| 稀土La掺杂对层状富锂锰基氧化物正极材料结构及电化学性能的影响 |
传统的石油天然气等能源储量有限,同时在使用过程中所带来的环境污染问题日益严重,越来越多的国家及研究机构开展了新型能源的开发工作,锂离子电池[1]以其高能量密度及长寿命等突出优点,广泛应用于手机、便携式电脑、电动汽车及智能电网等领域.目前以钴酸锂[2-6]、磷酸铁锂[7-12]及三元材料[13-19]为代表的正极材料可逆容量均在200 mAh/g以下,难以满足国家对新能源汽车能量密度提出的要求.近阶段,一类基于Li2MnO3的高比容量(大于250 mAh/g)的层状富锂锰基正极材料[20-24]zLi2MnO3·(1-z)LiMO2(0 < z < 1, M=Mn0.5Ni0.5, MnxNiyCo(1-x-y),0 < x, 1 < y, 0 < x+y < 1)成为满足这一要求的最有希望的材料之一.
为改善层状富锂锰基正极材料首次充放电不可逆容量损失大,循环性能不佳的问题,研究者做了大量的工作[25-30],但目前对于稀土元素掺杂改善富锂锰基正极材料电化学性能的报道较少.大量研究[31-35]表明:稀土La掺杂对钴酸锂,锰酸锂及磷酸铁锂等正极材料的充放电容量及循环性能均有一定的提升作用.以稀土元素La掺杂Li1.2Mn0.54-xNi0.13Co0.13LaxO2(x=0, 0.1, 0.3, 0.5)富锂锰基正极材料,考察不同La掺杂量对正极材料的结构及电化学性能的影响.
1 实验 1.1 样品制备采用碳酸盐共沉淀法制备La掺杂的富锂前驱体,以MnSO4·H2O, CoSO4·7H2O, NiSO4·6H2O, La(NO3)3·6H2O为原料,按化学计量比将原料溶于去离子水中,配成浓度为2 mol/L的硫酸盐混合溶液,以Na2CO3为沉淀剂,溶入去离子水中配成浓度为2 mol/L的Na2CO3水溶液,同时在Na2CO3水溶液加入适量NH3·H2O作为络合剂.在连续搅拌(800 r/mim)的过程中将上述2种混合溶液同时滴入容器中,严格控制溶液中的pH为7.8,反应温度为50 ℃,反应时间为6 h;反应结束后陈化4 h,搅洗,过滤,真空干燥12 h即可获得类球形形貌的La掺杂的富锂前驱体.取适量前驱体按化学计量比加入Li2CO3球磨混合均匀后,置于马弗炉中550 ℃预烧6 h,900 ℃高温煅烧12 h,获得不同La含量掺杂的富锂锰基正极材料.
1.2 样品表征采用荷兰帕纳科X’Pert Powder型号X射线衍射仪进行晶体结构测试,扫描电子显微镜(Scanning Electron Microscopy, SEM)和X射线能量散射谱(Energy-Dispersive Spectrometry, EDS)测试采用捷克TESCAN MIRA3 LMH场发射扫描电子显微镜.
1.3 电池的组装及电化学性能测试以NMP(N-甲基吡咯烷酮)为分散剂,按质量比80:10:10将正极材料、PVDF(聚偏氟乙烯)、乙炔黑混合均匀后,在涂布机上制得正极片.在水含量及氧含量均低于0.1×10-6的氩气保护的手套箱中,采用锂片为负极,celgard2400微孔聚丙烯膜为隔膜,电解液采用1 mol/L的LiPF6溶于1:1(V/V)的碳酸乙烯酯(EC)和碳酸二甲酯(DMC)的混合溶液组装CR2032纽扣电池.采用LAND(CT2001A)型电池测试仪对组装好的纽扣电池进行恒流充放电测试,测试电压范围为2.0~4.6 V;采用CHI660B型电化学工作站对组装的扣式电池进行阻抗测试.
