溃疡性结肠炎(ulcerative colitis, UC)是一种迁延难愈、易于复发、临床诊断和治疗都较为复杂的慢性非特异性炎症性肠病, 被世界卫生组织公认为难治愈性疾病之一[1]。UC在亚洲国家中的发病率为7.6/100 000~14.3/100 000, 呈逐年上升趋势, 已成为临床常见病[2, 3]。然而其发病机制尚未完全明确, 目前治疗UC的药物常以氨基水杨酸类、糖皮质激素类和免疫抑制剂类为主, 但因其疗效不稳定、停药后易复发、不良反应较大等问题使广大患者难以接受[4-6]。中医药在治疗UC有着辨证论治、不良反应小、疗效显著和复发率低等优势[7]。
附子理中丸(Fuzi-Lizhong pill, FLP)源于《太平惠民和剂局方》, 治脾胃冷弱, 心腹绞痛, 呕吐泄利, 呕哕不止, 并皆治之, 是我国临床常用的经典制剂之一, 由附子(制)、干姜、党参、炒白术和甘草组成[8, 9]。方中附子辛甘, 散寒止痛; 干姜辛热, 具温中散寒之功效; 党参甘平, 具补脾益肺之功效; 白术苦温, 可健脾燥湿; 甘草味甘性平, 有益气补中, 缓急止痛, 兼和药性。现代研究表明, FLP具有治疗慢性结肠炎、慢性胃炎、肠易激综合征、功能性消化不良、脾肾阳虚型五更泻和慢性腹泻等临床应用[10-15], 在治疗脾肾阳虚证UC和慢性UC中有着疗效显著、不良反应小等优势, 但其作用机制有待深入研究[16, 17]。
本研究采用网络药理学研究策略, 构建“药物成分-作用靶点-作用通路”网络, 探讨其治疗UC的多成分、多靶点和多通路作用特征, 预测FLP对于UC的潜在靶点及其作用机制, 为其进一步研究奠定基础。本研究思路流程图见图 1。
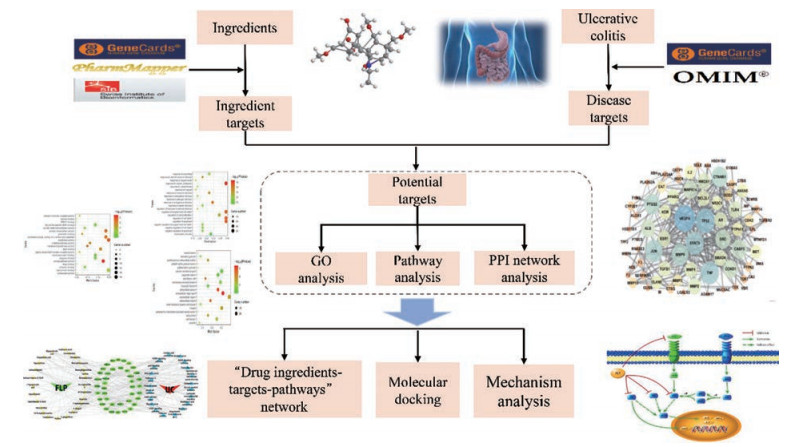
|
Figure 1 Flow chart of research |
成分收集 中药发挥药效作用的物质基础是化学成分的组合, 中药中虽含有众多成分, 但只有被吸收入血的成分才能产生作用[18], 因此, 基于本课题组前期实验的结果[19], 选取FLP中26个入血成分(23个原型化合物及3个代谢产物)为研究对象(表 1), 并通过PubChem数据库及Chem Bio Draw软件获得26个入血成分的化学结构。
| Table 1 Information sheet of chemical compounds. *Indicates metabolites |
预测和筛选潜在靶点 以26个入血成分为研究对象, 通过反向药效团匹配数据库PharmMapper (http://www.lilab-ecust.cn/pharmmapper/)[20]、SwissTarget Prediction平台(http://www.swisstargetprediction.ch/)[21]及GeneCards数据库(https://www.genecards.org/)进行靶点蛋白的预测。再通过UniProt数据库(https://www.uniprot.org/)将得到的靶点蛋白进行名称规范。选取各数据库中与化合物对接得分排名前10的靶点, 收集整理, 得到FLP入血成分的作用靶点。通过GeneCards和OMIM数据库(http://omim.org/)中输入关键词“ulcerative colitis”进行检索, 汇总整理, 与入血成分靶点进行交集, 获得FLP治疗UC的潜在靶点。
蛋白相互作用(PPI)网络构建 为了更好地分析靶点蛋白间的相互作用, 将潜在靶点导入String数据库, 限定物种为人, 为确保数据的可靠性, 选择0.7的高置信度, 保存结果。将结果中的node 1、node 2和结合分数(combined score)信息导入Cytoscape 3.7.0软件构建PPI网络, 对其网络进行分析, 并将节点(node)大小和颜色设置用于反映度值(degree)的大小, 边(edge)的粗细设置用于反映结合分数的大小。
GO分类富集分析与通路分析 将潜在靶点导入Davidv6.7数据库(https://david-d.ncifcrf.gov/), identifier设置为official gene symbol, list type设置为gene list, 物种设置为人类, 进行GO分析和KEGG通路分析(P < 0.05), 并结合KEGG数据库和Reactome数据库进行通路注释分析。
“药物成分-作用靶点-作用通路”网络构建 将上述FLP的入血成分、靶点预测结果、通路分析及对应疾病, 在Excel表中分别建立药物-成分、成分-靶点、靶点-通路和通路-疾病之间的对应关系, 导入Cytoscape软件, 构建“药物成分-作用靶点-作用通路”网络图。
分子对接 采用AutoDock vina软件对PPI网络中度值前3的靶点蛋白与26个入血成分进行分子对接验证。并用传统治疗药物5-氨基水杨酸[22](5-amino salicylic acid, 5-ASA)进行对照分析。从RCSB PDB蛋白质结构数据库和PubChem数据库中分别获得靶点蛋白的三维结构和5-ASA的化学结构, 采用AutoDock Tools对上述蛋白受体和配体进行常规处理, 再用其插件Autogrid得到对接活性位点, 进行分子对接, 得到结合能(affinity)。查阅文献[23-25], 本研究以结合能≤ -5.0 kJ·mol-1为分子与靶点结合性较好。
