Five new mexicanolide type limonoids from Heynea trijuga
Abstract
Five new mexicanolide-type limonoids, heytrijunolides A-E(1-5) were isolated from the branches and leaves of Heynea trijuga. The structures of these new compounds were elucidated on the basis of extensive spectroscopic analysis. Compound 3 showed weak cytotoxicity against HL-60, SMMC-7721 and A-549 human tumor cell lines with the IC50 values of 21.88, 20.66 and 12.70 μM, respectively.Keywords
Heynea trijuga Meliaceae limonoids mexicanolide-type heytrijunolideIntroduction
Limonoids are highly oxygenated and modified nortriterpenoids mainly found in the plants of the Meliaceae and Rutaceae families, which either containing or derived from a precursor with a 4, 4, 8-trimethyl-17-furanyl steroid skeleton, and have attracted continuous attention due to their diverse structures and significant biological activities.1, 2The bioactivity, such as antimalarial, antimicrobial, cytotoxic, insects growth-regulating, insects antifeeding, insecticidal, and antiphytopathogen activities has been reported.2 Till now, about 35 carbon frameworks have been isolated from Meliaceae family.2 Heynea trijuga Roxburgh (previously named: Trichilia connaroides var. microcarpa Bentvelzen) (Meliaceae) is distributed mainly in southern of China.3 Previous investigation on the chemical constituents of the genus Heynea has yielded a series of new limonoids, including trijugin-type, 30-nortrijugin-type, phragmalin-type, and mexicanolide-type.4-12 In our continuing effort to search for novel limonoids from Meliaceae family, five new mexicanolide-type limonoids (1–5) were isolated from the branches and leaves of H. trijuga collected from Hainan province of China. Herein we describe the isolation, structural elucidation and bioactivity assays of these compounds.

|
Results and Discussion
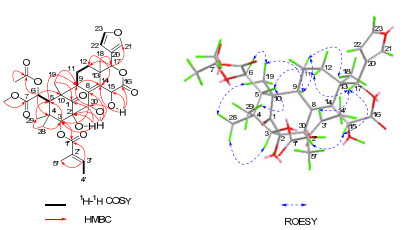
Heytrijunolide A (1) was isolated as white and amorphous powder. The molecular formula, C34H42O13, was deduced from the positive HRESIMS ion at m/z=681.2524 ([M + Na]+, calcd for C34H42O13Na, 681.2523). Its IR absorption bands showed the presence of hydroxyl (3442 cm–1) and ketone groups (1728 cm–1). The observation of proton signals for a β-substituted furan ring (δH 7.58 (1H, s, H-21), 6.48 (1H, s, H-22), and 7.44 (1H, s, H-23)), a methoxy group (δH 3.75, 3H, s), four tertiary methyls (δH 1.01 (3H, s, H-18), 1.25 (3H, s, H-19), 1.06 (3H, s, H-28), and 0.83 (3H, s, H-29)), and a characteristic low-field H-17 proton at δ 5.38 (1H, s) in the 1H NMR spectrum, as well as the characteristic carbonyl group at C-1 (δC 213.0) in the 13C NMR spectrum, strongly suggested that 1 was a mexicanolide-type limonoid.13, 14The 1H and 13C NMR data (Tables 1 and 2) of 1 including the fully substituted olefinic resonances at δC 135.8 and 140.4 due to C-8 and C-14, respectively, were similar to those of augustineolide.15 The major differences between them were the absence of isobutyryl group in compound 1, and the locations of the substituent. Detailed analysis of the 2D NMR spectra (HSQC, 1H-1H COSY, and HMBC) of compound 1, especially the key HMBC cross-peaks of H-3 (δH 5.02, 1H, s)/C-1′ (δC 166.8), H-6 (δH 5.46 (1H, s))/C-1″ (δC 170.0), OH-2 (δH 4.23 (1H, s))/C-1, C-2 (δC 79.4), C-3 (δC 85.8), OH-15 (δH 3.42 (1H, s))/C-14 (δC 140.4), C-15 (δC 65.2), C-16 (δC 173.9), and OH-30 (2.58 (1H, br. s))/C-2, C-8 (δC 135.8), C-30 (δC 73.9) indicated that the tigloyl, acetoxy, and three hydroxy groups were placed at C-3, C-6, C-2, C-15, and C-30, respectively.