2 实验结果与分析 2.1 微观组织结构图 1所示为不同La含量掺杂的Li1.2Mn0.54-x·Ni0.13Co0.13LaxO2(x=0, 0.01, 0.03, 0.05)富锂锰基正极材料的SEM图谱,由图 1可以看出,所有的样品均是由一次颗粒堆垛形成的二次颗粒,并且颗粒均分散均匀,但二次颗粒出现不同程度的团聚现象.值得注意的是,掺杂La后的样品并未改变掺杂前样品的形貌,均是由类球形的二次颗粒形貌.随着La掺杂量的升高,试样Li1.2Mn0.54-xNi0.13Co0.13LaxO2(0.01≤x≤0.05)的颗粒尺寸呈现了减小的趋势,所有样品的颗粒尺寸均低于10 μm.
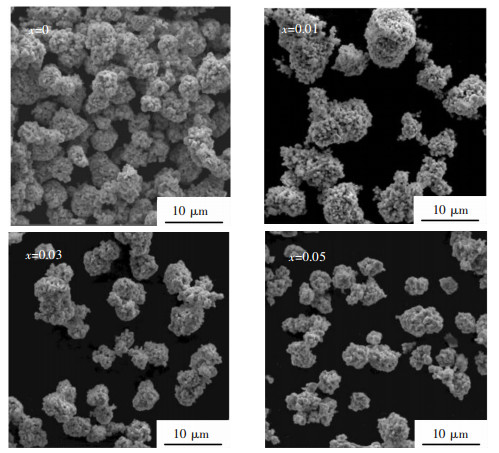 |
| 图 1 Li1.2Mn0.54-xNi0.13Co0.13LaxO2(x=0, 0.01, 0.03, 0.05)富锂锰基正极材料的SEM图谱 Fig. 1 SEM patterns of Li1.2Mn0.54-xNi0.13Co0.13LaxO2(x=0, 0.01, 0.03, 0.05) Li-rich Mn-based cathodes |
图 2所示为不同La含量掺杂的Li1.2Mn0.54-x·Ni0.13Co0.13LaxO2(x=0, 0.01, 0.03, 0.05)富锂锰基正极材料的EDS图谱.由图 2可知,所有样品均检测到Mn,Co,Ni 3种元素,当La掺杂量x=0.01时,EDS图谱中出现了微弱的La峰,随着La掺杂量的增加,EDS图谱中La的峰强度呈现出增强的趋势.为表征掺杂元素La在正极材料中的分布情况,元素的面分布图谱如图 3所示.由图 3可知,所含元素Mn,Co,Ni,La,O均被成功检测到且分布均匀.
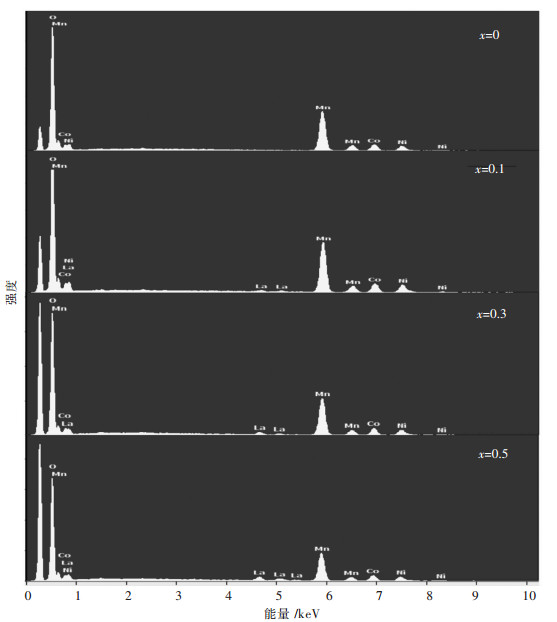 |
| 图 2 Li1.2Mn0.54-xNi0.13Co0.13LaxO2(x=0, 0.01, 0.03, 0.05)富锂锰基正极材料的EDS图谱 Fig. 2 EDS patterns of Li1.2Mn0.54-xNi0.13Co0.13LaxO2(x=0, 0.01, 0.03, 0.05) Li-rich Mn-based cathodes |
 |
| 图 3 Li1.2Mn0.54-xNi0.13Co0.13LaxO2(x=0.03)富锂锰基正极材料的元素面分布图谱 Fig. 3 EDS mapping patterns of Li1.2Mn0.54-xNi0.13Co0.13LaxO2(x=0.03) Li-rich Mn-based cathodes |
