature gradients, tidal fluctuations and anoxic soil conditions, with these plants possessing many chemical compounds, which protect them from these destructive elements1. Even though extracts from mangroves and mangrove-dependent species possess therapeutic activity against humans, animal and plant pathogens, the specific metabolites responsible for these bioactivities remains to be elucidated.
1 Rhizophoraceae
The global mangrove plant have 84 species belonging to 24 genera and 16 families2. Among them, 70 species are true mangroves pertaining to sixteen genera and eleven families whereas fourteen species are semi mangroves belonging to eight genera and five families. According to Wu et. al, suggests the family Rhizophoraceae belongs to true mangrove family, which contains 21 species in four genera2. In contrast three more species; Rhizophora annamalayana3, Kandelia obovata4 and Ceriops zeppeliana blume5 become 24 species in four genera in the Rhizophoraceae family of true mangroves. Thus the family Rhizophoraceae include: Bruguiera which contains seven species, Ceriops (five species), Kandelia (two species) and Rhizophora (ten species). The distribution of species in Rhizophoraceae family is detailed in Table 1. 54 studies can achieve the validity of ethnomedicines as well as apply the use of mangrove plants in the development of new drugs.
Mangroves of Rhizophoraceae family of true mangroves
In this review, the compounds identified from this family were listed, and their reported biological activities were compiled. Also chemotaxonamy and importance of further phytochemical research is discussed.
2 Chemical Constituents
2.1 Bruguiera
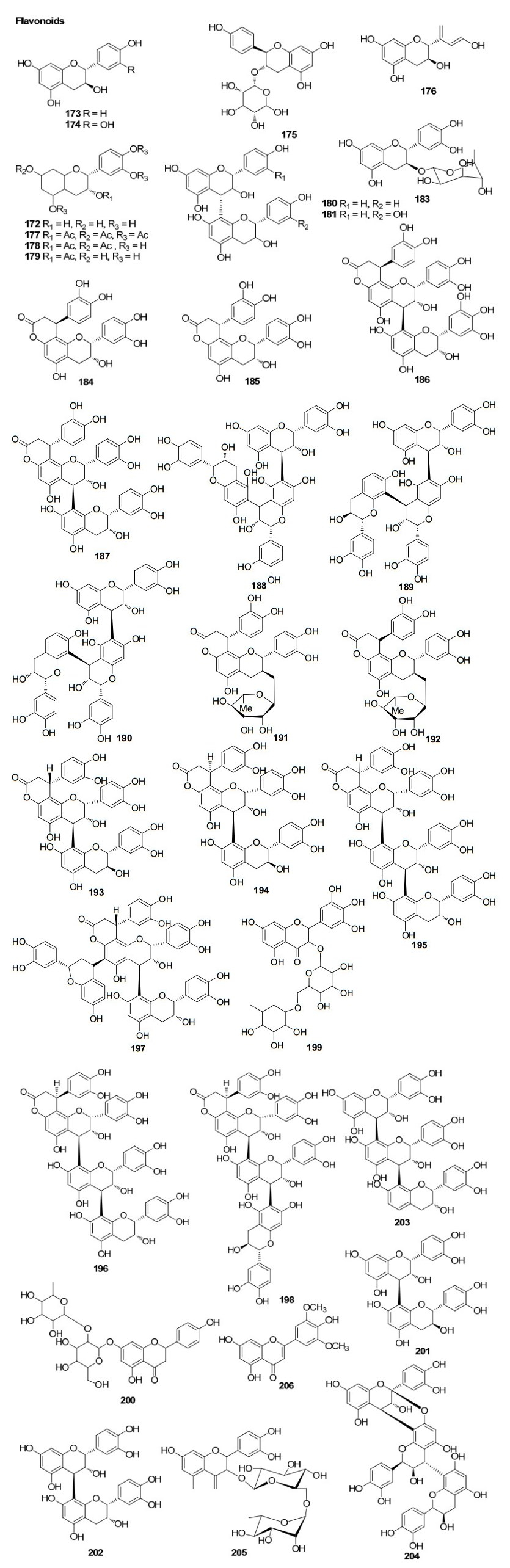
The genus Bruguiera has six species and one hybrid species which are derived from B. sexangula, including B. cylindrica, B. exarista, B. gymnorrhiza, B. hainessi, B. parviflora, B. sexangula and B. sexangula var. rhynchopetala. The metabolic pattern of this genus has been extensively characterised by a suite of diterpenes and triterpenes. In addition, these species also produce flavonoids, tropane derivatives and cyclic polysulphides. These include 22 metabolites from B. cylindrica, 54 metabolites from B. gymnorrhiza, nine metabolites from B. exaristata, six metabolites from B. parviflora, two metabolites from B. sexangula and 40 metabolites from B. sexangula var rhynchopetala were identified so far. A total of 114 metabolites have been reported form this genus including. A detailed list of chemical compounds identified from Bruguiera is recorded in Table 2.
Chemical constituents from the genus Bruguiera
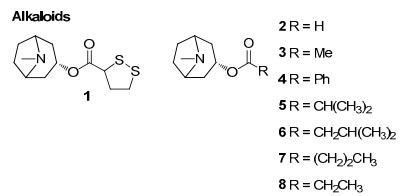
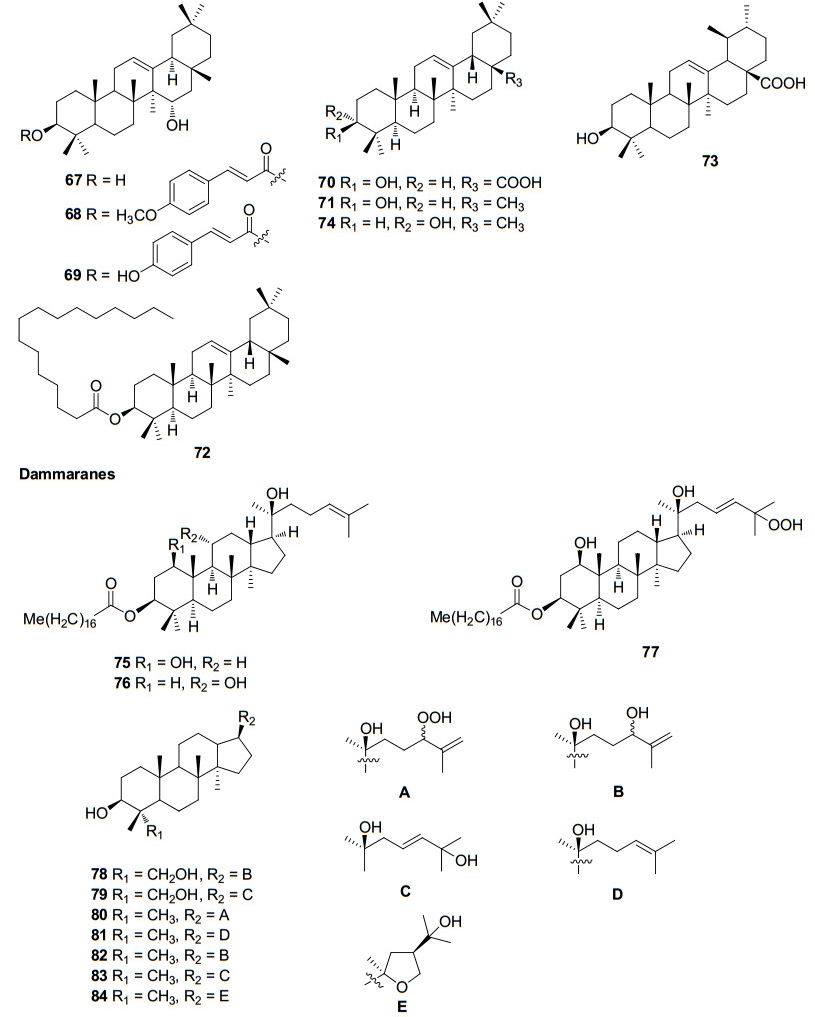
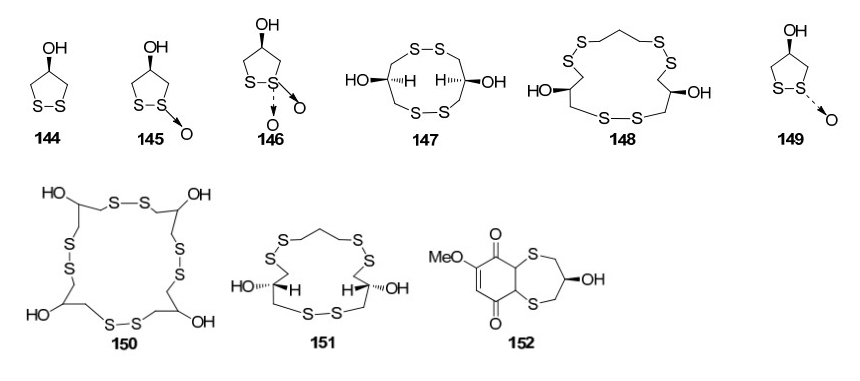
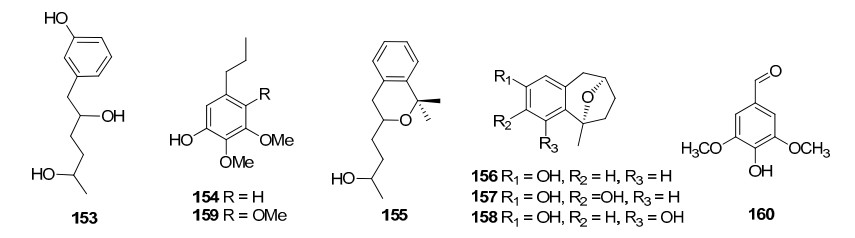
