2. 中国科学院大学附属肿瘤医院 (浙江省肿瘤医院), 浙江 杭州 310005
2. Cancer Hospital of The University of Chinese Academy of Sciences (Zhejiang Cancer Hospital), Hangzhou 310005, China
肿瘤是全球死亡率最高的疾病之一[1]。尽管近年来肿瘤的治疗手段取得了重大进展, 但癌症相关的总死亡率无显著改善[2], 肿瘤治疗仍然是目前研究的热点领域[3]。抗肿瘤药物是常用的治疗手段, 然而治疗相关的不良反应和耐药, 对患者生活质量和预后产生重要的影响。因此, 寻找更有效、更安全的个体化抗肿瘤的治疗方法迫在眉睫。
定量系统药理学(quantitative systems pharmacology, QSP) 结合了系统生物学和定量药理学的研究方法, 应用体外、体内实验数据和模型仿真技术, 定量分析药物和生物系统之间的动态相互作用, 探索药物的活性、靶点和效应之间的关系[4]。QSP研究方法的成功运用, 可有效预测药物的效应机制、疗效、毒性及筛选优势治疗人群, 促进肿瘤患者的精准治疗, 提高治疗有效率、降低不良反应的发生。本文对QSP在抗肿瘤药物研究中的应用和进展进行综述, 为抗肿瘤药物的研发和肿瘤患者的精准治疗提供借鉴。
1 系统生物学与定量系统药理学 1.1 系统生物学随着基因组学、转录组学、蛋白质组学和代谢组学等高通量检测技术的进步, 人们对生物体细胞、组织和器官的基础知识认识不断加深, 从而促进了新兴学科——系统生物学(systems biology) 的形成[5]。2000年以后该学科获得了生物医学界的广泛关注[6]。杨胜利院士对系统生物学概念作了如下定义[7]: “系统生物学是在细胞、组织、器官和生物体水平上研究结构和功能各异的生物分子及其相互作用, 并通过计算生物学定量阐明和预测生物功能、表型和行为”。通过系统生物学可确定系统的结构、分析系统的行为。
1.2 定量系统药理学QSP将系统生物学模型与药动学/药效学(pharmacokinetics/pharmacodynamics, PK/PD) 模型相结合(图 1), 定量分析药物的作用靶点和治疗效果, 从而设计精准的治疗方案[8]。2011年, 美国国立卫生研究院(National Institutes of Health, NIH) 首次提出该术语[9], 将药理学与系统生物学相结合, 用于药物的研发和临床应用。NIH在白皮书中[10]指出: QSP是一门新兴的转化医学的核心学科, 通过数学建模的方法, 纳入多维度的时间和空间数据, 创建跨分子、细胞、组织和人体的多维模型。模型侧重于描述生物分子、细胞、组织等之间的相互作用, 旨在精确地描述并预测药物的行为: ①如何在空间和时间维度上调节细胞网络; ②如何影响人体生理和病理状态, 从而预测药物的治疗与毒性作用。2021年, 美国食品药品监督管理局(FDA) 的Bai等[11]提出: 基于人体生物学的重要通路, QSP模型可定量表征药物靶点和药效学反应之间的机制性关系。其优势在于可同时对多个疗效或安全性生物标志物的动态变化进行预测。因此, QSP是针对药物和生物系统间动态相互作用的定量分析, 将“系统”作为一个整体, 而非单个组分或个体行为所开展的研究。
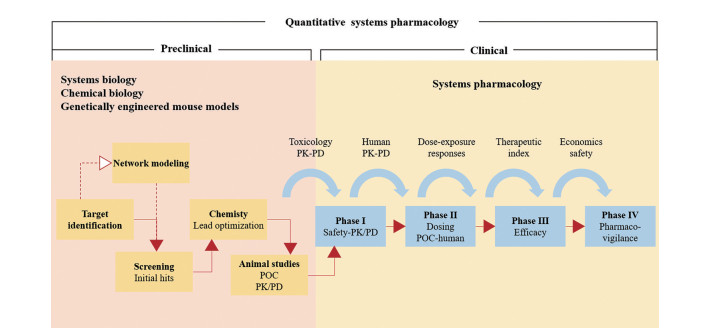
|
Figure 1 Quantitative systems pharmacology (QSP)[10]. POC: Proof of concept |
采用检索词“(systems pharmacology model) OR (quantitative systems pharmacology) OR (QSP model)”在PubMed数据库中进行检索, 检索时间为自建库至2021年11月。检索结果可见: 自2011年以来, QSP相关论文的发表数量逐年增多(图 2), 涉及了中枢神经系统疾病、肿瘤、自身免疫性疾病、糖尿病与血栓栓塞性疾病等。其中, 肿瘤是QSP应用的重点领域。QSP模型可将PK/PD与临床结局相链接, 定量考察药物与疾病之间的关系[12], 在药物研发和临床应用中具有极大的发展潜力。
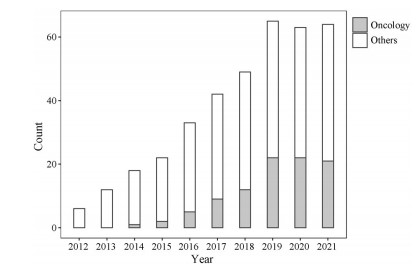
|
Figure 2 PubMed citations by year for QSP |
