2. 中国海洋大学医药学院, 山东 青岛 266003
2. College of Medicine and Pharmacy, Ocean University of China, Qingdao 266003, China
细胞凋亡又称细胞程序性死亡[1],在生物生长发育及病理生理过程中具有十分重要的作用[2-4]。细胞凋亡信号通路的异常、凋亡信号的缺失或抗凋亡信号的增强都会导致各种病理改变,如癌症的发生、转移及化疗的失败[5, 6]。现有的化疗药物大多数是通过细胞凋亡途径抑制肿瘤生长[3, 7-10]。细胞凋亡过程是由多种caspases级联反应介导的[11, 12]。哺乳动物中存在两条凋亡通路,即外源的死亡受体通路[13-16]和内源的线粒体通路[11, 17, 18]。两者之间存在交叉,许多信号分子可以同时参与这两条凋亡通路。
目前,临床上治疗使用的肿瘤化疗药物60% 以上是天然产物或基于天然产物合成的小分子药物。如长春新碱、伊立替康、鬼臼乙叉甙、紫杉醇和喜树碱等都是从陆地植物中获得的天然产物或其衍生物。由于海洋环境的特殊性,海洋生物中含有许多特有的生物活性物质。从海洋生物中分离到的具有抗肿瘤作用的化合物,包含生物碱、甾体化合物、萜烯类、大环内酯类、酮类和多肽等[19-24],其中部分化合物已经进入临床不同的试验阶段[25]。
诱导肿瘤细胞凋亡的海洋化合物可以通过靶向不同的信号分子诱导细胞凋亡 (图 1)。通过JNK/p38/ MAPK通路激活可以导致细胞色素C (Cyt C) 从线粒体中释放; 抗凋亡基因BCL-2与凋亡相关基因BAX之间的平衡在维持细胞存活中起关键作用,因此,抑制BCL-2和诱导BAX表达成为诱导肿瘤细胞凋亡的重要途径[26]; caspases是凋亡过程的关键分子,激活caspases是大多数海洋抗肿瘤药物发挥作用的主要方式[27, 28]; 一些海洋抗肿瘤药物可促进Cyt C从线粒体中释放,从而诱导凋亡细胞。凋亡是一个非常复杂的过程,涉及许多信号分子参与,而细胞凋亡激活的障碍是癌症化疗失败的一种主要原因。开发靶向诱导细胞凋亡的化合物成为发展新型抗癌药物的重要策略。
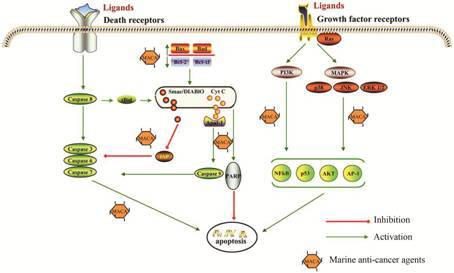
|
Figure 1 Schematic depiction of common target genes for marine proapoptotic products |
BCL-2家族成员包含促凋亡因子和抗凋亡因子两类,肿瘤细胞的凋亡取决于BCL-2家族成员间的平衡,BCL-2和BAX是抗癌药物的重要靶点[29]。在线粒体途径的凋亡过程中,BCL-2家族中至少有18个促/抗凋亡蛋白参与[26, 30]。此外,这些蛋白因子可能产生直接或间接的拮抗或协同作用。BAX作为BCL-2家族中的促凋亡成员,可以通过调节线粒体功能影响肿瘤细胞凋亡[26, 30]。
已发现了多种海洋化合物可以通过调节BCL-2家族成员间的平衡诱导细胞凋亡。从海洋无脊椎动物苔藓虫Bugula neritina中获得了多种具有大环内酯结构的化合物bryostatins[31]。Bryostatin 1不仅可以激 活蛋白激酶C (PKC),还能通过线粒体途径诱导细胞凋亡。Bryostatin 1上调凋亡因子BAX表达,下调抗凋亡因子BCL-2表达,导致BAX/BCL-2比率升高,从而诱导肿瘤细胞凋亡[32]。从乌贼墨中分离得到一种三肽化合物sepia ink oligopeptide (SIO),可以显著抑制肿瘤细胞增殖,SIO也可通过下调BCL-2和上 调BAX蛋白表达,提高BAX/BCL-2比率诱导细胞 凋亡[33]。Fucoxanthinol是从海鞘Halocynthia roretzi中分离得到的一种岩藻黄素衍生物,能抑制不同类型的肿瘤细胞生长,包括人类白血病细胞、乳腺癌细胞和结肠癌细胞。这种抑制作用与BCL-2表达水平的降低和Cyt C释放有关[34]。
1.2 通过激活JNK/p38/MAPK通路诱导肿瘤细胞凋亡JNK/p38/MAPK通路在肿瘤细胞的凋亡过程具有重要作用[35, 36]。JNK/p38/MAPK通路激活可以引起Cyt C的释放,并激活caspases级联反应[35]。Aplidine是从海鞘Aplidium albicans中分离到的一种环肽,可以通过线粒体的凋亡途径抑制淋巴瘤、乳腺癌和前列腺癌细胞的生长[37]。Aplidine可以诱导JNK和p38 MAPK两种激酶磷酸化,激活caspases级联反应和蛋白激酶δ (PKCδ),随之释放Cyt C[38-40]。Aplidine也可通过抑制血管内皮生长因子 (VEGF) 的释放和基质金属蛋白酶 (MMP-2和MMP-9) 的表达抑制新生血管形成[41, 42]。Ⅰ期临床试验表明,Aplidine具有良好的抗耐药作用[43, 44]。从海洋青铜色小单孢菌中分离
得到的FW523-3是一种新型脂肽化合物,具有抑制肿瘤细胞分化和诱导凋亡的特点。其凋亡机制系通过降低p38和ERK的表达,诱导肿瘤细胞凋亡[45]。新近发现的几种化合物,如从海洋海绵Mycale sp.中得到的onnamide A和theopederin B可以诱导HeLa细胞凋亡,p38/JNK通路激活是上述化合物诱导细胞凋亡的主要机制[46]。
1.3 通过激活caspases诱导肿瘤细胞凋亡Caspases与细胞凋亡密切相关,诱导肿瘤细胞凋亡的化合物大多可以激活不同的caspases。Ascididemin是一种从地中海海鞘Cystodytes dellechiajei中分离的四环芳香族类生物碱。Ascididemin既可以激活caspase-2,也可以通过激活JNK诱导凋亡[47]。Spisulosine (ES285) 是从蛤类海洋生物Mactromeris polynyma中分离的 化合物,ES285可以通过激活caspase-3和caspase-12诱导细胞凋亡,并促进p53磷酸化[48, 49]。从海绵中获得的naamidine A可使线粒体膜破裂,激活caspase-3、8、9[50]。Ircinin-1是一种从海洋海绵Sarcotragus sp.中分离的二倍半萜类衍生物,能抑制人体内黑色素腺瘤细胞的生长,阻滞细胞周期停滞在G1期。Ircinin-1诱导细胞凋亡涉及释放Cyt C、激活caspase-3和caspase-9、上调死亡因子Fas和Fas-L[51]。Porphyrans是硫酸多糖Porphyra的主要成分,在胃癌细胞AGS中通过激活caspase-3表现很强的诱导凋亡活性[52]。Pardaxin是从海洋鱼类Pardachirus marmoratus中得到的一种由33个氨基酸残基组成的多肽化合物,pardaxin除具有很强的抗菌活性外,也呈现良好的抗肿瘤活性,在舌鳞癌SCC-4细胞中可以激活caspase-3、增加p53表达诱导细胞凋亡[53]。