图 4所示为不同La含量掺杂的Li1.2Mn0.54-x·Ni0.13Co0.13LaxO2(x=0, 0.01, 0.03, 0.05)富锂锰基正极材料的XRD衍射图谱.由图 4可知,所有La掺杂的样品均呈现出层状富锂锰基正极材料的特征衍射峰,主体部分表现为层状R-3m型LiCoO2类似的α-NaFeO2的标准特征峰.可以发现,所有的样品在20°~25°之间均出现了微弱的衍射峰,这主要是由于材料组分中Li2MnO3存在于过渡金属层中,从而出现LiMn6型的超晶格的特征标志[36-38].值得注意的是,在图 4中所有样品的(006)与(012)和(018)与(110)特征峰对明显,由此可以看出所有La掺杂的样品均具有良好的晶体结晶性.随着La掺杂含量的增加,主相的峰并未发生明显的变化,依旧保持了明显的层状富锂锰基正极材料特征峰.当La掺杂量为1%时,即x=0.01,样品的XRD图谱中出现了微弱的二相衍射峰,经鉴定分析,该物相为La掺杂诱导形成的LaMnO3,由图 4可以清晰看到,LaMnO3的衍射峰强度随着La掺杂量的增加而显著增强,这与图 2中EDS图谱La峰强度随掺杂量增加变化的规律一致.
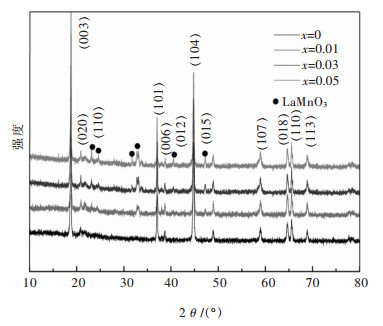 |
| 图 4 Li1.2Mn0.54-xNi0.13Co0.13LaxO2(x=0, 0.01, 0.03, 0.05)富锂锰基正极材料的XRD图谱 Fig. 4 XRD patterns of Li1.2Mn0.54-xNi0.13Co0.13LaxO2(x=0, 0.01, 0.03, 0.05) Li-rich Mn-based cathodes |
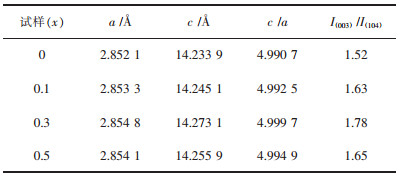
前期研究结果表明,材料的晶格常数出现变化可以证实掺杂元素是否进入材料的晶格[39],采用Jade软件计算样品的晶格常数及(003)/(104)峰强比,计算结果见表 1.从表 1中数据可以看出,掺杂后的样品晶格常数a值及c值均比未掺杂的样品大.结合图 2的EDS结果及图 4的XRD结果可知,稀土La掺杂后主要是以LaMnO3二相的形式存在,可能存在少部分La离子掺杂进入至材料的晶格中,La掺杂后拓宽了正极材料的嵌脱锂扩散通道,这主要是由于La离子半径(1.03Å)大于Mn离子半径(0.53Å)所致,该结果与文献[40-41]报道的类似.值得注意的是,随着La掺杂量的增加,样品的晶格常数并未出现线性的增长,这可能是由于La与O具有较强的结合力,当掺杂量达到一定程度时,La与O的强结合力使得层间距出现收缩[42].由掺杂前后材料的晶格常数变化可以证实La离子成功掺入至富锂锰基正极材料层状结构中,结合图 1的XRD图谱可知,未完全掺入材料晶格中的La离子以LaMnO3二相形式存在,LaMnO3二相的存在可能会对后续材料的电化学反应起到一定的促进作用.