Three sulphur compounds along with an alkaloid brugine were reported from the stem and bark of B. cylindrica by Japanese scientists of during 1975–1976. 20 years later, a number of oleananes and lupanes (triterpenoids) and one kaurane (diterpenoid) were reported. The first report on the chemical constituents of B. gymnorrhiza dates back to 1978 in which Sarkar and Ganguly reported a new triterpenoid called gymnorhizol (3-epi-δ-amyrin). Since then various triterpenoids (lupanes, oleananes, ursanes, dammaranes) and diterpenoids (kauranes, pimaranes, beyaranes) along with sulphur compounds, sterols and aromatic compounds were reported from this plant.
Only two reports are available regarding the chemical constituents of B. exaristata. As part of their investigation on tumor inhibitory plants, in 1969 Loder and Russell identified the presence of alkaloids (brugine, tropine and tropine esters of acetic, iosbutyric, iosvaleric, propionic, n-butyric and benzoic acids) in the bark extracts of B. exaristata6 while a pronounced accumulation of 1-d-1-O-methyl-muco-inositiol in the young leaves of B. exaristata was reported later by Richter and his team7.
In a continuing search for bioactive constituents from Thai medicinal plants, Chumkaew and his team isolated and elucidated a new triterpenoid ester 3-(Z)-caffeoyllupeol along with five other tritepenoids; lupeol caffeate, 3-(Z)-coumaroyllupeol, dioslupecin A, lupeol and lupenone from the fruits of B. Parviflora8. The earliest work regarding the chemical constituents of mangroves deals with the isolation and characterization of the tropine 1, 2-dithiolane-3-carbonylate named as bruguine from the stem bark of B. sexangula9. Later the same team identified additional alkaloids from the same plant as part of their investigation on tumor inhibitory plants6. In a study focusing on the marine fauna and flora from Chinese coasts, Li and his coworkers collected samples of the mangrove B. sexangula from Hainan Province, China. On separation of an EtOAc-soluble fraction of a methanol extract of the title plant, they isolated a new triterpene, named sexangulic acid10.
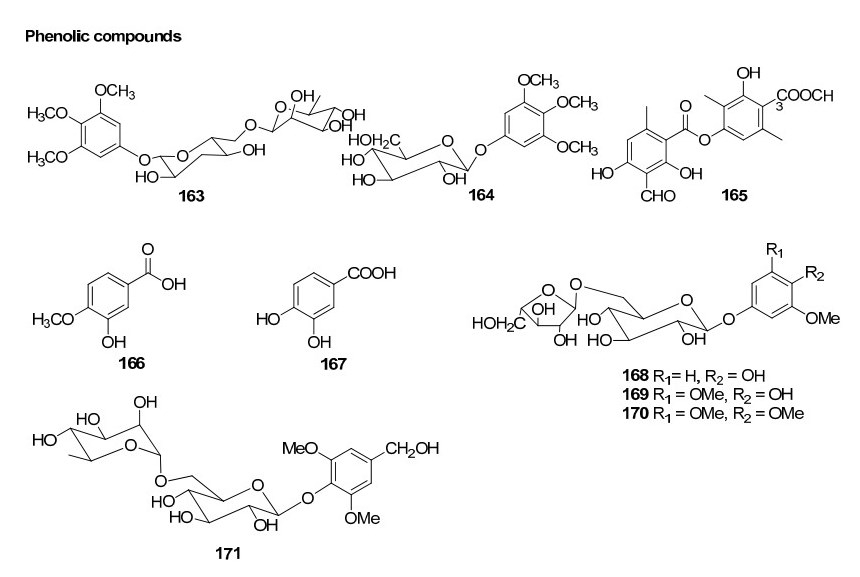
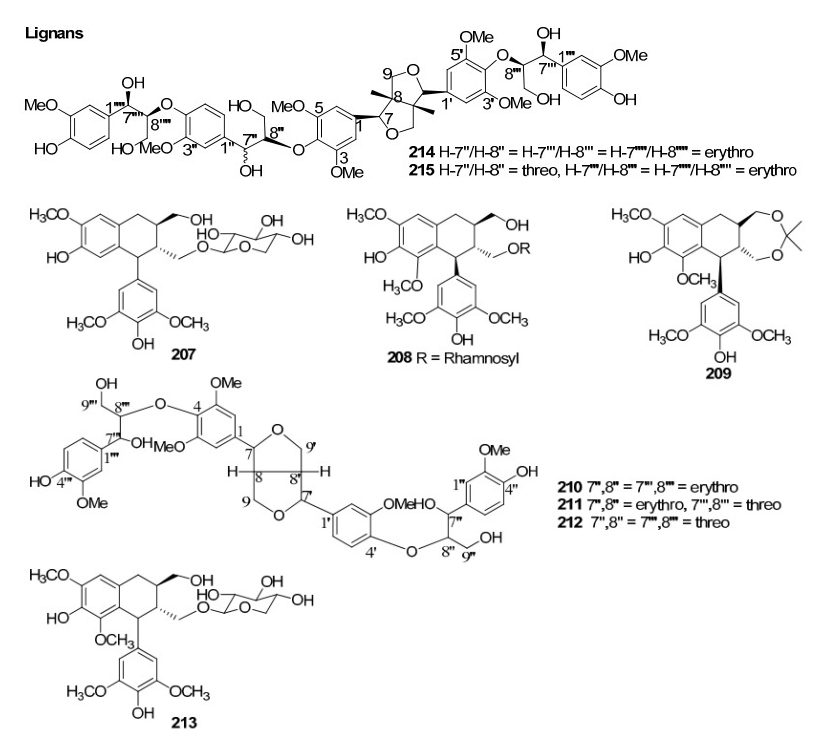
Investigation of Chinese mangrove plants led to the isolation and characterisation of 13 compounds; three new diterpenes; six known diterpenes, a new dithiobenzoquinone two cyclic disulfides and 2, 6-dimethoxy-1, 4-benzoquinone from the EtOH extract of the stem of B. sexangula var. rhynchopetala11. Further several triterpenoids and sterols were reported12. Recently, a continuous investigation for chemical diversity of B. sexangula var. rhynchopetala led to the isolation and characterization of six new phenolic constituents named rhyncosides A–F, together with twelve known compounds including two phenolic glycosides, four flavonoids, and six lignan derivatives13.
