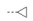2. 西南大学生命科学学院, 重庆 400715
2. College of Life Science, Southwest University, Chongqing 400715, China
结核病(tuberculosis, TB) 是由结核分枝杆菌(Mycobacterium tuberculosis, MTB) 引起的慢性致死性传染性疾病。在冠状病毒(COVID-19) 大流行之前, 结核病是单一传染病的主要死亡原因, 排名高于艾滋病。全世界约1/4的人口感染了MTB[1], 且约90% TB患者是成年人, 男性病例多于女性。TB依然是全球的公共健康问题。
世界卫生组织(WHO) 根据抗结核药物作用效果及不良反应的大小将其分为一线和二线抗结核治疗药物。一线抗结核药物链霉素(streptomycin, SM, 1944年)[2]、异烟肼(isoniazid, INH, H, 1952年)[3]、吡嗪酰胺(pyrazinamide, PZA, Z, 1954年)[4]、利福平(rifampicin, RIF, R, 1965年) 和乙胺丁醇(ethambutol, EMB, E, 1968年)[5]疗效显著、不良反应较少、能够同时杀灭快速增殖期和慢速繁殖期的结核杆菌, 是TB治疗的首选品种; 二线抗结核药物种类较多, 氟喹诺酮类(FQs)、大环内酯类、吩噻嗪类、氨基糖苷类、噁唑烷酮类、环丝氨酸、对氨基水杨酸(p-aminosalicylic acid, PAS) 是其代表, 但其不良反应更多、疗效相对较差。由于TB的特殊性, 敏感TB的治疗总是采用多药同时服用的联合治疗方案, 大约85%的TB患者采用包含利福平的2HRZE+4HR 6月治疗方案, 一般能够治疗成功[6, 7]。虽然敏感TB的治疗取得了巨大成就, 但耐药结核病(drug-resistant tuberculosis, DR-TB), 包括耐多药结核病和广泛耐药结核病, 在世界范围内依然是最严峻的挑战[8, 9]。多药耐药且耐药率高为复治肺结核患者主要特征, 即使采取WHO推荐的多种药物联合治疗方式, 还是存在治疗周期长、产生耐药性等临床问题, 原因之一是患者依从性差。将两种或多种抗结核分子偶联为单一分子, 既可能具有同样疗效并且可减少服药种类和/或数量, 是很有价值的药物研究课题。
PAS是继SM之后研发成功的第二款抗结核药物。1950年英国医学研究委员会(British Medical Research Council) 使用SM和PAS联合治疗TB, 发现其疗效比单一药物更好, 同时还可以减缓其他药物耐药的发生[10, 11]。前人在对PAS改构中发现, PAS的3个官能团都可修饰, 在这些位置合理衍生都可获得高活性分子[12-14]。本研究以PAS为原料, 对PAS羧基、羟基、氨基3个官能团进行修饰。这种策略的优势之一, 在于PAS既可视作联结分子(Linker)、又可作为一种抗结核药物, 由此设计的分子, 既将3种药物片段缀合在同一分子(3-in-1), 又可将目标分子的相对分子质量降低, 在降低合成难度的优势下还可能提高整个分子的成药性。
INH自1952年用于TB治疗以来, 至今仍然是TB治疗最有效的药物之一。INH是一种前药, 通过被动运输进入MTB内部, 由MTB的过氧化氢酶-过氧化物酶(KatG) 激活[15, 16], 形成异烟酰基自由基, 随后与NAD+结合, 形成IN-NAD加合物, 进而抑制烯酰-酰基载体蛋白还原酶(InhA), 干涉分枝菌酸的合成, 从而破坏细菌细胞壁[17, 18]。根据其作用机制, 异烟酰肼的结构片段可以为异烟酰胺或异烟酸酯。
抗菌药物FQs治疗TB的临床试验始于1985年。研究结果表明, FQs药物对敏感TB和DR-TB都有很好的治疗效果[19], 与其他抗结核药物联用的疗效比其单用更为显著[20, 21]。FQs药物靶向DNA解旋酶, 抑制MTB复制周期, 导致细胞死亡[22]。FQs骨架是成药性最高的结构之一。分析上市药物和文献中的活性分子发现, 保留FQs基本骨架、改变C-7杂环或对其修饰最多且也最成功[23, 24], 结合本实验室长期研究经验[25], 本研究选择克林沙星(CLX)、诺氟沙星(NOR)、环丙沙星(CIP)、沙拉沙星(SAR)、依诺沙星(ENX)、巴洛沙星(BAL)、洛美沙星(LOM)、加替沙星(GAT) 8个FQs药物进行修饰。
基于PAS的前期研究结果[26, 27], 在PAS的氨基和羟基上连接修饰基团, 最有可能获得高活性分子。因此, 可以将PAS衍生为对应羧酸酯, 留下氨基和羟基与异烟酸(INA) 和FQs连接, 设计3-in-1目标分子。作为一种尝试, 选择羟基连接FQs、氨基连接INA, 由此设计了目标分子模式(图 1)。为了实现上述目标, 需要合适的Linker。Linker的选择是药效团拼接法的重要内容。Linker必须拥有将两个或多个片段连接的功能, 同时满足相对分子质量尽可能小、分子刚柔性适度、代谢片段无毒等要求。Linker1是连接INA的羧基和PAS的氨基, Linker2是连接PAS的羟基和FQs的胺基, 基于分子无毒、结构简单、可连接羟基和胺基等设想, 选定卤代酰氯作为Linker1和Linker2的试剂, 据此进一步设计了图 1所示的目标分子模式。
|
Figure 1 Design of target molecules. PAS: p-Aminosalicylic acid; FQs: Fluoquinolones |
基于相对分子质量尽可能小的要求, 本研究进一步设计了Linker分别为乙酰基、丙酰基的目标分子TM1/TM2 (图 2, 简写为TM1/2); 通过条件探索, 成功合成了16个目标分子; 通过耻垢分枝杆菌及人致病菌活性测试, 发现了高活性分子。

|
Figure 2 General structure of target molecule |
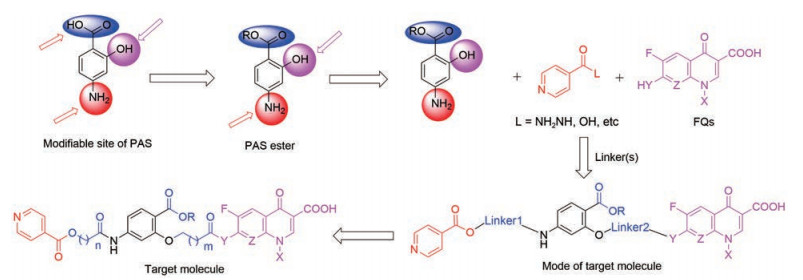
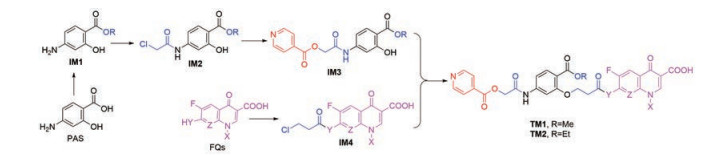
目标化合物TM1/2由INA、PAS和FQs 3种结构片段和2个Linker构成, 其合成路线很多, 本研究设计了如下4条合成路线(合成路线1~4)。
|
Scheme 1 Synthetic route 1 of TM1/2 |
|
Scheme 2 Synthetic route 2 of TM1/2. INA: Isonicotinic acid |
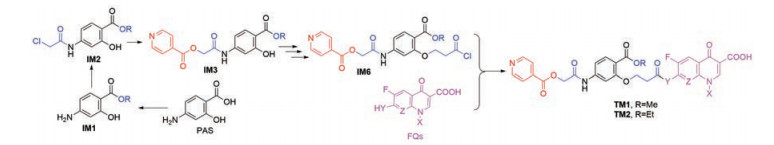
|
Scheme 3 Synthetic route 3 of TM1/2 |
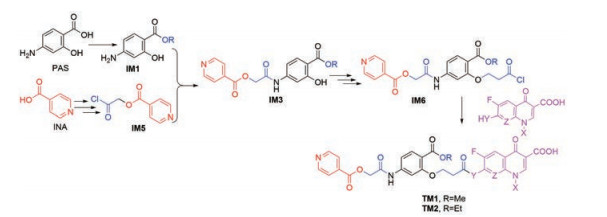
|
Scheme 4 Synthetic route 4 of TM1/2 |
路线1是先将PAS的羧基酯化的中间体IM1与Linker1通过酰胺键连接得到中间体IM2, IM2再与INA通过酯基连接得到共同中间体IM3, 预计IM1和IM2合成容易、IM3合成相对较难。FQs与Linker2反应, 既可引入Linker又可大大增加沙星的溶解性, 因此设计了中间体IM4; 此步为3-氯丙酰氯与脂肪胺基反应, 预计收率高。随后两片段连接, 得到目标分子TM1/2, 此路线预计能够成功。
路线2和路线1的差别, 在于中间体IM3是通过两个片段IM1和IM5连接, 看起来似乎更好, 但INA生成IM5, 其原料应该是卤代乙酸酯, 所得产物是有两个酯键的中间体, 要将乙酸酯水解而异烟酸酯不水解, 这是有难度的; 随后, 水解产物转化为酰氯IM5, 再与IM2偶联为IM3, 不仅路线长而且得到需要的IM5有难度。因此, 路线2不可取。
路线3是一条采用逐步合成法合成目标分子的典型路线, 且有共同中间体IM6; 只要IM6和FQs连接顺利, 就是一条好的合成路线。不幸的是, FQs溶解性差, 预计和IM6反应时间长、杂点多、收率低; 其次, IM6的合成预计有难度, 因为IM3要得到IM6, 通常需要采用卤代丙酸酯与IM3反应, 然后进行丙酸酯的酸碱催化水解(其羧酸酯为甲酯或乙酯) 或TFA型试剂脱保护(其羧酸酯为叔丁酯或芳甲基酯), 对于有3个酯基的分子要实现1个酯基的选择性脱保护, 预计有难度, 更何况随后还要进行酯基的活化, 才能与溶解性差的FQs连接。
