2. 南京中医药大学附属医院, 江苏 南京 210036;
3. 江苏省药物研究所, 江苏 南京 210009
2. Affiliated Hospital of Nanjing University of Chinese Medicine, Nanjing 210036, China;
3. Jiangsu Provincial Institute of Materia Medica, Nanjing 210009, China
Selaginella pulvinata (Hook. et Grev.) Maxim. (Selaginellaceae),is a perennial herb widely distributed in China. As one of the two qualified species listed in Chinese Pharmacopoeia (2010 edition)[1],it has been widely used in traditional Chinese medicine for the treatment of cancer,dysmenorrhea,asthma,and traumatic injury[2]. The pharmacological investigations of the genus Selaginella revealed that it has numerous biological activities,including anticancer[3,4],antivirus[5],anti-inflammation[6,7,8],anti-diabetes[9],and vascular endothelial protective effect[10]. In addition to biflavonoids,a series of interesting selaginellin derivatives[11,12,13,14] featuring unique alkynyl and p-quinone methide functionalities have been discovered from this genus. Continued investigation of the title plant led to the isolation of a new selaginellin derivative named as selaginellin S (1),together with a known compound (2,selaginellin M) (Figure 1). Compound 2 is first reported in this plant. Herein,we report the isolation and structural elucidation of the new compound by extensive spectroscopic analyses.
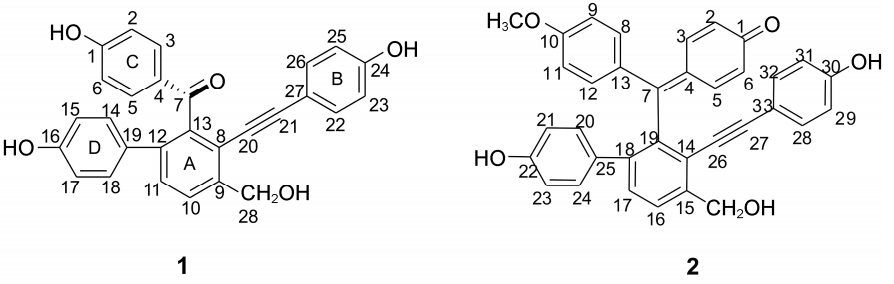 |
Figure 1 The structures of compounds 1-2 |
Selaginellin S (1) was obtained as a red powder; its molecular formula was determined as C28H20O5 by HR-ESI-MS with an ion peak at m/z 437.138 15 [M+ H]+ (calcd. 436.687 14),which indicated 19 degrees of unsaturation. The UV absorption at 282 nm was indicative of an aromatic system. The 1H NMR spectral data (Table 1) indicated the presence of two ortho- coupled aromatic signals δH 7.67 and δH 7.41 (1H,d,J = 8.4 Hz),attributed to the tetra-substituted A-ring; protons of three AA'XX' systems representing the respective p- substituted rings B,C,and D,respectively. Differentiation between the above spin systems,were achieved via 1H-1H COSY spectrum. The 13C NMR spectrum showed a total of 28 carbon signals,including one carbonyl,one oxygenated methylene,two acetylenic and twenty-four aromatic carbons. The NMR spectra of 1 were similar to those of selaginellin; instead of a semi-quinone unit,there was a carbonyl carbon.
|
|
Table 1 1H and 13C NMR (DMSO-d6) data for 1. J in Hz |
The apparent HMBC correlations from H-14/18 to C-12,and from H-11 to C-19,linked the A-ring to C- 12,which was further supported by ROESY correlation between H-11 and H-18. The location of a hydroxy- methyl [δH/δC 4.76 (2H,s)/60.9,C-28] group at C-9 (A) was confirmed using a combination of HMBC and ROESY experiments as depicted in Figure 2. Two characteristic signals at δC 98.7 and 83.2 in the 13C NMR (DEPT) spectrum implied the presence of an alkynyl,which was further confirmed by the absorption at 2 202 cm-1 inthe IR spectrum. HMBC correlations from H-22/26 to C-21 (δC 98.7) connected the B-ring to the alkynyl,whereas those from H-3/5 to C-7 (δC 195.3) placed the ketone group at C-4. In particular,the chemical shifts for C-8 (δC 117.7) and C-13 (δC 141.0) established the connections of C-8/C-20 and C-13/C-7,respectively,as is the case for reported selaginellins,thus defining the planar structure of 1 as drawn.
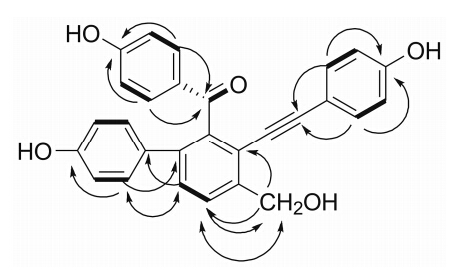 |
Figure 2 1H-1H COSY (—),key HMBC (→),and ROESY (↔) of 1 |
Despite extensive effort,attempts to produce suitable crystals of 1 for X-ray diffraction were unsuccessful. Alternatively,electronic circular dichroism (ECD) analysis was utilized as an indirect determination of absolute configuration. The theoretical ECD was calculated with a time-dependent density functional theory (TD-DFT) method at the B3LYP/6-311+G(d) level. The calculated ECD spectrum was then produced by SpecDis software[15]. As shown in Figure 3,the calculated ECD curve matched very well with the experimental CD curve (determined in CH3CN),indicating that 1 has an R configuration. Apparently,selaginellin S is a key intermediate in the biosynthesis pathway of selaginellins.
