真核延伸因子2激酶 (eukaryotic elongation factor 2 kinase,eEF2K) 是一种Ca2+/calmudulin依赖性蛋白激酶,由Nairn等[1]于1985年首次发现。研究[2, 3, 4, 5, 6, 7, 8, 9, 10]表明,eEF2K与肿瘤的关系十分密切,在多种肿瘤细胞中高度活化或者过量表达,促进肿瘤的发生、发展。
1 eEF2K的结构及其调控
1.1 eEF2K的分子结构
真核细胞中eEF2K分子的一级结构已经被基本确定,其相对分子质量约为81 499 Da,共由724个氨基酸组成[11,12]。eEF2K并不是常见的真核蛋白激酶超家族中的一员,而是属于具有“α-kinase”催化域的激酶小家族中的一员。eEF2K包括N-端的α-kinase催化结构域、N-末端的CaM结合位点、以及位于CaM结合域前75个氨基酸残基的区域,后者表现出对eEF2K活性的抑制作用。C-端包括“SEL1”结构域,这一结构通常能够为蛋白-蛋白相互作用提供平台,C-末端还存在一结构域,能够帮助eEF2K招募其底物eEF2,并帮助eEF2发生磷酸化。在C-端和N-端结构域间存在着一个可能的“linker”结构域[13,14] (图 1)。
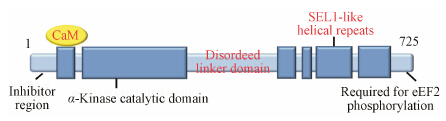
|
Figure 1 Structure of eEF2K |
1.2 eEF2K的调控
1.2.1 自身磷酸化对eEF2K活性的影响
据报道[15, 16, 17, 18, 19],eEF2K的多处Ser/Thr残基都能够发生自身磷酸化。自身磷酸化反应启动依赖于Ca2+和CAM,反应极为迅速。已经鉴定出许多eEF2K的自身磷酸化位点,包括: Ser61[18]、Ser66[18]、Ser78[18]、Thr348[19]、Thr353[18]、Ser366[18]、Ser445[18]、Ser474[19]、Ser491[18]和Ser500[19]。这些位点对eEF2K的活性有着重要影响,例如将Ser78、Thr348和Ser366位点突变为非磷酸化的Ala残基后能够明显降低eEF2K的活性[18]。Proud等[15]和Mitsui等[20]实验证明eEF2K的自身磷酸化能够提高其活性的2~3倍。到目前为止eEF2K的自身磷酸化位点还没有被完全鉴定出来,其不同位点自身磷酸化如何调控eEF2K的活性亦没有详细的报道。
1.2.2 其他位点磷酸化对eEF2K活性的调控
除了自身磷酸化,许多激酶可使得eEF2K的Ser位点发生磷酸化,从而促进或抑制eEF2K的活性。研究表明,mTORC1能够使得eEF2K的Ser78、Ser359和Ser366位点发生磷酸化而抑制eEF2K的活性[21]。例如,mTORC1能够磷酸化并激活p70S6k,导致eEF2K的Ser366位点磷酸化而抑制其活性。Wang等[22]报道MAPK能够通过p90RSKs使eEF2K的Ser366位点磷酸化而失活。Knebel等[23]还发现MAPK家族成员p38δ能够使eEF2K的Ser359位点发生磷酸化从而抑制其活性。除了mTORC1和MAPK信号通路外,其他蛋白也能使eEF2K发生磷酸化失活,例如Smith等[24]发现cdc2/cyclinB复合物能够使eEF2K的Ser359位点发生磷酸化而抑制其活性。
PKA和AMPK是报道最多的能够激活eEF2K的两种激酶[21, 25, 26, 27]。其中,Diggle等[21]研究发现PKA能够使得eEF2K的Ser499位点发生磷酸化进而激活eEF2K的活性。Browne等[27]研究表明,AMPK能够通过eEF2K的Ser398位点发生磷酸化激活eEF2K。除了这两种常见的激酶,也有研究[28]表明TRPM7 (A channel-kinase) 能够导致eEF2K的Ser77位点发生磷酸化进而激活eEF2K (图 2)。
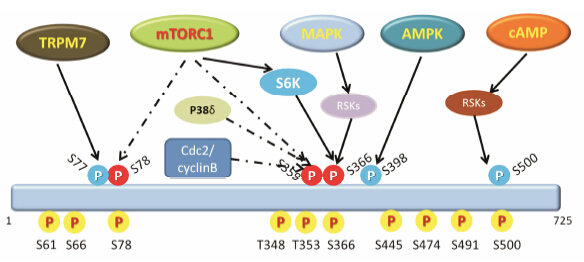
|
Figure 2 Schematic illustration of the autophosphorylation sites and regulation of eEF2K. The yellow cycle represents autophosphorylation sites,the red cycle represents inactivating sites and the blue represents activating sites. Further details are provided in the text |
2 eEF2K与肿瘤的关系
研究表明,eEF2K表达水平或者活性的改变将影响细胞许多生理进程,例如,细胞周期、细胞分化和细胞凋亡等[29, 30, 31, 32, 33, 34]。许多类型的癌细胞中eEF2K呈现高表达,尤其是在胶质瘤、乳腺癌、胰腺癌中最为明显[3, 4, 6, 7, 8, 9, 10]。
2.1 eEF2K与细胞周期
研究发现,eEF2K的活性改变影响细胞周期。在G1期,蛋白合成量很少,eEF2K的Ser359或Ser366位点磷酸化而失活[35,36]。在S期,蛋白合成急剧增加,eEF2K被一些激酶活化[36]。例如,PKA能够使eEF2K的Ser499位点发生磷酸化而发生活化[21]。在G2/M期,eEF2K又失活,抑制性的Ser366位点磷酸化又恢复到高水平[36]。Parmer等[6]和Carberg等[37]测定了MCF-7细胞、艾式腹水瘤 (Ehrlich ascites) 细胞不同时期细胞内eEF2K活性,发现在S期eEF2K的活性明显增加。eEF2K抑制剂NH125能够导致胶质瘤细胞株中S期细胞数量明显减少而G0/G1期细胞数增多[38]。而通过戈尔德霉素 (GA) 破坏HSP90和eEF2K间的相互作用后eEF2K表达降低,导致胶质瘤细胞 (C6和T98G) 不能进入S期[33]。