1.4 通过促进CytC释放诱导肿瘤细胞凋亡 Cyt C从线粒体释放到细胞质的过程是启动细胞凋亡的关键步骤[54]。Cyt C释放到细胞质中,与凋亡蛋白酶活化因子 (Apaf-1) 结合,进而激活caspase-9,诱导肿瘤细胞凋亡[55]。LAQ824是一种组蛋白去乙酰化酶 (histone deacetylase,HDAC) 抑制剂,最初从海绵Psammaplysilla sp.中得到,LAQ824可以引起肿瘤细胞周期阻滞,诱导细胞凋亡[56]。最近的研究表明,LAQ824能通过触发线粒体释放Cyt C,诱导肿瘤细胞凋亡。经LAQ824处理的A549肿瘤细胞内的凋亡诱导因子(apoptosis inducing factor,AIF)、核酸内切酶G (Endo G)、Cyt C的表达都有所增加[57]。另外,研究发现LAQ824也能促进caspase-3、8、9的活化[57]。从海蛞蝓Elysia ornate提取分离的lamellarins是一 类多环生物碱类。Lamellarin D可以通过靶向作用于拓扑异构酶Ⅰ诱导线粒体途径的细胞凋亡[58, 59]。Lamellarin D引起S期和G2/M期细胞周期阻滞,降低线粒体膜通透性,破坏线粒体内跨膜电位,促进Cyt C释放,抑制肿瘤细胞的生长[60]。
1.5 通过NF-κB信号通路诱导肿瘤细胞凋亡NF-κB转录因子是细胞凋亡的重要调节因子。NF-κB常常通过激活抗凋亡蛋白和抗氧化分子拮抗细胞凋亡[61, 62],抑制性激酶IκB kinase (IKK) 的磷酸化是激活NF-κB的重要过程。从海洋真菌Diaporthe sp.中分离获得的mycoepoxydiene (MED) 是一种聚酮类化合物,在胆管癌中MED可以通过抑制IKK磷酸化,减少MMP-9表达从而抑制NF-κB的激活,诱导细胞凋亡[63]。Microsclerodermin A是从海绵Microscleroderma sp.中分离获得的一种环肽化合物,是一种新的NF-κB抑制剂,可以抑制NK-κB活化诱导细胞凋亡[64]。
1.6 通过多靶点途径诱导肿瘤细胞凋亡许多海洋来源的化合物可以影响细胞凋亡通路的不同步骤,调控不同靶点发挥诱导凋亡的作用。如ascididemin不仅可以激活caspases,也可以影响JNK通路[47]; LAQ842在肿瘤细胞中不仅诱导线粒体促凋亡因子的释放,还能激活caspases活性; Ircinin-1诱导细胞 凋亡包含内源性和外源性两条通路[51]。从海洋蠕虫Cephalodiscus gilchristi中提取的cephalostatins是 一种二萜类化合物。Cephalostatin 1选择性引起线 粒体释放信号分子Smac/DIABLO诱导肿瘤细胞凋 亡[65]。进一步研究表明,cephalostatin 1还可以促进JNK磷酸化[66],其诱导细胞凋亡主要涉及caspase-4和caspase-9的激活[67]。Jaspamide可以通过激活caspase-3、降低BCL-2和MCL-2蛋白水平、增加 BAX蛋白表达诱导细胞凋亡[68]。从海洋生物毛蚶Arca subcrenata中分离到的多肽P2有很好的抗癌活性。在宫颈癌HeLa细胞中,P2不仅改变BCL-2/BAX比率,还可以激活caspase-3,7,9活性,增加DNA修复酶 (poly ADP-ribose polymerase,PARP) 表达量; 还能影响JNK/38/MAPK和ERK1/2通路,从而显示促凋亡活性[69]。
Cryptophycin 52是迄今为止发现的最强的微管蛋白抑制剂[70]。Cryptophycin 52诱导凋亡需要caspases的激活,同时还受BCL-2家族成员和p53调节[70]。Dideoxypetrosynol A是一种从海洋海绵Petrosia sp.中得到的聚乙炔类化合物,具有很强的细胞毒性[71]。Dideoxypetrosynol可以通过多种途径诱导细胞凋亡,包括上调BAX、下调BCL-2的表达、激活caspase-3与caspase-9、促进PARP的裂解和cIAP-1的下调[71]。
从海洋海绵Jaspis sp.中提取分离的jaspine B是一种鞘氨醇衍生物,其可以引起Cyt C的释放和caspases的激活,诱导黑色素瘤细胞凋亡[72]。Somocystinamide A (ScA) 可以通过内源性和外源性途径诱导多肿瘤细胞和血管内皮细胞凋亡[73]。
1.7 通过未知机制诱导细胞凋亡某些海洋化合物诱导肿瘤细胞死亡显示细胞凋亡的特征,如DNA片段化、细胞核固缩和细胞膜肿胀等,然而它们诱导凋亡的机制尚不明确。例如,从海洋蓝藻cyanobacterium Lyngbya sp.分离的一种乙炔脂肽jahanyne经证实具有很好的诱导凋亡作用和很强的细胞毒性,对HeLa细胞和HL60细胞的半数抑制浓度 (IC50)值分别是1.8和0.63 μmol·L-1,但是其促凋亡作用机制尚不清楚[74]。本课题组发现了多种具有抗肿瘤活性的海洋多肽,Mere15是从海洋生物文蛤Meritrix meritrix中分离的多肽。Mere15可以通过增加细胞通透性和抑制微管蛋白聚合显示其抗肿瘤活性[75]。最新的研究结果表明,Mere15也可以诱导p53依赖性细胞凋亡[76, 77]。CS5931是从海鞘Ciona savignyi中分离的抗肿瘤多肽,它可以引起线粒体途径的肿瘤细胞凋亡[78]。CS5931具有良好的体内抗肿瘤活性,对人肺癌A549细胞裸鼠移植性肿瘤的抑瘤率达到75% 以上。
