2. 中国药科大学中药学院, 江苏 南京 210009;
3. 中国药科大学药学院, 江苏 南京 210009
2. School of Chinese Materia Medica, China Pharmaceutical University, Nanjing 210009, China;
3. School of Pharmacy, China Pharmaceutical University, Nanjing 210009, China
高血糖性肾损伤(hyperglycemic kidney injury, HKI) 是糖尿病患者常见的严重并发症之一, 其主要特点是出现蛋白尿、肾小球肥大、细胞外基质过度积累和肾小球基底膜增厚, 最终导致肾功能衰竭[1]。有关HKI的发生机制至今没有弄清, 目前的主要治疗手段是通过胰岛素和肾素-血管紧张素抑制剂调节血糖和血压, 以及使用钠-葡萄糖协同转运蛋白2 (sodium-glucose cotransporter 2, SGLT-2) 抑制剂[2]。然而, 这些药物的临床疗效并不令人满意, 且SGLT-2抑制剂的不良反应众多。
5-羟色胺(5-hydroxytryptamine, 5-HT) 广泛存在于中枢和外周[3]。目前认为, 外周5-HT主要来源于肠嗜铬细胞, 以色氨酸为底物, 经色氨酸羟化酶1 (tryptophan hydroxylase 1, Tph1)、芳香族氨基酸脱羧酶(aromatic amino acid decarboxylase, AADC) 两步催化形成[4]。5-HT被线粒体外膜上的单胺氧化酶A (monoamine oxidase A, MAO-A) 降解失活[5], 此过程会产生活性氧(reactive oxygen species, ROS)[6]。5-HT还可作用于其受体(5-HT receptor, 5-HTR), 发挥生物学作用。5-HTR可分为7大类, 其中2A受体(5-HT2AR) 在脂代谢、糖代谢和炎症反应中发挥明显作用[7]。研究表明, 高血糖导致血管内皮细胞损伤, 进而激活凝血系统, 导致血小板释放5-HT, 使毛细血管处于收缩及高凝状态[7], 加重糖尿病及其并发症的发生和发展。
作者发现, 5-HT系统在2型糖尿病(type 2 diabetes mellitus, T2DM) 或应激导致肝脏脂代谢紊乱、炎症及纤维化[8, 9], 以及T2DM导致骨骼肌代谢异常、ATP产生减少和身体疲劳[10]时起关键作用。肝脏及骨骼肌均存在5-HT合成酶Tph1和AADC、5-HT2AR、MAO-A表达, T2DM时其表达上调, 从而不仅导致脂代谢紊乱, 还导致5-HT在线粒体的降解增加、ROS产生增多, 使细胞产生氧化应激、线粒体ATP合成减少, 由此还可引起细胞的炎症信号通路激活、炎症因子产生等异常[8, 10]。作者推测, HKI可能也与肾脏5-HT系统相关。本研究通过长期高脂饲料(high fat diet, HFD) 喂养联合链脲佐菌素(streptozotocin, STZ) 刺激建立小鼠T2DM模型, 再经长期HFD喂养导致HKI[11]。通过高糖诱导人肾小球系膜细胞(human glomerular mesangial cells, HMC) 损伤, 并经动物和细胞实验研究来验证推测。
材料与方法主要药品和试剂 动物实验用盐酸沙格雷酯(sarpogrelate hydrochloride, SH, 上海乾劲化工科技有限公司) 和卡比多巴(carbidopa, CDP, 浙江手心制药有限公司); 细胞实验用SH、CDP和氯吉兰(clorgyline, CGL) (上海CSNpharm公司); STZ (北京博爱港商贸中心); 5-HT (Celleck公司); D-葡萄糖(D-glucose, D-Glu, 美国Sigma-Aldrich公司); 丙二醛(malondialdehyde, MDA)、总超氧化物歧化酶(total superoxide dismutase, T-SOD)、肌酐(creatinine, CRE)、尿素氮(blood urea nitrogen, BUN)、白蛋白(albumin, Alb) 和还原型谷胱甘肽(glutathione, GSH) 检测试剂盒(南京建成生物工程研究所); 5-HT、白介素1β (interleukin-1β, IL-1β)、肿瘤坏死因子-α (tumor necrosis factor α, TNF-α) 和胶原Ⅳ (collagen Ⅳ, Col Ⅳ) ELISA检测试剂盒(上海酶联生物科技有限公司); ROS荧光探针(2, 7-dichlorodi-hydrofluorescein