Alkaloids from Rhizophoraceae mangroves
2.2 Ceriops
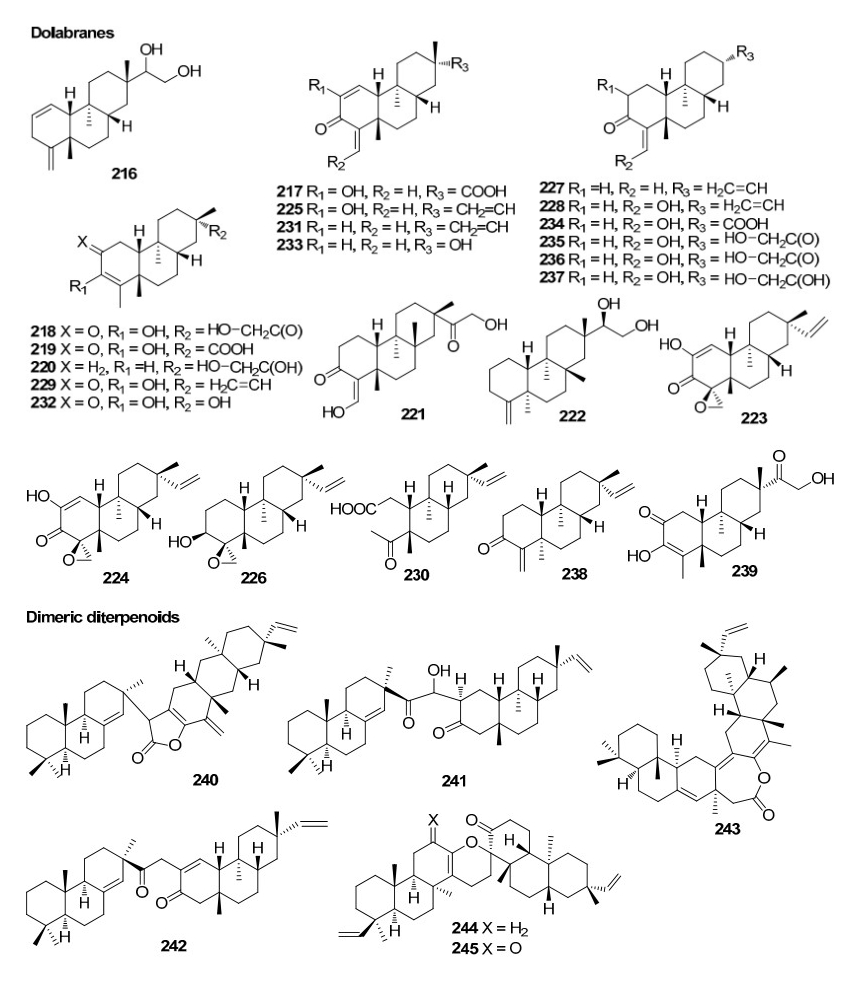
The genus of Ceriops has two species and two varieties, namely C. tagal (Perr.), C. decandra, C. tagal var. australasica and C. tagal var. typical. These plants are valued for their rich tannin content and are a rich source of pentacyclic triterpenoids17. To date, 30 metabolities from C. decandra and 72 metabolites from C. tagal are known. Thus a total of 92 metabolites including 45 diterpenoids (23 dolabranes, six dimeric diterpenoids, four beyeranes, five kauranes, and seven pimaranes) and 45 tritepenoids (35 lupanes, seven dammaranes, one oleanane, one ursane, and one abietane) along with two steroids have been reported so far from this genus.
On examination of the roots of C. decandra collected from the Kauvery estuary (Parangipettai coast), Anjaneyulu and his team isolated and characterised twelve diterpenoids30, 31, 32. Subsequently, two novel triterpene esters were isolated from the leaves of C. decandra in addition to 16 known triterpenes33 by scientists from Thailand. Dolabranes (diterpenoids) are the marker metabolites of C. tagal. These compounds can be used as chemotaxonomic markers of this plant. Dimeric diterpenoids (tetraterpenoids) and triterpenoids of lupane, dammarane, pimarane groups are also found in this plant. One abietane and an oleanane triterpenoid were also isolated from the stems and twigs of C. tagal. The chemical constituents identified from ceriops are listed in Table 3.
Chemical constituents of Ceriops
Chemical constituents of the bark of Kandelia candel
2.3 Kandelia
There are two species in the mangrove genus Kandelia: K. candel and K. obovata. Only one report is available regarding the chemical constituents of plants of this genus. A few tannin compounds have been reported from K. candel. Investigation of K. obovata for its chemical constituents remains to be observed. 24 phenolic compounds including three propelargonidin dimmers, three procyanidin trimers, fourteen proanthocyanidins and four flavan-3-ols have been isolated from the bark of K. candel Druce46.
2.4 Rhizhophora
The mangrove genus Rhizophora has ten species: R. apiculata, R. harrisonii, R. lamarckii, R. mangle, R. mucronata, R. racemosa, R. samoesis, R. selala, R. stylosa and R. annamalayana. Of these ten species, chemical constituents have only been reported in R. apiculata, R. mangle, R. mucronata, and R. stylosa. These reports reveal a total of 34 metabolites from R. apiculata, two metabolites from R. mangle, 23 metabolites from R. mucronata and 25 metabolites from R. stylosa, thus a total of 81 different metabolites from the genera Rhizophora, with details shown in Table 5.
Chemical constituents of the genus Rhizophora
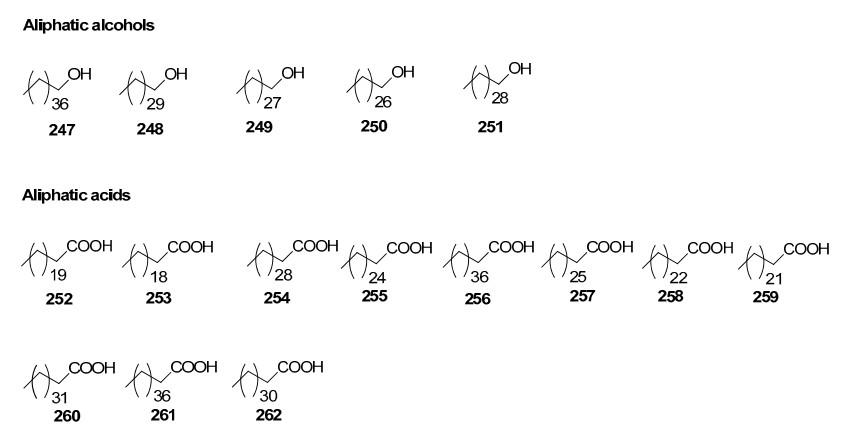
The chemical investigation carried out by Majumdar and Patra in 1976 resulted in the isolation of β-amyrin, β-amyrone, taraxerol, β-sitosterol, and triacantanol from R. apiculata47. Later in early nineties Kokpol and his team had identified three terpenoids, five long chain aliphatic alcohols, eleven long chain aliphatic saturated carboxylic acids, three steroids, 2, 6-dimethoxy-p-benzoquinone, syringaldehyde and sitosteryl 3-glucoside from this plant species. Also, five kauranes, one labdane and one pimarane diterpenoids and one lupane triterpenoid are reported so far from R. apiculata.