2 海洋药物发展存在的问题与发展前景不同海洋来源的化合物诱导凋亡途径见表 1。从表中可见,许多海洋来源天然化合物都可以通过诱导细胞凋亡发挥抗肿瘤作用,其诱导机制多样、涉及信号通路复杂。很多化合物除具有诱导细胞凋亡的 活性外,还能通过自噬、抑制血管形成和诱导细胞坏死等途径发挥抗肿瘤作用。因此,可以认为海洋化合物对肿瘤细胞生长的抑制作用涉及了细胞死亡的不同环节。但是由于海洋环境的特殊性,受深海生物采集技术的限制,现已发现的海洋活性产物占全部化合物的比例很低,证实具有抗癌活性并进入临床试验研究的就更少,能最终投入临床治疗使用的就少之又少。
| Table 1 Asummary of marine products and their targets on apoptotic pathway |
海洋中蕴含丰富的生物资源,我国海洋资源丰富、物种多样,许多海洋天然产物的作用机制尚不明确,从海洋中发现新的抗肿瘤药物具有广阔的前景。应加强对海洋生物资源的研究,发展抗癌活性强、不良反应小的海洋药物。随着科学研究的不断深入以及现代海洋采集技术的发展,有理由相信更多的海洋化合物会被发现,更多的海洋来源的抗癌药物将应用于临床治疗。
| [1] | Degterev A, Boyce M, Yuan J. A decade of caspases[J]. Oncogene , 2003, 22 :8543–8567. DOI:10.1038/sj.onc.1207107 |
| [2] | Rowinsky EK. Targeted induction of apoptosis in cancer management: the emerging role of tumor necrosis factor-related apoptosis-inducing ligand receptor activating agents[J]. J Clin Oncol , 2005, 23 :9394–9407. DOI:10.1200/JCO.2005.02.2889 |
| [3] | Call JA, Eckhardt SG, Camidge DR. Targeted manipulation of apoptosis in cancer treatment[J]. Lancet Oncol , 2008, 9 :1002–1011. DOI:10.1016/S1470-2045(08)70209-2 |
| [4] | Iannolo G, Conticello C, Memeo L, et al. Apoptosis in normal and cancer stem cells[J]. Crit Rev Oncol Hematol , 2008, 66 :42–51. DOI:10.1016/j.critrevonc.2007.09.004 |
| [5] | Burz C, Berindan-Neagoe I, Balacescu O, et al. Apoptosis in cancer: key molecular signaling pathways and therapy targets[J]. Acta Oncol , 2009, 48 :811–821. DOI:10.1080/02841860902974175 |
| [6] | Fulda S, Pervaiz S. Apoptosis signaling in cancer stem cells[J]. Int J Biochem Cell Biol , 2010, 42 :31–38. DOI:10.1016/j.biocel.2009.06.010 |
| [7] | Ghobrial IM, Witzig TE, Adjei AA. Targeting apoptosis pathways in cancer therapy[J]. CA Cancer J Clin , 2005, 55 :178–194. DOI:10.3322/canjclin.55.3.178 |
| [8] | Eberle J, Fecker LF, Forschner T, et al. Apoptosis pathways as promising targets for skin cancer therapy[J]. Br J Dermatol , 2007, 156 (Suppl 3:) :18–24. |
| [9] | Ziegler DS, Kung AL. Therapeutic targeting of apoptosis pathways in cancer[J]. Curr Opin Oncol , 2008, 20 :97–103. DOI:10.1097/CCO.0b013e3282f310f6 |
| [10] | Qiao L, Wong BC. Targeting apoptosis as an approach for gastrointestinal cancer therapy[J]. Drug Resist Updat , 2009, 12 :55–64. DOI:10.1016/j.drup.2009.02.002 |
| [11] | Reed JC. Mechanisms of apoptosis[J]. Am J Pathol , 2000, 157 :1415–1430. DOI:10.1016/S0002-9440(10)64779-7 |
| [12] | Oliver L, Vallette FM. The role of caspases in cell death and differentiation[J]. Drug Resist Updat , 2005, 8 :163–170. DOI:10.1016/j.drup.2005.05.001 |
| [13] | Wang W, Li J, Wen Q, et al. 4EGI-1 induces apoptosis and enhances radiotherapy sensitivity in nasopharyngeal carcinoma cells via DR5 induction on 4E-BP1 dephosphorylation[J]. Oncotarget , 2016 . DOI:10.18632/oncotarget.7824 |