diacetate, DCFH-DA, 上海碧云天生物技术有限公司); 5-HT2AR抗体和MAO-A抗体(美国Santa Cruz Biotechnology公司); Tph1抗体、AADC抗体和α-平滑肌肌动蛋白(α-smooth muscle actin, α-SMA) 抗体(美国Signalway Antibody公司); 3-磷酸甘油醛脱氢酶(glyceraldehyde-3-phosphate dehydrogenase, GAPDH) 抗体、胶原Ⅰ (Col I) 抗体(武汉三鹰生物技术有限公司); 核因子κB (nuclear factor kappa B, NF-κB) p65抗体、核因子κB抑制因子(inhibitor of NF-κB, IκB) α抗体及其磷酸化抗体, 以及基质金属蛋白酶-2 (matrix metalloproteinases-2, MMP-2) 抗体(沈阳万类生物公司)。
实验动物 无特定病原体(specific pathogen free, SPF) 级C57BL/6J雄性小鼠, 体重20 ± 2 g, 合格证编号: NO. 201904706 (扬州大学比较医学中心)。动物饲养于中国药科大学动物实验中心的清洁动物房。实验规程和方案符合中国药科大学药学动物实验中心伦理审查标准。
小鼠T2DM模型的建立 将小鼠按体重随机分成两组: 对照组(control, Ctrl) 和T2DM模型组。Ctrl组用普通饲料喂养; T2DM模型组用HFD喂养8周后连续腹腔注射(ip) STZ (55 mg·kg-1·day-1) 5天。Ctrl组ip等体积生理盐水。造模1周后禁食6 h, 测定空腹血糖(fasting blood glucose, FBG), FBG ≥ 11.1 mmol·L-1作为成模标准。
小鼠HKI模型建立、分组及给药 T2DM小鼠继续HFD喂养7周后, 选择FBG ≥ 18 mmol·L-1的小鼠作为HKI动物模型。小鼠随机分组: 模型组(model, Mod), 灌胃(ig) 0.5%羧甲基纤维素钠(CMC-Na) 溶液; SH给药组(SH), ig SH, 每次50 mg·kg-1; CDP给药组(CDP), ig CDP, 每次50 mg·kg-1; SH + CDP联合给药组(SC), ig SH + CDP, 每次50 mg·kg-1, 其中SH∶CDP = 2∶1 [依据前期研究结果[8], 该比率复方疗效最佳, 其中SH、CDP接近等摩尔剂量(SH、CDP分子量分别为465、226)]。Ctrl组也ig等体积0.5% CMC-Na溶液。每天给药2次, 连续给药9周。给药期间, 小鼠继续用HFD喂养。实验期间Mod组小鼠死亡则用备用动物补充, 而给药组在5周内死亡用备用动物补充、否则不再补充。连续给药9周后, 动物经戊巴比妥钠麻醉(45 mg·kg-1), 取血浆(血液加抗凝剂提取血浆)、肾脏、胃肠, 在-80 ℃冷冻备用。
细胞培养与处理 HMC细胞购自中国科学院干细胞库。常规细胞培养, 用含10%胎牛血清的DMEM培养, 待细胞生长至占培养皿80%~90%时进行传代。实验加药时采用无血清培养基。用高浓度的D-Glu连续刺激48 h建立HMC损伤模型, 分别用SH、CDP和CGL抑制5-HT2AR、AADC及MAO-A, 每24 h换药1次。
生化指标检测 采用比色法测定CRE、BUN、Alb、MDA、T-SOD和GSH水平, 采用ELISA法检测5-HT、TNF-α、IL-1β和Col Ⅳ, 酶标仪(M200 Pro, 瑞士Tecan Group Ltd.公司) 测量吸光度A值。
蛋白表达检测 采用Western blot法检测组织及细胞内5-HT2AR、AADC、Tph1、MAO-A、NF-κB p65炎症信号通路、Col I、MMP-2和α-SMA表达。全自动化学发光仪(Tanon 5200, 上海天能有限公司) 检测条带灰度。
免疫组化、PAS染色和Masson染色 取用福尔马林固定的肾脏制成3 μm厚的石蜡切片, 应用免疫组化、PAS染色和Masson染色后, 进行光镜观察(IX51型荧光倒置显微镜, Olympus公司), 按免疫组化两步法检测肾组织相关蛋白表达。Image-Pro Plus 6.0图像分析软件计算肾小球面积及肾小球内纤维化面积(纤维化面积/%=肾小球内Masson染色面积/肾小球总面积×100%)。
细胞内ROS含量测定 采用ROS荧光探针DCFH-DA检测细胞内ROS含量, 激光共聚焦显微镜(LSM700, 德国ZEISS公司) 观察并拍照。