1H NMR spectral data of compounds 1–5 in CDCl3 (J in Hz)
13C NMR spectral data of compounds 1–5 in CDCl3
The relative configuration of 1 was deduced from the analysis of its ROESY correlations. As shown in Figure 1, the observed ROESY correlations of Me-29/H-5, H-5/H-12β, H-12β/H-17, H-17/H-15, H-17/H-3′, and H-15/H-30 indicated that these protons and the C-3 tigloyl group were all β-oriented, whereas the ROESY correlations of Me-28/H-3, Me-28/Me-19, Me-19/H-9, H-9/H-11α, and H-11α/Me-18 revealed their α-orientations. Therefore, the structure of compound 1 was finally established.
Selected 2D NMR correlations of 1
Compound 2 was determined to be the 15-O-acetyl derivative of 1 according to the following information. Comparing the NMR (Tables 1 and 2) and MS data of 1 and 2, one more acetyl group was present in 2. The acetoxy group was located at C-15 in 2 on the basis of the HMBC correlation of H-15 (δH 6.37 (1H, s))/Ac-15 (δC 169.3). Furthermore, compound 3 possessed the molecular formula C38H46O15 as determined by positive HRESIMS, with 42 mass units more than that of 2. Detailed studies of its 1D and 2D NMR spectra indicated that 3 was the 30-O-acetyl derivative of 2. Moreover, this was confirmed by the HMBC correlation of H-30 (δH 5.66 (1H, s))/Ac-30 (δC 168.1).
Heytrijunolide D (4) was obtained as a white amorphous powder. The positive HRESIMS displayed a molecular formula, C31H38O10 by the ion peak at m/z 593.2362 ([M + Na]+, calcd for C31H38O10Na, 593.2362). Inspection of the 1H and 13C NMR spectra revealed the characteristic NMR resonances of mexicanolide type limonoids 13, 14 with a furan ring (δH 7.47 (1H, s, H-21), δC 141.2, C-21; 7.41 (1H, br. s, H-23), δC 143.4, C-23; 6.43 (1H, br. s, H-22), δC 110.4, C-22; and δC 120.3, C-20), C-7 carbomethoxy ester (δH 3.71 (3H, s), δC 52.7; δC 174.3, C-7), four quaternary methyl singlet resonances, as well as the carbonyl group at C-1 (δC 213.5). Comparison of its spectroscopic data with those of 3-angeloyl-3-detigloylruageanin B16 showed a close similarity, suggesting that 4 was an analogue of the latter. And the main difference between them was that an (E)-α, β-unsaturated butyroxy located at C-3 replaced an angeloyl in compound 4, which was further confirmed by the HMBC cross-peak of H-3 (δH 5.11 (1H, s))/C-1′ (δC 165.4). Moreover, the coupling constants of H-2′ (dd, J=15.6, 1.7 Hz) and H-3′ (dq, J=15.6, 6.9 Hz) revealed an E-geometry for the ∆2′(3′) double bond. The ROESY experiments indicated that the relative configuration of 4 was the same as that of 3-angeloyl-3-detigloylruageanin B.