| 表 1 Li1.2Mn0.54-xNi0.13Co0.13LaxO2(x=0, 0.01, 0.03, 0.05)富锂锰基正极材料的的晶格常数 Table 1 Lattice parameter patterns of Li1.2Mn0.54-xNi0.13Co0.13LaxO2(x=0, 0.01, 0.03, 0.05) Li-rich Mn-based cathodes |
 |
| 点击放大 |
一般来说,晶格常数c/a值可以作为评价材料的层状结构特征指数,c/a值越高,材料具有更优的层状结构特征.由表 1数据可知,经La掺杂后的样品的c/a值均大于未掺杂的试样,表明La掺杂后的样品较未掺杂的具有更好的层状结构特征.此外,I(003)/I(104)峰强比与正极材料层状结构中Li+与Ni2+阳离子混排有关,所有La掺杂的样品的I(003)/I(104)峰强比均比未掺杂的高,由此可知,经La掺杂后的样品均在一定程度上抑制了材料层状结构中Li+与Ni2+阳离子混排.
2.2 电化学性能所有样品在2.0~4.6 V电压期间25 mA/g(0.1C)的首次充放电曲线及电化学数据分别如图 5及表 2所列.由图 5可知,所有样品均呈现出类似的充放电曲线特征:充电曲线由4.5 V以下的斜线及4.5 V左右平台构成,分别对应于镍,钴的氧化及Li2MnO3活化.随着La掺杂量的升高,样品的充电容量依次降低,然而样品的放电量则先升高后降低,当掺杂量x=0.03时,样品的充放电量最高,分别为324.1 mAh/g和285.3 mAh/g.
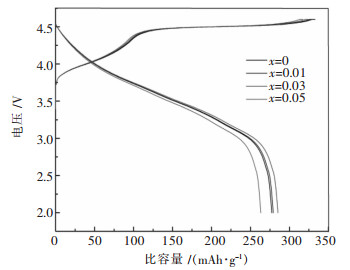 |
| 图 5 Li1.2Mn0.54-xNi0.13Co0.13LaxO2(x=0, 0.01, 0.03, 0.05)富锂锰基正极材料的初始充放电曲线 Fig. 5 The initial charge-discharge curves of Li1.2Mn0.54-xNi0.13Co0.13LaxO2(x=0, 0.01, 0.03, 0.05) Li-rich Mn-based cathodes |
| 表 2 Li1.2Mn0.54-xNi0.13Co0.13LaxO2(x=0, 0.01, 0.03, 0.05)富锂锰基正极材料的的晶格常数 Table 2 Lattice parameter patterns of Li1.2Mn0.54-xNi0.13Co0.13LaxO2(x=0, 0.01, 0.03, 0.05) Li-rich Mn-based cathodes |
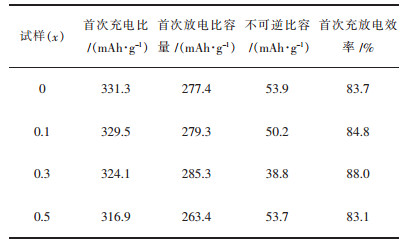 |
| 点击放大 |
由表 2可知,对比未掺杂La(x=0)的样品,La掺杂量为0.03时,其放电容量由277.4 mAh/g提高至285.3 mAh/g,不可逆容量损失由53.9 mAh/g降低至38.8 mAh/g,首次充放电效率由83.7 %提高至88.0 %.产生此现象的原因可能主要是:适量的La掺杂不仅可以拓宽锂离子的传输通道,而且能稳定材料的结构,掺杂La生成的微量LaMnO3可以保护首次充电时产生的氧空位,在一定程度上阻碍电解液对活性材料的浸蚀,同时减少锂空位在正极材料表面结构转变过程中的消失[39];然而掺杂量过少对材料的结构及电化学性能的改善作用有限,过量的La掺杂并未使La完全进入层状材料晶格,并且产生了过多的LaMnO3二相,该杂相可能会阻碍锂离子的传输,从而恶化了材料的电化学性能.