| [14] | Ozören N, El-Deiry WS. Cell surface death receptor signaling in normal and cancer cells[J]. Semin Cancer Biol , 2003, 13 :135–147. DOI:10.1016/S1044-579X(02)00131-1 |
| [15] | Peter ME, Krammer PH. The CD95 (APO-1/Fas) DISC and beyond[J]. Cell Death Differ , 2003, 10 :26–35. DOI:10.1038/sj.cdd.4401186 |
| [16] | Thorburn A. Death receptor-induced cell killing[J]. Cell Signal , 2004, 16 :139–144. DOI:10.1016/j.cellsig.2003.08.007 |
| [17] | Kroemer G. Mitochondrial control of apoptosis: an introduc-tion[J]. Biochem Biophys Res Commun , 2003, 304 :433–435. DOI:10.1016/S0006-291X(03)00614-4 |
| [18] | Gupta S, Kass GE, Szegezdi E, et al. The mitochondrial death pathway: a promising therapeutic target in diseases[J]. J Cell Mol Med , 2009, 13 :1004–1033. DOI:10.1111/jcmm.2009.13.issue-6 |
| [19] | Kinghorn AD, Chin YW, Swanson SM. Discovery of natural product anticancer agents from biodiverse organisms[J]. Curr Opin Drug Discov Devel , 2009, 12 :189–196. |
| [20] | Mayer AM, Gustafson KR. Marine pharmacology in 2003-2004: anti-tumour and cytotoxic compounds[J]. Eur J Cancer , 2006, 42 :2241–2270. DOI:10.1016/j.ejca.2006.05.019 |
| [21] | Mayer AM, Gustafson KR. Marine pharmacology in 2005-2006: antitumour and cytotoxic compounds[J]. Eur J Cancer , 2008, 44 :2357–2387. DOI:10.1016/j.ejca.2008.07.001 |
| [22] | Adrian TE. Novel marine-derived anti-cancer agents[J]. Curr Pharm Des , 2007, 13 :3417–3426. DOI:10.2174/138161207782360500 |
| [23] | Gulder TA, Moore BS. Chasing the treasures of the sea-bacterial marine natural products[J]. Curr Opin Microbiol , 2009, 12 :252–260. DOI:10.1016/j.mib.2009.05.002 |
| [24] | Molinski TF, Dalisay DS, Lievens SL, et al. Drug develop-ment from marine natural products[J]. Nat Rev Drug Discov , 2009, 8 :69–85. DOI:10.1038/nrd2487 |
| [25] | Singh R, Sharma M, Joshi P, et al. Clinical status of anticancer agents derived from marine sources[J]. Anticancer Agents Med Chem , 2008, 8 :603–617. DOI:10.2174/187152008785133074 |
| [26] | Yip KW, Reed JC. Bcl-2 family proteins and cancer[J]. Oncogene , 2008, 27 :6398–6406. DOI:10.1038/onc.2008.307 |
| [27] | Okun I, Balakin KV, Tkachenko SE, et al. Caspase activity modulators as anticancer agents[J]. Anticancer Agents Med Chem , 2008, 8 :322–341. DOI:10.2174/187152008783961914 |
| [28] | Ghavami S, Hashemi M, Ande SR, et al. Apoptosis and cancer: mutations within caspase genes[J]. J Med Genet , 2009, 46 :497–510. DOI:10.1136/jmg.2009.066944 |
| [29] | Kang MH, Reynolds CP. Bcl-2 inhibitors: targeting mitochondrial apoptotic pathways in cancer therapy[J]. Clin Cancer Res , 2009, 15 :1126–1132. DOI:10.1158/1078-0432.CCR-08-0144 |
