当机体受到外来微生物感染(pathogen-associated molecular patterns, PAMPs)或来自本身的损伤信号(danger-associated molecular patterns, DAMPs)刺激时, 便会启动先天性免疫系统通过其表面的模式识别受体或感应系统激活炎症反应, 促进type I interferons (interferon-α和interferon-β)或炎症因子IL-1β、IL-18和IL-33等释放。经典的NLRP3炎症小体活化由两种信号共同刺激激活。第一信号激活TLR4 (toll like receptor 4)信号通路, 促进核转录因子κB (NF-κB)激活介导IL-1β和IL-18等前体产生; 第二信号促进NLRP3/ASC/pro-caspase-1蛋白复合物组装, pro-caspase-1自剪切成活化形式, 活化的caspase-1将IL-1β和IL-18前体剪切活化释放到胞外[1]。非经典的NLRP3炎症小体活化不依赖于TLR4信号通路活化, 它是由caspase-11介导的细胞炎性死亡, 即pyroptosis, 导致病原微生物再次被释放及识别。最新研究表明, caspase-11直接识别胞内的lipopolysaccharide (LPS), 启动NLRP3炎症小体活化, 促进gasdermin D的活化与释放从而介导细胞死亡[2]。人体依靠与caspase-11有类似功能的caspase-4和caspase-5蛋白介导非经典NLRP3炎症小体活化[3]。NLRP3炎症小体活化所介导的促炎症因子的产生及细胞死亡是机体的一种自我保护性措施, 它与机体抵御病原微生物感染、维持自身稳态密切相关。因此了解其调控方式对于疾病治疗与预防有重大意义。
1 NLRP3 priming阶段调控在静息状态下, 细胞中NLRP3表达量处于极低水平, 需要激活的NF-κB转录出NLRP3 mRNA, 再进行翻译及翻译后修饰等, 此过程即称为NLRP3 priming[4]。调控NF-κB活性的分子可以间接影响NLRP3 priming。Ng等[5]报道, 细胞表面相关黏蛋白1 (cell surface associated mucin 1, MUC1)抑制白介素1受体相关激酶1 (interleukin-1 receptor-associated kinase 1, IRAK1)的磷酸化从而负调控NF-κB活性抑制NLRP3的表达。值得一提的是, IRAK1在快速炎症小体反应过程中可以绕过NLRP3 priming过程, 作为TLRs和NLRP3炎症小体桥梁直接启动炎症反应[6]。A20抑制NF-κB必需调节蛋白(NF-κB-essential modulator, NEMO)泛素化降解负调控NF-κB介导的NLRP3转录[7]。IL-1β K133位泛素化被证明在IL-1β剪切成熟过程有重要作用, A20抑制IL-1β K133位泛素化调控炎症反应[8]。
Caspase-8作为启动凋亡的蛋白酶, 参与介导死亡受体配体(Fas ligand, FASL)和肿瘤坏死因子(tumor necrosis factor, TNF)诱导的细胞凋亡[9]。2009年Nature杂志首次报道: caspase-8缺失导致伤口愈合过程中NLRP3表达上调, 促进caspase-1介导的interleukin-1β (IL-1β)释放, 从而促进炎症[10]。这与caspase-8以往在细胞凋亡中扮演的角色不同, 然而其对炎症的调节作用又不一致。Fas相关死亡域蛋白(Fas-associating protein with a novel death domain, FADD)及caspase-8敲除的小鼠IL-1β水平明显下降, 原因是caspase-8缺失导致了NLRP3表达下降从而抑制了caspase-1的活化[11]。在树突状细胞中, caspase-8缺失则增强了炎症小体复合组的组装, 其原因是caspase-8可以抑制受体相互作用蛋白激酶1 (receptor-interacting serine/threonine-protein kinase 1, RIPK1)和RIPK3的活性, 从而抑制炎症小体活化[12, 13]。
芳香烃受体(aryl hydrocarbon receptor, AhR)是胞浆中存在的转录因子。静息状态下其与一些伴侣分子结合且不具备生理活性, 然而在一些化学物质如2, 3, 7, 8-tetrachlorodibenzo-p-dioxin (TCDD)等作用下, AhR脱离伴侣分子进入细胞核与AhR nuclear translocator (ARNT)形成二聚体, 结合到NLRP3启动子的外源反应元件(xenobiotic response element, XRE), 抑制NLRP3转录[14]。
microRNA (miRNA)一类长度约为20~24个核苷酸的内源小RNA, 其介导的转录后调控在基因表达的控制中发挥重要作用。Bauernfeind等[15]发现, miR-223能够特异性靶向于NLRP3 mRNA的3'-UTR (untranslated region)区, 从而抑制NLRP3的翻译表达。在病毒入侵时, 为了避免引起机体免疫反应, 会释放microRNA抑制NLRP3炎症小体激活。如EB病毒(Epstein-Barr virus, EBV)来源的miR-BART15可以结合到与miR-223相同的NLRP3 3'-UTR区抑制其表达[16]。此外, miR-7和miR-155也参与NLRP3炎症小体活化调控, 然而机制尚未明确[17, 18]。
研究表明NLRP3活化依赖于其去泛素化。一方面, TLR4/MyD88信号的激活会导致NLRP3去泛素化, 该过程依赖于线粒体来源ROS的产生; 另一方面, 三磷酸腺苷(ATP)的刺激也会使NLRP3发生不依赖于活性氧(reactive oxygen species, ROS)的去泛素化[19]。Py等[20]采用去泛素化酶(deubiquitinase)小分子抑制剂进行筛选, 发现小分子G5可以显著活化NLRP3炎症小体进而证实乳腺癌基因1/2复合物亚基3 (breast cancer 1/2-containing complex, subunit 3, BRCC3)可介导NLRP3的去泛素化过程。