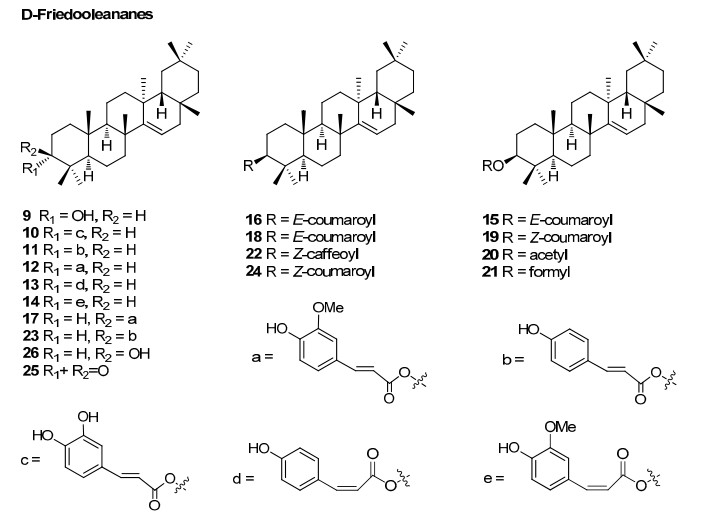
The study conducted by Williams et. al51 reported the isolation and chemical characterisation of taraxerol and cinnamoyllupeol, two triterpenoids from the leaves and stems of Rhizophora mangle L. A variety of steroids, diterpenoids and triterpenoids were reported from the leaves and bark of R. mucronata. A few beyeranes (diterpenoids) were identified from this plant, and are unique to this species. Only triterpenoids of the classes oleananes and D-friedooleananes from R. stylosa are reported to date.
D-friedooleananes (triterpenoids) from Rhizophoraceae mangroves
3 Bioactivities
3.1 Bioactivities of Compounds Identified
With stably transfected HepG2 cells, three new dammarane triterpenes; bruguierins A–C and a new cyclic 4-hydroxy-dithiosulfonatebruguiesulfurol as well as two known 4-hydroxydithiolane-1-oxides; brugierol and isobrugierol, were isolated from the flowers of Bruguiera gymnorrhiza. These phytochemicals activated an antioxidant response element (ARE luciferase activation) with EC50 values of 7.8, 9.4, 15.7, 56.7, 3.7 and 1.8 µM, respectively. Furthermore, bruguierin A, brugierol and isobrugierol also inhibited phorbol ester-induced NFκB (nuclear factor-κB) luciferase activation with an IC50 value of 1.4, 85.0 and 14.5 µM respectively, while bruguierin A and brugierol selectively inhibited cyclooxygenase-2 (COX-2) activity with an IC50 value of 0.37 and 6.1 µM respectively18, 26.
The compounds 16α-17, 19-ent-kauranediol; 13-hydroxy-16-ent-kaurene-19-ol and 16-ent-kaurene-19-ol showed promising activity against K-562 (human chronic myeloid leukemia) and L-929 (mouse fibroblasts) of which 16-ent-kaurene-19-ol showed the greatest selectivity for K-562 (IC50 6.8 µg/mL)22. (5R, 9S, 10R, 13S, 15S)ent-8(14)-pimarene-1-oxo-15, 16-diol showed moderate cytotoxic activities against L-92925.
The 15 membered macrocyclic polysulfide, gymnorrhizol, possesses an novel carbon skeleton which was isolated from B. gymnorrhiza and exhibited potent inhibitory activity against protein tryrosine phosphatase 1B (PTP1B). PTP1B is an enzyme involved in the regulation of insulin signaling and which is regarded as a key for treatment of type Ⅲ diabetes and obesity27. One of the aromatic compounds extracted from the stem of B. gymnorrhiza, bruguierol C showed moderate activity against gram-positive and gram-negative bacteria including mycobacteria and resistant strains (MICs 12.5 μg/mL)28.
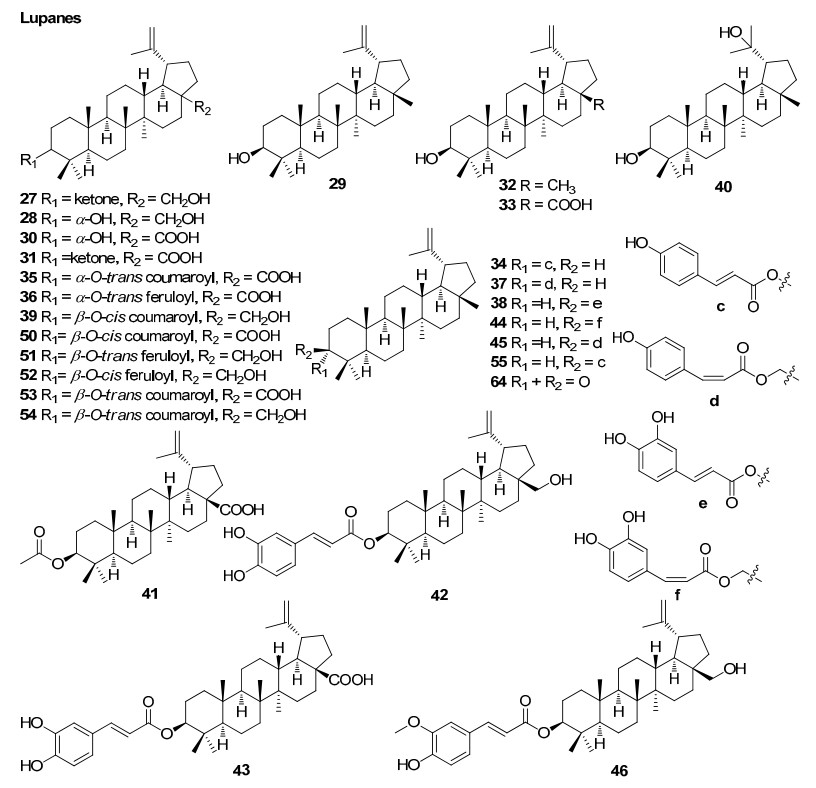
The lupane caffeoyl ester, 3-(Z)-caffeoyllupeol extracted from B. parviflora exhibited antimalarial activity with an EC50 value of 8.6 µg/mL8. Sexangulic acid obtained from B. sexangula showed moderate in vitro cytotoxicity against human lung cancer (A-549) and human luekaemic (H-L60) cell lines at a concentration of 5 µg/ml10. Tagalsin C found in C. tagal was found to exhibit moderate cytotoxicity against HeLa human cervical carcinoma cell lines35. The dimeric diterpenoid, 8(14)-enyl-pimar-2'(3')-en-4'(18')-en-15'(16')-endolabr-16, 15, 2', 3'-oxoan-16-one and the other terpenoids; tagalsin C, tagalsin I, lup-20(29)-ene-3β, 28-diol, 3-oxolup-20(29)-en-28-oic acid and 28-hydroxy-lup-20(29)-en-3-one isolated from the roots of the mangrove plant Ceriops tagal exhibited antifouling activity against cyprid larvae of the barnacle without significant toxicity36. The other nontoxic antifouling compounds identified were ethoxy-ent-8(14)-pimarenely-15-one, ent-8(14)-pimarene-15R, 16-diol, stigmasterol and β-sitosterol45. Tagalsins Q, R and U showed moderate antifeedant activity against the third instar larvae of Brontispa longissima at a concentration of 1 mg/mL38. Dolabr-4(17), 15(16)-dien-3-one, isopimar-8(14)-en-15, 16-diol, isopimar-8(14)-en-16-hydroxy-15-one, lupeol, lup-20(29)-en-3β, 28-diol and lup-20(29)-en-3β-hydroxy-28-oic acid were isolated from the roots of marine mangrove C. tagal which were evaluated for the activation of caspase-3 enzyme using caspase-3 colourimetric assay. Caspase-3 enzyme was activated by all compounds in cleaving pNA from Ac-DEVD-pNA in the presence of caspase-3-inhibitor; Ac-DEVD-CHO39.
