2. 成都中医药大学药学院, 四川 成都 610000;
3. 首都医科大学附属北京友谊医院, 北京 100050;
4. 中国中医科学院中药研究所, 北京 100700;
5. 国家食品药品监督管理总局药品评价中心, 北京 100045;
6. 解放军302医院中西医结合肝病诊疗与研究中心, 北京 100039
2. School of Pharmacy, Chengdu University of Traditional Chinese Medicine, Chengdu 610000, China;
3. Beijing Friendship Hospital of Capital Medical University, Beijing 100050, China;
4. Institute of Chinese Meteria Medica, China Academy of Chinese Medical Sciences, Beijing 100700, China;
5. Center for Drug Reevaluation, SFDA, Beijing 100045, China;
6. Integrative Medicine Center, 302 Military Hospital, Beijing 100039, China
何首乌为蓼科植物何首乌 (Polygonum multiflorum Thunb.) 的块根,为著名传统补益类中药。生品解 毒、消痈、截疟、润肠通便,炮制品补肝肾、益精血、乌须发[1]。何首乌广泛用于补肝肾、乌发、降血脂的中药制剂、保健食品,大众日常餐饮、食疗保健,以及洗护发用品等。据统计我国含首乌的中成药约500个,保健食品约200个。近年来国内外有关何首乌不良反应的报道大量增加,包括服用生首乌、制首乌、含何首乌的复方、中成药或保健食品等。国内外文献报道有关何首乌肝损伤病例约150例[2, 3, 4, 5]。2006年,英国和澳大利亚药监部门先后发布了何首乌肝损伤警告信息; 2013年,我国药监部门也发布了含首乌制剂肝损伤警示,何首乌肝损伤的问题引起国内外高度关注。因此,亟待阐明何首乌肝损伤的物质基础及相关科学机制,为临床合理用药、趋利避害提供科学依据。尽管何首乌致肝损伤的准确发病率数据尚不 清楚,根据国家不良反应中心的数据,以及302医院药物性肝损伤临床数据库的统计,推测何首乌肝损伤的总体发病率较低,可能存在高危人群,因此考虑何首乌肝损伤可能类似于特异质肝损伤。有关何首 乌特异质肝损伤的评价研究尚未见报道。本文采用 目前较广泛采用的基于内毒素 (脂多糖) 的特异质肝损伤动物模型,以何首乌为模型评价药物,考察何首乌对大鼠肝脏的损伤作用及其量-毒关系,以期为何首乌特异质肝损伤评价模型和科学机制的研究提供参考。
材料与方法 动物雄性SD大鼠,SPF级,200 g左右,购于 军事医学科学院实验动物中心 (合格证号SCXK-(军) 2012-0004)。分笼饲养,自由饮水及进食。室温 (25 ± 2) ℃,通风良好、环境安静,室内保持12 h照明,12 h 黑暗,并定期用紫外灯光消毒。
药物与试剂何首乌 (批号: 13101701) 购于北京绿野药业有限公司,经解放军第302医院肖小河研究员鉴定为蓼科植物何首乌 (Polygonum multiflorum Thunb.) 的干燥块根。脂多糖 (lipopolysaccharide,LPS) 购于Sigma公司(100 mg/支,批号113M4068V)。 戊巴比妥钠 (Sigma公司,批号57-33-0)。ALT、AST试剂盒均购自南京建成生物技术有限公司。其他所用试剂均为分析纯。
仪器SynergyH2全功能微孔板检测仪 (美国BioTek); 爱华KPJ-1A生物组织摊片烤片机 (天津天利航空有限公司); Leica 2016石蜡切片机 (上海莱卡仪器有限公司); Nikon E200光学显微镜。
药品制备根据前期实验,50% 乙醇冷浸提取何首乌的肝细胞毒性较强,本文采取50% 乙醇冷浸制备样品[6]。称取适量生何首乌 (PM) 饮片,加8倍量的50% 乙醇冷浸提取,共提取2次,每次48 h,合并提取液,减压浓缩回收乙醇,真空干燥得粗提物,临用前用去离子水配制成相应浓度 (按生药量配制)。
LPS给药剂量的确定将SD大鼠60只随机 分成6组 (n = 10): 正常对照组、PM组 (给药剂量为75.6 g·kg-1)、LPS (给药剂量为1.4 mg·kg-1) 组、LPS (给药剂量为2.8 mg·kg-1) 组、LP S (给药剂量为1.4 mg·kg-1) + PM组、LPS (给药剂量为2.8 mg·kg-1) + PM组。将大鼠称重,记录,其中PM通过灌胃给予,3 h后参考文献[7]尾静脉注射2.8 mg·kg-1的LPS,在7 h后,使用1% 戊巴比妥钠 (给药剂量为50 mg·kg-1) 将所有大鼠麻醉,下腔静脉取血,离心并采集血浆样本和肝组织样本[7]。
高剂量PM联合LPS的量-毒实验将SD大鼠80只随机分为8组 (n = 10): 正常对照组、LPS组、PM单独给药3个剂量组、LPS联合PM给药3个剂量 组。3个给药组剂量分别为: 18.9、37.8、75.6 g·kg-1。大鼠称重,灌胃不同剂量的何首乌提取物。其余同上述“LPS给药剂量的确定”实验部分。
低剂量PM联合LPS的量-毒实验何首乌3个给药组剂量分别为: 0.54、1.08、2.16 g·kg-1。其余同上述“LPS给药剂量的确定”实验部分。
肝功能指标检测采集大鼠血清,采用试剂盒 (酶标仪法) 检测肝功能指标ALT、AST。
肝脏病理学检查取大鼠肝组织1.5 cm × 1 cm × 0.5 cm,10% 甲醛固定,常规病理切片,HE染色,光镜下观察。
统计学分析采用SPSS13.0进行统计分析,计量资料采用单因素方差分析 (ANOVA)。