结果 1 潜在靶点的预测将PharmMapper数据库、SwissTargetPrediction平台及GeneCards数据库得到的所有靶点, 删除重复, 整合得到FLP入血成分作用靶点305个。将GeneCards和OMIM数据库得到的所有靶点, 删除重复后得到与UC相关基因794个。利用Venn作图工具对其进行交集(图 2), 得到82个治疗UC相关的潜在靶点。
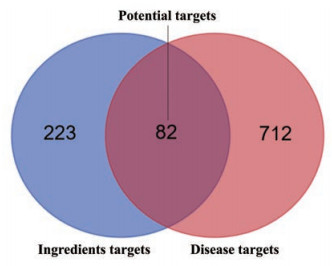
|
Figure 2 Venn's diagram of ingredient-disease of Fuzi-Lizhong pill (FLP) |
将String数据库中获取的靶点蛋白相互作用关系数据导入Cytoscape软件绘制PPI网络图, 见图 3。PPI网路中共有76个节点(靶点蛋白)、340条边(蛋白相互作用)。节点大小和颜色表示该节点度值的大小, 节点越大, 由橙色变蓝色对应的度值越大。边的粗细表示结合分数, 边越粗结合分数值越大。结果表明, 血管内皮生长因子A (VEGFA, 35)、肿瘤抑制基因P53 (TP53, 31)和信号转导与转录激活因子3 (STAT3, 28)靶点蛋白度值排名靠前, 为3个关键靶点。
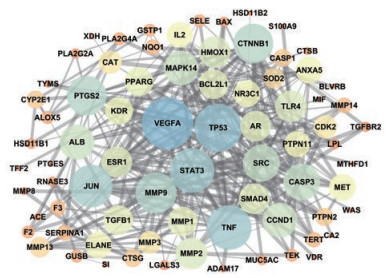
|
Figure 3 Protein-protein interaction (PPI) network of potential targets for FLP treatment of ulcerative colitis (UC) |
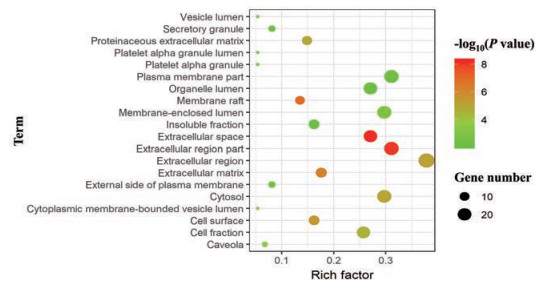
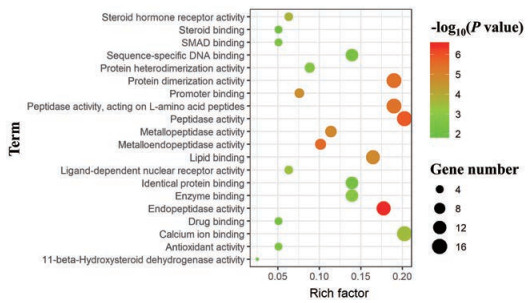
利用平台Davaid 6.7对FLP治疗UC的潜在靶点进行GO分析, 根据P value得到基因GO本体条目592条(P < 0.05)。GO富集分析分为3类:生物过程(BP)、细胞组分(CC)和分子功能(MF)。BP相关的条目最多, 有520条, 主要涉及有机物反应、细胞凋亡的调节、细胞程序性死亡的调控、细胞死亡的调节、类固醇激素刺激反应、内源性刺激反应、外部刺激反应的调节和激素刺激反应等方面; CC相关的条目33条, 主要涉及细胞间隙、胞外区和细胞膜等方面。MF相关的条目39条, 主要涉及肽链内切酶活性、肽酶的活动、金属内肽酶活性、蛋白质二聚活动、肽酶的活性——作用于L-氨基酸的肽和脂质结合等方面。将基因GO本体条目按P值进行排序, 分别选取BP、CC和MF的前20绘制气泡图(图 4~6)。
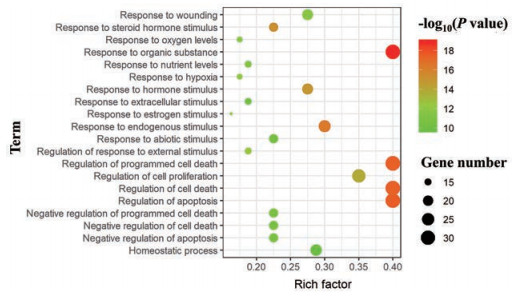
|
Figure 4 Biological process enrichment analysis diagram |
|
Figure 5 Cellular component enrichment analysis diagram |
|
Figure 6 Molecular function enrichment analysis diagram |
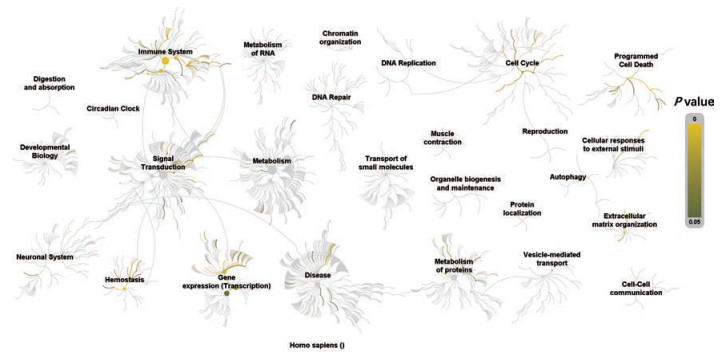
利用Davaid 6.7平台对FLP治疗UC的潜在靶点进行KEGG通路富集分析(P < 0.05), 并利用KEGG数据库和Reactome数据库进行通路注释分析, 见图 7和表 2。图 7显示FLP的潜在靶点主要涉及免疫系统、基因表达、信号转导、细胞凋亡、细胞周期和止血等多个生物过程, 其中免疫系统涉及最多, 这表明FLP通过调控多个复杂的生物过程来治疗UC, 图中黄色至白色线条代表潜在靶点富集的通路, 且颜色越黄P值越小。表 2中得到21条通路的富集, 主要涉及疾病通路、炎症相关通路及代谢相关通路等。疾病通路主要包括癌症、结直肠癌、肌萎缩性脊髓侧索硬化症(ALS)和胰腺癌等通路; 炎症相关通路主要包括血管内皮生长因子(VEGF)和丝裂原活化蛋白激酶(MAPK)等通路; 代谢相关通路包括花生四烯酸(AA)代谢和亚油酸代谢等通路。这也说明大多数癌症的发生与炎症有着密不可分的联系[26]。
|
Figure 7 Biological function analysis of potential targets for FLP treatment of UC |
| Table 2 Enrich KEGG pathways analysis of potential targets for FLP treatment of UC. VEGF: Vascular endothelial growth factor; GnRH: Gonadotropin-releasing hormone; MAPK: Mitogen-activated protein kinase |
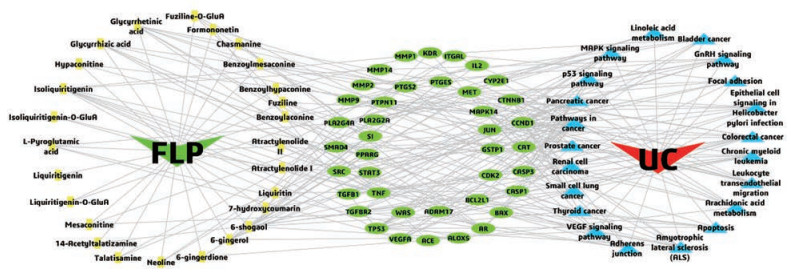
