Tribbles (TRB)蛋白是在果蝇体内发现的一类蛋白激酶样基因家族, TRB由于缺少激酶激活区域及ATP的结合位点被称为假性激酶[1, 2]。人类TRB家族包括TRB1、TRB2和TRB3, 其中TRB3 (也称为TRIB3、NIPK或SKIP3)定位于人类染色体20p13~p12.2, 长约17 kb, 包含4个外显子和3个内含子, 编码358个氨基酸大小的蛋白产物[3-5]。TRB3基因广泛存在于多种生物的不同器官组织细胞中, 并且表达水平存在明显差异。蛋白质序列分析表明TRB3具有3个功能序列, 即中心激酶样结构域以及具有不同功能的N-末端和C-末端蛋白结合结构域(图 1)。TRB的C端包含丝裂原活化蛋白激酶(mitogen-activated protein kinase, MAPK) (又称为MEK1域)结合位点和组成型光形态建成蛋白-1 (constitutive photomorphogenesis protein 1, COP1)结合位点[6-8]。近年来的研究表明, TRB3具有广泛的生物学功能, 并且TRB3作为关键的“压力调节开关”, 将体内平衡、代谢性疾病和癌症等联系起来, 并作为很多疾病的生物标志物和潜在的治疗靶点。本文就近年来有关TRB3的生物学功能研究做一概述。

|
Figure 1 Functional domains of tribbles homologous protein 3 (TRB3) |
2002年Bowers等[9]首次发现TRB3在人类肿瘤中过表达并且与细胞功能障碍直接相关。随后的研究发现TRB3在多种肿瘤组织中, 如甲状腺癌、肺癌、肝癌、乳癌、卵巢癌、结肠癌、胰腺癌和食管癌中表达较高, 而在子宫内膜癌和卵巢癌中表达相对较低[10-16]。但总体上, 肿瘤组织TRB3的表达都明显高于相应的正常组织。高表达TRB3的患者易于复发原肿瘤疾病, 并且表现出比低表达TRB3患者更差的总体存活率[11]。肿瘤血管化不良所导致的缺氧应激会增加TRB3基因的表达[9, 13]。缺氧介导的血管生成增加对于肿瘤生长是必不可少的, 而且缺氧通过诱导eIF2a的磷酸化影响对肿瘤的治疗[17, 18]。研究发现高表达TRB3蛋白的肿瘤细胞对缺氧敏感, 而低表达TRB3蛋白的肿瘤更耐缺氧从而改善癌细胞的缺氧存活[13, 19, 20]。因此, 通过调控TRB3的表达增加肿瘤细胞对低氧微环境的敏感性可能是TRB3定向抗肿瘤治疗中非常可行的方法。
肿瘤细胞受到来自周围微环境的连续旁分泌信号的影响, PI3K/AKT/mTOR级联反应是致癌作用的关键驱动因子[21]。研究表明过表达TRB3可以有效抑制AKT/mTOR活性从而抑制肿瘤的发生(图 2), 而缺失TRB3后通过mTORC2复合物对AKT磷酸化的失调, 增强AKT以及转录因子FOXO3的磷酸化, 最终促进肿瘤的发生[22, 23]。已进入临床试验的抗肿瘤药物ABTL0812通过上调TRB3的表达抑制AKT/ mTORC1通路发挥抑制肿瘤的作用(图 2)[24]。除了PI3K/AKT/mTOR通路外, TRB3还调节重要的癌症信号传导途径, 例如ERK、TGF-β和JAG1/Notch信号通路。TRB3在非小细胞肺癌(non-small cell lung cancer, NSCLC)中的表达上调, 与患者的肿瘤转移、疾病复发和生存率低有关[25]。在侵袭性癌细胞中JAG1表达和Notch活化均导致肿瘤治疗的预后不良, 研究显示在乳腺癌和肺癌细胞系中TRB3通过ERK和TGF-β通路下调Notch 1的表达(图 2), 可以显著抑制肿瘤细胞体外侵袭和细胞增殖, 以及体内转移和肿瘤生长, 提示TRB3表达可能对肺癌和乳腺癌的预后和治疗有潜在的意义[26, 27]。另外, 在肿瘤细胞特别是侵袭性癌细胞中同时靶向自噬和NF-κB途径使TRB3能够稳健快速增加, 从而起到抗肿瘤的作用[28-31]。具有自噬诱导作用的HIV蛋白酶抑制剂奈非那韦(nelfinavir)和NF-κB抑制剂姜黄素共同作用上调前列腺癌(CRPC)细胞中TRB3表达, 诱导肿瘤细胞凋亡从而发挥抗肿瘤的作用[32]。除了抑制肿瘤外, 有研究报道TRB3同样具有促进肿瘤的作用, 如TRB3的激酶样结构域(kinase-like domain, KD)通过与Smad3的MH2域相互作用促进了Smad3的核定位以及Smad3介导的转录活性的调控, 促进肿瘤细胞的迁移和入侵[33]。这可能是因为在不同的研究体系中, TRB3对肿瘤调控起着不同的作用。急性早幼粒细胞白血病(APL)由癌蛋白PML-RARα驱动, 其诱导分化阻滞和转录抑制, 并促进APL起始细胞自我更新。研究表明TRB3表达升高后通过与PML-RARα相互作用, 抑制PML的SUMO化和泛素化降解, 从而促进APL进展和治疗抵抗[34]。总之, TRB3与肿瘤的发生和发展密切相关, 可能成为抗肿瘤治疗和药物研发的潜在靶点。
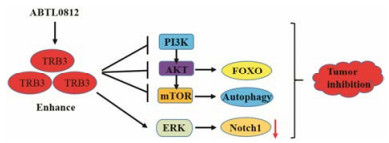
|
Figure 2 TRB3 inhibits tumor signaling pathways |
胰岛素抵抗是2型糖尿病发展的主要标志, 其特点是胰岛素不能促进肌肉中葡萄糖摄取和抑制肝脏中的葡萄糖生成。AKT作为丝氨酸/苏氨酸蛋白激酶是胰岛素信号通路上的重要分子, 其正常生理功能需要Thr308和Ser473位点的磷酸化来启动下游相关底物蛋白级联反应。研究发现, TRB3通过直接结合AKT的PH区域, 抑制其Thr308和Ser473位点的磷酸化从而干扰胰岛素信号转导通路, 导致胰岛素抵抗的发生。敲除TRB3可以增加AKT磷酸化, 降低胰岛素抵抗[35, 36]。叉头转录因子(forkheadboxO1, FoxO1)作为AKT的下游基因, 参与胰岛素调控的糖原合成和脂质代谢。研究发现, 外源表达FoxO1可以下调TRB3基因的mRNA和蛋白表达水平, 间接提高AKT磷酸化活性, 促进糖原合成和脂质沉积, 提高小鼠肝细胞对胰岛素的敏感性[37]。长期过量的摄入乙醇是引发2型糖尿病的重要危险因素, 研究发现长期用高浓度乙醇饲养大鼠显著上调肝脏中TRB3的表达, 下调AKT的活性。乙醇通过诱导TRB3表达, 还可以抑制糖原合成酶激酶-3β (glycogen synthase kinase-3 beta, GSK3β)和核型固醇调节元件结合蛋白-1 (nuclear sterol regulatory element binding protein 1, nSREBP-1)活性, 阻断胰岛素信号通路(图 3)[36]。研究发现, HEK293细胞在葡萄糖缺乏条件下, 胰岛素样生长因子结合蛋白2 (insulin-like growth factor binding proteins-2, IGFBP2)的表达减少会加重葡萄糖不足时的细胞死亡。上调TRB3的表达可促进IGFBP2表达从而增强细胞活力, 沉默IGFBP2则会消除TRB3的促生存效应[38]。总之, TRB3与胰岛素抵抗存在着密切的关系, 有可能成为糖尿病治疗的潜在靶点。
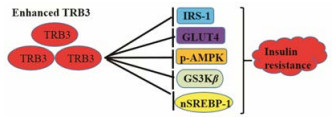
|
Figure 3 TRB3 regulates insulin resistance |