2.2 eEF2K与肿瘤的侵袭和转移
Zhang等[9]研究比较了敲除和未敲除eEF2K的胶质瘤细胞系 (T98G和LN229) 的侵袭和转移能力,结果发现通过SiRNA将eEF2K敲除后,胶质瘤细胞的侵袭、转移能力明显降低。Tekedereli等[39]也发现了相同的现象,他们利用SiRNA将乳腺癌细胞系 (MDA- MB-231) 中的eEF2K敲除后,侵袭、转移能力明显下降,而通过再次转染eEF2K使细胞中的eEF2K过表达则又能够增加乳腺癌细胞的侵袭和转移能力。
2.3 eEF2K与应激保护
研究表明,eEF2K活性增强是肿瘤细胞应对环境应激下的一种自我保护机制。尤其是细胞面临营养缺乏时,细胞内eEF2K在很大程度上被激活,使得其底物eEF2磷酸化而抑制蛋白合成[40, 41]。因为从氧化磷酸化途径向有氧糖酵解途径的转换使得大多数肿瘤细胞具有新陈代谢脆弱性,因此当肿瘤细胞面对环境应激时为了生存必须迅速终止蛋白质合成以保存能量; Leprivier等[40]证明AMPK-eEF2K级联信号通路在细胞处于营养缺乏条件下起着重要的作用,体内实验也发现,eEF2K的过表达能够帮助小鼠的肿瘤成功度过营养缺乏期,而敲除eEF2K后的肿瘤细胞对营养缺乏变得非常敏感,更容易引发凋亡和坏死,抑制肿瘤生长。同样地,研究发现在化疗药物应激作用下,许多肿瘤细胞的eEF2K活性也明显增强,抑制eEF2K则消除肿瘤细胞应对环境应激时的自我保护,从而选择性地杀死肿瘤细胞,增加化疗药物的疗效。White等[42]报道经阿霉素处理后,细胞内eEF2的磷酸化水平明显提升。Tekedereli等[39]研究发现静脉注射eEF2K的脂质体SiRNA后,能够明显下调体内eEF2K的表达,增加肿瘤细胞对化疗药物阿霉素的敏感性,抑制异位移植瘤的生长。类似,将eEF2K干扰或抑制其活性后与rottlerin[5, 10]、姜黄素[43]、bortezomib[43]、吉非替尼[8]、拉帕替尼[8]等联合作用也能够明显增加它们的抗肿瘤作用。
2.4 eEF2K与自噬
细胞自噬 (autophagy) 普遍存在于真核细胞中,有利于帮助细胞维持蛋白代谢平衡以及细胞内环境的稳定,当细胞受到内外因素刺激时如氨基酸缺乏、微生物入侵等都能够导致自噬的发生[44]。许多研究表明eEF2K与自噬存在着一定的联系,在大多数条件下eEF2K的过表达或者活性增加能增强细胞自噬水平,而通过SiRNA敲除eEF2K或者通过小分子抑制剂抑制eEF2K活性则能够抑制自噬降低细胞的生存能力,尤其是在胶质瘤、乳腺癌中最为明显[7, 8, 45, 46, 47]。其作用的分子机制可能是eEF2K位于mTORC1的下游,而mTORC1能够负向调控自噬的活性。因此,eEF2K可能在mTORC1和自噬调控中起着连接作用[7, 47]。然而并不是所有科学家都认同这一观点[48, 49, 50],最近Xie等[50]证明敲除人结肠癌细胞中的eEF2K后能够导致自噬,而且细胞活性增强,说明eEF2K在不同的细胞系中可能扮演不同的角色。因此,eEF2K与自噬的关系需要更进一步的研究。
3 eEF2K的抑制剂
eEF2K参与一些肿瘤的进展和应激保护,而且Ryazanov等[48]也表明eEF2K敲除后不影响小鼠及线虫正常的生长繁殖,提示eEF2K可能是一个潜在的治疗靶标,而天然产物来源是寻找其特异靶向抑制剂的重要途径之一。
目前发现的大部分eEF2K抑制剂都是非特异性的,包括rottlerin (图 3a)[5, 15, 51]、GA (图 3b)[52]、1,3- selenazine衍生物TS2 (图 3c)[53]、2-羟基亚芳-4-环戊烯-1,3-二酮衍生物TX-1918 (图 3d)[54]、噻吩[2,3-b]吡啶类似物34 (图 3e)[55]。比较特异的抑制剂只有两个,即吡啶[2,3-d]嘧啶-2,4-二酮衍生物A484954 (图 3f)[56, 57]和1-苯甲基-3-十六烷基-2-甲基咪唑碘化物NH125 (图 3g)[37, 49]。特异性抑制剂A484954通过和eEF2K的ATP结构域结合,在体外很低的浓度 (IC50为280 nmol·L-1) 即可抑制eEF2K的活性,但是细胞内抑制eEF2K需要很高的浓度 (约为100 μmol·L-1),难以体内实验,没有药物开发价值。由于其细胞毒性很低,是目前体外实验中常用的eEF2K抑制剂[57]。NH125曾被作为eEF2K特异性抑制剂而广泛应用,其体外IC50为60 nmol·L-1 [37, 49]。但是最近有报道认为NH125并不特异性抑制eEF2K的活性,Devkota等[56]甚至报道NH125对于肿瘤的抑制作用是通过使eEF2的磷酸化水平增高实现的,并不抑制eEF2K的活性。
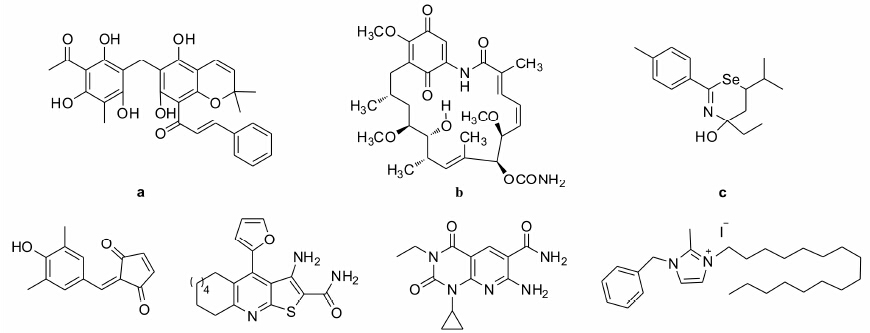
|
Figure 3 Structures of eEF2K inhibitors. a: Rottlerin; b: GA; c: TS2; d: TX-1918; e: Compound 34; f: A484954: g: NH125 |
