药物转运体是位于细胞膜上的功能性膜蛋白,在药物或化学异物的吸收、分布、代谢和排泄等体内过程中起非常重要的作用,是影响药物效应及产生药物-药物相互作用的重要因素。药物转运体主要分为可溶性物质载体 (solute carrier,SLC) 和ATP结合盒转运体 (ATP binding cassette,ABC)。
ABC转运体是由49个已知基因结构的家族成员构成的蛋白超家族,包括P-糖蛋白 (P-glycoprotein,P-gp)、多药耐药相关蛋白 (multidrug resistance protein,MRP1) 和乳腺癌耐药蛋白 (breast cancer resistance protein,BCRP) 等,主要发挥外排转运的作用[1, 2]。
BCRP是继P-gp和MRP1耐药蛋白之后发现的第三大外排转运蛋白[3]。作为外排性转运体,BCRP在机体中分布广泛,作用底物多样,不仅是引起肿瘤化疗药物耐药的重要因素,也影响其他非抗癌药的体内过程。鉴于其对药物代谢动力学的重要作用,CFDA、FDA等均建议在进行新药的临床申报时,应研究该化合物与BCRP转运体的相互作用。
自1998年BCRP转运体被发现以来,国内外已有许多相关文章发表,发现数百种药物或化学异物均为BCRP转运体的底物或抑制剂[4]。本文从BCRP转运体的发现、生理功能和转运机制、底物和抑制剂以及对药物代谢动力学特征的影响等方面总结了关于BCRP转运体的最新研究进展,以期为药物转运体BCRP与药物代谢动力学的研究提供借鉴。
1 BCRP的发现药物转运体介导的药物外排是引起多药耐药的重要因素。19世纪70年代,人们普遍认为P-gp和MRP1是产生药物耐药现象的主要外排蛋白。直到20世纪末,Ross等[5]从白血病患者体内发现一种新型耐药细胞,而P-gp和MRP1都不是引起该细胞株耐药的原因。Doyle等[6]进一步分离得到一种新的依赖ATP的转运蛋白,由于是从乳腺癌耐药细胞株 (drug- resistant breast cancer cell) MCF/AdrVp细胞株中发 现的,因此命名为BCRP。同时,另外两个实验室的Allikmets等[7]和Miyake等[8]也分别从人胎盘细胞 (human placenta cell) 和米托蒽醌耐药细胞株 (mitoxantrone-resistant cell) 中分离到该蛋白,因此又被命名为ABCP和MXR。BCRP、ABCP和MXR都是指同一种转运蛋白,只是个别位点的氨基酸不同。另外,BCRP的编码基因为ABC转运体超家族中G亚家族的第二个成员,因此又被称为ABCG2。为区别人和其他种属,人乳腺癌耐药蛋白命名为BCRP/ABCG2,啮齿类动物 (大鼠/小鼠) 乳腺癌耐药蛋白命名为Bcrp/Abcg2。
多个BCRP转染细胞研究表明其与某些抗癌药物的多药耐药程度高度相关,减少抗癌药物在细胞中的累积量,提示BCRP是特定类型抗癌药物耐药的耐药蛋白[9-13]。BCRP存在于体内多个重要器官,如小肠、胎盘、肝脏、肾脏等,对药物的吸收 (小肠)、分布 (胎盘)、代谢 (肝脏) 和排泄 (肾脏) 具有重要意义。
2 BCRP的组织分布了解药物转运体在不同组织中的分布趋势,有助于预测和解释药物在体内的吸收、分布、代谢和排泄过程。Maliepaard等[14]首次对BCRP转运体在人 体正常组织中的表达进行了研究,结果表明BCRP 转运体在胎盘合胞体滋养层 (placental syncytiotrophoblasts) 表达量最高,小肠和结肠上皮细胞顶侧膜 (apical membrane) 以及肝胆小管膜次之。Huls等[15]报道BCRP在人肾近曲小管细胞顶侧膜也有表达,不过表达量明显低于小肠和肝组织。随着研究的深入,人们逐渐发现BCRP在视网膜毛细血管内皮细胞和各种血-组织屏障 (blood-tissue barriers) 如血脑屏障 (blood-brain barriers)、血睾屏障 (blood-testis barriers)、血-胎盘屏障 (blood-placental barriers) 和血脊髓屏障(blood-spinal cord barriers) 中也有表达[16-19]。与人相比,小鼠肾组织中BCRP表达量较高,胎盘表达水平一般,存在一定的种属差异[20]。
3 BCRP的生理结构及转运机制BCRP是分子质量为72 kD的跨膜蛋白,由655个氨基酸组成,编码基因为ABCG2。由于BCRP只有1个核苷酸结合区 (nucleotide binding domain,NBD) 和1个6次跨膜区 (transmembrane domain,TMD),因此,又被称为半转运体 (half-transporter) BCRP[6]。此外,和大多数ABC转运体的结构相反,BCRP转运体的ATP结合部位在 -NH2端,跨膜区在 -COOH端(图 1)[21]。由于ABC转运体发挥转运功能需要两个NBD结构,因此,BCRP常以二聚体或多聚体的形式存在[22-24]。研究表明,BCRP多聚体的形成与603位半胱氨酸形成二硫键有关[25, 26]。BCRP结构中只有一个N糖基化结合位点,即位于5,6跨膜结构域的596位天冬氨酸 (Asp)[27]。
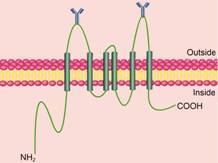
|
Figure 1 The membrane topology model of transporter breast cancer resistance protein (BCRP) |
作为ABC转运体家族的一员,BCRP与P-gp类似,也是利用ATP水解提供能量,将底物从细胞内转运至细胞外。NBD负责结合ATP; TMD作为底物的结合部位,决定转运底物的特异性。与P-gp转运机制不同的是,BCRP进行转运时需要由二硫键形成二聚体或多聚体[28]。
一般来说,ABC转运体均具有多个底物的结合位点。由于目前尚未获得高分辨率的BCRP 3D结构,人们对BCRP与底物的结合位点知之甚少。Mao等[29]报道Arg482和Pro485可影响BCRP对部分底物的转运,但对于是直接影响与底物的结合,还是间接通过稳定结构或诱导构象改变而影响BCRP与底物的结合还需要进一步确认。Clark等[30]通过亲和动力学研究发现米托蒽醌和哌唑嗪与BCRP的结合位点明显不同。Giri等[31]通过对BCRP与抑制剂的相互作用研究证实,齐多夫定和阿巴卡韦与BCRP的结合位点与哌唑嗪不完全重合。有学者[32]应用BCRP的同源模型推测,BCRP 6次跨膜区域形成的内部空腔足以适应多种底物与BCRP的结合,但是BCRP的准确结合位点还需高分辨的BCRP 3D结构进行确认。
4 BCRP的底物和抑制剂 4.1 BCRP的底物自BCRP转运体发现以来,已知底物超过200个,代表性经典底物见表 1[33-57]。由于BCRP发现于肿瘤耐药细胞,因此最初报道的BCRP底物大多是经典的肿瘤化疗药物,如米托蒽醌 (mitoxantrone)、喜树碱衍生物 (camptothecin derivates)、甲氨蝶呤 (methotrexate) 等。米托蒽醌是最早发现的经典BCRP底物,常被用来做为研究BCRP转运体的阳性对照药[20, 33, 34]。喜树碱衍生物包括喜 树碱类抗肿瘤药如拓扑替康 (topotecan)、伊立替康 (irinotecan)、SN-38 (伊立替康活性代谢产物) 等。上述药物在肿瘤细胞的耐药性与BCRP的表达水平密切相关[36-38]。需要指出的是,喜树碱本身以及其类似物包括DX9851f和9-硝基喜树碱却不是BCRP的 转运底物[37, 58, 59]。随着BCRP转运体的深入研究,更多的化疗药物被发现是BCRP的底物,如酪氨酸激 酶抑制剂 (tyrosine kinase inhibitors,TKIs): 伊马替尼 (imatinib); 光敏剂 (photosensitizers): 脱镁叶绿酸A (pheophorbide A,PhA)、原卟啉IX (protoporphyrin) 等[40-42]。
当然,BCRP转运体的底物不仅仅限于肿瘤治 疗药,部分非肿瘤治疗药物也是其底物。包括一些 抗病毒药 (antivirals)、调血脂药 (statins)、抗生素 (antibiotics)、钙离子通道阻滞剂 (calcium channel blockers) 如齐多夫定、西伐他汀、环丙沙星、尼群地平等[43, 48, 50, 60]。除此之外,某些荧光探针也是BCRP的底物,如BODIPY-FL prazosin、Hoechst 33342、PhA等,可作为体外研究BCRP转运体活性的重要工具[41, 61, 62]。
BCRP个别位点的氨基酸突变可影响其转运底物的特异性。蒽环霉素 (anthracycline) 在BCRP高表达的MCF-7/AdrVp3000细胞株和S1-M1-80细胞株中高度耐药,而在其他10种BCRP高表达的细胞株中则无耐药现象[63]。进一步研究发现,MCF-7/AdrVp3000细胞中BCRP cDNA编码的482号氨基酸为Thr,S1-M1-80为Gly (均为突变型),而在其他癌细胞和正常细胞中为Arg (野生型)。值得一提的是,罗丹明123 (rhodamine 123) 和Lysotracker只是突变型BCRP的底物,而非野生型BCRP的底物[64]。
| Table 1 Summary of substrates of BCRP |
虽然目前已发现200余种BCRP转运体的底物,但是人们对BCRP转运体与底物之间的构效关系 (structure-activity relationship,SAR) 却不甚明了。BCRP与喜树碱类化合物相互作用的SAR研究发现,喜树碱类化合物C-10和C-11的极性越大,与BCRP转运体的亲和力越强[65]。Hazai等[66]根据已知263个BCRP底物运用support vector machine (SVM) 方法构建了BCRP底物的预测模型,准确率约为76%。
4.2 BCRP的抑制剂抑制剂是研究药物转运体的重要工具。对于大多数转运体来说,研究一个药物是否是转运体的底物,最常用的方法就是抑制剂法。因此,了解转运体抑制剂的类型和种类,对于研究药物与转运体的相互作用具有重要意义。