利用Cytoscape软件构建了“药物成分-作用靶点-作用通路”网络模型图(图 8)并用其插件Network Analyzer进行分析。该网络有89个节点(包括1个药材、26个入血成分、40个靶点、21条通路和1个疾病)和279条边。度值越大表明与之相连的节点数越多, 在整个网络中调控作用越大。其中度值较大的入血成分有6-姜烯酚(6-shogaol, 9)、异甘草素(isoliquiritigenin, 9)、6-姜酚(6-gingerol, 8)和甘草次酸(glycyrrhetinic acid, 8)等。度值较大的靶点蛋白有TP53 (14)、细胞周期蛋白D1 (CCND1, 11)、间质上皮转化因子(MET, 10)和VEGFA (7)等, 可能是FLP干预UC发挥作用的靶点。
|
Figure 8 Network of drug ingredients-targets-pathways. The green v-shaped node represents drug, the red v-shaped node represents disease, the yellow squares represent absorbed ingredients in blood, the light blue triangle nodes represent pathways, the green round nodes represent targets |
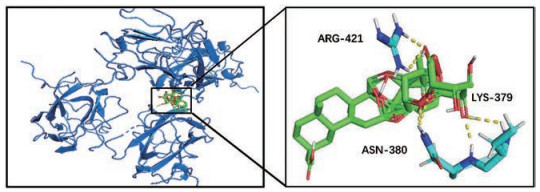
将PPI网络中排名前3的靶点蛋白分别与5-ASA和入血成分进行分子对接验证, 结果见表 3。若结合能 < 0, 表明配体分子均能和受体蛋白自发地结合, 结合能 < -5.0 kJ·mol-1, 表明其结合性好, 结合能越小对接越好[27]。表中93.6%入血成分都能与靶点蛋白较好地结合, 且75.6%的入血成分结合性均好于阳性对照药5-ASA。其中甘草酸(glycyrrhizic acid)与VEGFA结合性最好(图 9), 甘草酸与VEGFA活性位点ARG 421、ASN 380和LYS 379形成氢键相互作用, 这是促使其结合到活性位点的主要作用力。结果表明, 这26种入血成分与蛋白靶点结合性能较好, 作者前期研究结果中这些成分又是能够入血的成分, 表明其生物活性较好。
| Table 3 Molecular docking result of absorbed ingredients of FLP in blood. 5-ASA: 5-Amino salicylic acid; VEGFA: Vascular endothelial growth factor A; STAT3: Signal transducer and activator of transcription 3; TP53: Cellular tumor antigen P53 |
|
Figure 9 Molecular docking of glycyrrhizic acid and VEGFA. The dashed yellow lines represent hydrogen bonds |
UC病变绝大多数累及直肠与结肠黏膜及黏膜下层, 且有研究表明UC患者更容易患结直肠癌[28-30]。但UC的病因尚未完全明确, 目前认为是由多因素相互作用导致主要包括免疫、遗传、环境、感染及内部肠道微生物群等[31, 32], 其中免疫功能紊乱被认为是重要致病因素之一[33], 白细胞介素(interleukin, IL)、肿瘤坏死因子(tumor necrosis factor, TNF)等与免疫功能密切相关的促炎细胞因子能介导UC的发病[34]。本研究结果亦表明, FLP治疗UC的潜在靶点主要涉及白细胞介素-2 (IL-2)、TNF等细胞因子和与其密切相关的免疫功能。
UC归属中医泄泻、肠澼、滞下、痢疾等范畴, 发病基础为脾胃本虚, 其中温中健脾和通腑化滞等治疗原则多为临床应用[35]。FLP堪称温中健脾经久良方, 五药合用, 共奏温中健脾、温肾助阳、缓急止痛和固本止泻之功。对该方所含药味的现代研究表明, 化学成分如6-姜烯酚能够有效修复受损结肠黏膜, 进而治疗UC[36]; 6-姜酚能够通过其抗氧化和抗炎作用对UC引起的睾丸损伤起到保护作用[37]; 甘草酸能有效地调节紧密连接蛋白的含量和降低引起肠道炎症的重要介质肿瘤坏死因子-α (tumor necrosis factor-α, TNF-α)和白细胞介素-6 (IL-6), 进而促进UC小鼠的肠黏膜屏障功能的修复[38]; 7-羟基香豆素通过抗炎作用对UC小鼠有缓解作用[39]。作为多成分多靶点且作用机制叠加的经典复方制剂, FZP治疗UC的作用机制研究具有重要意义。