统计学分析 数据用均值±标准差(x±s) 表示, SPSS 17软件进行统计分析。“肾小球面积”和“肾小球纤维化面积”用Mann-Whitney U检验; 其他多组间比较数据用单因素方差分析, 选择LSD检验(方差齐) 或Games-Howell检验(方差不齐); 两组间比较用Student-t检验。P < 0.05表示有显著统计学差异, P < 0.01表示有非常显著统计学差异。
结果 1 抑制外周5-HT2AR或/和5-HT合成对HKI小鼠肾功能的影响作者已在T2DM小鼠骨骼肌ATP合成减少、产生疲劳的研究中报道了SH、CDP以及两者联合给药对T2DM小鼠进食量、体重、血糖及血脂的影响[10]。本研究所用小鼠与已报道所用小鼠无本质区别, 仅选择了FBG更高、HFD喂养时间更长的T2DM小鼠。两次研究结果相似, 故数据省略。简要说明, SH、CDP和SC可减少HKI小鼠HFD喂养时的进食量, 但可升高体重、降血糖和血脂。然而, 降血糖、血脂效果是轻度的, 尤其对血糖的影响中, SH、CDP和SC组间无显著差异。实验期间, 模型组死亡动物3只, 用备用动物补充; SH组及CDP组分别在给药第3、4周各死亡动物1只, 用备用动物补充; SH和CDP联合给药(SC) 组未见动物死亡。小鼠死亡应是血糖过高、过度的失重等糖尿病性原因造成。
尿糖(图 1A)、24 h尿量(图 1B)、24 h尿蛋白(图 1C) 检测结果表明, 上述指标在Mod组小鼠明显升高, 同时也检测到血浆CRE (图 1D)、BUN (图 1E) 明显升高, 及Alb (图 1F) 明显降低, 提示肾功能损伤。SH、CDP及SC治疗明显改善肾功能指标。除尿糖、尿蛋白外, 其他肾功能指标在SC组均改善更明显。结果表明, SH、CDP治疗明显改善HKI小鼠肾功能, 依据SH、CDP及SC组的给药剂量(相同剂量), 及SC组中SH、CDP的用量(分别只有SH、CDP组的2/3及1/3), 可推测SH和CDP联合给药的疗效具有“1+1 > 2”的效果, 即协同效应, SC组几乎完全恢复了血浆CRE、BUN指标。
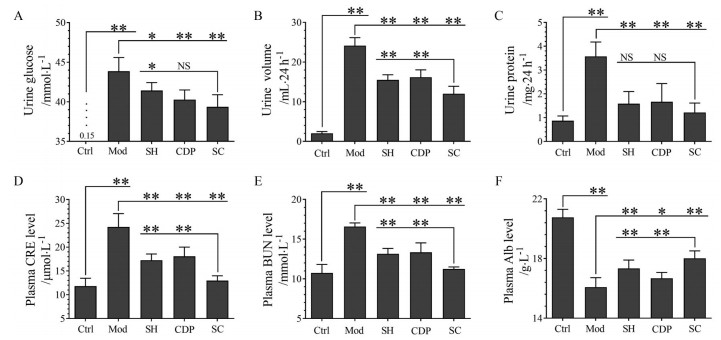
|
Figure 1 The effects of sarpogrelate hydrochloride (SH) and carbidopa (CDP) (alone or in combination) on the renal function indexes of hyperglycemic kidney injury (HKI) mice. Urine glucose (A), urine volume (B), 24 h urine protein (C), plasma creatinine (CRE, D), blood urea nitrogen (BUN, E), and albumin (Alb, F) levels in the control (Ctrl) and HKI model (Mod) mice, as well as SH or CDP-treated and both co-treated (SC) HKI mice were shown. n = 10, x±s. *P < 0.05, **P < 0.01. NS: Not significant |
免疫组化(图 2A) 和Western blot分析(图 2B) 结果表明, 小鼠肾小球基底膜和肾小管上皮细胞均存在5-HT2AR、Tph1和AADC表达, 而肾小球内毛细血管上皮细胞没有它们的表达, 且在HKI小鼠的表达量显著升高; 小肠(十二指肠) 的Tph1、AADC表达在HKI小鼠中也明显上调(图 2C)。同时, 与Ctrl比较, HKI小鼠肾组织MAO-A表达上调(图 2D)、血浆及肾脏5-HT水平升高(图 2E、F)。SH及CDP治疗可明显降低HKI小鼠肾组织MAO-A表达、血浆和肾脏的5-HT水平, 且SC组水平更低。HKI小鼠肾脏指数升高(图 2G), 而SH、CDP和SC治疗则降低肾指数, 且SC最明显。同时, HKI小鼠肾脏发生氧化应激, 表现为肾组织MDA (图 2H) 含量升高、SOD (图 2I) 活力下降、GSH (图 2J) 含量降低; HKI小鼠肾脏也发生炎症, 表现为炎症因子TNF-α (图 2K) 和IL-1β (图 2L) 含量明显升高, NF-κB p65和IκBα的磷酸化表达上调、而IκBα总蛋白表达下调(图 2M)。SH或/和CDP治疗则明显降低肾脏氧化应激和炎症, 且联合给药显示协同效应。
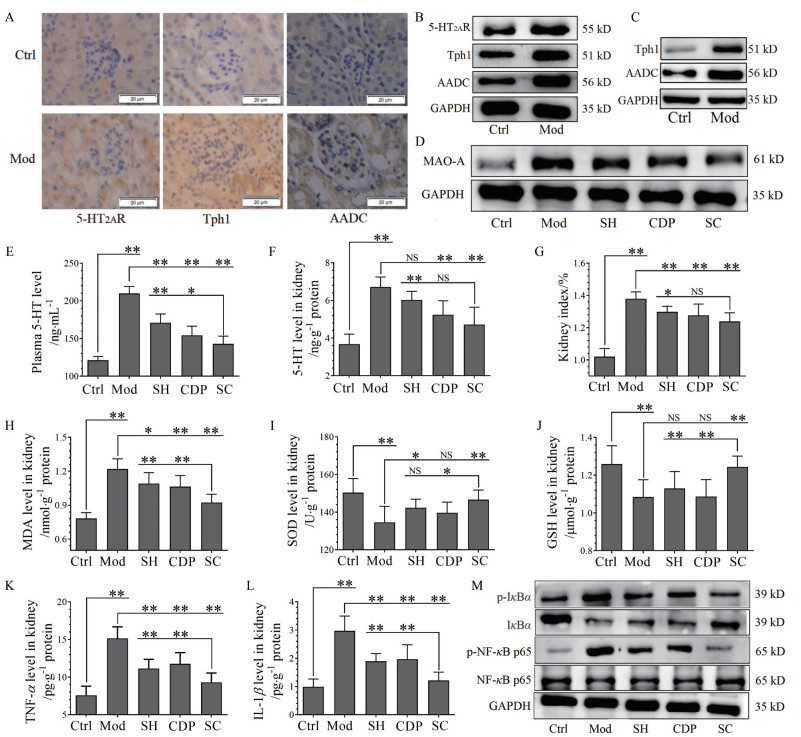
|
