Two novel fomannosane-type sesquiterpenoids from the culture of the basidiomycete Agrocybe salicacola
Abstract
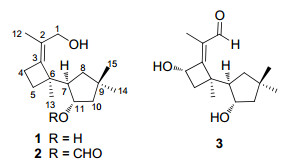
Two novel fomannosane-type sesquiterpenoids, agrocybins H(1) and I(2), together with a known compound illudosin(3), were isolated from the culture broth of the mushroom Agrocybe salicacola. Their structures were elucidated by extensive spectroscopic analysis. The relative stereochemistry of 1 was determined by the use of single crystal X-ray crystallographic diffraction.Keywords
Agrocybe salicacola fomannosane-type sesquiterpenoids agrocybinsIntroduction
Fomannosane-type sesquiterpenoids (FS) were biogenetically belonging to the largest group of sesquiteroenes derived from humulene, a monocyclic C-15 hydrocarbon.1, 2 The typical feature of FS is the existence of a cyclobutane and a cyclopentane connecting by the C–C bond.2, 3 So far, fewer than ten compounds of this kind have been reported with antibacterial, antimicrobial, and cytotoxic activities.3-6 The fungus Agrocybe salicacola (Chinese name, Yang-Liu-Tian-Tou-Gu) is a delicate edible mushroom endemic to Yunnan province, China, which grows at trunks of poplars and willows from September to October.7, 8 In the previous study, seven new sesquiterpene compounds, together with three known components, were isolated from the culture broth of A. salicacola.9 In a continuing investigation of this fungus, two novel fomannosane-type sesquiterpenoids, agrocybin H and I (1 and 2), together with the known illudosin (3)10, were obtained. The structures of compounds 1 and 2 were determined on the basis of extensive spectroscopic analysis and the X-ray crystallographic diffraction.
Results and Discussion
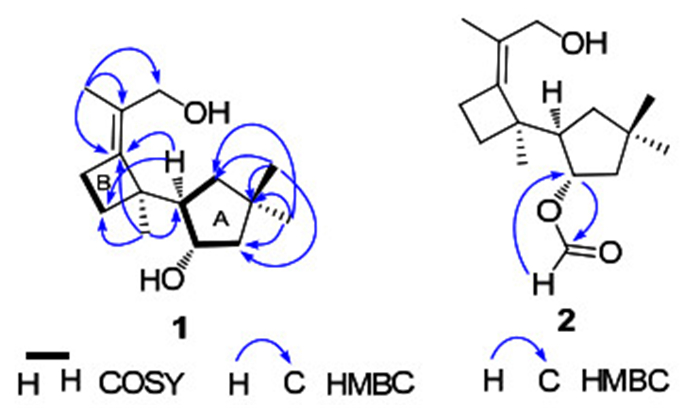
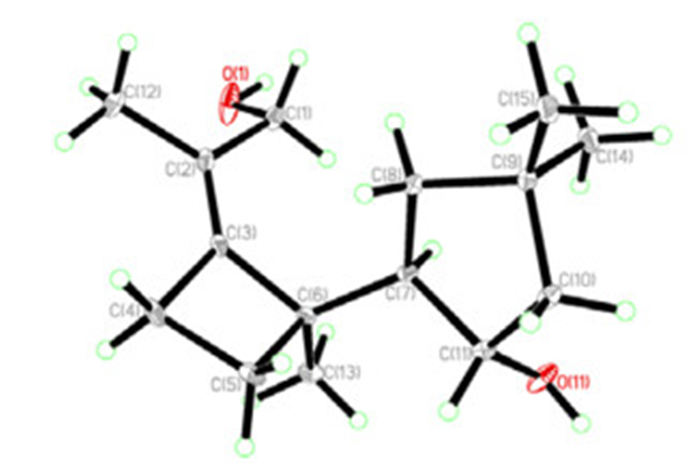
Compound 1, colorless needles, was assigned the molecular formula C15H26O2 on the basis of the positive HRESIMS at m/z 261.1833 ([M + Na]+), required three degrees of unsaturation. Inspection of the 13C NMR spectrum revealed the existence of fifteen carbon signals, including four methyls, five methylene groups (one oxymethylene), two methines (one oxygenated), and four quarternary carbons (including two sp2 ones). These data suggested that 1 was a bicyclic sesquiterpenoid. Ring A was elucidated on the basis of 1H-1H COSY correlations of δH 2.23 (1H, m, H-7) with δH 1.59 (1H, dd, J = 12.3, 6.4 Hz, H-8a), 1.24 (1H, t, J = 12.3 Hz, H-8b), and 3.89 (1H, m H-11), and of H-11 with H-10 [δH 1.72 (1H, dd, J = 13.4, 7.9 Hz, H-10a), and 1.49 (1H, dd, J = 13.4, 4.1 Hz, H-10b)], coupled with HMBC correlations from δH 1.10 (3H, s, H-14) and 1.00 (3H, s, H-15) to δC 37.3 (s, C-9), 44.6 (t, C-8), and 52.3 (t, C-10) (Figure 1). Ring B was determined to be a four-membered ring, as supported by the 1H-1H COSY and HMBC spectra. Specifically, the proton signals at δH 2.50 (1H, m, H-4a) and 2.33 (1H, m, H-4b) correlated with signals at δH 1.87 (1H, ddd, J = 10.9, 10.8, 6.2 Hz, H-5a) and 1.56 (1H, ddd, J = 10.9, 10.2, 6.3 Hz, H-5b) in the 1H-1H COSY spectrum; moreover, H-4a and H-4b