2 NLRP3炎症小体复合物组装的调控炎症小体的中心分子一般都含有PYCARD区, 因此含有这样区域结合域的蛋白一般都很有可能竞争结合这些分子, 从而阻止复合物的组装。这样的蛋白有POPs (PYD-only proteins)、COPs (CARD-only proteins)、热蛋白pyrin和PYNOD (NLRP10)等。POPs研究最多的有POP1和POP2。Stehlik等[21, 22]研究表明, POP1与ASC相互作用抑制复合物的组装, 从而抑制NLRP3炎症小体活化。POP2虽然也能与ASC结合但明显弱于POP1。COPs家族包含Iceberg、COP1/PseudoICE、INCA和caspase-12等分子, 它们与caspase-1的CARD区相似度很高, 从而与caspase-1结合以抑制ASC与CASP1的结合[23]。热蛋白Pyrin也具有负调炎症小体的作用, Pyrin不但能干扰NLRP3与ASC之间PYD依赖的信号传递过程, 还能够与其他炎症小体直接结合, 抑制caspase-1的活化[24]。PYNOD蛋白即NLRP10, 含有PYD区和NACHT区, 缺少LRR区, 虽然PYNOD的NACHT区在自身寡聚化后与ASC结合, 但不募集活化pro-caspase-1, 从而抑制IL-1β的产生[25]。
此外, 其他一些蛋白也陆续被报道可以调控NLRP3炎症小体复合物组装。富含亮氨酸重复序列的相互作用蛋白(leucine-rich repeat flightless-interacting protein, LRRFIP2)通过招募caspase-1底物抑制蛋白flightless-I负调控NLRP3炎症小体活化[26]。核转录因子κB激酶α (nuclear factor kappa-B kinase subunit α, IKKα)通过负调控ASC出核, 负调控NLRP3炎症小体活化[27]。小异源二聚体伴侣(small heterodimer partner, SHP)作为NLRP3炎症小体的负调控因子, 与NLRP3相互作用共同被招募至线粒体与ASC竞争性结合NLRP3[28]。细胞Fas-相关性死亡结构域样白介素-lβ转换酶抑制蛋白(cellular Fas-associated death domain-like interleulin-1β converting enzyme inhibitory protein, c-FLIP)可以与NLRP3和pro-caspase-1相互作用, 促进NLRP3复合物组装[29]。
3 内源性代谢物质和离子对NLRP3炎症小体的调控一些内源性的生理物质也可以调控NLRP3炎症小体活化。多巴胺[30]、Omega-3不饱和脂肪酸[31]、β-羟基丁酸[32]和一氧化氮[33]抑制NLRP3炎症小体活化, 而血清淀粉样蛋白A(Aβ)则促进NLRP3炎症小体活化[34]。其他一些诱导NLRP3炎症小体活化介导疾病的危险信号如尿酸结晶盐、饱和脂肪酸和胆固醇结晶等已有较多报道就不多赘述了。值得一提的是, 在临床上IFN-β被用来作为治疗多发性硬化症(multiple sclerosis, MS)的一线药物已有15年。然而只有NLRP3炎症小体介导的MS患者才对IFN-β治疗有效, 其机制为信号转录激活因子1 (signal transducers and activators of transcription 1, STAT1)抑制NLRP3炎症小体活化[35]。
高糖可以诱导NLRP3炎症小体活化。硫氧还蛋白相互作用蛋白(thioredoxin interacting protein, TXNIP)是一个NLRP3结合蛋白。在正常环境下, TXNIP与环氧硫蛋白结合, 而在高糖诱导ROS的环境下其从环氧硫蛋白上解离下来, 与NLRP3相互作用, 促进NLRP3活化。有趣的是, 高糖可以诱导胰岛细胞中TXNIP的高表达但并不影响巨噬细胞中TXNIP的表达。这两个机制共同作用, 促进了2型糖尿病的产生[36, 37]。但是与此相矛盾的是, 在IAPP的刺激下, TXNIP并不参与NLRP3炎症小体活化的调控[38]。因此, TXNIP依赖的NLRP3活化是在特定的疾病条件和特定的细胞中发生的。
细胞内低浓度钾离子可以诱导NLRP3炎症小体活化[39]。钾离子流对于经典和非经典NLRP3炎症小体都有调控作用[40]。钙离子作为第二信使在细胞信号传递中扮演重要角色。钙离子流紊乱将对细胞带来灾难性的后果, 因此细胞设置多种手段控制细胞内钙离子水平。钙离子受体激活磷脂酶C促进1, 4, 5-三磷酸肌醇表达诱导钙离子从内质网释放从而激活NLRP3炎症小体活化[41]。线粒体可以接受内质网释放的钙离子, 然而当线粒体内钙离子含量超负荷时会导致线粒体功能丧失[42]。小G蛋白GPRC6A也被报道可以介导钙离子诱导的NLRP3炎症小体活化[43]。线粒体损伤导致NAD+离子减少, 从而诱导α微管蛋白(α-tubulin)介导的ASC-NLRP3相互作用, 促进其活化[44]。
4 亚细胞结构器—线粒体对NLRP3炎症小体的调控NLRP3的线粒体定位对于NLRP3炎症小体活化至关重要。胞浆膜相关和内含体上的TLRs以及RIG样螺旋酶(RIG-I和MDA-5)在病毒感染时被线粒体外膜蛋白MAVS招募启动type I interferon反应。起初人们认为在胞浆中组装活化的NLRP3炎症小体似乎不太可能会被招募至线粒体。Zhou等[45]研究证明静息状态下的NLRP3主要定位于内质网, 然而在NLRP3炎症小体激活剂作用下, NLRP3与ASC被重新分配于核周边定位于簇集的内质网和线粒体上。进一步研究表明, 线粒体相关适配蛋白(mitochondria-associated adaptor protein, MAVS)是NLRP3炎症小体发挥其最佳活性的必要条件。在炎症小体活化过程中, MAVS可与NLRP3 N端相互作用[46, 47]。因此, MAVS不仅介导抵抗病毒的type I interferon反应, 同时可作为线粒体对NLRP3炎症小体调控的桥梁。除了MAVS, c-FLIP[29]和线粒体上的心磷脂[48, 49]对NLRP3线粒体定位也有调控作用。乙酰化的α-tubulin介导肌动蛋白依赖的线粒体上ASC传递, 使线粒体上的ASC与内质网上的NLRP3相互作用[44]。
随着自噬相关蛋白缺失对炎症影响的发现, 越来越多的研究显示自噬可以负调控炎症小体活性。自噬相关基因16L1 (autophagy gene 16L1, Atg16L1)缺失促进内毒素诱导的炎症[50], 自噬蛋白微管相关蛋白1轻链-3B (autophagy protein microtubule-associated protein 1 light chain-3B, LC3B)和膜突蛋白样BCL2作用蛋白1 (moesin-like BCL2-interacting protein, Beclin1)缺失导致线粒体功能障碍及线粒体DNA释放到胞浆, 促进NLRP3炎症小体活化[51]。因此自噬相关蛋白可以维持线粒体完整性, 抑制NLRP3炎症小体活化。在流感病毒感染过程中, RIPK2蛋白促进线粒体自噬抑制NLRP3炎症小体活化[52]。值得一提的是, Cell杂志上最新研究报道[53], LPS诱导的NF-κB活化促进p62/SQSTM1表达, 介导线粒体自噬抑制NLRP3炎症小体活化。线粒体分裂与融合介导了线粒体动态平衡, 线粒体分裂被抑制则加剧炎症小体活化[54]。Mitofusin 2是介导线粒体融合的蛋白, 它与NLRP3相互作用促进RNA病毒感染诱导的NLRP3炎症小体活化[55]。此外, RIP1-RIP3复合物调控DRP1活化, 介导线粒体分裂负调控NLRP3炎症小体活化[56, 57]。然而与这些研究相矛盾的是, 科罗索酸阻止内皮细胞中线粒体分裂和NADPH氧化酶2 (NOX2)表达可以抑制NLRP3炎症小体活化[58]。作者认为此矛盾的产生可能是小分子多靶性导致的, 需要基因敲除的验证。