作为肿瘤多药耐药蛋白,以BCRP为靶标逆转多药耐药的抑制剂研究较为广泛。表 2[67-82]总结了部分经典抑制剂。烟曲霉毒素 (fumitremorgin C,FTC) 是第一个被发现的BCRP特异性抑制剂,从烟曲霉原变种 (Aspergillus fumigatus) 菌落中提取分离,IC50约为1 μmol·L-1 [67, 68]。然而,由于FTC具有较强的神 经毒作用,人们以其为先导物,发现了多种对BCRP有抑制作用的类似物,如Ko132、ko134和Ko143等(IC50为100~200 nmol·L-1)[69]。虽然Ko143在过去十几年中一直作为研究药物与BCRP相互作用的阳性对照药,但最近报道指出,Ko143并非BCRP转运体的特异性抑制剂,其对ABCB1和ABCC1也有一定抑制作用。因此,应用Ko143研究药物与BCRP转运体的相互作用时需考虑其特异性问题[83]。
| Table 2 Summary of inhibitors of BCRP. ND: Not detected |
自FTC发现以后,陆续发现了多个结构类型不同的BCRP转运体的抑制剂,包括新生霉素 (novobiocin)、三氧苯胺 (tamoxifen)、利托那韦 (ritonavir)、吉非替尼 (gefitinib) 等[72-75],均为BCRP的非特异性抑制剂。直到目前为止,还未有报道BCRP的特异性抑制剂。
根据BCRP转运体抑制剂的作用机制不同,可 分为以下3类: ① 抑制ATP酶活性,从而抑制BCRP转运活性,如FTC和Ko143; ② 抑制剂本身为BCRP的底物,竞争性抑制BCRP的转运,如双嘧达莫; ③ 抑制剂虽不是BCRP的底物,但可与BCRP结合而 改变其构象,进而影响BCRP的转运,如利托那 韦[51, 67, 75, 77]。由于BCRP有多个结合位点,因此②和③类抑制剂只抑制部分药物的转运。Giri等[31]发现 那非那韦 (nelfinavir) 可抑制BCRP对齐多夫定和阿巴卡韦的转运,但不抑制哌唑嗪和伊马替尼的转运。
与BCRP的底物研究不同,对BCRP与抑制剂的SAR和定量构效关系 (QSAR) 的研究较为深入[84-86]。黄酮类化合物和FTC类似物的亲脂性是抑制作用的关键因素[87, 88]。此外,抑制剂的平面结构、杂环上C-NH2键以及潜在的氢键对BCRP的抑制也有重要影响[86, 89]。有学者构建了不同SAR和QSAR模型用于预测BCRP的抑制剂,由于目前对BCRP与底物和抑制剂的相互作用机制还不是十分清楚,因此,上述模型只能进行内部数据集的预测[29]。
5 BCRP的单核苷酸多态性基因突变在自然界中普遍存在,药物转运体也不例外。基因突变会导致mRNA表达水平、翻译效率以及蛋白功能的改变。由于药物转运体对药物的 吸收、分布和排泄有重要影响,因此药物转运体的基因突变会引起底物药物在不同个体的药代动力学差异,最终影响药物的有效性和毒性。目前,人们已从人不同器官的DNA样品中发现了大约80种ABCG2基因的单核苷酸基因多态性 (SNPs)。其中,34G > A (V12M) 和421C > A(Q141K) 的突变频率最高,在东亚人群中约为30%~60%,而在高加索人和美洲人群中约为5%~10%。其他SNPs的发生频率均较低,约为1%[29]。有研究表明,421C > A导致的Q141K突变可引起细胞表面的BCRP表达减少,进而降低其外排活性; 而34G > A导致的V12M突变对BCRP表达以及耐药程度无明显影响[90, 91]。其他SNPs如114T > C,369C > T,474C > T,564A > G,1098G > A和1425A > G不引起氨基酸的改变,但是否会影响BCRP的表达和活性还未有报道[92]。
有学者研究了BCRP的SNPs与其底物药物的药物代谢动力学的相关性,发现421C > A的基因突变对他汀类药物如罗素伐他汀、氟伐他汀和辛伐他汀 的药物代谢动力学特征有重要影响。联合口服给药BCRP抑制剂GF120918后,上述他汀类药物在BCRP基因表型为421AA纯合子的受试者体内的AUC或Cmax明显升高,而在421CA杂合子受试者体内则无明显变化[93, 94]。
6 BCRP对药物体内过程的影响由上所述,BCRP转运体存在于体内多个重要组织,因此其对药物和化学异物在体内过程的各个环节: 吸收 (小肠)、分布 (胎盘)、消除 (肝脏和肾脏) 均可能有重要影响。
6.1 BCRP对药物口服生物利用度的影响Jonker等[95]报道,ABCB1/ABCG2双抑制剂依克利达 (GF120918) 可显著提高拓扑替康在mdr1a/mdr1b-/-小鼠的生物利用度,提示拓扑替康生物利用度的增加可能来自小肠部位吸收的增加和肝脏消除的减少。Yamagata等[96]发现,Abcg2基因敲除小鼠拓扑替康的吸收速率增加了2倍,体内暴露量增加了3.6倍,进一步证明了BCRP转运体对拓扑替康的口服生物利用度的作用。此后,应用基因敲除Abcg2小鼠的研究多次报道了BCRP转运体对药物或化学异物在小肠吸收的影响,如抗生素类药物、抗炎药柳氮磺胺嘧啶、CDK抑制剂JNJ-7706621等[97-99]。此外,BCRP还可影响多种食物成分的摄取,如2-氨基-3甲基咪唑并 [4,5-f] 喹啉 (IQ)、3-甲基-1,4二甲基-5H-吡啶并 [4,3-b] 吲哚 (Trp-P-1) 等[100]。
值得注意的是,尽管体外研究证明多种药物是BCRP的底物,但在Abcg2基因敲除小鼠模型未证 实BCRP对生物利用度的调节作用。如依托泊苷 (etoposide) 曾被多次报道为BCRP的底物,然而有 研究[101, 102]发现在Mdr1a/Mdr1b-/-小鼠中依克立达 (BCRP抑制剂) 对其口服生物利用度和清除率并无显著性影响。同样的药物还有抗艾滋病药物阿巴卡 韦和齐多夫定,其在野生和Abcg2-/-小鼠中的药代动力学参数无显著性差异[71]。
6.2 BCRP对药物透过血脑屏障的影响血脑屏障是药物或化学异物进入大脑的重要屏障,对大脑内环境稳定起重要的保护作用。BCRP在大脑微血管内皮细胞中的表达提示其可调节药物进入中枢系统。BCRP转运体特异性底物如单曲林、木黄酮、香豆雌酚等在Abcg2-/-小鼠中脑内的累积量明显高于野生型小鼠[103]。但是由于血脑屏障结构的复杂性以及存在多种药物转运体如P-糖蛋白、ABCB1等,BCRP对非特异性底物透过血脑屏障的作用机制比较复杂。如静脉注射伊马替尼后,与野生型小鼠相比,Abcg2-/-小鼠大脑透过量增加2.5倍,而Mdr1a/Mdr1b-/-小鼠大脑透过量增加3.6倍,提示BCRP和MDR1转运 体均对伊马替尼透过血脑屏障产生影响。然而在体 脑灌流实验发现,伊马替尼低浓度 ( < 1 μmol·L-1)
时,MDR1对其转运起主要作用,BCRP对其吸收几 乎没有影响。高浓度 (1~20 μmol·L-1) 时,BCRP 明显影响伊马替尼的吸收[104, 105]。又如野生型小鼠和Abcg2-/-小鼠短时间在体脑灌流致癌物1-氨基-1-甲基-6-苯咪基并[4,5-b]吡啶 (PhIP),脑内累积量无明显差异,而随灌流时间的延长,Abcg2-/-小鼠PhIP脑内累积量明显高于野生鼠[106]。BCRP转运体对药物或化学异物跨血脑屏障的影响与药物浓度、暴露时间以及药物或化学异物与转运体的亲和力密切相关。
6.3 BCRP对药物透过胎盘屏障的影响BCRP在胎盘绒毛合胞体滋胚层顶侧膜高表达,阻止药物或化学异物进入胎盘。Keskitalo等[93]首次报道了BCRP对拓扑替康通过胎盘屏障的影响。Mdr1a/Mdr1b-/-小鼠给予依克立达后,胎儿体内拓扑替康浓度增加两倍。Zhang等[107]发现静脉注射BCRP底物呋喃妥英后,Abcg2-/-受孕小鼠胎儿体内呋喃妥因浓度是野生受孕鼠的5倍。另外,BCRP转运体对抗糖尿病药格列本脲、致癌物PhIP和甲硝基二甲胺等透过胎盘屏障也有明显影响[108-110]。
6.4 BCRP对药物进入乳腺的影响与其他屏障作用相反,BCRP可增加药物或化学异物进入乳腺。Alcorn等[111]研究发现,野生型小鼠阿昔洛韦的血浆/母乳比为5∶1,而在Abcg2-/-小鼠中阿昔洛韦的乳腺累积量显著降低。同样,BCRP转运体也可增加西咪替丁、呋喃妥英和PhIP等在乳腺中的吸收[112-114]。
6.5 BCRP对药物肝脏胆汁排泄的影响已知BCRP表达于肝细胞的胆小管膜,提示可能影响药物的胆汁排泄[115]。研究报道,甲氨蝶呤在Abcg2-/-小鼠的清除率明显低于野生型小鼠,而7-OH甲氨蝶呤却无此现象[75, 116]; 抗血脂药匹伐他汀在Abcg2-/-小鼠的胆汁排泄量是野生型小鼠的1/10[117]。Sparreboom等[39]利用Abcg2-/-小鼠研究了BCRP转运体对喹诺酮类抗生素环丙沙星、格帕沙星、氧氟沙星和尤利沙星胆汁排泄的影响。
6.6 BCRP对临床药物相互作用的影响目前已有临床研究证实BCRP在药物-药物相互作用 (drug- drug interactions) 中的重要作用。Kruijtzer等[118]首 次报道联合口服给药GF120918后,拓扑替康的生物利用度由40% 增加到97%。近年来,人们又多次发 现临床上BCRP的底物药物与其抑制剂合用后,会 发生明显的DDIs,包括阿托伐他汀与利托那韦、罗素伐他汀与利托那韦、辛伐他汀与GSK1292263,甲氨蝶呤和奥美拉唑合用等[119-121]。
7 结语近年来,药物转运体对药物体内过程的影响一直是药物代谢动力学研究的热点之一。BCRP转运体作为ABC转运体家族中的重要一员,对药物的吸收、分布、代谢和排泄等过程都起着重要作用。然而,目前对于BCRP转运体的分子转运机制尚不完全明了。因此,获得高分辨率BCRP 3D结构,明确BCRP转运体与底物或抑制剂的SAR,构建计算机预测的BCRP底物或抑制剂模型都将有助于深入了解BCRP转运体在药物代谢动力学和药物-药物相互作用中的意义,为新药研发提供依据。
| [1] | Borst P, Evers R, Kool M, et al. A family of drug transporters: The multidrug resistance-associated proteins[J]. J Natl Cancer Inst , 2000, 92 :1295–1302. DOI:10.1093/jnci/92.16.1295 |
| [2] | Dean M, Rzhetsky A, Allikmets R. The human ATP-binding cassette (ABC) transporter superfamily[J]. Genome Res , 2001, 11 :1156–1166. DOI:10.1101/gr.GR-1649R |
