作者贡献:田蓉、吴昊星、孙洪宝提出研究思路, 设计研究方案; 王晓蒙、李杰、潘立立负责进行实验; 沈国华负责采集、分析数据; 王晓蒙负责论文起草; 吴昊星、孙洪宝、沈国华负责最终修订版本。
利益冲突:作者声明无任何利益冲突。
2. 四川大学华西医院磁共振研究中心, 四川 成都 610041
2. Huaxi MR Research Center, West China Hospital, Sichuan University, Chengdu 610041, China
以四嗪生物正交反应为代表的逆电子需求Diels-Alder (IEDDA)反应相较于其他生物正交反应, 具有更快的反应动力学和优良的荧光调控能力等特点, 其在活细胞选择性标记以及生命体系中重要分子的动态精准检测上发挥着关键作用[1]。近年来, 除了经典的连接反应(tetrazine ligation)[2, 3], 基于四嗪生物正交化学的点击-释放反应越来越受到研究者的关注, 一系列修饰于亲二烯体的探针分子在四嗪生物正交反应后, 通过串联反应历程实现了释放[4]。其中, 反式环辛烯(TCO)与四嗪之间的点击-释放反应, 具有反应快速、无需催化以及副产物(N2和CO2)无毒[4]等优点, 使其在前药制备[5, 6]、药物递送[7]以及抗体-药物偶联[8, 9]等体内药物释放领域发挥着巨大的应用潜力[10, 11]。起初, 四嗪点击-释放反应主要应用于含胺类药物的释放, 例如TCO氨基甲酸酯类前药在体外和体内释放多柔比星(doxorubicin, Dox)[12](图 1a)。随后, Devaraj课题组[13]首先发现将乙烯基醚作为亲二烯体, 可以通过与四嗪的生物正交点击-释放反应实现苯酚类化合物的释放并开启荧光(图 1b)。Bernardes课题组[14]则将乙烯基醚作为氨基酸、单糖、荧光基团和细胞毒性药物多卡霉素等含醇分子的保护基团, 实现对蛋白质或药物活性的精确控制(图 1b)。自此, 四嗪与各种亲二烯体之间通过生物正交点击-释放反应实现不同官能团的释放愈发受到广泛关注[5]。
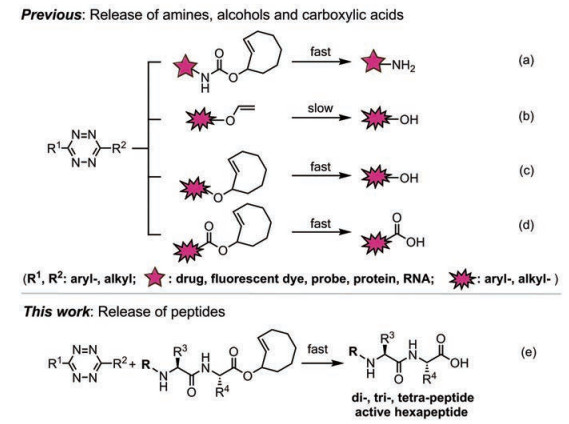
|
Figure 1 "Click-to-release" reaction of tetrazine |
肽是涉及生物体内多种细胞功能的生物活性物质[15-17]。多肽类化合物与传统小分子化合物和抗体相比, 具有合成和修饰简单、靶向性好、组织穿透性强、体内清除快以及毒副作用小等优点[18-20], 可作为抗癌药物、细胞毒性药物载体、疫苗、激素和放射性核素载体等, 被广泛应用于多种肿瘤的精准诊疗[21-24]。近年来, 生物正交反应已成为探索多肽、蛋白质和抗体等生物活性分子在生物系统中作用机制和功能的有效工具[25, 26], 特别是四嗪生物正交点击-释放反应, 被广泛应用于体内蛋白荧光标记成像[27, 28]和靶向蛋白功能调控[29]等方面。陈鹏课题组[30]首先利用四嗪生物正交脱笼反应(decaging reaction), 实现基因编码的非天然氨基酸单个激酶胞内高效激活。Bradley课题组[31]开发了易与四嗪发生IEDDA反应的氨基保护基(vinyl ether benzyloxycarbonyl, VeZ)来保护赖氨酸侧链氨基, 为Fmoc固相多肽合成方法提供了新的正交保护基策略。四嗪生物正交点击-释放反应的应用扩展了肽化学的范畴[32-34]。
近期, Robillard课题组[35]发现, TCO的酯类、碳酸酯类和醚类衍生物可通过四嗪生物正交点击-释放反应实现对醇或羧酸的释放(图 1c, 1d)。此外, Bernardes课题组[36]证明该反应可用于含羧酸类药物酮洛芬的释放(图 1d)。四嗪点击-释放反应具有反应条件温和、官能团兼容性好等优点, 目前可用于胺类、醇类或羧酸类基团的释放, 这些应用揭示了生物正交点击-释放反应对靶向小分子药物激活的应用潜力。但是, 能否将该类反应用于多肽合成, 并在温和条件下实现多肽的固相修饰, 从而赋予多肽新的功能仍有待探索。本研究通过TCO-四嗪点击-释放反应, 实现多肽的碳端释放, 并对传统多肽固相合成步骤中多肽释放步骤进行优化。与经典多肽固相合成(SPPS)中利用氢氟酸或三氟乙酸等强酸试剂相比, 在树脂上利用生物正交反应释放多肽具有反应条件温和以及官能团兼容性好等优点, 有利于在固相合成中对多肽进行进一步修饰。本研究将TCO-四嗪点击-释放反应从小分子药物扩展到多肽化合物(图 1e), 并将此种生物正交点击-释放反应作为工具, 开发了一种简单、温和、高效的肽分子探针释放方法, 以期改变目前多肽分子探针制备繁琐的现状。
根据陈鹏课题组[37]的发现, 在四嗪生物正交点击-释放反应中, 强吸电子取代基能够加速环加成步骤, 同时位阻较小的取代基能促进后续的释放步骤。鉴于此, 在本课题组有关四嗪类化合物合成研究[38]的基础上, 设计了在3位和6位分别引入一个吸电子基团和一个烷基基团的4个四嗪化合物S2~S5, 吸电子取代基固定在一侧以加速初始环加成反应, 另一侧引入位阻较小的烷基以促进后续的消除反应, 并制备了对称的四嗪S1作为对比(图 2)。设计合成TCO-二肽(TCO-Gly-Gly)作为模型底物, 分别与5个四嗪化合物反应, 利用LC-MS监测反应进程, 从中筛选释放效率最好的四嗪。
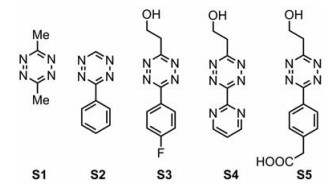
|
Figure 2 Design of tetrazine derivatives |
根据文献报道合成肽链的常用方法[39], 合成13个TCO-多肽化合物。为了研究氨基酸侧链对四嗪生物正交点击-释放反应的影响, 合成了将天冬氨酸、谷氨酰胺、酪氨酸和苯丙氨酸作为第一个氨基酸, 甘氨酸作为第二个氨基酸的5条TCO-二肽, 以及第一个氨基酸为甘氨酸, 第二个氨基酸分别为天冬氨酸和苯丙氨酸的2条TCO-二肽; 为了评价和比较肽链长度对释放效率的影响, 合成了4条TCO-三肽和2条TCO-四肽。所合成的氨基酸序列及化学结构式如图 3所示。
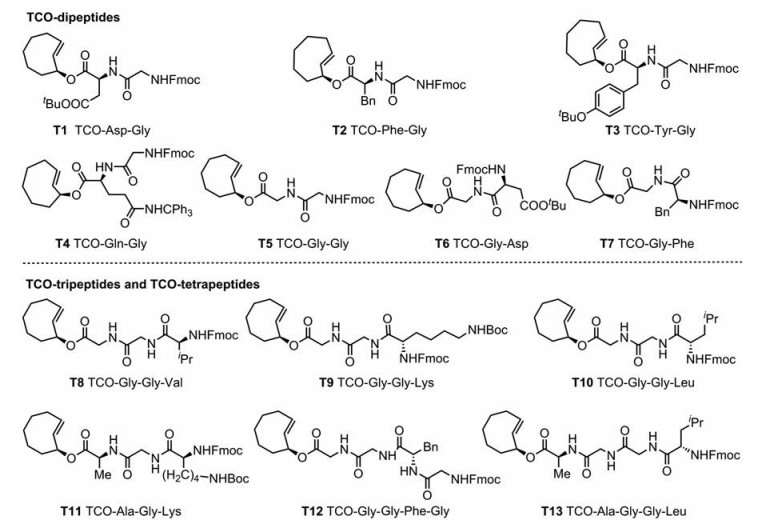
|
Figure 3 Designed and synthesized TCO peptides |
为了将TCO-四嗪点击-释放反应应用于多肽固相合成中的释放步骤, 需要设计合成一种双功能化的TCO类似物, 即在TCO的6位或7位引入一个含羧基的支链, 使其能够与固相合成树脂连接, 并且在烯丙位存在一个羟基用于连接多肽。如图 4所示, 设计了一条基于2, 6-环辛二烯-1-羟基的环氧化开环实现双功能化衍生的合成路线。首先将2, 6-环辛二烯-1-羟基用TBS基团保护, 然后进行环氧化反应, 选择性地将三元环安装在6位上, 再通过还原开环得到6位和7位羟基化合物4a和4b。化合物4a和4b分离较为困难, 可不经分离直接进行下一步官能团转化, 随后与己二酸单甲酯反应, 得到化合物6a和6b (可以分离纯化)。脱掉化合物6a和6b烯丙位的TBS保护基, 然后分别将化合物7a和7b进行254 nm紫外光照射, 得到8a1/a2和8b1/b2四种双功能化TCO衍生物, 并通过NOESY鉴定4种化合物烯丙位羟基空间构型[8]。
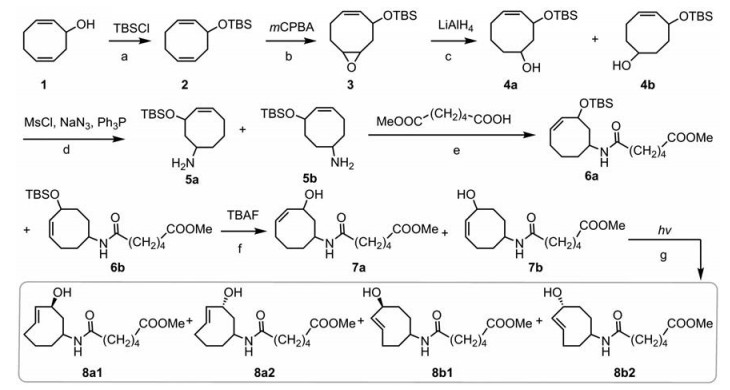
|
Figure 4 Synthesisof bifunctional TCO derivatives. Reagents and conditions: a) Imidazole, DCM, r.t., 2 h, 90.0%; b) DCM, 0 ℃-r.t., 18 h, 70.0%; c) THF, 70 ℃, 4 h, 70.0%; d) 1) MsCl, Et3N, DCM, r.t., 2 h; 2) NaN3, DMF, 70 ℃, 48 h; 3) Ph3P, DCM, r.t., 12 h, 50.0%; e) EDCI, HOBT, DIPEA, DCM, r.t., 4 h, 70.0%; f) THF, r.t., 2 h, 80.0%; g) 254 nm UV, EA, Et2O, r.t., 12 h, 8a1/8a2: 20.0%, 8b1/8b2: 23.0% |
TCO-四嗪点击-释放反应的反应条件与固相合成条件具有很好的相容性, 在探明该反应与常见氨基酸的兼容性和释放效率的基础上, 进一步扩展其在多肽固相合成中多肽释放步骤的应用, 使其应用于固相合成中释放活性六肽GIRLRG (图 5)。GIRLRG肽(Gly-Ile-Arg-Leu-Arg-Gly)是通过体内噬菌体展示肽库筛选鉴定出来的活性肽, 可与在多种癌细胞中高表达的葡萄糖调节蛋白受体(GRP78)特异性结合, 被广泛用于体外癌细胞特异性结合和体内无创肿瘤成像[40]。开发温和高效的合成该类活性多肽的方法具有很好的应用价值。设计如图 5所示合成路线, 首先分别合成TCO-Gly-Arg-Fmoc、Leu-Arg-Fmoc和Ile-Gly-Fmoc 3个肽段, 然后将3个肽段拼接, 合成一条由双功能化TCO修饰的六肽链。随后, 在较为温和碱性条件下(氯化钙和氢氧化锂的水溶液作为碱)或者三甲基氢氧化锡条件下, 脱去TCO-GIRLRG的甲酯[41, 42], 然后连接至Rink amide MBHA固相合成树脂上, 并在六肽甘氨酸末端氨基处用5(6)-羧基荧光素(fluorescein)修饰, 最后利用TCO-四嗪点击-释放反应释放带有荧光素的活性六肽GIRLRG。
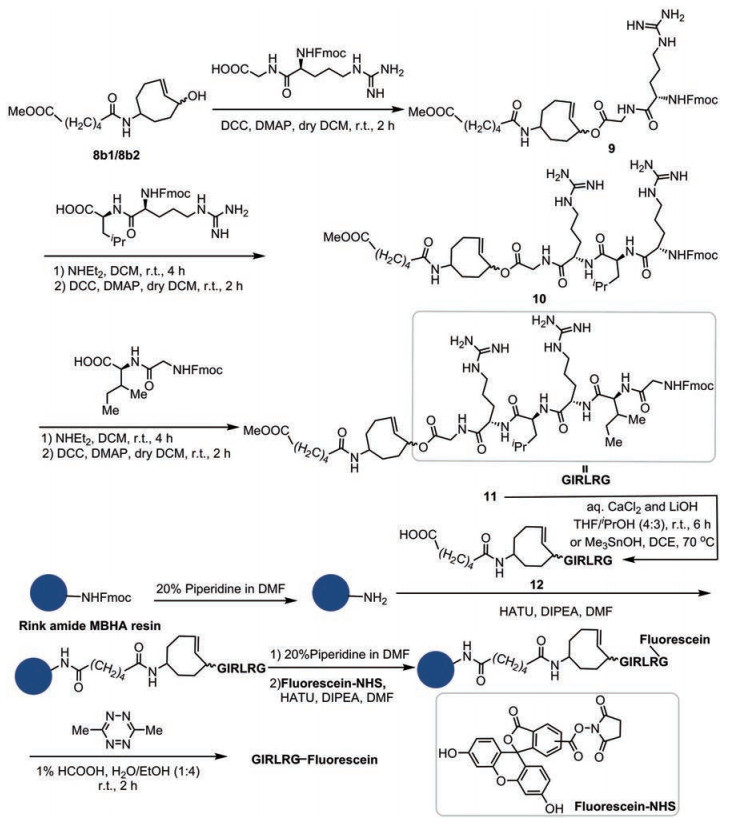
|
Figure 5 Synthesis of TCO-GIRLRG and release of GIRLRG-fluorescein |