showed HMBC correlations with δC 49.6 (s, C-6), 127.1 (s, C-2), and 144.9 (s, C-3), and H-5a and H-5b showed HMBC correlations with C-3 and C-6. Furthermore, the HMBC correlations from the proton at δH 2.23 (1H, m, H-7) to δC 49.6 (s, C-6), 26.5 (t, C-5), and 144.9 (s, C-3) revealed that ring A connected with ring B by C-6 and C-7 as shown in Figure 1. The X-ray crystallographic diffraction analysis confirmed the above elucidation, and determined the relative stereoconfiguration of 1 (Figure 2).
|
Key 2D NMR correlations of compounds 1 and 2
The X-ray stereoconfiguration of compound 1
Compound 2 was obtained as colorless oil. The positive HRESIMS showed a pseudo molecular ion at m/z 289.1772 ([M + Na]+), corresponding to the formula C16H26O3 that required four degrees of unsaturation. The 1D NMR data of 2 were highly similar with those of 1 (Table 1), except for the additional formyl group in 2. In the HMBC spectrum, the formyl group at δH 8.08 (1H, s) showed a strong correlation with δC 77.9 (d, C-11) (Figure 1), suggesting that 2 is the 11-O-formyl derivative of 1.
1H (400 MHz) and 13C (100 MHz) NMR spectroscopic data for compounds 1 and 2
The known compound illudosin (3) had been reported previously by Arnone et al., 10 whose absolute stereochemistry was determined by 1D and 2D NMR experiments and exciton chirality method. Illudosin was supposed to be an intermediate in the biosynthesis of the susquiterpene fomannosin.
Compounds 1–3 were tested for their cytotoxicity against five human cancer cell lines (HL-60, SMMC-7712, A-549, MCF-7, and SW480) by the MTT method, with DDP and taxol as positive controls. None of the compounds exhibited obvious activity at the concentration of 40 μM.
Experimental Section
General Experimental Procedures. Melting point was surveyed with an X-4 microscopic melting point meter. Optical rotations were measured on a Horiba SEPA-300 spectropolarimeter. UV spectra were recorded on a Shimadzu double-beam 210A spectrometer. IR spectra were obtained on a Bruker Tensor 27 FT-IR spectrometer using KBr pellets. NMR spectra were acquired on Bruker DRX-500 and AM-400 instruments at room temperature with TMS as an internal standard. Chemical shifts (δ) were expressed in ppm with reference to the solvent signals. Mass spectra (MS) were recorded on an API QSTAR time-of-flight spectrometer or a VG Autospec-3000 spectrometer. X-ray crystallographic data were collected on a Bruker APEX DUO diffractometer with graphite-monochromated Mo Kα radiation. Silica gel (200– 300 mesh, Qingdao Marine Chemical Inc., China), Sephadex LH-20 (Amersham Biosciences, Sweden), and RP-18 gel (40–75 μm, Fuji Silysia Chemical Ltd. Japan) were used for column chromatography (CC). Preparative HPLC (Prep-HPLC) was performed on an Agilent 1100 liquid chromatography system equipped with a Zorbax SB-C18 column (9.4 mm × 150 mm). Pre-coated silica gel GF254 plates (Qingdao Marine Chemical Inc., China) were used for TLC. Fractions were monitored by TLC, and spots were visualized by heating silica gel plates sprayed with 10% H2SO4 in ethanol.