| [3] | Meyer zu Schwabedissen HE, Kroemer HK. In vitro and in vivo evidence for the importance of breast cancer resistance protein transporters (BCRP/MXR/ABCP/-ABCG2)[J]. Handb Exp Pharmacol , 2011, 201 :327–371. |
| [4] | Natarajan K, Xie Y, Baer MR, et al. Role of breast cancer resistance protein (BCRP/ABCG2) in cancer drug resistance[J]. Biochem Pharmacol , 2012, 83 :1084–1103. DOI:10.1016/j.bcp.2012.01.002 |
| [5] | Ross DD, Doyle LA, Schiffer CA, et al. Expression of multidrug resistance-associated protein (MRP) mRNA in blast cells from acute myeloid leukemia (AML) patients[J]. Leukemia , 1996, 10 :48–55. |
| [6] | Doyle LA, Yang W, Abruzzo LV, et al. A multidrug resistance transport from human MCF-7 breast cancer cells[J]. Proc Natl Acad Sci USA , 1998, 95 :15665–15670. DOI:10.1073/pnas.95.26.15665 |
| [7] | Allikmets R, Schriml LM, Hutchinson A, et al. A human placenta-specific ATP-binding cassette gene (ABCP) on chromosome 4q22 that is involved in multidrug resistance[J]. Cancer Res , 1998, 58 :5337–5339. |
| [8] | Miyake K, Mickley L, Litman T, et al. Molecular cloning of cDNAs which are highly overexpressed in mitoxantrone- resistant cells: demonstration of homology to ABC transport genes[J]. Cancer Res , 1999, 59 :8–13. |
| [9] | Rocchi E, Khodjakov A, Volk EL, et al. The product of the ABC half-transporter gene ABCG2 (BCRP/MXR/ABCP) is expressed in the plasma membrane[J]. Biochem Biophys Res Commun , 2000, 271 :42–46. DOI:10.1006/bbrc.2000.2590 |
| [10] | Maliepaard M, Scheffer GL, Faneyte IF, et al. Subcellular localization and distribution of the breast cancer resistance protein transporter in normal human tissue[J]. Cancer Res , 2001, 61 :3458–3464. |
| [11] | Allen JD, Schinkel AH. Multidrug resistance and pharma-cological protection mediated by the breast cancer resistance protein (BCRP/ABCG2)[J]. Mol Cancer Ther , 2002, 1 :427–434. |
| [12] | Doyle LA, Ross DD. Multidrug resistance mediated by the breast cancer tesistance protein BCRP (ABCG2)[J]. Oncogene , 2003, 22 :7340–7358. DOI:10.1038/sj.onc.1206938 |
| [13] | Bates SE, Robey RW, Miyake K, et al. The role of half-transporter in multidrug resistance[J]. J Bioenerg Biomembr , 2001, 33 :503–511. DOI:10.1023/A:1012879205914 |
| [14] | Maliepaard M, Scheffer GL, Faneyte IF, et al. Subcellular localization and distribution of the breast cancer resistance protein transporter in normal human tissues[J]. Cancer Res , 2001, 61 :3458–3464. |
| [15] | Huls M, Brown CD, Windass AS, et al. The breast cancer resistance protein transporter ABCG2 is expressed in the human kidney proximal tubule apical membrane[J]. Kidney Int , 2008, 73 :220–225. DOI:10.1038/sj.ki.5002645 |
| [16] | Cooray HC, Blackmore CG, Maskell L, et al. Localisation of breast cancer resistance protein in microvessel endothelium of human brain[J]. Neuroreport , 2002, 13 :2059–2063. DOI:10.1097/00001756-200211150-00014 |
| [17] | Asashima T, Hori S, Ohtsuki S, et al. ATP-binding cassette transporter G2 mediates the efflux of phototoxins on the luminal membrane of retinal capillary endothelial cells[J]. Pharm Res , 2006, 23 :1235–1242. DOI:10.1007/s11095-006-0067-2 |
| [18] | Robillard KR, Hoque T, Bendayan R. Expression of ATP-binding cassette membrane transporters in rodent and human Sertoli cells: relevance to the permeability of antiretroviral therapy at the blood-testis barrier[J]. J Pharmacol Exp Ther , 2012, 340 :96–108. DOI:10.1124/jpet.111.186916 |
| [19] | Jablonski MR, Jacob DA, Campos C, et al. Selective increase of two ABC drug efflux transporters at the blood-spinal cord barrier suggests induced pharmacoresistance in ALS[J]. Neurobiol Dis , 2012, 47 :194–200. DOI:10.1016/j.nbd.2012.03.040 |
| [20] | Allen JD, Brikhuis RF, Wijnholds J, et al. The mouse Bcrp1/Mxr/Abcp gene: amplification and overexpression in cell lines selected for resistance to topotecan, mitoxantrone, or doxorubicin[J]. Cancer Res , 1999, 59 :4237–4241. |
| [21] | Polgar O, Robey RW, Bates SE. ABCG2: Structure, function and role in drug response[J]. Expert Opin Drug Metab Toxicol , 2008, 4 :1–15. DOI:10.1517/17425255.4.1.1 |
| [22] | Kage K, Tsukahara S, Sugiyama T, et al. Dominant-negative inhibition of breast cancer resistance protein as drug efflux pump through the inhibition of S-S dependent homodimeriza-tion[J]. Int J Cancer , 2002, 97 :626–630. DOI:10.1002/(ISSN)1097-0215 |
| [23] | Nakanishi T, Doyle A, Hassel B, et al. Functional characteri-zation of human breast cancer resistance protein (BCRP, ABCG2) expressed in the oocytes of Xenopus laevis[J]. Mol Pharmacol , 2003, 64 :1452–1462. DOI:10.1124/mol.64.6.1452 |