将TCO二肽(TCO-Gly-Gly)作为模型底物, 利用LC-MS测定其与5种四嗪发生点击-释放反应后二肽(Gly-Gly)碳端释放效率, 从而筛选出最佳反应条件。鉴于乙醇在生物医学研究和临床应用中的广泛性, 选择乙醇作为溶剂。在水溶液中TCO和四嗪通过疏水相互作用能够显著加快IEDDA反应[43], 并且在微酸性条件下, 质子化效应同样可以加速反应[44], 因此加入水作为共溶剂, 并加入微量酸促进反应。经过条件筛选优化, 最终选择含1%甲酸的水-乙醇(1:4)作为四嗪和TCO-肽的反应溶剂。鉴于多肽固相合成是在室温下进行的, 所以反应温度设为室温[38]。分别将四嗪S1~S5 (1.5eq.)与TCO-Gly-Gly在上述条件下进行反应, 利用LC-MS监测反应进程。结果表明, 取代基位阻最小的双甲基四嗪衍生物S1, 释放效率最高, 为90.7%;四嗪衍生物S2的活性最低, 释放效率仅为41.0% (图 6)。说明在该反应体系中, 四嗪上取代基的位阻作用非常明显。因此, 在进一步的TCO-四嗪点击-释放反应释放多肽的研究中选择使用四嗪S1。通过以上研究, 确定了TCO-肽和四嗪反应的最佳条件为:在含1%甲酸的水-乙醇(1:4)溶剂中, 室温下与四嗪S1 (1.5eq.)反应。
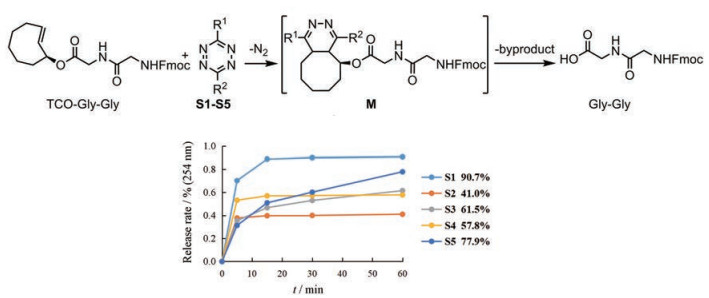
|
Figure 6 Determination of reaction release rates of TCO-Gly-Gly with different tetrazines. Reaction conditions: tetrazines (1.5 eq.), PhCOMe (1.0 eq.) as internal standard, 1% HCOOH in H2O/EtOH (1:4) (0.2 mmol·L-1), r.t.; M: Only one isomer was exhibited |
为探究TCO-四嗪点击-释放反应与多肽侧链官能团及肽链长度之间的相容性, 利用所合成的13个TCO-肽在含1%甲酸的水-乙醇(1:4)溶剂中与四嗪S1 (1.5eq.)在室温下反应1 h, 并用LC-MS监测反应进程。实验结果表明: ①无论第一个或第二个氨基酸的侧链是否相同, TCO-二肽的释放效率都很高, 产率均在90%以上, 说明不同种类氨基酸不会影响TCO-肽点击-释放反应的高效性(图 7A/B); ②无论是TCO-三肽还是TCO-四肽均能达到90%以上的释放率, 其中TCO-四肽T13释放率最高, 为97.7% (图 7C/D), 说明肽链长度同样不影响该反应高效释放多肽。本实验证明了TCO-四嗪点击-释放反应用于释放多肽的高效性、广泛性和实用性, 为TCO-四嗪生物正交反应用于固相合成中多肽释放奠定了基础。
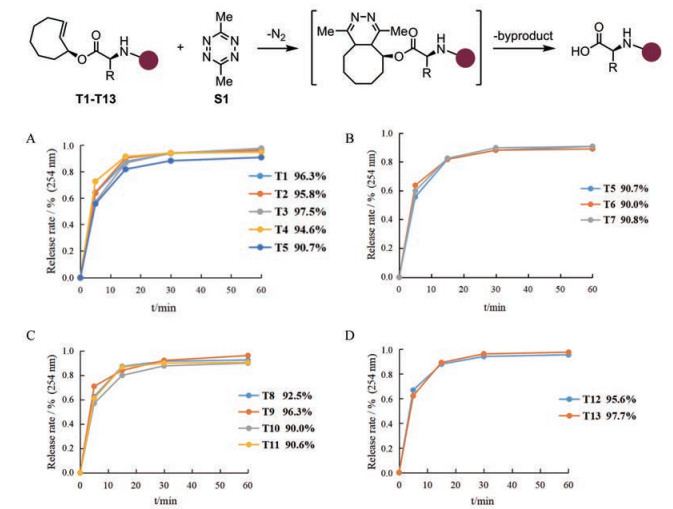
|
Figure 7 Determination of the reaction release efficiency of TCO-peptides with tetrazine S1 [reaction conditions: tetrazine S1 (1.5 eq.), PhCOMe (1.0 eq.) as internal standard, 1% HCOOH in H2O/EtOH (1:4) (0.2 mmol·L-1), r.t.]. A: The release rates of TCO-dipeptides of which the first amino acid is different; B: The release rates of TCO-dipeptides of which the second amino acid is different; C: The release rates of TCO-tripeptides; D: The release rates of TCO-tetrapeptides |
以Rink amide MBHA树脂为载体, 双功能化TCO衍生物作为连接体, 将六肽GIRLRG连接于固相载体, 随后在六肽甘氨酸末端氨基处修饰荧光素, 最后通过TCO-四嗪生物正交反应释放六肽-荧光素产物[在含1%甲酸的水-乙醇(1:4)溶剂中与四嗪S1 (0.9eq.)室温下反应1 h]。实验结果表明(图 8), 在荧光显微镜下, 可发现连接了六肽GIRLRG的树脂经荧光素标记后有明显的荧光信号(图 8A到图 8B); 通过生物正交反应将多肽从树脂切除后, 切割液可观察出有明显的荧光信号(图 8D), 而反应后固相合成树脂几乎无荧光信号(图 8C)。将切割液进行高分辨质谱分析证实切割液分子量为被荧光基团修饰的六肽分子GIRLRG-Fluorescein分子[ESI-HRMS (m/z): 1 029.478 9 [M+H]+]。该实验证明TCO-四嗪点击-释放反应可以应用于多肽固相合成的肽链释放步骤中, 且这种释放条件温和、官能团兼容性好, 能高效地对功能化的多肽实现释放。优化了传统固相多肽合成中多肽释放的方法, 对于酸敏多肽化合物的合成及释放提供了新的思路。
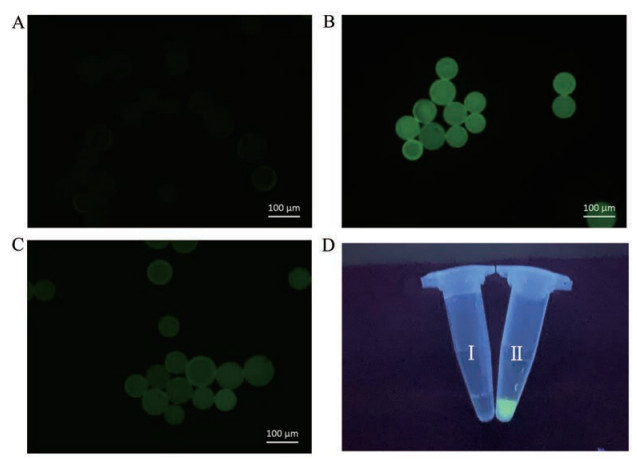
|
Figure 8 Fluorescence imaging. A: TCO-GIRLRG-resin material; B: TCO-GIRLRG-resin materialattached with fluorescein; C: Resin material after treated with tetrazine S1; D: Fluorescence of cleavage fluid: Ⅰ) H2O/EtOH (1:4) as control, Ⅱ) cleavage fluid |
本文设计合成了13个TCO-肽类化合物, 以TCO-二肽(TCO-Gly-Gly)作为模型底物, 与5种四嗪化合物发生点击-释放反应, 均能温和切除二肽分子的碳端连接, 其中与四嗪S1反应效率最高, 1 h释放产率达到90.7%。随后的实验结果表明, 13个TCO-肽类化合物都能够和四嗪S1高效发生反应, 多肽碳端释放率都在90%以上, 最高可达97.7% (TCO-四肽T13), 同时也证明了该释放反应与多肽侧链官能团及肽链长度具有很好兼容性。最后, 将此种生物正交点击-释放反应作为工具, 引入多肽固相合成的释放步骤中, 实现TCO-四嗪点击-释放反应的应用从小分子药物扩展到分子量 > 1 000的多肽药物中。本文实现了将双功能化的TCO衍生物作为连接体, 一端连接固相合成树脂, 一端连接GIRLRG六肽的碳端, 利用TCO-四嗪生物正交点击-释放反应释放多肽的策略。此种多肽释放方法可以在标准多肽固相合成Fmoc策略下顺利进行, 温和地从固相树脂上切割多肽产物, 且不影响其他侧链基团, 具有较好的实用性和应用前景。在此过程中, 拓展了四嗪生物正交点击-释放策略的应用范围。同时, 优化了固相多肽合成中释放多肽的方法, 对于酸敏多肽化合物的固相合成提供了可选择方法。
实验部分核磁共振图谱采用布鲁克AMX 400 M型核磁共振波谱仪记录, 氘代氯仿或TMS为内标; 高分辨质谱: Waters Q-TOF Premier; EYELA N-1300型旋转蒸发仪; 高效液相-质谱联用(LC-MS, 安捷伦科技有限公司); YZUV-12型254 nm紫外光反应仪; ZEISS-Observer.D1型荧光倒置显微镜; 2-羟基-反式环辛烯1 (轴向型)根据文献制备[45]; 5(6)-羧基荧光素琥珀酰亚胺酯(fluorescein-NHS, CAS: 117548-22-8, 96%异构体混合物)购自上海阿拉丁生化科技股份有限公司。在目标化合物合成过程中, 若无特殊说明, 所使用的试剂均为分析纯; 溶剂购自泰坦科技有限公司; 化学试剂购自安耐吉化学有限公司、上海毕得科技有限公司和百灵威科技有限公司。
1 TCO-肽的合成根据文献报道合成肽链的常用方法[39]: ①将2-羟基-反式环辛烯1 (20.0 mg, 0.16 mmol)、DCC (66.0 mg, 0.32 mmol)、DMAP (1.2 mg, 0.01 mmol)和相应的Fmoc保护的氨基酸(0.32 mmol)置于10 mL的反应试管内, 氮气保护。随后加入干燥的二氯甲烷(DCM)溶剂2 mL, 室温条件下反应2 h。TLC监测反应, 待反应完全后, DCM萃取, 饱和氯化钠洗涤, 收集有机相, 用无水硫酸钠干燥、浓缩, 经硅胶柱洗脱分离(PE:EA = 4:1), 减压浓缩, 得到产物。②将上一步TCO-氨基酸产物置于10 mL的反应瓶内, 加入2 mL的DCM溶剂使其完全溶解, 然后加入0.2 mL的二乙胺, 于室温条件下反应4 h。TLC监测反应, 待反应完全后, 反应液直接减压蒸馏浓缩, 所得粗品直接用于下一步。③将上步所得粗品、Fmoc保护的氨基酸或者二肽、三肽(0.32 mmol)、EDCI (61.3 mg, 0.32 mmol)、HOBT (43.2 mg, 0.32 mmol)置于10 mL的反应试管内, 氮气保护, 加入3 mL干燥的DCM溶剂, 随后缓慢滴加DIPEA (41.4 mg, 0.32 mmol), 于室温条件下反应2 h。TLC监测反应, 待反应完全后, DCM稀释反应液, 饱和氯化钠洗涤, 收集有机相, 用无水硫酸钠干燥、浓缩, 经硅胶柱洗脱分离(PE:EA = 1:1), 减压浓缩, 得到相应的目标产物。
