Induced biosyntheses of a novel butyrophenone and two aromatic polyketides in the plant pathogen Stagonospora nodorum
Abstract
Fungal aromatic compounds comprise an important and structurally diverse group of secondary metabolites. Several genome sequencing projects revealed many putative biosynthetic gene clusters of fungal aromatic compounds, but many of these genes seem to be silent under typical laboratory culture conditions. To gain access to this untapped reservoir of natural products, we utilized chemical epigenetic modifiers to induce the expression of dormant biosynthetic genes. As a result, the concomitant supplementation of the histone deacetylase inhibitors suberoylanilide hydroxamic acid (500 μM) and nicotinamide (50 μM) to the culture medium of a fungal pathogen, Stagonospora nodorum, resulted in the isolation of three aromatic compounds (1-3), including a novel natural butyrophenone, (+)-4'-methoxy-(2S)-methylbutyrophenone (1), and two known polyketides, alternariol (2) and (-)-(3R)-mellein methyl ether (3).Keywords
Stagonospora nodorum butyrophenone polyketides epigenetic manipulationIntroduction
Fungal aromatic compounds are structurally and functionally diverse, as exemplified by the mycotoxin aflatoxin, the pigment emodin, and the antifungal drug griseofulvin.1-3 Some of them are produced by polyketide synthases (PKSs), and have been linked to their biosynthetic genes. However, there are a large number of PKS genes with products that have not been identified in several fungal genome sequences, indicating that numerous cryptic biosynthetic pathways of natural products remain to be discovered. Stagonospora nodorum is a destructive wheat pathogen.4 It belongs to Dothideomycetes, a class of filamentous fungi that includes many plant pathogens, and thus important as a model for pathology study. Previous studies aiming toward the comprehension of plant diseases demonstrated the critical roles of host-specific proteinaceous toxins and various primary metabolic pathways for effective fungal pathogenicity.5-9 Nevertheless, only a few papers regarding the capacity of this strain to produce secondary metabolites have been published, except for one mycotoxin, alternariol.8 Recently, the whole genome sequence of S. nodorum revealed that it contains 19 genes encoding PKSs, 8 genes encoding non-ribosomal peptide synthetases (NRPSs), and one gene encoding a PKS-NRPS hybrid, to produce cryptic natural products that outnumber the currently known metabolites from this fungus.10 However, many of these putative biosynthetic genes seem to be silent under a variety of laboratory culturing conditions. To trigger the expression of these gene clusters, an effective method is to use epigenetic modifying agents, including histone deacetylase (HDAC) and DNA methyltransferase inhibitors, for manipulating the fungal epigenome.11 In our study, screening of the secondary metabolites produced by S. nodorum, grown in the presence of these inhibitors, revealed that the concomitant supplementation of the HDAC inhibitors to the culture medium induced significant changes in the production of metabolites. This finding led to the isolation of a novel natural butyrophenone, (+)-4'-methoxy-(2S)-methylbutyrophenone (1), along with two known aromatic polyketides, alternariol (2) and (–)-(3R)-mellein methyl ether (3). Details of the isolation and structural elucidation of 1–3 are reported herein.