| [24] | Xu J, Liu Y, Yang Y, et al. Characterization of oligomeric human half-ABC transporter ATP-binding cassette G2[J]. J Biol Chem , 2004, 279 :19781–19789. DOI:10.1074/jbc.M310785200 |
| [25] | Henrisksen U, Fog JU, Litman T, et al. Identification of intra-and intermolecular disulfide bridges in the multidrug resistance transporter ABCG2[J]. J Biol Chem , 2005, 280 :36926–36934. DOI:10.1074/jbc.M502937200 |
| [26] | Wakabayashi K, Nakagawa H, Tamura A, et al. Intramolecular disulfide bond is a critical check point determining degradative fates of ATP-binding cassette (ABC) transporter ABCG2 protein[J]. J Biol Chem , 2007, 282 :27841–27846. DOI:10.1074/jbc.C700133200 |
| [27] | Dipo NK, Hrycyna CA. N-linked glycosylation of the human ABC transporter ABCG2 on asparagine 596 is not essential for expression, transport activity, or trafficking to the plasma membrane[J]. Biochemistry , 2005, 44 :5420–5429. DOI:10.1021/bi0479858 |
| [28] | Nakagawa H, Wakabayashi-Nakao K, Tamura A, et al. Dis-ruption of N-linked glycosylation enhances ubiquitin-mediated proteasomal degradation of the human ATP-binding cassette transporter ABCG2[J]. FEBS J , 2009, 276 :7237–7252. DOI:10.1111/j.1742-4658.2009.07423.x |
| [29] | Mao Q, Unadkat JD. Role of the breast cancer resistance protein (BCRP/ABCG2) in drug transport-an update[J]. AAPS J , 2015, 17 :65–82. DOI:10.1208/s12248-014-9668-6 |
| [30] | Clark R, Kerr ID, Callaghan R, et al. Multiple drug binding site on the R482G isoform of the ABCG2 transporter[J]. Br J Pharmacol , 2006, 149 :506–515. DOI:10.1038/sj.bjp.0706904 |
| [31] | Giri N, Agarwal S, Shaik N, et al. Substrate-dependent breast cancer resistance protein (Bcrp1/Abcg2)-mediated interactions: consideration of multiple binding sites in in vitro assay design[J]. Drug Matab Dispos , 2009, 37 :560–570. DOI:10.1124/dmd.108.022046 |
| [32] | Ni Z, Bikadi Z, Cai X, et al. Transmembrane helices 1 and 6 of the human breast cancer resistance protein (BCRP/ABCG2): identification of polar residues important for drug transport[J]. Am J Physiol Cell Physiol , 2010, 299 :1100–1109. |
| [33] | Litman T, Brangi M, Hudson E, et al. The multidrug-resistant phenotype associated with overexpression of the new ABC half-transporter, MXR (ABCG2)[J]. J Cell Sci , 2000, 113 :2011–2021. |
| [34] | Minderman H, Suvannasankha A, O'Loughlin KL, et al. Flow cytometric analysis of breast cancer resistance protein expression and function[J]. Cytometry , 2002, 48 :59–65. DOI:10.1002/(ISSN)1097-0320 |
| [35] | Chen ZS, Robey RW, Belinsky MG, et al. Transport of meth-otrexate, methotrexate polyglutamates, and 17β-estradiol 17-(β-D-glucuronide) by ABCG2: effects of acquired mutations at R482 on methotrexate transport[J]. Cancer Res , 2003, 63 :4048–4054. |
| [36] | Yang CH, Schneider E, Kuo ML, et al. BCRP/MXR/ABCP expression in topotecan-resistant human breast carcinoma cells[J]. Biochem Pharmacol , 2000, 60 :831–837. DOI:10.1016/S0006-2952(00)00396-8 |
| [37] | Ishii M, Iwahana M, Mitsui I, et al. Growth inhibitory effect of a new camptothecin analog, DX-8951f, on various drug-resistant sublines including BCRP-mediated camptothecin derivative-resistant variants derived from the human lung cancer cell line PC-6[J]. Anticancer Drugs , 2000, 11 :353–362. DOI:10.1097/00001813-200006000-00005 |
| [38] | Kawabata S, Oka M, Shiozawa K, et al. Breast cancer resistance protein directly confers SN-38 resistance of lung cancer cells[J]. Biochem Biophys Res Commun , 2001, 280 :1216–1223. DOI:10.1006/bbrc.2001.4267 |
| [39] | Sparreboom A, Gelderblom H, Marsh S, et al. Diflomotecan pharmacokinetics in relation to ABCG2 421C>A genotype[J]. Clin Pharmacol Ther , 2004, 76 :38–44. DOI:10.1016/j.clpt.2004.03.003 |
| [40] | Burger H, Van Tol H, Boersma AW, et al. Imatinib mesylate (STI571) is a substrate for the breast cancer resistance protein (BCRP)/ABCG2 drug pump[J]. Blood , 2004, 104 :2940–2942. DOI:10.1182/blood-2004-04-1398 |
| [41] | Jonker JW, Buitelaar M, Wagenaar E, et al. The breast cancer resistance protein protects against a major chlorophyll-derived dietary phototoxin and protoporphyria[J]. Proc Natl Acad Sci USA , 2002, 99 :15649–15654. DOI:10.1073/pnas.202607599 |
| [42] | Robey RW, Steadman K, Polgar O, et al. ABCG2-mediated transport of photosensitizers: potential impact on photodynamic therapy[J]. Cancer Biol Ther , 2005, 4 :187–194. |
| [43] | Pan G, Giri N, Elmquist WF. Abcg2/Bcrp1 mediates the polarized transport of antiretroviral nucleosides abacavir and zidovudine[J]. Drug Metab Dispos , 2007, 35 :1165–1173. DOI:10.1124/dmd.106.014274 |