PPI网络图中可见靶点间存在多种相互作用, 连接度越大, 表明FLP通过该靶点治疗UC的可能性越大。在PPI网络中按度值排名筛选出3个关键靶点VEGFA、TP53及STAT3, 且在分子对接结果中入血成分都能较好地与靶点结合。VEGFA是最主要的VEGF, VEGF可促进间质血管内皮细胞的增生, 在血液的冲击下, 幼稚的血管内皮细胞形成管腔, 演变为成熟的毛细血管并具有血管的功能[40, 41]。Liao等[42]使用ELISA法检测到UC患者的组织中VEGF表达升高, Jiang等[43]采用免疫组化法在UC患者黏膜中检测到VEGF表达显著升高。这与UC病变炎症反应有密切关联, 炎症反应中需要大量的营养和氧, 但重度炎症抑制血液供应, 使局部表现出明显的缺血状态, 营养和氧均不足, 间质微血管灌注更不足, 尤其在伴有微血栓形成后, 缺血的表现更明显, 此时需要促进血管生成VEGF等细胞因子参与, 改善缺氧环境[42]。STAT3为信号转导与转录激活因子(STATs)家族成员之一, 是一种能与靶基因调控区DNA结合的胞浆蛋白, 负责调控细胞的生长、增殖、分化及凋亡等一系列重要的生理过程, 在维持稳态中必不可少, 尤其对损伤的上皮组织再生中起着重要作用[44-46]。有研究[47]通过蛋白免疫印迹法观察到UC大鼠的结肠组织中STAT3蛋白表达水平显著升高。TP53基因是目前发现的与肿瘤相关性最高的一种抑癌基因, 其编码产生的p53蛋白与细胞周期的调控、细胞凋亡和衰老等重要的生物学功能息息相关, 通过抑制细胞的生长, 从而预防癌症的发展[48, 49]。这些结果与网络药理学及分子对接结果共同表明, VEGFA、STAT3、TP53可能是FLP治疗UC的关键靶点, 也间接验证了网络药理学预测靶点的准确性。
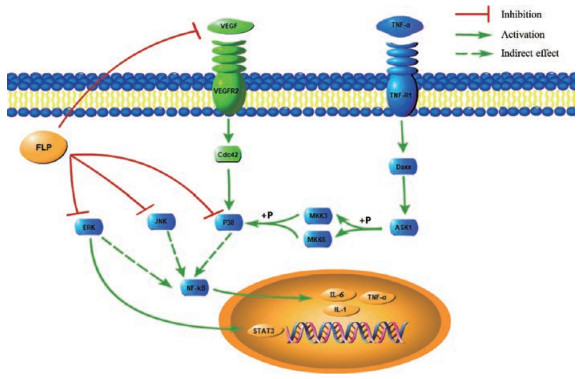
根据KEGG分析, FLP治疗UC的主要炎症通路为MAPK和VEGF等通路, 其作用机制图如图 10所示。MAPK通路可被炎性细胞因子和生长因子等多种胞外刺激因素激活, 之后调节激酶(ERK)、c-Jun氨基末端激酶(JNK)和p38丝裂原活化蛋白激酶(p38MAPK) 3条级联反应途径, 激活核转录因子-κB (NF-κB), 进而调控白细胞介素-1 (IL-1)、TNF-α等促炎因子的转录, 导致肠道黏膜炎症的发生[50]。有研究表明[51-53], 通过降低MAPK信号通路中的p38、JNK及ERK的表达水平, 抑制TNF-α和IL-6等促炎因子, 进而减轻小鼠UC症状及巨噬细胞炎症反应。同时结合前期对脾虚泄泻型大鼠的免疫细胞因子的研究结果[54], 提示FLP可能通过抑制MAPK的级联反应, 减少IL-1、IL-6和TNF-α等促炎因子的释放, 从而起到抗炎作用, 并改善UC症状。近年来, 有研究[55, 56]发现在UC患者中, 血清中VEGF的水平显著高于健康人群, 提示UC诱导的VEGF信号通路被激活。VEGF在活动性UC的炎性组织中表达增强, 可以影响肠黏膜微循环, 加重肠黏膜缺氧损伤。这提示FLP可能通过调控VEGF信号通路, 从而下调细胞分裂周期蛋白42 (Cdc42)的表达以改善结肠黏膜缺氧损伤, 防治UC。
|
Figure 10 Potential mechanisms of FLP for treatment of UC |
花生四烯酸(AA)是人体必需的游离脂肪酸之一, 在炎症和免疫反应中起到重要的调节作用, 肠道中AA的代谢产物、细胞因子等活性过高会造成免疫系统功能明显受损, 使得肠道癌变的可能性增加[57-59]。有研究[60]证实, AA代谢过度和氧化损伤可直接损伤结肠组织, 加剧炎症反应, 是UC发病的重要致病环节。这提示FLP可能通过抑制AA代谢过度, 从而减轻结肠炎症反应与损伤, 进而治疗UC。
综上所述, 本研究以FLP中入血成分为研究对象, 通过网络药理学和生物信息学方法, 对FLP治疗UC的潜在作用靶点及作用机制进行了研究, 为治疗发病机制复杂的UC提供了新的参考, 也为FLP分子模拟对接及作用机制的深入研究奠定了基础。
作者贡献:张臻和傅超美负责本实验研究的设计; 黄友负责实验执行, 杨莎莎、林夏、赵生嘉、魏馨怡参与实验研究; 张臻和黄友分析实验数据和撰写论文; 所有作者阅读和修改本论文。
利益冲突:本文所有作者均声明不存在利益冲突。
| [1] |
Ma XR, Wang YL, Zou DX, et al. Comparison of intestinal bacteria and inflammatory cytokine expression in rats with ulcerative colitis after treatment of three regiments[J]. Acta Pharm Sin (药学学报), 2019, 54: 1241-1250. |
| [2] |
Lani P, Michael AK, Perter PDC, et al. Inflammatory bowel disease in Asia:a systematic review[J]. J Gastroenterol Hepatol, 2012, 27: 1266-1280. DOI:10.1111/j.1440-1746.2012.07150.x |
| [3] |
Sheng H, Tang ZP, Tang XD, et al. Clinical practice guideline of TCM for common digestive system diseases:ulcerative colitis (edition for primary physician)[J]. China J Tradit Chin Med Pharm (中华中医药杂志), 2019, 34: 4155-4160. |