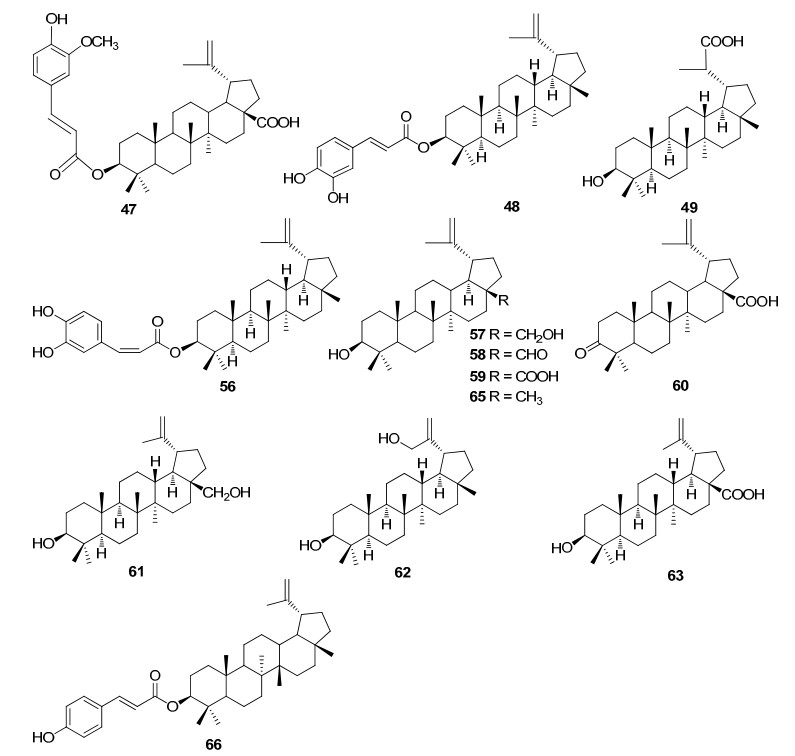
Lupanes from Rhizophoraceae mangroves
2, 6-dimethoxy-p-benzoquinone isolated from R. apiculata was identified as an active constituent component against fungi, bacteria and boll weevils55. Taraxerol and cinnamoyllupeol, are two triterpenoids derived from the leaves and stems of Rhizophora mangle L, were found exhibit insecticidal activity towards Cylas formicarius: one of the most destructive pests of the sweet potato51.
Among the compounds isolated from the leaves of Rhizophora stylosa, taraxerol has been confirmed to have growth inhibitory effects of Hela and BGC-823 with IC50 of 73.4 µmol/L and 73.3 µmol/L, respectively, while cis-careaborin may inhibit the growth of BGC-823 and MCF-7 with IC50 of 45.9 µmol/L and 116.0 µmol/L, respectively. Furthermore, the presence of astilbin and rutin were initially reported to stimulate the proliferation of mice splenic lymphocytes markedly in a dose-dependent manner50. The compounds, (–)-epicatechin, (–)-catechin, 3-O-acetyl-(–)-epicatechin, 3, 7-O-acetyl-(–)-epicatechin, (+)-afzelechin, cinchonain 1b and proanthocyanidin B2 were isolated from the same plant displayed DPPH radical scavenging activity which were comparable to that of the positive control butylated hydroxytoluene (BHT). Proanthocyaninidin B2 showed the strongest activity with IC50 4.3 µg/mL, being four fold greater than the positive control, BHT (IC50 18.0 µg/mL). The antioxidant flavan-3-ol glycosides from R. stylosa showed an increase in their radical scavenging activities with increase in number of catechol moieties present in the molecules60.
Lupanes from Rhizophoraceae mangroves
3.2 Bioactivities of Mangrove Extracts
Various publications have reported the biological activities of mangrove extracts. The components of crude alkaloid mixtures from B. sexangula and B. exarista were identified as tumor inhibitors6. A polysaccharide extracted from the leaves of B. cylindrica, R. apiculata and R. mucronata of Rhizophoraceae along with some other mangrove plants exhibited positive activity against human immunodefiency viruses (HIV)61. All parts of Ceriops decandra have proven antiviral activity61. It also possess promising antibacterial62, antiinflammatory63, and antidiabetic activity64. The leaves and bark extract of C. tagal shows antibacterial activity65. Phenolics are important components of the leaf extract and hypocotyls of K. candel and show excellent antioxidant activities66, 67. Therefore, K. candel can be a good candidate for further development as an antioxidant medicine. During the study on the antibacterial activities of mangrove extracts against two antibiotic resistant pathogenic bacteria Staphylococcus aureus and Proteus sp., it was observed that the ethyl acetate extract of B. Sexangula and R. apiculata also possessed promising antibacterial activity68. This antibacterial activity was also reported in a study showing that gallic acid was extracted from hydrolysable tannin from the barks of R. apiculata. The gallic acid possessed a significant antiyeast (anticandidal) activity towards some yeast species of medical importance. It is anticipated that gallic acid from R. apiculata is a novel antiyeast agent which may be useful in the treatment of candidiasis69. Alcoholic extract of the leaves of Rhizophora apiculata from the mangrove forest of Sunderbans, West Bengal, India were prepared and displayed manglycemic/anti-hyperglycemic activity in streptozotocin induced diabetic rats fed a glucose bolus. The results of this study revealed that this plant extract had potential hypoglycemic action70. The cholinesterase inhibition activity of R. lamarckii was established by Natarajan et. al 2009. The antihyperglycaemic effect of R. mangle was studied71. The leaf extracts of three mangrove plants of Rhizophoraceae family; Rhizophora mucronata, R. apiculata and R. annamalayana were found to have potential antidiabetic capacity due to the presence of an insulin-like protein72. The various studies mentioned, provide scientific support for the use of the mangroves in folklore medicine for the treatment of diabetes.
Oleananes, ursanes and dammaranes from Rhizophoraceae mangroves
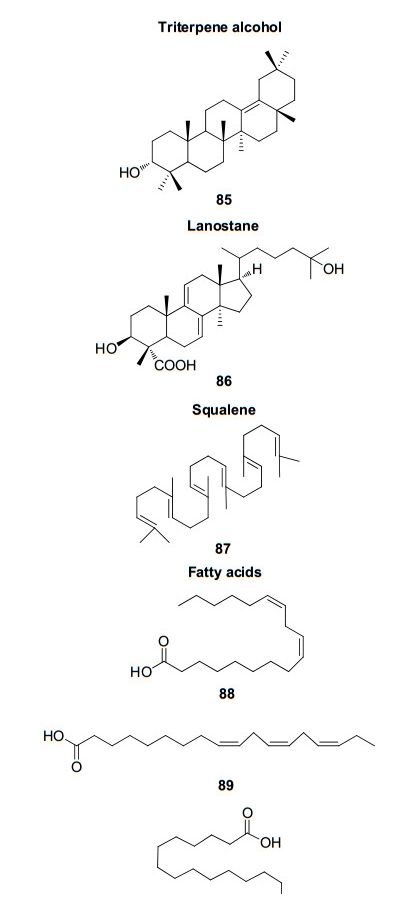
Triterpene alcohol, lanostane, squalene and fatty acids from Rhizophoraceae mangroves
Various mangrove plants were tested for their antioxidant capacity65, 73. It was found that the Rhizophoraceae mangroves showed comparatively higher antioxidant capacity which can be attributed to their higher phenolic content. Additionally, the mangrove plants of Rhizophoraceae family are the source of potent antiviral substances61.