| [44] | Kim HS, Sunwoo YE, Ryu JY, et al. The effect of ABCG2 V12M, Q141K and Q126X, known functional variants in vitro, on the disposition of lamivudine[J]. Br J Clin Pharmacol , 2007, 64 :645–654. DOI:10.1111/bcp.2007.64.issue-5 |
| [45] | Huang L, Wang Y, Grimm S. ATP-dependent transport of rosuvastatin in membrane vesicles expressing breast cancer resistance protein[J]. Drug Metab Dispos , 2006, 34 :738–742. DOI:10.1124/dmd.105.007534 |
| [46] | Fujino H, Saito T, Ogawa S, et al. Transporter-mediated influx and efflux mechanisms of pitavastatin, a new inhibitor of HMG-CoA reductase[J]. J Pharm Pharmacol , 2005, 57 :1305–1311. DOI:10.1211/jpp.57.10.0009 |
| [47] | Matsushima S, Maeda K, Kondo C, et al. Identification of the hepatic efflux transporters of organic anions using double-transfected Madin-Darby canine kidney II cells expressing human organic anion-transporting polypeptide 1B1 (OATP1B1)/ multidrug resistance-associated protein 2, OATP1B1/multidrug resistance 1, and OATP1B1/breast cancer resistance protein[J]. J Pharmacol Exp Ther , 2005, 314 :1059–1067. DOI:10.1124/jpet.105.085589 |
| [48] | Ando T, Kusuhara H, Merino G, et al. Involvement of breast cancer resistance protein (ABCG2) in the biliary excretion mechanism of fluoroquinolones[J]. Drug Metab Dispos , 2007, 35 :1873–1879. DOI:10.1124/dmd.107.014969 |
| [49] | Merino G, Alvarez AI, Pulido MM, et al. Breast cancer resistance protein (BCRP/ABCG2) transports fluoroquinolone antibiotics and affects their oral availability, pharmacokinetics, and milk secretion[J]. Drug Metab Dispos , 2006, 34 :690–695. DOI:10.1124/dmd.105.008219 |
| [50] | Chearwae W, Shukla S, Limtrakul P, et al. Modulation of the function of the multidrug resistance-linked ATP-binding cassette transporter ABCG2 by the cancer chemopreventive agent curcumin[J]. Mol Cancer Ther , 2007, 5 :1995–2006. |
| [51] | Shukla S, Robey RW, Bates SE, et al. The calcium channel blockers, 1,4-dihydropyridines, are substrates of the multidrug resistance-linked ABC drug transporter, ABCG2[J]. Bio-chemistry , 2006, 45 :8940–8951. |
| [52] | Jonker JW, Merino G, Musters S, et al. The breast cancer resistance protein BCRP (ABCG2) concentrates drugs and carcinogenic xenotoxins into milk[J]. Nat Med , 2005, 11 :127–129. DOI:10.1038/nm1186 |
| [53] | Zhao R, Raub TJ, Sawada GA, et al. Breast cancer resistance protein interacts with various compounds in vitro, but plays a minor role in substrate efflux at the blood-brain barrier[J]. Drug Metab Dispos , 2006, 37 :1251–1258. |
| [54] | Lagas JS, van der Kruijssen CM, van de Wetering K, et al. Transport of diclofenac by breast cancer resistance protein (ABCG2) and stimulation of multidrug resistance protein 2 (ABCC2)-mediated drug transport by diclofenac and benzbro-marone[J]. Drug Metab Dispos , 2009, 37 :129–136. DOI:10.1124/dmd.108.023200 |
| [55] | Gedeon C, Behravan J, Koren G, et al. Transport of glyburide by placental ABC transporters: implications in fetal drug exposure[J]. Placenta , 2006, 27 :1096–1102. DOI:10.1016/j.placenta.2005.11.012 |
| [56] | Merino G, Jonker JW, Wagenaar E, et al. Transport of anthelmintic benzimidazole drugs by breast cancer resistance protein (BCRP/ABCG2)[J]. Drug Metab Dispos , 2005, 33 :614–618. DOI:10.1124/dmd.104.003319 |
| [57] | Kis E, Nagy T, Jani M, et al. Leflunomide and its metabolite A771726 are high affinity substrates of BCRP: implications for drug resistance[J]. Ann Rheum Dis , 2009, 68 :1201–1207. DOI:10.1136/ard.2007.086264 |
| [58] | Maliepaard M, van Gastelen MA, de Jong LA, et al. Overex-pression of the BCRP/MXR/ABCP gene in a topotecan-selected ovarian tumor cell line[J]. Cancer Res , 1999, 59 :4559–4563. |
| [59] | Rajendra R, Gounder MK, Saleem A, et al. Differential effects of the breast cancer resistance protein on the cellular accumulation and cytotoxicity of 9-aminocamptothecin and 9-nitrocamptothecin[J]. Cancer Res , 2003, 63 :3228–3233. |
| [60] | Keskitalo JE, Pasanen MK, Neuvonen PJ, et al. Different effects of the ABCG2 c.421C>A SNP on the pharmacokinetics of fluvastatin, pravastatin and simvastatin[J]. Pharmacoge-nomics , 2009, 10 :1617–1624. |
| [61] | Robey RW, Honjo Y, van de Laar A, et al. A functional assay for detection of the mitoxantrone resistance protein, MXR (ABCG2)[J]. Biochim Biophys Acta , 2001, 1512 :171–182. DOI:10.1016/S0005-2736(01)00308-X |