Results and Discussion
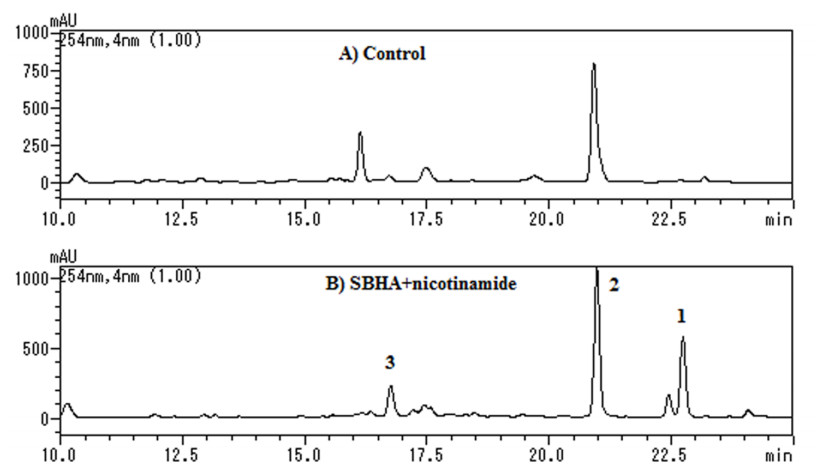
S. nodorum FGSC 10173 was incubated in 100 mL of potato dextrose broth (PDB), in the presence and absence of 500 μM SBHA and 50 μM nicotinamide. The ethyl acetate extract of the culture filtrate was analyzed by high performance liquid chromatography (HPLC). As a result, the production of two new products (1 and 3) was induced, and the intensity of one peak for compound 2 was enhanced (Figure 1).
HPLC analyses of culture broths of S. nodorum: (A) Incubated in PDB, (B) Incubated in PDB containing SBHA (500 μM) + nicotinamide (50 μM). Elutions were monitored by UV absorption at 254 nm.
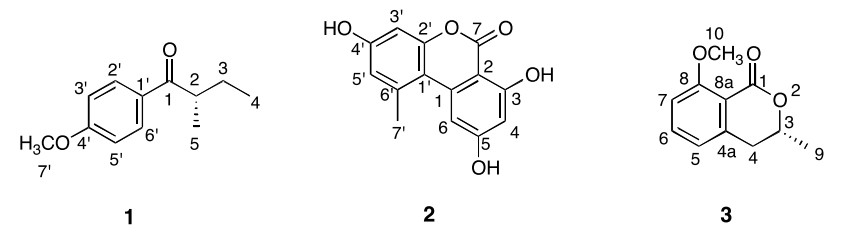
Compound 1 was obtained as a white solid. Its molecular formula was established as C12H16O2, by a pseudomolecular ion peak at m/z 193.1234 [M + H]+ (calcd for C12H17O2, 193.1223) in the positive HRESIMS, with 5 degrees of unsaturation. The 1H NMR analysis revealed the presence of a 1, 4-disubstituted aromatic ring (δH 7.71 (2H, d, J = 8.5 Hz), 6.80 (2H, d, J = 8.5 Hz)), three methyl groups (δH 3.85 (3H, s), 1.25 (3H, d, J = 6.5 Hz), 0.89 (3H, t, J = 7.5 Hz)), one methine group (δH 3.15 (1H, m)), and one methylene group (δH 1.60 (1H, m), 1.85 (1H, m)). Analyses of its 13C NMR, HSQC and 1 H-1H COSY data further revealed the presence of a 1, 4-disubstituted aromatic ring (δC 128.0 (s, C-1'), 132.7 (d, C-2', and 6'), 114.9 (d, C-3', and 5'), 163.3 (s, C-4')), one methoxy carbon (δC 52.9 (q, C-7')), and a 2-methylbutan-1-one moiety (δC 191.5 (s, C-1), 36.5 (d, C-2), 27.8 (t, C-3) 11.0 (q, C-4), 17.4 (q, C-5)). Further detailed interpretation of the HMBC correlations from H-2' and H-6' to C-1, and from H-7' to C-4', allowed us to assign the methoxy group and the 2-methylbutan-1-one moiety to C-4' and C-1', respectively. Finally, compound 1 was identified as (+)-4'-methoxy-(2S)-methylbutyrophenone (Figure 2), which was previously reported as a synthetic compound.12 The absolute configuration of C-2 was determined to be S, by comparing its optical rotation to the reported value.12
The structures of compounds 1–3
Compound 2 was obtained as a white solid. Its molecular formula was established as C14H10O5, by a pseudomolecular ion peak at m/z 259.0612 [M + H]+ (calcd for C14H11O5, 259.0601) in the positive HRESIMS. Analyses of its 13C NMR and HSQC data revealed 14 carbon resonances, including one ester carbonyl carbon, four aromatic methine carbons, eight aromatic quaternary carbons, and one methyl carbon. The 1H NMR spectrum also displayed four sp2 methines (δH 6.34 (1H, d, J = 1.7 Hz, H-4), 7.21 (1H, d, J = 1.7 Hz, H-6), 6.61 (1H, d, J = 2.3 Hz, H-3'), 6.69 (1H, d, J = 2.3 Hz, H-5')), featuring two 1, 2, 3, 5-tetrasubstituted aromatic rings deduced from their coupling constants, and one methyl (δH 2.68 (3H, s, H-7')). Thus, its NMR data were identical to those of alternariol, a known mycotoxin metabolite from the mold Alternaria tenuis.13
Compound 3 was obtained as a white solid. Its molecular formula was established as C11H12O3, by a pseudo-molecular ion peak at m/z 193.0837 [M + H]+ (calcd for C11H13O3, 193.0859) in the positive HRESIMS. Analysis of the 1H NMR spectrum revealed the presence of 1, 2, 3-trisubstituted aromatic ring signals (δH 6.85 (1H, d, J = 7.5 Hz, H-5), 7.47 (1H, dd, J = 8.5, 7.5 Hz, H-6), 7.00 (1H, d, J = 8.5 Hz, H-7)), one oxygenated methine (δH 4.45 (1H, m, H-3)), one methylene (δH 2.87 (2H, m, H-4)), and two methyls (δH 1.31 (3H, d, J = 7.0 Hz, H-9), 3.77 (3H, s, H-10)). The 13C NMR spectrum revealed 11 carbon signals, due to one ester carbonyl, three aromatic methine carbons, three aromatic quaternary carbons, one oxygenated methine carbon, one methylene carbon, and two methyl carbons. A comparison of the NMR data and the optical rotation value revealed that 3 is identical to (–)-(3R)-mellein methyl ether, previously isolated from the mangrove-derived fungus Xylaria cubensis (Figure 2).14