4 Chemotaxonomy
The chemical constituents of mangrove plants of the three true mangrove genera (Rhizophoraceae); Bruguiera, Ceriops and Rhizophora, are the diterpenoid class kauranes exist in the genera Bruguiera and Ceriops, however kauranes are absent in the genus Rhizophora. The genus Bruguiera is characterised by the presence of disulphides and polydisulphides which are unique to the genus. Thus they can be considered as significant chemotaxonamic markers of this genus. Also, it was observed that ent-pimarane coexists with isopimarane in the genus Bruguiera. Interestingly, dolabranes only exist in the genus Ceriops making it a significant chemotaxonomic marker. Similarly, labdane was found only in the genus Rhizophora, making it a significant chemotaxonomic marker of that specific genus.
Furthermore, extensive investigation is needed to identify and classify the chemical constituents of mangrove plants to construct a thorough basis for the chemotaxonamic studies of these versatile plants.
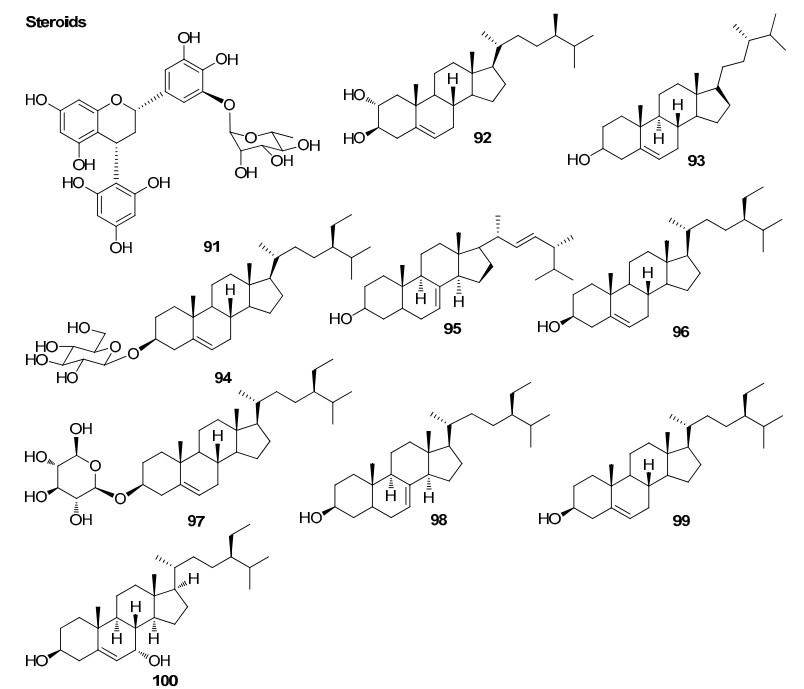
Steroids from Rhizophoraceae mangroves
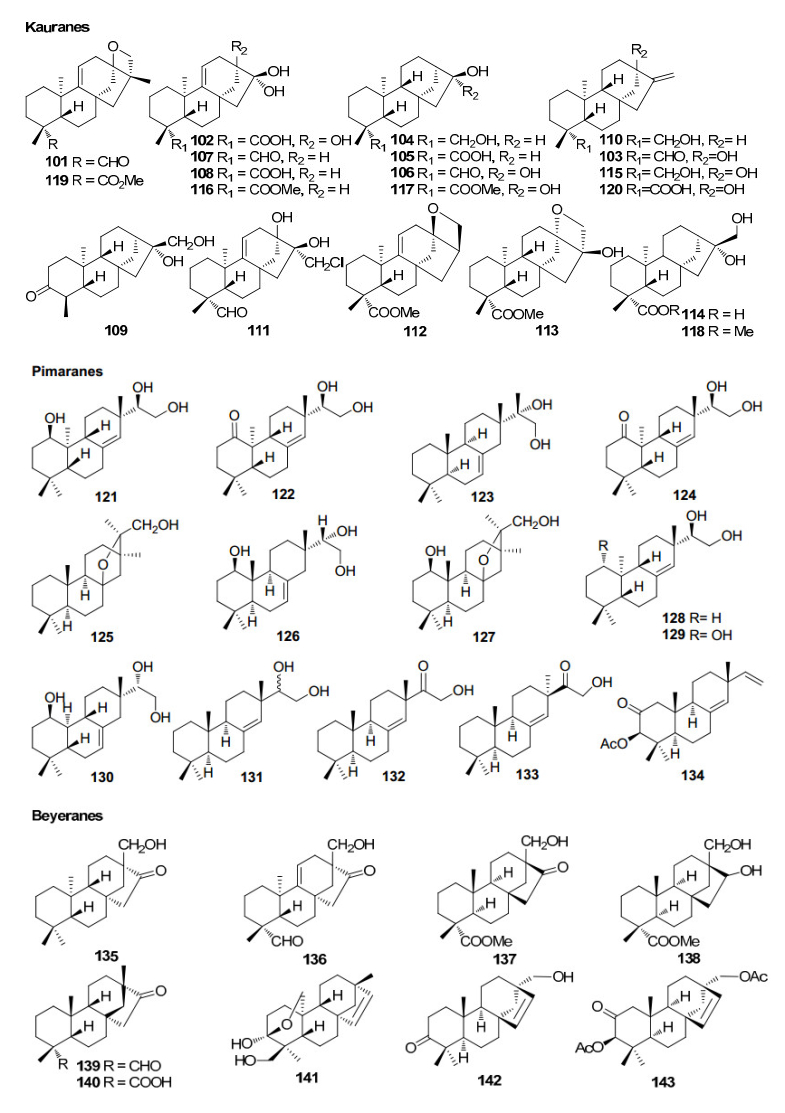
Kauranes, pimaranes and beyeranes (diterpenoids) from Rhizophoraceae mangroves
Sulphur compounds from Rhizophoraceae mangroves
Sulphur compounds from Rhizophoraceae mangroves
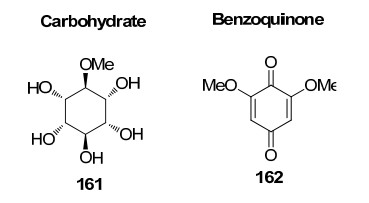
Carbohydrate and benzoquione identified from Rhizophoraceae mangroves
Phenolic compounds from Rhizophoraceae mangroves
Flavonoids from Rhizophoraceae mangroves
Lignans from Rhizophoraceae mangroves
Dolabranes and dimeric diterpenoids from Rhizophoraceae mangroves
Aliphatic alcohols and acids in Rhizophoraceae mangroves
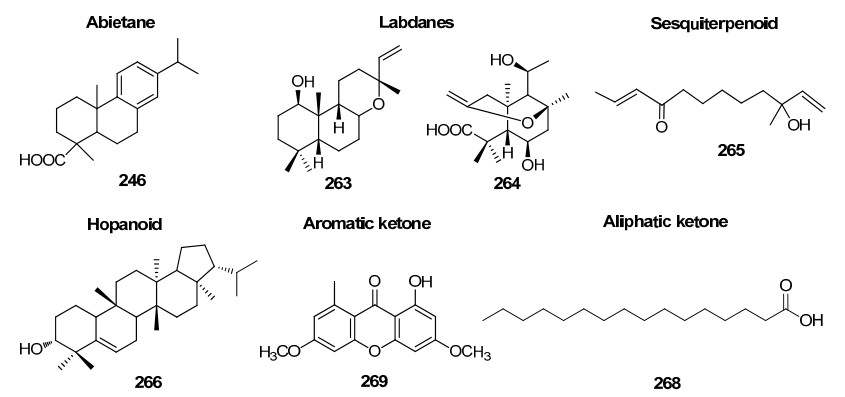
Terpenoids, abietane, labdanes, hopanoid, a sesquitepenoid, aromatic and aliphatic ketones in Rhizophoraceae mangroves
5 Conclusions
In this review, the chemistry and bioactivities of mangroves plants of Rhizophoraceae family have been summarised. Two types of diterpenoids; beyerane and pimarane, and three types of triterpenoids; lupane, oleanane and dammarane, are common chemical constituents ubiquitously found in this family, including 268 metabolites. To date, the chemical constituents from all the mangrove plants of this family have not been investigated. It is clear that mangrove plants can provide a new bank of phytochemical substances that are biologically active substances, with novel structures. It is essential to systematically conserve the biodiversity in the mangrove ecosystem and for the proper of this ecosystem for the future use of humanity.