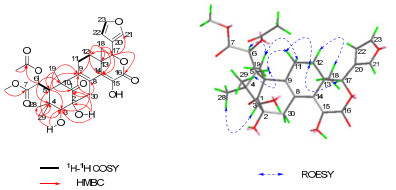
Heytrijunolide E (5) was isolated as a white, amorphous powder. The molecular formula was assigned as C29H34O11 from its HRESIMS peak at m/z 581.1994 ([M + Na]+, calcd for C29H34O11Na, 581.1998). The IR spectrum showed strong absorption bands at 3432, 1762, and 1713 cm–1, suggesting the presence of hydroxyl, and carbonyl. 1H, 13C, and DEPT NMR data of 5 (Tables 1 and 2) revealed a β-furan moiety, four methyl singlets, a carbomethoxy group, and a keto carbonyl at C-1 (δC 213.0). The information above strongly suggested that 5 was a mexicanolide-type limonoid.13, 14 The key HMBC cross-peaks of Hβ-30/C-8, C-9, H-18/C-13, C-14 and H-17/C-16 revealed a conjugated vinyl-vinyl-lactone unit from C-9 to C-16. The planar structure of 5 was further confirmed by detailed 2D NMR analysis (Figure 2). An acetoxy group was located at C-6 by the HMBC correlation of H-6/C-1″, and two hydroxyls were located at C-2 and C-3 by the HMBC correlations of OH-3 (δH 2.42 (1H, s))/C-3 (δC 83.5), C-4 (δC 39.5), and OH-2 (δH 3.99 (1H, s))/C-1 (δC 213.0), C-2 (δC 78.2), C-3 (δC 83.5). Furthermore, there was one more hydroxyl in 5 according to its molecular formula. The 13C and DEPT spectra totally revealed five olefinic quaternary carbons, but only four of those were already established in the structural segment. So the remaining hydroxy group must be located at C-15. Therefore, the hydroxyl and the conjugated vinyl-vinyl-lactone unit formed a conjugated vinyl-enollactone unit.
Selected 2D NMR correlations of 5
The relative configuration of 5 was deduced from the analysis of its ROESY correlations. As shown in Figure 2, the observed correlations of Me-29/H-5, H-5/H-11β, H-11β/H-12β, and H-12β/H-17 indicated that these protons were all β-oriented. Furthermore, the observed correlations of Me-28/H-3, H-12α/Me-18, and H-11α/Me-19 indicated that these protons were all α-oriented. Therefore, the structure of 5 was established.
Compounds 1 and 3 were selected to evaluate insecticidal activity for Artemia salina L. (brine shrimp).17 The results showed that 3 displayed activity at 100 ppm, with the corrected mortality 64.96%. Moreover, compound 3 was further tested in vitro for inhibitory activities against the HL-60, SMMC-7721, A-549, MCF-7, and SW480 human tumor cell lines, using the MTT method.18 The results indicated 3 had weak cytotoxicity against HL-60, SMMC-7721 and A-549 cells with the IC50 values of 21.88, 20.66 and 12.70 μM, respectively.
Experimental Section
General Experimental Procedures. Optical rotations were measured with a JASCO P-1020 digitsl polarimeter. UV spectrawere recorded with a Shimadzu UV-2401 PC spectrophotometer. IR spectra were recorded on a Bruker Tensor-27 infrared spectrometer with a KBr disk. 1H and 13C NMR spectra were recorded on a Bruker AM-400 spectrometer and 2D NMR spectra were recorded on a Bruker DRX-500 instrument and a Bruker Avance-600 spectrometer. Chemical shifts were reported using TMS as the internal standard. ESIMS, HRESIMS and HREIMS spectra were measured with a Brucker HCT Esquire 3000, API QSTAR Pulsarspectrometer and Waters Auto Premier P776 spectrom, respectively. Column chromatography was performed on silica gel (90–150 µm; Qingdao Marine Chemical Inc.), Sephadex LH-20 (40–70 µm; Amersham Pharmacia Biotech AB, Uppsala, Sweden), and Lichroprep RP-18 gel (20–45 µm; Merck, Darmstadt, Germany). Precoated silica gel GF254 and HF254 plates (Qindao Haiyang Chemical Plant, Qingdao, China) were used for thinlayer chromatography. Semipreparative HPLC was performed on a Hypersil gold column (i.d. 10 × 250 mm; thermo fisher scientific Co., Ltd). Fractions were monitored by TLC, and spots were visualized by heating thin-layer chromatography sprayed with 10% H2SO4.
