2. 山东中医药大学药学院, 山东 济南 250355
2. College of Pharmacy, Shandong University of Traditional Chinese Medicine, Jinan 250355, China
光动力学疗法(photodynamic therapy, PDT) 是一种无创、高选择性的治疗方法, 其原理是特定光激发光敏剂(photosensitizer, PS) 到激发态, 激发态光敏剂将能量传递给周围的氧, 生成活性氧(reactive oxygen species, ROS), 其中包括单线态氧(1O2), 进而对周围组织和细胞产生毒性[1]。PDT已广泛应用于食管癌和皮肤癌等肿瘤治疗。但由于光敏剂大多溶解度低, 缺乏靶向性, 同时激发光源(波长范围一般在400~700 nm) 对皮肤和组织穿透力不足, 限制了PDT应用于深层组织疾病如深部肿瘤的治疗[2]。
纳米给药系统(nanoscale drug delivery systems) 的优点众多, 已成为近年药物递送领域研究热点, 能提高难溶性药物溶解度, 同时递送多个药物, 可实现肿瘤靶向等。载光敏剂纳米给药系统结合二者优势, 能增强光动力学抗肿瘤效果。本综述从光动力学疗法作用机制、载光敏剂纳米制剂及光动力学疗法与其他方法联合应用等方面, 概括了基于光动力学疗法抗肿瘤纳米给药系统的研究进展, 希望为其临床应用提供参考。
1 PDT作用机制 1.1 PDT三要素PDT三要素为光敏剂、光和组织内氧含量。光能激发光敏剂和穿透生物组织, 其辐照方式、波长和强度直接影响疗效。临床常用光源(辐射源) 包括发光二极管(light emitting diode, LED)、X射线、自然光、近红外光(near infrared light, NIR) 和在体发光等。自然光治疗面积大, 操作简单, 是临床治疗光角化病的常用光源, 但对人体组织穿透深度小, 仅适用于浅表疾病治疗; NIR生物组织吸收较低, 组织穿透深度较大; X射线组织穿透深度不受限制, 突破了传统PDT治疗深度受限的问题, 但需较高辐照强度才可达到有效光量子产率, 易引起正常组织损伤; 在体发光可从根本上解决传统PDT中光源穿透深度的限制, 但与外部激发光源相比发光强度较弱, 影响PDT实际治疗效果, 故未来研究方向是如何提高在体发光的发光效率; LED相比于其他光源, 具有成本低、寿命长、功耗低和易推广等优点, 且带宽相对较窄, 光谱范围覆盖从紫外光到红外光, 可与不同光敏剂最佳吸收波长匹配[3]。
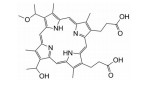
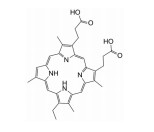
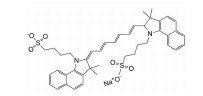
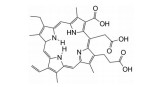
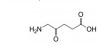
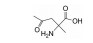
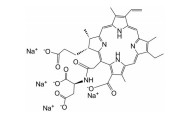
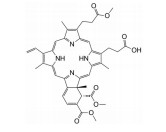
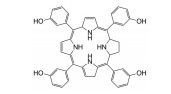
光敏剂是PDT过程中的活性氧生成催化剂, 是决定PDT疗效的重要因素。光敏剂的选择与疾病类型、给药途径有关[4]。目前, 光敏剂可分为三代: 第一代以血卟啉衍生物为代表, 有效成分主要是双血卟啉醚或酯; 第二代包括酞菁类、卟啉类衍生物和稠环醌类化合物, 相比第一代光敏剂, 采用了更长波长的激发光, 组织穿透能力强, 治疗深度加深, ROS产率更高, 提高了治疗效果; 第三代光敏剂由第二代光敏剂与靶向配体结合形成, 具体包括免疫靶向光敏剂、表皮生长因子受体靶向光敏剂和mRNA靶向光敏剂等, 进一步提高了光敏剂靶向性及PDT的高效、安全性。部分光敏剂及其应用见表 1[5-17]。
| Table 1 Some photosensitizers and their applications |
PDT有效治疗的一个必要条件是组织内要有足够的氧, 而肿瘤微环境一般为缺氧状态, 不利于PDT; 同时PDT会进一步消耗氧气, 加重了肿瘤内乏氧。因此可通过增加肿瘤组织氧供应来增加PDT疗效, 如用全氟化碳和血红蛋白等载体将氧气输送到肿瘤部位, 或用过氧化氢酶分解肿瘤细胞产生的H2O2得到O2, 以提高PDT效率[18]。
1.2 PDT作用机制光敏剂分布于病变部位后, 使用特定波长、足够强度的光照射光敏剂, 光敏剂分子吸收光子能量, 从基态变为激发态, 然后将能量传递给周围氧分子。具体机制包括I型和II型反应。I型反应为激发态光敏剂将自身电子转移至氧分子中形成超氧化物, 或把电子转移到其他电子受体使之成为自由基。II型反应是激发态光敏剂将自身电子能量转移给基态氧分子, 使之成为单线态氧[19, 20]。上述生成的活性成分统称为活性氧。PDT发挥效应的具体机制包括: ①活性氧的强氧化性能直接氧化蛋白质、脂质和核酸[1]; ②调节机体免疫反应[21]; ③损伤周围血管和组织, 造成肿瘤缺血性死亡[22]。
2 载光敏剂纳米给药系统粒径处于纳米级范围的给药系统可统称为纳米给药系统, 纳米级别在药学领域常被规定为1~1 000 nm[23]。纳米给药系统具有载药能力大、靶向、防止药物降解、体内循环时间长和缓控释等优点, 特别适用于分子质量大、稳定性差、难吸收、需靶向或缓控释的药物。
作为PDT中重要的一环, 光敏剂由于其本身缺陷导致应用受限, 如光敏剂多为脂溶性、靶向性差, 具有潜在光毒性等。采用纳米给药系统递送光敏剂, 可扬长避短, 能减少其在正常组织中的非特异性累积, 降低机体光敏性, 减少光毒性等。用功能基团或靶向基团进行表面修饰后, 可进一步加强靶向性, 降低使用剂量和不良反应, 提高PDT疗效[24]。将疏水光敏剂通过非共价键或共价键与适当纳米载体连接形成纳米光敏剂, 可被动或主动靶向到目标细胞, 有效增强活性氧产生的能力, 并在封闭的恶性肿瘤中选择性累积而不损害健康细胞。功能性纳米光敏剂还可响应特定内部或外部刺激, 如pH、酶、磁场、光、热和超声等产生智能化释药[25, 26]。内部刺激触发的纳米粒依赖于药物释放目标的生物环境变化, 外部因素调节的载光敏剂纳米粒可精确控制释放实现智能化给药, 主要依赖于外部刺激强度或持续时间[27, 28]。
