Chemical components of Dendrobium crepidatum and their neurite outgrowth enhancing activities
Abstract
15 compounds, including two new ones crepidatuols A (1) and B (2) were isolated from the stems of Dendrobium crepidatum. The planar structures of these compounds were elucidated by spectroscopic methods (NMR, MS, UV, and IR) and comparison with those from literatures. 10 compounds were send for enhancing activities on nerve growth factor (NGF) medicated neurite outgrowth in PC12 cells and the results indicated that crepidatuol A (1), confusarin and 3-(2-acetoxy-5-methoxy)-phenylpropanol showed enhancing activities at the concentration of 10.0 μM.Keywords
Orchidaceae Dendrobium crepidatum bibenzyl neurite outgrowth enhancing activityIntroduction
The stems of several Dendrobium species (Orchidaceae) are used as "Shi-Hu" in traditional Chinese medicine for a long time for the purpose of being beneficial to the stomach and promoting the production of body fluid, nourishing yin and clearing heat.1, 2 Inspired by this theory, "Shi-Hu" is mainly used for thrombotic diseases and health care especially for those older. D. crepidatum belongs to Sect. Dendrobium and the stems of this species used as a biological source of "ShiHu" and its flowers are very beautiful and get the name "Rosa Shi-Hu" in Chinese.3 Alkaloidal part of this species has been detected by Elander etc. and five octahydroindolizine alkaloids have been isolated and elucidated.4 To continue our research on "Shi-Hu"5 and provide chemical evidence for this traditional Chinese medicine, D. crepidatum was selected as our plant material and 15 compounds, including two new compounds, crepidatuols A (1) and B (2) (Figure 1) were isolated and elucidated herein, furthermore, 10 compounds were sent to assay their neurite outgrowth enhancing activities and the results indicated that three of them showed neuroprotective effects.
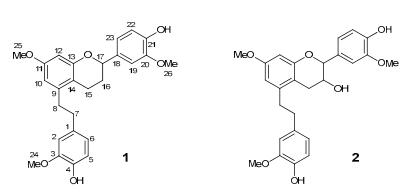
Structures of compounds 1 and 2
Results and Discussion
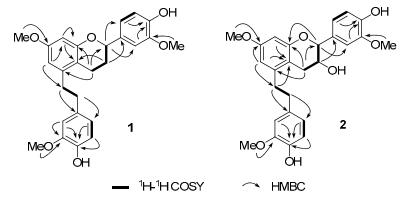
Compound 1 was obtained as yellow oil. Its molecular formula was established as C26H28O6 by the HREIMS showing a molecular ion peak at m/z 436.1882 (calcd. for C26H28O6 436.1886), indicating thirteen degrees of unsaturation. The UV spectra at λmax (log ε): 282.5 (3.9) nm indicated an extended conjugated system. The 1H and 13C NMR spectra data of 1 (Table 1) showed the existence of three methoxy groups, four aliphatic methylenes, one aliphatic methine and three aromatic rings. Two methylene carbons at δC 36.2 (C-7) and 35.0 (C-8) correlated with four benzylic protons at δH 2.82 (4H, m, H-7 and H-8) in the HSQC spectrum of 1 indicated a bibenzyl nucleus, 6 which was confirmed by the correlations [H-7, H-8/C-1, C-2, C-6, C-9, C-10, C-14] in the HMBC spectrum (Figure 2). Three aromatic hydrogen at δH 6.63 (1H, d, 1.8), 6.85 (1H, d, 8.1) and 6.71 (1H, dd, 8.1, 1.8) indicated a 1, 3, 4-trisubstituted phenyl, moreover, two others at δH 6.42 (1H, d, 2.5) and 6.39 (1H, d, 2.5) showed another phenyl with two meta-coupling hydrogen for the bibenzyl nucleus. The correlations [H-16/H-15, H-17] in the COSY spectrum indicated a structural moiety of -CH2-CH2-CH-(C-15, C-16, C-17), furthermore, the correlations [H-15/C-14 and C-13, H 16/C-14 and C-18, H-17/C-18, C-19 and C-23] in the HMBC spectrum indicated that the partial structure and the related aromatic ring were linked with the bibenzyl nucleus as trigonopol B5a except hydride reduction at C-16. Three methoxy groups could be observed that δH 3.92 (3H, s) belongs to δC 55.9, δH 3.76 (3H, s) belongs to δC 55.2, δH 3.86 (3H, s) belongs to δC 55.8 in the HSQC spectrum respectively, furthermore, cross peaks of δH 3.92 (3H, s) with δC 146.6, δH 3.86 (3H, s) with δC 146.2, δH 3.76 (3H, s) with δC 158.6 in the HMBC spectrum determined the position of three methoxy groups as shown in Figure 1. Thus, compound 1 was identified as 4-(5-(4-hydroxy-3-methoxyphenethyl)-7-methoxychroman-2-yl)-2-methoxyphenol, named as crepidatuol A.
