动脉粥样硬化(atherosclerosis, AS)是一种以动脉壁脂质和炎症细胞蓄积为特征的、复杂的血管炎症性疾病[1, 2]。巨噬细胞在AS发生发展中扮演重要角色, 参与慢性炎症过程, 促进活化巨噬细胞凋亡, 从而有效抑制AS的早期发展[3]。由此可见, 药物干预活化巨噬细胞凋亡对防护AS具有积极的临床意义。金雀异黄素(genistein, GEN)又称为染料木素或染料木黄酮, 是异黄酮类化合物中活性最强的一种, 大豆是其最丰富的来源。WHO-CARDIAC等临床研究显示, GEN可以抑制AS进程, 对心脑血管调节功能有益, 具有开发成心血管保护药物的性质[4], 但其作用机制尚未完全明确。鉴于此, 本课题组前期一直围绕GEN及其衍生物抗AS的药理学作用机制进行有关研究, 通过体内(新西兰兔、Apoe-/-小鼠)、体外实验证实GEN可以明显抑制AS[5-7]。本研究以小鼠巨噬细胞RAW264.7细胞为研究对象, 以脂多糖(lipopolysaccharides, LPS)为刺激因素, 探究GEN对活化巨噬细胞内质网应激凋亡相关靶点的影响及作用机制, 为GEN的临床应用提供实验依据。
材料与方法试剂和抗体 GEN, 纯度≥98%, Sigma公司; LPS, 纯度≥99%, Solarbio公司; 胎牛血清、DMEM培养基、青霉素/链霉素、磷酸盐缓冲液(phosphate buffered saline, PBS), BI公司; CCK8试剂盒、Annexin V-FITC/PI细胞凋亡检测试剂盒、Trizol、HiScript Ⅱ Q RT SuperMix for qPCR (+gDNA wiper)、miRNA 1st Strand cDNA Synthesis Kit (by stem-loop)、AceQ qPCR SYBR Green Master Mix、GAPDH抗体, Vazyme公司; RIPA细胞裂解液、BCA蛋白浓度测定试剂盒, Beyotime公司; 环氧合酶-2 (cyclooxygenase-2, COX-2)抗体和诱导型一氧化氮合酶(inducible nitric oxide synthases, iNOS)抗体, Affinity公司; CHOP抗体, Cell Signaling Technology公司; caspase-3抗体, ImmunoWay公司; β-actin抗体, CMCTAG公司; 鼠IgG和兔IgG, ComWin Biotech公司; 慢病毒颗粒, Genechem公司。
细胞 RAW264.7细胞购于中国科学院细胞库, 常规培养于含10%胎牛血清和100 U·mL-1青霉素/链霉素的DMEM完全培养基, 置于37 ℃、5% CO2细胞培养箱中培养, 所有实验均在细胞处于对数生长期时进行。将RAW264.7细胞接种于12孔板中(每毫升5×104个), 培养24 h, 分别按照慢病毒感染说明书加入miR-21 up-NC (阴性对照)、miR-21 up、miR-21 down-NC (阴性对照)、miR-21 down的慢病毒颗粒。
qRT-PCR 使用Trizol法提取总RNA, 分光光度计检测RNA纯度和浓度。根据mRNA和miRNA逆转录试剂说明书进行逆转录, 采用SYBR Green荧光染料法进行相对定量检测。基因表达水平以2-△△CT法进行分析。引物序列见表 1。
| Table 1 Primer sequence. TNF-α: Tumor necrosis factor-α; IL-6: Interleukin 6 |
Western blot 使用蛋白裂解液裂解细胞并离心(12 000 ×g, 4 ℃)提取蛋白, 应用BCA蛋白定量试剂盒测定蛋白浓度后将其变性。制备12% SDS-PAGE凝胶, 取40 µg样品, 电泳后转膜, 将蛋白转移至PVDF膜(polyvinylidene difluoride membranes, PVDF)上, 用5%脱脂牛奶-TBST (TBS包含0.05% Tween-20)室温封闭1 h, TBST洗涤3次。然后加入一抗4 ℃孵育过夜, 一抗稀释倍数按照说明书进行[iNOS (1︰2 000)、COX-2 (1︰2 000)、CHOP (1︰1 000)、caspase-3 (1︰ 1 000)、GAPDH (1︰10 000)和β-actin (1︰10 000)], GAPDH和β-actin为内参, TBST洗涤3次。之后加入二抗, 室温孵育1 h, TBST洗涤3次, 使用ECL发光剂和化学发光成像系统显影。