Compounds 1–3 were tested for their antifungal activities against three fungal strains, including Fusarium solani, Aspergillus terreus and Candida albicans.15 The results showed that these three compounds displayed moderate activities against the three tested fungal strains (Table 1).
Antifungal effect of compounds 1–3
Although S. nodorum contains 19 genes encoding PKSs, few polyketides were discovered from this fungus. Interestingly, these PKS gene clusters are dormant under normal culture conditions, but the concomitant supplementation of the HDAC inhibitors SBHA (500 μM) and nicotinamide (50 μM) to the culture medium of S. nodorum facilitated the production of three aromatic compounds (1–3). Upon further isolation, we identified the novel natural butyrophenone (1), together with two known aromatic polyketides (2 and 3). Our findings confirmed that chemical epigenetic manipulation is an effective strategy to access the biosynthetic potential of this fungus.
The novel (+)-4'-methoxy-(2S)-methylbutyrophenone (1) could be derived from the polyketide biosynthetic pathway. A related compound, 1-(2, 4, 6-trihydroxyphenyl)butan-1-one, was isolated from the endophytic fungus Paeonia delavayi, 16 which led us to assume that the phloroglucinol precursor is possibly converted into the para-substituted phenol scaffold of 1 by reductases. Furthermore, the (2S)-methylbutyryl moiety of 1 could be generated by a diketide-producing PKS, as in the case of the diketide side chain formation by LovF in lovastatin biosynthesis, 17 and then transferred to another PKS that catalyzes three chain extensions and a Dieckmann condensation, to produce the phloroglucinol scaffold. In fact, we found SNOG_09623, encoding a PKS with the same domain organization as those of LovF, and SNOG_09622, encoding a type Ⅲ PKS, in the S. nodorum genome. The two genes are clustered in the chromosome, but without any methyltransferase gene nearby. The polyketide chain transfer between two PKSs was reported for the bacterial PKS system of the alkylresorcinol and alkylpyrone biosynthesis in Azotobacter vinelandii18, and for the formation of phlorocaprophenone in Dictyostellium discoidium (type Ⅲ PKS Steely 2).19
On the other hand, although (–)-(3R)-mellein methyl ether (3) is widely distributed in fungi, its biosynthetic enzymes have not been reported. Recently, SACE5532 from the actinobacterium, Saccharopolyspora erythraea, which consists of ketosynthase (KS), acyltransferase (AT), thioester hydrolase (TH), and ketoreductase (KR) domains, was identified as a PKS that produces (R)-(–)-mellein.20 Therefore, a PKS with identical domains to those of SACE5532 in S. nodorum should be a candidate for a mellein PKS. Two PKS genes exist in S. nodorum, SNOG_00477 and SNOG_14927, and they encode PKSs with identical domain structures to those of SACE5532. Thus, one of them is likely to be the PKS that generates mellein. This information illustrates the application of chemical epigenetic manipulation to a genomesequenced strain, to provide helpful information toward identifying biosynthetic genes.