| [30] | Wong WW, Puthalakath H. Bcl-2 family proteins: the sentinels of the mitochondrial apoptosis pathway[J]. IUBMB Life , 2008, 60 :390–397. DOI:10.1002/(ISSN)1521-6551 |
| [31] | Mutter R, Wills M. Chemistry and clinical biology of the bryostatins[J]. Bioorg Med Chem , 2000, 8 :1841–1860. DOI:10.1016/S0968-0896(00)00150-4 |
| [32] | Lopez-Campistrous A, Song X, Schrier AJ, et al. Bryostatin analogue-induced apoptosis in mantle cell lymphoma cell lines[J]. Exp Hematol , 2012, 40 :646–656. DOI:10.1016/j.exphem.2012.03.002 |
| [33] | Huang F, Yang Z, Yu D, et al. Sepia ink oligopeptide induces apoptosis in prostate cancer cell lines via caspase-3 activation and elevation of Bax/Bcl-2 ratio[J]. Mar Drugs , 2012, 10 :2153–2165. DOI:10.3390/md10102153 |
| [34] | Konishi I, Hosokawa M, Sashima T, et al. Halocynthiaxanthin and fucoxanthinol isolated from Halocynthia roretzi induce apoptosis in human leukemia, breast and colon cancer cells[J]. Comp Biochem Physiol C Toxicol Pharmacol , 2006, 142 :53–59. DOI:10.1016/j.cbpc.2005.10.005 |
| [35] | Dhanasekaran DN, Reddy EP. JNK signaling in apoptosis[J]. Oncogene , 2008, 27 :6245–6251. DOI:10.1038/onc.2008.301 |
| [36] | Wagner EF, Nebreda AR. Signal integration by JNK and p38 MAPK pathways in cancer development[J]. Nat Rev Cancer , 2009, 9 :537–549. DOI:10.1038/nrc2694 |
| [37] | Urdiales JL, Morata P, Núñez De Castro I, et al. Antiproli-ferative effect of dehydrodidemnin B (DDB), a depsipeptide isolated from Mediterranean tunicates[J]. Cancer Lett , 1996, 102 :31–37. DOI:10.1016/0304-3835(96)04151-1 |
| [38] | Garcia-Fernández LF, Losada A, Alcaide V, et al. Aplidin induces the mitochondrial apoptotic pathway via oxidative stress-mediated JNK and p38 activation and protein kinase C delta[J]. Oncogene , 2002, 21 :7533–7544. DOI:10.1038/sj.onc.1205972 |
| [39] | Cuadrado A, Garcia-Fernandez LF, Gonzalez L, et al. Aplidin induces apoptosis in human cancer cells via glutathione depletion and sustained activation of the epidermal growth factor receptor, Src, JNK, and p38 MAPK[J]. J Biol Chem , 2003, 278 :241–250. |
| [40] | Gonzalez-Santiago L, Suárez Y, Zarich N, et al. Aplidin induces JNK-dependent apoptosis in human breast cancer cells via alteration of glutathione homeostasis, Rac1 GTPase activation, and MKP-1 phosphatase downregulation[J]. Cell Death Differ , 2006, 13 :1968–1981. DOI:10.1038/sj.cdd.4401898 |
| [41] | Broggini M, Marchini SV, Galliera E, et al. Aplidine, a new anticancer agent of marine origin, inhibits vascular endothelial growth factor (VEGF) secretion and blocks VEGF-VEGFR-1 (flt-1) autocrine loop in human leukemia cells MOLT-4[J]. Leukemia , 2003, 17 :52–59. DOI:10.1038/sj.leu.2402788 |