化合物T1 (TCO-Asp-Gly), 收率64.3%; 1H NMR (400 MHz, CDCl3) δ 7.76 (d, J = 8.0 Hz, 2H), 7.60 (d, J = 3.9 Hz, 2H), 7.40 (t, J = 8.1 Hz, 2H), 7.31 (t, J = 8.0 Hz, 2H), 6.99 (t, J = 7.8 Hz, 1H), 5.84~5.71 (m, 1H), 5.60~5.39 (m, 3H), 4.91~4.85 (m, 1H), 4.40 (d, J = 8.2 Hz, 2H), 4.23 (t, J = 8.0 Hz, 1H), 3.96 (s, 2H), 3.03~2.95 (m, 1H), 2.77~2.82 (m, 1H), 2.45~2.46 (m, 1H), 2.06~1.99 (m, 3H), 1.87~1.73 (m, 1H), 1.70~1.66 (m, 2H), 1.48~1.46 (m, 1H), 1.41 (s, 9H), 1.09~1.00 (m, 1H), 0.83~0.77 (m, 1H); 13C NMR (101 MHz, CDCl3) δ 170.03, 169.68, 168.78, 156.53, 143.95, 141.42, 132.81, 129.94, 127.85, 127.23, 125.25, 120.10, 82.08, 75.22, 67.49, 48.91, 47.24, 44.49, 40.52, 37.36, 36.04, 36.01, 29.11, 28.13, 24.33; ESI-HRMS (m/z): 599.273 2 [M+Na]+。
化合物T2 (TCO-Phe-Gly), 收率69.5%; 1H NMR (400 MHz, CDCl3) δ 7.75 (d, J = 7.8 Hz, 2H), 7.58 (d, J = 8.0 Hz, 2H), 7.40 (t, J = 8.1 Hz, 2H), 7.30 (t, J = 8.0 Hz, 2H), 7.26~7.18 (m, 3H), 7.13 (t, J = 7.9 Hz, 2H), 6.66~6.60 (m, 1H), 5.77~5.67 (m, 1H), 5.57 (s, 1H), 5.47 (s, 1H), 5.38 (s, 1H), 4.96 (s, 1H), 4.38 (d, J = 4.1 Hz, 2H), 4.21 (t, J = 7.8 Hz, 1H), 3.88~3.87 (m, 2H), 3.14~3.13 (m, 2H), 2.46~2.43 (m, 1H), 2.02~1.83 (m, 4H), 1.70~1.58 (m, 2H), 1.51~1.44 (m, 1H), 1.04~0.93 (m, 1H), 0.81~0.73 (m, 1H); 13C NMR (101 MHz, CDCl3) δ 170.54, 168.68, 156.60, 143.90, 141.40, 135.80, 133.18, 132.81, 129.84, 129.37, 128.66, 127.84, 127.20, 125.20, 120.09, 75.11, 67.42, 53.39, 47.18, 44.49, 40.50, 38.23, 36.10, 35.91, 29.06, 24.21; ESI-HRMS (m/z): 575.252 2 [M+Na]+。
化合物T3 (TCO-Tyr-Gly), 收率70.2%; 1H NMR (400 MHz, CDCl3) δ 7.75 (d, J = 8.0 Hz, 2H), 7.58 (d, J = 7.9 Hz, 2H), 7.40 (t, J = 8.1 Hz, 2H), 7.31 (t, J = 7.7 Hz, 2H), 7.01 (t, J = 8.0 Hz, 2H), 6.86 (d, J = 11.6 Hz, 2H), 6.41 (dd, J = 19.7, 8.1 Hz, 1H), 5.79~5.72 (m, 1H), 5.52~5.45 (m, 2H), 5.38 (s, 1H), 4.94~4.88 (m, 1H), 4.39 (d, J = 7.8 Hz, 2H), 4.22 (t, J = 8.1 Hz, 1H), 3.92~3.86 (m, 2H), 3.15~3.09 (m, 2H), 2.46~2.45 (m, 1H), 2.03~1.83 (m, 4H), 1.68~1.65 (m, 2H), 1.50~1.46 (m, 1H), 1.30 (s, 9H), 1.05~0.96 (m, 1H), 0.82~0.74 (m, 1H); 13C NMR (101 MHz, CDCl3) δ 170.52, 168.50, 156.59, 154.74, 143.93, 141.44, 132.83, 130.47, 129.92, 129.88, 127.88, 127.24, 125.23, 124.29, 120.13, 78.53, 75.12, 67.47, 53.49, 47.23, 44.56, 40.57, 37.64, 36.15, 36.03, 29.10, 28.97 24.28; ESI-HRMS (m/z): 647.309 2 [M+Na]+。
化合物T4 (TCO-Gln-Gly), 收率88.2%; 1H NMR (400 MHz, CDCl3) δ 7.74 (d, J = 7.6 Hz, 2H), 7.56~7.54 (m, 2H), 7.39 (t, J = 8.1 Hz, 2H), 7.30~7.19 (m, 17H), 7.13~7.08 (m, 1H), 6.93 (s, 1H), 5.84~5.73 (m, 1H), 5.53~5.40 (m, 2H), 5.26~5.24 (m, 1H), 4.58~4.54 (m, 1H), 4.33 (d, J = 7.8 Hz, 2H), 4.18 (t, J = 8.0 Hz, 1H), 3.76 (s, 2H), 2.46~2.39 (m, 2H), 2.31~2.22 (m, 1H), 2.05~1.94 (m, 3H), 1.89~1.83 (m, 1H), 1.73~1.63 (m, 4H), 1.50~1.44 (m, 1H), 1.10~1.00 (m, 1H), 0.82~0.74 (m, 1H); 13C NMR (101 MHz, CDCl3) δ 171.49, 170.83, 169.23, 156.52, 144.64, 143.95, 141.41, 132.75, 129.94, 128.82, 128.10, 127.84, 127.22, 125.26, 120.09, 74.89, 70.84, 67.33, 52.55, 47.23, 44.40, 40.64, 36.08, 35.94, 33.39, 29.13, 27.32, 24.28; ESI-HRMS (m/z): 798.352 1 [M+Na]+。
化合物T5 (TCO-Gly-Gly), 收率72.0%; 1H NMR (400 MHz, CDCl3) δ 7.74 (d, J = 8.0 Hz, 2H), 7.58 (d, J = 3.7 Hz, 2H), 7.39 (t, J = 8.1 Hz, 2H), 7.30 (t, J = 7.8 Hz, 2H), 6.60 (s, 1H), 5.84~5.76 (m, 1H), 5.58 (s, 1H), 5.48 (d, J = 19.5 Hz, 1H), 5.45 (s, 1H), 4.42 (d, J = 8.3 Hz, 2H), 4.22 (t, J = 8.0 Hz, 1H), 4.10 (t, J = 4.2 Hz, 2H), 3.92 (s, 2H), 2.48~2.45 (m, 1H), 2.04~1.99 (m, 3H), 1.96~1.88 (m, 1H), 1.74~1.66 (m, 2H), 1.52~1.48 (m, 1H), 1.11~1.02 (m, 1H), 0.83~0.75 (m, 1H); 13C NMR (101 MHz, CDCl3) δ 169.32, 169.00, 156.75, 143.87, 141.44, 132.75, 130.09, 127.86, 127.22, 125.18, 120.12, 75.08, 67.37, 47.26, 44.53, 41.55, 40.57, 36.04, 36.00, 29.11, 24.19; ESI-HRMS (m/z): 485.205 0 [M+Na]+。
化合物T6 (TCO-Gly-Asp), 收率84.6%; 1H NMR (400 MHz, CDCl3) δ 7.75 (d, J = 8.1 Hz, 2H), 7.58 (d, J = 7.5 Hz, 2H), 7.40 (t, J = 8.0 Hz, 2H), 7.31 (t, J = 7.6 Hz, 2H), 7.02 (s, 1H), 5.98 (d, J = 8.3 Hz, 1H), 5.85~5.78 (m, 1H), 5.52~5.46 (m, 2H), 4.59 (s, 1H), 4.42 (d, J = 7.9 Hz, 2H), 4.23 (d, J = 8.0 Hz, 1H), 4.10~4.01 (m, 2H), 2.89 (d, J = 16.3 Hz, 1H), 2.64 (dd, J = 15.9 Hz, 4.1 Hz, 1H), 2.48~2.46 (m, 1H), 2.07~1.96 (m, 3H), 1.91~1.85 (m, 1H), 1.73~1.61 (m, 2H), 1.54~1.48 (m, 1H), 1.45 (s, 9H), 1.12~1.03 (m, 1H), 0.83~0.75 (m, 1H); 13C NMR (101 MHz, CDCl3) δ 171.19, 170.85, 168.57, 156.19, 143.87, 141.45, 132.72, 130.13, 127.88, 127.25, 125.22, 120.15, 82.10, 74.89, 67.50, 51.22, 47.29, 41.81, 40.61, 37.54, 36.05, 35.99, 29.14, 28.18, 24.19; ESI-HRMS (m/z): 615.245 6 [M+Na]+。