CCK8检测细胞活力 使用DMEM完全培养基轻柔吹打RAW264.7细胞, 制备每毫升5×104个单细胞悬液, 将其接种于96孔板, 每孔100 µL。置于37 ℃、5% CO2细胞培养箱中培养24 h后, 加不同浓度GEN (10、20、40 μmol·L-1)预处理2 h, 再与1 000 ng·mL-1 LPS共孵育24 h。然后小心吸弃上清, PBS洗涤2次, 加入90 μL新鲜完全培养基和10 µL CCK8溶液, 轻轻摇晃培养板使试剂充分混匀, 继续放入培养箱内孵育2 h。使用多功能酶标仪(BioTek Instruments)检测各孔在450 nm处的吸光度, 并设置空白孔、对照孔, 每组3个复孔。细胞活力%= (处理孔-空白孔)/ (对照孔-空白孔)×100%。
AnnexinV-FITC/PI双染法检测细胞凋亡 制备每毫升5×104个RAW264.7单细胞悬液, 将其接种于12孔板, 每孔1 mL。在37 ℃、5% CO2细胞培养箱中培养24 h后, 加不同浓度GEN (10、20、40 μmol·L-1)预处理2 h, 再与1 000 ng·mL-1 LPS共孵育24 h。使用无EDTA的胰蛋白酶消化细胞, 并收集于离心管中离心(800 r·min-1, 7 min), PBS洗涤2次; 用200 μL结合缓冲液重悬细胞至浓度为每毫升4×105个, 取195 μL细胞(约8×104个细胞)悬液加入5 μL AnnexinV-FITC, 轻轻混匀后室温避光孵育10 min; 用200 μL结合缓冲液洗涤细胞(800 r·min-1, 7 min); 再用190 μL结合缓冲液重悬细胞, 加入5 μL碘化丙啶溶液, 轻轻混匀后上流式细胞仪检测各组细胞凋亡率。
统计学分析 数据均采用均数±标准差表示, 采用SPSS20.0统计软件和GraphPad Prism 5进行t检验或单因素方差分析。P < 0.05表示差异具有统计学意义。
结果 1 GEN对LPS活化的RAW264.7细胞活力与凋亡的影响 1.1 构建LPS活化的RAW264.7细胞模型LPS(1 000 ng·mL-1, 24 h)组细胞肿瘤坏死因子(tumor necrosis factor-α, TNF-α)、白细胞介素-6 (interleukin 6, IL-6) mRNA表达及COX-2、iNOS蛋白表达均高于对照组(P < 0.05), 见图 1。由此, 本研究选取1 000 ng·mL-1 LPS孵育24 h作为活化RAW264.7细胞的最佳浓度和时间。
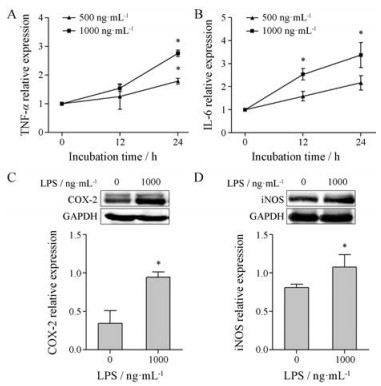
|
Figure 1 The effect of lipopolysaccharide (LPS) on RAW264.7 cells. RAW264.7 cells were activated by LPS (500 or 1 000 ng·mL-1) for 12 h or 24 h. qRT-PCR detected the level of TNF-α (A) and IL-6 (B) mRNA. Western blot detected the protein expression of COX-2 (C) and iNOS (D). n = 3, x± s. *P < 0.05 vs control group. COX: Cyclooxygenase-2; iNOS: Inducible nitric oxide synthases |
分别使用10、20、40 µmol·L-1 GEN预处理细胞2 h, 再与LPS共孵育24 h。与对照组相比, LPS明显增加细胞活力(P < 0.05); GEN则呈浓度依赖性下调LPS活化的RAW264.7细胞活力, 并促进凋亡, 其中LPS组凋亡率为5.3%, GEN组(10、20、40 µmol·L-1)凋亡率依次为5.8%、14%、36.1% (图 2A、B)。此外, LPS下调CHOP、caspase-3 mRNA和蛋白表达, 而GEN则上调其表达(P < 0.05, 图 2C~E)。由此, 本研究选择10 µmol·L-1 GEN进行后续实验。
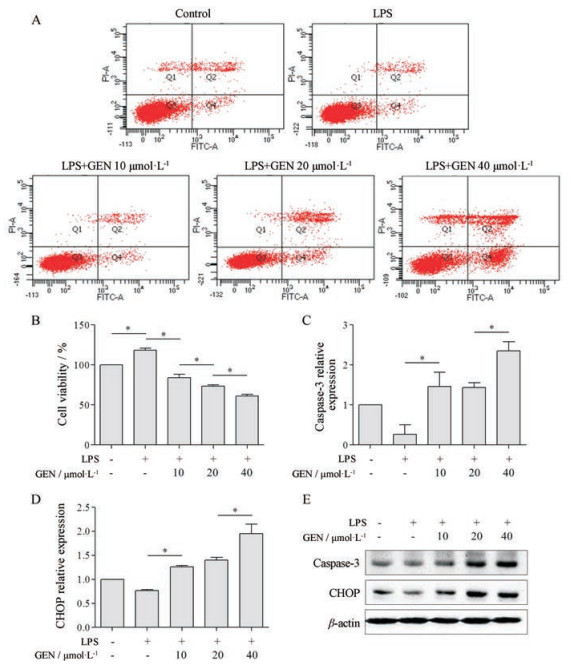