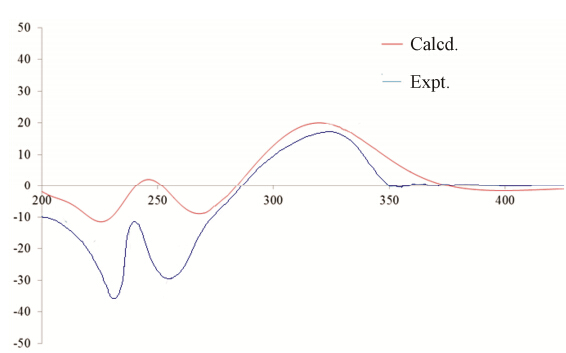 |
Figure 3 Experimental and calculated CD of (R)-1 |
General experimental procedures NMR spectra were recorded on BRUKER AV-500 spectrometers. UV/Vis spectra were recorded using a Shimadzu UV-2500PC spectrophotometer equipped with a 1 cm pathlength cell. IR spectra were obtained using a BRUKER Tensor 27 spectrometer with KBr disks. MS and HR-ESI-MS were measured on a VG Auto Spec-3000 mass spectrometer and an LTQ Orbitrap XL mass spectrometer (Thermo Scientific). Optical rotations were measured with a Jasco P-1020 polarimeter equipped with a 1 dm pathlength cell. CD spectra were recorded with a Jasco J-810 spectrometer with a 1 mm pathlength cell. Column chromatography was performed over silica gel H (200-300 mesh and 10-40 μm),and Sephadex LH-20 (50 μm; Uppsala,Sweden). TLC was conducted on precoated silica gel plates GF254 (Qingdao Marine Chemical Ltd.,Qingdao,People’s Republic of China).
Plant material The whole plants of Selaginella pulvinata were collected from Yunnan Province,P.R.China in October 2011 and identified by Dr. Prof. Jin-ao Duan. A voucher specimen (2011-10-018) was deposited at Jiangsu Collaborative Innovation Center of Chinese Medicinal Resources Industrialization,Nanjing University of Chinese Medicine.
Extraction and isolation The air-dried and powdered plants of S. pulvinata (5 kg) were extracted by 95% EtOH at room temperature for three times (50 L × 3),once per week. The EtOH solution was collected together and evaporated under reduced pressure to give a residue (65 g). The residue was suspended in water,and partitioned by n-hexane (10 L × 3),EtOAc (10 L × 3),and n-BuOH (10 L × 3). The EtOAc layer was chromatographed over silica gel eluted with CHCl3- CH3OH gradients (from 99∶1 to 1∶1) to afford five fractions (A-E),based on their TLC characteristics. Fraction C was further separated b y silica gel column eluted with CHCl3-CH3OH gradients (from 9∶1 to 1∶1) to afford five subfractions (C1-C5). Fraction C2 was isolated repeatedly by Sephadex LH-20 gel column (CH3OH,CH3OH-H2O) to yield compounds 1 (7 mg) and 2 (3 mg).
Selaginellin S (1): Red powder; [α]D25= +40.4 (c 0.1,CH3CN); UV 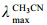 nm (logε): 282 (4.83); IR
nm (logε): 282 (4.83); IR  cm-1: 3 396,2 806,2 202,1 642,1 606,1 588,1 512,1 219,1 151,829; HR-ESI-MS m/z 437.138 15 [M+H]+ (calcd. for C28H20O5,436.687 14); 1H NMR (500 MHz,DMSO- d6) and 13C NMR (125 MHz,DMSO-d6) see Table 1; CD
cm-1: 3 396,2 806,2 202,1 642,1 606,1 588,1 512,1 219,1 151,829; HR-ESI-MS m/z 437.138 15 [M+H]+ (calcd. for C28H20O5,436.687 14); 1H NMR (500 MHz,DMSO- d6) and 13C NMR (125 MHz,DMSO-d6) see Table 1; CD 332 nm.
332 nm.