| [42] | Barboza NM, Medina DJ, Budak-Alpdogan T, et al. Plitidepsin (Aplidin) is a potent inhibitor of diffuse large cell and Burkitt lymphoma and is synergistic with rituximab[J]. Cancer Biol Ther , 2012, 13 :114–122. DOI:10.4161/cbt.13.2.18876 |
| [43] | Faivre S, Chièze S, Delbaldo C, et al. Phase I and pharma-cokinetic study of aplidine, a new marine cyclodepsipeptide in patients with advanced malignancies[J]. J Clin Oncol , 2005, 23 :7871–7880. DOI:10.1200/JCO.2005.09.357 |
| [44] | Maroun JA, Belanger K, Seymour L, et al. Phase I study of aplidine in a daily×5 one-hour infusion every 3 weeks in patients with solid tumors refractory to standard therapy. A National Cancer Institute of Canada Clinical Trials Group study: NCIC CTG IND 115[J]. Ann Oncol , 2006, 17 :1371–1378. DOI:10.1093/annonc/mdl165 |
| [45] | Xie JJ, Zhou F, Li EM, et al. FW523-3, a novel lipopeptide compound, induces apoptosis in cancer cells[J]. Mol Med Rep , 2011, 4 :759–763. |
| [46] | Lee KH, Nishimura S, Matsunaga S, et al. Inhibition of protein synthesis and activation of stress-activated protein kinases by onnamide A and theopederin B, antitumor marine natural products[J]. Cancer Sci , 2005, 96 :357–364. DOI:10.1111/cas.2005.96.issue-6 |
| [47] | Dirsch VM, Kirschke SO, Estermeier M, et al. Apoptosis signaling triggered by the marine alkaloid ascididemin is routed via caspase-2 and JNK to mitochondria[J]. Oncogene , 2004, 23 :1586–1593. DOI:10.1038/sj.onc.1207281 |
| [48] | Faircloth G, Cuevas C. Kahalalide F and ES285: potent anticancer agents from marine mollusks[J]. Prog Mol Subcell Biol , 2006, 43 :363–379. DOI:10.1007/978-3-540-30880-5 |
| [49] | Salcedo M, Cuevas C, Alonso JL, et al. The marine sphin-golipid-derived compound ES 285 triggers an atypical cell death pathway[J]. Apoptosis , 2007, 12 :395–409. DOI:10.1007/s10495-006-0573-z |
| [50] | LaBarbera DV, Modzelewska K, Glazar AI, et al. The marine alkaloid naamidine A promotes caspase-dependent apoptosis in tumor cells[J]. Anticancer Drugs , 2009, 20 :425–436. DOI:10.1097/CAD.0b013e32832ae55f |
| [51] | Choi HJ, Choi YH, Yee SB, et al. Ircinin-1 induces cell cycle arrest and apoptosis in SK-MEL-2 human melanoma cells[J]. Mol Carcinog , 2005, 44 :162–173. DOI:10.1002/(ISSN)1098-2744 |
| [52] | Kwon MJ, Nam TJ. Porphyran induces apoptosis related signal pathway in AGS gastric cancer cell lines[J]. Life Sci , 2006, 79 :1956–1962. DOI:10.1016/j.lfs.2006.06.031 |
| [53] | Han Y, Cui Z, Li YH, et al. In vitro and in vivo anticancer activity of pardaxin against proliferation and growth of oral squamous cell carcinoma[J]. Mar Drugs , 2016, 14 :2. |
| [54] | Scorrano L. Opening the doors to cytochrome c: changes in mitochondrial shape and apoptosis[J]. Int J Biochem Cell Biol , 2009, 41 :1875–1883. DOI:10.1016/j.biocel.2009.04.016 |
