2. 扬州大学医学院, 江苏 扬州 225001
2. Yangzhou University Medical Academy, Yangzhou 225001, China
哮喘是多种细胞及细胞成分参与的气道慢性炎症反应性疾病。变应性哮喘是以免疫球蛋白E(immunoglobulin E, IgE)过量合成、嗜酸性粒细胞数(eosinophils number, EOS)和肥大细胞浸润、气道反应性增高以及气道重塑为特征的慢性气道炎症性疾病, 是全球范围内高发疾病之一[1]。糖皮质激素是目前控制哮喘气道炎症的首选药。重度哮喘患者长期大剂量反复使用激素, 可引发高血压、高血糖和骨质疏松等全身不良反应, 因此, 寻找新型控制哮喘药物及其靶点亟待解决。他汀类药物系羟甲戊二酰辅酶A还原酶抑制剂, 临床上具有降低胆固醇、抗氧化、抗炎和免疫调节等药理学作用。有研究表明口服他汀类药物能降低哮喘患者住院率[2], 且对慢性阻塞性肺部疾病有预防功效[3]。此外, 哮喘患者口服他汀类药物可增强吸入性皮质激素的效果, 同时减少唾液中巨噬细胞数, 改变巨噬细胞中色氨酸降解酶吲哚胺2, 3-二加氧酶(indoleamine 2, 3-oxygenase 2, IDO)活性[4]。但口服他汀类药物对哮喘的疗效尚有争议, 有临床试验报道[5]使用辛伐他汀(simvastatin, Sim)每日10或40 mg口服, 对哮喘患者临床症状、炎症生物学标志与肺功能未获得一致性疗效。临床研究中Sim最高日剂量仅为40 mg, 动物实验使用超大剂量的Sim也不能排除临床疗效差的原因与患者口服Sim剂量太低有关, 而将动物实验剂量换算成人用量用于临床可能引起严重不良反应, 尤其是横纹肌溶解症[6]。雾化吸入给药可大幅度提高药物在肺部浓度, 降低血药浓度, 进而减少不良反应, 为肺部疾病治疗创造有利条件。本研究探讨雾化吸入Sim后, 在较低血药浓度下对哮喘模型小鼠气道炎症的影响。
材料与方法 动物与试剂BALB/c小鼠(6周龄, 体重20 ± 2 g), 雌性, 许可证号SCXK (苏) 2012-0004, 同系交配且无特殊病原体。饲养温度: 23 ± 2 ℃, 湿度: (50 ± 10) %, 12 h白昼交替。小鼠自由进食和饮水。实验前8 h禁食不禁水。在致敏第0天, 新鲜配制氢氧化铝凝胶加入0.2%卵白蛋白(OVA, Grade V, Sigma公司), 于小鼠腹股沟、脚掌、腹腔、颈部皮下和背部皮下多点注射致敏。每只小鼠共注射含OVA凝胶溶液0.5 mL。第14天再腹腔注射加强致敏1次, 第21天开始攻击, 每天1次, 每次15 min, 连续7天。
Sim雾化剂制备准确称取Sim溶解于50%二甲基亚砜和50%生理盐水的混合溶液, 制成1、5和20 mg·mL-1Sim溶液。
不同给药途径给予Sim对哮喘模型小鼠气道炎症的抑制作用BALB/c小鼠随机分为6组, 每组10只。分别为:空白对照(NS-vehicle)组、模型(OVA-vehicle)组、Sim-OVA (40 mg·kg-1, ip)组、Sim-OVA (40 mg·kg-1, ig)组、Sim-OVA (5 mg·mL-1, ih)组和地塞米松(DXM)-OVA (1 mg·kg-1, ip)组。将干预组小鼠放入塑料箱中, 分别以腹腔注射、灌胃和雾化吸入途径给予Sim (持续15 min), 从第21天至第28天连续干预7天。Sim干预1 h后, 小鼠雾化吸入OVA (10 mg·mL-1, 溶解于生理盐水)、持续30 min, 从第21天至第28天连续OVA攻击7天。模型组与空白对照组小鼠吸入等量50%二甲基亚砜和50%生理盐水, 阳性对照组为DXM, 连续7天。
雾化吸入不同剂量Sim对哮喘小鼠气道炎症的影响BALB/c小鼠分为6组, 每组10只。分别为: NS-vehicle组、OVA-vehicle组、Sim-OVA (1、5和20 mg·mL-1, ih)组和DXM-OVA (1 mg·kg-1, ip)组。小鼠从第21天至第28天连续吸入OVA 7天后, 分别按1、5和20 mg·mL-1雾化吸入Sim (持续15 min)。模型组及空白对照组小鼠吸入等量溶媒。Sim干预1 h, 小鼠雾化吸入OVA (10 mg·mL-1, 溶解于生理盐水)持续30 min, 从第21天至第28天连续OVA攻击7天。
收集小鼠肺泡灌洗液(BALF)最后一次OVA攻击后24 h制备小鼠BALF。乌拉坦(2 g·kg-1, ip)麻醉, 剖开小鼠颈部皮肤, 切开气管作肺泡灌洗(PBS溶液, 含1%小牛血清、5 000 u·L-1肝素), 每次注入制备好的灌洗液0.5 mL, 连续3次共收取1.5 mL。将收集到的BALF液于4 ℃、500×g离心10 min, 上清液于-80 ℃保存。BALF白细胞计数和分类计数:混匀收集的BALF与白细胞稀释液按1:1稀释, 用细胞计数板计数白细胞总数, 涂片, 用EOS特殊染色后在光学显微镜下作白细胞分类计数。