构建高效的eEF2K抑制剂筛选模型,尽快发现特异的eEF2K抑制剂对于新药开发乃至eEF2K结构和功能的研究都十分必要。Ryazanov等[58]报道了一种传统方法,他们采用放射性同位素标记的方法筛选eEF2K抑制剂。但因有放射性而难以普及。最近文 献[59]报道了两种应用于高通量筛选的方法。一种是背景低且能实时监测的Sox-based荧光实验,eEF2K的底物磷酸化后荧光增加,通过检测荧光变化而反映eEF2K活性来进行筛选,但该方法昂贵且需要特定的底物。另一种是平行性好、操作简单的基于化学发光的实验,eEF2K使底物发生磷酸化会消耗ATP,通过检测剩余ATP或者生成ADP的量来反映eEF2K的活性进行药物筛选。笔者实验室成功地表达、纯化eEF2K以及CaM,并优化了筛选模型中激酶、底物、ATP浓度以及作用时间,避免eEF2K自磷酸化对实验的干扰,建立了基于化学发光原理的eEF2K抑制剂筛选模型。天然天物是发现新药的重要途径之一, 而这一筛选模型的建立将有助于发现海洋天然产物中的eEF2K抑制剂。
4 小结
eEF2K的分子结构已经基本清楚,细胞内许多激酶以及信号通路与eEF2K的联系也得到阐明,特别是eEF2K的异常表达与活化及一些肿瘤的进程不断被揭示,提示eEF2K是一个抗肿瘤药物的新的潜在靶标。可以推测,随着对eEF2K的结构和功能的进 一步了解,特别是对不同磷酸化位点对eEF2K调控的理解,以及eEF2K晶体结构的成功解析,将有助于发现和设计出更加特异性靶向eEF2K的化合物,开发治疗癌症更有效的候选药物。
| [1] | Nairn AC, Bhagat B, Palfrey HC. Identification of calmodulin-dependent protein kinase III and its major Mr 100 000 substrate in mammalian tissues [J]. Proc Natl Acad Sci USA, 1985, 82: 7939-7943. |
| [2] | Chafouleas JG, Pardue RL, Brinkley BR, et al. Regulation of intracellular levels of calmodulin and tubulin in normal and transformed cells [J]. Proc Natl Acad Sci USA, 1981, 78: 996-1000. |
| [3] | Bagaglio DM, Hait WN. Role of calmodulin-dependent phosphorylation of elongation-factor-2 in the proliferation of rat glial-cells [J]. Cell Growth Differ, 1994, 5: 1403-1408. |
| [4] | Nilsson A, Nygard O. Phosphorylation of eukaryotic elongation factor 2 in differentiating and proliferating HL-60 cells [J]. Biochim Biophys Acta, 1995, 1268: 263-268. |
| [5] | Parmer TG, Ward MD, Hait WN. Effects of rottlerin, an inhibitor of calmodulin-dependent protein kinase III, on cellular proliferation, viability, and cell cycle distribution in malignant glioma cells [J]. Cell Growth Differ, 1997, 8: 327-334. |
| [6] | Parmer TG, Ward MD, Yurkow EJ, et al. Activity and regulation by growth factors of calmodulin dependent protein kinase III (elongation factor 2-kinase) in human breast cancer [J]. Br J Cancer, 1999, 79: 59-64. |
| [7] | Wu H, Yang JM, Jin S, et al. Elongation factor-2 kinase regulates autophagy in human glioblastoma cells [J]. Cancer Res, 2006, 66: 3015-3023. |
| [8] | Cheng Y, Li HJ, Ren XC, et al. Cytoprotective effect of the elongation factor-2 kinase-mediated autophagy in breast cancer cells subjected to growth factor inhibition [J]. PLoS One, 2010, 5: e9517. |
| [9] | Zhang L, Zhang Y, Liu XY, et al. Expression of elongation factor-2 kinase contributes to anoikis resistance and invasion of human glioma cells [J]. Acta Pharmacol Sin, 2011, 32: 361-367. |