线粒体是ROS的主要来源, 其参与NLRP3炎症小体调控。然而对于ROS在NLRP3炎症小体中的作用却颇有争议。一方面, 认为ROS介导NLRP3炎症小体活化[45, 51, 59]; 另一方面, 认为ROS促进NLRP3 priming, 但是对NLRP3炎症小体活化无影响[60]。
溶酶体作为真核细胞中分解各种外源和内源的大分子物质的一种细胞器, 也参与NLRP3炎症小体活化的调节。当二氧化硅、明矾或β-淀粉样蛋白刺激后, 引发细胞吞噬小体不稳定, 溶酶体组织蛋白酶B释放到细胞质中, 可引起NLRP3活化。另外, 内吞的结晶或特殊的分子可能直接破坏溶酶体膜, 导致吞噬微粒扩散进入胞浆, 这些吞噬微粒可能会直接与炎症小体相关蛋白作用促进NLRP3的活化[61, 62]。
5 细胞间的调控已知NLRP3炎症小体活化过程中释放的IL-1β及IL-18分别促进Th17和Th1的分化[63], 同样其他细胞释放的细胞因子也可以调控NLRP3炎症小体活化。NLRP3炎症小体活化介导微生物感染清除, 然而持续的炎症会导致机体损伤。研究发现效应和记忆CD4+ T细胞依赖于细胞间相互接触, 可以终止NLRP3炎症小体活化[64]。Foxp3- type 1 regulatory T (Tr1)细胞也被报道依赖于IL-10抑制NLRP3炎症小体活化[65]。其原因可能是IL-10可以抑制NLRP3表达[66]。此外, 自然杀伤T细胞释放TNF-α促进NLRP3 priming, 从而正调控NLRP3炎症小体活化[67]。
6 小分子化合物对NLRP3炎症小体的调控随着NLRP3炎症小体活化机制研究深入, 大量小分子化合物被发现可以调节NLRP3炎症小体活化改善疾病。格列本脲靶向ATP敏感的钾离子通道选择性抑制NLRP3炎症小体[68]。小分子化合物MCC950抑制NLRP3炎症小体活化改善小鼠败血症、实验性脑脊髓炎和CAPS综合症[69]。小分子化合物3, 4-methylenedioxy-β-nitrostyrene和砷酸也可以抑制NLRP3炎症小体活化[70, 71]。本实验室近年来着力于靶向NLRP3炎症小体的炎症疾病的治疗。Fc11a-2 (1-ethyl-5-methyl-2-phenyl-1H-benzo[d]-imidazole)抑制NLRP3炎症小体复合物组装改善硫酸葡聚糖(dextran sodium sulfate, DSS)诱导的小鼠溃疡性结肠炎[72]。穿心莲内酯诱导线粒体自噬抑制NLRP3炎症小体活化改善小鼠结肠炎及结肠炎-癌转化[73]。区别于右奥硝唑, 左奥硝唑抑制NLRP3炎症小体改善小鼠结肠炎和LPS诱导的败血症[74]。作者的研究一方面发现了作用于NLRP3炎症小体活化改善炎症的候选化合物, 另一方面期望以化合物为出发点, 通过其作用机制和靶蛋白的探索发现新的NLRP3炎症小体调控机制。
与此同时, NLRP3炎症小体活化与很多疾病药物治疗中的耐药密切相关。Nature Medcine杂志[75]报道了大肠癌患者对5-氟尿嘧啶(5-Fu)耐药归因于其化疗过程中5-Fu促进了MDSC中的组织蛋白酶B (cathepsin B)活化, 从而激活髓样抑制性细胞(myeloid derived suppress cells, MDSC)中NLRP3炎症小体活化, 产生大量IL-1β, 促进Th17细胞分化, 从而促进肿瘤耐药的产生。白血病患者对糖皮质激素的抵抗可能归因于NLRP3介导的caspase-1活化, 活化的caspase-1可以剪切糖皮质激素受体从而使机体对糖皮质激素脱敏[76]。
7 小结综上所述, 在不同的病理过程、不同的活化刺激下, NLRP3炎症小体活化调控呈多样性, 同时NLRP3炎症小体在疾病中发挥或正或反的作用。上文提及的NLRP3炎症小体的调控因素总结于图 1中。NLRP3炎症小体介导多发性硬化症、败血症、肥胖、糖尿病和动脉粥样硬化等炎症性疾病和代谢性疾病的发生发展。对于NLRP3介导的疾病治疗, 通常使用针对IL-1β和IL-18的中和抗体, 然而效果并不理想。这提示, 靶向炎症小体的疾病治疗不应仅针对其下游产物, 而应考虑炎症小体活化的上游环节[77]。因此, 对NLRP3炎症小体调控网络及作用机制的探讨, 不仅有助于了解炎症的生理病理过程, 还有助于为相关疾病提供靶向治疗的实验证据。
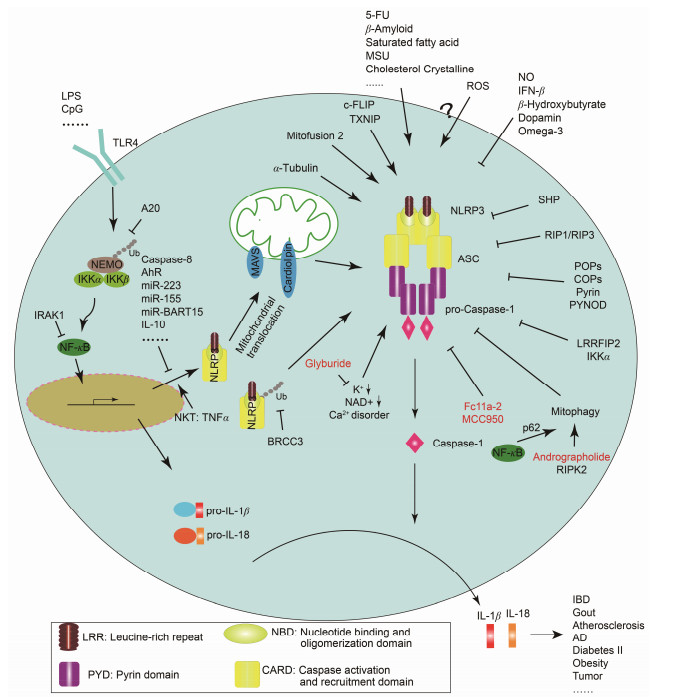
|
Figure 1 Mechanisms of NLRP3 inflammasome regulation |
| [1] | Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes[J]. Nat Rev Immunol , 2013, 13 :397–411. DOI:10.1038/nri3452 |
| [2] | Shi J, Zhao Y, Wang K, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death[J]. Nature , 2015, 526 :660–665. DOI:10.1038/nature15514 |
| [3] | Viganò E, Diamond CE, Spreafico R, et al. Human cas-pase-4 and caspase-5 regulate the one-step non-canonical inflammasome activation in monocytes[J]. Nat Commun , 2015, 6 :8761. DOI:10.1038/ncomms9761 |
| [4] | Qiao Y, Wang P, Qi JN, et al. TLR-induced NF-κB acti-vation regulates NLRP3 expression in murine macrophages[J]. FEBS Lett , 2012, 586 :1022–1026. DOI:10.1016/j.febslet.2012.02.045 |