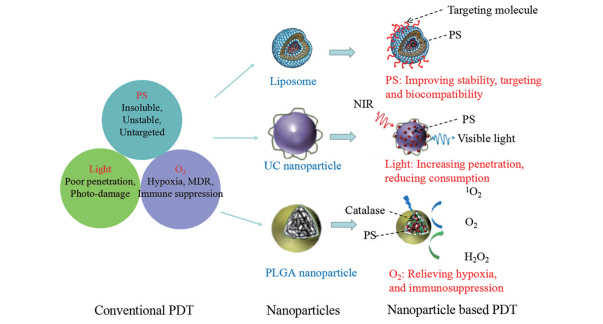
常用载光敏剂纳米给药系统包括脂质体、纳米粒[上转换纳米粒(upconversion nanoparticle, UCNP)]、胶束、纳米乳和纳米凝胶等(图 1), 上述载体在制备工艺、靶向性、PDT效率和给药方式等方面各有特点(表 2)[29-32]。
|
Figure 1 Nanoparticle-based photodynamic therapy (PDT) for enhanced anti-tumor treatment. PS: Photosensitizer; UC: Upconversion; NIR: Near infrared light; MDR: Multidrug resistance; PLGA: Poly (lactic-co-glycolic acid) |
| Table 2 Preparation methods and PDT efficiency of the nanocarriers. DDS: Drug delivery systems; ROS: Reactive oxygen species |
脂质体是由胆固醇和磷脂双分子层构成的囊泡, 其结构和组成与生物膜相似, 因此容易与细胞膜融合, 生物相容性好, 不良反应小。脂质体结构中同时含有亲水性和疏水性区域, 可用于亲水性和疏水性光敏剂的包载和递送[33]。
近年来, 使用载光敏剂脂质体的研究较多。亲水型光敏剂5-氨基酮戊酸皮肤渗透性差, 使用脂质体包载后可有效增强皮肤渗透性, 增加PDT渗透深度[34]。载酞菁脂质体可避免酞菁聚集, 增加单线态氧产率, 显著抑制胶质母细胞瘤生长, 对皮肤和黏膜恶性肿瘤疗效显著[35, 36]。半卟啉二甲酸二嗪是一种卟啉类光敏剂, 光学性能良好, 单线态氧产生率高, 但游离半卟啉二甲酸二嗪的细胞毒性大且稳定性差。中性、阴离子和阳离子脂质体分别载半卟啉二甲酸二嗪衍生物-S型半卟啉二甲酸二嗪后, 发现阳离子脂质体细胞毒性远低于游离化合物, 单线态氧产率高, 光激发后对两种口腔鳞状癌细胞(CAL27、HSC-3) 和人宫颈癌上皮细胞(HeLa) 均有良好杀伤效果[37]。近红外荧光染料光敏剂吲哚菁绿(indocyanine green, ICG) 已成功应用于临床, 如心脏病学、肝病学和荧光引导手术等, 但其在水溶液中不稳定, 皮肤渗透性不佳, 使用受限。载ICG壳聚糖脂质体可增强其皮肤渗透性, 显著增强B16-F10黑色素瘤细胞对ICG的摄取和光细胞毒性[38]。脂质体作为光敏剂载体需关注的问题包括光敏剂在脂质体中的包封率及如何从脂质体中完全释放等问题。
2.2 纳米粒(nanoparticles) 2.2.1 聚合物纳米粒(polymeric nanoparticles)聚合物纳米粒可定义为由天然高分子或合成聚合物构成的纳米粒[39], 前者包括白蛋白、壳聚糖和透明质酸; 后者有聚丙烯酰胺、聚乳酸和聚乳酸羟基乙酸等其他嵌段或接枝共聚物等。
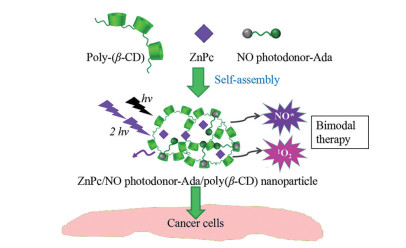
倍他环糊精(β-cyclodextrins, β-CD) 聚合物、阴离子型四磺酸基锌酞菁(zinc phthalocyanine, ZnPc) 和NO供体(NO photodonor)-硝基苯胺金刚烷胺衍生物(adamantyl-nitroaniline derivative, Ada) 混合后形成了超分子自组装纳米粒, 粒径仅为35 nm, 呈现双光子荧光成像和双模式治疗。聚β-CD作为载体包裹硝基苯胺衍生物, ZnPc分布其中, 在405和633 nm可见光激发下(分别激发NO供体和ZnPc), 同时释放具有细胞毒性的NO自由基和单线态氧, 高效杀死肿瘤细胞[40] (图 2)。
|
Figure 2 Molecular structure of poly(β-CD), ZnPc, an NO photodonor attached to an adamantine moiety (NO photodonor-Ada), and corresponding ZnPc/NO photodonor-Ada/poly(β-CD) nanoparticles. β-CD: β-Cyclodextrins; ZnPc: Zinc phthalocyanine; Ada: Adamantyl-nitroaniline derivative |
羧甲基壳聚糖纳米粒(carboxymethyl chitosan nanoparticles, CMC NPs) 包载光敏剂甲苯蓝(methylbenzene blue, MB) 制备的羧甲基壳聚糖纳米粒CMC-MBNP具有pH响应性释放的特点。体外研究表明, CMC-MBNP能在弱酸环境中抑制耐药性人乳腺癌细胞(MCF-7/ADR) 的生长[41], 推测其在肿瘤微酸环境下能释放甲苯蓝发挥抗肿瘤作用。二氧化锰包裹的血卟啉单甲醚PLGA纳米粒(PLGA/HMME@MnO2 NP) 被肿瘤细胞摄取后, 在肿瘤细胞内高浓度谷胱甘肽作用下, MnO2被还原为Mn2+, 促进血卟啉单甲醚释放, 发挥细胞内PDT作用。同时, PLGA/HMME@MnO2纳米粒可降低胞内谷胱甘肽水平, 减轻肿瘤乏氧状态, 提高PDT疗效[42]。
近红外二区光源(1 000~1 700 nm) 比近红外一区光源的组织穿透力更强, 成像深度更大。两性聚苯乙烯-共-氯甲基苯乙烯-接枝-聚乙二醇纳米粒通过自组装包载近红外二区氟硼二吡咯类(boron-dipyrromethene, BODIPY) 光敏剂BDP-I-N后, 再以PD-L1单克隆抗体修饰。该纳米粒在近红外二区光激发下可实现免疫检查点PD-L1实时成像, 单线态氧产率高, 可消除原发性肿瘤。该纳米粒除可对PD-L1表达及MC38肿瘤进行分析外, 还具有体内分子成像功能。其在808 nm激光激发后可产生1 200 nm以上发射波长, 肿瘤与正常组织信号比(T/NT) 约为14.1, 可实现成像。PDT和免疫疗法联合应用30天可使小鼠MC38肿瘤消除, 且40天内不复发[43]。因此充分利用近红外二区光源的穿透优势激活光敏剂, 可进一步改善PDT对深层肿瘤的治疗效果。开发生物相容性好、毒性低和单线态氧产率高的载近红外二区光敏剂纳米粒, 在分子成像和光动力学/免疫联合肿瘤治疗中非常有价值。聚合物纳米粒一般对光敏剂包封率较高, 且粒径较均匀, 需关注问题包括光敏剂完全释放及聚合物在体内降解性等。