| [10] | Ashour AA, Abdel-Aziz AA, Mansour AM, et al. Targeting elongation factor-2 kinase (eEF-2K) induces apoptosis in human pancreatic cancer cells [J]. Apoptosis, 2014, 19: 241-258. |
| [11] | Redpath NT, Price NT, Proud CG. Cloning and expression of cDNA encoding protein synthesis elongation factor-2 kinase [J]. J Biol Chem, 1996, 271: 17547-17554. |
| [12] | Ryazanov AG, Ward MD, Mendola CE, et al. Identification of a new class of protein kinases represented by eukaryotic elongation factor-2 kinase [J]. Proc Natl Acad Sci USA, 1997, 94: 4884-4889. |
| [13] | Pigott CR, Mikolajek H, Moore CE, et al. Insights into the regulation of eukaryotic elongation factor 2 kinase and the interplay between its domains [J]. Biochem J, 2012, 442: 105-118. |
| [14] | Mittl PR, Schneider-Brachert W. Sel1-like repeat proteins in signal transduction [J]. Cell Signal, 2007, 19: 20-31. |
| [15] | Redpath NT, Proud CG. Purification and phosphorylation of elongation factor-2 kinase from rabbit reticulocytes [J]. Eur J Biochem, 1993, 212: 511-520. |
| [16] | Clark K, Middelbeek J, Morrice NA, et al. Massive autophosphorylation of the Ser/Thr-rich domain controls protein kinase activity of TRPM6 and TRPM7 [J]. PLoS One, 2008, 3: e1876. |
| [17] | Crawley SW, Gharaei MS, Ye Q, et al. Autophosphorylation activates dictyostelium myosin II heavy chain kinase A by providing a ligand for an allosteric binding site in the alpha-kinase domain [J]. J Biol Chem, 2011, 286: 2607-2616. |
| [18] | Pyr Dit Ruys S, Wang X, Smith EM, et al. Identification of autophosphorylation sites in eukaryotic elongation factor-2 kinase [J]. Biochem J, 2012, 442: 681-692. |
| [19] | Tavares CD, O'Brien JP, Abramczyk O, et al. Calcium/calmodulin stimulates the autophosphorylation of elongation factor 2 kinase on Thr-348 and Ser-500 to regulate its activity and calcium dependence [J]. Biochemistry, 2012, 51: 2232-2245. |
| [20] | Mitsui K, Brady M, Palfrey HC, et al. Purification and characterization of calmodulin-dependent protein kinase III from rabbit reticulocytes and rat pancreas [J]. J Biol Chem, 1993, 268: 13422-13433. |
| [21] | Diggle TA, Subkhankulova T, Lilley KS, et al. Phosphorylation of elongation factor-2 kinase on serine 499 by cAMP-dependent protein kinase induces Ca2+/calmodulin-independent activity [J]. Biochem J, 2001, 353: 621-626. |
| [22] | Wang XM, Li W, Williams M, et al. Regulation of elongation factor 2 kinase by p90(RSK1) and p70 S6 kinase [J]. EMBO J, 2001, 20: 4370-4379. |