| [5] | Ng GZ, Menheniott TR, Every AL, et al. The MUC1 mucin protects against Helicobacter pylori pathogenesis in mice by regulation of the NLRP3 inflammasome[J]. Gut , 2016, 65 :1087–1099. DOI:10.1136/gutjnl-2014-307175 |
| [6] | Lin KM, Hu W, Troutman TD, et al. IRAK-1 bypasses priming and directly links TLRs to rapid NLRP3 inflam-masome activation[J]. Proc Natl Acad Sci U S A , 2014, 111 :775–780. DOI:10.1073/pnas.1320294111 |
| [7] | Vande Walle L, Van Opdenbosch N, Jacques P, et al. Nega-tive regulation of the NLRP3 inflammasome by A20 protects against arthritis[J]. Nature , 2014, 512 :69–73. |
| [8] | Duong BH, Onizawa M, Oses-Prieto JA, et al. A20 re-stricts ubiquitination of pro-interleukin-1β protein complexes and suppresses NLRP3 inflammasome activity[J]. Immunity , 2015, 42 :55–67. DOI:10.1016/j.immuni.2014.12.031 |
| [9] | Kischkel FC, Lawrence DA, Chuntharapai A, et al. Apo2L/TRAIL-dependent recruitment of endogenous FADD and caspase-8 to death receptors 4 and 5[J]. Immunity , 2000, 12 :611–620. DOI:10.1016/S1074-7613(00)80212-5 |
| [10] | Lee P, Lee DJ, Chan C, et al. Dynamic expression of epidermal caspase 8 simulates a wound healing response[J]. Nature , 2009, 458 :519–523. DOI:10.1038/nature07687 |
| [11] | Gurung P, Anand PK, Malireddi RK, et al. FADD and caspase-8 mediate priming and activation of the canonical and noncanonical Nlrp3 inflammasomes[J]. J Immunol , 2014, 192 :1835–1846. DOI:10.4049/jimmunol.1302839 |
| [12] | Kang S, Fernandes-Alnemri T, Rogers C, et al. Caspase-8 scaffolding function and MLKL regulate NLRP3 inflam-masome activation downstream of TLR3[J]. Nat Commun , 2015, 6 :7515. DOI:10.1038/ncomms8515 |
| [13] | Kang TB, Yang SH, Toth B, et al. Caspase-8 blocks kinase RIPK3-mediated activation of the NLRP3 inflammasome[J]. Immunity , 2013, 38 :27–40. DOI:10.1016/j.immuni.2012.09.015 |
| [14] | Huai WW, Zhao R, Song H, et al. Aryl hydrocarbon receptor negatively regulates NLRP3 inflammasome activity by inhibiting NLRP3 transcription[J]. Nat Commun , 2014, 5 :4738. DOI:10.1038/ncomms5738 |
| [15] | Bauernfeind F, Rieger A, Schildberg FA, et al. NLRP3 inflammasome activity is negatively controlled by miR-223[J]. J Immunol , 2012, 189 :4175–4181. DOI:10.4049/jimmunol.1201516 |
| [16] | Haneklaus M, Gerlic M, Kurowska-Stolarska M, et al. Cutting edge:miR-223 and EBV miR-BART15 regulate the NLRP3 inflammasome and IL-1β production[J]. J Immunol , 2012, 189 :3795–3799. DOI:10.4049/jimmunol.1200312 |
| [17] | Fan Z, Lu M, Qiao C, et al. MicroRNA-7 enhances subven-tricular zone neurogenesis by inhibiting NLRP3/caspase-1 axis in adult neural stem cells[J]. Mol Neurobiol , 2015 . DOI:10.1007/s12035-015-9620-5 |
| [18] | Chen S, Smith BAH, Iype J, et al. MicroRNA-155-deficient dendritic cells cause less severe GVHD through reduced migration and defective inflammasome activation[J]. Blood , 2015, 126 :103–112. DOI:10.1182/blood-2014-12-617258 |