2.2.2 无机纳米粒(inorganic nanoparticles)药物可通过物理吸附或共价键结合在无机纳米粒表面, 也可包埋在多孔无机载体中[44]。但无机纳米粒通常生物相容性较差, 可通过在其表面构建有机壳层, 既能减小无机纳米粒毒性, 也能为一些生物分子(如配体连接) 提供功能化位点, 以增强对受体和靶分子的亲和力和选择性[45]。
光的组织穿透深度和波长密切相关, 波长越长, 穿透组织深度越深, 但波长较长的光能量较小, 可能难以激发光敏剂。UCNP可将长波长激发光转变成多重短波光释放[46], 用光转换作用增加PDT治疗渗透深度[47]。UCNP通常由三价镧系离子嵌入在适当的无机宿主晶格中构成[48]。
基于上转换材料的纳米给药系统UCSiAuO-2由具有上转换发光特性的UCNP、多孔SiO2和具有氧生成功能的氧化金(Au2O3) 组成, 可作为二氢卟酚e6的载体。Au2O3经近红外光激发, 在UCNP帮助下通过荧光共振能量转移(fluorescence resonance energy transfer, FRET) 的方式产生氧。这种光控、自供式产氧模式能为光敏剂二氢卟酚e6提供足够氧以产生活性氧发挥细胞毒作用[49]。
NaGdF4是一种掺杂稀土元素Gd的空心上转换纳米粒, 可作为姜黄素(curcumin, CUR) 载体, 脱铁铁蛋白(apoferritin, AFn) 是一种能载多柔比星(doxorubicin, DOX) 的蛋白, 叶酸(folic acid, FA) 修饰的双载药上转换纳米粒CUR/NaGdF4-DOX/AFn-FA对肿瘤细胞具有良好靶向性, 并能短时间内实现两种药物的释放, 对MCF-7细胞具有明显生长抑制作用[50]。无机纳米粒的粒径均匀, 可高效实现光穿透和能量传递, 但需重点关注其长期应用安全性。
2.3 聚合物胶束(polymeric micelles)聚合物胶束一般由两亲性聚合物自发构成, 包括疏水性内核和亲水性外壳, 其热力学和动力学稳定性较高, 生物相容性好, 能同时包载抗肿瘤药物和光敏剂, 实现PDT和化疗联合应用。
聚乳酸-聚乙二醇共聚物在水中可自组装形成胶束, 包裹疏水性光敏剂-焦脱镁叶绿酸a和新型强双光子吸收化合物, 抗肿瘤作用显著[51]。载多柔比星和二氢卟吩e6的硝基咪唑聚合物胶束到达肿瘤组织后, 硝基咪唑转化为亲水性氨基咪唑, 导致胶束分解和药物快速释放[52]。载光敏剂金丝桃素的Pluronic P84胶束可增强药物渗透性, 提高药物稳定性, 增加黑色素瘤对金丝桃素的吸收[53]。载光敏剂全氟化聚合物胶束Ce6-PFOC-PEI-M (photosensitizer Ce6-loaded fluorinated polymeric micelle) 具有类似于全氟化碳的携氧能力, 与非氟化聚合物胶束Ce6-OC-PEI-M相比, 提高了携氧水平, 对肿瘤细胞具有明显毒性[54]。含二硫键的卟啉衍生物和金刚烷胺首先形成超分子(TPPC6-SS-Ada, 图 3), PEG400-β-CD再与TPPC6-SS-Ada基于主客分子相互作用在水溶液中自组装形成球形胶束, 其粒径小且均一, 约为72 nm, 并且二硫键能在肿瘤细胞内高还原性微环境中断裂释放光敏剂, 具有微环境响应性[55]。尽管聚合物胶束粒径均一, 易制备, 但其进入血液循环后能否耐受大量血液稀释是需要关注的问题。
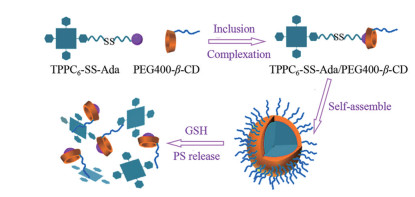
|
Figure 3 Self-assembly and disaggregation process of TPPC6-SS-Ada/PEG400-β-CD micelles. GSH: Glutathione |
纳米乳是由水、油、表面活性剂和助表面活性剂自发形成的、粒径为1~100 nm的热力学稳定、各向同性的均相胶体分散体系, 一般粒径 < 200 nm, 外观透明, 属热力学稳定体系。将光敏剂氯铝酞菁制成纳米乳后, 可被人胶质母细胞瘤细胞吞噬, 并均匀分布于细胞质中, 单线态氧产率高且激发态保持时间长[56]。采用临床使用的碘化油注射液溶解厌氧溶瘤菌Clostridium novyi-NT和掺杂稀土元素Gd、Tb及Ce的多功能发光剂NaGdF4: Tb, Ce@NaGdF4并制备成纳米乳。核壳结构型NaGdF4: Tb, Ce@NaGdF4可用于X射线引导下的常氧状态下肿瘤周边的PDT治疗, 而厌氧溶瘤菌可用于乏氧状态下肿瘤治疗。该纳米乳在影像导航下注射到瘤内, 可大大增加瘤中心和周围的肿瘤细胞凋亡[57]。载磁性纳米粒和光敏剂chlorine 6的纳米乳在激光照射下产生单线态氧, 同时在交变磁场条件下产热, 因此可同时利用磁热疗法和PDT增强抗肿瘤作用, 体外研究还证明其对表达低密度脂蛋白受体的MCF-7乳腺癌细胞具有靶向性[58]。尽管纳米乳包裹脂溶性光敏剂有一定优势, 但需关注外界环境对其稳定性的影响、光敏剂完全释放及单线态氧产率等问题。
2.5 纳米凝胶(nanogel)纳米凝胶是亲水性或两亲性聚合物以物理或化学方式交联形成的三维网状结构[59], 其特点包括: ①含水量高, 可响应外界条件发生收缩或膨胀; ②药物包封于网状结构中, 受外界环境影响小; ③粒径在20~200 nm之间, 有肿瘤被动靶向性[60]; ④易进行化学修饰和功能化, 实现药物控释[61]。因此, 载光敏剂纳米凝胶不仅可保护光敏剂, 还可提高其肿瘤靶向性, 实现缓控释。
聚光敏剂纳米凝胶可同时作为纳米光敏剂和药物载体。纳米凝胶-Ce6自身可作为纳米光敏剂, 无需释放游离光敏剂Ce6, 即可发挥光动力作用。纳米凝胶-Ce6还可进一步载组蛋白去乙酰化酶抑制剂, 通过抑制肿瘤细胞HIF-1和VEGF通路提高前列腺癌治疗效果[62]。