生理药动学(physiologically based pharmacokinetics, PBPK) 是建立在生物体解剖、生理、生化和药物理化性质等基础上的PK研究方法。利用PBPK模型, 可研究药物在体内的处置过程, 预测组织器官中药物与代谢产物的经时过程, 定量描述机体的病理、生理参数的变化对药物处置过程的影响[13]。尽管PBPK与QSP均可描述药物的PK/PD行为、进行预测剂量、评估药效学相互作用、研究生物标志物等, 但是二者亦有区别(表 1)。
| Table 1 Comparison of physiologically based pharmacokinetics (PBPK) and QSP. 1: Primary; 2: Complementary; 3: Secondary; NA: Not applicable[9] |
PBPK用于考察药物PK相互作用、评估体内外的相关性, 而QSP可实现药理机制的验证和疾病进展的评估。PBPK模型表征了药物在解剖生理局部的药理反应, 而QSP表征了药物在多维度的器官-组织-细胞-蛋白质和代谢物-基因的动态变化, 其中的数学关系更为复杂[9]。
3 定量系统药理学在肿瘤治疗中的应用 3.1 概述肿瘤治疗领域中, QSP在免疫治疗药物研究的应用最为受到关注, 包括免疫检查点抑制剂[14-24]、T细胞接合器[25-28]、嵌合抗原受体T细胞免疫疗法(chimeric antigen receptor T-cell immunotherapy, CAR-T)[29, 30]。在分子靶向药物方面, QSP的应用也是研究重点, 包括小分子激酶抑制剂[31-40]、蛋白水解活化抗体(probody therapeutics, Pb‐Tx)[41, 42]、抗体偶联药物[43, 44]、自噬调节剂[45]、表观遗传学药物[46, 47] (表 2[14-52]) 等。免疫治疗药物和分子靶向药物在延长总生存期或无进展生存期、无病生存期方面具有显著优势, 是近年发展最为迅猛的新型抗肿瘤药物[53]。其他抗肿瘤药物如化疗药物[48-51]、光动力疗法[52]等, QSP的研究和应用相对较少。
| Table 2 Application types of QSP in antitumor drugs. -: None |
欧洲制药工业协会联合会(European federation of pharmaceutical industriesand associations, EFPIA) 颁布的模型引导的药物研发良好规范(model-informed drug discovery and development, MID3) 中, 归纳了“模型”应用的八大领域, 包括探索效应机制、制订给药方案、选择优势人群、筛选候选化合物、优化试验方案、预测和表征PK、预测临床反应、与标准疗法的比较[54]。抗肿瘤药物研究中QSP的应用主要涉及了前三个方面(表 2), 以下将分别介绍。
3.2.1 效应机制的探索相较于传统的药物机制研究, QSP可整合多个重要通路, 更全面地探索药物疗效和毒性机制, 分析各通路在药物效应中所起的作用。例如, 酪氨酸激酶抑制剂(tyrosine kinase inhibitors, TKI) 可引起心力衰竭等心脏毒性, 但机制尚不明确。Vaidya等[35]建立了达沙替尼和索拉非尼引起线粒体心脏毒性的QSP模型, 表征达沙替尼和索拉非尼的心脏损伤机制: ①达沙替尼和索拉非尼抑制细胞凋亡通路中AKT8病毒癌基因细胞同源物(AKT8 virus oncogene cellular homolog, Akt) 的磷酸化, 导致抗凋亡蛋白B细胞淋巴瘤-2 (B-cell lymphoma 2 protein, Bcl-2) 的表达下调, 促进细胞凋亡因子半胱天冬氨酸蛋白酶-9 (caspase-9) 和半胱天冬氨酸蛋白酶-3 (caspase-3) 表达升高, 引起心肌细胞凋亡; ②达沙替尼和索拉非尼通过Akt/雷帕霉素靶蛋白(mammalian target of rapamycin, mTOR) 通路抑制心肌细胞自噬; ③索拉非尼促进c-Jun氨基末端激酶(c-Jun N-terminal kinase, JNK) 磷酸化, 促进Bcl-2相关死亡启动子(Bcl-2-associated death promoter, BAD) 的激活, 并抑制caspase-9和caspase-3的表达; ④索拉非尼直接提升Bcl-2表达水平, 促进心肌细胞自噬, 并通过下调Akt、直接抑制心肌细胞生长(图 3)。