| [19] | Juliana C, Fernandes-Alnemri T, Kang S, et al. Non-transcriptional priming and deubiquitination regulate NLRP3 inflammasome activation[J]. J Biol Chem , 2012, 287 :36617–36622. DOI:10.1074/jbc.M112.407130 |
| [20] | Py BF, Kim MS, Vakifahmetoglu-Norberg H, et al. Deubiqui-tination of NLRP3 by BRCC3 critically regulates inflam-masome activity[J]. Mol Cell , 2013, 49 :331–338. DOI:10.1016/j.molcel.2012.11.009 |
| [21] | Stehlik C, Dorfleutner A. COPs and POPs:modulators of inflammasome activity[J]. J Immunol , 2007, 179 :7993–7998. DOI:10.4049/jimmunol.179.12.7993 |
| [22] | de Almeida L, Khare S, Misharin AV, et al. The PYRIN domain-only protein POP1 inhibits inflammasome assembly and ameliorates inflammatory disease[J]. Immunity , 2015, 43 :264–276. DOI:10.1016/j.immuni.2015.07.018 |
| [23] | Le HT, Harton JA. Pyrin-and CARD-only proteins as regulators of NLR functions[J]. Front Immunol , 2013, 4 :275. |
| [24] | Matusiak M, Van Opdenbosch N, Lamkanfi M. CARD-and pyrin-only proteins regulating inflammasome activation and immunity[J]. Immunol Rev , 2015, 265 :217–230. DOI:10.1111/imr.12282 |
| [25] | Wang YT, Hasegawa M, Imamura R, et al. PYNOD, a novel Apaf-1/CED4-like protein is an inhibitor of ASC and caspase-1[J]. Int Immunol , 2004, 16 :777–786. DOI:10.1093/intimm/dxh081 |
| [26] | Jin J, Yu Q, Han CF, et al. LRRFIP2 negatively regulates NLRP3 inflammasome activation in macrophages by promoting Flightless-I-mediated caspase-1 inhibition[J]. Nat Commun , 2013, 4 :2075. |
| [27] | Martin BN, Wang CH, Willette-Brown J, et al. IKKα negatively regulates ASC-dependent inflammasome activa-tion[J]. Nat Commun , 2014, 5 :4977. DOI:10.1038/ncomms5977 |
| [28] | Yang CS, Kim JJ, Kim TS, et al. Small heterodimer partner interacts with NLRP3 and negatively regulates activation of the NLRP3 inflammasome[J]. Nat Commun , 2015, 6 :6115. DOI:10.1038/ncomms7115 |
| [29] | Wu YH, Kuo WC, Wu YJ, et al. Participation of c-FLIP in NLRP3 and AIM2 inflammasome activation[J]. Cell Death Differ , 2014, 21 :451–461. DOI:10.1038/cdd.2013.165 |
| [30] | Yan YQ, Jiang W, Liu L, et al. Dopamine controls systemic inflammation through inhibition of NLRP3 inflammasome[J]. Cell , 2015, 160 :62–73. DOI:10.1016/j.cell.2014.11.047 |
| [31] | Yan YQ, Jiang W, Spinetti T, et al. Omega-3 fatty acids prevent inflammation and metabolic disorder through inhibition of NLRP3 inflammasome activation[J]. Immunity , 2013, 38 :1154–1163. DOI:10.1016/j.immuni.2013.05.015 |
| [32] | Youm YH, Nguyen KY, Grant RW, et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease[J]. Nat Med , 2015, 21 :263–269. |
| [33] | Mao KR, Chen SZ, Chen MK, et al. Nitric oxide suppresses NLRP3 inflammasome activation and protects against LPS-induced septic shock[J]. Cell Res , 2013, 23 :201–212. DOI:10.1038/cr.2013.6 |