逆转录聚合酶链式反应1 mL TRIzol提取肺组织RNA。应用M-MLV逆转录酶逆转录。建立25 μL体系, PCR扩增: 1.25 U LATap DNA聚合酶、0.2 μmol·L-1正义和反义引物和400 μmol·L-1 dATP、dTTP、dGTP及dCTP。PCR产物检测采用1.5%琼脂糖凝胶电泳, 经凝胶成像分析系统成像、条带密度扫描, 以吸光度(OD) 细胞因子与ODβ-actin比值进行半定量分析。小鼠引物见表 1。
| Table 1 The gene primers (5'-3'), annealing temperature and cycle |
将保存于液氮中的BALF置冰上溶解后, 于4 ℃、2 000×g离心60 min。用ELISA试剂盒测定BALF上清中IL-4和IL-5浓度。
数据分析计量资料采用x±s统计, 运用SigmaStat软件统计。采用ANOVA和Student-Newman-Keuls多项比较试验法分析细胞因子mRNA表达及BALF中细胞因子水平。P < 0.05表示有统计学差异。
结果 1 不同途径给予Sim对哮喘小鼠BALF白细胞总数和EOS的影响OVA-vehicle组BALF中白细胞总数和EOS升高, 分别是空白对照组的4.5倍和85倍。与模型组相比, 不同途径给予Sim (5 mg·mL-1, ih、40 mg·kg-1, ig和40 mg·kg-1, ip)可显著降低哮喘小鼠气道白细胞总数, 分别下降了29.3%、46.6%和51.1% (图 1); OVA致敏小鼠经不同途径给予Sim预处理7天, EOS显著降低, 分别下降了50.7%、68%和75.5%。与灌胃和腹腔注射组相比, Sim雾化吸入组气道BALF白细胞总数与EOS减少较明显。结果见图 1。
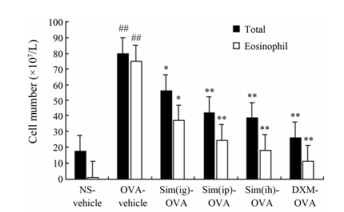
|
Figure 1 The effects of simvastatin (Sim) delivered via the different routes on the airway inflammatory cells in alveolar lavage fluid (BALF). BALF was harvested 24 h after the final ovalbumim (OVA) challenge. The treatments were as follows: Sim at 5 mg·mL-1 via inhalation (ih) for 15 min, 40 mg·kg-1 by intragastric injection (ig) or 40 mg·kg-1 by intraperitoneal injection (ip); dexamethasone (DXM) at 1 mg·kg-1 by ip, daily, for 7 consecutive days. n = 9-10, x±s. ##P < 0.01 vs NS-vehicle group; *P < 0.05, **P < 0.01 vs OVA-vehicle group |
OVA-vehicle组BALF中白细胞总数和EOS升高, 分别是空白对照组的6.8倍和363.8倍。与模型组相比, 雾化吸入Sim (1、5和20 mg·mL-1)预处理可显著降低哮喘小鼠气道白细胞总数, 分别下降了53.2%、56.1%和62.4%; OVA致敏小鼠经不同剂量Sim预处理7天, EOS显著降低, 分别下降了73.2%、74.2%和74.9%。结果见图 2。
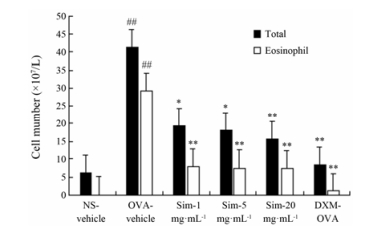
|
Figure 2 The dose-response of Sim delivered via inhalation route on the airway inflammatory cells in the BALF. n = 9-10, x±s. ##P < 0.01 vs NS-vehicle group; *P < 0.05, **P < 0.01 vs OVA-vehicle group |
最后一次OVA攻击24 h后检测小鼠肺组织IL-4和IL-5 mRNA表达。与空白对照组比较, OVA-vehicle组小鼠肺组织IL-4和IL-5 mRNA表达显著增强。Sim (5, 20 mg·mL-1, ih)显著减弱肺组织中IL-4和IL-5 mRNA表达。结果见图 3。