Plant Materal. The branches and leaves of H. trijuga Roxburgh were collected from Changjiang County, Hainan Province, China in December, 2010. The plant was identified by Dr. Guangwan Hu (Kunming Institute of Botany, Chinese Academy of Sciences). And its voucher specimen (H20101203) was deposited at the State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, CAS.
Extraction and Isolation. The air-dried powder of the plant material (12.0 kg) was extracted three times with 90% EtOH (25 L × 3, 4 h/time) under reflux to give a crude extract, which was suspended in water and then extracted successively with petroleum ether (PE) (8 L × 3), EtOAc (8 L × 6) to give two parts. The EtOAc part (180.0 g) was separated on a silica gel column (100–200 mesh, 10 × 100 cm, 1.0 kg) eluted with petroleum ether-Me2CO (100:0→0:100, each 20 L) to give seven fractions (Fr. 1→Fr. 7). After decoloration of Fr. 4 (35.3g) by MCI chromatography (75–150 μm) eluted with gradient MeOH-H2O (20% to 100%, each 10L), all fractions were detected by TLC. The fraction eluted with 80% (Fr. 4E) MeOH-H2O was detected containing Limonoids. Fr. 4E (7.0g) was purified by Sephadex LH-20 (eluted by CHCl3-MeOH 1:1, 3.2 × 140 cm) to get three fractions (Fr. 4E1→Fr. 4E3). The fraction Fr. 4E1 (2.3g) purified by Sephadex LH-20 (eluted by MeOH, 2.0 × 140 cm) and further by semipreparative HPLC to afford 3 (120.3 mg, MeOH-H2O, 63:37). The fraction Fr. 4E2 (1.0g) purified by Sephadex LH-20 (eluted by MeOH, 2.0 × 140 cm), RP-18 Si gel column (20-45 μm, 2 × 40 cm, 20 g) using a gradient system of Acetone-H2O (V/V = 10:90, 30:70, 50:50, 70:30, 90:10 each 4L) and semipreparative HPLC eluted with MeOH-H2O to produce compounds 1 (6.4 mg, MeOH-H2O, 65:35), 2 (2.2 mg, MeOH-H2O, 60:40), 4 (4.1 mg, MeOH-H2O, 70:30), and 5 (1.2 mg, MeOH-H2O, 60:40).
Heytrijunolide A (1): white powder; [α]D15 - 62.4 (c 0.11, CHCl3); UV (MeOH) λmax (log ε): 290 (2.19), 210 (3.59) nm; IR (KBr) νmax 3442, 2956, 2923, 2853, 1728 cm–1; 1H NMR (600 MHz, CDCl3), see Table 1, 13C NMR (150 MHz, CDCl3), see Table 2; positive-ion ESIMS, m/z 681 [M + Na]+; HRESIMS, m/z 681.2524 ([M + Na]+, calcd for C34H42O13Na, 681.2523).
Heytrijunolide B (2): white powder; [α]D22 – 48.8 (c 0.22, CH3OH); UV (MeOH) λmax (log ε): 211 (3.50) nm; IR (KBr) νmax 3439, 2929, 1757, 1728 cm–1; 1H NMR (600 MHz, CDCl3), see Table 1, 13C NMR (150 MHz, CDCl3), see Table 2; positive-ion ESIMS, m/z 723 [M + Na]+; HREIMS, m/z 700.2729 ([M]+, calcd for C36H44O14, 700.2731).
Heytrijunolide C (3): white powder; [α]D21 – 65.42 (c 0.24, CHCl3); UV (MeOH) λmax (log ε): 211 (3.69) nm; IR (KBr) νmax 3455, 2954, 1755 cm–1; 1H NMR (400 MHz, CDCl3), see Table 1, 13C NMR (100 MHz, CDCl3), see Table 2; positiveion ESIMS, m/z 765 [M + Na]+; HRESIMS, m/z 765.2737 ([M + Na]+, calcd for C38H46O15Na, 765.2734).