| [34] | Yu N, Liu S, Yi X, et al. Serum amyloid A induces inter-leukin-1β secretion from keratinocytes via the NACHT, LRR and PYD domains-containing protein 3 inflammasome[J]. Clin Exp Immunol , 2015, 179 :344–353. DOI:10.1111/cei.12458 |
| [35] | Guarda G, Braun M, Staehli F, et al. Type I interferon inhibits interleukin-1 production and inflammasome activation[J]. Immunity , 2011, 34 :213–223. DOI:10.1016/j.immuni.2011.02.006 |
| [36] | Zhou RB, Tardivel A, Thorens B, et al. Thiore-doxin-interacting protein links oxidative stress to inflammasome activation[J]. Nat Immunol , 2010, 11 :136–140. DOI:10.1038/ni.1831 |
| [37] | Schroder K, Zhou RB, Tschopp J. The NLRP3 inflam-masome:a sensor for metabolic danger?[J]. Science , 2010, 327 :296–300. DOI:10.1126/science.1184003 |
| [38] | Hui STY, Andres AM, Miller AK, et al. Txnip balances metabolic and growth signaling via PTEN disulfide reduction[J]. Proc Natl Acad Sci U S A , 2008, 105 :3921–3926. DOI:10.1073/pnas.0800293105 |
| [39] | Pétrilli V, Papin S, Dostert C, et al. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration[J]. Cell Death Differ , 2007, 14 :1583–1589. DOI:10.1038/sj.cdd.4402195 |
| [40] | Rivers-Auty J, Brough D. Potassium efflux fires the canon:potassium efflux as a common trigger for canonical and noncanonical NLRP3 pathways[J]. Eur J Immunol , 2015, 45 :2758–2761. DOI:10.1002/eji.201545958 |
| [41] | Lee GS, Subramanian N, Kim AI, et al. The cal-cium-sensing receptor regulates the NLRP3 inflammasome through Ca2+ and cAMP[J]. Nature , 2012, 492 :123–127. DOI:10.1038/nature11588 |
| [42] | Horng T. Calcium signaling and mitochondrial destabiliza-tion in the triggering of the NLRP3 inflammasome[J]. Trends Immunol , 2014, 35 :253–261. DOI:10.1016/j.it.2014.02.007 |
| [43] | Rossol M, Pierer M, Raulien N, et al. Extracellular Ca2+ is a danger signal activating the NLRP3 inflammasome through G protein-coupled calcium sensing receptors[J]. Nat Commun , 2012, 3 :1329. DOI:10.1038/ncomms2339 |
| [44] | Misawa T, Takahama M, Kozaki T, et al. Micro-tubule-driven spatial arrangement of mitochondria promotes activation of the NLRP3 inflammasome[J]. Nat Immunol , 2013, 14 :454–460. |
| [45] | Zhou RB, Yazdi AS, Menu P, et al. A role for mitochondria in NLRP3 inflammasome activation[J]. Nature , 2011, 469 :221–225. DOI:10.1038/nature09663 |
| [46] | Subramanian N, Natarajan K, Clatworthy MR, et al. The adaptor MAVS promotes NLRP3 mitochondrial localization and inflammasome activation[J]. Cell , 2013, 153 :348–361. DOI:10.1016/j.cell.2013.02.054 |
| [47] | Park S, Juliana C, Hong S, et al. The mitochondrial anti-viral protein MAVS associates with NLRP3 and regulates its inflammasome activity[J]. J Immunol , 2013, 191 :4358–4366. DOI:10.4049/jimmunol.1301170 |
| [48] | O'Neill LA. Cardiolipin and the Nlrp3 inflammasome[J]. Cell Metab , 2013, 18 :610–612. DOI:10.1016/j.cmet.2013.10.013 |
| [49] | Iyer SS, He Q, Janczy JR, et al. Mitochondrial cardiolipin is required for Nlrp3 inflammasome activation[J]. Immunity , 2013, 39 :311–323. DOI:10.1016/j.immuni.2013.08.001 |