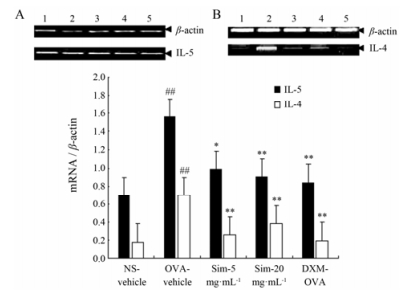
|
Figure 3 The effects of Sim via inhalation on the mRNA expression of IL-4 and IL-5 in the lungs of asthmatic mice. A: IL-5; B: IL-4. 1: NS-vehicle; 2: OVA-vehicle; 3: Sim (5 mg·mL-1); 4: Sim (20 mg·mL-1); 5: DXM-OVA. Twenty-four hours after the final OVA challenge, 100 mg of lung tissue from each mouse was harvested for the isolation of total RNA, and 1 μg aliquots of the total RNA sample were used for reverse transcriptional synthesis of first-strand that was used as template for the PCR amplification. The PCR products were analyzed by electrophoresis on 1.5% agarose gels, and specific bands were digitized using an imaging system. The PCR reactions were performed in triplicates. C: Relative expression of the mRNA from the independent triplicate experiments was normalized by dividing the IL-4 and IL-5 values by the β-actin values. n = 9-10, x±s. ##P < 0.01 vs NS-vehicle group; *P < 0.05, **P < 0.01 vs OVA-vehicle group |
ELISA法测定BALF中IL-4和IL-5水平。OVA-vehicle组小鼠BALF中IL-4和IL-5水平升高, 分别为空白对照组的9.0倍和18.6倍。与模型组相比, Sim雾化吸入预处理组(1、5和20 mg·mL-1)小鼠BALF中IL-4分别下降了35%、64.6%和76.7%; IL-5分别下降了80.2%、84.5%和91.2%。其中5和20 mg·mL-1剂量组小鼠显著减弱了BALF中IL-4和IL-5水平; 1 mg·mL-1剂量组小鼠BALF的IL-4水平略有下降但差异无显著性, IL-5水平下降幅度低于5和20 mg·mL-1剂量组。结果见图 4。
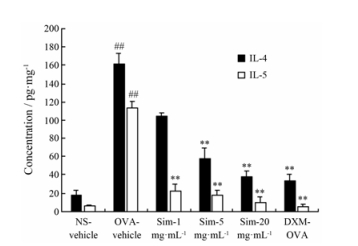
|
Figure 4 The effects of Sim via inhalation on the protein level of IL-4 and IL-5 in the BALF of asthmatic mice. The levels of IL-4 and IL-5 in the BALF were analyzed by ELISA. n = 9-10, x±s. ##P < 0.01 vs NS-vehicle group; **P < 0.01 vs OVA-vehicle group |
随着工业化发展以及生活方式与环境的改变, 哮喘发生率正逐年升高。吸入性糖皮质激素是哮喘治疗的主要手段, 但一些重度哮喘患者产生糖皮质激素抵抗, 且频繁口服大剂量糖皮质激素产生的严重不良反应。因此, 急需寻找新型抗炎药物[7]。目前, 临床上Sim被广泛用作调脂药, 研究表明他汀类亦具抗炎、抗氧化和免疫调节作用, 该作用与他汀类降脂活性不同。已有研究显示他汀类在治疗哮喘和其他气道炎症性疾病具有良好的应用前景。但临床口服高剂量的他汀类药物可能造成严重不良反应, 如横纹肌溶解症和肝损害等后果。探讨他汀类新的给药途径尤为重要, 如雾化吸入途径[6]。