路线4采用路线2的方法合成IM3, 采用路线3的方法合成IM6; 正如前述, IM3和IM6合成都有难度, 路线4可以认为不可行。
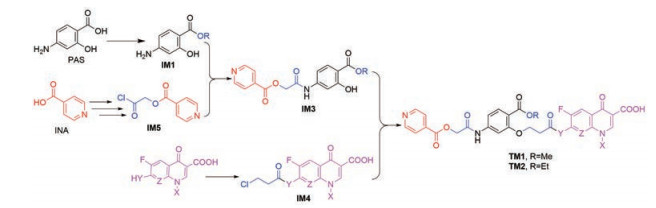
综上所述, 路线1是TM1/2合成的最佳路线, 据此给出了合成路线5所示的反应路线。
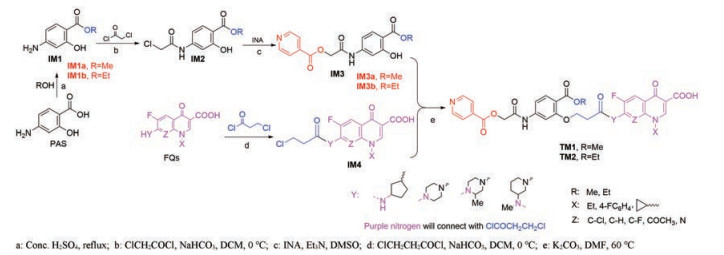
|
Scheme 5 Synthetic routes of TM1/2 |
PAS分别与MeOH和EtOH反应合成IM1a及IM1b, 实验过二氯亚砜、浓硫酸、对甲苯磺酸, 发现浓硫酸催化最佳, 放大实验的收率超过70%; IM1与氯乙酰氯合成IM2, 虽然涉及反应选择性, 但参照本实验室方法[28], 在优化的条件下收率超过98%。IM3的合成是酸INA和氯代烃的酯化反应, 未见文献报道, 用Et3N作碱、DMSO为溶剂、温度控制在60 ℃时反应最好, 收率超过70%。IM4的合成和IM2的合成类似, 参照IM2的合成方法可顺利合成IM4, 收率高(85.6%~92.7%), 收率的差别在于黄色油状粗产物纯化的难易。
1.2 目标分子TM1/2的合成IM3与IM4反应生成TM1/2为典型的酚羟基的烷基化反应。酚羟基烷基化的方法很多, 文献报道有碳酸钾为碱、DMF为溶剂的室温反应法[29]; 碳酸钾作碱、丁酮作溶剂、室温反应的碘化钾催化法[30]; 碳酸钾作碱、丙酮作溶剂、碘化钾催化的回流反应法[31]; 氢氧化钾为碱、甲醇为溶剂的回流反应法[32]。首先选择CH3CN、DMF、N-甲基吡咯烷酮(NMP)、DMSO为溶剂, K2CO3为碱进行反应条件探究。表 1显示, 溶剂为DMF、NMP可反应完全, 但有杂点生成; DMF为溶剂, 碱为Et3N、Na2CO3或DMAP时, IM3与IM4不反应; 选择溶剂为DMF、碱为K2CO3探索温度的影响, 发现温度升高到80 ℃或100 ℃时, 反应能够完全但杂点明显增多, 但若降温至60 ℃, 反应能够完全、杂点明显较少但反应时间更长。优化条件下加入催化量NaI (Entry10), TLC显示反应没有明显改变。
| Table 1 Conditional optimization of synthesis TM1/2. Note: Entry10 added catalytic amount NaI |
基于DMF为溶剂、K2CO3为碱、温度60 ℃、IM3∶IM4∶K2CO3投料比在1∶1.2∶1.5的优化条件, 实现了目标分子TM1/2的合成, 结果见表 2。
| Table 2 Synthetic results of TM1/2 |
TM1/2收率在33.3%~70.1%, 与反应完成程度、杂质的多少及纯化难易直接相关; 甲酯的收率高于乙酯, 可能是前者对邻位羟基的空间位阻相对较小之故。
2 生物活性评价 2.1 抗耻垢分枝杆菌活性本研究的主要目标是发现抗结核活性强的3-in-1目标分子。采用已有的测试方法[33, 34], 测试目标分子TM1/2对耻垢分枝杆菌(Mycobacterium smegmatis, MS) 的抑制活性, 结果见表 3。
| Table 3 Inhibitory activity of TM1/2 against Mycobacterium smegmatis (MIC value, μg·mL-1 and μmol·mL-1). *The values of tPSA, LogP, and CLogP are calculated by ChemDraw soft. CLX: Clinafloxacin; NOR: Norfloxacin; CIP: Ciprofloxacin; SAR: Sarafloxacin; ENX: Enoxacin; BAL: Balofloxacin; LOM: Lomeflolacin; GAT: Gatifloxacin |
表 3数据显示, 对测试的野生型MS, 8种FQs药物的MIC为0.001 1~0.024 μmol·mL-1, 16个TM1/2中有7个分子的MIC ≤ 0.005 4 μmol·mL-1, 显示很强的抑菌活性。目标化合物TM1f/TM1h/TM2h (MIC ≈ 0.005 1 μmol·mL-1; 后续括号内数据均为MIC值) 和阳性对照药物NOR、SAR、ENX的MIC (0.005 1~0.006 1 μmol·mL-1) 相当; TM2b的抑制活性(0.004 3 μmol·mL-1) 是CIP (0.024 μmol·mL-1) 的5.6倍; TM1a (0.001 3 μmol·mL-1) 的抑制活性与超强沙星CLX (0.001 1 μmol·mL-1) 相当, 是SAR (0.005 1 μmol·mL-1) 的4倍, 是CIP (0.023 6 μmol·mL-1) 的18倍; TM2a (0.000 63 μmol·mL-1) 显示了最强活性, 是CLX的1.7倍, 是NOR、SAR、ENX的8.1~9.7倍, 是CIP的37倍。结果表明, PAS、INA与FQs杂合, 可以得到对MS具有高抑制活性的化合物, 验证了设计思想。
构效关系分析发现, PAS和INA连接CLX的衍生物(TM1a/2a), 其抗结核活性超越所有FQs, 显示最强的抗MS活性; 乙酯化衍生物TM2 (0.000 63~0.14 μmol·mL-1) 的抑制活性强于甲酯化衍生物TM1 (0.001 3~0.14 μmol·mL-1); 总体而论, X为环丙基的沙星活性较好。从物理参数tPSA、LogP、CLogP来看, 活性好的化合物, 其tPSA大多数在193左右、LogP在1.9~2.31、CLogP在3.48~4.14, 提示化合物的抗结核活性可能和物理参数有关。
2.2 抗人致病菌活性许多细菌性感染疾病很难治疗, 往往需要几轮抗生素治疗才能清除相同的细菌感染。此外, 长期服用抗生素, 细菌可能产生耐药性, 进而导致长期的反复的感染, 甚至致使治疗失败[35]。目前治疗细菌感染主要采用化学药物治疗法。
为了考察目标分子的抗菌谱, 进一步测试了TM1/2对大肠杆菌(Escherichia coli ATCC25922)、沙门氏菌(Salmonella enteritidis ATCC13076)、鲍曼不动杆菌(Acinetobacter baumannii ATCC19606)、铜绿假单胞菌(Pseudomonas aeruginosa ATCC27853)、藤黄微球菌(Micrococcus luteus)、金葡菌(Staphyloccocus aureus ATCC25129, ATCC14125) 的抑制活性, 相关结果见表 4。
| Table 4 Inhibitory activity of TM1/2 against human pathogenic bacteria (MIC value, μg·mL-1, or μg·mL-1/μmol·mL-1) |
表 4实验结果表明, 目标化合物TM1/2的活性总体强于母核PAS和中间体IM3a/3b; PAS甲酯的抑制活性大于PAS乙酯。
对大肠杆菌, TM1/2抑制活性整体弱于FQs但强于PAS; 11个TM1/2的MIC为8~64 μg·mL-1, 其中3个化合物的MIC值为16 μg·mL-1, TM1a甚至低至8 μg·mL-1; 总体抑制活性TM1 > TM2。
对沙门氏菌, 总体抑制活性TM1 > TM2; 6个TM1/2的MIC值≤ 32 μg·mL-1, 强于阳性对照药物NOR (64 μg·mL-1) 和PAS; 尤其是TM1a/1b, 其MIC值分别为0.002 67及0.002 84 μmol·mL-1, 强于所测试的FQs (0.005 14~0.200 μmol·mL-1)。
对鲍曼不动杆菌, 8个阳性药物FQs, 5个的MIC值为256 μg·mL-1、2个为128 μg·mL-1; 16个TM1/2, 13个的MIC值为256 μg·mL-1、3个为128 μg·mL-1。总体而言, FQs和TM1/2的抑制活性都很差。