化合物T7 (TCO-Gly-Phe), 收率68.2%; 1H NMR (400 MHz, CDCl3) δ 7.76 (d, J = 8.1 Hz, 2H), 7.52 (t, J = 7.8 Hz, 2H), 7.39 (t, J = 8.0 Hz, 2H), 7.31~7.21 (m, 7H), 6.43 (s, 1H), 5.82~5.75 (m, 1H), 5.52~5.47 (dd, J = 19.8, 4.1 Hz, 1H), 5.44 (s, 2H), 4.50 (s, 1H), 4.41 (t, J = 8.5 Hz, 1H), 4.32 (s, 1H), 4.17 (t, J = 8.0 Hz, 1H), 4.05~3.92 (m, 2H), 3.11 (s, 2H), 2.48~2.46 (m, 1H), 2.04~1.96 (m, 3H), 1.91~1.83 (m, 1H), 1.73~1.62 (m, 2H), 1.55~1.44 (m, 1H), 1.10~1.03 (m, 1H), 0.83~0.76 (m, 1H); 13C NMR (101 MHz, CDCl3) δ 171.19, 168.68, 156.14, 143.87, 141.43, 136.44, 132.69, 130.12, 129.40, 128.86, 127.84, 127.23, 127.20, 125.17, 120.10, 74.98, 67.22, 56.23, 47.25, 41.61, 40.57, 38.58, 36.03, 55.99, 29.11, 24.19; ESI-HRMS (m/z): 575.251 6 [M+Na]+。
化合物T8 (TCO-Gly-Gly-Val), 收率67.3%; 1H NMR (400 MHz, CDCl3) δ 7.74 (d, J = 8.1 Hz, 2H), 7.58 (t, J = 7.8 Hz, 2H), 7.38 (t, J = 8.0 Hz, 2H), 7.29 (t, J = 8.4 Hz, 2H), 7.09 (s, 1H), 7.02 (s, 1H), 5.81~5.72 (m, 1H), 5.65 (s, 1H), 5.46 (dd, J = 15.7 Hz, 4.0 Hz, 1H), 5.39 (s, 1H), 4.43~4.33 (m, 2H), 4.19 (t, J = 8.1 Hz, 1H), 4.05~3.97 (m, 5H), 2.44~2.41 (m, 1H), 2.15~2.11 (m, 1H), 2.01~1.93 (m, 3H), 1.89~1.81 (m, 1H), 1.68~1.59 (m, 2H), 1.52~1.42 (m, 1H), 1.08~0.94 (m, 7H), 0.80~0.72 (m, 1H); 13C NMR (101 MHz, CDCl3) δ 172.14, 169.08, 169.05, 156.86, 143.94, 141.44, 132.67, 130.13, 127.87, 127.25, 125.21, 120.11, 74.97, 67.26, 60.93, 47.32, 43.03, 41.52, 40.56, 36.03, 35.99, 30.91, 29.11, 24.17, 19.40, 18.14; ESI-HRMS (m/z): 584.273 3 [M+Na]+。
化合物T9 (TCO-Gly-Gly-Lys), 收率70.0%; 1H NMR (400 MHz, CDCl3) δ 7.74 (d, J = 8.0 Hz, 2H), 7.58 (s, 2H), 7.38 (t, J = 7.8 Hz, 2H), 7.29 (t, J = 8.4 Hz, 2H), 7.12 (s, 1H), 7.05 (s, 1H), 5.82~5.72 (m, 2H), 5.46 (dd, J =16.1, 4.0 Hz, 1H), 5.37 (s, 1H), 4.76 (s, 1H), 4.38 (d, J = 4.6 Hz, 2H), 4.20~4.13 (m, 2H), 4.03 (s, 2H), 3.99 (s, 2H), 3.08 (s, 2H), 2.44~2.40 (m, 1H), 2.01~1.93 (m, 3H), 1.89~1.82 (m, 2H), 1.68~1.58 (m, 3H), 1.48~1.42 (m, 14H), 1.08~0.99 (m, 1H), 0.80~0.72 (m, 1H); 13C NMR (101 MHz, CDCl3) δ 172.70, 169.27, 169.13, 156.86, 156.48, 143.92, 141.42, 132.63, 130.16, 127.86, 127.23, 125.22, 120.10, 79.33, 74.93, 67.26, 55.46, 47.27, 43.04, 41.46, 40.54, 39.91, 36.01, 35.97, 31.67, 29.75, 29.10, 28.57, 24.16, 22.61; ESI-HRMS (m/z): 713.351 9 [M+Na]+。
化合物T10 (TCO-Gly-Gly-Leu), 收率78.0%; 1H NMR (400 MHz, CDCl3) δ 7.73 (d, J = 8.0 Hz, 2H), 7.56 (t, J = 8.1 Hz, 2H), 7.37 (t, J = 8.5 Hz, 2H), 7.28 (t, J = 7.9 Hz, 2H), 7.22 (s, 1H), 7.12 (s, 1H), 5.81~5.71 (m, 1H), 5.66 (t, J = 4.7 Hz, 1H), 5.42 (d, J = 15.5 Hz, 1H), 5.37 (s, 1H), 4.42 (d, J = 8.0 Hz, 2H), 4.21~4.16 (m, 2H), 4.07~3.92 (m, 4H), 2.43~2.40 (m, 1H), 2.01~1.93 (m, 3H), 1.87~1.80 (m, 1H), 1.67~1.41 (m, 6H), 1.07~0.99 (m, 1H), 0.92~0.86 (m, 6H), 0.79~0.71 (m, 1H); 13C NMR (101 MHz, CDCl3) δ 173.24, 169.35, 169.11, 156.68, 143.91, 141.41, 132.62, 130.15, 127.84, 127.21, 125.17, 120.08, 74.90, 67.18, 53.97, 47.28, 43.07, 41.46, 40.53, 36.00, 35.96, 29.08, 24.83, 24.15, 23.04, 21.97; ESI-HRMS (m/z): 598.289 5 [M+Na]+。
化合物T11 (TCO-Ala-Gly-Lys), 收率65.9%; 1H NMR (400 MHz, MeOD) δ 7.83 (d, J = 8.0 Hz, 2H), 7.69 (d, J = 7.6 Hz, 2H), 7.44 (t, J = 8.2 Hz, 2H), 7.35 (t, J = 8.3 Hz, 2H), 5.94~5.88 (m, 1H), 5.57 (dd, J = 19.6, 8.2 Hz, 1H), 5.39 (t, J = 4.0 Hz, 1H), 4.53~4.37 (m, 3H), 4.28~4.25 (m, 1H), 4.07~3.86 (m, 3H), 3.09 (t, J = 7.8 Hz, 2H), 2.48~2.44 (m, 1H), 2.08~1.97 (m, 3H), 1.92~1.81 (m, 1H), 1.77~1.71 (m, 2H), 1.68~1.63 (m, 1H), 1.56~1.39 (m, 18H), 1.22~1.11 (m, 1H), 0.92~0.85 (m, 1H); 13C NMR (101 MHz, MeOD) δ 175.43, 173.19, 171.26, 158.70, 158.57, 145.22, 142.61, 133.31, 131.72, 128.80, 128.20, 126.18, 120.94, 79.87, 75.81, 67.97, 56.91, 43.31, 41.31, 41.02, 36.93, 36.82, 32.16, 30.73, 30.58, 30.02, 28.81, 26.90, 25.23, 24.12, 17.56; ESI-HRMS (m/z): 727.368 3 [M+Na]+。
化合物T12 (TCO-Gly-Gly-Phe-Gly), 收率60.9%; 1H NMR (400 MHz, CDCl3) δ 7.74 (d, J = 8.2 Hz, 2H), 7.57 (d, J = 7.6 Hz, 2H), 7.41~7.33 (m, 3H), 7.31~7.25 (m, 3H), 7.25~7.07 (m, 6H), 5.97 (s, 1H), 5.75 (t, J =16.5 Hz, 1H), 5.41 (d, J =12.8 Hz, 1H), 5.37 (s, 1H), 4.82 (s, 1H), 4.35 (d, J = 8.3 Hz, 2H), 4.19 (t, J = 8.1 Hz, 1H), 4.03~3.93 (m, 6H), 3.12~2.99 (m, 2H), 2.42~2.39 (m, 1H), 2.00~1.80 (m, 4H), 1.66~1.60 (m, 2H), 1.46~1.43 (m, 1H), 1.05~0.97 (m, 1H), 0.78~0.70 (m, 1H); 13C NMR (101 MHz, CDCl3) δ 171.43, 169.70, 169.23, 169.07, 156.91, 143.92, 141.42, 136.40, 132.68, 130.16, 129.42, 128.73, 127.88, 127.22, 127.17, 125.25, 120.13, 75.01, 67.44, 54.76, 47.21, 44.60, 43.11, 41.58, 40.53, 38.55, 36.01, 35.96, 29.08, 24.16; ESI-HRMS (m/z): 689.295 2 [M+Na]+。