| [4] |
Xu YY, Cai H, Duan Y, et al. Research progress of Baizhu Shaoyao powder in treating ulcerative colitis[J]. China J Chin Mater Med (中国中药杂志), 2017, 42: 856-862. |
| [5] |
Xu HY, Wang YL, Wang DF, et al. Effect of Huangqin Tang on the gut microbiota in rats with ulcerative colitis model determined by high-throughput sequencing[J]. Acta Pharm Sin (药学学报), 2017, 52: 1673-1682. |
| [6] |
Chen BY, Liu K, Ge F, et al. Exploring potential mechanism of " Fu Zheng" rule of ulcerative colitis based on network pharmacology[J]. Chin J Exp Tradit Med Form (中国实验方剂学杂志), 2019, 25: 191-196. |
| [7] |
Mao TY, Hu LM, Sun ZM, et al. Progress in the treatment of ulcerative colitis with traditional Chinese medicine[J]. J Liaoning Univ Tradit Chin Med (辽宁中医药大学学报), 2018, 20: 59-62. |
| [8] |
Gan JH, Wang C, Song ZQ, et al. Effect of refined honey amount on pharmacokinetics of 9 different alkaloids in Fuzi Lizhongwan[J]. Chin J Exp Tradit Med Form (中国实验方剂学杂志), 2018, 24: 90-96. |
| [9] |
Hu ZC, Hou HF. Treatment of 56 cases of chronic enteritis with spleen yang deficiency by Fuzi Lizhong pill[J]. World Latest Med Inf (世界最新医学信息文摘), 2019, 19: 16-22. |
| [10] |
Liu XW, Yang DJ. Treatment of 89 cases of chronic colitis with Fuzi Lizhong pill combined with acupuncture[J]. J Pract Tradit Chin Med (实用中医药杂志), 2002, 18: 7. |
| [11] |
Yuan SR. Discussion on the clinical effect of plus and minus Fuzi Lizhong pill on chronic gastritis[J]. Pharmacol Clin Chin Mater Med (中药药理与临床), 2015, 31: 246-248. |
| [12] |
Ye SJ. The treatment of 62 cases of diarrhea-type irritable bowel syndrome with Sishen pill combined with Fuzi Lizhong pill[J]. Shandong J Tradit Chin Med (山东中医杂志), 2010, 29: 310. |
| [13] |
Li Y. Treatment of functional dyspepsia with Fuzi Lizhong pill:50 cases[J]. Guangming J Chin Med (光明中医), 2010, 25: 794-795. |
| [14] |
Yang AL. Effect of Fuzi Lizhong pill on spleen and kidney Yang deficiency type Wugeng diarrhea:a report of 132 cases[J]. Asia Pacific Tradit Med (亚太传统医药), 2012, 8: 77-78. |
| [15] |
Chne DZ. Treatment of 35 cases of chronic diarrhea with Fuzi Lizhong pill[J]. Henan Tradit Chin Med (河南中医), 2011, 31: 1312. |
| [16] |