对铜绿假单胞菌, FQs的MIC值为0.005 2~0.100 μmol·mL-1; 所有TM1/2的抑制活性都强于NOR (0.100 μmol·mL-1); TM1c/2c/2h的MIC值为8 μg·mL-1, 强于BAL; TM1h/2a的MIC值低至4 μg·mL-1, 抑制活性相当于或强于所测试FQs; TM1a/1b的MIC值低至2 μg·mL-1, 抑制活性强于所测试FQs。
对藤黄微球菌, 8个阳性药物FQs的抑制活性差别很大, MIC值为0.001 09~0.802 μmol·mL-1; TM1/2的MIC值为0.002 62~0.087 0 μmol·mL-1, 抑制活性差别相对较小; 带有CLX结构单元的TM1a/2a, MIC值分别达到了0.002 67和0.002 62 μmol·mL-1, 抑制活性是NOR (0.802 μmol·mL-1) 的300倍、是GAT (0.005 36 μmol·mL-1) 的2倍。
对金葡菌ATCC25129, 目标分子整体活性弱于FQs; 所有TM1/2的MIC值为2~64 μg·mL-1, 其中有12个TM1/2的MIC值≤ 16 μg·mL-1。值得注意的是, TM1a/2h的MIC值低至0.002 67及0.002 58 μmol·mL-1, 抑制活性是NOR (0.100 μmol·mL-1) 的38倍、是GAT (0.005 36 μmol·mL-1) 的2倍。对金葡菌ATCC14125, 8个FQs的MIC值为2~4 μg·mL-1, 而TM1/2的MIC值为0.125~32 μg·mL-1; 整体活性TM1 > TM2。有6个TM1/2的MIC值在0.000 167~0.002 84 μmol·mL-1, 强于所有FQs; 特别地, TM1a和TM1h的MIC值分别低至0.000 167和0.000 329 μmol·mL-1, 远远强于所有的阳性对照药物FQs (0.005 36~0.012 5 μmol·mL-1), 值得后续深入研究。
2.3 溶血性测试溶血是指红细胞(RBC) 破裂溶解现象, 很多理化因素都可以引起溶血, 如低渗溶液、机械性强力振荡、突然低温冷冻(-20 ℃~25 ℃) 或突然化冻、过酸或过碱, 以及酒精、乙醚、胆碱盐, 缺氧等[36]。
为了考察活性好的目标化合物TM1a/2a的安全性, 用健康人的新鲜血液分离的人红细胞离心测定化合物的溶血活性, 阴性对照样品采用生理盐水, 阳性对照选用0.1% Triton X-100生理盐水, 37 ℃孵育4 h, 并对不同浓度下的TM1a/2a进行评估, 结果见图 3。
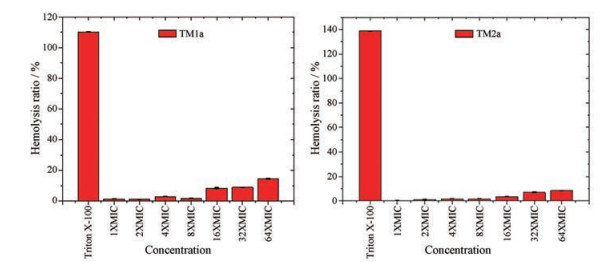
|
Figure 3 Hemolysis ratio of TM1a/2a |
图 3表明, TM1a/2a的溶血率随着化合物浓度的升高而升高。在1~64 μg·mL-1测试浓度下, TM1a和TM2a的溶血率分别在1.32%~14.40%及0.001 5%~8.20%。TM1a/2a的测试浓度分别为8及32 μg·mL-1时, 其溶血率(hemolysis) 分别为3.73%和3.19%, 均低于5%。按照国际标准, TM1a/2a在对应浓度下具有相对安全性。在相同浓度下, TM2a的溶血率低于TM1a, 亦即PAS乙酯衍生物的溶血率低于PAS甲酯衍生物, 这可能与PAS羧基酯化的烷基链越长其脂溶性越大有关。
3 小结采用片段拼接原理, 本研究将PAS、INA与FQs通过Linker拼接成单一分子, 设计并合成了16个新分子, 收率31.7%~70.1%。抗耻垢分枝杆菌活性显示, TM1a和TM2a的MIC值分别低至0.001 3及0.000 63 μmol·mL-1, 相当或强于所测试的FQs; 对沙门氏菌和铜绿假单胞菌, TM1a/1b的活性强于所测试的FQs; 对于金葡菌ATCC14125, TM1a/1h的MIC值分别低至0.000 167和0.000 329 μg·mL-1, 远强于所测试的FQs。高活性分子TM1a/2a的溶血性实验结果表明, TM1a/2a分别在8和32 μg·mL-1浓度以下时具有相对安全性。本研究发现了三分子药效团拼接的高活性分子, 可为抗菌药物的研发提供新的研究思路。
实验部分核磁共振仪(Bruker, ADVANCE ⅢTM 600 MHz, TMS为内标); 高分辨质谱仪(QTOF-MS, Bruker Impact Ⅱ, Germany); 熔点测定仪(X-6, 北京福凯仪器有限公司)。
PAS (上海泰坦有限公司, 98%); INA、浓硫酸、甲醇、乙醇、碳酸氢钠、碳酸钾(重庆川东化工有限公司); GAT、CLX、CIP、BAL、SAR、ENX (郑州克尔泰生化科技有限公司, > 95%); NOR、LOM (上海达瑞精细化工有限公司, AR); 氯乙酰氯、3-氯丙酰氯(重庆钛新化工有限公司); 其余试剂均为市售化学纯或分析纯产品, 未经纯化直接使用。
1 目标化合物的合成 1.1 IM1a/IM1b合成的操作示例于100 mL圆底烧瓶中加入PAS 1.53 g (10 mmol)、甲醇/乙醇10 mL, 室温搅拌, 恒压滴液漏斗缓慢滴加浓硫酸1.06 mL (20 mmol), 滴毕, 油浴回流反应, TLC监测直至反应结束。冰浴冷却, 饱和碳酸钠溶液调节pH = 8~9, 析出大量固体, 抽滤, 饱和NaCl溶液洗涤, 干燥, 得灰白色固体中间体IM1a/IM1b, 收率分别为74.1%、82.5%, mp分别为100.3~104.0 ℃、113.2~113.9 ℃。
1.2 IM2a/IM2b合成的操作示例于100 mL反应瓶中加入IM1a/IM1b (10 mmol)、二氯甲烷(DCM) 10 mL, 室温搅拌, 固体溶解后, 加入NaHCO3 (30 mmol, 2.522 g), 冰浴冷却, 滴加氯乙酰氯(12 mmol, 1 mL)。滴毕, 继续冰浴搅拌反应, TLC监测直至反应结束。旋除DCM, 加水20 mL, 抽滤, 滤饼用饱和NaCl溶液(15 mL×3)、水(15 mL×3) 洗涤, 真空干燥, 得白色固体中间体IM2a、IM2b, 收率分别为99.3%、98.8%, mp分别为180.7~181.5 ℃、189.9~190.7 ℃。
1.3 IM3a/IM3b合成的操作示例于100 mL反应瓶中加入IM2a/IM2b (5 mmol)、5 mL DMSO, 室温搅拌, 快速溶解, 加入Et3N (7.5 mmol), 继续搅拌0.5 h后加入INA (7.5 mmol, 0.931 g), 60 ℃油浴搅拌反应, TLC监测直至反应结束。加水, 析出固体, 抽滤, 滤饼用饱和NaCl溶液(15 mL×3)、水(5 mL×3) 洗涤, 真空干燥, 得粗品。柱色谱(PE: EA = 5∶1~1∶1, v/v) 得中间体IM3a/IM3b, 收率分别为72.7%、85.2%, mp分别为207.3~208.0 ℃、186.5~187.3 ℃。
1.4 IM4a~IM4h合成的操作示例于100 mL圆底烧瓶中加入沙星(10 mmol)、DCM 20 mL, 搅拌均匀后加入碾细的NaHCO3 (40 mmol), 室温搅拌均匀。移至0 ℃冰盐浴, 滴加3-氯丙酰氯(25 mmol) 的DCM (2.5 mL) 溶液。滴加完毕, 继续冰浴搅拌反应, TLC监测反应完全后, 加入饱和NaCl溶液20 mL, 2 mol·L-1 HCl调节pH = 2~3, 静置分层, 分液, 有机相用冰冷饱和NaCl溶液洗涤至水相无荧光点, 收集有机相, 干燥, 旋除溶剂, 得固体粗品。加入EA 15 mL搅拌30 min, 加入PE 40 mL进行分散, 静置, 抽滤, TLC监测固体, 如发现存在杂点, 重复多次进行分散, 真空干燥, 称重。当原料是巴洛沙星和加替沙星时, 得油状粗品, 加DCM 20 mL重结晶, 析出黄色固体, 抽滤, 得纯品, 收率在85.6%~92.7%。