图 6展示了La掺杂Li1.2Mn0.54-xNi0.13Co0.13LaxO2(x=0, 0.03)富锂锰基正极材料活化前的电化学阻抗图谱,由图 6可知,La掺杂量为0和0.03均呈现出了标准的阻抗图谱,在高频区呈现一个半圆,在低频区为一条斜线.锂离子电池正极材料中高频区出现的半圆直径为电荷转移阻抗,低频区对应的斜线为锂离子扩散的Warburg阻抗.由图 6可以明显发现,当La掺杂量为0.3时,试样的电荷转移阻抗明显低于未掺杂的试样,表明经La掺杂后可以降低材料的电荷转移阻抗,电荷转移阻抗的降低进一步改善了材料的电化学性能,结果与图 5的电化学性能具有较好的吻合.
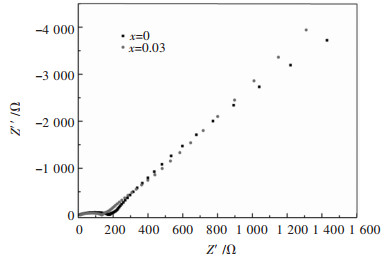 |
| 图 6 Li1.2Mn0.54-xNi0.13Co0.13LaxO2(x=0, 0.03)富锂锰基正极材料的阻抗曲线 Fig. 6 The impetance curves of Li1.2Mn0.54-xNi0.13Co0.13LaxO2(x=0, 0.03) Li-rich Mn-based cathodes |
图 7总结了La掺杂Li1.2Mn0.54-xNi0.13Co0.13LaxO2(x=0, 0.03)富锂锰基正极材料在25 mA/g(0.1 C)电流下的放电循环数据.由图 7可以看出,La掺杂量为0和0.03的样品的放电比容量均出现此起彼伏的趋势,这种容量衰减又升高的现象主要是层状富锂氧化物正极材料晶格中氧、锂空位的减少和高电位循环下电解液腐蚀共同作用的结果[43-44].当正极材料中未掺杂La时,其首次放电容量为277.4 mAh/g,循环50次后,放电容量由277.4 mAh/g减少至238.7 mAh/g,容量保持率为86.1 %.而当La掺杂量为0.03时,样品由首次的放电容量285.3 mAh/g降低为260.5 mAh/g,放电容量仅减少24.8 mAh/g,50次循环后的放电容量保持率为91.3 %.可以看出,未掺入的微量La所形成的LaMnO3能有效抑制电解液对活性材料的浸蚀和分解,提升了材料层状结构的稳定性,同时La掺杂后降低了材料的电荷转移阻抗,从而改善了材料的循环性能.
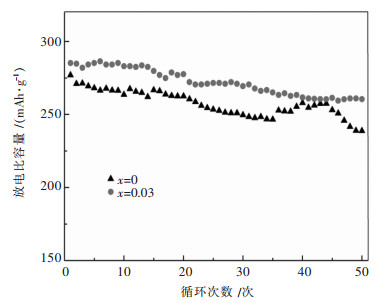 |
| 图 7 Li1.2Mn0.54-xNi0.13Co0.13LaxO2(x=0, 0.03)富锂锰基正极材料0.1C下的循环放电容量曲线 Fig. 7 0.1C rate cycling ability of Li1.2Mn0.54-xNi0.13Co0.13LaxO2 (x=0, 0.03) Li-rich Mn-based cathodes |
3 结论
1)所有样品均是由一次颗粒堆垛形成的二次颗粒,颗粒分散均匀,随着La掺杂量的增加,颗粒形貌未发生明显转变;不同La含量掺杂的Li1.2Mn0.54-xNi0.13Co0.13LaxO2(x=0, 0.01, 0.03, 0.05)富锂锰基正极材料均保持层状R-3m型LiCoO2类似的α-NaFeO2结构,经La掺杂后样品晶格常数均大于未掺杂的,所有La掺杂的样品的I(003)/I(104)峰强比均比未掺杂的高,由此表明,La掺杂后的样品具有更有的层状结构.
