2. 中国科学院大学药学院, 北京 100049
2. School of Pharmacy, University of Chinese Academy of Sciences, Beijing 100049, China
机体的免疫系统具有免疫监视的功能, 可以识别、杀伤并清除体内突变细胞。基于肿瘤与免疫系统的相互作用, 可以将肿瘤的进展分为“清除、平衡、逃逸”三个阶段[1]。在清除阶段, 免疫系统识别并清除肿瘤细胞; 而一些突变的肿瘤细胞可以进入平衡阶段, 处于较为稳定的状态; 最后, 肿瘤细胞通过降低免疫原性和增强免疫耐受逃避免疫监视和免疫杀伤, 肿瘤与免疫系统的相互作用失去平衡, 即进入逃逸阶段。这时, 肿瘤细胞可以分泌免疫抑制因子和招募免疫抑制性细胞建立免疫抑制性肿瘤微环境(tumor microenvironment, TME), 进一步抑制抗肿瘤免疫应答, 从而导致肿瘤不受控制的生长, 最终危及生命健康。
随着对肿瘤发生发展机制及其与免疫系统相互作用的深入研究, 免疫疗法成为继传统治疗方法如化疗、放疗后最有前景的策略[2]。现有的免疫治疗主要包括免疫增强化和免疫正常化两种策略[3], 免疫增强化策略包括利用免疫系统的效应分子或细胞直接攻击肿瘤的被动免疫疗法(如基因工程T细胞) 和激活内源性抗肿瘤免疫应答的主动免疫疗法(如疫苗); 免疫正常化策略则是通过纠正肿瘤进展过程中免疫反应的缺陷从而恢复天然的抗肿瘤免疫能力。尽管免疫治疗已经成为人类对抗癌症的有力武器, 但是临床阳性响应率较低。此外, 高昂的价格及药物非肿瘤部位分布导致的免疫相关不良反应(immune-related adverse events, irAE) 也极大限制其安全性和临床应用潜力[4, 5]。因此, 迫切需要开发改善免疫治疗药物瘤内分布的新策略以增强免疫应答和减少irAE[6]。
利用具有特定生物学性质及功能的材料构建的纳米递药系统(nano drug delivery systems, NDDS) 可实现延长药物血液循环时间、增强药物在肿瘤部位的穿透和蓄积、促进肿瘤细胞对其摄取和控制药物释放等多重目标, 为提高瘤内递药效率提供了新策略[7]。自20世纪80年代末以来, 增强的渗透与滞留(enhanced permeability and retention, EPR) 效应一直被认为是纳米载体增加药物在肿瘤部位分布的机制[8]; 然而, 最近研究发现, 纳米粒主要通过受体介导的胞吞转运而非内皮间隙穿透肿瘤[9]。通过对纳米粒进行修饰使其与靶细胞(如肿瘤细胞或免疫细胞) 表面特异性受体相结合可以有效提高靶向能力, 因此, 将研究重心从EPR效应介导的被动靶向型纳米粒转移至设计主动靶向型NDDS可能会更具成效[10]。
较游离药和被动靶向型纳米药物而言, 主动靶向肿瘤的纳米递药系统(active tumor-targeting nano drug delivery systems, aNDDS) 可以提高治疗效果并减少不良反应[11]。目前, 已有多项研究构建aNDDS用于免疫治疗, 并在动物模型上取得较好的治疗效果。本文将依据促进抗肿瘤免疫应答的原理和介导主动靶向的机制, 对近几年开发的用于免疫疗法的aNDDS进行介绍, 并提出该领域的局限性和未来的发展方向。
1 肿瘤免疫逃逸的机制抗肿瘤免疫应答主要由适应性免疫系统完成, 在这一过程中, 抗原呈递细胞如树突状细胞(dendritic cells, DC) 或巨噬细胞捕获肿瘤抗原, 并对其进行加工; 随后, 抗原呈递细胞迁移至淋巴器官将肿瘤抗原提呈给初始T细胞并诱导其活化, 形成效应T细胞; 肿瘤特异性的效应T细胞进入外周血并迁移到肿瘤组织, 识别并杀伤肿瘤细胞; 最后, 效应T细胞死亡, 并生成肿瘤特异性的记忆T细胞[12]。然而, 在“逃逸”阶段, 肿瘤只表达少量甚至不表达肿瘤抗原, 从而对免疫系统“不可见”[13]。
此外, 肿瘤细胞的新陈代谢独具特点, 可以促进免疫抑制性TME的形成[14]。首先, 异常的脉管系统无法供应充足的氧气, 而快速生长的肿瘤细胞又会消耗大量的氧气, 导致实体瘤缺氧, 促进了免疫抑制性细胞的招募, 并通过分泌腺苷和诱导PD-L1的表达抑制免疫应答[15, 16]。其次, 肿瘤细胞的有氧糖酵解导致TME较低的pH值和积累的乳酸, 可以抑制抗肿瘤效应细胞的浸润和活性[17]。另外, T细胞受体的表达和抗原特异性T细胞应答可被精氨酸酶介导的精氨酸耗竭抑制, 从而降低具有抗肿瘤活性的免疫细胞的瘤内浸润[18]。
除肿瘤细胞自身的原因外, TME中浸润的免疫细胞在肿瘤进展中发挥关键作用。调节性T细胞(regulatory T cells, Treg)、髓源抑制细胞(myeloid-derived suppressor cells, MDSC)、M2型巨噬细胞和N2型中性粒细胞等会促进肿瘤发展[19]。肿瘤进展过程中, 肿瘤细胞可以分泌免疫抑制因子如血管内皮生长因子(vascular endothelial growth factor, VEGF)、转化生长因子-β (transforming growth factor-β, TGF-β)、吲哚胺2, 3-二加氧酶(indoleamine-2, 3-dioxygenase, IDO) 等, 并招募Treg等免疫抑制细胞, 阻碍抗肿瘤免疫应答[20]。
以细胞毒性T淋巴细胞相关蛋白4 (cytotoxic T lymphocyte-associated antigen-4, CTLA-4) 和程序性死亡受体1 (programmed cell death 1, PD-1) 为代表的免疫检查点是近几年最受关注的肿瘤免疫逃逸机制[21]。CTLA-4表达于T细胞表面, 当其与DC表面的CD80或CD86结合后, 为T细胞活化提供负反馈信号, 从而抑制T细胞抗肿瘤免疫应答[22]。PD-1是CD28超家族的共抑制受体之一, 可在T细胞、B细胞和髓系细胞中被炎症反应诱导表达, PD-1配体(programmed cell death ligand 1, PD-L1) 在抗原呈递细胞、内皮细胞和肿瘤细胞等多种类型的细胞中表达以防止被细胞毒性T细胞(cytotoxic T lymphocytes, CTL) 识别, 从而逃避免疫监视[23]。除适应性免疫应答外, 肿瘤细胞高表达的CD47还可以与髓系细胞表面的信号调节蛋白α (signal regulatory protein α, SIRPα) 相互作用, 以逃避巨噬细胞和中性粒细胞的吞噬[24]。基于免疫检查点在抗肿瘤免疫应答中的作用, 已经开发出一系列针对CTLA-4、PD-1/PD-L1和CD47的单克隆抗体药物, 通过阻断免疫检查点, 重新激活抗肿瘤免疫应答, 实现有效的抗肿瘤免疫疗法[25]。
2 主动靶向肿瘤的纳米递药系统(aNDDS)通过主动靶向作用, 纳米载体可以减少药物的非特异性分布, 增加药物在瘤内的蓄积, 提高治疗的安全性和有效性[26]。通过表面偶联配体与靶细胞膜受体相互作用介导的胞吞, aNDDS能够提高药物在靶细胞内的浓度[27]。根据肿瘤细胞膜上特异性高表达的受体或蛋白抗原, 目前已经开发出一系列具有高特异性和亲和力的配体、抗体、抗体片段或短肽修饰的纳米载体[28]。例如, 转铁蛋白可以与转移性肿瘤和耐药肿瘤细胞膜上的转铁蛋白受体相结合, 介导药物的内吞[29], Sykes等[30]制备了一系列尺寸的纳米粒, 分别由聚乙二醇(polyethylene glycol, PEG) 和转铁蛋白修饰, 发现粒径在60 nm左右时, 转铁蛋白修饰的纳米粒(主动靶向) 较PEG修饰的纳米粒(被动靶向) 而言, 具有更快的肿瘤蓄积速度(快5倍) 和更高的瘤内浓度(高2倍); 抗CD47抗体修饰的氧化铁纳米粒被证明可以促进胰腺癌细胞对吉西他滨的摄取, 并提高化疗药物的细胞毒性[31]; 精氨酸-甘氨酸-天冬氨酸(RGD) 三肽已被证明可以靶向肿瘤血管和肿瘤细胞表面上调的整合素αvβ3并有效提高药物的递送效率[32]。除蛋白类配体外, 糖类配体也受到关注, 例如, 透明质酸(hyaluronic acid, HA) 是一种可以与CD44受体特异性结合的糖胺聚糖, 可以促进CD44高表达的肿瘤细胞对药物的摄取[33]。此外, 叶酸受体在多种肿瘤细胞中高表达, 多项研究证实叶酸修饰可以增强纳米药物的递送效率和安全性[34]。有研究显示核酸适体也可以结合肿瘤细胞表面特异性的受体, 将其偶联在纳米递送平台上可以介导药物的高效摄取[35]。
除了人工修饰的配体外, 某些特定类型的细胞因具备肿瘤归巢和血液中长循环等能力而被视为潜在的药物递送载体, 通过提取上述细胞的细胞膜并与纳米载体结合, 已经开发出一系列具有良好生物相容性并继承了源细胞优势的仿生纳米药物[36]。例如, 肿瘤细胞膜可以被用于纳米粒的表面功能化, 从而赋予其同源靶向的能力, 实现肿瘤特异性的药物递送[37]。再比如, 由于癌症发展过程中涉及一系列炎症反应, 多种免疫细胞会被招募到肿瘤部位, 这启发研究人员使用免疫细胞的细胞膜包覆纳米药物以获得主动靶向肿瘤的能力。已有研究证实, 基于单核/巨噬细胞膜、中性粒细胞膜和淋巴细胞膜的NDDS可以提高抗肿瘤药物的递送效率[38-41]。此外, 血小板可以与循环肿瘤细胞(circulating tumor cells, CTC) 上的黏附分子如P-选择素配体相互作用, 从而被用于构建主动靶向CTC的药物递送平台[42], 可被招募至肿瘤组织的各类干细胞也可以赋予纳米药物主动靶向肿瘤的能力[43]。
3 aNDDS激活抗肿瘤免疫应答由于免疫系统分布广泛, 且机体内时刻都有免疫反应的发生, 在免疫治疗中, 常规的全身给药策略难以避免对非抗肿瘤的免疫应答产生不必要的激活或放大作用, 从而引起一系列irAE。利用肿瘤靶向型纳米平台递送的免疫治疗药物, 可以有效提高药物的瘤内分布比例, 实现精准、特异的靶向给药, 改善治疗效果(图 1)。作者将根据aNDDS激活抗肿瘤免疫应答的机制对现有的策略进行分类阐述。
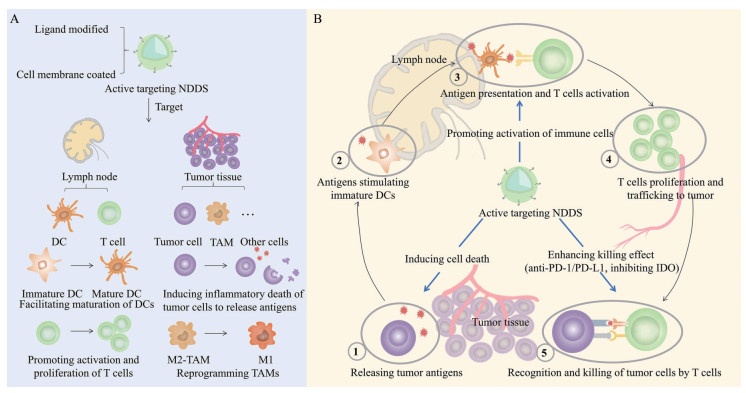
|
Figure 1 Nano drug delivery systems (NDDS) with the active targeting ability promoting anti-tumor immune responses. A: Ligand/antibody-modified or cell membrane-coated NDDS can actively target lymph nodes or tumor tissues to activate anti-tumor immune responses; B: The cancer-immunity cycle and the role of active targeting NDDS. DC: Dendritic cell; TAM: Tumor-associated macrophage; IDO: Indoleamine 2, 3-dioxygenase |
肿瘤抗原表达的缺失是免疫逃逸的重要机制之一, 肿瘤细胞通过下调强排斥抗原的表达、下调主要组织相容性抗原I (major histocompatibility antigen I, MHC I) 水平和削弱抗原呈递功能来逃避免疫系统的杀伤[44]。利用aNDDS将药物特异性递送至肿瘤细胞, 并诱导特定类型的细胞死亡, 会导致肿瘤相关抗原(tumor-associated antigen, TAA) 的释放, 进而被免疫细胞识别, 激活抗肿瘤免疫应答。
3.1.1 诱导免疫原性细胞死亡肿瘤细胞在特定化疗药物、光疗或放疗等诱导下可以发生免疫原性细胞死亡(immunogenic cell death, ICD), 释放TAA和损伤相关分子模式(damage associated molecule pattern, DAMP), 使“冷”肿瘤转变成“热”肿瘤, 促进免疫细胞的瘤内浸润[45]。此外, 触发的抗肿瘤免疫应答不仅可以抑制原发灶肿瘤的生长, 还可以发挥远端效应, 杀伤转移的癌细胞。
肝配蛋白受体A2 (EphA2) 在非小细胞肺癌、肝癌和乳腺癌等肿瘤细胞表面高表达, Drummond团队[46]证实针对该受体的抗体单链可变区片段(scFv) 介导的主动靶向可以改善载多西他赛脂质体的药物体内分布和瘤内穿透能力。多西他赛可以诱导肿瘤细胞高表达钙网蛋白和MHC-I, 显著提高了CD8+ T细胞介导的杀伤活性[47]。与免疫检查点抑制剂联合使用时, 在EMT-6小鼠乳腺癌模型中抑制了93%的肿瘤生长。而游离的多西他赛无论是单独使用还是与抗PD-1抗体的联合治疗, 都不能提高荷瘤小鼠的中位生存期。由此可见, 药物的有效递送对其疗效有不可忽视的影响, aNDDS递送细胞毒性药物联合免疫检查点抑制剂为激活抗肿瘤免疫响应提供了可行的方案。
利用计算生物学技术, 根据抗原设计抗体模拟物用于修饰纳米载体有效提高了亲和力和选择性, Au等[48]采用选择性高亲和力配体(selective high affinity ligand, SHAL) 功能化的纳米粒递送多柔比星, 其可与淋巴瘤表达的人白细胞抗原D相关抗原结合。研究表明, 该功能化纳米粒在肿瘤内的蓄积量是游离多柔比星的两倍, 能更有效地抑制肿瘤的生长。多柔比星与后续的放疗协同诱导肿瘤细胞发生ICD, 实现有效的化学免疫放射疗法的同时没有发生明显不良反应。