将Sim作为研究对象基于以下药动学性质。首先, Sim吸收差, 生物利用度较低。药动学实验表明口服40 mg·kg-1 Sim的生物利用度仅5%, 口服全身吸收易造成不良反应[8], 而肺部局部浓度仍然很低。若吸入给药则优势明显, 吸入性的糖皮质激素与长效M受体阻断剂即为成功研究案例[8, 9]; 其次, 他汀类的血浆蛋白结合率高达94%~98%, 口服后体内游离药物浓度较低; 第三, Sim亲脂性强, 能穿透肺泡上皮屏障到达靶细胞发挥作用[10]。因此, Sim适合作为雾化吸入用干粉剂或压力定量吸入剂[11]。
重度哮喘患者血液与痰液EOS水平提高与肺功能受损、肺疾患恶化率和生活质量下降呈正相关[12], EOS是参与哮喘气道炎症最主要的效应细胞, EOS募集入肺并释放损伤性介质是气道炎症的中心环节。因此控制肺组织IL-4、IL-5及EOS水平成为治疗哮喘的重要靶标。临床前研究表明, 口服Sim通过诱导哮喘小鼠细胞自噬和细胞凋亡, 抑制气道炎症和气道重塑[13], 但雾化吸入是否影响炎症细胞及炎症因子尚不明确。本实验表明不同途径给予Sim显著降低哮喘小鼠BALF白细胞总数和/或EOS数量; 不同剂量Sim (1、5和20 mg·mL-1, ih)预处理, 均可减少BALF中白细胞总数和EOS数量; 不同剂量Sim (5和20 mg·mL-1, ih)预处理可降低小鼠肺组织中IL-4和IL-5 mRNA的表达水平; Sim (5和20 mg·mL-1, ih)预处理可显著降低BALF中IL-4和IL-5水平, 但1 mg·mL-1预处理组小鼠BALF的IL-4水平略有下降, 差异无显著性; IL-5水平与模型组相比有显著差异, 但下降幅度低于Sim (5和20 mg·mL-1, ih)组。IL-4和IL-5分别在Th1和Th2介导的过敏性炎症反应和气道高反应中具有重要作用。IL-4通过调节肺组织中eotaxin、IL-5产生而激活EOS特异性黏附通路, 共同对EOS产生作用。IL-5可调节EOS生长、活化和存活, 并能为EOS从骨髓迁移至肺部及其他器官提供重要信号。通过抑制IL-4/IL-13路径与IL-5/eotaxin路径可减少EOS, 降低气道高反应性[14], 耗竭eotaxin与IL-5可显著减少肺部气道炎症、气道高反应性与气道重塑[15]。
综上, 研究表明雾化吸入不同剂量Sim能抑制气道炎症, 抑制BALF中炎症细胞因子和肺组织趋化因子mRNA表达。因此雾化吸入Sim在气道炎症性疾病中优势明显, 可避免因口服大剂量Sim而产生的毒副反应使临床疗效降低。
| [1] | Zhang BY, Chi S, Sun Y. Modulation of Toll-like signal path of allergic asthma by CpG-ODNs from Bordetella pertussis[J]. Acta Pharm Sin (药学学报), 2011, 46: 285–292. |
| [2] | Huang CC, Chan WL, Chen YC, et al. Statin use in patients with asthma -a nationwide population-based study[J]. Eur J Clin Invest, 2011, 41: 507–512. DOI:10.1111/j.1365-2362.2010.02434.x |
| [3] | Wang W, Le W, Ahuja R, et al. Inhibition of inflammatory mediators:role of statins in airway inflammation[J]. Otolaryngol Head Neck Sur, 2011, 144: 982–987. DOI:10.1177/0194599811400367 |
| [4] | Maneechotesuwan K, Ekjiratrakul W, Kasetsinsombat K, et al. Statins enhance the anti-inflammatory effects of inhaled corticosteroids in asthmatic patients through increased induction of indoleamine 2, 3-dioxygenase[J]. J Allergy Clin Immunol, 2010, 126: 754–762. DOI:10.1016/j.jaci.2010.08.005 |
| [5] | Ostroukhova M, Kouides RW, Friedman E. The effect of statin therapy on allergic patients with asthma[J]. Ann Allergy Asthma Immunol, 2009, 103: 463–468. DOI:10.1016/S1081-1206(10)60261-X |