还原敏感性PEG化多肽纳米凝胶与溴原子修饰的活性光敏剂氟化硼二吡咯琥珀酰亚胺酯NHS-BODIPY-Br结合后形成纳米凝胶P-BODIPY。载多柔比星P-BODIPY具有还原敏感性药物释放特性, 能在10 mmol·L-1谷胱甘肽作用下释放多柔比星, 同时仅需较低强度激光照射(25 mW·cm-2, 10~15 J·cm-2) 及较低剂量多柔比星(3~5 μg·mL-1) 即可有效抑制HepG2肝癌细胞生长[63]。
3 载光敏剂纳米给药系统与其他方法联用治疗肿瘤近年来载光敏剂纳米给药系统与其他肿瘤疗法联用成为研究热点, 如化疗、放疗和免疫治疗等。与单独PDT比较, 联合疗法能发挥协同作用, 在提高疗效的同时减少单一疗法的不良反应, 实现治疗效果的最大化和最优化。
3.1 光动力纳米给药系统与化疗联用化疗是肿瘤主要治疗方法之一, 但大部分化疗药物呈现非肿瘤细胞特异性, 易出现耐药性, 临床使用受限。载光敏剂纳米给药系统通过单线态氧直接破坏肿瘤细胞, 不产生耐药性; 与化疗药物联用还可能产生协同作用, 具有减少化疗药物剂量、提高肿瘤组织对化疗药物的敏感性、克服化疗药物多重耐药性和提高肿瘤晚期患者疗效的优点。
FA修饰的牛血清白蛋白(bovine serum albumin, BSA)-氧化镍纳米粒(nickel oxide nanoparticle, NOP) 核-壳结构单线态氧响应载多柔比星纳米给药系统NOP-DOX@BSA-FA可提高多柔比星的肿瘤靶向性, 实现高效低毒[64]。原卟啉IX (PpIX) 和多柔比星局部用透明质酸多功能水凝胶经近红外光照射后可产生ROS, 破坏凝胶结构释放多柔比星(图 4)[65]。以光敏剂四羧基锌酞菁为核心制备载多柔比星pH敏感型四臂星形共聚物, 静脉注射后可靶向肿瘤, 基于肿瘤内低pH环境响应释放, 效果明显[66]。将抗肿瘤单克隆抗体与光敏剂二氢卟吩e6偶联后, 肿瘤靶向性增强, 协同治疗HER2阳性乳腺癌效果显著[9]。
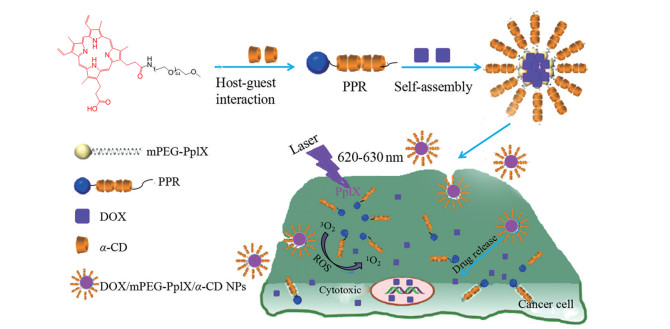
|
Figure 4 Illustration of polypseudorotaxane doxorubicin (DOX)/mPEG-PpIX/α-CD nanoparticles with the dual PDT/chemotherapy effects. PPR: Polypseudorotaxane |
载光敏剂纳米给药系统联合化疗还可克服多药耐药(multidrug resistance, MDR)。光敏剂亚甲蓝与多柔比星联用, 可增加多柔比星在肿瘤细胞中浓度, 同时下调P-gp转运表达, 使ROS大量积累, 导致耐药肿瘤细胞坏死或凋亡[66]。
3.2 载光敏剂纳米给药系统与免疫疗法联用载光敏剂纳米给药系统可导致肿瘤组织坏死或凋亡, 释放出大量肿瘤抗原物质, 从而诱发抗肿瘤免疫反应(图 5), 与免疫检查点抑制剂联合应用能进一步增强原位和复发肿瘤的治疗效果。PpIX与免疫检查点抑制剂1-甲基色氨酸(1-methyltryptophan, 1MT) 结合得到一种嵌合肽PpIX-1MT, 制成纳米粒后靶向至肿瘤组织, 经光源激发后产生ROS, 诱导肿瘤细胞凋亡, 促进caspase-3表达和肿瘤抗原产生, 引发强烈免疫反应; 而释放的1MT可进一步增强免疫, 激活CD8+ T细胞发挥抗肿瘤作用, 有效抑制原发性和肺转移性肿瘤[67]。光敏剂焦脱镁叶绿酸和IDO抑制剂(inhibitor of indoleamine 2, 3-dioxygenase) NLG919共价结合后制备成透明质酸纳米粒, 可通过CD44受体实现肿瘤靶向, 经近红外激光照射后释放ROS, 激发T淋巴细胞的抗肿瘤作用, 可有效治疗免疫功能正常小鼠的CT26大肠肿瘤[68]。
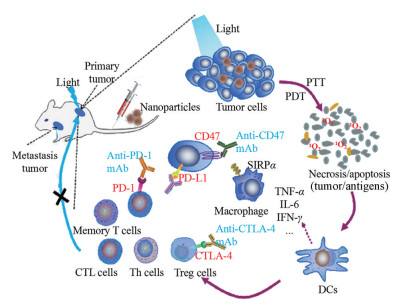
|
Figure 5 Antitumor immune responses induced by nanoparticles-based PDT. Nanoparticles enter the tumor site through passive or active targeting. Tumor cells are killed by PDT. Then, cell debris and tumor-associated antigens were released to induce immune effector cells, including the activation and redistribution of DCs and T lymphocytes, together with the expression and secretion of cytokines. The combined use of checkpoint inhibitors can enhance antitumor immunity for the treatment of primary and metastatic tumors. PD-1: Programmed cell death-1; PD-L1: Programmed cell death-ligand 1; CTL cells: Cytotoxic T lymphocyte cells; Th cells: Helper T cells; Treg cells: Regulatory cells; CD47: Cluster of differentiation 47; CTLA-4: Cytotoxic T-lymphocyte-associated antigen 4; SIRPα: Signal regulatory protein α; TNF-α: Tumor necrosis factor-α; IL-6: Interleukin-6; IFN-γ: Interferon γ; DCs: Dendritic cells; PTT: Photothermal therapy |