| [23] | Knebel A, Morrice N, Cohen P. A novel method to identify protein kinase substrates: eEF2 kinase is phosphorylated and inhibited by SAPK4/p38 delta [J]. EMBO J, 2001, 20: 4360-4369. |
| [24] | Smith EM, Proud CG. CDC2-cyclin B regulates eEF2 kinase activity in a cell cycle-and amino acid-dependent manner [J]. EMBO J, 2008, 27: 1005-1016. |
| [25] | Redpath NT, Proud CG. Cyclic AMP-dependent protein kinase phosphorylates rabbit reticulocyte elongation factor-2 kinase and induces calcium-independent activity [J]. Biochem J, 1993, 293 (Pt 1): 31-34. |
| [26] | Diggle TA, Redpath NT, Heesom KJ, et al. Regulation of protein-synthesis elongation-factor-2 kinase by cAMP in adipocytes [J]. Biochem J, 1998, 336: 525-529. |
| [27] | Browne GJ, Finn SG, Proud CG. Stimulation of the AMP-activated protein kinase leads to activation of eukaryotic elongation factor 2 kinase and to its phosphorylation at a novel site, serine 398 [J]. J Biol Chem, 2004, 279: 12220-12231. |
| [28] | Perraud AL, Zhao XY, Ryazanov AG, et al. The channel-kinase TRPM7 regulates phosphorylation of the translational factor eEF2 via eEF2-k [J]. Cell Signal, 2011, 23: 586-593. |
| [29] | End D, Hanson M, Hashimoto S, et al. Inhibition of the phosphorylation of a 1000, 000-dalton soluble protein in whole cells and cell-free extracts of PC12 pheochromocytoma cells following treatment with nerve growth factor [J]. J Biol Chem, 1982, 257: 9223-9225. |
| [30] | Brady MJ, Nairn AC, Wagner JA, et al. Nerve growth factor-induced down-regulation of calmodulin-dependent protein kinase-Ⅲ in PC12 cells involves cyclic AMP-dependent protein-kinase [J]. J Neurochem, 1990, 54: 1034-1039. |
| [31] | Celis JE, Madsen P, Ryazanov AG. Increased phosphorylation of elongation factor 2 during mitosis in transformed human amnion cells correlates with a decreased rate of protein synthesis [J]. Proc Natl Acad Sci USA, 1990, 87: 4231-4235. |
| [32] | Severinov KV, Melnikova EG, Ryazanov AG. Downregulation of the translation elongation factor 2 kinase in Xenopus laevis oocytes at the final stages of oogenesis [J]. New Biol, 1990, 2: 887-893. |
| [33] | White-Gilbertson S, Kurtz DT, Voelkel-Johnson C. The role of protein synthesis in cell cycling and cancer [J]. Mol Oncol, 2009, 3: 402-408. |