Figure 2 The relevance of expression of 5-HT2A receptor (5-HT2AR), 5-HT synthetases tryptophan hydroxylase 1 (Tph1), aromatic amino acid decarboxylase (AADC), and monoamine oxidase A (MAO-A) with oxidative stress and inflammation in kidney tissues of HKI mice. Immunohistochemistry staining (40×; scale bar: 20 μm; A), Western blot analysis of 5-HT2AR, Tph1, and AADC in the kidney tissues (B), and small intestine tissues (C) of Ctrl and Mod mice. Expression of renal MAO-A (D), levels of plasma 5-HT (E) and renal 5-HT (F), kidney index (kidney weight as % of body weight, G), levels of renal malondialdehyde (MDA, H), superoxide dismutase (SOD, I), glutathione (GSH, J), tumor necrosis factor α (TNF-α, K), and interleukin-1β (IL-1β, L), and phosphorylation (p-) and expression of renal inhibitor of NF-κB (IκBα), nuclear factor kappa B (NF-κB) p65, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH, M) in the Ctrl and Mod mice, as well as SH or CDP-treated and SC HKI mice. n = 10, x±s. *P < 0.05, **P < 0.01 |
结果表明, 不仅小肠存在5-HT合成, 肾小球内和肾小管上皮细胞也存在5-HT合成, 且在HKI小鼠被上调, 导致肾脏内及血浆5-HT水平升高。HKI小鼠还出现肾脏5-HT2AR和MAO-A表达上调, 提示5-HT2AR的激活及5-HT降解的增多; 同时, 肾脏发生氧化应激和炎症。通过抑制外周5-HT2AR和5-HT合成, 可明显抑制HKI时肾脏的氧化应激及炎症, 且联合抑制有协同效应。
3 HKI小鼠肾小球病变与5-HT2AR和5-HT合成的关系肾组织切片PAS染色结果显示(图 3A), Mod小鼠肾小球内出现明显的糖原沉积和基底膜增厚, 且肾小球面积明显增大(图 3A)。SH、CDP治疗明显降低肾小球内糖原沉积并抑制基底膜增厚, 且使肾小球面积明显缩小, 联合给药显示协同效应。蛋白表达检测结果表明, Mod组肾脏MMP-2、α-SMA和ColⅠ的表达显著上调(图 3B); 且Masson染色(图 3C) 显示肾小球内和肾小管间质出现明显纤维化, 计算表明肾小球内纤维组织面积增大(图 3C) 且Col Ⅳ (图 3D) 含量升高。SH、CDP治疗可明显改善肾脏纤维化, 且联合治疗有协同效应。
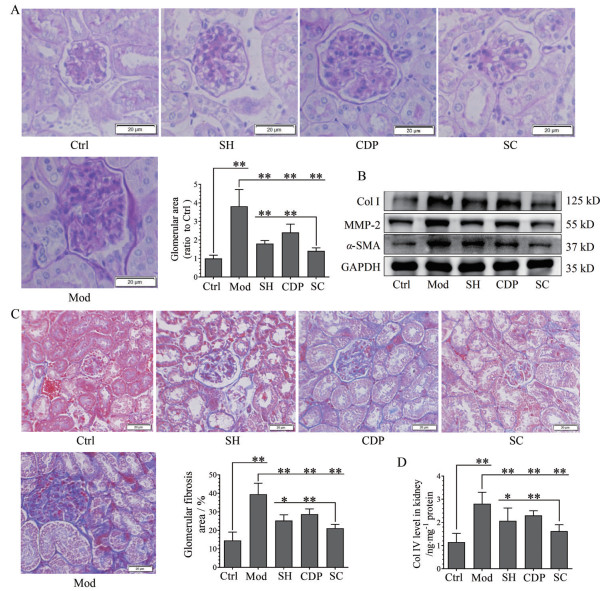
|
Figure 3 The relevance of 5-HT2A receptor, 5-HT synthesis with the lesions of kidney glomerular basement membrane and fibrosis in HKI mice. PAS staining and the analysis of glomerular area (40×; n = 8, A), expression of Col Ⅰ, MMP-2, α-SMA, and GAPDH (B), Masson staining (40×) and the analysis of glomerular fibrosis area (n = 8, C), and levels of Col Ⅳ (n = 10, D) in the renal tissues of Ctrl and Mod mice, as well as SH or CDP-treated and SC HKI mice. Scale bar: 20 μm. x±s. *P < 0.05, **P < 0.01. Col Ⅰ, Collagen I; MMP-2: Matrix metalloproteinases-2; α-SMA: α-Smooth muscle actin |