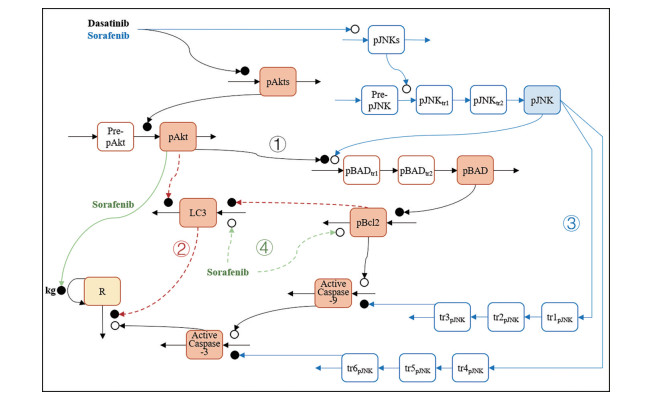
|
Figure 3 Model structure for dasatinib and sorafenib induced toxicity in AC16 cells. Orange and blue highlighted compartments represent measured protein expression and the yellow highlighted compartment represents measured cellular response. Open and closed circles represent stimulation and inhibition processes. Solid arrows represent connections of the apoptotic pathway and dashed arrows represent the autophagy pathway |
构建的QSP模型表征了蛋白信号和细胞反应的动态趋势, 且通过了体外实验的外部数据集的验证。研究结果显示: 达沙替尼在75%人类心肌细胞(AC16细胞) 活力抑制浓度时, 线粒体凋亡是引起心脏毒性的主要机制, 自噬的作用较弱。而索拉非尼致心脏毒性的机制中, 自噬和线粒体凋亡均发挥了重要的作用。建立的QSP模型可阐明达沙替尼和索拉非尼的心脏毒性机制, 为其他小分子靶向治疗药物引起心脏毒性的机制研究提供了参考, 有助于优化此类药物的联合用药策略, 降低心脏毒性不良反应的发生。
3.2.2 给药方案的制订基于临床前研究数据建立的QSP模型, 实现体内外或动物与人体的桥接, 模拟药物在人体中的剂量-暴露-效应关系、从而制订给药方案, 提高临床试验成功率, 加速新药的获批上市。例如, 奥西替尼及其活性代谢产物AZ5104是表皮生长因子受体(epidermal growth factor receptor, EGFR) 突变不可逆抑制剂。Yates等[40]建立了奥西替尼与AZ5104在不同EGFR突变小鼠的QSP模型, 表征了奥西替尼与AZ5104抑制肿瘤增殖的机制, 包括: ①肿瘤细胞增殖和自我凋亡; ②异种移植肿瘤体积随磷酸化EGFR降低而缩小; ③磷酸化EGFR合成的限速过程; ④药物不可逆结合磷酸化EGFR使磷酸化EGFR比例减少。该模型还可描述奥西替尼与AZ5104在小鼠体内的PK特征(图 4), 准确预测不同给药方案下异种移植肿瘤体积的动态变化。
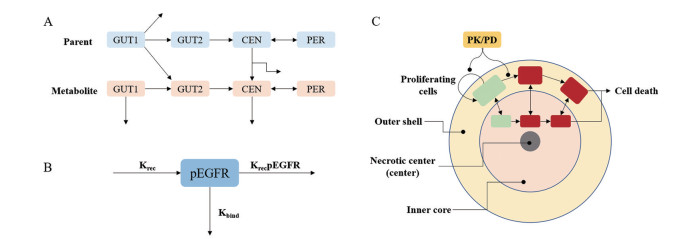
|
Figure 4 Schematics of QSP model[40]. A: Osimertinib-AZ5104 pharmacokinetic module; B: Irreversible binder pharmacodynamic module; C: Tumor module used to describe the efficacy data observed in tumor xenografts |
随后, Yates[55]将临床前QSP模型与奥西替尼和AZ5104的临床药动学研究结果相结合, 预测80 mg奥西替尼对TKI耐药突变的非小细胞肺癌患者的抗肿瘤效果最大, 进一步增加奥西替尼的剂量, 疗效不再增强。随后的临床试验进一步证实: 接受每日80 mg奥西替尼治疗的患者可达59%的客观缓解率(ORR)[56]。因此, 奥西替尼获得了FDA的加速批准。QSP模型为奥西替尼的剂量选择提供了重要的证据[57]。
3.2.3 生物标志物的筛选QSP模型可包含更多的机制通路, 以描述器官、组织、细胞、蛋白、基因等水平的细节, 为生物标志物的识别和筛选提供有价值的信息。例如, 逾半数的三阴性乳腺癌患者, 无法从免疫检查点抑制剂的治疗中获益。通过生物标志物筛选优势治疗人群, 可提高治疗的有效率。Wang等[23]建立了阿替利珠单抗联合紫杉醇(白蛋白结合型) 治疗三阴性乳腺癌的QSP模型。该模型表征了免疫检查点抑制剂和紫杉醇(白蛋白结合型) 治疗三阴性乳腺癌的作用机制, 包括: ①肿瘤细胞增殖和自我凋亡; ②效应T细胞杀伤肿瘤细胞; ③肿瘤细胞释放抗原, 抗原递呈细胞(antigen presenting cells, APC) 提呈抗原, 促使CD8+和CD4+ T细胞激活; ④ T细分布至淋巴结室、中央室、外周室和肿瘤室; ⑤免疫检查点、调节性T细胞(Treg)、骨髓来源的抑制性细胞(myeloid-derived suppressor cells, MDSCs) 和抗炎细胞因子介导免疫逃逸; ⑥阿替利珠单抗阻断PD-1受体与PD-L1配体的结合, 紫杉醇(白蛋白结合型) 直接杀伤肿瘤细胞及肿瘤组织的血管内皮细胞(图 5)。