结果 1 不同LPS剂量对正常和LPS模型大鼠肝功能指标的影响差异从图 1可以看出,与正常对照组及LPS组的血 浆ALT、AST活力相比较,单用PM (给药剂量为 75.6 g·kg-1) 组没有显著性差异 (P > 0.05); 单用1.4和2.8 mg·kg-1的LPS组也没有显著性差异 (P > 0.05); 1.4 mg·kg-1LPS联用PM组ALT (P < 0.01),AST (P < 0.05) 均有显著性差异,2.8 mg·kg-1 LPS联用 PM组均有显著性差异 (P < 0.01)。从ALT与AST 活力值升高的幅度可以看出,2.8 mg·kg-1组比1.4 mg·kg-1组明显,故最终确定LPS造模的剂量为2.8 mg·kg-1。

|
Figure 1 Influence of co-treatment with different lipopolysaccharide (LPS) and Polygonum multiflorum (PM) on plasma alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities. For LPS-treated groups,“-”,“1.4” and “2.8” represent intravenous administration of saline and 1.4 mg·kg-1,2.8 mg·kg-1 of LPS,respectively. For PM-treated groups,“-” and “+” represent intragastric administration of saline and 75.6 g·kg-1 of PM,respectively. The saline-treated group served as a control group and the LPS-treated group served as a model group. n = 10,x± s. P < 0.05,**P < 0.01 vs control group; ▲P < 0.05,▲▲P < 0.01 vs model group |
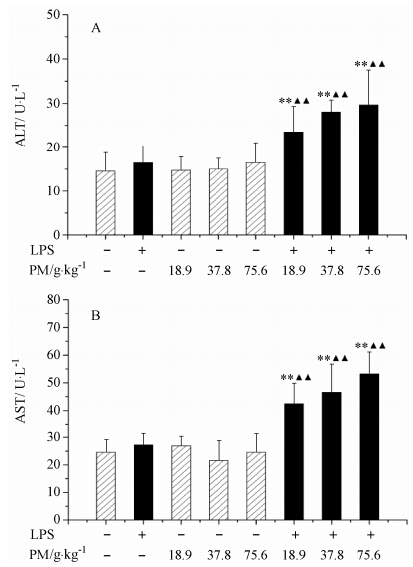
与正常对照组及LPS组比较,单独给药LPS、单独给药不同剂量何首乌组的ALT和AST活力均未 显著性升高 (P > 0.05); LPS联合18.9、37.8和75.6 g·kg-1何首乌组的ALT和AST活力均有显著性升高 (P < 0.01),且表现出明显的量-毒关系 (图 2)。
|
Figure 2 Influence of co-treatment with LPS and high doses of PM on plasma ALT and AST activities. For LPS-treated groups,“-” and “+” represent intravenous administration of saline and 2.8 mg·kg-1 of LPS,respectively,and for PM-treated groups,“-”,“18.9”,“37.8” and “75.6” represent intragastric administration of saline and 18.9,37.8 and 75.6 g·kg-1 of PM,respectively. Saline-treated group served as a control group and LPS-treated group served as a model group. n = 10,x± s. **P < 0.01 vs control group; ▲▲P < 0.01 vs model group |
与正常对照组及LPS组比较,单独给药LPS组与何首乌 (PM) 的2个剂量组的ALT、AST活力均无显著性改变 (P > 0.05); LPS联合1.08 g·kg-1何首乌组的ALT活力显著性升高 (P < 0.05),LPS联合2.16 g·kg-1何首乌组的ALT活力显著性升高 (P < 0.01),LPS联合1.08和2.16 g·kg-1何首乌组的AST活力显著性升高 (P < 0.01),而LPS联合0.54 g·kg-1何首乌组无显著性改变 (P > 0.05),说明作为特异质肝损伤研究模型可靠 (图 3)。

|
Figure 3 Influence of co-treatment with LPS,close to clinical equivalent doses of PM on plasma ALT and AST activities. For LPS-treated groups,“-” and “+” represent intravenous administration of saline and 2.8 mg·kg-1 of LPS,respectively and for PM-treated groups,“-”,“0.54”,“1.08” and “2.16” represent intragastric administration of saline and 0.54,1.08 and 2.16 g·kg-1 of PM,respectively. Saline-treated group served as a control group and LPS-treated group served as a model group. n = 10,x± s. P < 0.05,**P < 0.01 vs control group; ▲P < 0.05,▲▲P < 0.01 vs model group |
与正常对照组相比,何首乌给药组 (PM) 无显著病理学改变; LPS组门管区有轻度的炎症细胞浸润; LPS+何首乌组可见中央静脉扩张、内膜脱落,中央静脉周肝细胞肿胀、坏死,汇管区有大量炎症细胞浸润,中间区部分肝细胞肿胀、坏死 (表 1与图 4)。