化合物T13 (TCO-Ala-Gly-Gly-Leu), 收率68.8%; 1H NMR (400 MHz, CDCl3) δ 7.72 (d, J = 8.1 Hz, 2H), 7.61~7.51 (m, 2H), 7.37 (t, J = 7.5 Hz, 2H), 7.34~7.22 (m, 4H), 7.14~7.04 (m, 1H), 5.81~5.70 (m, 2H), 5.48~5.41 (m, 1H), 5.36 (s, 1H), 4.59 (t, J = 8.4 Hz, 1H), 4.43~4.39 (m, 2H), 4.26 (s, 1H), 4.18 (t, J = 8.2 Hz, 1H), 4.04~3.92 (m, 4H), 2.44~2.41 (m, 1H), 2.05~1.94 (m, 3H), 1.89~1.81 (m, 1H), 1.68~1.63 (m, 4H), 1.54~1.48 (m, 1H), 1.41 (d, J = 8.0 Hz, 2H), 1.37 (d, J = 8.2 Hz, 2H), 1.08~0.99 (m, 1H), 0.90 (s, 6H), 0.80~0.72 (m, 1H); 13C NMR (101 MHz, CDCl3) δ 173.61, 172.20, 169.44, 168.62, 156.80, 143.95, 141.42, 132.77, 130.48, 127.86, 127.25, 125.20, 120.11, 74.87, 67.21, 53.84, 48.44, 47.29, 43.44, 42.99, 41.23, 40.59, 36.04, 35.97, 29.13, 24.82, 24.14, 23.07, 22.05, 18.37; ESI-HRMS (m/z): 669.325 8 [M+Na]+。
2 双功能化TCO的合成 2.1 化合物2的合成在500 mL圆底烧瓶中加入1-羟基-2, 6-环辛二烯(10.0 g, 80.0 mmol)、叔丁基二甲基氯硅烷(18.0 g, 120.0 mmol)、咪唑(10.9 g, 160.0 mmol)和200 mL的DCM, 室温下反应3 h。TLC (PE:DCM = 3:1)监测反应进程。反应结束后加入DCM 100 mL稀释反应液, 饱和氯化钠洗涤两次。收集有机相, 用无水硫酸钠干燥, 抽滤, 浓缩, 经硅胶柱洗脱分离(PE), 减压浓缩, 得到淡黄色油状液体17.3 g, 收率90.0%。1H NMR (400 MHz, CDCl3) δ 5.57~5.54 (m, 2H), 5.47 (s, 2H), 5.05~5.00 (m, 1H), 2.57 (d, 1H), 2.43~2.27 (m, 3H), 2.24~2.10 (m, 2H), 0.89 (s, 9H), 0.08 (s, 6H); 13C NMR (101 MHz, CDCl3) δ 135.13, 128.83, 127.22, 126.09, 77.48, 70.16, 38.86, 28.43, 26.08, 18.47, -4.51。
2.2 化合物3的合成在250 mL圆底烧瓶中加入化合物2 (10.0 g, 42.0 mmol)和DCM 50 mL, 室温搅拌5 min, 将间氯过氧苯甲酸(8.0 g, 46.2 mmol)溶于80 mL的DCM, 在0 ℃下缓慢滴加于反应液中, 约30 min滴完。室温搅拌18 h, TLC监测显示反应基本完全。分别用饱和亚硫酸氢钠水溶液(100 mL×3)、饱和碳酸氢钠水溶液(100 mL×3)、饱和氯化钠(100 mL×3)洗涤, 收集有机相, 用无水硫酸钠干燥, 抽滤, 浓缩, 经硅胶柱洗脱分离(PE:EA = 60:1), 减压浓缩, 得到淡黄色油状液体7.5 g, 收率70.0%。1H NMR (400 MHz, CDCl3) δ 5.54~5.41 (m, 2H), 4.81~4.71 (m, 1H), 3.18~2.92 (m, 2H), 2.40 (s, 1H), 2.19 (s, 3H), 2.10 (s, 1H), 1.74 (s, 1H), 0.85 (s, 9H), 0.08 (s, 6H); 13C NMR (101 MHz, CDCl3) δ 135.81, 125.88, 66.92, 57.38, 54.91, 38.92, 28.83, 25.98, 24.30, 18.38, -4.44。
2.3 化合物4a和4b的合成在装有回流冷凝器的500 mL双颈烧瓶中, 加入氢化铝锂粉末(4.5 g, 117.0 mmol), 抽真空氮气保护, 并将其置于冰浴中冷却, 随后加入干燥的四氢呋喃150 mL。将化合物3 (10.0 g, 39.0 mmol)溶于50 mL干燥的四氢呋喃中, 在0 ℃下缓慢滴加, 室温下搅拌20 min, 后置于70 ℃回流反应4 h。反应结束后, 将反应瓶冷却至0 ℃, 缓慢滴加水(4.5 mL)、15%的氢氧化钠溶液(4.5 mL)、水(13.5 mL)。在室温下搅拌4 h, 至灰色的沉淀变为白色, 然后加入过量硅藻土和无水硫酸钠, 充分搅拌30 min。抽滤, 有机相减压浓缩, 得到的粗品为混合物7.0 g (3a:3b = 2:3), 收率70.0%。无需进一步纯化, 直接用于下一步反应。
2.4 化合物5a和5b合成① 在250 mL圆底烧瓶中, 将化合物4a和4b混合物(5.0 g, 19.0 mmol)与三乙胺(8.2 mL, 58.0 mmol)在100 mL DCM中混合, 冷却至0 ℃, 缓慢滴加甲磺酰氯(9.1 mL, 58.0 mmol), 在0 ℃下搅拌反应30 min, 然后升温至室温继续搅拌2 h。反应结束后, 减压浓缩, 得到的粗品无需进一步纯化, 直接用于下一步反应。②将上步粗产品溶于50 mL DMF溶液, 将固体NaN3 (3.8 g, 58.0 mmol)小心加入其中。然后加热到60 ℃, 搅拌2天。冷却至室温后, 加入150 mL水淬灭反应, 用乙醚(100 mL×3)萃取, 合并有机相, 用水洗涤一次, 然后用无水硫酸钠干燥有机层, 减压浓缩, 经硅胶柱洗脱分离(PE), 减压浓缩, 得到淡黄色油状液体3.1 g, 收率55.0%。③将上步产物(3.0 g, 7.8 mmol)和三苯基膦(36.7 g, 140.0 mmol)溶于THF (100 mL)中, 在室温下反应过夜。TLC监测反应完全后, 减压浓缩除去溶剂, 加入正己烷后有固体析出, 过滤除去固体, 滤液减压浓缩, 经硅胶柱洗脱分离(DCM:CH3OH = 20:1), 减压浓缩, 得到淡黄色油状液体1.4 g, 收率50%。5a: 1H NMR (400 MHz, CDCl3) δ 5.47 (t, J = 2.3 Hz, 2H), 4.57~4.54 (m, 1H), 3.77~3.73 (m, 1H), 2.31 (s, 1H), 1.97~1.93 (m, 1H), 1.93~1.61 (m, 4H), 1.59~1.48 (m, 2H), 0.88 (s, 9H), 0.06 (s, 6H); 13C NMR (101 MHz, CDCl3) δ 135.87, 132.59, 70.54, 66.15, 47.39, 38.44, 32.07, 29.81, 26.42, 18.39, -4.66。5b: 1H NMR (400 MHz, CDCl3) δ 5.65 (t, J = 2.0 Hz, 2H), 4.77~4.74 (m, 1H), 3.80~3.77 (m, 1H), 2.25~1.95 (m, 3H), 1.91~1.68 (m, 4H), 1.49~1.46 (m, 1H), 0.88 (s, 9H), 0.06 (s, 6H); 13C NMR (101 MHz, CDCl3) δ 135.28, 132.15, 74.48, 72.85, 37.32, 35.94, 33.81, 28.82, 25.96, 22.51, -4.54。