多肽是常用的靶向配体之一, Wang等[49]通过微流体技术将肿瘤靶向肽RGD偶联在聚合物上形成尺寸均一的纳米复合物, 可以增加在癌细胞中的摄取, 从而提高对4T1乳腺癌细胞的光热毒性。光热疗法(photothermal therapy, PTT) 是一种热消融的局部疗法, 可以诱导TAA的释放。当用该纳米复合物介导的PTT治疗荷瘤小鼠后, 肿瘤部位CD4+和CD8+ T细胞的浸润大幅提高, 促炎细胞因子的分泌也相应增加, 表明该疗法触发了强烈的抗肿瘤免疫反应。
利用HA与CD44的结合能力, He等[50]将HA与多柔比星偶联, 并与装载氯尼达明二聚体(LTPT) 的三苯基膦纳米粒组装形成纳米复合物(图 2A), 该纳米复合物具有长循环和高肿瘤蓄积的特点, 在肿瘤内, 透明质酸酶降解HA, 使得利于细胞摄取的阳离子基团暴露出来, 在胞内, 三苯基膦携带LTPT靶向至线粒体, 诱导细胞色素c释放及下游凋亡信号激活。释放的多柔比星诱导肿瘤细胞凋亡和ICD, 激活免疫反应, 与抗PD-L1抗体协同激活CTL (图 2B)。在给荷4T1乳腺癌小鼠注射纳米复合物36 h后, 多柔比星的荧光是未偶联HA制剂的1.4倍, 且实现了较长时间的瘤内蓄积, 体现了HA修饰的优越性(图 2C、D)。双药化疗与免疫治疗诱导了大多数肿瘤细胞的凋亡, 抑制了原位瘤和转移瘤的生长, 并诱导长期免疫记忆, 延长荷瘤小鼠的生存时间(图 2E~M)。这种级联靶向的NDDS大幅提高了治疗的有效性和安全性, 为联合用药提供了新的策略。
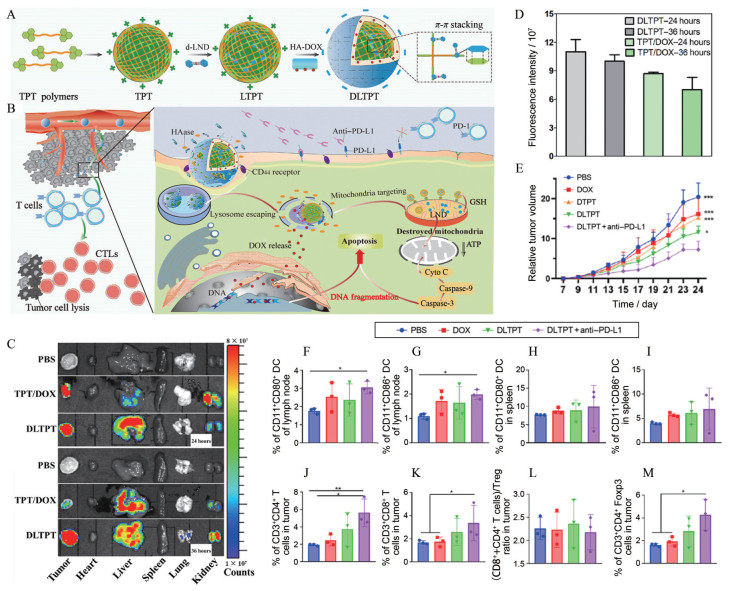
|
Figure 2 A dual-drug-loaded HA-decorated enzyme-sensitive nanoparticle for chemo-immune therapy of breast cancer. A: Schematic illustration for the self-assembly process of the cascade-targeting enzyme-sensitive hierarchical nanoplatform; B: Schematic illustration of the combinational effects of chemotherapy with the anti-PD-L1 therapy for activating the immune system; C: Ex vivo fluorescence distribution of different tissues; D: Quantitative analysis of tumor tissues after administration with phosphate buffered saline (PBS), triphenylphosphonium derivative nanoparticle (TPT)/doxorubicin (DOX), and DOX and lonidamine (LND)-loaded TPT (DLTPT) for 24 and 36 h in 4T1 tumor model mice; E: Relative tumor volumes of mice with PBS, DOX, DOX-loaded TPT (DTPT), DLTPT, and DLTPT + anti-PD-L1 formulations at the end of each treatment. n = 6, x±s. *P < 0.05, ***P < 0.001 vs DLTPT + anti-PD-L1; F: CD11c+ CD80+ cells in the lymph node; G: CD11c+ CD86+ cells in the lymph node; H: CD11c+ CD80+ cells in the spleen; I: CD11c+ CD86+ cells in the spleen; J: CD4+ T cells; K: CD8+ T cells; L: The ratio of (CD8+ T and CD4+ T cells) and CD4+ Foxp3 T cells (Treg); M: Treg in tumors. n = 3, x±s. *P < 0.05, **P < 0.01. LTPT: LND-loaded TPT; CTL: Cytotoxic T lymphocytes; Treg: Regulatory T cell; HAase: Hyaluronidase; GSH: Glutathione; ATP: Adenosine triphosphate. (Adapted from Ref. 50 with permission. Copyright © 2021 American Association for the Advancement of Science) |
铁死亡是近年来发现的一种新的细胞死亡形式, 可以提高肿瘤免疫原性。现有的诱导肿瘤细胞发生铁死亡的试剂通常面临易被机体免疫系统清除和难以靶向肿瘤细胞两大问题。Jiang等[51]通过挤压法将装载柳氮磺吡啶(SAS) 的磁性氧化铁纳米粒(Fe3O4 NP) 包覆上血小板膜, 获得具有较好稳定性的仿生纳米粒Fe3O4-SAS@PLT (图 3A)。血小板膜上的P-选择素与4T1肿瘤细胞表面的CD44的相互作用可以增强细胞对纳米粒的摄取, 并促进纳米粒在转移性肿瘤中蓄积。被摄取的磁性氧化铁和柳氮磺吡啶协同诱导铁死亡, 暴露TAA, 激活抗肿瘤免疫应答。仿生纳米粒诱导的DC熟化率增加至69.9%, 显著高于未包覆血小板膜的纳米粒(28.4%) (图 3B~D)。PD-1抑制剂和该仿生纳米粒被联合用于治疗荷瘤小鼠, 使76%的小鼠生存期达到80天, 而其他对照组的小鼠均在50天内死亡。这种利用细胞膜的天然配体将药物特异性地递送至肿瘤部位的方法为增强抗肿瘤免疫提供了可行的策略, 同时, 来自细胞膜的出色的生物相容性提高了临床转化的可能性。
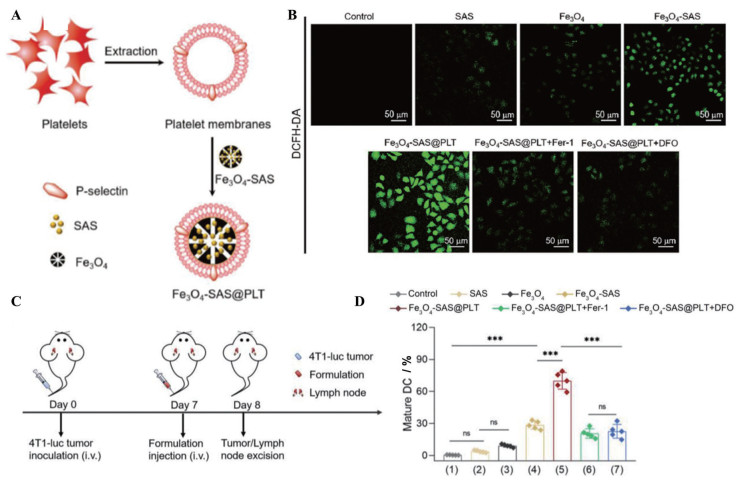
|
Figure 3 Magnetic nanoparticles camouflaged with platelet membranes triggered ferroptosis for enhancing immunotherapy. A: Preparation of sulfasalazine (SAS)-loaded mesoporous magnetic nanoparticles (Fe3O4) and platelet (PLT) membrane camouflage (Fe3O4-SAS@PLT); B: Representative confocal laser scanning microscope images of 4T1 cells stained with 2, 7-dichlorodi-hydrofluorescein diacetate (DCFH-DA) in different groups [SAS, Fe3O4, Fe3O4-SAS, Fe3O4-SAS@PLT, Fe3O4-SAS@PLT + ferrostatin-1 (Fer-1), and Fe3O4-SAS@PLT + deferoxamine (DFO)]; C: Schematic illustration of dosing regimens of Fe3O4-SAS@PLT in 4T1 metastatic tumor-bearing mice; D: Quantification of mature DC (CD86+/CD80+, gated on CD11c+ cells) in the lymph nodes of 4T1 metastatic tumor-bearing mice at 24 h after mice received different treatments including ① untreated 4T1 cells, ② SAS, ③ Fe3O4, ④ Fe3O4-SAS, ⑤ Fe3O4-SAS@PLT, ⑥ Fe3O4-SAS@PLT + Fer-1, and ⑦ Fe3O4-SAS@PLT + DFO. n = 5, x±s. ***P < 0.001. (Adapted from Ref. 51 with permission. Copyright © 2020 WILEY-VCH) |
细胞焦亡作为一种高度促炎性的细胞死亡, 具有逆转肿瘤免疫抑制的潜力。为了减轻肿瘤对诱导焦亡的化疗药物的耐药性及化疗药物对正常细胞的毒性, 亟需开发将诱导细胞焦亡的药物靶向递送至肿瘤部位的新策略。为了获得肿瘤细胞的低免疫原性和同源黏附特性, Zhao等[52]使用4T1乳腺癌细胞膜包覆聚乳酸-聚乙醇酸共聚物[poly(lactic-co-glycolic acid), PLGA] 纳米粒, 并封装光敏剂吲哚菁绿(ICG) 和地西他滨, 形成仿生纳米粒(BNP)。由于肿瘤细胞膜的伪装, 4T1细胞可以更有效地摄取BNP。随后, ICG介导的光疗和地西他滨协同诱导肿瘤细胞发生细胞焦亡, 释放细胞色素c, 活化半胱氨酸天冬氨酸蛋白酶3。BNP联合光照提高了小鼠血液中白介素-6和肿瘤坏死因子α的水平, 并在原位瘤和肿瘤引流淋巴结中诱导DC的成熟, 表明该治疗促进了肿瘤的抗原呈递, 触发了全身性的抗肿瘤免疫应答。此外, 接受BNP给药联合光疗的小鼠转移瘤中CD8+和CD4+ T细胞显著增加而Treg浸润明显减少, 证明了细胞焦亡可以激活效应T细胞, 实现有效的抗肿瘤免疫治疗。
3.2 调节抗肿瘤免疫相关的免疫细胞TME的免疫细胞相互影响, 并在肿瘤进展过程中逐渐演变, 通过免疫编辑而维持肿瘤的免疫抑制[19]。通过一系列方法重塑免疫细胞的组成, 提高抗肿瘤免疫细胞的比例并降低免疫抑制性细胞的比例, 可以逆转TME的免疫抑制。
3.2.1 促进DC熟化DC加工肿瘤抗原, 并将其呈递给初始T细胞, 诱导T细胞的活化, 在抗肿瘤免疫应答中起到承上启下的作用。由于内源性肿瘤的低免疫原性无法诱导DC熟化, 科研人员设计了一系列方法将疫苗或佐剂递送至淋巴结的DC从而激活抗肿瘤免疫反应。
为了将癌症疫苗有效地递送到淋巴结的CD8+ DC, 研究人员构建了修饰CD205的肿瘤细胞膜, 用于包覆吸附了免疫佐剂CpG寡脱氧核苷酸(CpG oligodeoxynucleotide, CpG-ODN) 的磁性纳米团簇(图 4A、B)[53]。磁场控制可使磁性纳米团簇滞留在淋巴结, 优先被DC识别, 肿瘤细胞膜上的多种蛋白质作为抗原, 与CpG联合激活DC, 激发抗肿瘤免疫(图 4C~F)。使用该治疗方案联合抗PD-1抗体, 在恶性转移瘤模型中实现了80天100%的生存率。
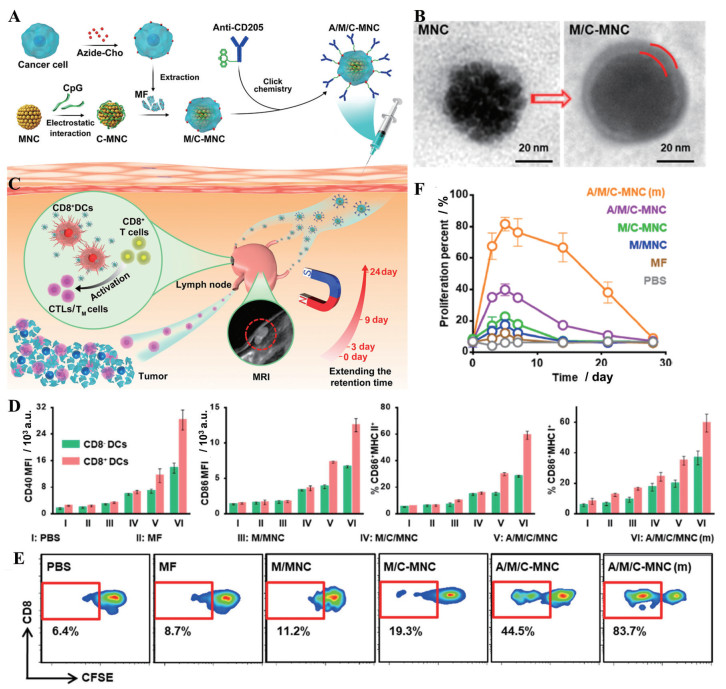
|
Figure 4 Engineering magnetosomes with the active targeting ability promoted DC maturation. A: Fabrication process of cancer-cell-membrane-coated CpG-ODN-loaded Fe3O4 magnetic nanoclusters (MNC) with anti-CD205 decoration (A/M/C-MNC); B: Transmission electron microscope images of MNC and cancer-cell-membrane-coated CpG-ODN-loaded MNC (M/C-MNC). The space between the red lines indicates the cancer cell membrane; C: Illustration of A/M/C-MNC-mediated cellular immune responses eliciting CTLs and memory T cells (TM cells) for cancer immunotherapy; D: Analysis of DC maturation markers CD40 and CD86, MHC-II, and the cross-presentation marker MHC-I in the lymph nodes after vaccination with different formulations: PBS, 4T1 cancer cell membrane fragments (MF), MNC with membranes camouflaging (M/MNC), M/C-MNC, A/M/C-MNC, A/M/C-MNC with magnetic retention [A/M/C-MNC (m)]. n = 3, x±s; E: Carboxyfluorescein-succinimidyl-aminoester (CFSE)-based flow cytometry of CD8+ T cell proliferation in vivo after different treatments; F: CTL proliferation percent at 4 weeks after vaccination with different formulations. n = 3, x±s. MRI: Magnetic resonance imaging. (Adapted from Ref. 53 with permission. Copyright © 2019 American Chemical Society) |