1.5 TM1/2合成的操作示例于100 mL反应瓶中加入IM3a/IM3b (1 mmol)、DMF 2 mL, 室温搅拌得淡黄色清亮溶液, 加入K2CO3 (1.5 mmol, 0.207 g), 搅拌0.5 h后加IM4a~IM4h (1.2 mmol)、NaI (0.01 mmol), 继续搅拌0.5 h后, 转入60 ℃油浴搅拌反应, TLC监测直至反应结束。加入冰冷的饱和NaCl溶液, 2 mol·L-1 HCl溶液调节pH = 3~4, 析出固体, 冷藏。抽滤, 滤饼用饱和NaCl溶液(10 mL×1)、冰水(15 mL×2) 洗涤, 滤饼真空干燥得粗品。柱色谱(DCM∶CH3OH = 150∶1~20∶1, v/v), 部分化合物还经薄层色谱(DCM∶CH3OH = 50∶1, v/v), 得目标化合物TM1/2。
TM1/2系列化合物的表征数据如下:
TM1a: 白色固体, mp: 228.3~229.2 ℃。1H NMR (600 MHz, DMSO-d6) δ 14.51 (s, 1H), 10.61 (s, 1H), 10.55 (s, 1H), 8.88~8.81 (m, 2H), 7.94 (d, J = 11.7 Hz, 1H), 7.91 (d, J = 5.6 Hz, 2H), 7.75 (d, J = 8.7 Hz, 1H), 7.35 (d, J = 1.1 Hz, 1H), 7.09 (d, J = 7.4 Hz, 1H), 5.73 (dd, J = 10.5, 1.8 Hz, 2H), 5.01 (s, 2H), 4.41 (d, J = 3.3 Hz, 1H), 4.02 (dt, J = 20.6, 10.4 Hz, 1H), 3.87 (s, 3H), 3.76 (s, 3H), 3.35 (s, 4H), 2.08 (d, J = 7.4 Hz, 1H), 1.20 (dd, J = 13.1, 6.9 Hz, 3H), 1.01 (s, 2H); 13C NMR (150 MHz, DMSO-d6) δ 176.59, 172.45, 169.15, 166.09, 165.56, 165.14, 164.89, 161.81, 156.91, 155.24, 153.12, 151.28, 145.14, 143.85, 143.76, 138.31, 136.80, 128.60, 127.98, 123.13, 120.30, 110.98, 108.41, 108.17, 106.85, 64.14, 61.59, 60.21, 55.27, 46.40, 41.99, 30.47, 21.44, 19.10, 14.45, 11.29。HR-MS [M+H]+ m/z C36H33ClFN5O10计算值750.197 3, 测量值750.197 2。
TM1b: 白色固体, mp: 216.2~217.0 ℃。1H NMR (600 MHz, DMSO-d6) δ 11.13 (s, 1H, H), 10.64 (s, 1H), 10.57 (s, 1H), 9.97 (s, 1H), 8.86 (d, J = 3.8 Hz, 2H), 7.92 (d, J = 4.2 Hz, 2H), 7.76 (d, J = 8.7 Hz, 1H), 7.72 (d, J = 8.1 Hz, 1H), 7.37 (s, 1H), 7.11 (d, J = 8.6 Hz, 1H), 5.68 (s, 2H), 5.00 (d, J = 28.9 Hz, 2H), 4.23 (t, J = 6.4 Hz, 2H), 4.03 (d, J = 5.7 Hz, 1H), 3.88 (s, 3H), 3.37 (s, 9H), 1.65 (t, J = 7.3 Hz, 3H); 13C NMR (150 MHz, DMSO-d6) δ 176.58, 172.15, 169.39, 166.06, 165.50, 165.08, 163.69, 161.63, 153.15, 148.88, 146.24, 145.44, 145.16, 143.86, 143.04, 138.33, 131.34, 128.66, 127.93, 120.32, 111.12, 111.02, 108.38, 108.19, 106.89, 64.21, 62.49, 52.67, 51.10, 46.38, 42.00, 35.77, 30.49, 19.11, 11.31。HR-MS [M+Na]+ m/z C35H34FN5O10计算值726.218 2, 测量值726.216 4。
TM1c: 白色固体, mp: 234.2~235.1 ℃。1H NMR (600 MHz, DMSO-d6) δ 15.19 (s, 1H, H-23), 10.62 (s, 3H, H-5), 10.57 (s, 2H, H-19), 8.85 (d, J = 4.9 Hz, 2H, H-9 and H-10), 8.66 (s, 1H), 7.91 (d, J = 5.4 Hz, 2H), 7.75 (d, J = 8.7 Hz, 1H), 7.57 (d, J = 6.9 Hz, 1H), 7.35 (s, 1H), 7.09 (d, J = 8.6 Hz, 1H), 5.01 (s, 2H), 4.23 (s, 4H), 3.87 (s, 3H), 3.81 (t, J = 16.1 Hz, 2H), 3.42 (s, 4H), 3.36 (s, 3H), 1.33 (d, J = 6.4 Hz, 1H), 1.21 (d, J = 30.7 Hz, 2H), 0.88~0.79 (m, 1H); 13C NMR (150 MHz, DMSO-d6) δ 176.57, 172.14, 169.14, 168.53, 166.04, 165.49, 165.08, 163.68, 161.81, 155.24, 153.13, 148.87, 146.24, 145.44, 145.16, 143.03, 138.31, 131.24, 130.94, 128.64, 127.93, 110.98, 108.38, 108.18, 106.85, 65.47, 64.19, 62.48, 61.57, 61.52, 55.32, 41.99, 35.77, 30.49, 14.46, 11.31。HR-MS [M+H]+ m/z C36H34FN5O10计算值716.236 2, 测量值716.235 9; [M+Na]+ m/z计算值738.218 2, 测量738.217 9。
TM1d: 白色固体, mp: 207.3~208.0 ℃。1H NMR (600 MHz, DMSO-d6) δ 15.11 (s, 1H), 10.63 (s, 1H), 10.57 (s, 1H), 8.85 (d, J = 5.3 Hz, 2H), 8.64 (s, 1H), 7.99 (d, J = 13.0 Hz, 2H), 7.91 (d, J = 5.4 Hz, 2H), 7.83~7.70 (m, 2H), 7.53 (t, J = 8.4 Hz, 1H), 7.35 (s, 1H), 7.09 (d, J = 8.6 Hz, 2H), 5.74 (s, 2H), 5.01 (s, 2H), 3.87 (s, 3H), 3.74~3.54 (m, 2H), 3.41 (d, J = 24.9 Hz, 8H); 13C NMR (150 MHz, DMSO-d6) δ 176.61, 172.49, 169.15, 166.11, 165.60, 165.16, 164.90, 161.79, 156.90, 155.23, 153.13, 151.27, 145.13, 143.85, 143.76, 138.31, 136.80, 131.92, 131.27, 129.09, 128.95, 128.57, 128.04, 123.51, 123.14, 120.29, 111.11, 110.98, 108.42, 108.14, 106.85, 64.13, 61.60, 55.26, 41.99, 30.46, 21.44, 19.09, 14.45。HR-MS [M+H]+ m/z C39H33F2N5O10计算值770.226 8, 测量值: 770.226 9。