| [6] | Barnes PJ. Future treatments for chronic obstructive pulmonary disease and its comorbidities[J]. Proc Am Thorac Soc, 2008, 5: 857–864. DOI:10.1513/pats.200807-069TH |
| [7] | Barnes PJ. Corticosteroid resistance in patients with asthma and chronic obstructive pulmonary disease[J]. J Allergy Clin Immunol, 2013, 131: 636–645. DOI:10.1016/j.jaci.2012.12.1564 |
| [8] | Cerasoli F Jr. Developing the ideal inhaled corticosteroid[J]. Chest, 2006, 130: 54S–64S. DOI:10.1378/chest.130.1_suppl.54S |
| [9] | Disse B, Speck GA, Rominger KL, et al. Tiotropium (Spiriva):mechanistical considerations and clinical profile in obstructive lung disease[J]. Life Sci, 1999, 64: 457–464. DOI:10.1016/S0024-3205(98)00588-8 |
| [10] | Knobloch J, Yakin Y, Körber S, et al. Simvastatin requires activation in accessory cells to modulate T-cell responses in asthma and COPD[J]. Eur J Pharm, 2016, 788: 294–305. DOI:10.1016/j.ejphar.2016.06.037 |
| [11] | Crim C, Pierre LN, Daley-Yates PT. A review of the pharmacology and pharmacokinetics of inhaled fluticasone propionate and mometasone furoate[J]. Clin Therapeut, 2001, 23: 1339–1354. DOI:10.1016/S0149-2918(01)80113-2 |
| [12] | Mukherjee M, Sehmi R, Nair P. Anti-IL5 therapy for asthma and beyond[J]. World Allergy Organ J, 2014, 7: 32. DOI:10.1186/1939-4551-7-32 |
| [13] | Gu W, Cui R, Ding T, et al. Simvastatin alleviates airway inflammation and remodelling through up-regulation of autophagy in mouse models of asthma[J]. Respirology, 2017, 22: 533–541. DOI:10.1111/resp.2017.22.issue-3 |
| [14] | Foster PS, Mould AW, Yang M, et al. Elemental signals regulating eosinophil accumulation in the lung[J]. Immunol Rev, 2001, 179: 173–181. DOI:10.1034/j.1600-065X.2001.790117.x |
| [15] | Halwani R, Alabri J, Beland M, et al. CC and CXC chemokines induce airway smooth muscle proliferation and survival[J]. J Immunol, 2011, 186: 4156–4163. DOI:10.4049/jimmunol.1001210 |
 2017, Vol. 52
2017, Vol. 52