肥胖与胰岛素抵抗相关, 在胰岛素抵抗脂肪细胞中, 胰岛素受体底物-1 (insulin receptor substrate 1, IRS-1)和葡萄糖转运蛋白4 (glucose transporter isoform 4, GLUT4)以及磷酸化AMP激活蛋白激酶(p-AMPK)的蛋白水平降低, 葡萄糖摄取显著减少, 而TRB3蛋白水平和甘油三酯水平显著增加。敲低TRB3减轻脂肪细胞的胰岛素抵抗, 且脂肪细胞中IRS-1、GLUT4和p-AMPK的蛋白质水平增加(图 3)。另外, 在糖尿病大鼠的附睾和棕色脂肪组织中, 敲除TRB3会增加p-AMPK水平, 降低甘油三酯, 同时增加糖原含量[39]。研究发现, 高脂饮食增加小鼠肌肉中的TRB3表达水平, TRB3通过阻止GLUT4转位抑制胰岛素介导的葡萄糖摄取, 增加胰岛素抵抗[40]。利用肌肉特异性TRB3过表达和敲除小鼠研究发现, 与野生型小鼠相比, TRB3过表达小鼠在体内表现出体重增加和更严重的胰岛素抵抗, 同时炎症和氧化应激增加, 脂质代谢基因表达上调。TRB3敲除小鼠则降低了葡萄糖诱导的胰岛素抵抗、糖氧化损伤[41]。血浆中高浓度的游离脂肪酸也可以诱导TRB3表达, 降低乙酰辅酶A羧化酶的活性, 最终促进游离脂肪酸的氧化活性。TRB3通过抑制转录因子CCAAT增强子结合蛋白(ccaat-enhancer-binding proteins, C/EBP)和过氧化物酶体增殖物激活受体(peroxisome proliferator activated receptors, PPARs)参与血糖代谢, 延缓胰岛素介导的脂肪细胞分化[42]。在糖尿病大鼠模型中敲除TRB3, 可上调PPARγ的活性, 通过IRS-1/ PI3K通路调节胰岛β细胞分泌胰岛素和胰岛素的敏感性[43]。另外, PPARγ辅助激活因子-1 (PPARγ coactivator-1, PGC-1)通过调节TRB3诱导肝细胞对胰岛素敏感性的降低[44]。脂质代谢紊乱是导致胰岛素抵抗的重要诱因, db/db肥胖小鼠肝脏中TRB3的mRNA和蛋白水平都显著高于正常的野生型小鼠, 高表达TRB3的转基因小鼠的脂肪组织含量明显减少, 即使是高脂饮食也不会引起肥胖, 进一步研究显示TRB3通过募集COP1蛋白促进乙酰辅A羧化酶的降解, 下调其活性, 增加脂质的氧化分解, 减少脂质的合成[45]。TRB3反义寡核苷酸处理会特异性降低小鼠肝脏和白色脂肪组织中TRB3表达, 提高PPARγ及其关键靶基因的表达, 使白色脂肪组织质量增加70%[43]。因此, TRB3对脂质代谢的调控可能是其影响胰岛素功能的另一条途径。
高同型半胱氨酸血症(HHcy)与胰岛素抵抗有关。在哺乳动物中, 血浆氨基酸浓度受饮食或病理状况的影响, 研究显示TRB3表达水平在喂食亮氨酸缺乏饮食小鼠的肝脏中显著上调[46]。在HHcy的小鼠中, 同型半胱氨酸诱导肝脏cAMP和cAMP反应元件结合蛋白磷酸化水平的增加, 从而上调PPAR-1和TRB3的表达, 同时伴随GSK3和AKT的去磷酸化[47]。因此, 同型半胱氨酸通过调节TRB3的表达来调控胰岛素抵抗。
TRB3存在基因多态性现象, 在人群中已证实有5种错义突变(Q84R、S146N、R153H、L178P、R181C)和2种同义突变基因型(Y111Y、A323A)。Prudente等[48]研究发现只有TRB3 Q84R位点的基因多态性在意大利的高加索人群中的异常表达明显降低AKT的磷酸化水平。TRB3 Q84R基因多态性将增加TRB3蛋白的表达并增强对AKT的抑制作用, 调节胰岛素信号通路。同时, TRB3 Q84R基因多态性会下调血管内皮细胞中胰岛素介导的NO水平, 并导致心脏血管的舒缩障碍[49, 50]。
3 TRB3与细胞应激反应及凋亡和自噬诱发内质网应激的因素包括营养物质缺乏、缺氧、氧化剂、病毒感染等。活化转录因子4 (activating transcription factor 4, ATF4)和C/EBP同源蛋白(CHOP)在哺乳动物细胞中广泛表达, 是内质网应激反应的重要标志蛋白。研究发现, ATF4和CHOP可以与TRB3启动子中特定的位点结合, 激活TRB3基因转录, 调节内质网应激相关的细胞凋亡等(图 4)[51-54]。另外, 在一些弱应激刺激条件下如营养物质缺乏或低糖条件下, TRB3表达的上调可以减少细胞死亡, 起到保护细胞的作用。有学者认为TRB3在强烈持续应激条件下, 主要表现为促凋亡作用, 而在弱及短暂刺激情况下, 其表达上调可对ATF4和CHOP起负反馈调节, 一定程度上限制应激反应进一步恶化。
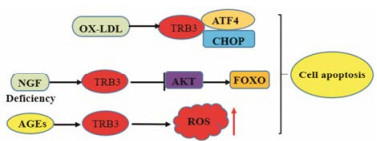
|
Figure 4 TRB3 regulates cell apoptosis |
TRB3最早发现与细胞凋亡相关是在神经元凋亡研究中, 研究观察到神经生长因子诱导神经元凋亡的过程中TRB3表达升高, TRB3过表达促进神经元死亡, 而敲除TRB3可以保护神经元[55, 56]。研究显示神经生长因子(nerve growth factor, NGF)缺失导致TRB3上调, 进一步抑制AKT活化和AKT底物FoxO去磷酸化的调节, FoxO被去磷酸化后发生核移位并激活促凋亡基因过表达, 最终引起神经元死亡(图 4)[57]。研究发现在高糖诱导胰岛β细胞系INS-1细胞和人内皮细胞发生凋亡时细胞TRB3表达水平升高, 而敲低TRB3表达可减少细胞的凋亡, 并且TRB3通过NF-κB介导内质网应激(endoplasmic reticulum stress, ER stress)诱导的细胞凋亡[1, 58, 59]。研究发现, 晚期糖基化终产物(AGEs)可能是导致胰腺β细胞衰竭因素之一。在INS-1细胞中, AGEs通过上调TRB3表达诱导INS-1细胞凋亡。AGEs还增加了胰腺β细胞中的ROS水平, 在敲低TRB3后, AGE诱导的细胞凋亡和细胞内ROS水平显著降低, 表明TRB3介导AGE诱导的细胞凋亡(图 4)[60]。Humphrey等[61, 62]研究发现, 混合谱系激酶-3 (mixed lineage kinases-3, MLK3)通过与TRB3结合可以稳定TRB3, TRB3抑制AKT从而介导细胞因子诱导的胰岛β细胞凋亡, 敲除了TRB3之后可以抑制细胞因子诱导的胰岛β细胞凋亡。
在人巨噬细胞中, 氧化低密度脂蛋白(OX-LDL)以剂量和时间依赖性方式上调ATF4和CHOP的表达, 从而诱导TRB3表达。TRB3表达上调促进巨噬细胞的脂质积累, 并且抑制细胞因子的表达(图 4)。另外, 上调TRB3的巨噬细胞的细胞存活率降低, 细胞凋亡增加。敲低TRB3可以抵抗OX-LDL诱导的巨噬细胞凋亡[63-65]。
自噬与凋亡在调控细胞存活中发挥重要作用, 二者相互转化, 互相影响。TRB3不仅与细胞凋亡关系密切, 同样参与细胞自噬的调控。TRB3与自噬“货车蛋白”P62存在相互作用, 相互作用的位点位于P62的LC3相互作用结构域(LC3-interacting region, LTR)和泛素结合结构域(ubiquitin-associate domain, UBA)。TRB3通过与LTR及UBA的相互作用阻碍P62与LC3以及与泛素化蛋白间的结合, 增加P62蛋白稳定性, 促进P62堆积, 抑制P62功能, 进而抑制自噬[66]。TRB3在正常人支气管上皮细胞中的过表达抑制LC3-Ⅰ/-Ⅱ转化并增加可溶性和不溶性SQSTM1的水平。相反, 沉默TRB3降低了人肝癌细胞系HepG2细胞中可溶性和不溶性SQSTM1的基础和IGF1诱导的积累。增强的TRB3诱导自噬通量的阻断, 导致自噬-溶酶体依赖性降解途径的减弱。淀粉样蛋白Aβ处理后神经元中TRB3表达上调, TRB3通过阻止unc-51样自噬激活激酶1 (unc-51 like autophagy activating kinase 1, Ulk1)的磷酸化间接激活Ulk1。Ulk1激活后增强自噬体形成和减少自噬通量。因为自噬通量被阻止, 自噬体积累在神经元中, 因此, TRB3是形成自噬体所必需的。沉默内源性TRB3可以保护神经元细胞免受淀粉样蛋白Aβ的损害[67]。总之, TRB3与细胞应激反应及细胞凋亡和自噬密切相关, 通过调控TRB3来调节细胞凋亡和自噬等生理过程, 对于与之相关疾病的调控起到重要作用。
4 TRB3与炎症炎症是机体对损伤因子所发生的防御反应, 炎症的发生伴随着大量炎症因子的产生, 从而引发细胞严重的应激反应, 甚至细胞死亡。TRB3对于炎症因子的反应在不同种类的细胞中各不相同, 研究发现IL-1b、IL-3和IL-6增加细胞中TRB3表达, 而IL-1刺激动脉平滑肌细胞和成纤维细胞时TRB3 mRNA却无显著变化, 抗炎剂地塞米松则通过转录因子CREB和FOXO-1降低TRB3表达[68-70]。另一方面, 研究表明TRB3可能作为促炎细胞因子和趋化因子的负调控因子, 控制炎症反应的程度。在RBL-CCR1细胞中沉默TRB3显著诱导肿瘤坏死因子TNF-α、IL-4、IL-6和趋化因子的表达[71]。单核细胞趋化蛋白-1 (monocyte chemotactic protein 1, MCP1)有利于肾病相关的炎性损伤, 研究表明TRB3可以抑制MCP1的表达, 从而减轻肾病相关的炎性损伤[72]。TRB3在糖尿病肾病中表达上调, 相对于野生型小鼠, TRB3敲除小鼠的肾皮质中产生更高水平的白蛋白尿并且增加炎性细胞因子和趋化因子mRNA的表达, 肾小管细胞中研究结果显示TRB3可以抑制IL-6的分泌[73]。总之, TRB3与炎症因子互相调节, 在疾病的发展过程中发挥重要的作用, 通过TRB3的调控达到减轻炎症作用, 对于很多疾病的治疗能起到很好的辅助作用。