TM1e: 白色固体, mp: 213.6~214.5 ℃。1H NMR (600 MHz, DMSO-d6) δ 15.30 (s, 1H), 10.62 (s, 1H), 10.57 (s, 1H), 8.96 (s, 1H), 8.85 (d, J = 4.4 Hz, 2H), 8.07 (d, J = 13.3 Hz, 1H), 7.91 (d, J = 4.4 Hz, 2H), 7.74 (d, J = 8.7 Hz, 1H), 7.35 (s, 1H), 5.01 (s, 2H), 4.56~4.42 (m, 2H), 3.87 (s, 3H), 3.77 (d, J = 35.3 Hz, 3H), 3.43 (s, 10H), 1.40 (t, J = 6.8 Hz, 3H); 13C NMR (150 MHz, DMSO-d6) δ 176.60, 172.16, 169.14, 166.06, 165.54, 165.10, 163.69, 161.80, 155.24, 153.15, 148.88, 146.24, 145.44, 145.15, 143.01, 131.26, 129.10, 128.62, 127.98, 111.26, 110.99, 108.40, 108.17, 106.86, 65.48, 64.19, 62.46, 61.58, 42.00, 35.76, 30.47, 19.10, 14.46, 11.30。HR-MS [M+H]+ m/z C34H33FN6O10计算值705.231 4, 测量值705.231 5。
TM1f: 黄色固体, mp: 297.9~298.8 ℃。1H NMR (600 MHz, DMSO-d6) δ 14.94 (s, 1H), 10.62 (s, 1H), 8.86 (d, J = 4.5 Hz, 1H), 8.69 (s, 2H), 7.91 (d, J = 4.5 Hz, 2H), 7.74 (d, J = 11.9 Hz, 4H), 4.56 (s, 2H), 4.17 (s, 3H), 4.03 (dd, J = 14.3, 7.2 Hz, 2H), 3.87 (s, 3H), 3.79 (s, 4H), 3.14 (dd, J = 28.2, 10.4 Hz, 3H), 3.00 (s, 3H), 2.92 (d, J = 14.52 Hz, 2H), 2.82 (m, 2H), 1.90 (Hz, 2H), 1.22~1.14 (m, 2H); 13C NMR (150 MHz, DMSO-d6) δ 176.74, 169.71, 169.39, 166.05, 162.76, 161.63, 155.35, 151.32, 150.76, 146.43, 145.17, 139.74, 139.66, 136.81, 134.49, 131.35, 131.04, 129.69, 127.62, 123.12, 121.41, 121.35, 111.00, 107.03, 106.86, 64.16, 63.23, 63.19, 60.20, 52.67, 51.00, 41.28, 36.61, 30.45, 27.29, 25.79, 14.53, 9.49, 9.27。HR-MS [M+Na]+ m/z C39H40FN5O11计算值796.260 1, 测量值796.260 8。
TM1g: 淡黄色固体, mp: 209.5~210.3 ℃。1H NMR (600 MHz, DMSO-d6) δ 15.08 (s, 1H), 10.61 (s, 1H), 9.95 (s, 1H), 8.93 (d, J = 8.6 Hz, 2H), 8.64 (s, 1H), 7.72 (d, J = 8.7 Hz, 1H), 7.87 (d, J = 8.7, 2H), 7.79 (s, 1H), 7.53 (dd, J = 8.6, 1.7 Hz, 1H), 5.72 (d, J = 10.7 Hz, 2H), 4.59 (d, J = 3.2 Hz, 8H), 3.87 (s, 3H), 3.51 (d, J = 9.7 Hz, 2H), 3.36 (d, J = 9.6 Hz, 3H), 1.45 (t, J = 6.7 Hz, 6H); 13C NMR (150 MHz, DMSO-d6) δ 176.61, 172.18, 169.39, 166.08, 165.56, 163.69, 161.62, 153.16, 148.88, 146.24, 145.44, 145.14, 143.01, 138.33, 131.36, 128.62, 128.00, 111.29, 111.12, 111.03, 110.97, 108.40, 108.16, 106.99, 106.90, 65.49, 64.20, 62.45, 52.68, 42.00, 41.05, 35.75, 30.47, 19.10, 13.95, 11.30。HR-MS [M+H]+ m/z C36H35F2N5O10计算值736.242 5, 测量值736.243 7。
TM1h: 黄色固体, mp: 225.6~226.3 ℃。1H NMR (600 MHz, DMSO-d6) δ 14.93 (s, 1H), 10.63 (s, 1H), 10.57 (s, 1H), 8.85 (d, J = 4.3 Hz, 2H), 8.72 (s, 1H), 7.91 (d, J = 4.3 Hz, 2H), 7.76 (d, J = 6.2 Hz, 1H), 7.35 (s, 1H), 7.09 (d, J = 8.5 Hz, 1H), 5.73 (d, J = 13.6 Hz, 4H), 5.01 (s, 2H), 4.21 (dd, J = 24.5, 17.9 Hz, 1H), 3.87 (s, 3H), 3.72 (s, 2H), 3.46 (s, 4H), 3.36 (s, 3H), 3.20 (d, J = 17.6 Hz, 1H), 1.28 (d, J = 45.5 Hz, 3H), 1.13 (s, 1H), 1.03 (s, 1H), 0.95~0.89 (m, 1H), 0.87~0.80 (m, 1H); 13C NMR (150 MHz, DMSO-d6) δ 176.74, 169.42, 166.09, 166.07, 161.64, 156.83, 155.17, 151.28, 150.95, 148.86, 146.23, 145.42, 145.15, 140.01, 139.94, 136.79, 134.56, 131.33, 127.88, 123.12, 111.03, 108.37, 107.18, 107.14, 106.90, 65.48, 64.18, 64.14, 64.07, 62.44, 55.27, 52.66, 50.95, 41.08, 30.47, 19.10, 13.93, 9.45。HR-MS [M+Na]+ m/z C38H38FN5O11计算值782.244 4, 测量值782.242 7。
TM2a: 黄色固体, mp: 236.3~237.1 ℃。1H NMR (600 MHz, DMSO-d6) δ 14.48 (s, 1H), 10.69 (s, 1H), 10.54 (s, 1H), 8.84 (d, J = 3.1 Hz, 2H), 8.82 (s, 1H), 7.90 (d, J = 2.5 Hz, 2H), 7.73 (d, J = 8.6 Hz, 1H), 7.33 (s, 1H), 7.08 (d, J = 8.3 Hz, 1H), 5.01 (s, 2H), 4.34 (q, J = 6.9 Hz, 2H), 3.74 (s, 2H), 3.42 (s, 8H), 3.32 (s, 3H), 1.32 (t, J = 6.9 Hz, 3H), 1.20 (d, J = 5.7 Hz, 2H), 1.00 (s, 1H), 0.87 (dd, J = 44.5, 6.3 Hz, 1H); 13C NMR (150 MHz, DMSO-d6) δ 176.59, 169.15, 166.08, 165.54, 164.89, 161.81, 156.91, 155.25, 153.11, 151.28, 145.14, 143.85, 143.76, 138.31, 136.80, 131.25, 128.61, 127.96, 123.12, 120.31, 111.11, 110.98, 108.41, 108.18, 106.86, 65.48, 64.14, 61.58, 55.27, 51.63, 51.08, 46.36, 41.98, 31.38, 30.47, 14.45, 11.29。HR-MS [M+Na]+ m/z C37H35ClFN5O10计算值786.194 9, 测量值786.193 6。