|
Figure 2 The effect of genistein (GEN) on the cell viability and apoptosis of LPS-activated RAW264.7 cells. RAW264.7 cells were pretreated with GEN (10、20、40 µmol·L-1) for 2 h, then incubated with LPS (1 000 ng·mL-1) for 24 h. Annexin V-FITC/PI kits and CCK8 kits detected cell apoptosis rate (A) and viability (B), respectively. qRT-PCR detected the level of caspase-3 (C) and CHOP (D) mRNA. Western blot detected the protein expression of caspase-3 and CHOP (E). n = 3, x±s. *P < 0.05 |
与对照组相比, LPS上调RAW264.7细胞miR-21表达; 与LPS组相比, GEN下调LPS活化的RAW264.7细胞miR-21表达(P < 0.05, 图 3)。
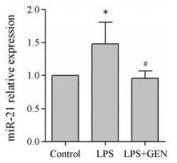
|
Figure 3 The effect of GEN on the level of miR-21 in LPS- activated RAW264.7 cells. RAW264.7 cells were pretreated with GEN (10 µmol·L-1) for 2 h, then incubated with LPS (1 000 ng·mL-1) for 24 h. qRT-PCR was used for the level of miR-21. n = 3, x± s. *P < 0.05 vs control group; #P < 0.05 vs LPS group |
将miR-21 up-NC和miR-21 up慢病毒颗粒分别感染至RAW264.7细胞, 培养72 h后, 荧光显微镜观察GFP表达的细胞比例达85%以上; 与miR-21 up-NC组相比, miR-21 up组miR-21表达上调约2.5倍, 可用于后续实验(图 4A、B)。与miR-21 up-NC组相比, miR-21 up抑制LPS活化的RAW264.7细胞CHOP、caspase-3 mRNA (P < 0.05)和蛋白表达(图 4C~E)。
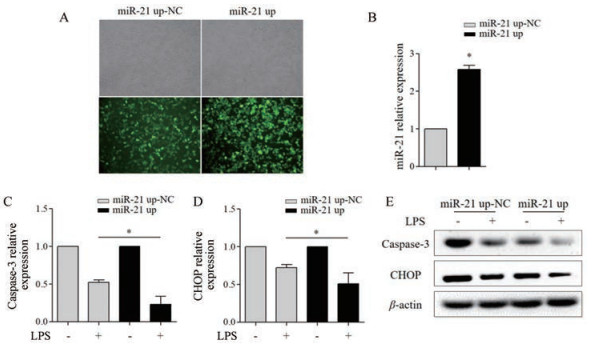
|
Figure 4 The effect of miR-21 up on the apoptosis of LPS (1 000 ng·mL-1)-activated RAW264.7 cells. A: RAW264.7 cells infected with miR-21 up-NC (multiplicity of infection, MOI = 50) and miR-21 up (MOI = 50) lentivirus with GFP were captured under phase contrast and fluorescence microscopy (×200); B: qRT-PCR detected the level of miR-21; qRT-PCR detected the level of caspase-3 (C) and CHOP (D) mRNA; E: Western blot detected the protein expression of caspase-3 and CHOP. n = 3, x± s. *P < 0.05 |
将miR-21 down-NC和miR-21 down慢病毒颗粒分别感染至RAW264.7细胞, 培养72 h后, 荧光显微镜观察GFP表达的细胞比例达85%以上; 与miR-21 down-NC组相比, miR-21 down组miR-21表达明显下调约70% (P < 0.05), 可用于后续实验(图 5A、B)。与miR-21 down-NC组相比, miR-21 down促进LPS活化的RAW264.7细胞CHOP、caspase-3 mRNA (P < 0.05)和蛋白表达(图 5C~E)。