2)样品的放电比容量随着La掺杂量的增加先升高后降低,当La掺杂量为0.03时在0.1 C电流下具有最高的放电比容量(285.3 mAh/g),最低的阻抗,经50次充放电循环后放电比容量为260.5 mAh/g,容量保持率为91.3 %,显著高于La未掺杂的样品容量保持率的86.1 %.
| [1] |
OZAWA K. Lithium-ion rechargeable batteries with LiCoO2 and car bon electrodes-the LiCoO2-C system[J].
Solid State Ionics, 1994, 69(3): 212–221. |
| [2] |
KEMP J, COX P. Electronic structure of LiCoO2 and related materials; photoemission studies[J].
Journal of Physics-Condensed Matter, 1990, 2(48): 9653–9667. DOI: 10.1088/0953-8984/2/48/018. |
| [3] |
SEKAI K, AZUMA H, OMARU A, et al. Lithium-ion rechargeable cells with LiCoO2 and carbon electrodes[J].
Journal of Power Sources, 1993, 43(1/2/3): 241–244. |
| [4] |
DELMAS C, BRACONNIER J J, HAGENMULLER P. A new variety of LiCoO2 with an unusual oxygen packing obtained by exchange reaction[J].
Materials Research Bulletin, 1982, 17(1): 117–123. DOI: 10.1016/0025-5408(82)90192-1. |
| [5] |
MOORE R J, WHITE J. Equilibrium relationships in the systems Li-Co-O and Li-Ni-O[J].
Journal of Materials Science, 1974, 9(1): 1401–1408. |
| [6] |
CHEN C, KELDER E M, SCHOONMAN J, et al. Morphology control of thin LiCoO2 films fabricated using the electrostatic spray deposition (ESD) technique[J].
Journal of Materials Chemistry, 1996, 6(5): 765–771. DOI: 10.1039/jm9960600765. |
| [7] |
WANG J J, SUN X L. Understanding and recent development of carbon coating on LiFePO4 cathode materials for lithium-ion batteries[J].
Energy Environmental Science, 2012, 5(1): 5163–5185. |
| [8] |
WANG D, LI H, SHI S, et al. Improving the rate performance of LiFePO4 by Fe-site doping[J].
Electrochimica Acta, 2005, 50(14): 2955–2958. DOI: 10.1016/j.electacta.2004.11.045. |
| [9] |
GELLER S, DURAND J. Refinement of the structure of LiMnPO4[J].
Acta Crystallographica, 1960, 13(4): 325–331. DOI: 10.1107/S0365110X60002521. |
| [10] |
BRAMNIK N N, BRAMNIK K G, BUHRMESTER T, et al. Electrochemical and structural study of LiCoPO4-based electrodes[J].
Journal of Solid State Electrochemistry, 2004, 8(8): 558–564. |
| [11] |
BAKENOV Z, TANIGUCHI I. Electrochemical performance of nanocomposite LiMnPO4/C cathode materials for lithium batteries[J].
Electrochemistry Communications, 2010, 12(1): 75–78. |
| [12] |
JIN B, GU H B, KIM K W. Effect of different conductive additives on charge/discharge properties of LiCoPO4/Li batteries[J].
Journal of Solid State Electrochemistry, 2008, 12(2): 105–111. |
| [13] |
OHZUKU T, MAKIMURA Y. Layered Lithium Insertion Material of LiNi1/3Co1/3Mn1/3O2 for Lithium-Ion Batteries[J].