前期研究已揭示, 高糖、饱和脂肪酸诱导的肝细胞、骨骼肌细胞线粒体ROS产生及相应的炎症信号通路激活是因为5-HT2AR、5-HT合成及5-HT降解激活[8, 10]。本研究用HMC细胞来观察高糖诱导肾小球损伤是否也是这种机制。MTT法检测表明, SH和CDP以5 μmol·L-1或CGL以10 μmol·L-1浓度可用于实验, D-Glu以33或66 mmol·L-1处理细胞48 h不降低细胞活力。用两种浓度D-Glu刺激HMC 48 h可使其5-HT2AR、Tph1和AADC表达上调, 且表达水平接近(图 4A), 故以下实验用33 mmol·L-1 D-Glu造模。
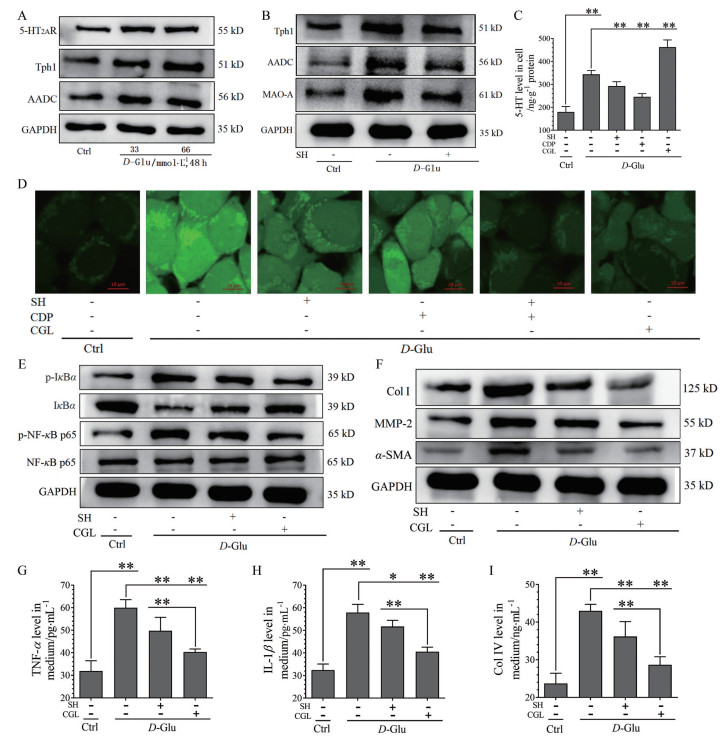
|
Figure 4 The relationship between 5-HT2AR, 5-HT synthesis, and 5-HT degradation and D-glucose (D-Glu)-induced ROS production, inflammation, and fibrosis in human glomerular mesangial cells (HMC). SH (5 μmol·L-1), CDP (5 μmol·L-1), and clorgyline (CGL, 10 μmol·L-1) were added to HMC. Except for Ctrl, the cells exposed to D-Glu were treated with or without drugs for 48 h. Expression of 5-HT2AR, Tph1, AADC, and GAPDH in the Ctrl and D-Glu (33 or 66 mmol·L-1)-treated cells (A). Expression of Tph1, AADC, MAO-A, and GAPDH in the Ctrl, D-Glu (33 mmol·L-1)-treated with or without SH-treated cells (B). Levels of intracellular 5-HT in the Ctrl, D-Glu (33 mmol·L-1)-treated with or without SH, CDP, and CGL-treated cells (C). Intracellular ROS distribution (63×) determined by fluorescent dye staining in the Ctrl, D-Glu (33 mmol·L-1)-treated with or without SH or CDP-treated (alone or in combination), and CGL-treated cells (D). Phosphorylation (p-) and expression of IκBα, NF-κB p65, and GAPDH (E), expression of Col Ⅰ, α-SMA, MMP-2, and GAPDH (F), and levels of medium TNF-α (G), IL-1β (H), and Col Ⅳ (I) in the Ctrl, D-Glu (33 mmol·L-1)-treated with or without SH and CGL-treated cells. n = 4, x±s. *P < 0.05, **P < 0.01 |
D-Glu诱导的Tph1、AADC和MAO-A表达上调可被SH抑制(图 4B); 而D-Glu诱导的5-HT含量升高可被SH和CDP抑制, 但被CGL (抑制MAO-A) 进一步升高(图 4C)。相应地, D-Glu诱导的细胞内ROS增多也可被SH、CDP和CGL明显抑制, 且SH + CDP及CGL均强烈抑制ROS产生(图 4D)。结果提示, 高糖诱导HMC细胞内ROS产生是通过增加5-HT合成及5-HT在线粒体的降解实现的; 其中, 5-HT2AR可通过介导5-HT合成及MAO-A表达, 从而调控5-HT降解, 与在肝细胞、骨骼肌细胞的作用机制相同。