|
Figure 5 QSP model of immunotherapy in triple-negative breast cancer[23]. Arg-I: Arginase I; aT: Activated T cell; CCL2: Chemokine ligand 2; IL-2: Interleukin-2; mAPC: Mature antigen presenting cell; MDSC: Myeloid-derived suppressor cell; NO: Nitric oxide; nT: Naive T cell; Teff: Effector T cell; TGF-β: Transforming growth factor-β. Th: T helper cell; Treg: Regulatory T cell |
构建的QSP模型还表征了阿替利珠单抗与紫杉醇(白蛋白结合型) 的PK特征。在转移性三阴性乳腺癌患者中, 基于模型的临床试验的仿真模拟, 准确预测了阿替利珠单抗单药以及与紫杉醇(白蛋白结合型) 联用时的ORR。联合治疗的患者中, 应答者肿瘤特异性T细胞克隆、PD-L1表达、CD8+和CD4+ T细胞密度、CD4+/Treg比值和Treg细胞密度的中位数显著高于非应答者; PD-L1表达、CD8+和CD4+ T细胞密度、CD4+/Treg水平高的亚组中具有更高的ORR。敏感性分析进一步表明: 两药联用时, CD8+和CD4+ T细胞密度是最具潜力的生物标志物。基于体内复杂组织、细胞、分子网络的QSP研究方法, 为生物标志物筛选及抗肿瘤药物的精准治疗提供了新的思路。
3.2.4 其他鉴于肿瘤靶向药物的复杂药理机制, 应用QSP模型还可准确预测候选化合物在生物网络中的作用机制及疗效, 筛选具有前景的抗肿瘤新药。例如, 针对肺癌中常见的KRAS突变, 通过表征激活Ras通路的QSP模型, 比较并验证了KRAS G12C抑制剂候选化合物的疗效, 加速了靶向药物的开发[34, 37]。
QSP还可预测和表征抗肿瘤药物的复杂药动学行为。例如, 单克隆抗体制剂通过结合靶向抗原产生疗效的过程中, 应用QSP模型描述了Pb‐Tx[42]、抗体偶联药物[43]与靶标在瘤内分布、消除等体内的药动学过程, 模拟了药物从体循环到肿瘤组织的分布以及肿瘤细胞对药物的摄取, 为预测疗效和制定给药方案提供参考。
此外, 当药物暴露量无法直接预测疗效时, QSP模型可基于机制量化浓度-响应关系, 用于预测临床反应。例如, 在CD3双特异性抗体用于结肠癌的治疗[25]、mTOR抑制剂依维莫司用于肝细胞癌的治疗中[31], QSP模型可准确预测临床疗效。
4 挑战与展望近年来QSP技术受到越来越广泛的关注, 但也面临着巨大的挑战。Bai等[58]评价了已发表的QSP模型, 发现普遍存在模型稳定性和可信性不佳等问题, 建议: ①模型构建时, 应适当简化模型、减少参数、并充分利用组学数据校准模型; ②模型验证时, 将基于模型的临床试验仿真与真实临床研究结果相比较, 优化模型, 提高QSP模型的可信度。
另外, 既往开发的QSP模型涉及多种建模工具, 如MATLAB、R、NONMEM、PK‐Sim/MoBi等, 建模技术和建模过程复杂, 限制了QSP模型的广泛应用。Kirouac等[59]建议应规范模型文件格式和运行脚本, 标准化模型注释方式, 以利于QSP模型的推广。近年来, Certara[60]、Lixoft[61]等软件的开发商正在研发QSP专用软件包, 构建一体化的建模工具, 简化QSP模型开发的流程, 提高QSP模型的可重复性和实用性。
目前肿瘤治疗的疗效、毒性、耐药机制尚不完全明确, 药物研发的失败率居高不下。QSP作为新兴的研究方法, 通过定量描述药物与体内分子、细胞和组织在时间与空间多维度相互作用的网络关系, 可以更好地探索抗肿瘤药物的作用机制、遴选合适生物标志物、有效预测药物的疗效、毒性、耐药, 筛选优势治疗人群, 可加速药物研究的进展。作为FDA模型引导的药物研发试点计划(model-informed drug development pilot program) 中的一部分, QSP在新药监管申报中的应用日益广泛, 大大加速了药物的获批上市。药物上市后, QSP可进一步优化治疗方案, 促进抗肿瘤药物的临床精准治疗。随着系统生物学和建模技术的不断发展, QSP的应用将更加广泛, 在肿瘤治疗领域中必将发挥更大的作用。