Lu BY, Shi RJ. Effect of mesalazine combined with Fuzilizhong pill on ulcerative colitis of spleen and kidney Yang deficiency and its effect on serum HIF-1α and SOCS-3 levels[J]. Chin Mod Doct (中国现代医生), 2019, 57: 30-33. |
| [17] |
Hu LQ, Zhan LX, Zhang LF. Observation and analysis of the curative effect of Fuzilizhong pill combined with five vitamins oral solution in the treatment of chronic ulcerative colitis[J]. Med Innov Chin (中国医学创新), 2012, 9: 123-124. |
| [18] |
Wang XJ. Research on serum pharmacochemistry of traditional Chinese medicine and its compound[J]. World Sci Technol-Mod Tradit Chin Med Mater Med (世界科学技术-中医药现代化), 2002(2): 1-4, 78. |
| [19] |
Zhang Z, Jiang MY, Wei XY, et al. Rapid discovery of chemical constituents and absorbed components in rat serum after oral administration of Fuzi-Lizhong pill based on high-throughput HPLC-Q-TOF/MS analysis[J]. Chin Med, 2019, 14: 6. DOI:10.1186/s13020-019-0227-z |
| [20] |
Liu XF, Ouyang SS, Yu B, et al. PharmMapper server:a web server for potential drug target identification using pharmacophore mapping approach[J]. Nucleic Acids Res, 2010, 38: W609-W614. DOI:10.1093/nar/gkq300 |
| [21] |
David G, Aurelien G, Matthias W, et al. SwissTargetPrediction:a web server for target prediction of bioactive small molecules[J]. Nucleic Acids Res, 2014, 42: W32-W38. DOI:10.1093/nar/gku293 |
| [22] |
Ou YQ. The treatment of ulcerative colitis[J]. Chin J Pract Intern Med (中国实用内科杂志), 2010, 30: 383-385. |
| [23] |
Xu SN, Zhang L, Zhai YY, et al. Material basis and mechanism of Erzhi pill for preventing osteoporosis based on network pharmacology[J]. Chin Pharm J (中国药学杂志), 2018, 53: 1913-1920. |
| [24] |
Yao CG, XI CL, Zhu X, et al. Expression, purification, evaluation of activity, and analysis of inhibitor docking of enterovirus 713C protease[J]. J Pathog Biol (中国病原生物学杂志), 2017, 12: 722-726. |
| [25] |
Shi HL, Feng XS, Mao XJ, et al. Pharmacological network-based study on interventional mechanism of Gu-Chang-Zhi-Xie pills for treatment of irritable bowel syndrome[J]. Acta Pharm Sin (药学学报), 2019, 54: 482-493. |
| [26] |
Luo XX, Chen GD, Jiao Y, et al. Research status of TLR4 in inflammatory cancer[J]. Chin J Immun (中国免疫学杂志), 2017, 11: 1735-1740. |
| [27] |
Zhang L, Zhai YY, Yao WF, et al. The mechanism study of protecting kidney of Erzhi pill based on network pharmacology[J]. Acta Pharm Sin (药学学报), 2019, 54: 877-885. |