| [50] | Saitoh T, Fujita N, Jang MH, et al. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1β pro-duction[J]. Nature , 2008, 456 :264–268. DOI:10.1038/nature07383 |
| [51] | Nakahira K, Haspel JA, Rathinam VA, et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome[J]. Nat Immunol , 2011, 12 :222–230. |
| [52] | Lupfer C, Thomas PG, Anand PK, et al. Receptor interact-ing protein kinase 2-mediated mitophagy regulates inflam-masome activation during virus infection[J]. Nat Immunol , 2013, 14 :480–488. DOI:10.1038/ni.2563 |
| [53] | Zhong ZY, Umemura A, Sanchez-Lopez E, et al. NF-κB restricts inflammasome activation via elimination of damaged mitochondria[J]. Cell , 2016, 164 :896–910. DOI:10.1016/j.cell.2015.12.057 |
| [54] | Park S, Won JH, Hwang I, et al. Defective mitochondrial fission augments NLRP3 inflammasome activation[J]. Sci Rep , 2015, 5 :15489. DOI:10.1038/srep15489 |
| [55] | Ichinohe T, Yamazaki T, Koshiba T, et al. Mitochondrial protein mitofusin 2 is required for NLRP3 inflammasome activation after RNA virus infection[J]. Proc Natl Acad Sci U S A , 2013, 110 :17963–17968. DOI:10.1073/pnas.1312571110 |
| [56] | Rayamajhi M, Miao EA. The RIP1-RIP3 complex initiates mitochondrial fission to fuel NLRP3[J]. Nat Immunol , 2014, 15 :1100–1102. DOI:10.1038/ni.3030 |
| [57] | Wang XQ, Jiang W, Yan YQ, et al. RNA viruses promote activation of the NLRP3 inflammasome through a RIP1-RIP3-DRP1 signaling pathway[J]. Nat Immunol , 2014, 15 :1126–1133. DOI:10.1038/ni.3015 |
| [58] | Li Y, Zhou ZH, Chen MH, et al. Inhibition of mitochon-drial fission and NOX2 expression prevent NLRP3 inflammasome activation in endothelium:the role of corosolic acid action in the amelioration of endothelial dysfunction[J]. Antioxid Redox Signal , 2016, 24 :893–908. DOI:10.1089/ars.2015.6479 |
| [59] | Shimada K, Crother TR, Karlin J, et al. Oxidized mitochon-drial DNA activates the NLRP3 inflammasome during apop-tosis[J]. Immunity , 2012, 36 :401–414. DOI:10.1016/j.immuni.2012.01.009 |
| [60] | Bauernfeind F, Bartok E, Rieger A, et al. Cutting edge:reactive oxygen species inhibitors block priming, but not activation, of the NLRP3 inflammasome[J]. J Immunol , 2011, 187 :613–617. DOI:10.4049/jimmunol.1100613 |
| [61] | Lamkanfi M, Dixit VM. Inflammasomes and their roles in health and disease[J]. Annu Rev Cell Dev Biol , 2012, 28 :137–161. DOI:10.1146/annurev-cellbio-101011-155745 |
| [62] | Cassel SL, Eisenbarth SC, Iyer SS, et al. The Nalp3 inflammasome is essential for the development of silicosis[J]. Proc Natl Acad Sci U S A , 2008, 105 :9035–9040. DOI:10.1073/pnas.0803933105 |
| [63] | Gris D, Ye ZM, Iocca HA, et al. NLRP3 plays a critical role in the development of experimental autoimmune enceph-alomyelitis by mediating Th1 and Th17 responses[J]. J Immunol , 2010, 185 :974–981. DOI:10.4049/jimmunol.0904145 |