作者贡献: 杨迪虹负责论文撰写与修改、图表制作; 王琛瑀提供相关资料的调研及文献整理; 方罗参与论文的审阅与修改; 焦正提供文章的框架设计, 负责全文的审阅与修改。
利益冲突: 所有作者均声明无利益冲突。
| [1] |
Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2018, 68: 394-424. DOI:10.3322/caac.21492 |
| [2] |
Murphy SL, Kochanek KD, Xu J, et al. Mortality in the United States, 2020[J]. NCHS Data Brief, 2021, 427: 1-8. |
| [3] |
Knight-Schrijver VR, Chelliah V, Cucurull-Sanchez L, et al. The promises of quantitative systems pharmacology modelling for drug development[J]. Comput Struct Biotechnol J, 2016, 14: 363-370. DOI:10.1016/j.csbj.2016.09.002 |
| [4] |
Pérez-Nueno VI. Using quantitative systems pharmacology for novel drug discovery[J]. Expert Opin Drug Discov, 2015, 10: 1315-1331. DOI:10.1517/17460441.2015.1082543 |
| [5] |
Aderem A. Systems biology: its practice and challenges[J]. Cell, 2005, 121: 511-513. DOI:10.1016/j.cell.2005.04.020 |
| [6] |
Kohl P, Crampin EJ, Quinn TA, et al. Systems biology: an approach[J]. Clin Pharmacol Ther, 2010, 88: 25-33. DOI:10.1038/clpt.2010.92 |
| [7] |
Yang SL. The progress of systems biology[J]. Bull Chin Acad Sci (中国科学院院刊), 2004, 1: 31-34. |
| [8] |
Tavassoly I, Goldfarb J, Iyengar R. Systems biology primer: the basic methods and approaches[J]. Essays Biochem, 2018, 62: 487-500. DOI:10.1042/EBC20180003 |
| [9] |
Azer K, Kaddi CD, Barrett JS, et al. History and future perspectives on the discipline of quantitative systems pharmacology modeling and its applications[J]. Front Physiol, 2021, 12: 637999. DOI:10.3389/fphys.2021.637999 |
| [10] |
Sorger PK. Quantitative and systems pharmacology in the postgenomic era: new approaches to discovering drugs and understanding therapeutic mechanisms [EB/OL]. NIH, 2011 [2022-01-05]. https://www.nigms.nih.gov/training/documents/systemspharmawpsorger2011.pdf.