1H and 13C NMR data (500/125 MHz, δ in ppm, J in Hz) for compounds 1 and 2 in CDCl3
Selected 1H-1H COSY and HMBC correlations of compounds 1 and 2
Compound 2 was obtained as yellow oil, its molecular formula was determined as C26H28O7 by the HRESIMS (m/z 452.1832 [M]+, calcd. for 452.1835), with thirteen degrees of unsaturation. The 13C NMR and DEPT spectrum exhibited 26 carbon signals, including three methoxy, three aliphatic methylenes, two aliphatic methines and three aromatic rings. The 1H and 13C NMR data of 2 (Table 1) were similar to those of trigonopol B5a as compound 1, indicating a similar bibenzyl core for compound 2. The threo-isomer (6–8 Hz) is larger than in the erythro-isomer (2–4 Hz)5e, from the chemical shifts and the coupling constants (J = 8.7) of compound 2 and trigonopol B (J = 6.4), it could be predicated that relative stereochemistry of C-16 and C-17 were similar in two compounds. One more methoxy in 2 than trigonopol B and correlations [H-24/C-3, H-25/C-11, H-26/C-20] in the HMBC spectrum (Figure 2) indicated that compound 2 was 5-(4-hydroxy-3-methoxyphenethyl)-2-(4-hydroxy-3-methoxyphenyl)-7-methoxychroman-3-ol as shown in figure 1, named as crepidatuol B.
The 13 known compounds were elucidated as erianin, 7 crepidatin, 8 moscatilin, 8 hircinol, 9 confusarin, 10 syringaldazine, 11 3-(2-acetoxy-5-methoxy)phenylpropanol, 11 kaempferol, 12 4, 6-dihydroxyisobenzofuran-1(3H)-one, 13 ergostan-7, 9, 22-trien-3, 5-diol, 14 ergosterol, 15 β-sitosterol and daucosterol by comparing with those from literature and TLC means.
For the purpose of evaluating the effect of neuroprotective effects of this species, the isolated 10 compounds including crepidatuol A (1), erianin, crepidatin, moscatilin, confusarin, 3-(2-acetoxy-5-methoxy)-phenylpropanol, kaempferol, 4, 6-dihydroxyisobenzofuran-1(3H)-one, ergostan-7, 9, 22-trien-3, 5-diol and ergosterol were evaluated for their enhancing activity on NGF-induced (nerve growth factor, R & D Systems Inc., 97%) neurite outgrowth in PC12 cells.16 The results indicated that the proportion of the NGF-induced (10 ng/mL) neurite-bearing cells were enhanced by crepidatuol A (1, 8.5%), confusarin (7.1%) and 3-(2-acetoxy-5-methoxy)-phenylpropanol (6.8%) at the concentration of 10 μM (Table 2). Others showed no activity on 10 ng/mL NGF-induced neurite outgrowth in PC12 cells at 10.0 μM.
The percentages of the neurite-bearing cells after 72 h incubation with compounds crepidatuol A, confusarin and 3-(2-acetoxy-5-methoxy)-phenylpropanol
Experimental Section
General Experimental Procedures
Optical rotations were measured using a JASCODIP-370 polarimeter. UV spectra were obtained with a Hitachi UV-3210. IR spectra were recorded on a Bio-Rad BRUKER TENSOR27 IR spectrophotometer as KBr disks. NMR spectra were run on Bruker AVANCE 400 NMR spectrometer (400 MHz for 1H NMR, 100 MHz for 13C NMR) and DRX-500 NMR spectrometer (500 MHz for 1H NMR, 125 MHz for 13C NMR) with TMS as internal standard. Mass spectra were performed on a AutoSpec Premier P776 mass spectrometer. Column chromatography was carried out on silica gel H60 (Qingdao Haiyang Chemical Group Corp., Qingdao, China), Sephadex LH-20 (Amersham Biosciences), and ODS (40–63 μm, Merck) as packing materials. Silica gel G was used for analytical TLC.
Plant Material
The fresh stems of D. crepidatum were collected in Yunnan Province in 2008 and identified by Professor Hong Yu of Yunnan University. A voucher specimen (Zsh-8) was deposited at the State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Science, China.