Experimental Section
HPLC Analysis of the Compounds Accumulated in the Broth of S. nodorum.S. nodorum FGSC 10173 was obtained from the Fungal Genetic Stock Center. The fungal strain was cultured on potato dextrose agar (PDA) at 28 ℃ for 7 days, and then inoculated into 100 mL of PDB containing 500 μM SBHA and/or 50 μM nicotinamide in 500 mL Erlenmeyer flasks, under shaking conditions at 28 oC for 7 days. The culture broths were extracted three times with ethyl acetate. After evaporation in vacuo, the crude extracts were subjected to an HPLC analysis. The HPLC analysis was performed at a flow rate of 0.8 mL/min on an ODS column (Cosmosil 5C18 PAQ Waters, 4.6 × 250 mm), with a mixture of H2O and CH3CN (0–30 min, 10–100% CH3CN; 30–50 min, 100% CH3CN; 50–55 min, 100–10% CH3CN).
Isolation of Compounds (1–3).To isolate the induced compounds, S. nodorum was inoculated in 20 × 100 mL of PDB, containing 500 μM SBHA and 50 μM nicotinamide, in 500 mL Erlenmeyer flasks under shaking conditions at 28 oC for 7 days. The culture broths were extracted three times with ethyl acetate. After evaporation in vacuo, the crude extract was directly separated by preparative HPLC (Shimadzu, LC-20AD and SPD-20A Prominence Diode Array Detector), using a Cosmosil 5C18-PAQ Waters column (10 × 250 mm, Nacalai Tesque, Kyoto) eluted with a mixture of H2O (solvent A) and CH3CN (solvent B), both containing 0.1% acetic acid, at a flow rate of 2.5 mL/min. The separation performance was equivalent to that of the analytical method, which led to the isolation of 1 (1.28 mg, tR = 21.1 min, 35% CH3CN over 15 min), 2 (2.28 mg, tR = 17.8 min, 35% CH3CN over 15 min), and 3 (6.96 mg, tR = 16.7 min, 35% CH3CN over 15 min).
Antifungal Activity Assays.Antifungal bioassays were conducted according to the previously reported procedure.15 The fungal strains, Fusarium solani, Aspergillus terreus and Candida albicans, were grown on PDA. Targeted microbes (3–4 colonies) were prepared from broth cultures (28 oC for 72 h), and the final fungal spore suspensions (in PDB) were 104 mycelial fragments/mL. Test samples (10 mg/mL as a stock solution in DMSO and serial dilutions) were transferred to a 96-well clear plate in triplicate, and the suspension of the test organisms was added to each well, to achieve a final volume of 100 μL (ketoconazole was used as the positive control). After incubation, the minimum inhibitory concentration (MIC) was defined as the lowest test concentration that completely inhibited the growth of the test organisms.