CTL是适应性免疫应答中发挥杀伤作用的效应细胞, 其在瘤内的浸润和活化不足也是现有的免疫疗法如抗PD-1/PD-L1疗法疗效受限的重要原因。因此, 使用主动靶向型NDDS促进T细胞的招募, 并提高其活性, 可以有效增强抗肿瘤免疫活力。
已有研究表明, TGF-β信号的抑制可以促进CD8+ T细胞的扩增和激活并限制Treg的活性; 此外, Toll样受体的激活可以增加淋巴细胞的瘤内浸润, 从而提高癌症患者对免疫治疗的反应性。为将TGF-β抑制剂或Toll样受体7/8激动剂靶向递送至T细胞, Schmid等[54]设计了一种由抗PD-1抗体帕博利珠单抗F(ab')2片段修饰的纳米载体递送TGF-β抑制剂SD-208或Toll样受体7/8激动剂雷西莫特。结果表明, 与未载药的空纳米载体或游离药物组相比, 仅载SD-208的纳米载体抑制了小鼠肿瘤的生长, 载雷西莫特的纳米载体上调了肿瘤浸润淋巴细胞。这种将免疫治疗药物靶向递送至免疫细胞亚群的方式在逆转免疫耐受、增加患者对免疫疗法敏感性方面有广阔的应用前景。
除直接将免疫激动剂递送给T细胞外, 也可以通过调节TME或肿瘤细胞内信号通路以及将能招募T细胞的趋化因子递送至肿瘤内来影响T细胞的浸润和活化。Cho等[55]使用RGD将小干扰RNA (siRNA) 靶向递送到肿瘤血管内皮细胞, 下调血管内皮生长因子受体2 (vascular endothelial growth factor receptor 2, VEGFR2) 的表达, 使肿瘤血管正常化, 缓解肿瘤缺氧, 有效提高T细胞的浸润, 为免疫检查点阻断疗法营造有利的环境。
3.2.3 诱导肿瘤相关巨噬细胞表型转化肿瘤相关巨噬细胞(tumor-associated macrophage, TAM) 是免疫抑制性TME的重要组成成分[56]。巨噬细胞的经典表型分为促炎、抗癌的M1型和抗炎、促癌的M2型。TAM通常为M2型, 因此, 将M2型TAM转化为M1型是恢复抗肿瘤免疫反应的可行方案。然而, 由于巨噬细胞分布的异质性及实体瘤中致密的细胞外基质, 如何实现有效且特异的药物递送成为诱导TAM表型转化治疗的一大难点。主动靶向巨噬细胞的纳米载体为转化TAM的药物递送提供了有潜力的平台。
靶向M2型巨噬细胞的多肽可被用于修饰递送促巨噬细胞转化药物的载体, 以实现对TAM特异的调控。信号转导和转录激活蛋白6 (signal transducer and activator of transcription 6, STAT6) 和核因子-κB (nuclear factor kappa-B, NF-κB) 通过不同的机制促进巨噬细胞的M2极化, 因此, 抑制这两个信号通路可能导致巨噬细胞的表型转换。Xiao等[57]构建了一种集TME响应和主动靶向双重特征于一体的纳米药物。首先使用pH敏感的PEG对M2靶向肽进行掩蔽, 随后用该靶向肽修饰包载STAT6抑制剂AS1517499 (AS) 和NF-κB上游分子NF-κB激酶抑制因子(inhibitor of nuclear factor kappa-B kinase β, IKKβ) siRNA的纳米药物, 获得纳米复合物(图 5A、B)。纳米复合物到达肿瘤后, 在TME弱酸条件下PEG保护壳脱落, 暴露出M2靶向肽, 将所载药物特异性地递送至M2型巨噬细胞, 诱导M2型巨噬细胞表型转化的同时, 减少不良反应的发生, 实现安全有效的免疫疗法(图 5C~E)。
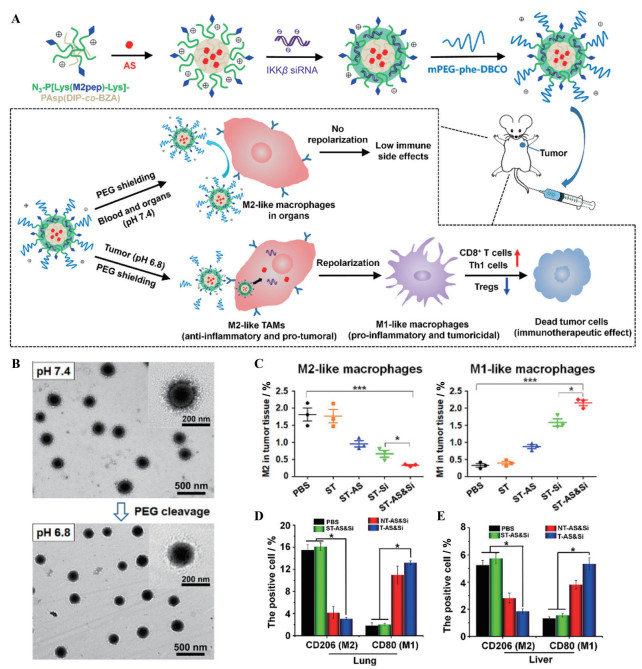
|
Figure 5 Targeting peptide-decorated nanocarriers delivered drugs for specifically reprogramming TAMs. A: Schematic illustration of a PEG-sheddable nanodrug targeting M2-like TAMs for anti-tumor immunotherapy; B: Morphology and size of the inhibitor of IKKβ siRNA (Si)-loaded M2-targeting micelle coating a sheddable PEG corona (ST-Si) (N/P = 15) at pH 7.4 and pH 6.8 under transmission electron microscope; C: Representative flow cytometric analysis displaying the absolute percentage of the tumor-infiltrating M2-like macrophages, M1-like macrophages in the tumors following various treatments [PBS, M2-targeting micelle coating a sheddable PEG corona (ST), AS-loaded M2-targeting micelle coating a sheddable PEG corona (ST-AS), ST-Si, AS & Si-coloaded M2-targeting micelle coating a sheddable PEG corona (ST-AS & Si)]; D, E: Amounts of M2- or M1-like macrophages in the lung and liver were analyzed by immunohistochemical assays to explore the inflammatory reaction. CD206-positive cells (M2) and CD80-positive cells (M1) counted in the lung (D) and liver (E) after different treatments [PBS, ST-AS & Si, AS & Si-coloaded M2-targeting micelle coating a nonsheddable PEG corona (NT-AS & Si), AS & Si-coloaded M2-targeting micelle (T-AS & Si)]. n = 3, x±s. *P < 0.05, ***P < 0.001. siRNA dose: 250 μg·kg-1; AS dose: 60 μg·kg-1. Th1 cells: Type 1 T helper cells. (Adapted from Ref. 57 with permission. Copyright © 2020 American Chemical Society) |
特定的配体, 例如与唾液酸结合免疫球蛋白型凝集素(外周血单核细胞和TAM表面高表达) 结合的唾液酸[58]、与甘露糖受体结合的甘露糖[59]、与CD44结合的HA[60], 均可有效提高纳米粒主动靶向TAM的能力。Song等[61]利用PEG保护的甘露糖修饰聚合物纳米粒, 以赋予其TME敏感及TAM靶向的双重功能, 最终有效地将VEGF siRNA和胎盘生长因子siRNA递送至TAM中, 减少了瘤内M2型巨噬细胞的比例。根据巨噬细胞表面的特定蛋白, Kulkarni等[62]通过计算生物学设计了一种能和SIRPα特异性结合并抑制集落刺激因子1受体(colony stimulating factor-1 receptor, CSF-1R) 的超分子, 该超分子可与特定化合物自组装成纳米粒。通过超分子靶向巨噬细胞, 阻断巨噬细胞SIRPα受体与CD47的相互结合, 并阻断CSF-1R信号通路, 使得TAM向M1型转化, 诱导针对肿瘤的固有免疫应答。除了使用单一的配体修饰纳米载体外, He等[63]使用甘露糖和HA共同修饰的载体递送CpG-ODN, 发现双靶向可以实现比单靶向更有效的药物递送, 并导致CD80在超过70%的巨噬细胞中表达, 证明巨噬细胞有效转化为M1型。
除直接提取的细胞膜外, 其他类型的细胞来源膜结构在主动靶向仿生纳米药物的设计中也得到了广泛应用。细胞外纳米囊泡(cellular nanovesicles, NV) 是一种从细胞膜衍生的纳米脂质囊泡, 具有较高的稳定性和安全性, 且含有源细胞的蛋白和脂质, 因此继承了源细胞的一系列功能。基于NV的上述优势, Rao等[64]使用血小板来源的NV (P-NV)、M1型巨噬细胞来源的NV (M1-NV) 和经SIRPα变体改造的癌细胞衍生的NV (C-NV) 合成三元杂化NV (hNV)。由于其脂质双层结构和部分血小板的特征, hNV具有延长的血液循环时间和靶向CTC的特点, 可以减少在其他器官的积累, 降低不良反应; M1-NV包含了M1型巨噬细胞的mRNA和miRNA, 可以传递促炎信号, 从而促使瘤内TAM从M2型转变为M1型; SIRPα变体可拮抗CD47, 激活巨噬细胞对肿瘤的吞噬作用。基于上述作用机制, hNV抑制了B16F10肿瘤的术后复发和转移, 为开发新型的激活抗肿瘤免疫应答策略提供了可参考的平台。除了hNV外, M1巨噬细胞衍生的外泌体自身即可作为免疫调节剂, 诱导TAM转化为M1型, 促进抗PD-L1抗体的治疗效果[65]。
3.2.4 调节多种免疫细胞抗肿瘤免疫应答由多种免疫细胞共同参与, 利用aNDDS共递送不同药物, 同时对多种免疫细胞进行调节, 有望实现更明显的抗肿瘤效应。其递送策略主要分为两类, 一是靶向肿瘤组织, 实现对TME中免疫细胞的调节; 二是靶向肿瘤引流淋巴结的免疫细胞, 增强对外周免疫器官的刺激, 实现全身性抗肿瘤效果。
为避免靶向肿瘤HA受体的载药系统被吞噬细胞摄取而在肝内蓄积, Shin等[66]使用基质金属蛋白酶-9 (matrix metalloproteinase-9, MMP-9) 敏感性多肽连接PEG和HA, 以临时掩蔽HA的靶向能力, PEG偶联的HA随后与外源抗原模型卵清蛋白(OVA) 连接, 形成MMP-9敏感型疫苗PEG-pep-HA-OVA。与未用多肽修饰的纳米药物PEG-HA-OVA相比, PEG-pep-HA-OVA可以更显著地增强抗原呈递效率和促进CTL的活化, 有效控制肿瘤体积增长。此外, HA还具有介导免疫细胞黏附和迁移的功能, Sun等[67]合成了HA修饰的纳米粒, 在实现靶向和深度穿透乳腺癌组织的同时, 招募淋巴细胞并诱导TAM表型转变, 为重塑TME、增强免疫治疗效果提供了新颖的无药物平台。另外, 使用黑色素瘤来源的肿瘤细胞膜构建的仿生纳米系统集抗原、佐剂和化疗药物于一体, 通过同源靶向功能蓄积到肿瘤部位, 实现了高效的化疗-免疫联合治疗[68]。
为了将肿瘤抗原靶向递送至淋巴结, Cao等[69]在金纳米粒上偶联了OVA和HA, 以实现近红外光(near infrared light, NIR) 触发内体/溶酶体破裂和胞质抗原释放(图 6A)。结果表明, HA的修饰导致纳米药物更有效地从注射部位分布到淋巴结, 且HA和NIR都能增强骨髓源性DC对OVA的摄取, 并在抗原的刺激下提高DC成熟率(图 6B)。使用主动靶向纳米载体递送的OVA联合NIR照射导致抗原特异性的CD8+ T细胞比例的升高, 是游离OVA组的3倍以上, 激活的免疫系统抑制肿瘤体积增长, 从而延长荷瘤小鼠的生存期(图 6C~E)。除了特异性的配体, 经靶向淋巴细胞表面特定蛋白的抗体修饰的纳米药物同样可以通过受体介导的胞吞作用提高递送效率[70]。
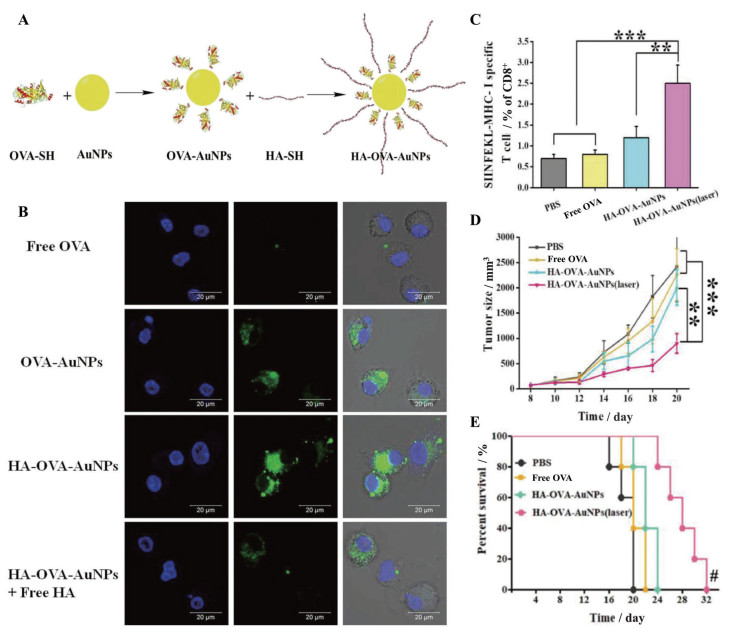
|
Figure 6 HA-decorated gold nanoparticles achieved photothermally controlled cytosolic antigen delivery for effective immunotherapy. A: Strategic illustration of the HA and ovalbumin (OVA)-decorated gold nanoparticle (AuNPs) (HA-OVA-AuNPs) complex as a nanovaccine; B: Cellular uptake images of fluorescein isothiocyanate (FITC)-labeled OVA-AuNPs with or without HA coating; C: The proportion of OVA-specific CD8+ T cells in the spleen determined by flow cytometry; D, E: Tumor volumes (D) and mice survival rates (E) during the treatment period. n = 3-5, x±s. **P < 0.01, ***P < 0.001; the Hashtag symbols indicated that differences between HA-OVA-AuNPs under laser irradiation and the other groups, #P < 0.05. (Adapted from Ref. 69 with permission. Copyright © 2018 WILEY-VCH) |