| [64] | Guarda G, Dostert C, Staehli F, et al. T cells dampen innate immune responses through inhibition of NLRP1 and NLRP3 inflammasomes[J]. Nature , 2009, 460 :269–273. DOI:10.1038/nature08100 |
| [65] | Yao Y, Vent-Schmidt J, McGeough MD, et al. Tr1 Cells, but not Foxp3+ regulatory T cells, suppress NLRP3 inflam-masome activation via an IL-10-dependent mechanism[J]. J Immunol , 2015, 195 :488–497. DOI:10.4049/jimmunol.1403225 |
| [66] | Gurung P, Li BF, Subbarao Malireddi RK, et al. Chronic TLR stimulation controls NLRP3 inflammasome activation through IL-10 mediated regulation of NLRP3 expression and caspase-8 activation[J]. Sci Rep , 2015, 5 :14488. DOI:10.1038/srep14488 |
| [67] | Chow MT, Duret H, Andrews DM, et al. Type I NKT-cell-mediated TNF-α is a positive regulator of NLRP3 inflammasome priming[J]. Eur J Immunol , 2014, 44 :2111–2120. DOI:10.1002/eji.201344329 |
| [68] | Lamkanfi M, Mueller JL, Vitari AC, et al. Glyburide in-hibits the cryopyrin/Nalp3 inflammasome[J]. J Cell Biol , 2009, 187 :61–70. DOI:10.1083/jcb.200903124 |
| [69] | Coll RC, Robertson AA, Chae JJ, et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases[J]. Nat Med , 2015, 21 :248–255. |
| [70] | He Y, Varadarajan S, Muñoz-Planillo R, et al. 3, 4-Methyl-enedioxy-β-nitrostyrene inhibits NLRP3 inflammasome activation by blocking assembly of the inflammasome[J]. J Biol Chem , 2014, 289 :1142–1150. DOI:10.1074/jbc.M113.515080 |
| [71] | Maier NK, Crown D, Liu J, et al. Arsenic trioxide and other arsenical compounds inhibit the NLRP1, NLRP3, and NAIP5/NLRC4 inflammasomes[J]. J Immunol , 2014, 192 :763–770. DOI:10.4049/jimmunol.1301434 |
| [72] | Liu W, Guo WJ, Wu J, et al. A novel benzo[d]imidazole derivate prevents the development of dextran sulfate so-dium-induced murine experimental colitis via inhibition of NLRP3 inflammasome[J]. Biochem Pharmacol, 2013, 85:1504-1512. |
| [73] | Guo WJ, Sun Y, Liu W, et al. Small molecule-driven mitophagy-mediated NLRP3 inflammasome inhibition is responsible for the prevention of colitis-associated cancer[J]. Autophagy , 2014, 10 :972–985. DOI:10.4161/auto.28374 |
| [74] | Wang XQ, Wang SY, Hu CH, et al. A new pharmacological effect of levornidazole:inhibition of NLRP3 inflammasome activation[J]. Biochem Pharmacol , 2015, 97 :178–188. DOI:10.1016/j.bcp.2015.06.030 |
| [75] | Bruchard M, Mignot G, Derangère V, et al. Chemotherapy-triggered cathepsin B release in myeloid-derived suppressor cells activates the Nlrp3 inflammasome and promotes tumor growth[J]. Nat Med , 2013, 19 :57–64. |
| [76] | Paugh SW, Bonten EJ, Savic D, et al. NALP3 inflam-masome upregulation and CASP1 cleavage of the glucocorticoid receptor cause glucocorticoid resistance in leukemia cells[J]. Nat Genet , 2015, 47 :607–614. DOI:10.1038/ng.3283 |
| [77] | Shao BZ, Xu ZQ, Han BZ, et al. NLRP3 inflammasome and its inhibitors:a review[J]. Front Pharmacol , 2015, 6 :262. |
 2016, Vol. 51
2016, Vol. 51