5 TRB3与纤维化心肌间质纤维化是指心脏在负荷增加或损伤等因素发生时的形态学变化, 是导致心脏衰竭的主要机制之一。心肌间质纤维化发生时心肌细胞间质中胶原浓度显著升高, 其中轻度心肌间质纤维化发生时以Ⅲ型胶原纤维增加为主, 重度心肌间质纤维化发生时以Ⅰ型胶原纤维增加为主。TRB3在胰岛素抵抗状态的心脏纤维化进展中起重要作用。促分裂原活化蛋白激酶(MAPK)是调控心肌间质纤维化的关键分子, 增加TRB3表达可显著增强ERK MAPK和JNK MAPK活性, 从而调节心肌间质的纤维化水平。在Ⅱ型糖尿病大鼠的模型中, 沉默TRB3可通过调节MAPK和AKT通路, 降低心肌纤维化和减少Ⅰ/Ⅲ胶原的比例, 延缓糖尿病心肌病的发生和进展[74]。
肾纤维化被认为是慢性肾病的大多数病例的常见途径。高脂饮食大鼠表现出胰岛素抵抗和TRB3过表达。上调TRB3表达可以诱导肾纤维化并伴随ERK MAPK的磷酸化增加。敲低TRB3可以改善肾纤维化, 伴随着ERK MAPK磷酸化水平降低[75]。糖尿病大鼠肾小管特别是近端小管TRB3表达上调, 蛋白超载可以增加TRB3的表达并诱导了细胞凋亡, 增加NRK-52E细胞中Ⅰ型胶原和纤连蛋白的分泌。沉默TRB3可以减轻糖尿病大鼠肾脏和白蛋白超载引起的NRK-52E细胞中Ⅰ型胶原和纤连蛋白的积累, 减轻细胞凋亡[76, 77]。
6 TRB3与细胞生长调控在果蝇的胚胎发育过程中, TRB通过促进蛋白酶体介导的Cdc25/String降解, 负调控细胞分裂, 使细胞生长周期发生阻滞[78]。研究发现TRB3与羧基末端结合蛋白(C-terminal-binding protein, CtBP)的相互作用蛋白(CtBP-interacting protein, CtIP)存在相互作用, 并且相互作用区域为两种蛋白质的C末端。TRB3和CtIP共定位于HeLa细胞的细胞核, 并呈现出独特的点状图案。由于CtIP在细胞周期检查点控制中起重要作用, 并且它与肿瘤发生有关, 表明TRB3可能通过与CtIP相互作用参与这些生物过程[79]。研究发现同型半胱氨酸治疗人脐静脉内皮细胞后通过环磷腺苷效应元件结合蛋白(cAMP-response element binding protein, CREB)上调TRB3的表达, 诱导G1期细胞周期阻滞[80]。巨核细胞生成是由激素促血小板生成素驱动的复杂分化过程, TRB3参与巨核细胞生成, 是巨核细胞生成的负调节剂。血小板生成素诱导的巨核细胞分化导致TRB3 mRNA的时间和剂量依赖性降低, 而TRB3的沉默促进巨核细胞分化[81]。NF-κB可以上调细胞周期调控蛋白cyclin D1的表达, 增加Rb蛋白磷酸化, 促进细胞增生。TRB3通过抑制NF-κB活化阻断细胞周期, 对细胞分化起到负调控作用。人骨髓间充质干细胞(hBMSCs)的成骨分化受多种因素的调控, 包括骨形态发生蛋白(bone morphogenetic protein, BMP)、Notch、生长激素和MAPKs。TRB3在hBMSCs成骨分化过程中被高度表达。研究显示TRB3是BMP信号通路的重要调控蛋白, 参与促进成骨细胞分化成熟, TRB3通过调节分化中期ERK MAPK活性在增殖和成骨分化中发挥重要作用, 在分化中期进行TRB3敲除会促进增殖和减少成骨分化[82]。
7 TRB3与其他疾病TRB3在哺乳动物中参与多种生物学过程, 除了以上的生物调节作用, TRB3在其他疾病中也有一定的作用[82]。TRB3在寻常型银屑病病变中升高并在体外介导HaCaT细胞增殖。TRB3在银屑病患者的病变中过度表达并可能参与角质形成细胞异常增殖。因此, TRB3可能是潜在的牛皮癣的治疗靶点[83]。在采用高脂饮食和低剂量链脲佐菌素诱导2型糖尿病大鼠模型中, 大鼠主动脉中层厚度增加, 胶原过度沉积, 弹性纤维减少, 血管顺应性减少。在糖尿病发生12周后, 沉默TRB3显著逆转病理性主动脉重构, 主动脉内侧厚度及血管周围纤维化明显降低, 减少胶原含量, 改善胶原/弹性蛋白的比例, 改善动脉依从性, 因此TRB3可能成为糖尿病血管并发症的潜在治疗靶点[84]。幽门螺杆菌引起慢性胃炎、消化性溃疡和胃癌。在幽门螺杆菌感染的人胃上皮细胞系和组织样品中, 幽门螺杆菌降低了C/EBP同源蛋白的表达并激活ATF4, 从而下调TRB3的表达。幽门螺旋杆菌对TRB3的调节可能是其诱发疾病的重要机制[85]。丙型肝炎病毒(hepatitis C virus, HCV)复制上调TRB3的mRNA和蛋白质水平, HCV诱导的ER应激增加TRB3的启动子活性。TRB3的沉默导致HCV的RNA和蛋白质水平增加, 而TRB3的过表达降低HCV复制。HCV NS3特异性地与TRB3相互作用, 并且阻断TRB3和AKT的结合, 因此, 在HCV感染的细胞中TRB3-AKT信号传导受损[86]。目前关于TRB3与不同疾病的研究尚处于起步阶段, TRB3对不同疾病的调节作用有待国内外学者的进一步研究。随着研究的不断深入, 研究方向的不断扩展, 相信TRB3会在众多不同领域的生物调节中起到重要作用。
8 结语TRB3参与肿瘤发生、胰岛素抵抗、炎症、细胞应激反应等过程, 具有广泛的细胞生物学功能。研究TRB3在不同疾病发生、发展过程中的功能, 可能有助于研究者进一步了解不同疾病发病的分子机制, 对于疾病的预防和控制, 乃至药物的研发至关重要。总之, TRB3可能成为众多疾病的治疗靶标, 对于TRB3生物学功能的研究仍旧需要不断扩展和深入。
| [1] | Salazar M, Lorente M, Orea-Soufi A, et al. Oncosuppressive functions of tribbles pseudokinase 3[J]. Biochem Soc Trans, 2015, 43: 1122–1126. DOI:10.1042/BST20150124 |
| [2] | Nicoletti Carvalho JE, Nogueira TC, Gorjao R, et al. UPR-mediated TRIB3 expression correlates with reduced AKT phosphorylation and inability of interleukin 6 to overcome palmitate-induced apoptosis in RINm5F cells[J]. J Endocrinol, 2010, 206: 183–193. DOI:10.1677/JOE-09-0356 |
| [3] | Wu M, Xu LG, Zhai Z, et al. SINK is a p65-interacting negative regulator of NF-κB-dependent transcription[J]. J Biol Chem, 2003, 278: 27072–27079. DOI:10.1074/jbc.M209814200 |
| [4] | Huang J, Teng L, Liu T, et al. Identification of a novel serine/threonine kinase that inhibits TNF-induced NF-κB activation and p53-induced transcription[J]. Biochem Biophys Res Commun, 2003, 309: 774–778. DOI:10.1016/j.bbrc.2003.08.069 |
| [5] | Kiss-Toth E, Bagstaff SM, Sung HY, et al. Human tribbles, a protein family controlling mitogen-activated protein kinase cascades[J]. J Biol Chem, 2004, 279: 42703–42708. DOI:10.1074/jbc.M407732200 |