|
|
Table 1 Effects of PM on morphological changes of rat liver obtained 7 h after LPS administration. n = 10,x± s. +: Mild; ++: Moderate; +++: Marked; -: Negative |

|
Figure 4 Representative microphotographs of livers isolated from rats. Saline-treated (control) (A) and PM (75.6 g·kg-1,ig)- treated (B) rats had no or minimal histopathological changes. Treatment with LPS (2.8 mg·kg-1,iv) alone caused slight infiltration of inflammatory cells in portal area but no evident hepatocytes injury (C). Co-treatment with LPS (2.8 mg·kg-1,iv) and PM (75.6 g·kg-1,ig) caused hepatocyte focal necrosis,loss of central vein intima and a large number of inflammatory cell infiltration in portal areas (D). HE staining (×200) |
药物的肝脏毒副反应可分为固有肝毒性 (intrinsic hepatotoxicity) 和特异质肝毒性 (idiosyncratic heaptotoxicity) 两类[8],其中前者是指药物肝损伤发生 与剂量、时间正相关,用药个体间差异不明显; 而后者是指临床上仅有小部分人群发生 (通常发生率1/ 1 000~1/10 000)[9],这些药物往往在动物安全性评价中难以发现明显肝损伤,甚至在临床试验阶段也难以发现,只有在上市后大量人群应用才能发现[10]。从实验毒理学来看,何首乌在正常动物水平需要在超大剂量 (40~60 g·kg-1)、长期给药 (4~12周) 情况下才能引起大鼠肝损伤[11, 12, 13, 14],与一般意义上的肝毒物相差甚远; 但从临床肝损伤病例来看,许多患者并没有超剂量服用,服用剂量是在药典规定范围 (生首乌3~6 g/日,制首乌6~12 g/日) 之内[4, 15, 16, 17, 18, 19]。因此仅从正常动物损伤评价结果难以解释和研究何首乌肝损伤问题。尽管何首乌肝损伤病例报道较多,但考虑到服用何首乌人群巨大,其发生率可能较低,结合在正常动物安全性评价表现出的肝损伤不强的特点,考虑何首乌可能为特异质肝损伤。因此,建立何首乌特异质肝损伤评价模型,对于深入研究何首乌肝损伤的物质基础和作用机制具有重要意义。
特异质肝毒性评价一直是国内外毒理学研究的难点和热点[9, 11, 20]。近年来研究表明,采用内毒素 (如脂多糖LPS) 免疫敏化的动物模型评价药物特异质肝损伤与临床实际情况较为近似。LPS是革兰阴性菌细胞壁的主要成分,广泛存在于人和动物的消化系统,当肠道屏障受损或相关炎症疾病时,可发生LPS通过门静脉入肝增加; LPS可以激活免疫系统和炎症细胞,释放的炎症因子可以放大炎症反应,如TNF-α、IL-1β和IL-6。微量的LPS可以引起温和的、无损伤的炎症反应,并不会发展为明显的肝细胞毒性; 但是伴随着炎症因子的释放,破坏组织内环境的稳定性,使肝脏对药物敏感性大大增强,表现出药物特异质肝损伤。已有较多文献报道证实,联合使用无毒剂量的LPS与一些药物共同作用,可以产生类似临床特异质肝损伤反应。如临床发现有特异质肝损伤的曲伐沙星在LPS模型上表现出肝损伤,而临床上没有特异质肝损伤的左氧氟沙星在LPS模型上则不能表现出肝损伤[21],证明LPS模型可以很好地评价临床药物特异质肝损伤。还有H2受体阻断剂的例子也证明LPS模型的应用价值,临床发现有特异质肝损伤的雷尼替丁在LPS模型上表现出损伤,而临床上没有特异质肝损伤的法莫替丁在LPS模型上则不能表现出肝损伤[22]。Deng等[23]报道LPS与双氯芬酸联合使用是通过激活免疫系统诱导的肝损伤,因此LPS模型可以作为研究特异质肝损伤的有效方法之一。
何首乌肝损伤问题已引起高度关注,但有关何首乌特异质肝损伤的研究方法尚未见报道。本文采用基于LPS的特异质肝损伤模型研究发现,何首乌在较低剂量 (1.08 g·kg-1,相当于人12 g/日的临床等效剂量) 即可引起大鼠肝功能损伤和组织病理学改变,而在正常动物上何首乌在该剂量的70倍 (75.6 g·kg-1) 给药仍未见明显肝损伤表现。由此可见,LPS可显著增强何首乌对实验动物的肝损伤。目前,关于何首乌肝损伤成分还不清楚,二苯乙烯苷[24]和大黄素[25]是其主要成分,关于二者究竟哪一个成分是毒性成分一直存在争议。基于该模型,通过目标成分敲出/敲入方法[26],将何首乌中不同类别成分 (组分) 敲出,与总提物对比损伤大小,探讨何首乌中贡献肝损伤的主要成分,以及不同类别成分间可能存在的协同增毒作用。此外,还可以利用该模型探讨何首乌特异质肝毒性的作用机制。在阐明特异质机制的基础上,就可以建立肝损伤高危人群识别方法,从而实现临床个体化安全用药[27],提高何首乌临床合理用药水平。
| [1] | China Pharmacopoeia Committee. Chinese Pharmacopoeia (中国药典) [M]. Beijing: China Medical Science Press, 2010: 122. |