在肿瘤进程中, 一些分子的表达上调导致免疫应答的抑制, 帮助肿瘤实现免疫逃逸。因此, 阻断肿瘤细胞与免疫系统负向调控的信号通路, 可以激活抗肿瘤免疫应答。例如, 表面修饰了CD47抗体和重组钙网蛋白的二氧化硅纳米粒, 可以阻断CD47/SIRPα通路, 激活巨噬细胞对肿瘤细胞的吞噬作用[71]。另一方面, IDO在肿瘤中上调, 可以将色氨酸代谢为犬尿氨酸, 抑制效应性T细胞活性, 促进Treg的增殖, 从而有助于肿瘤逃避免疫系统的攻击[72]。因此, 阻断IDO通路的药物也具有阻断肿瘤免疫逃逸的潜力。通过aNDDS递送上述药物, 可避免在非肿瘤部位免疫系统的过度激活, 减少irAE[73]。
3.3.1 阻断PD-1/PD-L1近年来, 已有多种NDDS被应用于抗PD-1/PD-L1的治疗[74]。通过在纳米载体表面修饰靶向抗体或配体, 可以提高免疫检查点抑制剂在肿瘤部位的有效浓度, 提高治疗方法的安全性和有效性。除了使用抗体外, 抑制PD-1/PD-L1上游信号通路同样可以实现免疫检查点的阻断。研究表明, β-连环素的敲除会导致肿瘤中PD-L1表达的下调。为了将能够敲除β-连环素的CRISPR-Cas9质粒有效导入肿瘤细胞, 将核仁素的适体AS1411和HA偶联, 赋予其靶向肿瘤细胞膜或细胞核的能力[75], 并同时将含有核定位序列的多肽与载体偶联, 用于增强其细胞穿透和核转运能力。多重修饰的纳米载体被肿瘤细胞高效摄取, 随后将质粒成功递送至细胞核, 实现了高达40.2%的基因编辑效率, 显著下调β-连环素和PD-L1的表达。处理过的肿瘤细胞可以促进CD8+ T细胞的增殖、细胞因子的释放及癌细胞溶解, 表明该纳米系统具有逆转肿瘤免疫抑制的潜力。此外, 经C-X-C基序趋化因子受体4 (C-X-C motif chemokine receptor 4, CXCR4) 靶向肽修饰的纳米粒通过递送丝裂原活化蛋白激酶/细胞外调节蛋白激酶激酶(mitogen-activated protein kinase/extracellular signal-regulated kinase kinase, MEK) 抑制剂可以有效抑制索拉菲尼导致的PD-L1的上调[76]。这些不同类型配体修饰的递药系统为阻断免疫检查点提供了多样的解决方案, 从阻断PD-1/PD-L1通路的药物选择、如何提高递送效率和特异性以及联合用药等方面为后续的研究提供了参考。
基于细胞膜的仿生纳米载体也可实现免疫检查点抑制剂的主动靶向递送。使用PD-L1抑制肽修饰的肿瘤细胞膜[77]、抗PD-L1抗体修饰的血小板细胞膜[78]和表达了PD-1的杂化细胞膜[79]制备仿生纳米载体都显示出有效的瘤内蓄积能力, 从而恢复T细胞对肿瘤细胞的杀伤。T细胞膜上存在多种可以与肿瘤细胞相互作用的蛋白(如黏性蛋白LFA-1), 并且表达PD-1, 因此, 选用T细胞膜作为药物递送平台, 无需对其进行额外的工程化便可实现主动靶向和免疫检查点阻断双重作用。Kang等[80]选择来源于EL4细胞系的T细胞膜包覆载药PLGA纳米粒来制备模拟T细胞的纳米粒(TCMNP), TCMNP可以通过释放所载药物起到抗肿瘤效果, 也可以通过T细胞膜表面的Fas配体(FasL) 直接杀伤肿瘤细胞, 并通过表面的PD-1阻断免疫检查点, 协助CTL恢复细胞毒性(图 7A~C)。在B16F10黑色素瘤小鼠模型中, 与游离抗PD-L1抗体相比, TCMNP有效地提高了CD8+ T细胞的浸润并降低Treg的比例, 在没有观察到明显毒性的情况下持续延缓了肿瘤的生长(图 7D~G)。
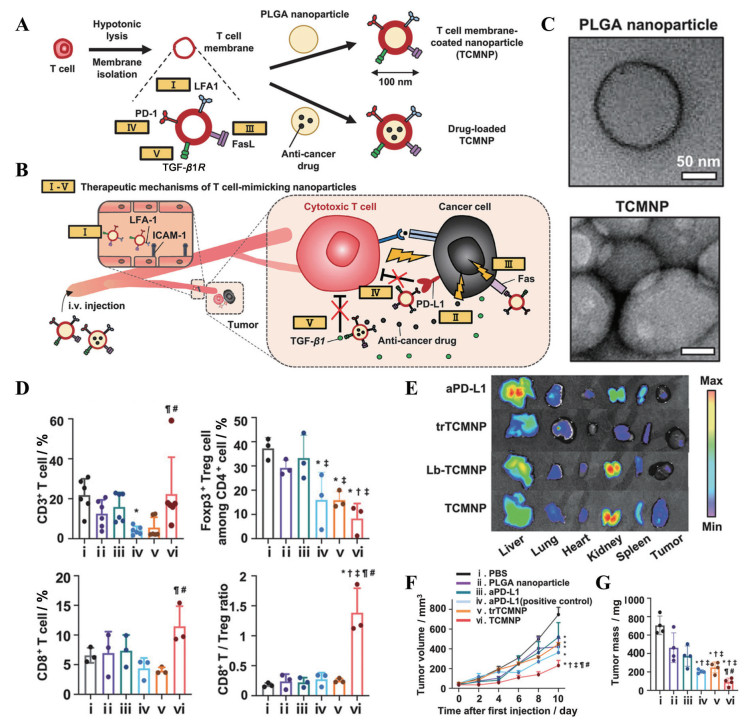
|
Figure 7 T-cell-membrane-coated nanoparticles achieved anti-tumor immunotherapy. A: Preparation of T-cell-membrane-coated nanoparticles (TCMNPs) and anti-cancer drug-loaded TCMNPs; B: Proposed therapeutic mechanisms of TCMNPs: Ⅰ) TCMNPs actively targeted the tumor by interactions between lymphocyte function associated antigen (LFA)-1 on TCMNPs and intercellular cell adhesion molecule (ICAM)-1 on inflamed endothelium in tumor tissues; Ⅱ) Anti-cancer drugs released from TCMNPs; Ⅲ) Fas ligand (FasL) on TCMNPs directly killed cancer cells. Unlike CTLs, TCMNPs were not influenced by immunosuppressive factors in TME and could avoid immunosuppressive molecule-mediated inhibition of anti-tumoral functions of CTLs. TCMNPs could: Ⅳ) block the PD-1/PD-L1 signal and Ⅴ) scavenge TGF-β1; C: Transmission electron microscopy images of PLGA nanoparticles and TCMNPs; D: The percentages of CD3+ T cells, regulatory T cells (Treg, CD3+ CD4+ Foxp3+), CD8+ T cells, and the ratio of CD8+ T cells to Treg in the tumor tissues following various treatments. n = 3-6, x±s; E: Representative ex vivo images and the quantification data of fluorescent signals in the major organs and tumors of mice at 24 h after intravenous injection of various Cy5.5-labeled nanoparticles or antibodies (n = 3, x±s), which demonstrated LFA-1-mediated tumor-targeting of TCMNPs. Lb-TCMNP: LFA-1-blocked TCMNP; trTCMNP: Trypsin-pretreated TCMNP; aPD-L1: Anti-PD-L1 antibody. The volumes (F) and mass (G) of tumors retrieved on day 10 after the first injection (n = 4). Data were represented as mean ± SD. Tumor growth data were mean ± SEM. *P < 0.05 vs PBS; †P < 0.05 vs PLGA: ‡P < 0.05 vs aPD-L1; ¶P < 0.05 vs aPD-L1 (positive control); #P < 0.05 vs trTCMNPs. (Adapted from Ref. 80 with permission. Copyright © 2020 Wiley-VCH) |
IDO是癌症免疫疗法的重要靶标。针对常用的IDO抑制剂NLG919水溶性差的问题, Han等[81]将其通过酯键与pH敏感的肽段连接, 再与RGD偶联, 形成的三元化合物可以自组装成纳米粒NLG-RGD NI (图 8A~C)。当NLG-RGD NI主动靶向到肿瘤组织并被摄取后, 溶酶体的酸性环境和酯酶协同裂解肽段, 导致药物的可控释放, 较游离NLG919而言IDO抑制作用更强(图 8D)。当NLG-RGD NI与抗PD-L1抗体联用时, 肿瘤抑制效率达57.4%, 是单独抗PD-L1抗体或抗体与游离NLG919混合物抑制效率的2倍左右(图 8E、F)。类似地, 使用HA或甘露糖修饰的纳米载体递送IDO抑制剂或IDO siRNA同样实现了有效的IDO通路阻断[82, 83]。
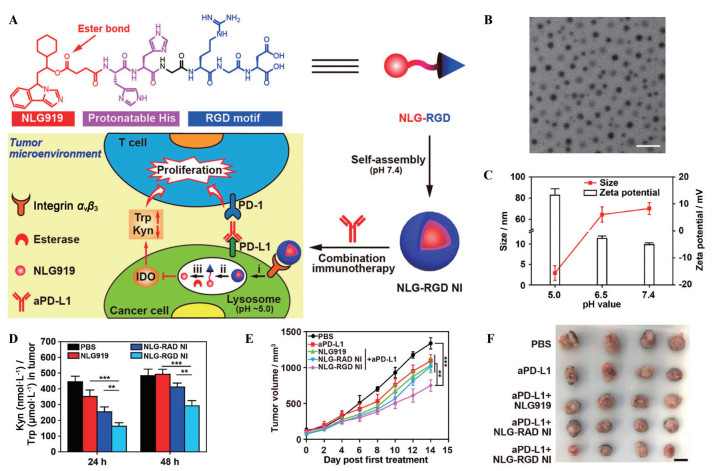
|
Figure 8 A nanoparticle modified with a targeting peptide inhibited the indoleamine 2, 3-dioxygenase (IDO) pathway for cancer immunotherapy. A: Schematic illustration of the modular design and self-assembly of NLG919 (NLG)-RGD and the proposed mechanism of combinatorial normalization immunotherapy by concurrent blocking IDO and PD-L1 using NLG-RGD NI and the anti-PD-L1 antibody (aPD-L1); B: The transmission electron microscope image of NLG-RGD NI at pH 7.4. Scale bar = 200 nm; C: Hydrodynamic size and ζ potential of NLG-RGD NI after equilibrium at pH 7.4, 6.5, or 5.0 for 2 h. n = 3, x±s; D: The kynurenine (Kyn)/tryptophan (Trp) ratios in tumors. n = 4, x±s. **P < 0.01, ***P < 0.001; E: Tumor growth curves. n = 4, x±s. **P < 0.01, ***P < 0.001; F: The photograph of excised tumors. Scale bar = 1 cm. NLG-RAD NI: NLG-arginyl-alanyl-aspartic acid (RAD) nanoinhibitor. (Adapted from Ref. 81 with permission. Copyright © 2020, American Chemical Society) |
由于抗肿瘤免疫应答涉及多个步骤, 对单一步骤进行调控可能无法得到满意的效果。因此, 同时激活免疫应答过程中的多个步骤可以发挥协同的抗肿瘤效果, 从而实现更有效的免疫疗法。aNDDS既可以实现特异性的药物递送, 也具备同时负载多种药物的功能, 因此成为联合免疫疗法的强大助力。
3.4.1 提高肿瘤免疫原性并激活免疫细胞诱导肿瘤细胞发生ICD的同时使用外源性物质激活免疫细胞, 可以协同ICD暴露的DAMP和抗原一起放大抗肿瘤免疫应答。光动力疗法(photodynamictherapy, PDT) 是一种常用的诱导肿瘤细胞发生ICD的方式, 敲除蛋白酪氨酸磷酸酶非受体2型(protein tyrosine phosphatase non receptor 2, Ptpn2) 可以活化CD8+ T细胞并促进IFN-γ介导的肿瘤抑制。为实现二者的协同作用, Yang等[84]将可以敲除Ptpn2的CRISPR-Cas9质粒与iRGD偶联, 随后通过静电吸附与光敏剂Ce6形成复合物, 最后, 使用HA包裹该复合物。HA有效提高了纳米系统的递送能力, 转染效率达75%, 远高于使用聚合物或仅用iRGD修饰的CRISPR-Cas9质粒, 也高于市售的质粒转染载体。为了实现更深的肿瘤穿透效果, 将HRP@CCP与可以降解细胞外基质(extracellular matrix, ECM) 的透明质酸酶同时给药, 在PDT、基因编辑和ECM降解的协同作用下, 肿瘤抑制率达到93.06%, 而未包含Ce6或未包含Ptpn2敲除质粒的纳米粒抑瘤率仅为4.13%和74.37%。此类关于使用HA修饰纳米载体以实现ICD与免疫激活剂的协同作用的报道还有很多, 均能有效抑制肿瘤生长, 体现了该策略的普适性[85, 86]。