TM2b: 黄色固体, mp: 201.6~201.3 ℃。1H NMR (600 MHz, DMSO-d6) δ 15.25 (s, 1H), 10.70 (s, 1H), 10.56 (s, 1H), 8.95 (s, 1H), 8.86 (d, J = 5.3 Hz, 2H), 7.91 (d, J = 5.7 Hz, 2H), 7.76 (d, J = 8.7 Hz, 1H), 7.35 (s, 1H), 7.19 (d, J = 6.2 Hz, 1H), 7.09 (d, J = 8.6 Hz, 1H), 5.02 (s, 2H), 4.59 (dd, J = 13.7, 6.7 Hz, 1H), 4.34 (q, J = 7.0 Hz, 2H), 3.89~3.64 (m, 4H), 3.35 (s, 8H), 2.92 (dd, J = 14.6, 8.2 Hz, 1H), 1.42 (t, J = 7.0 Hz, 3H), 1.33 (t, J = 7.0 Hz, 3H); 13C NMR (150 MHz, DMSO-d6) δ 176.74, 169.39, 166.07, 164.88, 162.78, 161.62, 156.98, 155.33, 151.31, 150.76, 146.41, 145.45, 145.17, 139.75, 139.67, 136.80, 134.48, 131.35, 131.04, 123.13, 121.38, 121.32, 111.00, 108.36, 107.01, 64.15, 63.22, 52.68, 51.00, 41.28, 36.59, 36.24, 31.24, 30.45, 25.78, 14.52。HR-MS [M+Na] m/z C36H36FN5O10计算值740.233 8, 测量值740.233 1。
TM2c: 白色固体, mp: 235.8~236.6 ℃。1H NMR (600 MHz, DMSO-d6) δ 15.18 (s, 1H), 10.70 (s, 1H), 10.56 (s, 1H), 8.86 (d, J = 4.4 Hz, 2H), 8.66 (s, 1H), 7.91 (d, J = 5.4 Hz, 2H), 7.75 (d, J = 8.7 Hz, 1H), 7.57 (d, J = 7.1 Hz, 1H), 7.35 (s, 1H), 7.09 (d, J = 8.4 Hz, 1H), 5.01 (s, 2H), 4.34 (q, J = 6.9 Hz, 2H), 3.80 (d, J = 20.1 Hz, 4H), 3.37 (s, 13H), 1.33 (t, J = 6.9 Hz, 3H); 13C NMR (150 MHz, DMSO-d6) δ 176.74, 169.42, 166.11, 166.07, 164.89, 163.68, 161.63, 155.16, 151.27, 150.94, 148.86, 146.23, 145.42, 145.13, 142.99, 140.01, 136.79, 134.55, 131.33, 127.92, 123.13, 111.02, 108.36, 107.16, 106.98, 106.89, 65.48, 64.17, 64.13, 64.07, 62.43, 55.27, 52.67, 41.09, 30.46, 19.09, 13.93, 9.44。HR-MS, [M+Na]+ m/z C37H36FN5O10计算值752.233 8, 测量值752.233 3。
TM2d: 黄色固体, mp: 212.1~213.0 ℃。1H NMR (600 MHz, DMSO-d6) δ 15.09 (s, 1H), 10.68 (s, 1H), 9.95 (s, 1H), 8.65 (s, 1H), 8.01 (d, J = 13.0 Hz, 2H), 7.79 (t, J = 6.0 Hz, 2H), 7.54 (t, J = 8.1 Hz, 2H), 7.15 (s, 1H), 6.80 (dd, J = 16.6, 10.5 Hz, 4H), 6.41 (d, J = 7.2 Hz, 1H), 5.69 (d, J = 10.8 Hz, 1H), 4.34 (dd, J = 13.7, 6.7 Hz, 2H), 4.02 (t, J = 6.3 Hz, 2H), 3.78 (t, J = 6.3 Hz, 2H), 3.65 (m, 8H), 1.33 (t, J = 6.9 Hz, 3H); 13C NMR (150 MHz, DMSO-d6) δ 176.75, 172.19, 169.78, 169.26, 169.14, 166.18, 166.12, 164.90, 161.78, 151.28, 150.75, 146.38, 145.38, 145.13, 139.74, 137.66, 136.80, 134.98, 134.46, 131.29, 130.99, 128.50, 123.14, 121.36, 121.30, 111.27, 110.99, 108.43, 108.10, 106.99, 106.85, 64.13, 63.21, 63.17, 61.61, 41.26, 36.57, 30.44, 25.75, 14.45。HR-MS [M+H]+ m/z C40H35F2N5O10计算值784.242 5, 测量值784.241 9。
TM2e: 白色固体, mp: 215.7~216.5 ℃。1H NMR (600 MHz, DMSO-d6) δ 15.28 (s, 1H), 10.70 (s, 1H), 10.55 (s, 1H), 8.97 (s, 1H), 8.86 (d, J = 3.6 Hz, 2H), 8.08 (d, J = 13.3 Hz, 1H), 7.91 (d, J = 3.5 Hz, 2H), 7.75 (d, J = 8.6 Hz, 1H), 7.35 (s, 1H), 5.01 (s, 2H), 4.50 (d, J = 6.6 Hz, 2H), 4.34 (q, J = 6.9 Hz, 2H), 4.23 (t, J = 6.8 Hz, 2H), 4.02 (t, J = 6.8 Hz, 2H), 3.96~3.63 (m, 8H), 1.40 (t, J = 6.6 Hz, 3H), 1.33 (t, J = 6.8 Hz, 3H); 13C NMR (150 MHz, DMSO-d6) δ 176.73, 169.16, 166.12, 166.06, 164.88, 163.67, 161.80, 151.22, 150.89, 148.85, 146.22, 145.41, 145.11, 142.97, 140.00, 139.92, 134.52, 131.22, 127.93, 123.14, 110.98, 108.37, 107.13, 106.85, 65.48, 64.87, 62.40, 61.60, 61.54, 55.24, 41.08, 30.45, 19.08, 14.42, 9.43。HR-MS [M+Na]+ m/z C35H35FN6O10计算值741.229 1, 测量值741.229 7。