| [62] | Kim M, Turnquist H, Jackson J, et al. The multidrug resistance transporter ABCG2 (breast cancer resistance protein 1) effluxes Hoechst 33342 and is overexpressed in hematopoietic stem cells[J]. Clin Cancer Res , 2002, 8 :22–28. |
| [63] | Honjo Y, Hrycyna CA, Yan QW, et al. Acquired mutations in the MXR/BCRP/ABCP gene alter substrate specificity in MXR/BCRP/ABCP-overexpressing cells[J]. Cancer Res , 2001, 61 :6635–6639. |
| [64] | Robey RW, Honjo Y, Morisaki K, et al. Mutations at amino-acid 482 in the ABCG2 gene affect substrate and antagonist specificity[J]. Br J Cancer , 2003, 89 :1971–1978. DOI:10.1038/sj.bjc.6601370 |
| [65] | Yoshikawa M, Ikegami Y, Hayasaka S, et al. Novel campto-thecin analogues that circumvent ABCG2-associated drug resistance in human tumor cells[J]. Int J Cancer , 2004, 110 :921–927. DOI:10.1002/(ISSN)1097-0215 |
| [66] | Hazai E, Hazai I, Ragueneau-Majlessi I, et al. Predicting substrates of the human breast cancer resistance protein using a support vector machine method[J]. BMC Bioinform , 2013, 14 :130–135. DOI:10.1186/1471-2105-14-130 |
| [67] | Rabindran SK, He H, Singh M, et al. Reversal of a novel multidrug resistance mechanism in human colon carcinoma cells by fumitremorgin C[J]. Cancer Res , 1998, 58 :5850–5858. |
| [68] | Rabindran SK, Ross DD, Doyle LA, et al. Fumitremorgin C reverses multidrug resistance in cells transfected with the breast cancer resistance protein[J]. Cancer Res , 2000, 60 :47–50. |
| [69] | Allen JD, van Loevezijn A, Lakhai JM, et al. Potent and specific inhibition of the breast cancer resistance protein multidrug transporter in vitro and in mouse intestine by a novel analogue of fumitremorgin C[J]. Mol Cancer Ther , 2002, 1 :417–425. DOI:10.4161/cbt.1.4.20 |
| [70] | Takada K, Imamura N, Gustafson KR, et al. Synthesis and structure-activity relationship of botryllamides that block the ABCG2 multidrug transporter[J]. Bioorg Med Chem Lett , 2010, 20 :1330–1333. DOI:10.1016/j.bmcl.2010.01.016 |
| [71] | Sim HM, Lee CY, Ee PL, et al. Dimethoxyaurones: potent inhibitors of ABCG2 (breast cancer resistance protein)[J]. Eur J Pharm Sci , 2008, 35 :293–306. DOI:10.1016/j.ejps.2008.07.008 |
| [72] | Ozvegy-Laczka C, Hegedus T, Varady G, et al. High-affinity interaction of tyrosine kinase inhibitors with the ABCG2 multidrug transporter[J]. Mol Pharmacol , 2004, 65 :1485–1495. DOI:10.1124/mol.65.6.1485 |
| [73] | Yang CH, Chen YC, Kuo ML. Novobiocin sensitizes BCRP/ MXR/ABCP overexpressing topotecan-resistant human breast carcinoma cells to topotecan and mitoxantrone[J]. Anticancer Res , 2003, 23 :2519–2523. |
| [74] | Sugimoto Y, Tsukahara S, Imai Y, et al. Reversal of breast cancer resistance protein-mediated drug resistance by estrogen antagonists and agonists[J]. Mol Cancer Ther , 2003, 2 :105–112. |
| [75] | Gupta A, Zhang Y, Unadkat JD, et al. HIV protease inhibitors are inhibitors but not substrates of the human breast cancer resistance protein (BCRP/ABCG2)[J]. J Pharmacol Exp Ther , 2004, 310 :334–341. DOI:10.1124/jpet.104.065342 |
| [76] | Weiss J, Rose J, Storch CH, et al. Modulation of human BCRP (ABCG2) activity by anti-HIV drugs[J]. J Antimicrob Chemother , 2007, 59 :238–245. |
| [77] | Zhang Y, Gupta A, Wang H, et al. BCRP transports dipyrida-mole and is inhibited by calcium channel blockers[J]. Pharm Res , 2005, 22 :2023–2034. DOI:10.1007/s11095-005-8384-4 |
| [78] | Gupta A, Unadkat JD, Mao Q. Interactions of azole antifungal agents with the human breast cancer resistance protein (BCRP)[J]. J Pharm Sci , 2007, 96 :3226–3235. DOI:10.1002/jps.20963 |
| [79] | Holland ML, Lau DT, Allen JD, et al. The multidrug transporter ABCG2 (BCRP) is inhibited by plant-derived cannabinoids[J]. Br J Pharmacol , 2007, 152 :815–824. |
| [80] | Breedveld P, Zelcer N, Pluim D, et al. Mechanism of the pharmacokinetic interaction between methotrexate and benzimidazoles: potential role for breast cancer resistance protein in clinical drug-drug interactions[J]. Cancer Res , 2004, 64 :5804–5811. DOI:10.1158/0008-5472.CAN-03-4062 |
| [81] | Ahmed-Belkacem A, Pozza A, Munoz-Martinez F, et al. Flavonoid structure-activity studies identify 6-prenylchrysin and tectochrysin as potent and specific inhibitors of breast cancer resistance protein ABCG2[J]. Cancer Res , 2005, 65 :4852–4860. DOI:10.1158/0008-5472.CAN-04-1817 |
| [82] | Yoshikawa M, Ikegami Y, Sano K, et al. Transport of SN-38 by the wild type of human ABC transporter ABCG2 and its inhibition by quercetin, a natural flavonoid[J]. J Exp Ther Oncol , 2004, 4 :25–35. |
| [83] | Weidner LD, Zoghbi SS, Lu SY, et al. The inhibitor Ko143 is not specific for ABCG2[J]. J Pharmacol Exp Ther , 2015, 354 :384–393. DOI:10.1124/jpet.115.225482 |