Chemistry Letters, 2001, 30(7): 642–644. DOI: 10.1246/cl.2001.642. |
| [14] |
SHAJU K M, RAO G V, CHOWDARI B. Performance of layered LiNi1/3Co1/3Mn1/3O2 as cathode for Li-ion batteries[J].
Electrochimica Acta, 2002, 48(2): 145–151. DOI: 10.1016/S0013-4686(02)00593-5. |
| [15] |
LI Y J, HAN Q, MING X Q, et al. Synthesis and characterization of LiNi0.5Co0.2Mn0.3O2 cathode material prepared by a novel hydrothermal process[J].
Ceramics International, 2014(40): 14933–14938. |
| [16] |
NOH M, CHO J. Optimized synthetic conditions of LiNi0.5Co0.2Mn0.3O2 cathode materials for high rate lithium batteries via Co-precipitation method[J].
Journal of The Electrochemical Society, 2012, 160(1): 105–111. |
| [17] |
YANG C F, HUANG J J, HUANG L G, et al. Electrochemical performance of LiCo1/3Co1/3Mn1/3O2 hollow spheres as cathode material forlithium ion batteries[J].
Journal of Power Sources, 2013, 226: 219–222. DOI: 10.1016/j.jpowsour.2012.10.089. |
| [18] |
LEE K S, MYUNG S T, PRAKASH J, et al. Optimization of microwave synthesis of Li[Ni0.4Co0.2Mn0.4]O2 as a positive electrode material for lithium batteries[J].
Electrochimica Acta, 2008, 53(7): 3065–3074. DOI: 10.1016/j.electacta.2007.11.042. |
| [19] |
FU C Y, ZHOU Z L, LIU Y H, et al. Synthesis and electrochemical properties of Mg-doped LiNi0.6Co0.2Mn0.2O2 cathode materials for Li-ion battery[J].
Journal of Wuhan University of Technology, 2011, 26(2): 211–215. DOI: 10.1007/s11595-011-0199-z. |
| [20] |
MYUNG S T, IZUMI K, KOMABA S, et al. Role of alumina coating on Li-Ni-Co-Mn-O particles as positive electrode material for lithium- ion batteries[J].
Chemistry of Materials, 2005, 17(14): 3695–3704. DOI: 10.1021/cm050566s. |
| [21] |
ZHENG J M, LI J, ZHANG Z R, et al. The effects of TiO2 coating on the electrochemical performance of Li[Li0.2Mn0.54Ni0.13Co0.13]O2 cathode material for lithium-ion battery[J].
Solid State Ionics, 2008, 179(27-32): 1794–1799. DOI: 10.1016/j.ssi.2008.01.091. |
| [22] |
WU Y, MURUGAN A V, MANTHIRAM A. Surface modification of high capacity layered Li[Li0.2Mn0.54Ni0.13Co0.13]O2 cathodes by AlPO4[J].
Journal of The Electrochemical Society, 2008, 155(9): A635–A641. DOI: 10.1149/1.2948350. |
| [23] |
LIU J, WANG Q Y. Carbon-coated high capacity layeredLi[Li0.2Mn0.54Ni0.13Co0.13]O2 cathodes[J].
Electrochemistry Communications, 2010, 12(6): 750–753. DOI: 10.1016/j.elecom.2010.03.024. |
| [24] |
LIU J, REEJA J B, MANTHIRAM A. Conductive surface modification with aluminum of high capacity layered Li[Li0.2Mn0.54Ni0.13Co0.13]O2 cathodes[J].
Journal of Physical Chemistry C, 2010, 114(20): 9528–9533. DOI: 10.1021/jp102050s. |
| [25] |
JIAO L F, ZHANG M, YUAN H T, et al. Effect of Cr doping on the structural, electrochemical properties of Li[Li0.2Ni0.2-x/2Mn0.6-x/2Crx]O2 (x=0, 0.02, 0.04, 0.06, 0.08) as cathode materials for lithium secondary batteries[J].