Fungal Material and Cultivation Conditions. The fungus A. salicacola was collected at the Botanic Garden of Kunming Institute of Botany, Chinese Academy of Sciences, China, in spring 2008, and identified by Prof. Mu Zang, Kunming Institute of Botany. The voucher specimen has been deposited in the Herbarium of Kunming Institute of Botany, Chinese Academy of Sciences. The liquid culture medium contained saccharine 5%, yeast powder 0.5%, peptone 0.15%, KH2PO4 0.05%, and MgSO4 0.05%. Inoculums of A. salicacola were prepared in a 15L-fermentor (Biostar, Shanghai Guo Qiang, China) for 6 days under the following conditions: culture temperature 24 ℃, initial pH 6.0, agitation speed 250 r/min, inoculation volume 10% (by volume), and aeration rate 1.0 vvm. Then, the liquid seed was transferred into a 100Lfermentation tank to be cultivated under the same conditions for 20 days to afford 80 L culture broth.
Extraction and Isolation. The entire culture broth of A. salicacola (80 L) was initially filtered, and the filtrate was extracted three times with EtOAc. The organic layer was concentrated under reduced pressure to give a crude extract (280 g), and further subjected to Column Chromatography (CC) over silica gel using a petroleum ether-Me2CO gradient (1:0 → 0:1) to afford fractions A–L. Fraction F (65 g) was loaded on CC over silica gel to provide four subfractions (F1– F4). Fraction F2 was first separated by silica gel CC (petroleum ether-Me2CO, 5:1), then purified by Prep-HPLC (MeCN/H2O, 2:8 → 5:5) to afford compound 2 (2 mg). Compound 1 (38 mg) was obtained from fraction F4 by repeated column chromatography over silica gel. Fraction H was performed on silica gel CC eluted using a chloroform-methanol gradient (10:1 → 1:1), and then purified by Sephadex LH-20 (chloroform:methanol 1:1) to afford compound 3 (5 mg).
Agrocybin H (1): colorless needles (acetone); mp 141– 142 ℃; [α]D17 + 88.2 (c 0.22, MeOH); UV (MeOH) λmax(log ε) 203 (3.41) nm; IR (KBr) νmax 3248, 2949, 2925 cm–1; 13C and 1H NMR data, see Table 1; ESIMS (positive) m/z 261 [M + Na]+; HRESIMS (positive) m/z 261.1833 (calcd. for C15H26O2Na, 261.1830).
Agrocybin I (2): colorless oil; [α]D13 + 19.9 (c 0.20, MeOH); 13C and 1H NMR data, see Table 1; ESIMS (positive) m/z 289 [M + Na]+; HRESIMS (positive) m/z 289.1772 (calcd. for C16H26O3Na, 289.1779).
Crystallographic Data of Agrocybin H (1): C30H52O4 (C15H26O2 × 2); M = 476.72; Orthorhombic; space group P21212; a = 13.4396(2) Å, b = 23.1684(3) Å, c = 9.27430(10) Å; α = β = γ = 90°, V = 2887.78(6) Å3; Z = 4; ρ = 1.096 g·cm–3; crystal dimensions 0.50 × 0.11 × 0.06 mm3; Shelxs97 with a graphite monochromator; Mo Kα radiation. The total number of reflections measured was 15344, of which 4961 were observed, I > 2σ (I); R1 = 0.0769, wR2 = 0.2074. Crystallographic data for agrocybin H (1) has been deposited at the Cambridge Crystallographic Data Centre as deposition number CCDC 866717. Copies of the data can be obtained free of charge on application to CCDC, 12 Union Road, Cambridge CB2 1EZ, UK (Tel: +44 (0)1223 762911, e-mail: deposit@ccdc.cam.ac.uk).