| [6] | Salomè M, Campos J, Keeshan K. TRIB2 and the ubiquitin proteasome system in cancer[J]. Biochem Soc Trans, 2015, 43: 1089–1094. DOI:10.1042/BST20150103 |
| [7] | Xu SS, Tong MH, Huang JQ, et al. TRIB2 inhibits Wnt/β-Catenin/TCF4 signaling through its associated ubiquitin E3 ligases, β-TrCP, COP1 and Smurf1, in liver cancer cells[J]. FEBS Lett, 2014, 588: 4334–4341. DOI:10.1016/j.febslet.2014.09.042 |
| [8] | Mondal D, Mathur A, Chandra PK. Tripping on TRIB3 at the junction of health, metabolic dysfunction and cancer[J]. Biochimie, 2016, 124: 34–52. DOI:10.1016/j.biochi.2016.02.005 |
| [9] | Bowers AJ, Scully S, Boylan JF. SKIP3, a novel Drosophila tribbles ortholog, is overexpressed in human tumors and is regulated by hypoxia[J]. Oncogene, 2003, 22: 2823–2835. DOI:10.1038/sj.onc.1206367 |
| [10] | Okamoto H, Latres E, Liu R, et al. Genetic deletion of Trb3, the mammalian Drosophila tribbles homolog, displays normal hepatic insulin signaling and glucose homeostasis[J]. Diabetes, 2007, 56: 1350–1356. DOI:10.2337/db06-1448 |
| [11] | Miyoshi N, Ishii H, Mimori K, et al. Abnormal expression of TRIB3 in colorectal cancer:a novel marker for prognosis[J]. British J Cancer, 2009, 101: 1664–1670. DOI:10.1038/sj.bjc.6605361 |
| [12] | Dong ST, Xia JL, Wang HQ, et al. Overexpression of TRIB3 promotes angiogenesis in human gastric cancer[J]. Oncol Rep, 2016, 36: 2339–2348. DOI:10.3892/or.2016.5017 |
| [13] | Wennemers M, Bussink J, Grebenchtchikov N, et al. TRIB3 protein denotes a good prognosis in breast cancer patients and is associated with hypoxia sensitivity[J]. Radiother Oncol, 2011, 101: 198–202. DOI:10.1016/j.radonc.2011.05.057 |
| [14] | Liang KL, Rishi L, Keeshan K. Tribbles in acute leukemia[J]. Blood, 2013, 121: 4265–4270. DOI:10.1182/blood-2012-12-471300 |
| [15] | Li Y, Zhu DX, Hou LD, et al. TRB3 reverses chemotherapy resistance and mediates crosstalk between endoplasmic reticulum stress and AKT signaling pathways in MHCC97H human hepatocellular carcinoma cells[J]. Oncol Lett, 2018, 15: 1343–1349. |
| [16] | Ma B, Zhang H, Wang Y, et al. Corosolic acid, a natural triterpenoid, induces ER stress-dependent apoptosis in humancastration resistant prostate cancer cells via activation of IRE-1/JNK, PERK/CHOP and TRIB3[J]. J Exp Clin Cancer Res, 2018, 37: 210. DOI:10.1186/s13046-018-0889-x |
| [17] | Palsson-McDermott EM, O'Neill LA. The Warburg effect then and now:from cancer to inflammatory diseases[J]. Bioessays, 2013, 35: 965–973. DOI:10.1002/bies.v35.11 |
| [18] | Blaustein M, Pérez-Munizaga D, Sánchez MA, et al. Modulation of the AKT pathway reveals a novel link with PERK/eIF2α, which is relevant during hypoxia[J]. PLoS One, 2013, 8: e69668. DOI:10.1371/journal.pone.0069668 |
| [19] | Wennemers M, Bussink J, Scheijen B, et al. Tribbles homolog 3 denotes a poor prognosis in breast cancer and is involved in hypoxia response[J]. Breast Cancer Res, 2011, 13: R82. DOI:10.1186/bcr2934 |
| [20] | Wennemers M, Bussink J, van den Beucken T, et al. Regulation of TRIB3 mRNA and protein in breast cancer[J]. PLoS One, 2012, 7: e49439. DOI:10.1371/journal.pone.0049439 |
| [21] | Yu JJ, Hua F, Hu ZW. Advances in the studies of the link between diabetes and cancer[J]. Acta Pharm Sin (药学学报), 2016, 51: 1017–1024. |
| [22] | Salazar M, Lorente M, García-Taboada E, et al. Loss of tribbles pseudokinase-3 promotes AKT-driven tumorigenesis via FOXO inactivation[J]. Cell Death Differ, 2015, 22: 131–144. DOI:10.1038/cdd.2014.133 |