进一步研究5-HT2AR和5-HT降解对高糖诱导HMC细胞内炎症和纤维化激活的调控作用。可发现, D-Glu诱导HMC细胞内NF-κB p65和IκBα的磷酸化表达上调、IκBα表达下调(图 4E), Col Ⅰ、MMP-2和α-SMA的表达上调(图 4F), 以及培养基中TNF-α (图 4G)、IL-1β (图 4H)、Col Ⅳ (图 4I) 浓度升高, 可被SH明显抑制(P < 0.01或P < 0.05), 而被CGL强烈抑制(P < 0.01), CGL的抑制效应明显好于SH, 表明高糖诱导HMC细胞的肌成纤维细胞化、炎症及纤维化病理改变是由5-HT降解导致的。
讨论本实验建立的小鼠HKI模型, 与临床患者相似[1], 出现多饮多食多尿、体重减轻、高血糖和尿糖等特征, 同时也检测到明显肾功能损伤及肾小球病变特征。作者发现, 高血糖不是HKI的直接原因。SH、CDP分别或联合给药的降血糖作用没有差异, 但对HKI的治疗效果明显不同, 联合给药有协同效应, 治疗效果明显。结合动物及细胞研究结果, 推测HKI的肾小球病变与高血糖刺激肾小球系膜细胞5-HT2AR、5-HT合成酶和MAO-A表达上调密切相关, 导致5-HT合成及5-HT在线粒体的降解增多、ROS产生增多, 从而使细胞发生氧化应激。这与T2DM时肝细胞及骨骼肌细胞病理损伤的机制是相同的[8, 10], 刺激因子可以是高糖或高浓度脂肪酸。因此, T2DM患者出现肝脏、骨骼肌和肾脏损伤具有相同机制, 是器官5-HT系统被激活的后果。同时, 小肠5-HT合成也被激活, 这可能是血浆5-HT浓度升高的主要原因(胃肠道是外周5-HT的主要来源); 而肾小管上皮细胞的5-HT系统激活, 可能是导致其间质纤维化的主要原因。
Kim等[12]报道, SH对HKI动物模型具有明显的肾脏保护作用, 且证实5-HT2AR在肾小球系膜细胞存在表达。Lee等[13]研究表明, SH可通过抗炎和抗纤维化作用来治疗HKI, 认为5-HT增加了HMC的增殖、细胞外基质(extracellular matrix, ECM)的合成和纤维化。但尚不清楚SH对HKI有益作用的确切机制。依据本研究及肝细胞和骨骼肌的研究[8, 10], 5-HT2AR通过调控5-HT合成、MAO-A表达介导线粒体的5-HT降解和ROS产生, 导致肾小球炎症及纤维化的发生, 其信号通路可能是G蛋白Gq/PLC (phospholipase C) 途径, 介导蛋白激酶Cε (PKCε) 的激活[8, 14]; PKCε激活后调控Tph1、AADC及MAO-A的表达。确切的机制有待研究。
本研究及文献都支持高糖刺激时肾小球系膜细胞ROS产生会增多, 从而使细胞发生氧化应激, 激活NF-κB信号通路引发炎症[15], 导致基底膜增厚、ECM蛋白合成增加及内皮功能障碍, 而ECM的主要成分就是Col Ⅰ和Col Ⅳ等。HKI发生时, 系膜细胞肌成纤维细胞化, α-SMA表达上调[16]。另外, MMP活性失调与纤维化发生也存在联系。MMP介导ECM更新, 激活与肾体积增大、肾细胞增生、肾瘢痕形成及纤维化有关的生长因子释放[17]。有研究认为, 过多葡萄糖的氧化造成线粒体过度负荷, 导致ROS产生过多[18]。然而, 本研究表明, 高糖诱导系膜细胞ROS产生的机制是激活了线粒体5-HT降解, 其机制是使5-HT2AR、5-HT合成酶及MAO-A表达同时上调, 导致线粒体ROS产生增多。
目前, HKI的新药开发遇到困难, 在Ⅲ期临床试验中极少成功, 常因疗效不佳、毒副作用而宣告失败[19]。本研究发现, 使用5-HT2AR拮抗剂和5-HT合成抑制剂联合给药可以有效地治疗HKI, 且具有协同效应, 这为新药开发提供了新思路。
作者贡献: 傅继华为本项目的课题负责人, 负责实验设计、实验指导及数据分析, 主导论文构思并参与论文写作及修改; 徐凡负责论文写作、修改及投稿, 并完成部分实验工作; 杨静为本论文实验工作的主要完成人; 张锐、闪雪纯、梁秀睿、张怡、赵希彤、郝宇轩、张誉馨、金佳琦和关晶均参与本论文实验工作。全体作者都阅读并同意最终的文本。
利益冲突: 所有作者均声明不存在利益冲突。
| [1] |
Zhu L, Han J, Yuan R, et al. Berberine ameliorates diabetic nephropathy by inhibiting TLR4/NF-κB pathway[J]. Biol Res, 2018, 51: 9. DOI:10.1186/s40659-018-0157-8 |
| [2] |