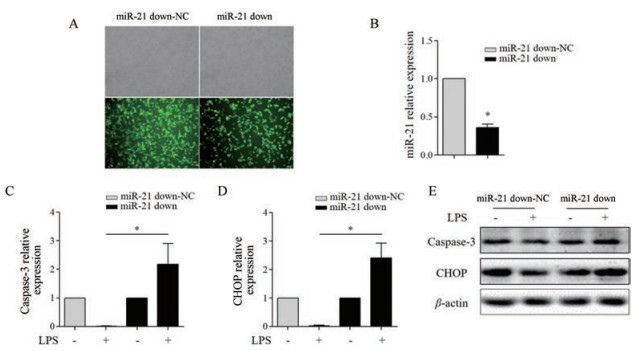
|
Figure 5 The effect of miR-21 down on the apoptosis of LPS (1 000 ng·mL-1)-activated RAW264.7 cells. A: RAW264.7 cells infected with miR-21 down-NC (MOI = 50) and miR-21 down (MOI = 50) lentivirus with GFP were captured under phase contrast and fluorescence microscopy (×200); B: qRT-PCR detected the level of miR-21; qRT-PCR detected the level of caspase-3 (C) and CHOP (D) mRNA; E: Western blot detected the protein expression of caspase-3 and CHOP. n = 3, x± s. *P < 0.05 |
与miR-21 up-NC+LPS+GEN组相比, miR-21 up+LPS+GEN组RAW264.7细胞CHOP、caspase-3 mRNA (P < 0.05)和蛋白表达均下调(图 6)。
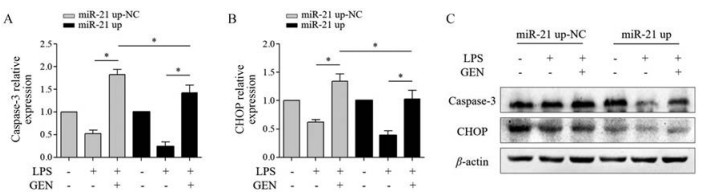
|
Figure 6 The effect of GEN (10 µmol·L-1) on the apoptosis of LPS (1 000 ng·mL-1)-activated RAW264.7 cells infected with miR-21 up (MOI = 50). qRT-PCR detected the level of caspase-3 (A) and CHOP (B) mRNA; C: Western blot detected the protein expression of caspase-3 and CHOP. n = 3, x± s. *P < 0.05 |
与miR-21 down-NC+LPS+GEN组相比, miR-21 down+LPS+GEN组RAW264.7细胞CHOP、caspase-3 mRNA (P < 0.05)和蛋白表达均上调(图 7)。
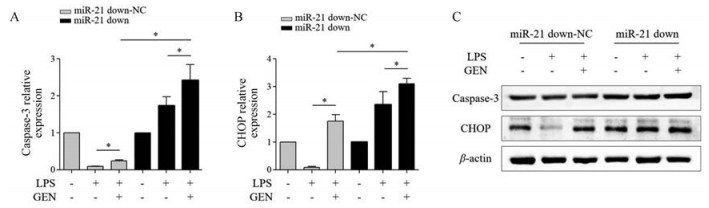
|
Figure 7 The effect of GEN (10 µmol·L-1) on the apoptosis of LPS (1 000 ng·mL-1)-activated RAW264.7 cells infected with miR-21 down (MOI = 50). qRT-PCR detected the level of caspase-3 (A) and CHOP (B) mRNA; C: Western blot detected the protein expression of caspase-3 and CHOP. n = 3, x± s. *P < 0.05 |
血管慢性炎症是促进AS发展的因素之一, 巨噬细胞作为主要的免疫细胞, 全程参与此过程[8]。LPS是革兰阴性细菌细胞壁的主要成分之一, 能够活化巨噬细胞, 引发损伤性炎症反应, 激活先天性免疫[9]。本研究结果显示, LPS能够刺激小鼠巨噬细胞RAW264.7产生大量炎症细胞因子和诱导型酶, 如TNF-α、IL-6、COX-2和iNOS, 同时也能够促进巨噬细胞增殖, 抑制凋亡相关蛋白CHOP和caspase-3的表达, 与Cheng和De Santis等[10, 11]的研究结果一致。以上结果表明, LPS可能通过抑制巨噬细胞凋亡, 促进慢性炎症反应。另有文献[12-14]报道, 促进活化巨噬细胞凋亡不仅可以减少炎症细胞因子, 如IL-6、单核细胞趋化蛋白-1 (monocyte chemotactic protein-1, MCP-1)等的释放, 避免炎症反应级联放大效应, 还可以减少巨噬细胞源性泡沫细胞的形成, 抑制AS早期斑块发生, 有效改善AS。