| [23] | Gu JL, Yan XQ, Dai XZ, et al. Metallothionein preserves AKT2 activity and cardiac function via inhibiting TRB3 in diabetic hearts[J]. Diabetes, 2018, 67: 507–517. DOI:10.2337/db17-0219 |
| [24] | Erazo T, Lorente M, López-Plana A, et al. The new antitumor drug ABTL0812 inhibits the AKT/mTORC1 axis by upregulating tribbles-3 pseudokinase[J]. Clin Cancer Res, 2016, 22: 2508–2519. DOI:10.1158/1078-0432.CCR-15-1808 |
| [25] | López-Ayllón BD, de Castro-Carpeño J, Rodriguez C, et al. Biomarkers of erlotinib response in non-small cell lung cancer tumors that do not harbor the more common epidermal growth factor receptor mutations[J]. Int J Clin Exp Pathol, 2015, 8: 2888–2898. |
| [26] | Zhou H, Luo Y, Chen JH, et al. Knockdown of TRB3 induces apoptosis in human lung adenocarcinoma cells through regulation of Notch 1 expression[J]. Mol Med Rep, 2013, 8: 47–52. DOI:10.3892/mmr.2013.1453 |
| [27] | Izrailit J, Berman HK, Datti A, et al. High throughput kinase inhibitor screens reveal TRB3 and MAPK-ERK/TGFβ pathways as fundamental Notch regulators in breast cancer[J]. Proc Natl Acad Sci U S A, 2013, 110: 1714–1719. DOI:10.1073/pnas.1214014110 |
| [28] | Hua F, Li K, Yu JJ, et al. The TRIB3-SQSTM1 interaction mediates metabolic stress-promoted tumorigenesis and progression via suppressing autophagic and proteasomal degradation[J]. Autophagy, 2015, 11: 1929–1931. DOI:10.1080/15548627.2015.1084458 |
| [29] | Johnson CE, Hunt DK, Wiltshire M, et al. Endoplasmic reticulum stress and cell death in mTORC1-overactive cells is induced by nelfinavir and enhanced by chloroquine[J]. Mol Oncol, 2015, 9: 675–688. DOI:10.1016/j.molonc.2014.11.005 |
| [30] | Matyszewski A, Czarnecka AM, Solarek W, et al. Molecular basis of carcinogenesis in diabetic patients (review)[J]. Int J Oncol, 2015, 46: 1435–1443. DOI:10.3892/ijo.2015.2865 |
| [31] | Fang N, Zhang WJ, Xu SQ, et al. TRIB3 alters endoplasmic reticulum stress-induced β-cell apoptosis via the NF-κB pathway[J]. Metabolism, 2014, 63: 822–830. DOI:10.1016/j.metabol.2014.03.003 |
| [32] | Mathur A, Abd Elmageed ZY, Liu X, et al. Subverting ER-stress towards apoptosis by nelfinavir and curcumin coexposure augments docetaxel efficacy in castration resistant prostate cancer cells[J]. PLoS One, 2014, 9: e103109. DOI:10.1371/journal.pone.0103109 |
| [33] | Hua F, Mu R, Liu JW, et al. TRB3 interacts with SMAD3 promoting tumor cell migration and invasion[J]. J Cell Sci, 2011, 124: 3235–3246. DOI:10.1242/jcs.082875 |
| [34] | Li K, Wang F, Cao WB, et al. TRIB3 promotes APL progression through stabilization of the oncoprotein PML-RARα and inhibition of p53-mediated senescence[J]. Cancer Cell, 2017, 31: 697–710.e7. DOI:10.1016/j.ccell.2017.04.006 |
| [35] | Du KY, Stephan H, Kulkarni RN, et al. TRB3:a tribbles homolog that inhibits AKT/PKB activation by insulin in liver[J]. Science, 2003, 300: 1574–1577. DOI:10.1126/science.1079817 |
| [36] | He L, Simmen FA, Mehendale HM, et al. Chronic ethanol intake impairs insulin signaling in rats by disrupting AKT association with the cell membrane. Role of TRB3 in inhibittion of AKT/protein kinase B activation[J]. J Biol Chem, 2006, 281: 11126–11134. DOI:10.1074/jbc.M510724200 |
| [37] | Matsumoto M, Han S, Kitamura T, et al. Dual role of transcription factor FoxO1 in controlling hepatic insulin sensitivity and lipid metabolism[J]. J Clin Invest, 2006, 116: 2464–2472. |
| [38] | Örd T, Örd D, Adler P, et al. TRIB3 enhances cell viability during glucose deprivation in HEK293-derived cells by upregulating IGFBP2, a novel nutrient deficiency survival factor[J]. Biochim Biophys Acta, 2015, 1853: 2492–2505. |