Ninčević V, Omanović Kolarić T, Roguljić H, et al. Kidney benefits of SGLT 2 inhibitors and GLP-1 receptor agonists: evidence supporting a paradigm shift in the medical management of type 2 diabetes[J]. Int J Mol Sci, 2019, 20: 5831. DOI:10.3390/ijms20235831 |
| [3] |
Hansson B, Medina A, Fryklund C, et al. Serotonin (5-HT) and 5-HT2A receptor agonists suppress lipolysis in primary rat adipose cells[J]. Biochem Biophys Res Commun, 2016, 474: 357-363. DOI:10.1016/j.bbrc.2016.04.110 |
| [4] |
Coates MD, Tekin I, Vrana KE, et al. Review article: the many potential roles of intestinal serotonin (5-hydroxytryptamine, 5-HT) signalling in inflammatory bowel disease[J]. Aliment Pharmacol Ther, 2017, 46: 569-580. DOI:10.1111/apt.14226 |
| [5] |
Manzella N, Santin Y, Maggiorani D, et al. Monoamine oxidase-A is a novel driver of stress-induced premature senescence through inhibition of parkin-mediated mitophagy[J]. Aging Cell, 2018, 17: 12811. DOI:10.1111/acel.12811 |
| [6] |
Oliver KH, Duvernay MT, Hamm HE, et al. Loss of serotonin transporter function alters ADP-mediated glycoprotein αIIbβ3 activation through dysregulation of the 5-HT2A receptor[J]. J Bio Chem, 2016, 291: 20210-20219. DOI:10.1074/jbc.M116.736983 |
| [7] |
Li X, Guo K, Li T, et al. 5-HT 2 receptor mediates high-fat diet-induced hepatic steatosis and very low density lipoprotein overproduction in rats[J]. Obes Res Clin Pract, 2018, 12: 16-28. |
| [8] |
Fu J, Li C, Zhang G, et al. Crucial roles of 5-HT and 5-HT2 receptor in diabetes-related lipid accumulation and pro-inflammatory cytokine generation in hepatocytes[J]. Cell Physiol Biochem, 2018, 48: 2409-2428. DOI:10.1159/000492656 |
| [9] |
Fu J, Ma S, Li X, et al. Long-term stress with hyperglucocorticoidemia-induced hepatic steatosis with VLDL overproduction is dependent on both 5-HT2A receptor and 5-HT synthesis in liver[J]. Int J Biol Sci, 2016, 12: 219-234. DOI:10.7150/ijbs.13062 |
| [10] |
Zhang YX, Zhang R, Yang J, et al. Study on relationship between fatigue caused by type 2 diabetes mellitus and 5-HT degradation in skeletal muscle[J]. Acta Pharm Sin (药学学报), 2021, 56: 190-200. |