(+)-4'-Methoxy-(2S)-methylbutyrophenone (1):white solid; [α]24 D + 7.5 (c 0.07, MeOH); UV (MeOH) λmax (log ε) 265, 284, 315 nm; 1H NMR (CD3OD, 500 MHz): δ 0.89 (3H, t, J = 7.5 Hz, H-4), 1.25 (3H, d, J = 6.5 Hz, H-5), 1.60 (1H, m, H-3a), 1.85 (1H, m, H-3b), 3.15 (1H, m, H-2), 3.85 (3H, s, H-7'), 6.80 (2H, d, J = 8.5 Hz, H-3' and 5'), 7.71 (2H, d, J = 8.5 Hz, H-2' and 6'); 13C NMR (125 MHz, CD3OD): δ 191.5 (s, C-1), 163.3 (s, C-4'), 132.7 (d, C-2' and 6'), 128.0 (s, C-1'), 114.9 (d, C-3' and 5'), 52.9 (q, C-7'), 36.5 (d, C-2), 27.8 (t, C-3), 17.4 (q, C-5), 11.0 (q, C-4). ESIMS (positive) m/z 193 [M + H]+; HRESIMS m/z 193.1234 (calcd for C12H17O2, 193.1223).
Alternariol (2): white solid; UV (MeOH) λmax (log ε) 255, 297, 335 nm; 1H NMR (DMSO-d6, 500 MHz): δ 2.68 (3H, s, H-7'), 6.34 (1H, d, J = 1.7 Hz, H-4), 6.61 (1H, d, J = 2.3 Hz, H-3'), 6.69 (1H, d, J = 2.3 Hz, H-5'), 7.21 (1H, d, J = 1.7 Hz, H-6); 13C NMR (125 MHz, DMSO-d6): δ 166.3 (s, C-5), 165.2 (s, C-7), 164.6 (s, C-3), 159.0 (s, C-4'), 153.2 (s, C-2'), 138.8 (s, C-6'), 138.1 (s, C-1), 118.1 (d, C-5'), 109.5 (s, C-1'), 105.0 (d, C-6), 102.1 (d, C-3'), 101.5 (d, C-4), 97.8 (s, C-2), 25.8 (q, C-7'). ESIMS (positive) m/z 259 [M + H]+. HRESIMS m/z 259.0612 (calcd for C14H11O5, 259.0601).
(–)-(3R)-Mellein methyl ether (3): white solid; [α]D24 – 324.0 (c 0.52, MeOH); UV (MeOH) λmax 243, 306 nm; 1H NMR (DMSO-d6, 500 MHz): δ 1.31 (3H, d, J = 7.0 Hz, H-9), 2.87 (2H, m, H-4), 3.77 (3H, s, H-10), 4.45 (1H, m, H-3), 6.85 (1H, d, J = 7.5 Hz, H-5), 7.00 (1H, d, J = 8.5 Hz, H-7), 7.47 (1H, dd, J = 8.5, 7.5 Hz, H-6); 13C NMR (125 MHz, CD3OD): δ 166.4 (s, C-1), 163.5 (s, C-8), 144.8 (s, C-4a), 137.3 (d, C-6), 121.6 (d, C-5), 115.1 (s, C-8a), 113.2 (d, C-7), 77.1 (d, C-3), 57.4 (q, C-10), 37.6 (t, C-4), 21.8 (q, C-9); ESIMS (positive) m/z 193 [M + H]+; HRESIMS m/z 193.0837 (calcd for C11H13O3, 193.0859).
Notes
Acknowledgements
This work was financially supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan, and by programs from the National Natural Science Foundation Province of China (21202033), the Natural Science Foundation of Hebei (C2012201047), and the Foundation of Hebei University (179).