| [84] | Ishikawa T, Hirano H, Saito H, et al. Quantitative structure-activity relationship (QSAR) analysis to predict drug-drug interactions of ABC transporter ABCG2[J]. Mini-Rev Med Chem , 2012, 12 :505–514. DOI:10.2174/138955712800493825 |
| [85] | Gandhi YA, Morris ME. Structure-activity relationships and quantitative structure-activity relationships for breast cancer resistance protein (ABCG2)[J]. AAPS J , 2009, 11 :541–552. DOI:10.1208/s12248-009-9132-1 |
| [86] | Nicolle E, Boumendjel A, Macalou S, et al. QSAR analysis and molecular modeling of ABCG2-specific inhibitors[J]. Adv Drug Deliv Rev , 2009, 61 :34–46. DOI:10.1016/j.addr.2008.10.004 |
| [87] | van Loevezijn A, Allen JD, Schinkel AH, et al. Inhibition of BCRP-mediated drug efflux by fumitremorgin-type indolyl diketopiperazines[J]. Bioorg Med Chem Lett , 2001, 11 :29–32. DOI:10.1016/S0960-894X(00)00588-6 |
| [88] | Zhang S, Yang X, Coburn RA, et al. Structure activity relationships and quantitative structure activity relationships for the flavonoid-mediated inhibition of breast cancer resis-tance protein[J]. Biochem Pharmacol , 2005, 70 :627–639. DOI:10.1016/j.bcp.2005.05.017 |
| [89] | Stacy AE, Jansson PJ, Richardson DR. Molecular pharma-cology of ABCG2 and its role in chemoresistance[J]. Mol Pharmacol , 2013, 84 :655–669. DOI:10.1124/mol.113.088609 |
| [90] | Imai Y, Nakane M, Kage K, et al. C421A polymorphism in the human breast cancer resistance protein gene is associated with low expression of Q141K protein and low-level drug resistance[J]. Mol Cancer Ther , 2002, 1 :611–616. |
| [91] | Vethanayagam RR, Wang H, Gupta A, et al. Functional analysis of the human variants of breast cancer resistance protein: I206L, N590Y, and D620N[J]. Drug Metab Dispos , 2005, 33 :697–705. DOI:10.1124/dmd.105.003657 |
| [92] | Noguchi K, Katayama K, Sugimoto Y. Human ABC trans-porter ABCG2/BCRP expression in chemoresistance: basic and clinical perspectives for molecular cancer therapeutics[J]. Pharmgenomics Pers Med , 2014, 7 :53–64. |
| [93] | Keskitalo JE, Zolk O, Fromm MF, et al. ABCG2 polymor-phism markedly affects the pharmacokinetics of atorvastatin and rosuvastatin[J]. Clin Pharmacol Ther , 2009, 86 :197–203. DOI:10.1038/clpt.2009.79 |
| [94] | Keskitalo JE, Pasanen MK, Neuvonen PJ, et al. Different effects of the ABCG2 c.421C>A SNP on the pharmacokinetics of fluvastatin, pravastatin and simvastatin[J]. Pharmacoge-nomics , 2009, 10 :1617–1624. |
| [95] | Jonker JW, Smit JW, Brinkhuis RF, et al. Role of breast cancer resistance protein in the bioavailability and fetal penetration of topotecan[J]. J Natl Cancer Inst , 2000, 92 :1651–1656. DOI:10.1093/jnci/92.20.1651 |
| [96] | Yamagata T, Kusuhara H, Morishita M, et al. Improvement of the oral drug absorption of topotecan through the inhibition of intestinal xenobiotic efflux transporter, breast cancer resistance protein, by excipients[J]. Drug Metab Dispos , 2007, 35 :1142–1148. DOI:10.1124/dmd.106.014217 |
| [97] | Merino G, Jonker JW, Wagenaar E, et al. The breast cancer resistance protein (BCRP/ABCG2) affects pharmacokinetics, hepatobiliary excretion, and milk secretion of the antibiotic nitrofurantoin[J]. Mol Pharmacol , 2005, 67 :1758–1764. DOI:10.1124/mol.104.010439 |
| [98] | Zaher H, Khan AA, Palandra J, et al. Breast cancer resistance protein (Bcrp/abcg2) is a major determinant of sulfasalazine absorption and elimination in the mouse[J]. Mol Pharm , 2006, 3 :55–61. DOI:10.1021/mp050113v |
| [99] | Seamon J, Rugg CA, Emanuel S, et al. Role of the ABCG2 drug transporter in the resistance and oral bioavailability of a potent cyclin-dependent kinase/Aurora kinase inhibitor[J]. Mol Cancer Ther , 2006, 5 :2459–2467. DOI:10.1158/1535-7163.MCT-06-0339 |
| [100] | van Erp NP, Eechoute K, van der Veldt AA, et al. Pharmaco-genetic pathway analysis for determination of sunitinib- induced toxicity[J]. J Clin Oncol , 2009, 27 :4406–4412. DOI:10.1200/JCO.2008.21.7679 |
| [101] | Kellner U, Hutchinson L, Seidel A, et al. Decreased drug accumulation in a mitoxantrone-resistant gastric carcinoma cell line in the absence of P-glycoprotein[J]. Int J Cancer , 2997, 71 :817–824. |
| [102] | Allen JD, van Dort SC, Buitelaar M, et al. Mouse breast cancer resistance protein (Bcrp1/Abcg2) mediates etoposide resistance and transport, but etoposide oral availability is limited primarily by P-glycoprotein[J]. Cancer Res , 2003, 63 :1339–1344. |