|
| [11] |
Bai JPF, Schmidt BJ, Gadkar KG, et al. FDA-industry scientific exchange on assessing quantitative systems pharmacology models in clinical drug development: a meeting report, summary of challenges/gaps, and future perspective[J]. AAPS J, 2021, 23: 60. DOI:10.1208/s12248-021-00585-x |
| [12] |
Vicini P, van der Graaf PH. Systems pharmacology for drug discovery and development: paradigm shift or flash in the pan?[J]. Clin Pharmacol Ther, 2013, 93: 379-381. DOI:10.1038/clpt.2013.40 |
| [13] |
Jiao Z, Li XG, Shang DW, et al. Model informed precision dosing: China expert consensus report[J]. Chin J Clin Pharmacol Ther (中国临床药理学与治疗学), 2021, 26: 1215-1228. |
| [14] |
Kosinsky Y, Dovedi SJ, Peskov K, et al. Radiation and PD-(L)1 treatment combinations: immune response and dose optimization via a predictive systems model[J]. J Immunother Cancer, 2018, 6: 17. DOI:10.1186/s40425-018-0327-9 |
| [15] |
Jafarnejad M, Gong C, Gabrielson E, et al. A computational model of neoadjuvant PD-1 inhibition in non-small cell lung cancer[J]. AAPS J, 2019, 21: 79. DOI:10.1208/s12248-019-0350-x |
| [16] |
Milberg O, Gong C, Jafarnejad M, et al. A QSP model for predicting clinical responses to monotherapy, combination and sequential therapy following CTLA-4, PD-1, and PD-L1 checkpoint blockade[J]. Sci Rep, 2019, 9: 11286. DOI:10.1038/s41598-019-47802-4 |
| [17] |
Wang H, Milberg O, Bartelink IH, et al. In silico simulation of a clinical trial with anti-CTLA-4 and anti-PD-L1 immunotherapies in metastatic breast cancer using a systems pharmacology model[J]. R Soc Open Sci, 2019, 6: 190366. DOI:10.1098/rsos.190366 |
| [18] |
Coletti R, Leonardelli L, Parolo S, et al. A QSP model of prostate cancer immunotherapy to identify effective combination therapies[J]. Sci Rep, 2020, 10: 9063. DOI:10.1038/s41598-020-65590-0 |
| [19] |
Balti A, Zugaj D, Fenneteau F, et al. Dynamical systems analysis as an additional tool to inform treatment outcomes: the case study of a quantitative systems pharmacology model of immuno-oncology[J]. Chaos, 2021, 31: 023124. DOI:10.1063/5.0022238 |
| [20] |
Gong C, Ruiz-Martinez A, Kimko H, et al. A spatial quantitative systems pharmacology platform spQSP-IO for simulations of tumor-immune interactions and effects of checkpoint inhibitor immunotherapy[J]. Cancers (Basel), 2021, 13: 3751. DOI:10.3390/cancers13153751 |
| [21] |
Kumar R, Thiagarajan K, Jagannathan L, et al. Beyond the single average tumor: understanding IO combinations using a clinical QSP model that incorporates heterogeneity in patient response[J]. CPT Pharmacometrics Syst Pharmacol, 2021, 10: 684-695. DOI:10.1002/psp4.12637 |
| [22] |
Voronova V, Peskov K, Kosinsky Y, et al. Evaluation of combination strategies for the A2AR inhibitor AZD4635 across tumor microenvironment conditions via a systems pharmacology model[J]. Front Immunol, 2021, 12: 617316. DOI:10.3389/fimmu.2021.617316 |
| [23] |
Wang H, Ma H, Sové RJ, et al. Quantitative systems pharmacology model predictions for efficacy of atezolizumab and nab-paclitaxel in triple-negative breast cancer[J]. J Immunother Cancer, 2021, 9: e002100. DOI:10.1136/jitc-2020-002100 |
| [24] |
Zhang S, Gong C, Ruiz-Martinez A, et al. Integrating single cell sequencing with a spatial quantitative systems pharmacology model spQSP for personalized prediction of triple-negative breast cancer immunotherapy response[J]. Immunoinformatics (Amst), 2021, 1-2: 100002. DOI:10.1016/j.immuno.2021.100002 |
| [25] |
Betts A, Haddish-Berhane N, Shah DK, et al. A translational quantitative systems pharmacology model for CD3 bispecific molecules: application to quantify T cell-mediated tumor cell killing by P-cadherin LP DART®[J]. AAPS J, 2019, 21: 66. DOI:10.1208/s12248-019-0332-z |
| [26] |
Ma H, Wang H, Sove RJ, et al. A quantitative systems pharmacology model of T cell engager applied to solid tumor[J]. AAPS J, 2020, 22: 85. DOI:10.1208/s12248-020-00450-3 |
| [27] |
Ma H, Wang H, Sové RJ, et al. Combination therapy with T cell engager and PD-L1 blockade enhances the antitumor potency of T cells as predicted by a QSP model[J]. J Immunother Cancer, 2020, 8: e001141. DOI:10.1136/jitc-2020-001141 |