金雀异黄素富含于豆制食品中, 既能通过激活雌激素受体发挥抗氧化作用, 又能抑制酪氨酸激酶家族发挥抗细胞增殖作用, 有效抵抗低密度脂蛋白氧化和血管炎症, 发挥抗AS的作用[15]。CHOP是抗凋亡向促凋亡转换的重要信号分子, 正常情况下主要存在于细胞浆中且表达量很低, 但在应激状态下其表达明显增加, 进而导致细胞周期停滞, 细胞最终死亡[16]。Caspase是细胞凋亡的核心成分, 位于执行性凋亡蛋白的最下游, 在细胞凋亡过程中必不可少[17]。本研究结果显示, GEN能够抑制LPS活化的巨噬细胞活力, 上调CHOP和caspase-3表达, 诱导活化巨噬细胞凋亡, 同时下调miR-21表达。这些结果提示, GEN可能下调miR-21表达后, 通过内质网应激途径促进LPS活化的巨噬细胞凋亡, 发挥抗炎作用。
miR-21高表达于心血管疾病, 在心血管系统中的作用非常复杂, 其通过作用于同源性磷酸酶-张力蛋白(phosphatase and tensin homolog, PTEN)基因, 抑制心肌干细胞凋亡[18], 也可以通过丝裂原活化蛋白激酶激酶3抑制巨噬细胞凋亡[1]。病理状态下, miR-21作为巨噬细胞中表达量最丰富的miRNAs之一, 参与细胞增殖、迁移、凋亡及炎症[19, 20]。本研究结果显示, LPS能够上调RAW264.7细胞miR-21表达, 与既往研究一致[21]。Canfránduque等[1]研究显示, 敲除miR-21能够有效促进巨噬细胞凋亡。本课题组使用慢病毒介导的miR-21构建稳定表达miR-21 up和miR-21 down的RAW264.7细胞株发现, 当miR-21表达上调时, 明显抑制LPS活化的巨噬细胞促凋亡蛋白的表达, 而miR-21 down结果与其相反, 这表明miR-21能够通过内质网应激途径抑制活化巨噬细胞的凋亡。此外, 还发现GEN与miR-21 down具有协同作用, 能够促进LPS活化的巨噬细胞凋亡。
综上推断, GEN可能下调miR-21表达, 通过激活内质网应激反应性途径, 促进LPS活化的巨噬细胞凋亡, 进而发挥抗AS的作用。但线粒体凋亡途径和死亡配体介导的外源性凋亡途径是否参与其中, 尚未得知。此外, Canfránduque和Feng等[1, 21]研究显示, miR-21可以调节巨噬细胞内脂质蓄积及细胞膜上CD36的表达, 但并无文献报道GEN对miR-21这些作用的干预研究。在后续实验中, 课题组将继续研究GEN通过调控miR-21对巨噬细胞凋亡、泡沫化及炎症反应的影响, 以进一步阐明GEN抗AS的作用机制, 为临床药物研究提供实验依据。
| [1] | Canfránduque A, Rotllan N, Zhang X, et al. Macrophage deficiency of miR-21 promotes apoptosis, plaque necrosis, and vascular inflammation during atherogenesis[J]. EMBO Mol Med, 2017, 9: 1244–1262. DOI:10.15252/emmm.201607492 |
| [2] | Laffont B, Rayner KJ. MicroRNAs in the pathobiology of atherosclerosis[J]. Can J Cardiol, 2017, 33: 313–324. DOI:10.1016/j.cjca.2017.01.001 |
| [3] | Schultze JL, Schmieder A, Goerdt S. Macrophage activation in human diseases[J]. Semin Immunol, 2015, 27: 249–256. DOI:10.1016/j.smim.2015.07.003 |
| [4] | Averill MM, Bennett BJ, Rattazzi M, et al. Neither antioxidants nor genistein inhibit the progression of established atherosclerotic lesions in older ApoE deficient mice[J]. Atherosclerosis, 2009, 203: 82–88. DOI:10.1016/j.atherosclerosis.2008.06.017 |