| [39] | Sun XY, Song M, Wang H, et al. TRB3 gene silencing activates AMPK in adipose tissue with beneficial metabolic effects in obese and diabetic rats[J]. Biochem Biophys Res Commun, 2017, 488: 22–28. DOI:10.1016/j.bbrc.2017.04.154 |
| [40] | Lantier L, Williams AS, Williams IM, et al. SIRT3 is crucial for maintaining skeletal muscle insulin action and protects against severe insulin resistance in high-fat-fed mice[J]. Diabetes, 2015, 64: 3081–3092. DOI:10.2337/db14-1810 |
| [41] | Zhang W, Wu MR, Kim TY, et al. Skeletal muscle TRIB3 mediates glucose toxicity in diabetes and high-fat diet-induced insulin resistance[J]. Diabetes, 2016, 65: 2380–2391. DOI:10.2337/db16-0154 |
| [42] | Jiang C, Sun J, Dai YF, et al. HIF-1A and C/EBPs transcriptionally regulate adipogenic differentiation of bone marrow-derived MSCs in hypoxia[J]. Stem Cell Res Ther, 2015, 6: 21. DOI:10.1186/s13287-015-0014-4 |
| [43] | Weismann D, Erion DM, Ignatova-Todorava I, et al. Knockdown of the gene encoding Drosophila tribbles homologue 3(Trib3) improves insulin sensitivity through peroxisome proliferator-activated receptor-γ (PPAR-γ) activation in a rat model of insulin resistance[J]. Diabetologia, 2011, 54: 935–944. DOI:10.1007/s00125-010-1984-5 |
| [44] | Koo SH, Satoh H, Herzig S, et al. PGC-1 promotes insulin resistance in liver through PPAR-alpha-dependent induction of TRB-3[J]. Nat Med, 2004, 10: 530–534. DOI:10.1038/nm1044 |
| [45] | Qi L, Heredia JE, Altarejos JY, et al. TRB3 links the E3 ubiquitin ligase COP1 to lipid metabolism[J]. Science, 2006, 312: 1763–1766. DOI:10.1126/science.1123374 |
| [46] | Carraro V, Maurin AC, Lambert-Langlais S, et al. Amino acid availability controls TRB3 transcription in liver through the GCN2/eIF2a/ATF4 pathway[J]. PLoS One, 2010, 5: e15716. DOI:10.1371/journal.pone.0015716 |
| [47] | Liu WJ, Ma LQ, Liu WH, et al. Inhibition of hepatic glycogen synthesis by hyperhomocysteinemia mediated by TRB3[J]. Am J Pathol, 2011, 178: 1489–1499. DOI:10.1016/j.ajpath.2010.12.052 |
| [48] | Prudente S, Hribal ML, Flex E, et al. The functional Q84R polymorphism of mammalian tribbles homolog TRB3 is associated with insulin resistance and related cardiovascular risk in Caucasians from Italy[J]. Diabetes, 2005, 54: 2807–2811. DOI:10.2337/diabetes.54.9.2807 |
| [49] | Andreozzi F, Formoso G, Prudente S, et al. TRIB3 R84 variant is associated with impaired insulin-mediated nitric oxide production in human endothelial cells[J]. Arterioscler Thromb Vasc Biol, 2008, 28: 1355–1360. DOI:10.1161/ATVBAHA.108.162883 |
| [50] | Formoso G, di Tomo P, Andreozzi F, et al. The TRIB3 R84 variant is associated with increased carotid intima-media thickness in vivo and with enhanced MAPK signalling in human endothelial cells[J]. Cardiovasc Res, 2011, 89: 184–192. DOI:10.1093/cvr/cvq255 |
| [51] | Ohoka N, Yoshii S, Hattori T, et al. TRB3, a novel ER stress-inducible gene, is induced via ATF4-CHOP pathway and is involved in cell death[J]. EMBO J, 2005, 24: 1243–1255. DOI:10.1038/sj.emboj.7600596 |
| [52] | Morse E, Schroth J, You YH, et al. TRB3 is stimulated in diabetic kidneys, regulated by the ER stress marker CHOP, and is a suppressor of podocyte MCP-1[J]. Am J Physiol Renal Physiol, 2010, 299: F965–F972. DOI:10.1152/ajprenal.00236.2010 |