| [28] |
Zhang Q, Sha SM, Xu B, et al. Prevalence of colorectal cancer in patients with ulcerative colitis:a retrospective, monocenter study in China[J]. J Cancer Res Ther, 2015, 11: 899. DOI:10.4103/0973-1482.143345 |
| [29] |
Matteo R, Marta T, Francesca DC, et al. Risk factors for locally advanced cancer associated with ulcerative colitis:results of a retrospective multicentric study in the era of biologics[J]. Dig Liver Dis, 2020, 52: 33-37. DOI:10.1016/j.dld.2019.08.024 |
| [30] |
Sawan B, Ashwin NA, Saurabh K, et al. Risk of colorectal cancer in Asian patients with ulcerative colitis:a systematic review and meta-analysis[J]. Lancet Gastroenterol Hepatol, 2017, 2: 269-276. DOI:10.1016/S2468-1253(17)30004-3 |
| [31] |
Zhang GX, Shi R. Progress in treatment of ulcerative colitis[J]. Mod J Integr Tradit Chin West Med (现代中西医结合杂志), 2019, 28: 2842-2847. |
| [32] |
Jin CX, Li F. Advance in research on immune molecules associated with ulcerative colitis and their clinical applications[J]. Chin J Immun (中国免疫学杂志), 2019, 35: 505-508, 514. |
| [33] |
Ai J, Wang CD. The role of genetic and environmental factors in the pathogenesis of inflammatory bowel disease[J]. Int J Dig Dis (国际消化病杂志), 2014, 34: 110-113. |
| [34] |
Cui CW, Sun ZR. Research progress on pathogenesis of ulcerative colitis[J]. Curr Immunol (现代免疫学), 2019, 39: 77-81. |
| [35] |
Liu SJ, Zhang XY, Ding XK, et al. Review on etiology and pathogenesis of ulcerative colitis[J]. Henan Tradit Chin Med (河南中医), 2019, 39: 799-801. |
| [36] |
Hui Y, Yan SG, Li JT, et al. Effect of berberine combined with 6-shogaol on notch signaling pathway in colon epithelial cells of ulcerative colitis mice[J]. Chin Tradit Herb Drugs (中草药), 2019, 50: 3147-3154. |
| [37] |
Farombi EO, Adedara IA, Ajayi BO, et al. 6-Gingerol improves testicular function in mice model of chronic ulcerative colitis[J]. Hum Exp Toxicol, 2018, 37: 358-372. DOI:10.1177/0960327117703689 |
| [38] |
Heng Y. Investigation for the Effects and Mechanism of Glycyrrhizin on Tight Junctional Protein to Repair the Intestinal Mucosal Barrier Injury with Ulcerative Colitis (甘草酸调节紧密连接蛋白修复溃疡性结肠炎肠黏膜屏障损伤的药理作用研究)[D]. Xian: Fourth Mil Med Univ (第四军医大学), 2017.