References
-
1.N. P. Keller, G. Turner, J. W. Bennett, Nat. Rev. Microbiol. 3, 937-947 (2005) CrossRef PubMed Google Scholar
-
2.C. Hertweck, A. Luzhetskyy, Y. Rebets, A. Bechthold, Nat. Prod. Rep. 24, 162-190 (2007) CrossRef PubMed Google Scholar
-
3.H. Zhou, Y. Li, Y. Tang, Nat. Prod. Rep. 27, 839-868 (2010) CrossRef PubMed Google Scholar
-
4.J. S. Bhathal, R. Loughman, J. Speijers, Eur. J. Plant Pathol. 109, 435-443 (2003) CrossRef PubMed Google Scholar
-
5.T. L. Friesen, C. G. Chu, Z. H. Liu, S. S. Xu, S. Halley, J. D. Faris, Theor. Appl. Genet. 118, 1489-1497 (2009) CrossRef PubMed Google Scholar
-
6.T. L. Friesen, Z. C. Zhang, P. S. Solomon, R. P. Oliver, J. D. Faris, Plant Physiol. 146, 682 (2008) PubMed Google Scholar
-
7.R. G. T. Lowe, M. Lord, K. Rybak, R. D. Trengove, R. P. Oliver, P. S. Solomon, Fungal Genet. Biol. 45, 1479-1486 (2008) CrossRef PubMed Google Scholar
-
8.K. C. Tan, R. D. Trengove, G. L. Maker, R. P. Oliver, P. S. Solomon, Metabolomics 5, 330-335 (2009) CrossRef PubMed Google Scholar
-
9.S. V. S. IpCho, K. C. Tan, G. Koh, J. Gummer, R. P. Oliver, R. D. Trengove, P. S. Solomon, Eukaryot. Cell 9, 1100-1108 (2010) CrossRef PubMed Google Scholar
-
10.J. K. Hane, R. G. T. Lowe, P. S. Solomon, K. C. Tan, C. L. Schoch, J. W. Spatafora, P. W. Crous, C. Kodira, B. W. Birren, J. E. Galagan, S. F. F. Torriani, B. A. McDonald, R. P. Oliver, The Plant Cell 19, 3347-3368 (2007) CrossRef PubMed Google Scholar
-
11.R. H. Cichewicz, Nat. Prod. Rep. 27, 11-22 (2010) CrossRef PubMed Google Scholar
-
12.O. Korver, Tetrahedron 27, 4643-4651 (1971) CrossRef PubMed Google Scholar
-
13.E. E. Stinson, W. B. Wise, R. A. Moreau, A. J. Jurewicz, P. E. Pfeffer, Can. J. Chem. 64, 1590-1594 (1986) CrossRef PubMed Google Scholar
-
14.S. Klaiklay, V. Rukachaisirikul, Y. Sukpondma, S. Phongpaichit, J. Buatong, B. Bussaban, Arch. Pharm. Res. 35, 1127-1131 (2012) CrossRef PubMed Google Scholar
-
15.D. T. Wicklow, B. K. Joshi, W. R. Gamble, J. B. Gloer, P. F. Dowd, Appl. Environ. Microbiol. 64, 4482-4484 (1998) PubMed Google Scholar
-
16.J. Hu, J. Wang, C. Miao, Q. Xuan, Y. Zhai, F. Song, Y. Chen, S. Wu, Zhongguo Zhong Yao Za Zhi 37, 1602-1606 (2012) PubMed Google Scholar
-
17.J. Kennedy, K. Auclair, S. G. Kendrew, C. Park, J. C. Vederas, C. R. Hutchinson, Science 284, 1368-1372 (1999) CrossRef PubMed Google Scholar
-
18.A. Miyanaga, N. Funa, T. Awakawa, S. Horinouchi, Proc. Natl. Acad. Sci. U.S.A. 105, 871-876 (2008) CrossRef PubMed Google Scholar
-
19.M. B. Austin, T. Saito, M. E. Bowman, S. Haydock, A. Kato, B. S. Moore, R. R. Kay, J. P. Noel, Nat. Chem. Biol. 2, 494-502 (2006) CrossRef PubMed Google Scholar
-
20.H. Sun, C. L. Ho, F. Ding, I. Soehano, X. W. Liu, Z. X. Liang, J. Am. Chem. Soc. 134, 11924-11927 (2012) CrossRef PubMed Google Scholar
Copyright information
© The Author(s) 2013
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.