2.5 化合物6a和6b的合成在100 mL圆底烧瓶中加入化合物5a和5b混合物(2.0 g, 7.8 mmol)、EDCI (3.0 g, 15.6 mmol)、HOBT (2.1 g, 15.6 mmol), 抽真空氮气保护, 加入20 mL干燥的DCM使反应物完全溶解, 然后分别加入己二酸单甲酯(2.3 mL, 15.6 mmol)和DIPEA (2.6 mL, 15.6 mmol), 在室温下反应4 h。TLC监测反应完全后, 用水洗涤两次, 收集有机相用无水硫酸钠干燥、浓缩, 经硅胶柱洗脱分离(PE::EA = 1:1), 减压浓缩, 得到淡黄色油状液体3.1 g, 收率70.0%。6a: 1H NMR (400 MHz, CDCl3) δ 5.84 (d, J = 7.3 Hz, 1H), 5.67~5.63 (m, 1H), 5.48~5.41 (m, 1H), 4.61~4.57 (m, 1H), 4.01~3.96 (m, 1H), 3.65 (s, 3H), 2.32~2.20 (m, 2H), 2.14~1.90 (m, 5H), 1.71~1.62 (m, 2H), 1.64~1.59 (m, 5H), 1.53~1.49 (m, 1H), 1.43~1.41 (m, 1H), 0.88 (s, 9H), 0.05 (s, 6H); 13C NMR (101 MHz, CDCl3) δ 174.01, 170.90, 137.28, 126.44, 68.73, 51.65, 46.96, 45.03, 36.55, 33.84, 32.10, 25.99, 25.43, 25.27, 25.09, 24.58, 18.30, -4.55; ESI-HRMS (m/z): 420.2545 [M+Na]+。6b: 1H NMR (400 MHz, CDCl3) δ 5.67 (d, J = 3.3 Hz, 1H), 5.64~5.59 (m, 1H), 5.44~5.40 (m, 1H), 4.65~4.59 (m, 1H), 3.87~3.82 (m, 1H), 3.63 (s, 3H), 2.32~2.28 (m, 1H), 2.25~2.18 (m, 2H), 2.15~2.01 (m, 4H), 1.82~1.77 (m, 2H), 1.62~1.54 (m, 5H), 1.52~1.49 (m, 2H), 0.82 (s, 9H), 0.08 (s, 6H); 13C NMR (101 MHz, CDCl3) δ 174.00, 170.97, 135.61, 127.73, 68.75, 51.61, 46.96, 44.87, 36.44, 33.77, 31.94, 25.96, 25.20, 24.45, 22.51, 18.31, -4.66; ESI-HRMS (m/z): 420.255 9 [M+Na]+。
2.6 化合物7a和7b的合成将化合物6a和6b的混合物(2.0 g, 5.0 mmol)溶于20 mL四氢呋喃溶液中, 随后加入5 mL的TBAF (1 mol·L-1四氢呋喃)溶液, 于室温下反应4 h。TLC监测反应完全后, 反应液浓缩后, 加入100 mL乙酸乙酯稀释, 用水洗涤一次。收集有机相用无水硫酸钠干燥、浓缩, 经硅胶柱洗脱分离(PE:EA = 1:3), 减压浓缩, 得到产物1.1 g (7a:7b = 4:5), 收率80.0%。7a: 1H NMR (400 MHz, CDCl3) δ 6.09 (d, J = 8.0 Hz, 1H), 5.68~5.64 (m, 1H), 5.50~5.43 (m, 1H), 4.64~4.61 (m, 1H), 3.91~3.89 (m, 1H), 3.63 (s, 3H), 2.33~2.28 (m, 2H), 2.23~2.02 (m, 5H), 1.85~1.71 (m, 2H), 1.66~1.57 (m, 5H), 1.53~1.49 (m, 1H), 1.44~1.39 (m, 1H); 13C NMR (101 MHz, CDCl3) δ 174.11, 171.16, 136.07, 127.15, 67.60, 51.66, 46.96, 44.88, 36.38, 34.93, 33.39, 25.75, 25.43, 24.58, 18.30; ESI-HRMS (m/z): 284.1861 [M+H]+。7b: 1H NMR (400 MHz, CDCl3) δ 5.75~5.68 (m, 2H), 5.49~5.45 (m, 1H), 4.78~4.68 (m, 1H), 3.90~3.86 (m, 1H), 3.64 (s, 3H), 2.30~2.26 (m, 2H), 2.22~2.05 (m, 4H), 1.96~1.85 (m, 1H), 1.82~1.75 (m, 1H), 1.71~1.68 (m, 1H), 1.62~1.57 (m, 5H), 1.52~1.46 (m, 2H), 1.40~1.33 (m, 1H); 13C NMR (101 MHz, CDCl3) δ 174.01, 170.98, 135.61, 127.73, 68.74, 51.61, 50.16, 36.44, 35.82, 35.25, 33.76, 31.94, 25.96, 24.96, 18.34; ESI-HRMS (m/z): 284.186 4 [M+H]+。
2.7 化合物8a1、8a2、8b1、8b2的合成将化合物7b (200.0 mg, 0.7 mmol)、苯甲酸甲酯(200.0 mg, 3.5 mmol)置于250 mL的石英管内, 加入100 mL的混合溶剂(Et2O:EA = 10:1)溶解, 在254 nm紫外光下照射, 每隔30 min用8.0 g的AgNO3硅胶柱过滤, 滤液继续光照, 总共光照12 h。收集AgNO3硅胶, 用混合溶剂(DCM:氨水=3:1) 100 mL将吸附在硅胶上的产物洗脱, 收集有机相, 水相用DCM萃取3次, 合并有机相, 用无水硫酸钠干燥、浓缩, 经硅胶柱洗脱分离(PE:EA = 1:1), 减压浓缩, 得到产物46.0 mg (8b1:8b2 = 5:3), 收率23.0%。同样的方法可以得到化合物8a1和8a2 (8a1:8a2 = 2:3), 收率20.0%。8a1: 1H NMR (400 MHz, CDCl3) δ 5.79 (d, J = 7.6 Hz, 1H), 5.71~5.67 (m, 1H), 5.57~5.50 (m, 1H), 4.78~4.66 (m, 1H), 4.02~3.95 (m, 1H), 3.67 (s, 3H), 2.35~2.32 (m, 2H), 2.27~2.19 (m, 1H), 2.15~2.10 (m, 4H), 1.87~1.75 (m, 2H), 1.70~1.62 (m, 6H), 1.57~1.53 (m, 1H), 1.48~1.41 (m, 1H); 13C NMR (101 MHz, CDCl3) δ 174.50, 170.89, 135.87, 127.69, 67.92, 51.72, 46.94, 44.79, 36.55, 33.81, 32.34, 25.41, 25.27, 25.11, 24.51; ESI-HRMS (m/z): 284.186 0 [M+H]+。8a2: 1H NMR (400 MHz, CDCl3) δ 7.12 (s, 1H), 6.00~5.93 (m, 1H), 5.70 (d, J = 15.4 Hz, 1H), 4.64 (s, 1H), 4.12~4.07 (m, 1H), 3.67 (s, 3H), 2.39~2.27 (m, 6H), 2.23~2.20 (m, 2H), 2.01~1.94 (m, 1H), 1.91~1.80 (m, 2H), 1.70~1.66 (m, 5H), 1.52~1.41 (m, 1H); 13C NMR (101 MHz, CDCl3) δ 174.23, 171.62, 133.97, 130.80, 72.41, 51.71, 47.04, 46.96, 36.42, 34.82, 33.89, 31.51, 29.40, 25.40, 24.70; ESI-HRMS (m/z): 284.186 3 [M+H]+。8b1: 1H NMR (400 MHz, CDCl3) δ 5.77~5.70 (m, 1H), 5.57 (d, J = 4.1 Hz, 1H), 5.48~5.41 (m, 1H), 4.31~4.20 (m, 2H), 3.68 (s, 3H), 2.38~2.35 (m, 3H), 2.28~2.23 (m, 3H), 2.20~2.12 (m, 4H), 2.08~2.01 (m, 1H), 1.75~1.63 (m, 5H), 1.59~1.50 (m, 1H); 13C NMR (101 MHz, CDCl3) δ 174.22, 171.76, 135.78, 131.55, 75.38, 51.81, 45.25, 41.07, 38.30, 36.57, 33.79, 30.64, 30.18, 25.44, 24.55; ESI-HRMS (m/z): 306.168 3 [M+Na]+。8b2: 1H NMR (400 MHz, CDCl3) δ 6.08~6.00 (m, 1H), 5.66 (d, J = 16.0 Hz, 1H), 5.59 (d, J = 3.8 Hz, 1H), 4.63 (s, 1H), 3.67 (s, 3H), 3.45~3.43 (m, 1H), 2.49~2.46 (m, 1H), 2.37~2.32 (m, 3H), 2.15~2.10 (m, 3H), 2.10~1.94 (m, 2H), 1.90~1.78 (m, 3H), 1.64~1.59 (m, 5H); 13CNMR (101 MHz, CDCl3) δ 174.12, 171.10, 134.98, 131.13, 70.31, 53.91, 51.71, 42.57, 39.99, 36.42, 34.04, 33.81, 33.79, 25.18, 24.51; ESI-HRMS (m/z): 306.168 1 [M+Na]+。
| [1] |
Oliveira BL, Guo Z, Bernardes GJL. Inverse electron demand Diels-Alder reactions in chemical biology[J]. Chem Soc Rev, 2017, 46: 4895-4950. DOI:10.1039/C7CS00184C |
| [2] |
Devaraj NK, Weissleder R, Hilderbrand SA. Tetrazine-based cycloadditions:application to pretargeted live cell imaging[J]. Bioconjug Chem, 2008, 19: 2297-2299. DOI:10.1021/bc8004446 |
| [3] |