| [34] | Bagaglio DM, Cheng EH, Gorelick FS, et al. Phosphorylation of elongation factor 2 in normal and malignant rat glial cells [J]. Cancer Res, 1993, 53: 2260-2264. |
| [35] | Proud CG. Signalling to translation: how signal transduction pathways control the protein synthetic machinery [J]. Biochem J, 2007, 403: 217-234. |
| [36] | Roberts EC, Hammond K, Traish AM, et al. Identification of G2/M targets for the MAP kinase pathway by functional proteomics [J]. Proteomics, 2006, 6: 4541-4553. |
| [37] | Carlberg U, Nilsson A, Skog S, et al. Increased activity of the eEF-2 specific, Ca2+ and calmodulin dependent protein kinase III during the S-phase in ehrlich ascites cells [J]. Biochem Biophys Res Commun, 1991, 180: 1372-1376. |
| [38] | Arora S, Yang JM, Kinzy TG, et al. Identification and characterization of an inhibitor of eukaryotic elongation factor 2 kinase against human cancer cell lines [J]. Cancer Res, 2003, 63: 6894-6899. |
| [39] | Tekedereli I, Alpay SN, Tavares CDJ, et al. Targeted silencing of elongation factor 2 kinase suppresses growth and sensitizes tumors to doxorubicin in an orthotopic model of breast cancer [J] PLoS One, 2012, 7: e41171. |
| [40] | Leprivier G, Remke M, Rotblat B, et al. The eEF2 kinase confers resistance to nutrient deprivation by blocking translation elongation [J]. Cell, 2013, 153: 1064-1079. |
| [41] | Kenney JW, Moore CE, Wang X, et al. Eukaryotic elongation factor 2 kinase, an unusual enzyme with multiple roles [J]. Adv Biol Regul, 2014, 55: 15-27. |
| [42] | White SJ, Kasman LM, Kelly MM, et al. Doxorubicin generates a proapoptotic phenotype by phosphorylation of elongation factor 2 [J]. Free Radic Biol Med, 2007, 43: 1313-1321. |
| [43] | Cheng Y, Ren XC, Zhang Y, et al. Integrated regulation of autophagy and apoptosis by EEF2K controls cellular fate and modulates the efficacy of curcumin and velcade against tumor cells [J]. Autophagy, 2013, 9: 208-219. |
| [44] | Yang YP, Liang ZQ, Gu ZL, et al. Molecular mechanism and regulation of autophagy [J]. Acta Pharm Sin (药学学报), 2005, 26: 1421-1434. |
| [45] | Cheng Y, Ren XC, Zhang Y, et al. eEF-2 kinase dictates cross-talk between autophagy and apoptosis induced by Akt inhibition, thereby modulating cytotoxicity of novel Akt inhibitor MK-2206 [J]. Cancer Res, 2011, 71: 2654-2663. |
| [46] | Hait WN, Wu H, Jin S, et al. Elongation factor-2 kinase: its role in protein synthesis and autophagy [J]. Autophagy, 2006, 2: 294-296. |