References
-
1.W. M. Bandaranayake, Wetl. Ecol. Manag. 10, 421-452 (2002) CrossRef PubMed Google Scholar
-
2.J. Wu, Q. Xiao, J. Xu, M. Y. Li, J. Y. Pan, M. H. Yang, Nat. prod. Rep. 25, 955-981 (2008) CrossRef PubMed Google Scholar
-
3.K. Kathiresan, Environ. Ecol. 13, 240-241 (1995) PubMed Google Scholar
-
4.C. Sheue, H. Liu, J. W. H. Yong, Taxon 52, 287-294 (2003) CrossRef PubMed Google Scholar
-
5.C. Sheue, S. M. A. Rashid, J. W. H. Yong, Y. Yang, Taiwania 55, 72-77 (2010) PubMed Google Scholar
-
6.J. W. Loder, G. B. Russell, Aust. J. Chem. 22, 1271-1275 (1969) CrossRef PubMed Google Scholar
-
7.A. Richter, B. Thonke, M. Popp, Phytochemistry 29, 1785-1786 (1990) CrossRef PubMed Google Scholar
-
8.P. Chumkaew, S. Kato, K. Chantrapromma, Chem. Pharm. Bull. 53, 95-96 (2005) CrossRef PubMed Google Scholar
-
9.J. W. Loder, G. B. Russell, Tetrahedron Lett. 51, 6527-6529 (1966) PubMed Google Scholar
-
10.L. Li, C. G. Huang, C. Y. Wang, Y. W. Guo, Nat. Prod. Res. 24, 1044-1049 (2010) CrossRef PubMed Google Scholar
-
11.S. Bao, Z. Deng, H. Fu, P. Proksch, W. Lin, Helv. Chim. Acta 88, 2757-2763 (2005) CrossRef PubMed Google Scholar
-
12.B. SY, W. Lin, Zhongguo Zhong Yao Za Zhi 31, 1168-1171 (2006) PubMed Google Scholar
-
13.S. Bao, Y. Ding, Z. Deng, P. Proksch, W. Lin, Chem. Pharm. Bull. 55, 1175-1180 (2007) CrossRef PubMed Google Scholar
-
14.A. Katu, J. Takahashi, Phytochemistry 5, 220-221 (1975) PubMed Google Scholar
-
15.S. Laphookhieo, C. Karalai, C. Ponglimanont, K. Chantrapromma, J. Nat. Prod. 67, 886-888 (2004) CrossRef PubMed Google Scholar
-
16.C. Karalai, S. Laphookhieo, Aust. J. Chem. 58, 556-559 (2005) CrossRef PubMed Google Scholar
-
17.A. Ghosh, S. Misra, A. K. Dutta, A. Choudary, Phytochemistry 24, 1725-1727 (1985) CrossRef PubMed Google Scholar
-
18.S. Homhual, N. Bunyapraphatsara, T. Kondratyuk, A. Herunsalee, W. Chaukul, J. M. Pezzuto, H. H. S. Fong, H. J. Zhang, J. Nat. Prod. 69, 421-424 (2006) CrossRef PubMed Google Scholar
-
19.S. N. Ganguly, A. Sarkar, Indian J. Chem. B. 16, 742-744 (1978) PubMed Google Scholar
-
20.R. W. Hogg, F. T. Gillan, Phytochemistry 23, 93-97 (1984) CrossRef PubMed Google Scholar
-
21.S. Achamadi, G. Syahbirin, E. T. Choong, W. R. Hemingway, Phytochemistry 35, 217-219 (1994) PubMed Google Scholar
-
22.L. Han, X. Huang, I. Sattler, H. M. Dahse, H. Fu, W. Lin, S. Grabley, J. Nat. Prod. 67, 1620-1623 (2004) CrossRef PubMed Google Scholar
-
23.C. Subrahmanyam, B. V. Rao, R. S. Ward, M. B. Hursthouse, D. E. Hibbs, Phytochemistry 83, 83-90 (1999) PubMed Google Scholar
-
24.A. W. Salae, S. Chantrapromma, H. K. Fun, C. Ponglimanont, Acta Crystallogr. E. 63, 1899-1901 (2007) CrossRef PubMed Google Scholar
-
25.L. Han, X. Huang, I. Sattler, H. M. Dahse, H. Fu, S. Grabley, W. Lin, Pharmazie 60, 705-707 (2005) PubMed Google Scholar
-
26.S. Homhual, H. J. Zhang, N. Bunyapraphatsara, T. Kondratyuk, B. D. Santarsiero, A. D. Mesecar, A. Herunsalee, W. Chaukul, J. M. Pezzuto, H. H. Fong, Planta Med. 72, 255-260 (2006) CrossRef PubMed Google Scholar
-
27.X. Y. Huang, Q. Wang, H. L. Liu, Y. Zhang, G. R. Xin, X. Shen, M. L. Dong, Y. W. Guo, Phytochemistry 70, 2096-100 (2009) CrossRef PubMed Google Scholar
-
28.L. Han, X. Huang, I. Sattler, U. Moellmann, H. Fu, W. Lin, S. Grabley, Planta Med. 71, 160-164 (2005) CrossRef PubMed Google Scholar
-
29.L. Han, X. Huang, I. Sattler, U. Moellmann, H. Fu, W. Lin, S. Grabley, J. Asian Nat. Prod. Res. 9, 327-331 (2007) CrossRef PubMed Google Scholar
-
30.A. S. R. Anjaneyulu, V. L. Rao, Phytochemistry 60, 777-782 (2002) CrossRef PubMed Google Scholar
-
31.A. S. R. Anjaneyulu, V. L. Rao, Phytochemistry 62, 1207-1211 (2003) CrossRef PubMed Google Scholar
-
32.A. S. R. Anjaneyulu, V. L. Rao, E. Lobkovsky, J. Clardy, J. Nat. Prod. 65, 592-594 (2002) CrossRef PubMed Google Scholar
-
33.C. Ponglimanont, P. Thongdeeying, Aust. J. Chem. 58, 615-618 (2005) CrossRef PubMed Google Scholar
-
34.Y. Zhang, Z. Deng, T. Gao, P. Proksch, W. Lin, Phytochemistry 66, 1465-1471 (2005) CrossRef PubMed Google Scholar
-
35.X. W. Ouyang, X. C. Wang, Q. X. Yue, L. H. Hu, Nat. Prod. Commun. 5, 9-12 (2010) PubMed Google Scholar
-
36.J. D. Chen, R. Z. Yi, Y. M. Lin, D. Q. Feng, H. C. Zhou, Z. C. Wang, Int. J. Mol. Sci. 12, 6517-6528 (2011) CrossRef PubMed Google Scholar
-
37.H. K. Fun, C. Pakhathirathien, S. Chantrapromma, C. Karalai, K. Chantrapromma, Acta Crystallogr. E. 62, 5539-5541 (2006) CrossRef PubMed Google Scholar
-