仿生载药系统也被用于ICD和免疫细胞调节的联合疗法。MDSC既可以逃避免疫系统的清除, 还可以通过细胞膜表面的蛋白受体接受肿瘤的趋化因子和细胞因子信号, 主动迁移至肿瘤。因此, Yu等[87]制备了MDSC细胞膜包覆磁性Fe3O4纳米粒(MNP) 的仿生磁性纳米粒MNP@MDSC。MNP@MDSC较MNP而言, 具有更低的巨噬细胞摄取和更高的肿瘤蓄积能力, 介导了有效的磁共振成像和PTT, PTT导致肿瘤细胞发生ICD, 释放出的MNP将M2型TAM诱导为M1型, 增强抗肿瘤免疫应答。此外, 自然杀伤细胞的细胞膜因具备肿瘤靶向和转化TAM的能力被用来包覆光敏剂形成仿生纳米粒, 通过联合PDT和免疫疗法延长荷瘤小鼠生存期[88]。
3.4.2 提高肿瘤免疫原性并阻断肿瘤免疫逃逸肿瘤发生ICD虽然有利于效应性T细胞的瘤内浸润, 但也会导致IFN-γ大量分泌进而促进IDO和PD-L1的表达, 引起对免疫杀伤的负反馈调节。使用aNDDS将ICD诱导剂与PD-1/PD-L1或IDO抑制剂联合, 具有肿瘤特异性和疗效协同性的双重优势, 可以更有效地促进抗肿瘤免疫治疗[89, 90]。
为实现PDT-免疫联合疗法, Sun等[91]将接枝了β-环糊精的HA、溴结构域蛋白4抑制剂JQ1前药和光敏剂焦磷酸a (PPa) 前药组装成可以靶向肿瘤细胞的多功能纳米复合物HCJSP (图 9A~C)。HCJSP可以借助HA主动靶向肿瘤; 被肿瘤细胞内吞后, 在NIR的照射下, PPa介导活性氧(reactive oxygen species, ROS) 的产生, 诱导细胞发生ICD, 促进CD8+ T细胞的浸润和活化; 同时, JQ1前药响应瘤内高含量的谷胱甘肽而活化, 下调PD-L1的表达, 恢复CD8+ T细胞对肿瘤细胞的杀伤能力(图 9D~J)。与未用HA包覆的纳米复合物相比, HCJSP的24 h瘤内积累量提高了2.5倍, 且在瘤内可以滞留48 h以上, 与激光治疗结合可基本控制小鼠肿瘤的生长和转移, 体现了联合疗法的优势。
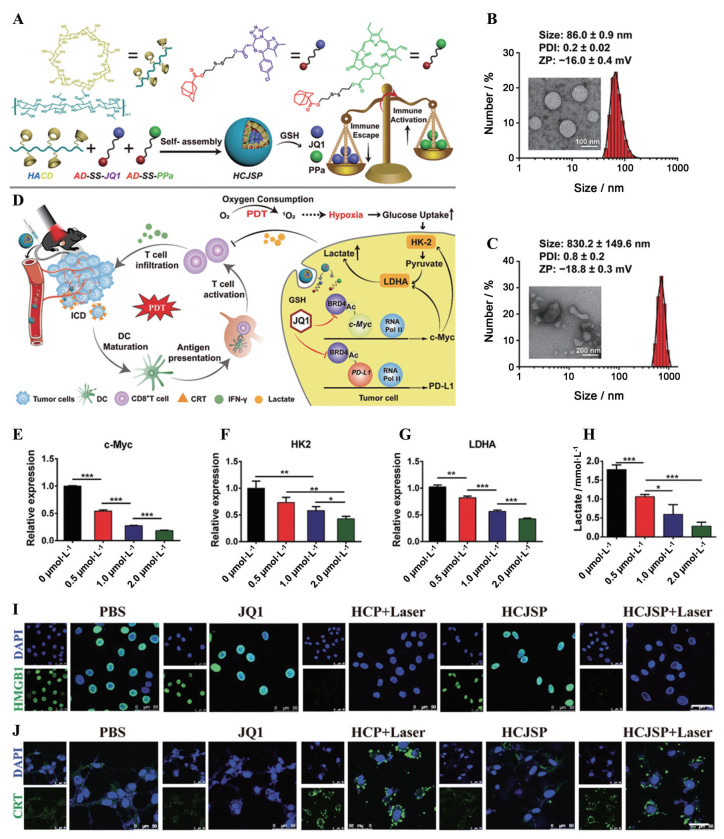
|
Figure 9 A Nanocomplex with HA-mediated active targeting ability promoted photo-immunotherapy of cancer. A: Schematic illustration of the supramolecular prodrug nanoparticles fabricated by complexing β-cyclodextrin-grafted hydronic acid (HA-CD) with the reduction-activatable heterodimer of adamantine-conjugated heterodimers of pyropheophorbide a (PPa) (AD-SS-PPa) and adamantine-conjugated heterodimers of JQ1 (AD-SS-JQ1) (HCJSP) prepared via the host-guest interaction between HA-CD and AD-SS-JQ1 and AD-SS-PPa; B, C: Transmission electron microscope images and dynamic light scattering-identified size distribution of HCJSP without (B) and with 10 mmol·L-1 GSH (C) treatment; D: Proposed mechanisms of HCJSP-based combinatory immunotherapy of pancreatic tumor by eliciting immunogenicity and overcoming adaptive immune resistance; E-G: Semi-quantitative analysis of the expression of c-Myc (E), hexokinase-II (HK-2, F) and lactate dehydrogenase A (LDHA, G) after treated with 0.5, 1.0 or 2.0 µmol·L-1 of JQ1 for 24 h; H: Lactate secretion in the cell culture supernatant derived from Panc02 cells incubated with different concentrations of JQ1 for 24 h. n = 3, x±s. *P < 0.05, **P < 0.01, ***P < 0.001; I, J: Confocal laser scanning microscope examination of PDT-induced surface calreticulin (CRT) expression (I) and nuclear high mobility group box 1 (HMGB1) efflux (J) in the Panc02 cells in vitro (scale bar = 50 µm). BRD4: Bromodomain and extraterminal protein 4; DAPI: 4', 6-Diamidino-2-phenylindole; HCP: A PPa-loaded supramolecular nanoparticle fabricated by host-guest interaction between HA-CD and AD-SS-PPa. (Adapted from Ref. 91 with permission. Copyright © 2021, Wiley-VCH) |
肿瘤免疫疗法是抗肿瘤治疗的重要手段, 但仍存在疗效差异大和不良反应多等亟待解决的问题。随着纳米技术和材料科学的发展, 多功能NDDS为提高抗肿瘤治疗的疗效并降低其不良反应提供了可行的解决策略[92, 93]。通过在纳米载体上修饰可与特定细胞表面受体结合的配体或抗体, 使载体通过配体-受体或抗体-抗原相互作用识别并进入靶细胞。此外, 由于不同来源的细胞膜上存在的多种生物大分子可作为天然配体, 使用细胞膜制备的仿生纳米药物同样具有主动靶向的功能。
可用于肿瘤免疫疗法的aNDDS体现了以下优势。首先, 可以将药物特异性递送到肿瘤细胞或免疫细胞, 提高药物的有效浓度。一系列研究表明, 游离药物或使用非靶向载体递送药物的靶细胞蓄积明显少于aNDDS; 第二, 增加药物的有效浓度意味着给药时可以降低剂量, 同时减少非靶向性分布, 均有利于减轻不良反应, 提高免疫疗法的安全性; 第三, aNDDS能够同时递送多种药物, 一方面, 通过同时激活抗肿瘤免疫应答途径中的多个环节, 实现协同的抗肿瘤效果, 级联放大抗肿瘤免疫响应, 有效控制肿瘤的发展; 另一方面, 通过设计智能递药载体, 可以控制不同药物释放的时间和位置; 最后, aNDDS还能提供更灵活的治疗方案, 例如, 可以将肿瘤细胞靶向和免疫细胞靶向结合, 或将化疗、光疗和免疫治疗联合等, 以进一步地提高疗效并降低不良反应。
为了实现上述策略的临床转化, 还需要进行更多必要的研究。第一, 载体设计应选择生物可降解和生物相容性好的材料, 以满足临床转化的安全性需求; 第二, 成分复杂的纳米药物较难开展大规模制备和实现精准质控, 因此, 需要探索aNDDS的功能多样性与规模化制备工艺的平衡; 第三, 还需要深入研究aNDDS与免疫系统的相互作用, 对纳米递药系统各个组分的功能进行确认, 以明确其在激活免疫应答和发挥抗肿瘤作用时的机制; 第四, 在使用aNDDS进行多种药物联合递送时, 需要考虑不同药物的理化性质差异及对不同药物的剂量比例进行斟酌, 使其最大程度地发挥作用; 最后, 还需要挖掘新型的主动靶向递送思路, 更进一步地提高药物的靶向性, 例如近期报道的在细胞和纳米载体表面修饰特定的基团, 通过发生点击反应介导的主动靶向药物递送策略[94, 95]。相信随着纳米技术的发展和肿瘤免疫疗法机制研究的不断深入, 基于aNDDS的免疫疗法会对肿瘤的临床治疗产生有力的推动作用。
作者贡献: 颜雯璐和郎天群提出并确定综述的主旨及提纲; 颜雯璐完成综述的撰写; 郎天群、尹琦和李亚平对综述进行修改。
利益冲突: 所有作者声明无利益冲突。
| [1] |
Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion[J]. Science, 2011, 331: 1565-1570. DOI:10.1126/science.1203486 |
| [2] |
Couzin-Frankel J. Breakthrough of the year 2013. Cancer immunotherapy[J]. Science, 2013, 342: 1432-1433. DOI:10.1126/science.342.6165.1432 |
| [3] |
Sanmamed MF, Chen L. A paradigm shift in cancer immunotherapy: from enhancement to normalization[J]. Cell, 2018, 175: 313-326. DOI:10.1016/j.cell.2018.09.035 |
| [4] |
Abdel-Wahab N, Alshawa A, Suarez-Almazor ME. Adverse events in cancer immunotherapy[J]. Adv Exp Med Biol, 2017, 995: 155-174. |
| [5] |
June CH, Warshauer JT, Bluestone JA. Is autoimmunity the Achilles' heel of cancer immunotherapy?[J]. Nat Med, 2017, 23: 540-547. DOI:10.1038/nm.4321 |
| [6] |
Riley RS, June CH, Langer R, et al. Delivery technologies for cancer immunotherapy[J]. Nat Rev Drug Discov, 2019, 18: 175-196. DOI:10.1038/s41573-018-0006-z |
| [7] |
Zhang P, Zhai Y, Cai Y, et al. Nanomedicine-based immunotherapy for the treatment of cancer metastasis[J]. Adv Mater, 2019, 31: e1904156. DOI:10.1002/adma.201904156 |
| [8] |
Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs[J]. Cancer Res, 1986, 46: 6387-6392. |
| [9] |
Sindhwani S, Syed AM, Ngai J, et al. The entry of nanoparticles into solid tumours[J]. Nat Mater, 2020, 19: 566-575. DOI:10.1038/s41563-019-0566-2 |
| [10] |
Pandit S, Dutta D, Nie S. Active transcytosis and new opportunities for cancer nanomedicine[J]. Nat Mater, 2020, 19: 478-480. DOI:10.1038/s41563-020-0672-1 |
| [11] |
Attia MF, Anton N, Wallyn J, et al. An overview of active and passive targeting strategies to improve the nanocarriers efficiency to tumour sites[J]. J Pharm Pharmacol, 2019, 71: 1185-1198. DOI:10.1111/jphp.13098 |
| [12] |
Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point[J]. Nature, 2017, 541: 321-330. DOI:10.1038/nature21349 |
| [13] |
Galon J, Bruni D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies[J]. Nat Rev Drug Discov, 2019, 18: 197-218. DOI:10.1038/s41573-018-0007-y |
| [14] |
Li X, Wenes M, Romero P, et al. Navigating metabolic pathways to enhance antitumour immunity and immunotherapy[J]. Nat Rev Clin Oncol, 2019, 16: 425-441. DOI:10.1038/s41571-019-0203-7 |
| [15] |