| [5] | Zhang Y, Li L, You J, et al. Effect of 7-difluoromethyl-5, 4'-dimethoxygenistein on aorta atherosclerosis in hyperlipidemia ApoE-/- mice induced by a cholesterol-rich diet[J]. Drug Des Devel Ther, 2013, 7: 233–242. |
| [6] | Cong L, Yang S, Zhang Y, et al. DFMG attenuates the activation of macrophages induced by coculture with LPS-injured HUVE-12 cells via the TLR4/MyD88/NF-κB signaling pathway[J]. Int J Mol Med, 2018, 41: 2619–2628. |
| [7] | Zhao H, Li C, Cao JG, et al. 7-Difluoromethyl-5, 4'-dimethoxygenistein, a novel genistein derivative, has therapeutic effects on atherosclerosis in a rabbit model[J]. J Cardiovasc Pharmacol, 2009, 54: 412–420. DOI:10.1097/FJC.0b013e3181bad280 |
| [8] | Koelwyn GJ, Corr EM, Erbay E, et al. Regulation of macrophage immunometabolism in atherosclerosis[J]. Nat Immunol, 2018, 19: 526–537. |
| [9] | Yuan Q, Zhang D, Liu C, et al. Chikusetsusaponin V inhibits LPS-activated inflammatory responses via SIRT1/NF-κB signaling pathway in RAW264.7 cells[J]. Inflammation, 2018. DOI:10.1007/s1075 |
| [10] | Cheng Y, Yang C, Luo D, et al. N-Propargyl caffeamide skews macrophages towards a resolving M2-like phenotype against myocardial ischemic injury via activating Nrf2/HO-1 pathway and inhibiting NF-κB pathway[J]. Cell Physiol Biochem, 2018, 47: 2544–2557. DOI:10.1159/000491651 |
| [11] | De Santis R, Liepelt A, Mossanen JC, et al. miR-155 targets caspase-3 mRNA in activated macrophages[J]. RNA Biol, 2016, 13: 43–58. DOI:10.1080/15476286.2015.1109768 |
| [12] | Erbilgin A, Seldin MM, Wu X, et al. Transcription factor Zhx2 deficiency reduces atherosclerosis and promotes macrophage apoptosis in mice[J]. Arterioscler Thromb Vasc Biol, 2018, 38: 2016–2027. DOI:10.1161/ATVBAHA.118.311266 |