Blackman ML, Royzen M, Fox JM. Tetrazine ligation:fast bioconjugation based on inverse-electron-demand Diels-Alder reactivity[J]. J Am Chem Soc, 2008, 130: 13518-13519. DOI:10.1021/ja8053805 |
| [4] |
Wu H, Devaraj NK. Inverse electron-demand Diels-Alder bioorthogonal reactions[J]. Top Curr Chem (Z), 2016, 374: 3. DOI:10.1007/s41061-015-0005-z |
| [5] |
Mejia Oneto JM, Khan I, Seebald L, et al. In vivo bioorthogonal chemistry enables local hydrogel and systemic pro-drug to treat soft tissue sarcoma[J]. ACS Cent Sci, 2016, 2: 476-482. DOI:10.1021/acscentsci.6b00150 |
| [6] |
Ji X, Zhou C, Ji K, et al. Click and release:a chemical strategy toward developing gasotransmitter prodrugs by using an intramolecular Diels-Alder reaction[J]. Angew Chem Int Ed, 2016, 55: 15846-15851. DOI:10.1002/anie.201608732 |
| [7] |
Akgun B, Hall DG. Boronic acids as bioorthogonal probes for site-selective labeling of proteins[J]. Angew Chem Int Ed, 2018, 57: 13028-13044. DOI:10.1002/anie.201712611 |
| [8] |
Rossin R, van Duijnhoven SMJ, Robillard MS, et al. Triggered drug release from an antibody-drug conjugate using fast "click-to-release" chemistry in mice[J]. Bioconjug Chem, 2016, 27: 1697-1706. DOI:10.1021/acs.bioconjchem.6b00231 |
| [9] |
Agarwal P, Bertozzi CR. Site-specific antibody-drug conjugates:the nexus of bioorthogonal chemistry, protein engineering, and drug development[J]. Bioconjug Chem, 2015, 26: 176-192. DOI:10.1021/bc5004982 |
| [10] |
Lang K, Chin JW. Bioorthogonal reactions for labeling proteins[J]. ACS Chem Biol, 2014, 9: 16-20. DOI:10.1021/cb4009292 |
| [11] |
Li J, Chen PR. Development and application of bond cleavage reactions in bioorthogonal chemistry[J]. Nat Chem Biol, 2016, 12: 129-137. DOI:10.1038/nchembio.2024 |
| [12] |
Versteegen RM, Rossin R, Robillard MS, et al. Click to release:instantaneous doxorubicin elimination upon tetrazine ligation[J]. Angew Chem Int Ed, 2013, 52: 14112-14116. DOI:10.1002/anie.201305969 |
| [13] |
Wu H, Alexander SC, Jin S, et al. A bioorthogonal near-infrared fluorogenic probe for mRNA detection[J]. J Am Chem Soc, 2016, 138: 11429-11432. DOI:10.1021/jacs.6b01625 |
| [14] |
Jiménez-Moreno E, Guo Z, Bernardes GJL, et al. Vinyl ether/tetrazine pair for the traceless release of alcohols in cells[J]. Angew Chem Int Ed, 2017, 56: 243-247. DOI:10.1002/anie.201609607 |
| [15] |
Xiang L, Yan Z, Hong GH. Different stapling-based peptide drug design:mimicking α-helix as inhibitors of protein-protein interaction[J]. Chin Chem Lett, 2018, 29: 1088-1092. DOI:10.1016/j.cclet.2018.01.018 |
| [16] |
Zhao Z, Bao XQ, Zhang D. Mechanisms of ferroptosis and its involvement in Parkinson's disease[J]. Acta Pharm Sin (药学学报), 2019, 54: 399-406. |
| [17] |
Lin JH, Chen G, Lin M, et al. Advances in research on mass spectrometry based chiral amino acid analysis for quality control of racemic peptide impurities[J]. Acta Pharm Sin (药学学报), 2019, 54: 1958-1964. |
| [18] |
Zhao X, Liu X, Li Y, et al. Injectable peptide hydrogel as intraperitonealtriptolide depot for the treatment of orthotopichepatocellular carcinoma[J]. Acta Pharm Sin B, 2019, 9: 1050-1060. DOI:10.1016/j.apsb.2019.06.001 |
| [19] |
He JY, Liang J, Xuan MS, et al. Effective strategies for improving the stability of peptides in vivo[J]. Acta Pharm Sin (药学学报), 2020, 55: 25-32. |
| [20] |
Han MY, Chen JJ, Zhu P, et al. Advances in the nonribosomal peptide synthetases[J]. Acta Pharm Sin (药学学报), 2018, 53: 1080-1089. |
| [21] |
Li M, Xu H, Wang J. Optimized functional and structural design of dual-target LMRAP, a bifunctional fusion protein with a 25-amino-acid antitumor peptide and GnRH Fc fragment[J]. Acta Pharm Sin B, 2020, 10: 262-275. DOI:10.1016/j.apsb.2019.10.010 |
| [22] |
Wang R, Shen Q, Liu M, et al. Efficacy of inverso isomer of CendR peptide on tumor tissue penetration[J]. Acta Pharm Sin B, 2018, 8: 825-832. DOI:10.1016/j.apsb.2018.06.006 |
| [23] |
Yang J, Hong YL, Yu YZ, et al. Macrocyclic peptides as regulators of protein-protein interactions[J]. Chin Chem Lett, 2018, 29: 1067-1073. DOI:10.1016/j.cclet.2018.05.028 |
| [24] |
Su H, Wang Y, Liu G, et al. Emerging transporter-targeted nanoparticulate drug delivery systems[J]. Acta Pharm Sin B, 2019, 9: 49-58. DOI:10.1016/j.apsb.2018.10.005 |
| [25] |