| [28] |
Ma H, Pilvankar M, Wang J, et al. Quantitative systems pharmacology modeling of PBMC-humanized mouse to facilitate preclinical immuno-oncology drug development[J]. ACS Pharmacol Transl Sci, 2020, 4: 213-225. |
| [29] |
Hardiansyah D, Ng CM. Quantitative systems pharmacology model of chimeric antigen receptor T-cell therapy[J]. Clin Transl Sci, 2019, 12: 343-349. DOI:10.1111/cts.12636 |
| [30] |
Mueller-Schoell A, Puebla-Osorio N, Michelet R, et al. Early survival prediction framework in CD19-specific CAR-T cell immunotherapy using a quantitative systems pharmacology model[J]. Cancers (Basel), 2021, 13: 2782. DOI:10.3390/cancers13112782 |
| [31] |
Ande A, Chaar M, Ait-Oudhia S. Multiscale systems pharmacological analysis of everolimus action in hepatocellular carcinoma[J]. J Pharmacokinet Pharmacodyn, 2018, 45: 607-620. DOI:10.1007/s10928-018-9590-0 |
| [32] |
Barrette AM, Bouhaddou M, Birtwistle MR. Integrating transcriptomic data with mechanistic systems pharmacology models for virtual drug combination trials[J]. ACS Chem Neurosci, 2018, 9: 118-129. DOI:10.1021/acschemneuro.7b00197 |
| [33] |
Shankaran H, Cronin A, Barnes J, et al. Systems pharmacology model of gastrointestinal damage predicts species differences and optimizes clinical dosing schedules[J]. CPT Pharmacometrics Syst Pharmacol, 2018, 7: 26-33. DOI:10.1002/psp4.12255 |
| [34] |
Stites EC, Shaw AS. Quantitative systems pharmacology analysis of KRAS G12C covalent inhibitors[J]. CPT Pharmacometrics Syst Pharmacol, 2018, 7: 342-351. DOI:10.1002/psp4.12291 |
| [35] |
Vaidya T, Kamta J, Chaar M, et al. Systems pharmacological analysis of mitochondrial cardiotoxicity induced by selected tyrosine kinase inhibitors[J]. J Pharmacokinet Pharmacodyn, 2018, 45: 401-418. |
| [36] |
Franco YL, Ramakrishnan V, Vaidya TR, et al. A quantitative systems pharmacological approach identified activation of JNK signaling pathway as a promising treatment strategy for refractory HER2 positive breast cancer[J]. J Pharmacokinet Pharmacodyn, 2021, 48: 273-293. DOI:10.1007/s10928-020-09732-x |
| [37] |
Sayama H, Marcantonio D, Nagashima T, et al. Virtual clinical trial simulations for a novel KRAS G12C inhibitor (ASP2453) in non-small cell lung cancer[J]. CPT Pharmacometrics Syst Pharmacol, 2021, 10: 864-877. DOI:10.1002/psp4.12661 |
| [38] |
Wilson JL, Lu D, Corr N, et al. An in vitro quantitative systems pharmacology approach for deconvolving mechanisms of drug-induced, multilineage cytopenias[J]. PLoS Comput Biol, 2020, 16: e1007620. DOI:10.1371/journal.pcbi.1007620 |
| [39] |
Sharan S, Woo S. Quantitative insight in utilizing circulating angiogenic factors as biomarkers for antiangiogenic therapy: systems pharmacology approach[J]. CPT Pharmacometrics Syst Pharmacol, 2014, 3: e139. DOI:10.1038/psp.2014.36 |
| [40] |
Yates JW, Ashton S, Cross D, et al. Irreversible inhibition of EGFR: modeling the combined pharmacokinetic-pharmacodynamic relationship of osimertinib and its active metabolite AZ5104[J]. Mol Cancer Ther, 2016, 15: 2378-2387. DOI:10.1158/1535-7163.MCT-16-0142 |
| [41] |
Stroh M, Green M, Millard BL, et al. Model-informed drug development of the masked anti-PD-L1 antibody CX-072[J]. Clin Pharmacol Ther, 2021, 109: 383-393. DOI:10.1002/cpt.1985 |
| [42] |
Stroh M, Sagert J, Burke JM, et al. Quantitative systems pharmacology model of a masked, tumor-activated antibody[J]. CPT Pharmacometrics Syst Pharmacol, 2019, 8: 676-684. DOI:10.1002/psp4.12448 |
| [43] |
Burton JK, Bottino D, Secomb TW. A systems pharmacology model for drug delivery to solid tumors by antibody-drug conjugates: implications for bystander effects[J]. AAPS J, 2019, 22: 12. |
| [44] |
Menezes B, Cilliers C, Wessler T, et al. An agent-based systems pharmacology model of the antibody-drug conjugate kadcyla to predict efficacy of different dosing regimens[J]. AAPS J, 2020, 22: 29. DOI:10.1208/s12248-019-0391-1 |
| [45] |