| [11] |
Breyer MD, Böttinger E, Brosius FC, et al. Mouse models of diabetic nephropathy[J]. J Am Soc Nephrol, 2005, 16: 27-45. DOI:10.1681/ASN.2004080648 |
| [12] |
Kim D, Choi B, Ku S, et al. Beneficial effects of sarpogrelate and rosuvastatin in high fat diet/streptozotocin-induced nephropathy in mice[J]. PLoS One, 2016, 11: e0153965. DOI:10.1371/journal.pone.0153965 |
| [13] |
Lee ES, Lee MY, Kwon MH, et al. Sarpogrelate hydrochloride ameliorates diabetic nephropathy associated with inhibition of macrophage activity and inflammatory reaction in db/db mice[J]. PLoS One, 2017, 12: e0179221. DOI:10.1371/journal.pone.0179221 |
| [14] |
Maroteaux L, Ayme-Dietrich E, Aubertin-Kirch G, et al. New therapeutic opportunities for 5-HT2 receptor ligands[J]. Pharmacol Ther, 2017, 170: 14-36. DOI:10.1016/j.pharmthera.2016.10.008 |
| [15] |
Turkmen K. Inflammation, oxidative stress, apoptosis, and autophagy in diabetes mellitus and diabetic kidney disease: the Four Horsemen of the Apocalypse[J]. Int Urol Nephrol, 2017, 49: 837-844. DOI:10.1007/s11255-016-1488-4 |
| [16] |
Huang F, Zhao Y, Wang Q, et al. Dapagliflozin attenuates kidney tubulointerstitial fibrosis associated with type 1 diabetes by regulating STAT1/TGFβ1 signaling[J]. Front Endocrinol (Lausanne), 2019, 10: 441. DOI:10.3389/fendo.2019.00441 |
| [17] |
Xu X, Xiao L, Xiao P, et al. A glimpse of matrix metalloproteinases in diabetic nephropathy[J]. Curr Med Chem, 2014, 21: 3244-3260. DOI:10.2174/0929867321666140716092052 |
| [18] |
Dong Z, Sun Y, Wei G, et al. Ergosterol ameliorates diabetic nephropathy by attenuating mesangial cell proliferation and extracellular matrix deposition via the TGF-β1/Smad2 signaling pathway[J]. Nutrients, 2019, 11: 483. DOI:10.3390/nu11020483 |
| [19] |
Chan GCW, Tang SCW. Diabetic nephropathy: landmark clinical trials and tribulations[J]. Nephrol Dial Transplant, 2016, 31: 359-368. DOI:10.1093/ndt/gfu411 |
 2021, Vol. 56
2021, Vol. 56