Wang K, Sachdeva A, Chin JW, et al. Optimized orthogonal translation of unnatural amino acids enables spontaneous protein double-labelling and FRET[J]. Nat Chem, 2014, 6: 393-403. DOI:10.1038/nchem.1919 |
| [26] |
Uttamapinant C, Barry NP, Chin JW, et al. Genetic code expansion enables live-cell and super-resolution imaging of site-specifically labeled cellular proteins[J]. J Am Chem Soc, 2015, 137: 4602-4605. DOI:10.1021/ja512838z |
| [27] |
Li Z, Wang D, Yao SQ, et al. "Minimalist" cyclopropene-containing photo-cross-linkers suitable for live-cell imaging and affinity-based protein labeling[J]. J Am Chem Soc, 2014, 136: 9990-9998. DOI:10.1021/ja502780z |
| [28] |
Šečkutė J, Yang J, Devaraj NK. Rapid oligonucleotide-templated fluorogenic tetrazine ligations[J]. Nucl Acid Res, 2013, 41: 148-157. DOI:10.1093/nar/gkt540 |
| [29] |
James ML, Gambhir SS. A molecular imaging primer:modalities, imaging agents, and applications[J]. Physiol Rev, 2012, 92: 897-965. DOI:10.1152/physrev.00049.2010 |
| [30] |
Zhang G, Li J, Chen PR, et al. Bioorthogonal chemical activation of kinases in living systems[J]. ACS Cent Sci, 2016, 2: 325-331. DOI:10.1021/acscentsci.6b00024 |
| [31] |
Staderini M, Gambardella A, Bradley M, et al. A tetrazine-labile vinyl ether benzyloxycarbonyl protecting group (VeZ):an orthogonal tool for solid-phase peptide chemistry[J]. Org Lett, 2018, 20: 3170-3173. DOI:10.1021/acs.orglett.8b00898 |
| [32] |
Asare-Okai PN, Agustin E, Royzen M, et al. Site-specific fluorescence labelling of RNA using bio-orthogonal reaction of trans-cyclooctene and tetrazine[J]. Chem Commun, 2014, 50: 7844-7847. DOI:10.1039/C4CC02435D |
| [33] |
Tang W, Becker ML. " Click" reactions:a versatile toolbox for the synthesis of peptide-conjugates[J]. Chem Soc Rev, 2014, 43: 7013-7039. DOI:10.1039/C4CS00139G |
| [34] |
Erak M, Bellmann-Sickert K, Beck-Sickinger AG, et al. Peptide chemistry toolbox-transforming natural peptides into peptide therapeutics[J]. Bioorg Med Chem, 2018, 26: 2759-2765. DOI:10.1016/j.bmc.2018.01.012 |
| [35] |
Versteegen RM, Hoeve W, Robillard MS, et al. Click-to-release from trans-cyclooctenes:mechanistic insights and expansion of scope from established carbamate to remarkable ether cleavage[J]. Angew Chem Int Ed, 2018, 57: 10494-10499. DOI:10.1002/anie.201800402 |
| [36] |
Davies S, Qiao L, Oliveira BL, et al. Tetrazine-triggered release of carboxylic-acid-containing molecules for activation of an anti-inflammatory drug[J]. Chembiochem, 2019, 20: 1541-1546. |
| [37] |
Fan X, Ge Y, Chen PR, et al. Optimized tetrazine derivatives for rapid bioorthogonal decaging in living cells[J]. Angew Chem Int Ed, 2016, 55: 14046-14050. DOI:10.1002/anie.201608009 |
| [38] |
Mao W, Shi W, Wu H, et al. Organocatalytic and scalable syntheses of unsymmetrical 1, 2, 4, 5-tetrazines by thiol-containing promotors[J]. Angew Chem Int Ed, 2019, 58: 1106-1109. DOI:10.1002/anie.201812550 |
| [39] |
Vlieghe P, Lisowski V, Khrestchatisky M, et al. Synthetic therapeutic peptides:science and market[J]. Drug Discov Today, 2010, 15: 40-56. DOI:10.1016/j.drudis.2009.10.009 |
| [40] |
Kapoor V, Dadey DYA, Hallahan DE, et al. Tumor-specific binding of radiolabeled PEGylated GIRLRG peptide:a novel agent for targeting cancers[J]. J Nucl Med, 2016, 57: 1991-1997. DOI:10.2967/jnumed.115.165118 |
| [41] |
Capicciotti CJ, Trant JF, Leclère M, et al. Synthesis of C-linked triazole-containing AFGP analogues and their ability to inhibit ice recrystallization[J]. Bioconjug Chem, 2011, 22: 605-616. DOI:10.1021/bc100394k |
| [42] |
Nicolaou KC, Estrada AA, Zak M, et al. A mild and selective method for the hydrolysis of esters with trimethyltin hydroxide[J]. Angew Chem Int Ed, 2005, 44: 1378-1382. DOI:10.1002/anie.200462207 |
| [43] |
Meijer A, Otto S, Engberts JBFN. Effects of the hydrophobicity of the reactants on Diels-Alder reactions in water[J]. J Org Chem, 1998, 63: 8989-8994. DOI:10.1021/jo981359x |
| [44] |
Palomo JM. Solid-phase peptide synthesis:an overview focusedon the preparation of biologically relevant peptides[J]. RSC Adv, 2014, 4: 32658-32672. DOI:10.1039/C4RA02458C |
| [45] |
Zhang J, Matta ME, Martinez H, et al. Precision vinyl acetate/ethylene (VAE) copolymersby ROMP of acetoxy-substituted cyclic alkenes[J]. Macromolecules, 2013, 46: 2535-2543. DOI:10.1021/ma400092z |
 2020, Vol. 55
2020, Vol. 55