| [103] | Enokizono J, Kusuhara H, Sugiyama Y. Effect of breast cancer resistance protein (Bcrp/Abcg2) on the disposition of phytoestrogens[J]. Mol Pharmacol , 2007, 72 :967–975. DOI:10.1124/mol.107.034751 |
| [104] | Breedveld P, Pluim D, Cipriani G, et al. The effect of Bcrp1 (Abcg2) on the in vivo pharmacokinetics and brain penetration of imatinib mesylate (Gleevec): implications for the use of breast cancer resistance protein and P-glycoprotein inhibitors to enable the brain penetration of imatinib in patients[J]. Cancer Res , 2005, 65 :2577–2582. DOI:10.1158/0008-5472.CAN-04-2416 |
| [105] | Bihorel S, Camenisch G, Lemaire M, et al. Influence of breast cancer resistance protein (Abcg2) and p-glycoprotein (Abcb1a) on the transport of imatinib mesylate (Gleevec) across the mouse blood-brain barrier[J]. J Neurochem , 2007, 102 :1749–1757. DOI:10.1111/jnc.2007.102.issue-6 |
| [106] | Enokizono J, Kusuhara H, Ose A, et al. Quantitative investi-gation of the role of breast cancer resistance protein (Bcrp/ Abcg2) in limiting brain and testis penetration of xenobiotic compounds[J]. Drug Metab Dispos , 2008, 36 :995–1002. DOI:10.1124/dmd.107.019257 |
| [107] | Zhang Y, Wang H, Unadkat JD, et al. Breast cancer resistance protein 1 limits fetal distribution of nitrofurantoin in the pregnant mouse[J]. Drug Metab Dispos , 2007, 35 :2154–2158. DOI:10.1124/dmd.107.018044 |
| [108] | Annola K, Heikkinen AT, Partanen H, et al. Transplacental transfer of nitrosodimethylamine in perfused human placenta[J]. Placenta , 2009, 30 :277–283. DOI:10.1016/j.placenta.2008.12.012 |
| [109] | Myllynen P, Kummu M, Kangas T, et al. ABCG2/BCRP decreases the transfer of a food-born chemical carcinogen, 2-amino-1-methyl-6-phenylimidazo[4,5-b] pyridine (PhIP) in perfused term human placenta[J]. Toxicol Appl Pharmacol , 2008, 232 :210–217. DOI:10.1016/j.taap.2008.07.006 |
| [110] | Pollex E, Lubetsky A, Koren G. The role of placental breast cancer resistance protein in the efflux of glyburide across the human placenta[J]. Placenta , 2008, 29 :743–747. DOI:10.1016/j.placenta.2008.05.001 |
| [111] | Alcorn J, McNamara PJ. Acyclovir, ganciclovir, and zidovudine transfer into rat milk[J]. Antimicrob Agents Chemother , 2002, 46 :1831–1836. DOI:10.1128/AAC.46.6.1831-1836.2002 |
| [112] | Jagerstad IM, Johansson MA, Karlsson AA, et al. Dose-dependent milk transfer and tissue distribution of the food mutagen PhIP in rats and their suckling pups[J]. Carcino-genesis , 1994, 15 :2479–2484. DOI:10.1093/carcin/15.11.2479 |
| [113] | McNamara PJ, Meece JA, Paxton E. Active transport of cimetidine and ranitidine into the milk of Sprague Dawley rats[J]. J Pharmacol Exp Ther , 1993, 277 :1615–1621. |
| [114] | Oo CY, Paxton EW, McNamara PJ. Active transport of nitrofurantoin into rat milk[J]. Adv Exp Med Biol , 2001, 501 :547–552. DOI:10.1007/978-1-4615-1371-1 |
| [115] | Liu ZH, Liu KX. Application of model-based approaches to evaluate hepatic transporter-mediated drug clearance: in vivo, in vitro and in vivo-in vitro extrapolation[J]. Curr Drug Metab , 2016, 17 :456–468. DOI:10.2174/1389200217666160111124139 |
| [116] | Vlaming ML, Pala Z, van Esch A, et al. Functionally overlapping roles of Abcg2 (Bcrp1) and Abcc2 (Mrp2) in the elimination of methotrexate and its main toxic metabolite 7-hydroxymethotrexate in vivo[J]. Clin Cancer Res , 2009, 15 :3084–3093. DOI:10.1158/1078-0432.CCR-08-2940 |
| [117] | Hirano M, Maeda K, Matsushima S, et al. Involvement of BCRP (ABCG2) in the biliary excretion of pitavastatin[J]. Mol Pharmacol , 2005, 68 :800–807. |
| [118] | Kruijtzer CM, Beijnen JH, Rosing H, et al. Increased oral bioavailability of topotecan in combination with the breast cancer resistance protein and P-glycoprotein inhibitor GF120918[J]. J Clin Oncol , 2002, 20 :2943–2950. DOI:10.1200/JCO.2002.12.116 |
| [119] | Pham PA, la Porte CJ, Lee LS, et al. Differential effects of tipranavir plus ritonavir on atorvastatin or rosuvastatin pharmacokinetics in healthy volunteers[J]. Antimicrob Agents Chemother , 2009, 53 :4385–4392. DOI:10.1128/AAC.00449-09 |
| [120] | Polli JW, Hussey E, Bush M, et al. Evaluation of drug interactions of GSK1292263 (a GPR119 agonist) with statins: from in vitro data to clinical study design[J]. Xenobiotica , 2013, 43 :498–508. DOI:10.3109/00498254.2012.739719 |
| [121] | Suzuki K, Doki K, Homma M, et al. Co-administration of proton pump inhibitors delays elimination of plasma meth-otrexate in high-dose methotrexate therapy[J]. Br J Clin Pharmacol , 2009, 67 :44–49. DOI:10.1111/bcp.2008.67.issue-1 |
 2016, Vol. 51
2016, Vol. 51