| [55] | Ledgerwood EC, Morison IM. Targeting the apoptosome for cancer therapy[J]. Clin Cancer Res , 2009, 15 :420–424. DOI:10.1158/1078-0432.CCR-08-1172 |
| [56] | Atadja P, Hsu M, Kwon P, et al. Molecular and cellular basis for the anti-proliferative effects of the HDAC inhibitor LAQ824[J]. Novartis Found Symp , 2004, 259 :249–266. DOI:10.1002/SERIES1767 |
| [57] | Wang S, Yan-Neale Y, Cai R, et al. Activation of mitochon-drial pathway is crucial for tumor selective induction of apoptosis by LAQ824[J]. Cell Cycle , 2006, 5 :1662–1668. DOI:10.4161/cc.5.15.3099 |
| [58] | Facompré M, Tardy C, Bal-Mahieu C, et al. Lamellarin D: a novel potent inhibitor of topoisomerase I[J]. Cancer Res , 2003, 63 :7392–7399. |
| [59] | Marco E, Laine W, Tardy C, et al. Molecular determinants of topoisomerase I poisoning by lamellarins: comparison with camptothecin and structure-activity relationships[J]. J Med Chem , 2005, 48 :3796–3807. DOI:10.1021/jm049060w |
| [60] | Kluza J, Gallego MA, Loyens A, et al. Cancer cell mitochondria are direct proapoptotic targets for the marine antitumor drug lamellarin D[J]. Cancer Res , 2006, 66 :3177–3187. DOI:10.1158/0008-5472.CAN-05-1929 |
| [61] | Tas SW, Vervoordeldonk MJ, Tak PP. Gene therapy targeting nuclear factor-κB: towards clinical application in inflammatory diseases and cancer[J]. Curr Gene Ther , 2009, 9 :160–170. DOI:10.2174/156652309788488569 |
| [62] | Yu Y, Wan Y, Huang C. The biological functions of NF-κB1 (p50) and its potential as an anti-cancer target[J]. Curr Cancer Drug Targets , 2009, 9 :566–571. DOI:10.2174/156800909788486759 |
| [63] | Li WJ, Li M, Su XH. Mycoepoxydiene induces apoptosis and inhibits TPA-induced invasion in human cholangiocarci-noma cells via blocking NF-κB pathway[J]. Biochimie , 2014, 101 :183–191. DOI:10.1016/j.biochi.2014.01.012 |
| [64] | Guzmán EA, Maers K, Roberts J. The marine natural product microsclerodermin A is a novel inhibitor of the nuclear factor kappa B and induces apoptosis in pancreatic cancer cells[J]. Invest New Drugs , 2015, 33 :86–94. DOI:10.1007/s10637-014-0185-3 |
| [65] | Dirsch VM, Muller IM, Eichhorst ST, et al. Cephalostatin 1 selectively triggers the release of Smac/DIABLO and subse-quent apoptosis that is characterized by an increased density of the mitochondrial matrix[J]. Cancer Res , 2003, 63 :8869–8876. |
| [66] | Muller IM, Dirsch VM, Rudy A, et al. Cephalostatin 1 inactivates Bcl-2 by hyperphosphorylation independent of M-phase arrest and DNA damage[J]. Mol Pharmacol , 2005, 67 :1684–1689. DOI:10.1124/mol.104.004234 |