Cytotoxicity Assay. The following human tumor cell lines were used: HL-60, SMMC-7712, A-549, MCF-7, and SW480. All the cells were cultured in RMPI-1640 or DMEM medium (Hyclone, Logan, UT), supplemented with 10% fetal bovine serum (Hyclone) at 37 ℃ in a humidified atmosphere with 5% CO2. Cell viability was assessed by conducting colorimetric measurements of the amount of insoluble formazan formed in living cells based on the reduction of 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) (Sigma, St. Louis, MO). Briefly, 100 μL of adherent cells were seeded into each well of a 96-well cell culture plate and allowed to adhere for 12 h before drug addition, while suspended cells were seeded just before drug addition, both with an initial density of 1 × 105 cells/mL in 100 μL of medium. Each tumor cell line was exposed to the test compounds at various concentrations in triplicate for 48 h, with DDP and toxal as positive controls. After the incubation, MTT (100 μg) was added to each well, and the incubation continued for 4 h at 37 ℃. The cells lysed with 200 μL SDS after removal of 100 μL of medium. The optical density of lysate was measured at 595 nm in a 96-well microtiter plate reader (Bio-Rad 680). The IC50 value of each compound was calculated by Reed and Muench's method.11
Notes
Electronic Supplementary Material
Supplementary material is available in the online version of this article at http://dx.doi.org/10.1007/s13659-012-0031-2 and is accessible for authorized users.
Acknowledgments
This project was supported by the National Basic Research Program of China (973 Program, 2009CB522300), and the National Natural Sciences Foundation of China (30830113, U1132607).
References
-
1.M. L. Burgess, K. D. Barrow, J. Chem. Soc., Perkin Trans. 1 17, 2461-2466 (1999) PubMed Google Scholar
-
2.W. R. Abraham, Curr. Med. Chem. 8, 583-606 (2001) CrossRef PubMed Google Scholar
-
3.G. R. Pettit, Y. Meng, R. K. Pettit, D. L. Herald, F. Hogan, Z. A. Cichacz, Bioorg. Med. Chem. 18, 4879-4883 (2010) CrossRef PubMed Google Scholar
-
4.T. C. McMorris, R. Lira, P. K. Gantzel, M. J. Kelner, R. Dawe, J. Nat. Prod. 63, 1557-1559 (2000) CrossRef PubMed Google Scholar
-
5.F. Rasser, T. Anke, O. Sterner, Tetrahedron 58, 7785-7789 (2002) CrossRef PubMed Google Scholar
-
6.T. C. McMorris, A. Kashinathama, R. Lira, H. Rundgrena, P. K. Gantzela, M. J. Kelnerb, R. Dawe, Phytochemistry 61, 395-398 (2002) CrossRef PubMed Google Scholar
-
7.Z. L. Yang, M. Zang, X. X. Liu, Acta Bot. Yunn. 15, 18-20 (1993) PubMed Google Scholar
-
8.H. M. Zhou, Y. C. Zhao, W. M. Chen, H. M. Chai, S. H. Li, J. Zhao, Acta Bot. Yunn. 32, 315-322 (2010) PubMed Google Scholar
-
9.L. Y. Liu, L. Zhang, T. Feng, Z. H. Li, Z. J. Dong, X. Y. Li, J. Su, Y. Li, J. K. Liu, Nat. Prod. Bioprospect. 1, 87-92 (2011) CrossRef PubMed Google Scholar
-
10.A. Alberto, C. Rosanna, N. Gianluca, V. P. Orso, J. Chem. Soc., Perkin Trans. 1 8, 1787-1791 (1991) PubMed Google Scholar
-
11.L. J. Reed, H. Muench, Am. J. Hyg. 27, 493-497 (1938) PubMed Google Scholar
Copyright information
© The Author(s) 2012
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.