放疗是肿瘤治疗常用方法之一。早期皮肤癌、宫颈癌等患者单独使用放疗治疗率高达90%以上, 5年生存率可达50%以上。晚期癌症患者姑息性放疗可减轻症状和疼痛, 延长生存时间[69]。但放疗的非特异性往往导致辐射场中正常组织损伤, 且肿瘤组织内乏氧细胞可能对放疗具有抗性。因此, 迫切需要提高肿瘤对放疗敏感性, 提高放疗效率, 缩短放疗时间或减少辐射剂量。
多数核素能产生切伦科夫辐射, 从而可作为内置光源激发光敏剂。但核素产生的辐射光子效率较低, 限制了其肿瘤治疗效果。基于此, 设计了放射性核素碘131标记的长余辉纳米递送系统用于PDT和放疗联合治疗肿瘤。使用半衰期较短的放射性药物18FDG作为内置光源, 激发ZGCs (ZnGa2O4: Cr3+) 长余辉纳米材料产生近红外光。碘131进一步激发ZGCs产生长时间荧光持续激发光敏剂四羧酸酞菁锌, 发挥PDT治疗效果。同时纳米递送系统高效递送治疗性核素碘131到达肿瘤细胞产生放疗[70]。
4 总结PDT利用特定光源激发光敏剂, 在肿瘤组织内部产生活性氧, 直接杀伤肿瘤细胞, 不会产生肿瘤耐药性, 在肿瘤治疗方面显示出独特优势。但PDT组织穿透性和光敏剂效率仍存在一定局限性, 目前仅限于表皮和浅表肿瘤治疗。纳米给药系统递送光敏剂可解决PDT光敏剂溶解度低、渗透性弱和靶向性差等问题, 二者结合可谓扬长避短。同时, PDT和其他多种抗肿瘤疗法具有协同作用, 可进一步提高抗肿瘤效果和安全性。基于纳米给药系统的PDT肿瘤治疗是未来研究热点, 具有较好的临床应用前景。
作者贡献: 贾学丽和刘一婧负责撰写; 李淼、杜丽娜和金义光负责设计与修改。
利益冲突: 作者声明不存在利益冲突。
| [1] |
Jiang XP, Dai ZF. Reactive oxygen species in photodynamic therapy[J]. Chin Sci Bull (科学通报), 2018, 63: 1783-1802. DOI:10.1360/N972018-00214 |
| [2] |
Oruba Z, Łabuz P, Macyk W, et al. Antimicrobial photodynamic therapy--a discovery originating from the pre-antibiotic era in a novel periodontal therapy[J]. Photodiagnosis Photodyn Ther, 2015, 12: 612-618. DOI:10.1016/j.pdpdt.2015.10.007 |
| [3] |
Liang FQ, Shen Y, Gu Y, et al. Recent advances in light sources for photodynamic therapy[J]. Acta Laser Biol Sin (激光生物学报), 2019, 28: 97-108. |
| [4] |
Xie SM, Ping FS. Therapeutic characteristics and clinical evaluation element of the photosensitive drugs[J]. Chin New Drugs J (中国新药杂志), 2008, 17: 618-624. |
| [5] |
Li X, Feng H, Li F. Progression of basic research, clinical application of photodynamic therapy and fluorescence-guided surgery in glioma treatment[J]. J Centr South Univ (Med Sci) (中南大学学报(医学版)), 2018, 43: 360-367. |
| [6] |
Wu H, Minamide T, Yano T. Role of photodynamic therapy in the treatment of esophageal cancer[J]. Dig Endosc, 2019, 31: 508-516. DOI:10.1111/den.13353 |
| [7] |
Chiu WT, Tran TV, Pan SC, et al. Cystic fibrosis transmembrane conductance regulator: a possible new target for photodynamic therapy enhances wound healing[J]. Adv Wound Care, 2019, 8: 476-486. DOI:10.1089/wound.2018.0927 |
| [8] |
Namvar MA, Vahedi M, Abdolsamadi HR, et al. Effect of photodynamic therapy by 810 and 940 nm diode laser on herpes simplex virus 1: an in vitro study[J]. Photodiagnosis Photodyn Ther, 2019, 25: 87-91. DOI:10.1016/j.pdpdt.2018.11.011 |
| [9] |
Kim KS, Kim J, Kim DH, et al. Multifunctional trastuzumab-chlorin e6 conjugate for the treatment of HER2-positive human breast cancer[J]. Biomater Sci, 2018, 6: 1217-1226. DOI:10.1039/C7BM01084B |
| [10] |
Matoba Y, Banno K, Kisu I, et al. Clinical application of photodynamic diagnosis and photodynamic therapy for gynecologic malignant diseases: a review[J]. Photodiagnosis Photodyn Ther, 2018, 24: 52-57. DOI:10.1016/j.pdpdt.2018.08.014 |