TM2f: 黄色固体, mp: 208.0~208.8 ℃。1H NMR (600 MHz, DMSO-d6) δ 14.95 (s, 1H), 10.70 (s, 1H), 10.56 (s, 1H), 8.86 (d, J = 4.7 Hz, 2H), 8.70 (s, 1H), 7.91 (d, J = 4.6 Hz, 2H), 7.76 (d, J = 8.5 Hz, 1H), 7.35 (s, 1H), 7.09 (d, J = 8.5 Hz, 1H), 5.01 (s, 2H), 4.34 (dd, J = 14.1, 7.0 Hz, 2H), 4.17 (t, J = 6.8 Hz, 2H), 4.02 (s, 3H), 3.79 (d, J = 6.5 Hz, 3H), 3.39 (s, 3H), 3.26 (s, 2H), 3.20~3.06 (m, 1H), 3.04~2.69 (m, 2H), 2.16 (dd, J = 29.1, 8.7 Hz, 1H), 1.78~1.91(m, 3H), 1.33 (t, J = 7.0 Hz, 3H), 1.26~0.94 (m, 4H); 13C NMR (150 MHz, DMSO-d6) δ 176.79, 172.17, 169.13, 166.11, 164.89, 162.65, 161.80, 151.33, 150.80, 146.40, 145.17, 139.75, 136.81, 134.50, 131.31, 130.99, 128.52, 123.13, 121.43, 111.28, 110.99, 108.44, 107.88, 107.05, 106.86, 64.16, 63.23, 63.19, 62.47, 61.58, 51.00, 50.01, 43.18, 41.28, 34.44, 30.45, 30.30, 25.78, 14.48, 9.26。HR-MS [M+H]+ m/z C40H42FN5O11计算值788.293 8, 测量值788.295 3。
TM2g: 白色固体, mp: 223.9~224.8 ℃。1H NMR (600 MHz, DMSO-d6) δ 14.88 (s, 1H), 10.71 (s, 1H), 10.57 (s, 1H), 8.92 (s, 1H), 8.85 (d, J = 4.6 Hz, 2H), 7.91 (d, J = 4.6 Hz, 2H), 7.87 (d, J = 11.6 Hz, 1H), 7.75 (d, J = 8.7 Hz, 1H), 7.34 (s, 1H), 5.74 (t, J = 18.2 Hz, 7H), 5.01 (s, 2H, H-7), 4.58 (s, 2H), 4.34 (q, J = 13.9, 6.9 Hz, 2H), 4.02 (t, J = 6.9 Hz, 4H), 1.45 (t, J = 6.6 Hz, 3H), 1.33 (t, J = 7.0 Hz, 6H); 13C NMR (150 MHz, DMSO-d6) δ 176.74, 172.20, 169.42, 166.12, 166.08, 164.89, 163.68, 161.63, 155.14, 151.28, 150.95, 148.88, 146.23, 145.43, 145.13, 142.97, 139.94, 136.77, 134.55, 131.34, 123.13, 111.00, 108.34, 107.13, 106.86, 65.49, 64.17, 64.08, 62.42, 55.28, 52.68, 51.00, 41.09, 30.46, 19.10, 13.94, 9.44。HR-MS [M+H]+ m/z C37H37F2N5O10计算值750.258 1, 测量值750.257 8。
TM2h: 黄色固体, mp: 233.2~234.0 ℃。1H NMR (600 MHz, DMSO-d6) δ 14.92 (s, 1H), 10.71 (s, 1H), 10.57 (s, 1H), 8.85 (d, J = 5.6 Hz, 2H), 8.71 (s, 1H), 7.91 (d, J = 5.6 Hz, 2H), 7.75 (d, J = 9.1 Hz, 2H), 7.35 (s, 1H), 5.74 (s, 4H), 5.01 (s, 2H), 4.34 (dd, J = 14.1, 7.0 Hz, 2H), 4.16 (s, 1H), 3.72 (s, 3H), 3.35 (d, J = 12.1 Hz, 7H), 1.33 (t, J = 7.1 Hz, 6H), 1.08 (m, 4H); 13C NMR (150 MHz, DMSO-d6) δ 176.72, 172.15, 169.16, 166.08, 166.05, 163.67, 161.82, 156.82, 151.26, 150.91, 148.85, 146.23, 145.42, 145.14, 143.00, 140.00, 139.92, 134.53, 131.23, 127.87, 123.12, 111.24, 110.98, 108.37, 106.85, 65.47, 64.17, 64.05, 62.44, 61.58, 61.53, 55.26, 50.97, 41.08, 30.47, 19.09, 14.43, 13.92, 9.44。HR-MS [M+H]+ m/z C39H40FN5O11计算值774.278 1, 测量值774.278 0。
2 化合物的生物活性测试 2.1 抗耻垢分枝杆菌活性采用本实验室常用的测试方法[33, 34], 测定了目标分子TM1/2抗耻垢分枝杆菌的活性, 相关结果见表 3。
2.2 抗人致病菌活性按照美国国家临床实验室标准化委员会(NCCLS) 推荐的微量稀释法, 采用本实验室常用的测试方法[33, 34], 测定大肠杆菌ATCC25922、沙门氏菌ATCC13076、鲍曼不动杆菌ATCC19606、铜绿假单胞菌ATCC27853、藤黄微球菌、金黄色葡萄球菌ATCC25129/ATCC14125的MIC, 相关结果见表 4。
2.3 溶血性测试用健康人的新鲜血液分离的人红细胞离心测定化合物的溶血活性。新鲜血液来自西南大学医院, 西南大学化学化工学院批准。用生理盐水(0.9% NaCl) 洗涤3次, 最终得到2%的红细胞悬液(红细胞200 μL, 生理盐水10 mL), 将红细胞悬液加入96孔板(每孔100 μL), 将待测化合物加入96孔板第一孔中, 进行连续二倍稀释。阴性对照样品采用生理盐水, 阳性对照选用0.1% Triton X-100的生理盐水, 37 ℃孵育4 h, 离心, 提取上清液, 测试吸光度(A540), 利用式1计算溶血率。其中A为被测试样品在540 nm处的吸光度值, A0为阴性对照样品的吸光度值, Atotal为阳性对照样品的吸光度值。
| $ {\rm{Hemolysis}} = \frac{A-{A}_{0}}{{A}_{\mathrm{t}\mathrm{o}\mathrm{t}\mathrm{a}\mathrm{l}}-{A}_{0}} $ | (1) |
致谢: 感谢西南大学药学院中医药学院徐兴然副教授对抗菌活性测试的帮助和本学院老师在核磁共振、质谱测试中提供的支持。
作者贡献: 任艳会负责化合物的合成与部分生物活性测试, 论文的撰写、修改和校对; 范莉负责部分分子的合成及文本修改; 代乐平、毛丹负责部分分子的合成; 许峻旗负责抗结核活性测试; 谢建平为抗结核活性测试责任人; 杨茜负责溶血性测试; 杨大成负责课题规划且为论文责任负责人。
利益冲突: 无利益冲突。
| [1] |
World Health Organization. Global Tuberculosis Report 2021[R/OL]. Geneva: WHO, 2021. https://www.who.int/publications/digital/global-tuberculosis-report-2021.