38.W. M. Hu, M. Y. Li, J. Li, Q. Xiao, G. Feng, J. Wu, J. Nat. Prod. 73, 1701-1705 (2010) CrossRef PubMed Google Scholar
-
39.M. Chacha, Int. J. Biol. Chem. Sci. 5, 402-409 (2011) PubMed Google Scholar
-
40.S. Chantrapromma, H. K. Fun, C. Pakhathirathien, C. Karalai, K. Chantrapromma, Acta Crystallogr. E. 63, 459-461 (2007) CrossRef PubMed Google Scholar
-
41.Y. Zhang, Y. Lu, L. Mao, P. Proksch, W. Lin, Org. Lett. 7, 3037-3040 (2005) CrossRef PubMed Google Scholar
-
42.J. D. Chen, Y. Qiu, Z. W. Yang, P. Lin, Y. M. Lin, Helv. Chim. Acta 91, 2292-2298 (2008) CrossRef PubMed Google Scholar
-
43.C. Pakhathirathien, C. Karalai, C. Ponglimanont, S. Subhadhirasakul, K. Chantrapromma, J. Nat. Prod. 68, 1787-1789 (2005) CrossRef PubMed Google Scholar
-
44.X. C. Wang, X. W. Ouyang, L. H. Hu, J. Asian Nat. Prod. Res. 12, 576-581 (2010) CrossRef PubMed Google Scholar
-
45.J. D. Chen, D. Q. Feng, Z. W. Yang, Z. C. Wang, Y. Qiu, Y. M. Lin, Molecules 13, 212-219 (2008) CrossRef PubMed Google Scholar
-
46.F. L. Hsu, G. I. Nonaka, I. Nishioka, Chem. Pharm. Bull. 33, 3142-3152 (1985) CrossRef PubMed Google Scholar
-
47.U. Kokpol, W. Chavasiri, J. Nat. Prod. 53, 953-955 (1990) CrossRef PubMed Google Scholar
-
48.D. L. Li, X. M. Li, B. G. Wang, Nat. Prod. Res. 22, 808-813 (2008) CrossRef PubMed Google Scholar
-
49.S. Laphookhieo, C. Karalai, C. Ponglimanont, Chem. Pharm. Bull. 52, 883-885 (2004) CrossRef PubMed Google Scholar
-
50.X. H. Yang, H. B. Li, H. Chen, P. Li, B. P. Ye, Yao Xue Xue Bao 43, 974-978 (2008) PubMed Google Scholar
-
51.L. Williams, Die Naturwissenschaften 86, 450-452 (1999) CrossRef PubMed Google Scholar
-
52.M. Z. Gao, X. Y. Yuan, M. C. Cheng, H. B. Xiao, S. X. Bao, J. Asian Nat. Prod. Res. 13, 776-779 (2011) CrossRef PubMed Google Scholar
-
53.R. M. Rohini, A. K. Das, Nat. Prod. Res. 24, 197-202 (2010) CrossRef PubMed Google Scholar
-
54.B. V. Rao, C. V. Rao, C. Subrahmanyam, M. A. J. Jairaj, Indian Chem. Soc. 82, 155-157 (2005) PubMed Google Scholar
-
55.U. Kokpol, W. Chavasiri, V. Chittawong, M. Bruce, G. N. Cunningham, D. H. Miles, Phytochemistry 33, 1129-1131 (1993) CrossRef PubMed Google Scholar
-
56.E. Saxena, H. S. Garg, Nat. Product Lett. 4, 149-154 (1994) CrossRef PubMed Google Scholar
-
57.A. S. R. Anjaneyulu, V. L. Rao, Nat. Product Lett. 15, 13-19 (2001) CrossRef PubMed Google Scholar
-
58.A. S. R. Anjaneyulu, V. Anjaneyulu, V. L. Rao, J. Asian Nat. Prod. Res. 4, 53-61 (2002) CrossRef PubMed Google Scholar
-
59.D. L. Li, X. M. Li, Z. Y. Peng, B. G. Wang, Molecules 12, 1163-1169 (2007) CrossRef PubMed Google Scholar
-
60.K. Takara, A. Kuniyoshi, K. Wada, K. Kinjyo, H. Iwasaki, Biosci. Biotech. Biochem. 72, 2191-2194 (2008) CrossRef PubMed Google Scholar
-
61.M. Premanathan, K. Kathiresan, H. Nakashima, South Pacific Study 19, 49-57 (1999) PubMed Google Scholar
-
62.M. Chandrasekaran, K. Kannathasan, V. Venkatesalu, K. Prabhakar, World J. Microb. Biotech. 25, 155-160 (2008) PubMed Google Scholar
-
63.H. Hossain, Sk. Moniruzzaman, I. Nimmi, H. Kawsar, A. Hossain, A. Islam, I. A. Jahan, Oriental Pharm. Exp. Med. 11, 215-220 (2011) CrossRef PubMed Google Scholar
-
64.M. A. Nabeel, K. Kathiresan, S. J. Manivannan, Diabetes 2, 97-103 (2010) CrossRef PubMed Google Scholar
-
65.N. Arivuselvan, D. Silambarasan, T. Govindan, K. Kathiresan, Adv. Biol. Res. 5, 251-254 (2011) PubMed Google Scholar
-
66.L. L. Zhang, Y. M. Lin, H. C. Zhou, S. D. Wei, J. H. Chen, Molecules 15, 420-31 (2010) CrossRef PubMed Google Scholar
-
67.S. D. Wei, H. C. Zhou, Y. M. Lin, Int. J. Mol. Sci. 11, 4080-4093 (2010) CrossRef PubMed Google Scholar
-
68.P. D. Abeysinghe, Indian J. Pharm. Sci. 72, 167-172 (2010) CrossRef PubMed Google Scholar
-
69.L. S. Hong, D. Ibrahim, J. Kassim, S. Sulaiman, J. Appl. Pharm. Sci. 1, 75-79 (2011) PubMed Google Scholar
-
70.T. K. Sur, T. Seal, S. Pandit, D. Bhattacharya, Nat. Prod. Sci. 10, 11-15 (2004) PubMed Google Scholar
-
71.F. J. Alarcon-Aguilara, R. Roman-Ramos, S. Perez-Gutierrez, A. Aguilar-Contreras, C. C. Contreras-Weber, J. L. Flores-Saenz, J. Ethnopharmacol. 61, 101-110 (1998) CrossRef PubMed Google Scholar
-
72.A. M. Nabeel, K. Kathiresan, M. Chinthamani, S. Manivannan, Nat. Prod. Res. 26, 1161-1166 (2012) CrossRef PubMed Google Scholar
-
73.G. Agoramoorthy, F. A. Chen, V. Venkatesalu, D. H. Kuo, P. C. Shea, Asian J. Chem. 20, 1311-1322 (2008) PubMed Google Scholar
Copyright information
© The Author(s) 2013
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.