Harris AL. Hypoxia——a key regulatory factor in tumour growth[J]. Nat Rev Cancer, 2002, 2: 38-47. DOI:10.1038/nrc704 |
| [16] |
Leone RD, Horton MR, Powell JD. Something in the air: hyperoxic conditioning of the tumor microenvironment for enhanced immunotherapy[J]. Cancer cell, 2015, 27: 435-436. DOI:10.1016/j.ccell.2015.03.014 |
| [17] |
Siska PJ, Singer K, Evert K, et al. The immunological Warburg effect: can a metabolic-tumor-stroma score (MeTS) guide cancer immunotherapy?[J]. Immunol Rev, 2020, 295: 187-202. DOI:10.1111/imr.12846 |
| [18] |
Geiger R, Rieckmann JC, Wolf T, et al. L-Arginine modulates T cell metabolism and enhances survival and anti-tumor activity[J]. Cell, 2016, 167: 829-842. DOI:10.1016/j.cell.2016.09.031 |
| [19] |
Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment[J]. Nat Immunol, 2013, 14: 1014-1022. DOI:10.1038/ni.2703 |
| [20] |
Vanichapol T, Chutipongtanate S, Anurathapan U, et al. Immune escape mechanisms and future prospects for immunotherapy in neuroblastoma[J]. Biomed Res Int, 2018, 2018: 1812535. |
| [21] |
Teillaud JL. Cancer immunotherapy crowned with Nobel prize in physiology or medicine awarded to James Allison and Tasuku Honjo[J]. Med Sci, 2019, 35: 365-366. |
| [22] |
Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade[J]. Science, 1996, 271: 1734-1736. DOI:10.1126/science.271.5256.1734 |
| [23] |
Iwai Y, Ishida M, Tanaka Y, et al. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade[J]. Proc Natl Acad Sci U S A, 2002, 99: 12293-12297. DOI:10.1073/pnas.192461099 |
| [24] |
Hayat SMG, Bianconi V, Pirro M, et al. CD47:role in the immune system and application to cancer therapy[J]. Cell Oncol, 2020, 43: 19-30. DOI:10.1007/s13402-019-00469-5 |
| [25] |
Kubli SP, Berger T, Araujo DV, et al. Beyond immune checkpoint blockade: emerging immunological strategies[J]. Nat Rev Drug Discov, 2021, 20: 899-919. DOI:10.1038/s41573-021-00155-y |
| [26] |
Sun Q, Zhou Z, Qiu N, et al. Rational design of cancer nanomedicine: nanoproperty integration and synchronization[J]. Adv Mater, 2017, 29: 1606628. DOI:10.1002/adma.201606628 |
| [27] |
Muhamad N, Plengsuriyakarn T, Na-Bangchang K. Application of active targeting nanoparticle delivery system for chemotherapeutic drugs and traditional/herbal medicines in cancer therapy: a systematic review[J]. Int J Nanomedicine, 2018, 13: 3921-3935. DOI:10.2147/IJN.S165210 |
| [28] |
Liu C, Lin W. Polymeric nanoparticles conjugate a novel heptapeptide as an epidermal growth factor receptor-active targeting ligand for doxorubicin[J]. Int J Nanomedicine, 2012, 7: 4749-4767. |
| [29] |
Hong M, Zhu S, Jiang Y, et al. Novel anti-tumor strategy: PEG-hydroxycamptothecin conjugate loaded transferrin-PEG-nanoparticles[J]. J Control Release, 2010, 141: 22-29. DOI:10.1016/j.jconrel.2009.08.024 |
| [30] |
Sykes EA, Chen J, Zheng G, et al. Investigating the impact of nanoparticle size on active and passive tumor targeting efficiency[J]. ACS Nano, 2014, 8: 5696-5706. DOI:10.1021/nn500299p |
| [31] |
Trabulo S, Aires A, Aicher A, et al. Multifunctionalized iron oxide nanoparticles for selective targeting of pancreatic cancer cells[J]. Biochim Biophys Acta Gen Subj, 2017, 1861: 1597-1605. DOI:10.1016/j.bbagen.2017.01.035 |
| [32] |
Jiang X, Sha X, Xin H, et al. Self-aggregated pegylated poly (trimethylene carbonate) nanoparticles decorated with c(RGDyK) peptide for targeted paclitaxel delivery to integrin-rich tumors[J]. Biomaterials, 2011, 32: 9457-9469. DOI:10.1016/j.biomaterials.2011.08.055 |
| [33] |
Huang G, Huang H. Hyaluronic acid-based biopharmaceutical delivery and tumor-targeted drug delivery system[J]. J Control Release, 2018, 278: 122-126. DOI:10.1016/j.jconrel.2018.04.015 |
| [34] |
Narmani A, Rezvani M, Farhood B, et al. Folic acid functionalized nanoparticles as pharmaceutical carriers in drug delivery systems[J]. Drug Dev Res, 2019, 80: 404-424. DOI:10.1002/ddr.21545 |
| [35] |
Li X, Zhu X, Qiu L. Constructing aptamer anchored nanovesicles for enhanced tumor penetration and cellular uptake of water soluble chemotherapeutics[J]. Acta Biomater, 2016, 35: 269-279. DOI:10.1016/j.actbio.2016.02.012 |
| [36] |
Lang T, Yin Q, Li Y. Progress of cell-derived biomimetic drug delivery systems for cancer therapy[J]. Adv Therapeut, 2018, 1: 1800053. DOI:10.1002/adtp.201800053 |
| [37] |
Chen Z, Zhao P, Luo Z, et al. Cancer cell membrane-biomimetic nanoparticles for homologous-targeting dual-modal imaging and photothermal therapy[J]. ACS Nano, 2016, 10: 10049-10057. DOI:10.1021/acsnano.6b04695 |
| [38] |
Cao H, Dan Z, He X, et al. Liposomes coated with isolated macrophage membrane can target lung metastasis of breast cancer[J]. ACS Nano, 2016, 10: 7738-7748. DOI:10.1021/acsnano.6b03148 |
| [39] |
Kang T, Zhu Q, Wei D, et al. Nanoparticles coated with neutrophil membranes can effectively treat cancer metastasis[J]. ACS Nano, 2017, 11: 1397-1411. DOI:10.1021/acsnano.6b06477 |
| [40] |
Krishnamurthy S, Gnanasammandhan MK, Xie C, et al. Monocyte cell membrane-derived nanoghosts for targeted cancer therapy[J]. Nanoscale, 2016, 8: 6981-6985. DOI:10.1039/C5NR07588B |
| [41] |
Parodi A, Quattrocchi N, van de Ven AL, et al. Synthetic nanoparticles functionalized with biomimetic leukocyte membranes possess cell-like functions[J]. Nat Nanotechnol, 2013, 8: 61-68. DOI:10.1038/nnano.2012.212 |
| [42] |
Hu Q, Sun W, Qian C, et al. Anticancer platelet-mimicking nanovehicles[J]. Adv Mater, 2015, 27: 7043-7050. DOI:10.1002/adma.201503323 |
| [43] |
Bagó JR, Alfonso-Pecchio A, Okolie O, et al. Therapeutically engineered induced neural stem cells are tumour-homing and inhibit progression of glioblastoma[J]. Nat Commun, 2016, 7: 10593. DOI:10.1038/ncomms10593 |
| [44] |