|
| [2] |
Schatz A, Waksman SA. Effect of streptomycin and other antibiotic substances upon Mycobacterium tuberculosis and related organisms[J]. Exp Biol Med, 1944, 57: 244-248. DOI:10.3181/00379727-57-14769 |
| [3] |
Tebrock HE, Fisher MM, Mamlok ER. The new drug: isoniazid[J]. Am J Nurs, 1952, 52: 1342-1344. |
| [4] |
Malone L, Schurr A, Lind HH, et al. The effect of pyrazinamide (aldinamide) on experimental tuberculosis in mice[J]. Am Rev Tuberc, 1952, 65: 511-518. |
| [5] |
Laboratories L. Ethambutol hydrochloride (myambutol tablets)[J]. Med Lett Drugs Ther, 1968, 9: 41-42. |
| [6] |
Rahman MU, Arfan M, Shah Z, et al. Nonlinear fractional mathematical model of tuberculosis (TB) disease with incomplete treatment under Atangana-Baleanu derivative[J]. Alex Eng J, 2021, 60: 2845-2856. DOI:10.1016/j.aej.2021.01.015 |
| [7] |
Siswantining T, Susilowati MHD, Purwandani N, et al. Geoinformatics of tuberculosis (TB) disease in Jakarta city Indonesia[J]. Int J Geomate, 2020, 19: 35-42. |
| [8] |
Pym AS, Diacon AH, Tang SJ, et al. Bedaquiline in the treatment of multidrug-and extensively drug-resistant tuberculosis[J]. Eur Respir J, 2016, 47: 564. DOI:10.1183/13993003.00724-2015 |
| [9] |
Juan P, Anandi M. Drug resistance mechanisms in Mycobacterium tuberculosis[J]. Antibiotics, 2014, 3: 317-340. DOI:10.3390/antibiotics3030317 |
| [10] |
Medical Research Council. Treatment of pulmonary tuberculosis with streptomycin and para-aminosalicylic acid: a medical research council investigation[J]. Br Med J, 1950, 2: 1073-1085. DOI:10.1136/bmj.2.4688.1073 |
| [11] |
Medical Research Council. Various combinations of isoniazid with streptomycin or with PAS in the trearment of pulmonary tuberculosis[J]. Br Med J, 1955, 1: 435-445. DOI:10.1136/bmj.1.4911.435 |
| [12] |
Jensen KA, Rosdahl KG, Ingvorsen H. Tuberculostatic derivatives of p-aminobenzoic acid; esters and amides of 4-aminosalicylic acid[J]. Acta Chem Scand, 1948, 2: 220-224. DOI:10.3891/acta.chem.scand.02-0220 |
| [13] |
Doub L, Schaefer JJ, Bambas LL, et al. Some derivatives of 4-amino-2-hydroxybenzoic acid (p-aminosalicylic acid)[J]. J Am Chem Soc, 1951, 73: 903-906. DOI:10.1021/ja01147a006 |
| [14] |
Patole J, Shingnapurkar D, Padhye S, et al. Schiff base conjugates of p-aminosalicylic acid as antimycobacterial agents[J]. Bioorg Med Chem Lett, 2006, 16: 1514-1517. DOI:10.1016/j.bmcl.2005.12.035 |
| [15] |
Sajal KH, Fatiha E. In silico identification of novel chemical compounds with antituberculosis activity for the inhibition of InhA and EthR proteins from Mycobacterium tuberculosis[J]. J Clin Tuberc Other Mycobact Dis, 2021, 24: 100246. DOI:10.1016/j.jctube.2021.100246 |
| [16] |
Pflégr V, Horváth L, Stolaříková J, et al. Design and synthesis of 2-(2-isonicotinoylhydrazineylidene) propanamides as InhA inhibitors with high antitubercular activity[J]. Eur J Med Chem, 2021, 223: 113668. DOI:10.1016/j.ejmech.2021.113668 |
| [17] |
Ionut D, Oana MD, Sangram K, et al. New isoniazid derivatives with improved pharmaco-toxicolog-ical profile: obtaining, characterization and biological evaluation[J]. Eur J Pharm Sci, 2019, 137: 104974. DOI:10.1016/j.ejps.2019.104974 |
| [18] |
Srivastava G, Tripathi S, Kumar A. Molecular investigation of active binding site of isoniazid (INH) and insight into resistance mechanism of S315T-MtKatG in Mycobacterium tuberculosis[J]. Tuberculosis, 2017, 105: 18-27. DOI:10.1016/j.tube.2017.04.002 |
| [19] |
Lee HW, Lee JK, Kim E, et al. The effectiveness and safety of fluoroquinolone-containing regimen as a first-line treatment for drug-sensitive pulmonary tuberculosis: a systematic review and meta-analysis[J]. PLoS One, 2016, 11: e0159827. DOI:10.1371/journal.pone.0159827 |
| [20] |
Verma RK, Agrawal AK, Singh AK, et al. New fluoroquinolones active against fluoroquinolones-resistant Mycobacterium tuberculosis strains[J]. Tuberculosis, 2013, 93: 405-411. DOI:10.1016/j.tube.2013.02.017 |
| [21] |
World Health Organization. Treatment of Tuberculosis: Guidelines[M]. Geneva: World Health Organization, 2010.
|
| [22] |
Sharma A, Rosa MD, Singla N, et al. Tuberculosis: an overview of the immunogenic response, disease progression, and medicinal chemistry efforts in the last decade toward the development of potential drugs for extensively drug-resistant tuberculosis strains[J]. J Med Chem, 2021, 64: 4359-4395. DOI:10.1021/acs.jmedchem.0c01833 |
| [23] |
Qu LB, Tian MC, Cheng SX, et al. Synthesis and in vitro antibacterial activity of 7-(4-acylamino-thioformyl-1-piperazinyl) quinolones[J]. Acta Pharm Sin (药学学报), 2003, 38: 264-267. |
| [24] |
Gao LZ, Li T, Xie YS, et al. Design and synthesis of[1, 2, 4] triazino-[3, 4-h] [1, 8] naphthalidine-8-keto-7-carboxylic acid derivatives and their antibacterial and anti-cell proliferation activities[J]. Acta Pharm Sin (药学学报), 2015, 50: 332-336. |
| [25] |
Pan JF, Sun XL, Fan L, et al. Study on the hybrid molecules of dihydroartemisinin and fluoroquinolones linked by L-homoserine derivative[J]. Acta Pharm Sin (药学学报), 2020, 52: 2157-2169. |
| [26] |
Kakemi K, Sezaki H, Kitazawa S, et al. Studies on the pharmaceutical potentiation of drugss. Ⅲ. Antagonistic effects of PABA on antitubercular activities of PAS derivatives[J]. Chem Pharm Bull, 1967, 15: 925-931. DOI:10.1248/cpb.15.925 |
| [27] |
Imramovsk A, Polanc S, Kocevar M, et al. A new modification of antitubercular active molecules[J]. Bioorg Med Chem, 2007, 15: 2551-2559. DOI:10.1016/j.bmc.2007.01.051 |
| [28] |
Yang DC, Ren YH, Fan L, et al. Fluoroquinolone derivatives of p-aminosalicylic acid and intermediates, preparation methods and applications thereof: CN, 2020110256397[P]. 2020-09-25.
|
| [29] |
Zhou H, Li X, Li Y, et al. Synthesis and bioevaluation of 1-phenylimidazole-4-carboxylic acid derivatives as novel xanthine oxidoreductase inhibitors[J]. Eur J Med Chem, 2020, 186: 111883. DOI:10.1016/j.ejmech.2019.111883 |
| [30] |
Huang L, Shi A, He F, et al. Synthesis, biological evaluation, and molecular modeling of berberine derivatives as potent acetylcholinesterase inhibitors[J]. Bioorg Med Chem, 2010, 18: 1244-1251. DOI:10.1016/j.bmc.2009.12.035 |
| [31] |
Markus F, David RL, Aleksandra Z, et al. Histamine H3 receptor antagonists with peptidomimetic (keto) piperazine structures to inhibit Aβ oligomerisation[J]. Bioorg Med Chem, 2021, 50: 116462. DOI:10.1016/j.bmc.2021.116462 |
| [32] |
Sharma VS, Patel SB, Sharma AS, et al. Calamitic-rod-shaped mesogens based on chalcone esters comprising varying alkoxy and lauryl ester chains: synthesis, mesomorphism and computational study[J]. Mol Cryst Liq Cryst, 2017, 556: 32-47. |
| [33] |
Yang DC, Pan JF, Fan L, et al. Fluoroquinolone derivatives of p-aminosalicylic acid and intermediates, preparation methods and applications thereof: CN, 2020110256626[P]. 2020-09-25.
|
| [34] |
Pan JF. Design, Synthesis and Bioactivity of p-Amino Salicylic Acid Derivatives (对氨基水杨酸衍生物的设计、合成及其生物活性研究)[D]. Chongqing: Southwest University, 2020.
|
| [35] |
Bridget G, Grzegorz G, Michaux C, et al. Bacterial persisters and infection: past, present, and progressing[J]. Annu Rev Microbiol, 2019, 73: 359-385. DOI:10.1146/annurev-micro-020518-115650 |
| [36] |
Lubka R, Pablo B, France P. The role of complement in post-transfusion hemolysis and hyper hemolysis reaction[J]. Transfus Med Rev, 2019, 33: 225-230. DOI:10.1016/j.tmrv.2019.09.007 |
 2022, Vol. 57
2022, Vol. 57