|
| [39] |
Wang CZ, Liu J, Zhou YC, et al. The effect of 7-hydroxycoumarin on dextran sulfate sodium-induced ulcerative colitis[J]. Basic Clin Pharmacol Toxicol, 2019, 124: 749. DOI:10.1111/bcpt.12948 |
| [40] |
Uluer ET, Inan S, Ozbilgin K, et al. The role of hypoxia related angiogenesis in uterine smooth muscle tumors[J]. Biotech Histochem, 2015, 90: 102-110. DOI:10.3109/10520295.2014.952339 |
| [41] |
Takamitsu S, Yasuhiko K, Toru N, et al. Inhibition of epidermal growth factor receptor and vascular endothelial growth factor receptor phosphorylation on tumor-associated endothelial cells leads to treatment of orthotopic human colon cancer in nude mice[J]. Neoplasia, 2007, 9: 1066-1077. DOI:10.1593/neo.07667 |
| [42] |
Liao RY, Yao P, Zhang Y. Expression and significance of eukaryotic initial factor A1 and vascular endothelial growth factor in ulcerative colitis[J]. Chin J Geriatr (中国老年学杂志), 2019, 39: 74-77. |
| [43] |
Jiang DS, Zhang J, Rong Z, et al. The expression and significance of VEGF in ulverative colitis[J]. Chin J Clin Gastroenterol (临床消化病杂志), 2010, 22: 25-27. |
| [44] |
Jimmy ZL, Suzanne VS, Huang HL, et al. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations[J]. Nat Genet, 2015, 47: 979-986. DOI:10.1038/ng.3359 |
| [45] |
Clemens N, Geethanjali P, Zheng Y, et al. Activation of epithelial STAT3 regulates intestinal homeostasis[J]. Cell Cycle, 2010, 9: 652-655. DOI:10.4161/cc.9.4.10615 |
| [46] |
Zou Y, Liu Y, Huang W, et al. Advances on anti-neoplastic STAT3 inhibitors[J]. Acta Pharm Sin (药学学报), 2018, 53: 1598-1608. |
| [47] |
Tang MF. Molecular Mechanism of Berberine Regulating miR-31-5p-Th17/Treg Immune Network in the Treatment of Ulcerative Colitis (小檗碱调控miR-31-5p-Th17/Treg免疫网络治疗溃疡性结肠炎分子机制研究)[D]. Xian: J Shaanxi Coll Tradit Chin Med (陕西中医药大学), 2019.
|
| [48] |
Vearonique B, Tatiana Z, Monique V, et al. Tissue and cell-specific expression of the p53-target genes:Bax, Fas, Mdm2 and Waf1/p21, before and following ionising irradiation in mice[J]. Oncogene, 2000, 19: 649-660. DOI:10.1038/sj.onc.1203366 |
| [49] |
Fei J, Lu YJ. Clinical features and prognosis of patients with lung adenocarcinoma harboring TP53 mutation[J]. J Clin Pulm Med (临床肺科杂志), 2019, 24: 1503-1506. |
| [50] |
Tang M, Lu Q, Liu H, et al. Research progress of ulcerative colitis related signaling pathways[J]. Chin Pharm Bull (中国药理学通报), 2018, 34: 1642-1647. |
| [51] |
Lian L, Zhang S, Yu ZL, et al. The dietary freeze-dried fruit powder of Actinidia arguta ameliorates dextran sulphate sodium-induced ulcerative colitis in mice by inhibiting the activation of MAPKs[J]. Food Funct, 2019, 10: 5768-5778. DOI:10.1039/C9FO00664H |
| [52] |
Li ZH, Wang J, Cai RL, et al. Effects of Shenling Baizhu powder on the expressions of AQP3 and AQP4 in UC rats via ERK/p38 MAPK signal pathway[J]. Chin Tradit Pat Med (中成药), 2015, 37: 1883-1888. |
| [53] |
Chen S, He YH, Wu Q, et al. The research on observing the effect of Baishaoqiwu granules acting mechanism in treating ulcerative colitis from NOXs-ROS-P38MAPK related signal pathway[J]. Lishizhen Med Mater Med Res (时珍国医国药), 2017, 28: 2826-2828. |
| [54] |
Shi JF, Jiang MY, Lin X, et al. Effect of Fuzi-Lizhong prescription on spleen deficiency and diarrhea and its key factors based on factor analysis[J]. Pharmacol Clin Chin Mater Med (中药药理与临床), 2019, 35: 22-26. |
| [55] |
He S, Liu K, Huang YK. Impacts of mesalazine, clostridium and montmorillonite powder on plasma PGE2, LT-B4, PAF and VEGF in rats with ulcerative colitis[J]. Chin J Immun (中国免疫学杂志), 2016, 32: 1212-1218. |
| [56] |
Alicja WD, Jerzy J, Anna P, et al. Pigment epithelium-derived factor in ulcerative colitis:possible relationship with disease activity[J]. Regul Pept, 2007, 140: 1-4. DOI:10.1016/j.regpep.2006.11.006 |
| [57] |
Wei WF, Liu H, Huo JH, et al. Study on mechanism of Qinbai Qingfei concentrated pellets in treatment of mycoplasma pneumonia mice model based on metabolomics[J]. Chin Tradit Herb Drugs (中草药), 2017, 48: 5211-5216. |
| [58] |
Yang GY, Taboada S, Liao J. Inflammatory bowel disease:a model of chronic inflammation-induced cancer[J]. Methods Mol Biol, 2009, 511: 193. |
| [59] |
Sun QH, Zhang J, Li ZZ, et al. Action mechanism of Mahuang Xixin Fuzi decoction for mice with influenza based on metabolomics information[J]. China J Chin Mater Med (中国中药杂志), 2017, 42: 763-771. |
| [60] |
Zhang J, Wan SQ. Effects of sodium ferulate on the oxidative damage and arachidonic acid metabolism in patients with ulcerative colitis[J]. Lishizhen Med Mater Med Res (时珍国医国药), 2008, 19: 1168-1169. |
 2020, Vol. 55
2020, Vol. 55