| [53] | Bromati CR, Lellis-Santos C, Yamanaka TS, et al. UPR induces transient burst of apoptosis in islets of early lactating rats through reduced AKT phosphorylation via ATF4/CHOP stimulation of TRB3 expression[J]. Am J Physiol Regul Integr Comp Physiol, 2011, 300: R92–R100. DOI:10.1152/ajpregu.00169.2010 |
| [54] | ÖrdD, MeeritsK, ÖrdT. TRB3 protects cells against the growth inhibitory and cytotoxic effect of ATF4[J]. Exp Cell Res, 2007, 313: 3556–3567. |
| [55] | Mayumi-Matsuda K, Kojima S, Suzuki H, et al. Identification of a novel kinase-like gene induced during neuronal cell death[J]. Biochem Biophys Res Commun, 1999, 258: 260–264. DOI:10.1006/bbrc.1999.0576 |
| [56] | Aimé P, Sun XT, Zareen N, et al. Trib3 is elevated in Parkinson's disease and mediates death in Parkinson's disease models[J]. J Neurosci, 2015, 35: 10731–10749. DOI:10.1523/JNEUROSCI.0614-15.2015 |
| [57] | Zareen N, Biswas SC, Greene LA. A feed-forward loop involving Trib3, AKT and FoxO mediates death of NGF-deprived neurons[J]. Cell Death Differ, 2013, 20: 1719–1730. DOI:10.1038/cdd.2013.128 |
| [58] | Qian B, Wang H, Men X, et al. TRIB3 is implicated in glucotoxicity and endoplasmic reticulum-stress-induced beta-cell apoptosis[J]. J Endocrinol, 2008, 199: 407–416. DOI:10.1677/JOE-08-0331 |
| [59] | Guo L, Guo ZX, Gong HP, et al. Tribbles homolog 3 is induced by high glucose and associated with apoptosis in human endothelial cells[J]. Mol Med Rep, 2015, 12: 1963–1970. DOI:10.3892/mmr.2015.3576 |
| [60] | Wang M, Zhang WJ, Xu SQ, et al. TRB3 mediates advanced glycation end product-induced apoptosis of pancreatic β-cells through the protein kinase C β pathway[J]. Int J Mol Med, 2017, 40: 130–136. DOI:10.3892/ijmm.2017.2991 |
| [61] | Humphrey RK, Newcomb CJ, Yu SM, et al. Mixed lineage kinase-3 stabilizes and functionally cooperates with tribbles-3 to compromise mitochondrial integrity in cytokine-induced death of pancreatic beta cells[J]. J Biol Chem, 2010, 285: 22426–22436. DOI:10.1074/jbc.M110.123786 |
| [62] | Humphrey RK, Ray A, Gonuguntla S, et al. Loss of TRB3 alters dynamics of MLK3-JNK signaling and inhibits cytokine-activated pancreatic beta cell death[J]. J Biol Chem, 2014, 289: 29994–30004. DOI:10.1074/jbc.M114.575613 |
| [63] | Steverson D Jr, Tian L, Fu Y, et al. Tribbles homolog 3 promotes foam cell formation associated with decreased proinflammatory cytokine production in macrophages:evidence for reciprocal regulation of cholesterol uptake and inflammation[J]. Metab Syndr Relat Disord, 2016, 14: 7–15. DOI:10.1089/met.2015.0037 |
| [64] | Shang YY, Zhong M, Zhang LP, et al. Tribble 3, a novel oxidized low-density lipoprotein-inducible gene, is induced via the activating transcription factor 4-C/EBP homologous protein pathway[J]. Clin Exp Pharmacol Physiol, 2010, 37: 51–55. DOI:10.1111/cep.2009.37.issue-1 |
| [65] | Shang YY, Wang ZH, Zhang LP, et al. TRB3, upregulated by ox-LDL, mediates human monocyte-derived macrophage apoptosis[J]. FEBS J, 2009, 276: 2752–2761. DOI:10.1111/ejb.2009.276.issue-10 |
| [66] | Hua F, Li K, Yu JJ, et al. TRB3 links insulin/IGF to tumour promotion by interacting with p62 and impeding autophagic/proteasomal degradations[J]. Nat Commun, 2015, 6: 7951. DOI:10.1038/ncomms8951 |
| [67] | Saleem S, Biswas SC. Tribbles pseudokinase 3 induces both apoptosis and autophagy in amyloid-induced neuronal death[J]. J Biol Chem, 2017, 292: 2571–2585. DOI:10.1074/jbc.M116.744730 |
| [68] | Örd T, Örd D, Kuuse S, et al. Trib3 is regulated by IL-3 and affects bone marrow-derived mast cell survival and function[J]. Cell Immunol, 2012, 280: 68–75. |