| [47] | Py BF, Boyce M, Yuan J. A critical role of eEF-2K in mediating autophagy in response to multiple cellular stresses [J]. Autophagy, 2009, 5: 393-396. |
| [48] | Ryazanov AG. Elongation factor-2 kinase and its newly discovered relatives [J]. FEBS Lett, 2002, 514: 26-29. |
| [49] | Chen ZH, Gopalakrishnan SM, Bui MH, et al. 1-Benzyl-3-cetyl-2-methylimidazolium iodide (NH125) induces phosphorylation of eukaryotic elongation factor-2 (eEF2) a cautionary note on the anticancer mechanism of an eEF2 kinase inhibitor [J]. J Biol Chem, 2011, 286: 43951-43958. |
| [50] | Xie CM, Liu XY, Sham KW, et al. Silencing of EEF2K (eukaryotic elongation factor-2 kinase) reveals AMPK-ULK1-dependent autophagy in colon cancer cells [J]. Autophagy, 2014, 10: 1495-1508. |
| [51] | Pavur KS, Petrov AN, Ryazanov AG. Mapping the functional domains of elongation factor-2 kinase [J]. Biochemistry, 2000, 39: 12216-12224. |
| [52] | Yang J, Yang JM, Iannone M, et al. Disruption of the EF-2 kinase/Hsp90 protein complex: a possible mechanism to inhibit glioblastoma by geldanamycin [J]. Cancer Res, 2001, 61: 4010-4016. |
| [53] | Cho SI, Koketsu I, Ishihara H, et al. Novel compounds, ‘1, 3-selenazine derivatives’ as specific inhibitors of eukaryotic elongation factor-2 kinase [J]. Biochim Biophys Acta, 2000, 1475: 207-215. |
| [54] | Hori H, Nagasawa H, Ishibashi M, et al. TX-1123: An antitumor 2-hydroxyarylidene-4-cyclopentene-1, 3-dione as a protein tyrosine kinase inhibitor having low mitochondrial toxicity [J]. Bioorg Med Chem, 2002, 10: 3257-3265. |
| [55] | Lockman JW, Reeder MD, Suzuki K, et al. Inhibition of eEF2-K by thieno[2, 3-b]pyridine analogues [J]. Bioorg Med Chem Lett, 2010, 20: 2283-2286. |
| [56] | Devkota AK, Tavares CDJ, Warthaka M, et al. Investigating the kinetic mechanism of inhibition of elongation factor 2 kinase by NH125: evidence of a common in vitro artifact [J]. Biochemistry, 2012, 51: 2100-2112. |
| [57] | Edupuganti R, Wang Q, Tavares CD, et al. Synthesis and biological evaluation of pyrido[2, 3-d]pyrimidine-2, 4-dione derivatives as eEF-2K inhibitors [J]. Bioorg Med Chem, 2014, 22: 4910-4916. |
| [58] | Ryazanov AG, Hait WN, Pavur KS. Elongation factor-2 kinase (EF-2 kinase), and methods of use therefor: US, 6346406 [P]. 2002-12-02.Devkota AK, Warthaka M, Edupuganti R, et al. High-throughput screens for eEF-2 kinase [J]. J Biomol Screen, 2014, 19: 445-452. |
 2015, Vol. 50
2015, Vol. 50