Heytrijunolide D (4): white powder;[α]D15 – 76.82 (c 0.17, CHCl3); UV (CH3OH) λmax (log ε): 208 (3.26) nm; IR (KBr) νmax 3442, 2924, 1732 cm–1; 1H NMR (600 MHz, CDCl3), see Table 1, 13C NMR (150 MHz, CDCl3), see Table 2; positiveion ESIMS, m/z 593 [M + Na]+; HRESIMS, m/z 593.2362 ([M + Na]+, calcd for C31H38O10Na, 593.2362).
Heytrijunolide E (5): white powder;[α]D15 + 126.98 (c 0.10, CHCl3); IR (KBr) νmax 3432, 2924, 1762, 1751, 1713 cm–1; 1H NMR (600 MHz, CDCl3), see Table 1, 13C NMR (150 MHz, CDCl3), see Table 2; positive-ion ESIMS, m/z 581 [M + Na]+; HRESIMS, m/z 581.1994 ([M + Na]+, calcd for C29H34O11Na, 581.1998).
Insecticidal Assay.17 The test compounds were dissolved in DMSO or water and then diluted with artificial seawater to the final concentrations of 100, 50, 10 ppm (mg/L), which were added to 96-well plates with each well of 15-25 Artemia salina. After cultivation at 28 ℃ for 24 h, the numbers of the dead A. salina were counted with a microscope. Each concentration was repeated in triplicate with toosendanin as the positive control. And the control group was treated in the same way without samples. The corrected mortality was calculated by the Abbot formula.
Corrected mortality = (the mortality of the A. salina with sample-the mortality of the A. salina of control group) / (1 –the mortality of the A. salina of control group) × 100%
Cytotoxicity Assay.18 HL-60, SMMC-7721, A-549, MCF-7 and SW480 were cultured in RPMI 1640 or DMEM medium (Hyclone, USA) supplemented with 10% fetal bovine serum (Hyclone, USA) at 37 ℃. The cytotoxicity assay was performed according to the MTT method. The IC50 were calculated by the Reed and Muench method. DDP was included as a positive control.
Notes
Electronic Supplementary Material
Supplementary material is available in the online version of this article at http://dx.doi.org/10.1007/s13659-012-0040-1 and is accessible for authorized users.
Acknowledgments
We thank Dr. Qiang Zhang, Northwest A & F University for insecticidal bioactivity testing. This work was financially supported by grants from the National Basic Research Program of China (973 Program, 2009CB522300 and 2009CB940900), National Natural Science Funding of China (31170332), and the Young Academic and Technical Leader Raising Foundation of Yunnan Province (2010CI047).
References
-
1.Silva M. F. da, G. F. das, O. R. Gottlieb, D. L. Dreyer, Biochem. Syst. Ecol. 12, 299-310 (1984) CrossRef PubMed Google Scholar
-