| [69] | Yacoub Wasef SZ, Robinson KA, Berkaw MN, et al. Glucose, dexamethasone, and the unfolded protein response regulate TRB3 mRNA expression in 3T3-L1 adipocytes and L6 myotubes[J]. Am J Physiol Endocrinol Metab, 2006, 291: E1274–E1280. DOI:10.1152/ajpendo.00117.2006 |
| [70] | Schneider CC, Ampofo E, Montenarh M. CK2 regulates ATF4 and CHOP transcription within the cellular stress response signalling pathway[J]. Cell Signal, 2012, 24: 1797–1802. DOI:10.1016/j.cellsig.2012.05.006 |
| [71] | Kuo CH, Morohoshi K, Aye CC, et al. The role of TRB3 in mast cells sensitized with monomeric IgE[J]. Exp Mol Pathol, 2012, 93: 408–415. DOI:10.1016/j.yexmp.2012.09.008 |
| [72] | Tripathi YB, Pandey V. Obesity and endoplasmic reticulum (ER) stresses[J]. Front Immunol, 2012, 3: 240–247. |
| [73] | Borsting E, Patel SV, Declèves AE, et al. Tribbles homolog 3 attenuates mammalian target of rapamycin complex-2 signaling and inflammation in the diabetic kidney[J]. J Am Soc Nephrol, 2014, 25: 2067–2078. DOI:10.1681/ASN.2013070811 |
| [74] | Ti Y, Xie GL, Wang ZH, et al. TRB3 gene silencing alleviates diabetic cardiomyopathy in a type 2 diabetic rat model[J]. Diabetes, 2011, 60: 2963–2974. DOI:10.2337/db11-0549 |
| [75] | Wang WW, Cheng J, Sun AL, et al. TRB3 mediates renal tubular cell apoptosis associated with proteinuria[J]. Clin Exp Med, 2015, 15: 167–177. DOI:10.1007/s10238-014-0287-4 |
| [76] | Ding WY, Li WB, Ti Y, et al. Protection from renal fibrosis, putative role of TRIB3 gene silencing[J]. Exp Mol Pathol, 2014, 96: 80–84. DOI:10.1016/j.yexmp.2013.12.003 |
| [77] | Wang WW, Sun AL, Lv W, et al. TRB3, up-regulated in kidneys of rats with type1 diabetes, mediates extracellular matrix accumulation in vivo and in vitro[J]. Diabetes Res Clin Pract, 2014, 106: 101–109. DOI:10.1016/j.diabres.2014.07.015 |
| [78] | Sakai S, Ohoka N, Onozaki K, et al. Dual mode of regulation of cell division cycle 25 A protein by TRB3[J]. Biol Pharm Bull, 2010, 33: 1112–1116. DOI:10.1248/bpb.33.1112 |
| [79] | Xu JM, Lv S, Qin Y, et al. TRB3 interacts with CtIP and is overexpressed in certain cancers[J]. Biochim Biophys Acta, 2007, 1770: 273–278. DOI:10.1016/j.bbagen.2006.09.025 |
| [80] | Zou T, Liu WJ, Li SD, et al. TRB3 mediates homocysteine-induced inhibition of endothelial cell proliferation[J]. J Cell Physiol, 2011, 226: 2782–2789. DOI:10.1002/jcp.22554 |
| [81] | Butcher L, Ahluwalia M, Örd T, et al. Evidence for a role of TRIB3 in the regulation of megakaryocytopoiesis[J]. Sci Rep, 2017, 7: 6684. DOI:10.1038/s41598-017-07096-w |
| [82] | Zhang C, Hong FF, Wang CC, et al. TRIB3 inhibits proliferation and promotes osteogenesis in hBMSCs by regulating the ERK1/2 signaling pathway[J]. Sci Rep, 2017, 7: 10342. DOI:10.1038/s41598-017-10601-w |
| [83] | Yu XJ, Song TJ, Zhang LW, et al. TRB3 is elevated in psoriasis vulgaris lesions and mediates HaCaT cells proliferation in vitro[J]. J Investig Med, 2017, 65: 1084–1088. DOI:10.1136/jim-2017-000453 |
| [84] | Ti Y, Xie GL, Wang ZH, et al. Tribbles 3:a potential player in diabetic aortic remodeling[J]. Diab Vasc Dis Res, 2016, 13: 69–80. DOI:10.1177/1479164115605645 |
| [85] | Smith SM, Moran AP, Duggan SP, et al. Tribbles 3:a novel regulator of TLR2-mediated signaling in response to Helicobacter pylori lipopolysaccharide[J]. J Immunol, 2011, 186: 2462–2471. DOI:10.4049/jimmunol.1000864 |
| [86] | Tran SC, Pham TM, Nguyen LN, et al. Nonstructural 3 protein of hepatitis C virus modulates the tribbles homolog 3/AKT signaling pathway for persistent viral infection[J]. J Virol, 2016, 90: 7231–7247. DOI:10.1128/JVI.00326-16 |
 2018, Vol. 53
2018, Vol. 53