Khong HT, Restifo NP. Natural selection of tumor variants in the generation of "tumor escape" phenotypes[J]. Nat Immunol, 2002, 3: 999-1005. DOI:10.1038/ni1102-999 |
| [45] |
Yan W, Lang T, Qi X, et al. Engineering immunogenic cell death with nanosized drug delivery systems improving cancer immunotherapy[J]. Curr Opin Biotechnol, 2020, 66: 36-43. DOI:10.1016/j.copbio.2020.06.007 |
| [46] |
Kamoun WS, Kirpotin DB, Huang ZR, et al. Antitumour activity and tolerability of an EphA2-targeted nanotherapeutic in multiple mouse models[J]. Nat Biomed Eng, 2019, 3: 264-280. DOI:10.1038/s41551-019-0385-4 |
| [47] |
Kamoun WS, Dugast AS, Suchy JJ, et al. Synergy between EphA2-ILs-DTXp, a novel EphA2-targeted nanoliposomal taxane, and PD-1inhibitors in preclinical tumor models[J]. Mol Cancer Ther, 2020, 19: 270-281. DOI:10.1158/1535-7163.MCT-19-0414 |
| [48] |
Au KM, Balhorn R, Balhorn MC, et al. High-performance concurrent chemo-immuno-radiotherapy for the treatment of hematologic cancer through selective high-affinity ligand antibody mimic-functionalized doxorubicin-encapsulated nanoparticles[J]. ACS Cent Sci, 2019, 5: 122-144. DOI:10.1021/acscentsci.8b00746 |
| [49] |
Wang Z, Guo B, Middha E, et al. Microfluidics-prepared uniform conjugated polymer nanoparticles for photo-triggered immune microenvironment modulation and cancer therapy[J]. ACS Appl Mater Interfaces, 2019, 11: 11167-11176. DOI:10.1021/acsami.8b22579 |
| [50] |
He Y, Lei L, Cao J, et al. A combinational chemo-immune therapy using an enzyme-sensitive nanoplatform for dual-drug delivery to specific sites by cascade targeting[J]. Sci Adv, 2021, 7: eaba0776. DOI:10.1126/sciadv.aba0776 |
| [51] |
Jiang Q, Wang K, Zhang X, et al. Platelet membrane-camouflaged magnetic nanoparticles for ferroptosis-enhanced cancer immunotherapy[J]. Small, 2020, 16: e2001704. DOI:10.1002/smll.202001704 |
| [52] |
Zhao P, Wang M, Chen M, et al. Programming cell pyroptosis with biomimetic nanoparticles for solid tumor immunotherapy[J]. Biomaterials, 2020, 254: 120142. DOI:10.1016/j.biomaterials.2020.120142 |
| [53] |
Li F, Nie W, Zhang F, et al. Engineering magnetosomes for high-performance cancer vaccination[J]. ACS Cent Sci, 2019, 5: 796-807. DOI:10.1021/acscentsci.9b00060 |
| [54] |
Schmid D, Park CG, Hartl CA, et al. T cell-targeting nanoparticles focus delivery of immunotherapy to improve antitumor immunity[J]. Nat Commun, 2017, 8: 1747. DOI:10.1038/s41467-017-01830-8 |
| [55] |
Cho R, Sakurai Y, Jones HS, et al. Silencing of VEGFR2 by RGD-modified lipid nanoparticles enhanced the efficacy of anti-PD-1 antibody by accelerating vascular normalization and infiltration of T cells in tumors[J]. Cancers, 2020, 12: 3630. DOI:10.3390/cancers12123630 |
| [56] |
Malfitano AM, Pisanti S, Napolitano F, et al. Tumor-associated macrophage status in cancer treatment[J]. Cancers, 2020, 12: 1987. DOI:10.3390/cancers12071987 |
| [57] |
Xiao H, Guo Y, Li B, et al. M2-like tumor-associated macrophage-targeted codelivery of STAT6 inhibitor and IKKβ siRNA induces M2-to-M1 repolarization for cancer immunotherapy with low immune side effects[J]. ACS Cent Sci, 2020, 6: 1208-1222. DOI:10.1021/acscentsci.9b01235 |
| [58] |
Qiu Q, Li C, Song Y, et al. Targeted delivery of ibrutinib to tumor-associated macrophages by sialic acid-stearic acid conjugate modified nanocomplexes for cancer immunotherapy[J]. Acta Biomater, 2019, 92: 184-195. DOI:10.1016/j.actbio.2019.05.030 |
| [59] |
Ortega RA, Barham WJ, Kumar B, et al. Biocompatible mannosylated endosomal-escape nanoparticles enhance selective delivery of short nucleotide sequences to tumor associated macrophages[J]. Nanoscale, 2015, 7: 500-510. DOI:10.1039/C4NR03962A |
| [60] |
Li C, Zhang Y, Dong X, et al. Artificially reprogrammed macrophages as tumor-tropic immunosuppression-resistant biologics to realize therapeutics production and immune activation[J]. Adv Mater, 2019, 31: e1807211. DOI:10.1002/adma.201807211 |
| [61] |
Song Y, Tang C, Yin C. Combination antitumor immunotherapy with VEGF and PIGF siRNA via systemic delivery of multi-functionalized nanoparticles to tumor-associated macrophages and breast cancer cells[J]. Biomaterials, 2018, 185: 117-132. DOI:10.1016/j.biomaterials.2018.09.017 |
| [62] |
Kulkarni A, Chandrasekar V, Natarajan SK, et al. A designer self-assembled supramolecule amplifies macrophage immune responses against aggressive cancer[J]. Nat Biomed Eng, 2018, 2: 589-599. DOI:10.1038/s41551-018-0254-6 |
| [63] |
He X, Liu B, Wu J, et al. A dual macrophage targeting nanovector for delivery of oligodeoxynucleotides to overcome cancer-associated immunosuppression[J]. ACS Appl Mater Interfaces, 2017, 9: 42566-42576. DOI:10.1021/acsami.7b13594 |
| [64] |
Rao L, Wu L, Liu Z, et al. Hybrid cellular membrane nanovesicles amplify macrophage immune responses against cancer recurrence and metastasis[J]. Nat Commun, 2020, 11: 4909. DOI:10.1038/s41467-020-18626-y |
| [65] |
Choo YW, Kang M, Kim HY, et al. M1 Macrophage-derived nanovesicles potentiate the anticancer efficacy of immune checkpoint inhibitors[J]. ACS Nano, 2018, 12: 8977-8993. DOI:10.1021/acsnano.8b02446 |
| [66] |
Shin J, Oh S, Kwon S, et al. A PEGylated hyaluronic acid conjugate for targeted cancer immunotherapy[J]. J Control Release, 2017, 267: 181-190. DOI:10.1016/j.jconrel.2017.08.032 |
| [67] |
Sun W, Yang J, Hou M, et al. A nano "immune-guide" recruiting lymphocytes and modulating the ratio of macrophages from different origins to enhance cancer immunotherapy[J]. Adv Funct Mater, 2021, 31: 2009116. DOI:10.1002/adfm.202009116 |
| [68] |
Wu M, Liu X, Bai H, et al. Surface-layer protein-enhanced immunotherapy based on cell membrane-coated nanoparticles for the effective inhibition of tumor growth and metastasis[J]. ACS Appl Mater Interfaces, 2019, 11: 9850-9859. DOI:10.1021/acsami.9b00294 |
| [69] |