2.(a) Fang, X.; Di, Y. T.; Hao, X. J. Curr. Org. Chem. 2011, 15, 1363-1391. (b) Tan, Q. G.; Luo, X. D. Chem. Rev. 2011, 111, 7437-7522. (c) Cai, J. Y.; Zhang, Y.; Luo, S. H.; Chen, D. Z.; Tang, G. H.; Yuan, C. M.; Di, Y. T.; Li, S. H.; Hao, X. J.; He, H. P. Org. Lett. 2012, 14, 2524-2527. (d) Han, M. L.; Zhang, H.; Yang, S. P.; Yue, J. M. Org. Lett. 2012, 14, 486-489. (e) Yin, S.; Wang, X. N.; Fan, C. Q.; Liao, S. G.; Yue, J. M. Org. Lett. 2007, 9, 2353-2356. (f) Ge, Y. H.; Zhang, J. X.; Mu, S. Z.; Chen, Y.; Yang, F. M.; Lu, Y.; Hao, X. J. Tetrahedron 2012, 68, 566-572. (g) Fowles, R.; Mootoo, B.; Ramsewak, R.; Khan, A.; Ramsubhag, A.; Reynolds, W.; Nair, M. Pest Manage. Sci. 2010, 66, 1298-1303. PubMed Google Scholar
-
3.H. Peng, J. M. David, Flora of China 11, 120-121 (2008) PubMed Google Scholar
-
4.Q. Zhang, Y. T. Di, H. P. He, X. Fang, D. L. Chen, X. H. Yan, F. Zhu, T. Q. Yang, L. L. Liu, X. J. Hao, J. Nat. Prod. 74, 152-157 (2011) CrossRef PubMed Google Scholar
-
5.X. Fang, Y. T. Di, Z. L. Geng, C. J. Tan, J. Guo, J. Ning, X. Hao, J. Eur. J. Org. Chem. 2010 (1381) PubMed Google Scholar
-
6.Z. L. Geng, X. Fang, Y. T. Di, Q. Zhang, Y. Zeng, Y. M. Shen, X. J. Hao, Tetrahedron Lett. 50, 2132-2134 (2009) CrossRef PubMed Google Scholar
-
7.X. N. Wang, C. Q. Fan, S. Yin, L. S. Gan, J. M. Yue, Phytochemistry 69, 1319-1327 (2008) CrossRef PubMed Google Scholar
-
8.H. P. Zhang, S. H. Wu, Y. M. Shen, Y. B. Ma, D. G. Wu, S. H. Qi, X. D. Luo, Can. J. Chem. 81, 253-257 (2003) CrossRef PubMed Google Scholar
-
9.A. Inada, M. Konishi, H. Murata, T. Nakanishi, J. Nat. Prod. 57, 1446-1449 (1994) CrossRef PubMed Google Scholar
-
10.V. Mathuram, A. B. Kundu, Indian J. Chem 29B, 970 (1990) PubMed Google Scholar
-
11.K. K. Purushothaman, M. Venkatanarasimhan, A. Sarada, J. D. Connolly, D. S. Rycroft, Can. J. Chem. 65, 35-37 (1987) CrossRef PubMed Google Scholar
-
12.Z. L. Geng, X. Fang, Y. T. Di, Q. Zhang, Y. M. Shen, X. J. Z. Hao, Naturforsch 62b, 1-3 (2010) PubMed Google Scholar
-
13.X. Fang, Y. T. Di, C. S. Li, Z. L. Geng, Z. Zhang, Y. Zhang, Y. Lu, Q. T. Zheng, S. Y. Yang, X. J. Hao, J. Nat. Prod. 72, 714-718 (2009) CrossRef PubMed Google Scholar
-
14.J. Ning, Y. T. Di, X. Fang, H. P. He, Y. Y. Wang, Y. Li, S. L. Li, X. J. Hao, J. Nat. Prod. 73, 1327-1331 (2010) CrossRef PubMed Google Scholar
-
15.B. S. Mootoo, A. Ali, R. Motilal, R. Pingal, A. Ramlal, A. Khan, W. F. Reynolds, S. McLean, J. Nat. Prod. 62, 1514-1517 (1999) CrossRef PubMed Google Scholar
-
16.P. H. Coombes, D. A. Mulholland, M. Randrianarivelojosia, Phytochemistry 66, 1100-1107 (2005) CrossRef PubMed Google Scholar
-
17.W. X. Liu, G. H. Tang, H. P. He, Y. Zhang, S. L. Li, X. Hao, J. Nat. Prod. Bioprospect. 2, 29-34 (2012) CrossRef PubMed Google Scholar
-
18.F. Zhu, Y. T. Di, X. Y. Li, L. L. Liu, Q. Zhang, Y. Li, X. J. Hao, H. P. He, Planta Med. 77, 1536-1541 (2011) CrossRef PubMed Google Scholar
Copyright information
© The Author(s) 2012
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.