| [67] | López-Antón N, Rudy A, Barth N, et al. The marine product cephalostatin 1 activates an endoplasmic reticulum stress-specific and apoptosome-independent apoptotic signaling pathway[J]. J Biol Chem , 2006, 281 :33078–33086. DOI:10.1074/jbc.M607904200 |
| [68] | Cioca DP, Kitano K. Induction of apoptosis and CD10/ neutral endopeptidase expression by jaspamide in HL-60 line cells[J]. Cell Mol Life Sci , 2002, 59 :1377–1387. DOI:10.1007/s00018-002-8515-6 |
| [69] | Hu X, Zhang Z, Liu T, et al. Polypeptide fraction from Arca subcrenata induces apoptosis and G2/M phase arrest in HeLa cells via ROS-mediated MAPKs pathways[J]. Evid Based Complement Alternat Med , 2015 . DOI:10.1155/2015/930249 |
| [70] | Drew L, Fine RL, Do TN, et al. The novel antimicrotubule agent cryptophycin 52 (LY355703) induces apoptosis via multiple pathways in human prostate cancer cells[J]. Clin Cancer Res , 2002, 8 :3922–3932. |
| [71] | Choi HJ, Bae SJ, Kim ND, et al. Induction of apoptosis by dideoxypetrosynol A, a polyacetylene from the sponge Petrosia sp., in human skin melanoma cells[J]. Int J Mol Med , 2004, 14 :1091–1096. |
| [72] | Salma Y, Lafont E, Therville N, et al. The natural marine anhydrophytosphingosine, Jaspine B, induces apoptosis in melanoma cells by interfering with ceramide metabolism[J]. Biochem Pharmacol , 2009, 78 :477–485. DOI:10.1016/j.bcp.2009.05.002 |
| [73] | Wrasidlo W, Mielgo A, Torres VA, et al. The marine lipopep-tide somocystinamide A triggers apoptosis via caspase 8[J]. Proc Natl Acad Sci U S A , 2008, 105 :2313–2318. DOI:10.1073/pnas.0712198105 |
| [74] | Iwasaki A, Ohno O, Sumimoto S. Jahanyne, an apoptosis-inducing lipopeptide from the marine cyanobacterium Lyngbya sp[J]. Org Lett , 2015, 17 :652–655. DOI:10.1021/ol5036722 |
| [75] | Bowman EJ, Gustafson KR, Bowman BJ, et al. Identification of a new chondropsin class of antitumor compound that selectively inhibits V-ATPases[J]. J Biol Chem , 2003, 278 :44147–44152. DOI:10.1074/jbc.M306595200 |
| [76] | Ning X, Zhao J, Zhang Y, et al. A novel anti-tumor protein extracted from Meretrix meretrix Linnaeus induces cell death by increasing cell permeability and inhibiting tubulin polym-erization[J]. Int J Oncol , 2009, 35 :805–812. |
| [77] | Liu M, Zhao XZ, Zhao J, et al. Induction of apoptosis, G0/G1 phase arrest and microtubule disassembly in K562 leukemia cells by Mere15, a novel polypeptide from Meretrix meretrix Linnaeus[J]. Mar Drugs , 2012, 10 :2596–2607. DOI:10.3390/md10112596 |
| [78] | Zhao J, Wei JT, Liu M, et al. Cloning, characterization and expression of a cDNA encoding a granulin-like polypeptide in Ciona savignyi[J]. Biochimie , 2013, 95 :1611–1619. DOI:10.1016/j.biochi.2013.05.001 |
 2016, Vol. 51
2016, Vol. 51