| [11] |
Ablon G. Combination 830-nm and 633-nm light-emitting diode phototherapy shows promise in the treatment of recalcitrant psoriasis: preliminary findings[J]. Photomed Laser Surg, 2010, 28: 141-146. DOI:10.1089/pho.2009.2484 |
| [12] |
Morton CA, Braathen LR. Daylight photodynamic therapy for actinic keratoses[J]. Am J Clin Dermatol, 2018, 19: 647-656. DOI:10.1007/s40257-018-0360-y |
| [13] |
Ikeda H, Ohba S, Egashira K, et al. The effect of photodynamic therapy with talaporfin sodium, a second-generation photosensitizer, on oral squamous cell carcinoma: a series of eight cases[J]. Photodiagnosis Photodyn Ther, 2018, 21: 176-180. DOI:10.1016/j.pdpdt.2017.11.016 |
| [14] |
Cruess AF, Zlateva G, Pleil AM, et al. Photodynamic therapy with verteporfin in age-related macular degeneration: a systematic review of efficacy, safety, treatment modifications and pharmacoeconomic properties[J]. Acta Ophthalmol, 2009, 87: 118-132. DOI:10.1111/j.1755-3768.2008.01218.x |
| [15] |
D'Cruz AK, Robinson MH, Biel MA. mTHPC-mediated photodynamic therapy in patients with advanced, incurable head and neck cancer: a multicenter study of 128 patients[J]. Head Neck, 2004, 26: 232-240. DOI:10.1002/hed.10372 |
| [16] |
Moore CM, Nathan TR, Lees WR, et al. Photodynamic therapy using meso tetra hydroxy phenyl chlorin (mTHPC) in early prostate cancer[J]. Lasers Surg Med, 2006, 38: 356-363. DOI:10.1002/lsm.20275 |
| [17] |
Carrion-Gutierrez M, Ramirez-Bosca A, Navarro-Lopez V, et al. Effects of Curcuma extract and visible light on adults with plaque psoriasis[J]. Eur J Dermatol, 2015, 25: 240-246. DOI:10.1684/ejd.2015.2584 |
| [18] |
Qin ZG, Liu D, Yang F, et al. Advances in nanomedicine for cancer photodynamic therapy[J]. Prog Pharm Sci (药学进展), 2017, 4: 812-823. |
| [19] |
Oniszczuk A, Wojtunik-Kulesza KA, Oniszczuk T, et al. The potential of photodynamic therapy (PDT)-experimental investigations and clinical use[J]. Biomed Pharmacother, 2016, 83: 912-929. DOI:10.1016/j.biopha.2016.07.058 |
| [20] |
Chilakamarthi U, Giribabu L. Photodynamic therapy: past, present and future[J]. Chem Rec, 2017, 17: 775-802. DOI:10.1002/tcr.201600121 |
| [21] |
Lan M, Zhao S, Liu W, et al. Photosensitizers for photodynamic therapy[J]. Adv Healthc Mater, 2019, 8: e1900132. DOI:10.1002/adhm.201900132 |
| [22] |
Tian G, Zhang X, Gu Z, et al. Recent advances in upconversion nanoparticles-based multifunctional nanocomposites for combined cancer therapy[J]. Adv Mater, 2015, 27: 7692-7712. DOI:10.1002/adma.201503280 |
| [23] |
Jin Y. Nanotechnology in Pharmaceutical Manufacturing[M]. New Jersey: John Wiley & Sons Inc, 2008: 1249-1288.