Selaginellin M (2): C35H26O5,red powder; [α]D25= 0 ( c 0.01,CH3OH); ESI-MS m/z 527 [M+H]+; 1H NMR (DMSO-d6,500 MHz) δH: 6.37 (1H,dd,J = 10.0,2.0 Hz,H-2),7.43 (1H,dd,J = 10.0,2.0 Hz,H-3),7.20 (1H,dd,J = 10.0,2.0 Hz,H-5),6.31 (1H,dd,J = 10.0,2.0 Hz,H-6),6.83 (4H,m,H-8,9,11,12),7.69 (1H,d,J = 8.0 Hz,H-16),7.34 (1H,d,J = 8.0 Hz,H-17),6.78 (2H,d,J = 8.4 Hz,H-20/24),6.59 (2H,d,J = 8.4 Hz,H-21/ 23),6.95 (2H,d,J = 8.4 Hz,H-28/32),6.71 (2H,d,J = 8.4 Hz,H-29/31),4.78 (2H,d,J = 5.4 Hz,H-34),3.71 (3H,s,H-35),5.59 (1H,br s),9.62 (1H,br s),10.16 (1H,br s); 13C NMR (DMSO-d6,125 MHz) δC: 185.0 (C-1),128.1 (C-2),138.5 (C-3),130.8 (C-4),139.5 (C-5),128.1 (C-6),159.0 (C-7),132.7 (C-8/12),113.9 (C-9/11),160.4 (C-10),130.7 (C-13),121.5 (C-14),142.9 (C-15),127.5 (C-16),129.7 (C-17),140.6 (C-18),140.1 (C-19),129.5 (C-20/24),114.6 (C-21/23),156.9 (C-22),130.7 (C-25),83.8 (C-26),98.7 (C-27),132.8 (C-28/32),115.8 (C-29/31),158.3 (C-30),112.8 (C-33),61.4 (C-34),55.4 (C-35). These data were consistent with those reported[14].
| [1] | Chinese Pharmacopoeia Commission. Pharmacopoeia of the People's Republic of China: Vol 1 (中华人民共和国药典: 第一卷) [S]. Beijing: China Medical Science Press, 2010: 210. |
| [2] | Editorial Board of Zhong Hua Ben Cao, State Administra-tion of Traditional Chinese Medicine of the People's Republic of China. Chinese Materia Medica: Vol 4 (中华本草: 第四卷) [M]. Shanghai: Shanghai Scientific and Technical Publishers, 1999: 387. |
| [3] | Chen JJ, Duh CY, Chen JF. New cytotoxic biflavonoids from Selaginella delicatula [J]. Planta Med, 2005, 71: 659-665. |
| [4] | Lin LC, Kou YC, Chou CJ. Cytotoxic biflavonoids from Selaginella delicatula [J]. J Nat Prod, 2000, 63: 627-630. |
| [5] | Ma SC, But PPH, Ooi VEC, et al. Antiviral amentoflavone from Selaginella sinensis [J]. Biol Pharm Bull, 2001, 24: 311-312. |
| [6] | Pokharel YR, Yang JW, Kim JY, et al. Potent inhibition of the inductions of inducible nitric oxide synthase and cyclooxygenase-2 by taiwaniaflavone [J]. Nitric Oxide, 2006, 15: 217-225. |
| [7] | Yang JW, Pokharel YR, Kim MR, et al. Inhibition of inducible nitric oxide synthase by sumaflavone isolated from Selaginella tamariscina [J]. J Ethnopharmacol, 2006, 105: 107-113. |
| [8] | Woo ER, Pokharel YR, Yang JW, et al. Inhibition of nuclear factor-κ B activation by 2', 8''-biapigenin [J]. Biol Pharm Bull, 2006, 29: 976-980. |
| [9] | Zheng XK, Zhang L, Wang WW, et al. Anti-diabetic activ-ity and potential mechanism of total flavonoids of Selaginella tamariscina (Beauv.) Spring in rats induced by high fat diet and low dose STZ [J]. J Ethnopharmacol, 2011, 137: 662- 668. |
| [10] | Zheng XK, Liu CX, Zhai YY, et al. Protection effect of amentoflavone in Selaginella tamariscina against TNF-α-induced vascular injure of endothelial cells [J]. Acta Pharm Sin (药学学报), 2013, 48: 1503-1509. |
| [11] | Zhang LP, Liang YM, Wei XC, et al. A new unusual natu-ral pigment from Selaginella sinensis and its noticeable physicochemical properties [J]. J Org Chem, 2007, 72, 3921-3924. |
| [12] | Cheng XL, Ma SC, Yu JD, et al. Selaginellin A and B, two novel natural pigments isolated from Selaginella tamariscina [J]. Chem Pharm Bull, 2008, 56: 982-984 |
| [13] | Cao Y, Chen JJ, Tan NH, et al. Antimicrobial selaginellin derivatives from Selaginella pulvinata [J]. Bioorg Med Chem Lett, 2010, 20: 2456-2460. |
| [14] | Zhang GG, Jing Y, Zhang HM, et al. Isolation and cyto-toxic activity of selaginellin derivatives and biflavonoids from Selaginella tamariscina [J]. Planta Med, 2012, 78: 390-392. |
| [15] | Bruhn T, Hemberger Y, Schaumloffel A, et al. SpecDis, version 1.51 [OL]. Germany: University of Wuerzburg, 2011. |
 2015, Vol. 50
2015, Vol. 50