| [2] | Kyoung AJ, Hyun JM, Seung SY, et al. Drug-induced liver injury: twenty five cases of acute hepatitis following ingestion of Polygonum multiflorum Thunb [J]. Gut Liver, 2011, 5: 493-499. |
| [3] | But PP, Tomlinson B, Lee KL. Hepatitis related to the Chinese medicine Shou-wu-pian manufactured from Polygonum multiflorum [J]. Vet Human Toxicol, 1996, 38: 280-282. |
| [4] | Park GJ, Mann SP, Ngu MC. Acute hepatitis induced by Shou-Wu-Pian, a herbal product derived from Polygonum multiflorum [J]. J Gastroenterol Hepatol, 2001, 16: 115-117. |
| [5] | Mazzanti G, Batinelli L, Daniele C, et al. New case of acute hepatitis following the consumption of Shou Wu Pian, a Chinese herbal product derived from Polygonum multiflorum [J]. Ann Intern Med, 2004, 140: W30. |
| [6] | Lü Y, Wang JB, Ji Y, et al. Influence of extracting solvent on hepatocytes toxicity of Polygonum multiflorum [J]. Chin J Exp Tradit Med Form (中国实验方剂学杂志), 2013, 20: 268-272. |
| [7] | Steven BY, Umesh MH, Bryan LC, et al. Endothelial cell injury and coagulation system activation during synergistic hepatotoxicity from monocrotaline and bacterial lipopolysaccharide coexposure [J]. Toxicol Sci, 2003, 74: 203-214. |
| [8] | Zhang SY, Zhu XG, Zhang GP, et al. Study on acute and chronic toxicities of Polygonimultiflori Radix Praeparata extracts [J]. J Toxicol August (毒理学杂志), 2013, 274: 261-264. |
| [9] | Victor JN, John RS. Drug-related hepatotoxicity [J]. New Engl J Med, 2006, 354: 731-739. |
| [10] | William ML. Drug-induced hepatotoxicity [J]. N Engl J Med, 2003, 349: 474-485. |
| [11] | Li Q, Zhao QJ, Zhao YL, et al. High dosage administration of Polygonum multiflorum alcohol extract caused the multi-organ injury in rats [J]. Global Tradit Chin Med (环球中医药), 2013, 6: 1-7. |
| [12] | Wang T, Wang JY, Jiang ZZ, et al. Study on hepatotoxicity of aqueous extracts of Polygonum multiflorum in rats after 28 play oral administration-analysis on correlation of cholestasis [J]. China J Chin Mater Med (中国中药杂志), 2012, 37: 1445-1450. |
| [13] | Hu XQ, Geng ZY, Li QL, et al. Experimental study of different doses of Polygoni Multiflori Preparata and the degree of liver injury in rats [J]. Shanxi J Tradit Chin Med (陕西中医), 2007, 28: 1420-1421. |
| [14] | Chang Q, Zhao HJ, Li C, et al. Effects of Radix Polygoni Multiflori Preparata and quantitative exercise on rat liver microcirculation and liver function [J]. Chin J Pharmacovigil (中国药物警戒), 2014, 11: 193-197, 202. |
| [15] | Panis B, Wong DR, Hooymans PM, et al. Recurrent toxic hepatitis in a Caucasian girl related to the use of Shou Wu Pian, a Chinese herbal preparation [J]. J Pediatr Gastroenterol Nutr, 2005, 41: 256-258. |