|
| [24] |
Qi JP, Lu Y, Dong XC, et al. In vivo fate study of drug nanocarriers: the applications of environment-responsive fluorescent dyes[J]. Acta Pharm Sin (药学学报), 2019, 54: 1965-1975. |
| [25] |
Su C, Liu Y, He Y, et al. Analytical methods for investigating in vivo fate of nanoliposomes: a review[J]. J Pharm Anal, 2018, 8: 219-225. DOI:10.1016/j.jpha.2018.07.002 |
| [26] |
Feliu N, Docter D, Heine M, et al. In vivo degeneration and the fate of inorganic nanoparticles[J]. Chem Soc Rev, 2016, 45: 2440-2457. DOI:10.1039/C5CS00699F |
| [27] |
He H, Jiang S, Xie Y, et al. Reassessment of long circulation via monitoring of integral polymeric nanoparticles justifies a more accurate understanding[J]. Nanoscale Horiz, 2018, 3: 397-407. DOI:10.1039/C8NH00010G |
| [28] |
Shi Y, van der Meel R, Theek B, et al. Complete regression of xenograft tumors upon targeted delivery of paclitaxel via Π-Π stacking stabilized polymeric micelles[J]. ACS Nano, 2015, 9: 3740-3752. DOI:10.1021/acsnano.5b00929 |
| [29] |
Tran TH, Nguyen HT, Le NV, et al. Engineering of multifunctional temperature-sensitive liposomes for synergistic photothermal, photodynamic, and chemotherapeutic effects[J]. Int J Pharm, 2017, 528: 692-704. DOI:10.1016/j.ijpharm.2017.06.069 |
| [30] |
de Paula CS, Tedesco AC, Primo FL, et al. Chloroaluminium phthalocyanine polymeric nanoparticles as photosensitisers: photophysical and physicochemical characterisation, release and phototoxicity in vitro[J]. Eur J Pharm Sci, 2013, 49: 371-381. DOI:10.1016/j.ejps.2013.03.011 |
| [31] |
Lamch L, Bazylińska U, Kulbacka J, et al. Polymeric micelles for enhanced Photofrin Ⅱ ® delivery, cytotoxicity and pro-apoptotic activity in human breast and ovarian cancer cells[J]. Photodiagnosis Photodyn Ther, 2014, 11: 570-585. DOI:10.1016/j.pdpdt.2014.10.005 |
| [32] |
Muehlmann LA, Rodrigues MC, Longo JP, et al. Aluminium-phthalocyanine chloride nanoemulsions for anticancer photodynamic therapy: development and in vitro activity against monolayers and spheroids of human mammary adenocarcinoma MCF-7 cells[J]. J Nanobiotechnology, 2015, 13: 36. DOI:10.1186/s12951-015-0095-3 |
| [33] |
Debele TA, Peng S, Tsai HC. Drug carrier for photodynamic cancer therapy[J]. Int J Mol Sci, 2015, 16: 22094-22136. DOI:10.3390/ijms160922094 |
| [34] |
Reina H, Yoshikazu T. Skin permeation of 5-aminolevulinic acid encapsulated in liposomes[J]. Toin Univ Yokohama Res Bull, 2019, 41: 57-61. |
| [35] |
Lantsova AV, Borisova LM, Meerovich GA, et al. Analysis of antitumor activity of the liposomal photosensitizer lipophthalocyan[J]. Bull Exp Biol Med, 2020, 168: 361-365. DOI:10.1007/s10517-020-04709-9 |
| [36] |
Miretti M, Tempesti TC, Prucca CG, et al. Zn phthalocyanines loaded into liposomes: characterization and enhanced performance of photodynamic activity on glioblastoma cells[J]. Bioorg Med Chem, 2020, 28: 115355. DOI:10.1016/j.bmc.2020.115355 |
| [37] |
Mlynarczyk DT, Piskorz J, Popenda L, et al. S-seco-porphyrazine as a new member of the seco-porphyrazine family - synthesis, characterization and photocytotoxicity against cancer cells[J]. Bioorg Chem, 2020, 96: 103634. DOI:10.1016/j.bioorg.2020.103634 |
| [38] |
Lee EH, Lim SJ, Lee MK. Chitosan-coated liposomes to stabilize and enhance transdermal delivery of indocyanine green for photodynamic therapy of melanoma[J]. Carbohydr Polym, 2019, 224: 115143. DOI:10.1016/j.carbpol.2019.115143 |
| [39] |
Palazzolo S, Bayda S, Hadla M, et al. The clinical translation of organic nanomaterials for cancer therapy: a focus on polymeric nanoparticles, micelles, liposomes and exosomes[J]. Curr Med Chem, 2018, 25: 4224-4268. DOI:10.2174/0929867324666170830113755 |
| [40] |
Kandoth N, Kirejev V, Monti S, et al. Two-photon fluorescence imaging and bimodal phototherapy of epidermal cancer cells with biocompatible self-assembled polymer nanoparticles[J]. Biomacromolecules, 2014, 15: 1768-1776. DOI:10.1021/bm500156z |
| [41] |
Sun L, Jiang W, Zhang H, et al. Photosensitizer-loaded multifunctional chitosan nanoparticles for simultaneous in situ imaging, highly efficient bacterial biofilm eradication, and tumor ablation[J]. ACS Appl Mater Interfaces, 2019, 11: 2302-2316. DOI:10.1021/acsami.8b19522 |
| [42] |
Hao Y, Zhang B, Zheng C, et al. Multifunctional nanoplatform for enhanced photodynamic cancer therapy and magnetic resonance imaging[J]. Colloids Surf B Biointerfaces, 2017, 151: 384-393. DOI:10.1016/j.colsurfb.2016.10.039 |
| [43] |
Liu Q, Tian J, Tian Y, et al. Near-infrared-Ⅱ nanoparticles for cancer imaging of immune checkpoint programmed death-ligand 1 and photodynamic/immune therapy[J]. ACS Nano, 2021, 15: 515-525. DOI:10.1021/acsnano.0c05317 |
| [44] |
Yi G, Hong SH, Son J, et al. Recent advances in nanoparticle carriers for photodynamic therapy[J]. Quant Imaging Med Surg, 2018, 8: 433-443. DOI:10.21037/qims.2018.05.04 |
| [45] |
Rotello VM. Interfacing inorganic nanoparticles with biology[J]. Bioconjug Chem, 2017, 28: 1-2. DOI:10.1021/acs.bioconjchem.6b00727 |
| [46] |
Hou X, Tao Y, Pang Y, et al. Nanoparticle-based photothermal and photodynamic immunotherapy for tumor treatment[J]. Int J Cancer, 2018, 143: 3050-3060. DOI:10.1002/ijc.31717 |