Cao F, Yan M, Liu Y, et al. Photothermally controlled MHC class Ⅰ restricted CD8+ T-cell responses elicited by hyaluronic acid decorated gold nanoparticles as a vaccine for cancer immunotherapy[J]. Adv Health Mater, 2018, 7: e1701439. DOI:10.1002/adhm.201701439 |
| [70] |
Yang YS, Moynihan KD, Bekdemir A, et al. Targeting small molecule drugs to T cells with antibody-directed cell-penetrating gold nanoparticles[J]. Biomater Sci, 2018, 7: 113-124. |
| [71] |
Zhang Y, Luo J, Zhang J, et al. Nanoparticle-enabled dual modulation of phagocytic signals to improve macrophage-mediated cancer immunotherapy[J]. Small, 2020, 16: e2004240. DOI:10.1002/smll.202004240 |
| [72] |
Selvan SR, Dowling JP, Kelly WK, et al. Indoleamine 2, 3-dioxygenase (IDO): biology and target in cancer immunotherapies[J]. Curr Cancer Drug Targets, 2016, 16: 755-764. DOI:10.2174/1568009615666151030102250 |
| [73] |
Günther J, Däbritz J, Wirthgen E. Limitations and off-target effects of tryptophan-related IDO inhibitors in cancer treatment[J]. Front Immunol, 2019, 10: 1801. DOI:10.3389/fimmu.2019.01801 |
| [74] |
Liu X, Wang D, Zhang P, et al. Recent advances in nanosized drug delivery systems for overcoming the barriers to anti-PD immunotherapy of cancer[J]. Nano Today, 2019, 29: 100801. DOI:10.1016/j.nantod.2019.100801 |
| [75] |
He X, Ren X, Peng Y, et al. Aptamer/peptide-functionalized genome-editing system for effective immune restoration through reversal of PD-L1-mediated cancer immunosuppression[J]. Adv Mater, 2020, 32: e2000208. DOI:10.1002/adma.202000208 |
| [76] |
Chen Y, Liu YC, Sung YC, et al. Overcoming sorafenib evasion in hepatocellular carcinoma using CXCR4-targeted nanoparticles to co-deliver MEK-inhibitors[J]. Sci Rep, 2017, 7: 44123. DOI:10.1038/srep44123 |
| [77] |
Meng X, Wang J, Zhou J, et al. Tumor cell membrane-based peptide delivery system targeting the tumor microenvironment for cancer immunotherapy and diagnosis[J]. Acta Biomater, 2021, 127: 266-275. DOI:10.1016/j.actbio.2021.03.056 |
| [78] |
Han X, Chen J, Chu J, et al. Platelets as platforms for inhibition of tumor recurrence post-physical therapy by delivery of anti-PD-L1 checkpoint antibody[J]. J Control Release, 2019, 304: 233-241. DOI:10.1016/j.jconrel.2019.05.008 |
| [79] |
Wang Z, Liu J, Yang F, et al. Tailor-made cell-based biomimetic nanoprobes for fluorescence imaging guided colorectal cancer chemo-immunotherapy[J]. ACS Appl Bio Mater, 2021, 4: 1920-1931. DOI:10.1021/acsabm.0c01553 |
| [80] |
Kang M, Hong J, Jung M, et al. T-cell-mimicking nanoparticles for cancer immunotherapy[J]. Adv Mater, 2020, 32: e2003368. DOI:10.1002/adma.202003368 |
| [81] |
Han X, Cheng K, Xu Y, et al. Modularly designed peptide nanoprodrug augments antitumor immunity of PD-L1 checkpoint blockade by targeting indoleamine 2, 3-dioxygenase[J]. J Am Chem Soc, 2020, 142: 2490-2496. DOI:10.1021/jacs.9b12232 |
| [82] |
Hu Y, Chen X, Xu Y, et al. Hierarchical assembly of hyaluronan coated albumin nanoparticles for pancreatic cancer chemoimmunotherapy[J]. Nanoscale, 2019, 11: 16476-16487. DOI:10.1039/C9NR03684A |
| [83] |
Zhang Y, Fu J, Shi Y, et al. A new cancer immunotherapy via simultaneous DC-mobilization and DC-targeted IDO gene silencing using an immune-stimulatory nanosystem[J]. Int J Cancer, 2018, 143: 2039-2052. DOI:10.1002/ijc.31588 |
| [84] |
Yang C, Fu Y, Huang C, et al. Chlorin e6 and CRISPR-Cas9 dual-loading system with deep penetration for a synergistic tumoral photodynamic-immunotherapy[J]. Biomaterials, 2020, 255: 120194. |
| [85] |
Uthaman S, Pillarisetti S, Hwang HS, et al. Tumor microenvironment-regulating immunosenescence-independent nanostimulant synergizing with near-infrared light irradiation for antitumor immunity[J]. ACS Appl Mater Interfaces, 2021, 13: 4844-4852. |
| [86] |
Wang L, Ding K, Zheng C, et al. Detachable nanoparticle-enhanced chemoimmunotherapy based on precise killing of tumor seeds and normalizing the growing soil strategy[J]. Nano Lett, 2020, 20: 6272-6280. |
| [87] |
Yu G, Rao L, Wu H, et al. Myeloid-derived suppressor cell membrane-coated magnetic nanoparticles for cancer theranostics by inducing macrophage polarization and synergizing immunogenic cell death[J]. Adv Funct Mater, 2018, 28: 1801389. |
| [88] |
Deng G, Sun Z, Li S, et al. Cell-membrane immunotherapy based on natural killer cell membrane coated nanoparticles for the effective inhibition of primary and abscopal tumor growth[J]. ACS nano, 2018, 12: 12096-12108. |
| [89] |
Hu X, Hou B, Xu Z, et al. Supramolecular prodrug nanovectors for active tumor targeting and combination immunotherapy of colorectal cancer[J]. Adv Sci, 2020, 7: 1903332. |
| [90] |
Peng J, Yang Q, Xiao Y, et al. Tumor microenvironment responsive drug-dye-peptide nanoassembly for enhanced tumor-targeting, penetration, and photo-chemo-immunotherapy[J]. Adv Funct Mater, 2019, 29: 1900004. |
| [91] |
Sun F, Zhu Q, Li T, et al. Regulating glucose metabolism with prodrug nanoparticles for promoting photoimmunotherapy of pancreatic cancer[J]. Adv Sci, 2021, 8: 2002746. |
| [92] |
Yang YQ, Gong F, Bai S, et al. Progress in tumor microenvironment responsive nano-platforms for cancer theranostics[J]. Acta Pharm Sin (药学学报), 2021, 56: 465-475. |
| [93] |
Hou B, Wang DG, Gao J, et al. Advances of microenvironment-activated nanosized drug delivery system for cancer immunotherapy[J]. Acta Pharm Sin (药学学报), 2019, 54: 1802-1809. |
| [94] |
Li W, Pan H, He H, et al. Bio-orthogonal T cell targeting strategy for robustly enhancing cytotoxicity against tumor cells[J]. Small, 2019, 15: e1804383. |
| [95] |
Qin H, Zhao R, Qin Y, et al. Development of a cancer vaccine using in vivo click-chemistry-mediated active lymph node accumulation for improved immunotherapy[J]. Adv Mater, 2021, 33: e2006007. |
 2022, Vol. 57
2022, Vol. 57