Extraction and Isolation
The air-dried stems of D. crepidatum (15 kg) were refluxed three times with 95% EtOH. After evaporation of solvent in vacuo, the residue was suspended in H2O and adjusted pH to 2 with hydrochloric acid. Then partitioned with chloroform and non-alkaloid fraction (1000 g) were obtained. Then the non-alkaloid fraction was subjected to column chromatography on silica gel (200–300 mesh, 6 kg) and eluted with petroleum ether/acetone (6:4→0:10) to afford 7 fractions. Fraction B (120g) was applied repeatedly to column chromatography over silica gel (petroleum ether/acetone, 10:1 to 1:1) and got 13 (267 mg), 14 (259 mg) and 16 (1.1 g), then residue were purified with Sephadex LH-20 (CHCl3-MeOH, 1:1) to afford compounds 1 (29 mg), 4 (1.73 g), 9 (9 mg) and 10 (11 mg). Fraction C (62.7 g) was chromatographed on silica gel (petroleum ether-acetone, 5:1 to 1:1) to afford 7 fractions. Each fraction was subjected repeatedly to column chromatography over silica gel and then passed over Sephadex LH-20 (MeOH) respectively to afford compounds 5 (43 mg), 7 (11 mg) and 12 (17 mg). Fraction D was treated the same as Fraction C to afford compounds 6 (28 mg), 8 (14 mg) and 11 (300 mg). Fraction E was subjected to column chromatography over silica gel and got 15 (400 mg) and then applied to ODS column (MeOH-2O, 3:7→5:5) and Sephadex LH-20 column (MeOH) to yield compounds 2 (5 mg) and 3 (4 mg).
Crepidatuol A (1)
yellow oil, [α] D16.8 – 11.89 (c 0.15, CHCl3), UV (CHCl3): λmax (log ε) 282.5 (3.9) nm; IR (KBr): νmax 3431, 2924, 2850, 1614, 1583, 1516, 1463, 1428, 1364, 1270, 1196, 1140, 1032 cm–1; 1H NMR (CDCl3, 500 MHz) and 13C NMR (CDCl3, 125 MHz) data: see Table 1; EIMS m/z 436 (92), 299 (28), 285 (46), 137 (100); HREIMS m/z 436.1882 [M]+ (calcd for C26H28O6, 436.1886).
Crepidatuol B (2)
yellow oil, [α] D11.9 + 0.24 (c 0.26, CHCl3), UV (CHCl3): λmax (log ε) 282.0 (3.87) nm; IR (KBr): νmax 3493, 3313, 2923, 2852, 1728, 1614, 1584, 1516, 1463, 1272, 1197, 1140, 1031 cm–1; 1H NMR (CDCl3, 500 MHz) and 13C NMR (CDCl3, 125 MHz) data: see Table 1; EIMS m/z 452 (37), 287 (63), 137 (100); HREIMS m/z 452.1832 [M]+ (calcd for C26H28O7, 452.1835).
Cell Culture and Evaluation of Neurite OutgrowthPromoting Activity
The neurotrophic activities of the test compounds were examined according to an assay using PC12 cells as reported.17 Briefly, PC12 cells were maintained in F12 medium supplemented with 12.5% horse serum (HS), and 2.5% fetal bovine serum (FBS), and incubated at 5% CO2 and 37 ℃. Test compounds were dissolved in DMSO. For the neurite outgrowth-promoting activity bioassay, PC12 cells were seeded at a density of 2 × 104 cells/mL in 48-well plate coated with poly-L-lysine. After 24 h, the medium was changed to that containing 10 μM of each test compounds plus 10 ng/mL nerve growth factor (NGF), or various concentrations of NGF (50 ng/mL for the positive control, 10 ng/mL for the negative control). The final concentration of DMSO was 0.1%, and the same concentration of DMSO was added into the negative control and blank. After 72 h incubation, the neurite outgrowth was assessed under a phasecontrast microscope. Neurite processes with a length equal to or greater than the diameter of the neuron cell body were scored as neurite bearing cells. The ratio of the neurite-bearing cells to total cells (with at least 100 cells examined/view area; 5 viewing area/well) was determined and expressed as a percentage.
Notes
Electronic Supplementary Material
Supplementary material is available in the online version of this article at http://dx.doi.org/10.1007/s13659-012-0103-3 and is accessible for authorized users.