| [47] |
Zhang L, Zeng L, Pan Y, et al. Inorganic photosensitizer coupled Gd-based upconversion luminescent nanocomposites for in vivo magnetic resonance imaging and near-infrared-responsive photodynamic therapy in cancers[J]. Biomaterials, 2015, 44: 82-90. DOI:10.1016/j.biomaterials.2014.12.040 |
| [48] |
Chen G, Qiu H, Prasad PN, et al. Upconversion nanoparticles: design, nanochemistry, and applications in theranostics[J]. Chem Rev, 2014, 114: 5161-5214. DOI:10.1021/cr400425h |
| [49] |
Niu N, Zhang Z, Gao X, et al. Photodynamic therapy in hypoxia: near-infrared-sensitive, self-supported, oxygen generation nano-platform enabled by upconverting nanoparticles[J]. Chem Eng J, 2018, 15: 818-827. |
| [50] |
Zhou J, Yao H, Meng L, et al. A hollow NaGdF(4)/AFn nanosystem based on "relay race" release for therapy[J]. ChemMedChem, 2017, 12: 1191-1200. DOI:10.1002/cmdc.201700295 |
| [51] |
Luo L, Liu HB, Yin Z, et al. Biodegradable micelles for two-photon photodynamic therapy in a mouse model of breast cancer[J]. Acta Pharm Sin (药学学报), 2019, 54: 927-936. |
| [52] |
Deng J, Liu F, Wang L, et al. Hypoxia- and singlet oxygen-responsive chemo-photodynamic micelles featured with glutathione depletion and aldehyde production[J]. Biomater Sci, 2018, 7: 429-441. |
| [53] |
Goncalves RS, Braga G, Oliveira ACV, et al. Hypericin delivery system based on P84 copolymeric micelles linked with N-(3-aminopropyl)-2-pyrrolidone for melanoma-targeted photodynamic therapy[J]. ACS Appl Energy Mater, 2020, 2: 1692-1701. |
| [54] |
Wang Q, Li JM, Yu H, et al. Fluorinated polymeric micelles to overcome hypoxia and enhance photodynamic cancer therapy[J]. Biomater Sci, 2018, 6: 3096-3107. DOI:10.1039/C8BM00852C |
| [55] |
Liu F, Ma Y, Xu L, et al. Redox-responsive supramolecular amphiphiles constructed via host-guest interactions for photodynamic therapy[J]. Biomater Sci, 2015, 3: 1218-1227. DOI:10.1039/C5BM00045A |
| [56] |
de Paula LB, Primo FL, Tedesco AC. Nanomedicine associated with photodynamic therapy for glioblastoma treatment[J]. Biophys Rev, 2017, 9: 761-773. DOI:10.1007/s12551-017-0293-3 |
| [57] |
Park W, Cho S, Kang D, et al. Tumor microenvironment targeting nano-bio emulsion for synergistic combinational X-ray PDT with oncolytic bacteria therapy[J]. Adv Healthc Mater, 2020, 9: e1901812. DOI:10.1002/adhm.201901812 |
| [58] |
Pellosi DS, Macaroff PP, Morais PC, et al. Magneto low-density nanoemulsion (MLDE): a potential vehicle for combined hyperthermia and photodynamic therapy to treat cancer selectively[J]. Mater Sci Eng C Mater Biol Appl, 2018, 92: 103-111. DOI:10.1016/j.msec.2018.06.033 |
| [59] |
Merino S, Martín C, Kostarelos K, et al. Nanocomposite hydrogels: 3D polymer-nanoparticle synergies for on-demand drug delivery[J]. ACS Nano, 2015, 9: 4686-4697. DOI:10.1021/acsnano.5b01433 |
| [60] |
Soni KS, Desale SS, Bronich TK. Nanogels: an overview of properties, biomedical applications and obstacles to clinical translation[J]. J Control Release, 2016, 240: 109-126. DOI:10.1016/j.jconrel.2015.11.009 |
| [61] |
Ahmed EM. Hydrogel: preparation, characterization, and applications: a review[J]. J Adv Res, 2015, 62: 105-121. |
| [62] |
Liu N, Liu H, Chen H, et al. Polyphotosensitizer nanogels for GSH-responsive histone deacetylase inhibitors delivery and enhanced cancer photodynamic therapy[J]. Colloids Surf B Biointerfaces, 2020, 188: 110753. DOI:10.1016/j.colsurfb.2019.110753 |
| [63] |
Liu L, Li T, Ruan Z, et al. Reduction-sensitive polypeptide nanogel conjugated BODIPY-Br for NIR imaging-guided chem/photodynamic therapy at low light and drug dose[J]. Mater Sci Eng C Mater Biol Appl, 2018, 92: 745-756. DOI:10.1016/j.msec.2018.07.034 |
| [64] |
Bano S, Nazir S, Munir S, et al. "Smart" nickel oxide based core-shell nanoparticles for combined chemo and photodynamic cancer therapy[J]. Int J Nanomedicine, 2016, 11: 3159-3166. DOI:10.2147/IJN.S106533 |
| [65] |
Xu X, Zeng Z, Huang Z, et al. Near-infrared light-triggered degradable hyaluronic acid hydrogel for on-demand drug release and combined chemo-photodynamic therapy[J]. Carbohydr Polym, 2020, 229: 115394. DOI:10.1016/j.carbpol.2019.115394 |
| [66] |
Zhang X, Li Q, Sun X, et al. Doxorubicin-loaded photosensitizer-core pH-responsive copolymer nanocarriers for combining photodynamic therapy and chemotherapy[J]. ACS Biomater Sci Eng, 2017, 3: 1008-1016. DOI:10.1021/acsbiomaterials.6b00762 |
| [67] |
Song W, Kuang J, Li CX, et al. Enhanced immunotherapy based on photodynamic therapy for both primary and lung metastasis tumor eradication[J]. ACS Nano, 2018, 12: 1978-1989. DOI:10.1021/acsnano.7b09112 |
| [68] |
Hu X, Hou B, Xu Z, et al. Supramolecular prodrug nanovectors for active tumor targeting and combination immunotherapy of colorectal cancer[J]. Adv Sci, 2020, 7: 1903332. DOI:10.1002/advs.201903332 |
| [69] |
Zhang Q, Li L. Photodynamic combinational therapy in cancer treatment[J]. J BUON, 2018, 23: 561-567. |
| [70] |
Wang Q, Liu N, Hou Z, et al. Radioiodinated persistent luminescence nanoplatform for radiation-induced photodynamic therapy and radiotherapy[J]. Adv Healthc Mater, 2021, 10: e2000802. DOI:10.1002/adhm.202000802 |
 2021, Vol. 56
2021, Vol. 56