Shi Q, Pei F, Silverman GA, et al. Mechanisms of action of autophagy modulators dissected by quantitative systems pharmacology analysis[J]. Int J Mol Sci, 2020, 21: 2855. DOI:10.3390/ijms21082855 |
| [46] |
Bouhaddou M, Yu LJ, Lunardi S, et al. Predicting in vivo efficacy from in vitro data: quantitative systems pharmacology modeling for an epigenetic modifier drug in cancer[J]. Clin Transl Sci, 2020, 13: 419-429. DOI:10.1111/cts.12727 |
| [47] |
Wang H, Sové RJ, Jafarnejad M, et al. Conducting a virtual clinical trial in HER2-negative breast cancer using a quantitative systems pharmacology model with an epigenetic modulator and immune checkpoint inhibitors[J]. Front Bioeng Biotechnol, 2020, 8: 141. DOI:10.3389/fbioe.2020.00141 |
| [48] |
Fornari C, Oplustil O'Connor L, Pin C, et al. Quantifying drug-induced bone marrow toxicity using a novel haematopoiesis systems pharmacology model[J]. CPT Pharmacometrics Syst Pharmacol, 2019, 8: 858-868. DOI:10.1002/psp4.12459 |
| [49] |
Mackey MC, Glisovic S, Leclerc JM, et al. The timing of cyclic cytotoxic chemotherapy can worsen neutropenia and neutrophilia[J]. Br J Clin Pharmacol, 2021, 87: 687-693. DOI:10.1111/bcp.14424 |
| [50] |
Paredes Bonilla RV, Nekka F, Craig M. A quantitative systems pharmacology framework for optimal doxorubicin granulocyte colony-stimulating factor regimens in triple-negative breast cancer[J]. Pharmacology, 2021, 106: 542-550. DOI:10.1159/000518037 |
| [51] |
Sang L, Yuan Y, Zhou Y, et al. A quantitative systems pharmacology approach to predict the safe-equivalent dose of doxorubicin in patients with cardiovascular comorbidity[J]. CPT Pharmacometrics Syst Pharmacol, 2021, 10: 1512-1524. DOI:10.1002/psp4.12719 |
| [52] |
Li M, Nguyen L, Subramaniyan B, et al. PBPK modeling-based optimization of site-specific chemo-photodynamic therapy with far-red light-activatable paclitaxel prodrug[J]. J Control Release, 2019, 308: 86-97. DOI:10.1016/j.jconrel.2019.07.010 |
| [53] |
Falcone R, Lombardi P, Filetti M, et al. Oncologic drugs approval in Europe for solid tumors: overview of the last 6 years[J]. Cancers (Basel), 2022, 14: 889. DOI:10.3390/cancers14040889 |
| [54] |
EFPIA MID 3 Workgroup, Marshall SF, Burghaus R, et al. Good practices in model-informed drug discovery and development: practice, application, and documentation[J]. CPT Pharmacometrics Syst Pharmacol, 2016, 5: 93-122. DOI:10.1002/psp4.12049 |
| [55] |
Yates JW. Dose Selection Using Preclinical PKPD: An Oncology Systems Pharmacology Approach to Dose Selection [R]. London: Dose Finding Workshop European Medicines Agency, 2014 [2022-03-09]. https://www.ema.europa.eu/en/documents/presentation/presentation-oncology-systems-pharmacology-approach-dose-selection-james-yates_en.pdf.
|
| [56] |
Ahn MJ, Tsai CM, Shepherd FA, et al. Osimertinib in patients with T790M mutation-positive, advanced non-small cell lung cancer: long-term follow-up from a pooled analysis of 2 phase 2 studies[J]. Cancer, 2019, 125: 892-901. DOI:10.1002/cncr.31891 |
| [57] |
Musuamba FT, Manolis E, Holford N, et al. Advanced methods for dose and regimen finding during drug development: summary of the EMA/EFPIA workshop on dose finding (London 4-5 December 2014)[J]. CPT Pharmacometrics Syst Pharmacol, 2017, 6: 418-429. DOI:10.1002/psp4.12196 |
| [58] |
Bai JPF, Earp JC, Pillai VC. Translational quantitative systems pharmacology in drug development: from current landscape to good practices[J]. AAPS J, 2019, 21: 72. DOI:10.1208/s12248-019-0339-5 |
| [59] |
Kirouac DC, Cicali B, Schmidt S. Reproducibility of quantitative systems pharmacology models: current challenges and future opportunities[J]. CPT Pharmacometrics Syst Pharmacol, 2019, 8: 205-210. DOI:10.1002/psp4.12390 |
| [60] |
Certara. Quantitative systems pharmacology (QSP) software: regulatory-ready software platforms for reproducible model development [EB/OL]. Certara [2022-03-26]. https://www.certara.com/software/quantitative-systems-pharmacology/.
|
| [61] |
Lixoft. QSP and mechanistic PK/PD modeling workshop [EB/OL]. Lixoft, 2020 [2022-03-26]. https://lixoft.com/blog/2020/02/14/2020qspc_ws/.
|
 2022, Vol. 57
2022, Vol. 57