Acknowledgments
This work was financially supported by National Natural and Science Foundations of China (No. 30800090), "Xi-BuZhi-Guang" project (2009–2012) from Chinese Academy of Science and the Fund of State Key Laboratory of Phytochemistry and Plant Resources in West China (P2010-ZZ012). The authors are grateful to the members of the analytical group of the State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, for all the spectral measurements.
References
-
1.Jiangsu New Medicinal University, Dictionary of Chinese Medicines; Shanghai Scientific and Technical Publishers: Shanghai, 1986; pp 586-590. PubMed Google Scholar
-
2.Chinese Pharmacopoeia Commission, Chinese Pharmacopoeia, Vol. Ⅰ. China Medical Science and Technology Press: Beijing, 2010; pp 85-87, 265-266 and 631-632. PubMed Google Scholar
-
3.Flora of China; Science Press: Beijing, 1999; Vol. 19, pp 109-110. PubMed Google Scholar
-
4.M. Elander, K. Leander, J. Rosenblom, E. Ruusa, Acta Chem. Scand. 27, 1907-1913 (1973) CrossRef PubMed Google Scholar
-
5.(a) Hu, J. M. ; Chen, J. J. ; Yu, H. ; Zhao, Y. X. ; Zhou, J. J. Asian Nat. Prod. Res. 2008, 10, 647-651. (b) Hu, J. M. ; Chen, J. J. ; Yu, H. ; Zhao, Y. X. ; Zhou, J. Planta Med. 2008, 74, 535-539. (c) Hu, J. M. ; Zhao, Y. X. ; Miao, Z. H. ; Zhou, J. Bull. Korean Chem. Soc. 2009, 30, 2098-2100. (d) Hu, J. M. ; Fan, W. W. ; Dong, F. W. ; Miao, Z. H. ; Zhou, J. Chin. J. Chem. 2012, 30, 1327-1330. [e] Wang, C. Z. ; Jia, Z. J. ; Shen, X. M. India J. Chem. 1997, 36, 150-153. PubMed Google Scholar
-
6.R. M. Letcher, L. R. M. Nhamo, J. Chem. Soc., Perkin Trans Ⅰ 23, 2941-2946 (1972) PubMed Google Scholar
-
7.G. X. Ma, G. J. Xu, L. S. Xu, Z. T. Wang, T. Kickuchi, Acta Pharm. Sin. 31, 222-225 (1996) PubMed Google Scholar
-
8.Y. Li, L. H. Qin, Bligh S. W. Annie, A. Bashall, C. F. Zhang, M. Zhang, Z. T. Wang, L. S. Xu, Bioorg. Med. Chem. 14, 3496-3501 (2006) CrossRef PubMed Google Scholar
-
9.G. N. Zhang, L. Y. Zhong, S. W. Annie Bligh, Y. L. Guo, C. F. Zhang, M. Zhang, Z. T. Wang, L. S. Xu, Phytochemistry 66, 1113-1120 (2005) CrossRef PubMed Google Scholar
-
10.G. X. Ma, Z. T. Wang, L. S. Xu, G. J. Xu, J. Chin. Pharm. Sci. 7, 59-61 (1998) PubMed Google Scholar
-
11.X. Zhang, H. Gao, N. L. Wang, X. S. Yao, Chin. Tradit. Herbal Drugs 37, 652-655 (2006) PubMed Google Scholar
-
12.Y. Li, Z. T. Wang, L. S. Xu, Biochem. Syst. Ecol. 34, 658-660 (2006) CrossRef PubMed Google Scholar
-
13.Y. J. Gao, Q. C. Zhao, P. Min, G. B. Shi, M. Yan, Chin. J. Med. Chem. 20, 47-49 (2010) PubMed Google Scholar
-
14.Z. L. Yu, Y. B. Wu, X. F. Chen, L. M. Gao, Z. H. Zheng, Chin. J. Magn. Reson. 20, 297-306 (2003) PubMed Google Scholar
-
15.X. Q. Zhang, Z. Q. Yin, W. C. Ye, S. X. Zhao, Chin. Tradit. Herbal Drugs 36, 1601-1603 (2005) PubMed Google Scholar
-
16.L. A. Greene, A. S. Tischler, Proc. Natl. Acad. Sci. U.S.A. 73, 2424-2428 (1976) CrossRef PubMed Google Scholar
-
17.M. C. Marcotullio, R. Pagiott, F. Maltese, Y. Obara, T. Hoshino, N. Nakahata, M. Curini, Planta Med. 72, 819-823 (2006) CrossRef PubMed Google Scholar
Copyright information
© The Author(s) 2013
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.