| [13] | Babaev VR, Yeung M, Erbay E, et al. Jnk1 deficiency in hematopoietic cells suppresses macrophage apoptosis and increases atherosclerosis in low-density lipoprotein receptor null mice[J]. Arterioscler Thromb Vasc Biol, 2016, 36: 1122–1131. DOI:10.1161/ATVBAHA.116.307580 |
| [14] | Sun X, Guo S, Wang W, et al. Potential involvement of the 18 kDa translocator protein and reactive oxygen species in apoptosis of THP-1 macrophages induced by sonodynamic therapy[J]. PLoS One, 2018, 13: e0196541. DOI:10.1371/journal.pone.0196541 |
| [15] | Zhang H, Zhao Z, Pang X, et al. miR-34a/sirtuin-1/foxo3a is involved in genistein protecting against ox-LDL-induced oxidative damage in HUVECs[J]. Toxicol Lett, 2017, 277: 115–122. DOI:10.1016/j.toxlet.2017.07.216 |
| [16] | Li Y, Guo Y, Tang J, et al. New insights into the roles of CHOP-induced apoptosis in ER stress[J]. Acta Biochim Biophys Sin, 2015, 47: 146–147. DOI:10.1093/abbs/gmu128 |
| [17] | Choudhary GS, Al-Harbi S, Almasan A. Caspase-3 activation is a critical determinant of genotoxic stress-induced apoptosis[J]. Methods Mol Biol, 2015, 1219: 1–9. DOI:10.1007/978-1-4939-1661-0 |
| [18] | Deng W, Wang Y, Long X, et al. miR-21 reduces hydrogen peroxide-induced apoptosis in c-kit+ cardiac stem cells in vitro through PTEN/PI3K/Akt signaling[J]. Oxid Med Cell Longev, 2016, 2016: 5389181. |
| [19] | Sun Q, Miao J, Luo J, et al. The feedback loop between miR-21, PDCD4 and AP-1 functions as a driving force for renal fibrogenesis[J]. J Cell Sci, 2018. DOI:10.1242/jcs.202317 |
| [20] | McClure C, McPeak MB, Youssef D, et al. Stat3 and C/EBPβ synergize to induce miR-21 and miR-181b expression during sepsis[J]. Immunol Cell Biol, 2017, 95: 42–55. DOI:10.1038/icb.2016.63 |
| [21] | Feng J, Li A, Deng J, et al. miR-21 attenuates lipopolysaccharide-induced lipid accumulation and inflammatory response:potential role in cerebrovascular disease[J]. Lipids Health Dis, 2014, 13: 27. DOI:10.1186/1476-511X-13-27 |
 2019, Vol. 54
2019, Vol. 54