| [16] | Jung KA, Min HJ, Yoo SS, et al. Drug-induced liver injury: twenty five cases of acute hepatitis following ingestion of Polygonum multiflorum Thunb [J]. Gut Liver, 2011, 5: 493- 499. |
| [17] | But PP, Tomlinson B, Lee KL. Hepatitis related to the Chinese medicine Shou-Wu-Pian manufactured from Polygonum multiflorum [J]. Vet Hum Toxicol, 1996, 38: 280-282. |
| [18] | Mazzanti G, Battinelli L, Daniele C, et al. New case of acute hepatitis following the consumption of Shou Wu Pian, a Chinese herbal product derived from Polygonum multiflorum [J]. Ann Intern Med, 2004, 140: W30. |
| [19] | Cárdenas A, Restrepo JC, Sierra F, et al. Acute hepatitis due to shen-min: a herbal product derived from Polygonum multiflorum [J]. J Clin Gastroenterol, 2006, 40: 629-632. |
| [20] | Wang Q, Mei H, Zhang YL, et al. The associations between idiosyncratic adverse drug reactions and HLA alleles and their underlying mechanism [J]. Acta Pharm Sin (药学学报), 2013, 48: 799-808. |
| [21] | Waring JF, Liguori MJ, Luyendyk JP, et al. Microarray analysis of lipopolysaccharide potentiation of trovafloxacin-induced liver injury in rats suggests a role for proinflammatory chemokines and neutrophils [J]. J Pharmacol Exp Ther, 2006, 16: 1080-1087. |
| [22] | Luyendyk JP, Lehman-McKeeman LD, Nelson DM, et al. Coagulation-dependent gene expression and liver injury in rats given lipopolysaccharide with ranitidine but not with famotidine [J]. J Pharmacol Exp Ther, 2006, 317: 635-643. |
| [23] | Deng X, Stachlewitz RF, Liguori MJ, et al. Modest inflammation enhances diclofenac hepatotoxicity in rats: role of neutrophils and bacterial translocation [J]. J Pharmacol Exp Ther, 2006, 319: 1191-1199. |
| [24] | Wang CY, Zhang LT, Yuan ZF, et al. Study on pharmacokinetics of stilbens glucoside in Polygonum multiflorum [J]. Acta Pharm Sin (药学学报), 2002, 37: 955-958. |
| [25] | National Toxicology Program. Toxicology and carcinogenesis studies of EMODIN (CAS NO. 518-82-1) feed studies in F344/N rats and B6C3F1 mice [J]. Natl Toxicol Program Tech Rep Ser, 2001, 493: 1-278. |
| [26] | Kong WJ, Wang JB, Zhang QC, et al. A novel “target constituent knock-out” strategy coupled with TLC, UPLC-ELSD and microcalorimetry for preliminary screening of antibacterial constituents in Calculus bovis [J]. J Chromatogr B Analyt Technol Biomed Life Sci, 2011, 879: 3565-3573. |
| [27] | Zhang W, Zhou HH. Translational approach for pharmacogenomics and personalized medicine [J]. Acta Pharm Sin (药学学报), 2011, 46: 1-5. |
 2015, Vol. 50
2015, Vol. 50