Journal of Power Sources, 2007, 167(1): 178–184. DOI: 10.1016/j.jpowsour.2007.01.070. |
| [26] |
PARK S H, SUN Y K. Synthesis and electrochemical properties of layered Li[Li0.25Ni(0.275-x/2)AlxMn(0.575-x/2)]O2 materials prepared by Sol-Gel method[J].
Journal of Power Sources, 2003, 119(21): 161–165. |
| [27] |
KANG S H, THACKERAY M M. Stabilization of xLi2MnO3·(1-x)LiMO2 electrode surfaces (M=Mn, Ni, Co) with mildly acidic, fluorinated solutions[J].
Journal of The Electrochemical Society, 2008, 155(4): A269–A275. DOI: 10.1149/1.2834904. |
| [28] |
KANG S H, AMINE K. Layered Li(Li0.2Ni0.15+0.5zCo0.10Mn0.55-0.5z)O2-zFz cathode materials for Li-ion secondary batteries[J].
Journal of Power Sources, 2005, 146(1/2): 654–657. |
| [29] |
钟盛文, 黎明旭, 张骞, 等. 富锂锰基正极材料的高温储存性能研究[J].
有色金属科学与工程, 2013, 4(3): 45–48.
|
| [30] |
徐宝和, 吴甜甜, 钟盛文, 等. Si4+掺杂对富锂Li[Li0.15Mn0.575Ni0.275]1-xSixO2材料性能的影响[J].
有色金属科学与工程, 2012, 3(2): 25–27.
|
| [31] |
GHULAM F, GHULAM M, MUHAMMAD U, et al. Effect of La-doping on the structural, morphological and electrochemical properties of LiCoO2 nanoparticles using Sol-Gel technique[J].
Materials Research Express, 2018, 5: 055044. DOI: 10.1088/2053-1591/aac4dc. |
| [32] |
张海朗, 安静. 稀土La元素掺杂对锂离子电池正极材料LiMn2O4性能的影响[J].
功能材料, 2009, 40(2): 507–508.
|
| [33] |
廖春发, 陈辉煌, 陈子发, 等. 稀土掺杂锂离子正极材料LiCoO2的影响研究[J].
江西有色金属, 2004, 18(2): 33–37.
DOI: 10.3969/j.issn.1674-9669.2004.02.010.
|
| [34] |
桂阳海, 胡国荣, 赵恒勤, 等. 稀土掺杂锂离子电池正极材料锂镍钴氧的研究[J].
矿产保护与利用, 2003, 4(2): 41–44.
DOI: 10.3969/j.issn.1001-0076.2003.02.012.
|
| [35] |
刘晓红, 项玲芳, 高军, 等. 稀土元素La掺杂材料LiCoPO4的性能研究[J].
矿冶工程, 2011, 31(3): 108–110.
DOI: 10.3969/j.issn.0253-6099.2011.03.028.
|
| [36] |
DENG H, BELHAROUAK I, SUN Y K, et al. LixNi0.25Mn0.75Oy (0.5≤x≤2, 2≤y≤2.75) compounds for high-energy lithium-ion batteries[J].
Journal of Materials Chemistry, 2009, 19(26): 4510–4516. DOI: 10.1039/b904098f. |
| [37] |
JARVIS K A, DENG Z, ALLARD L F, et al. Atomic structure of a lithium-rich layered oxide material for lithium-ion batteries: evidence of a solid solution[J].
Journal of Materials Chemistry, 2011, 23(16): 3614–3621. DOI: 10.1021/cm200831c. |
| [38] |
LU Z, MACNEIL D D, DAHN J R. Layered cathode materials Li[NixLi(1/3-2x/3)Mn(2/3-x/3)]O2 for llithium-ion batteries[J].
Electrochemical and Solid State Letters, 2001, 4(11): A191–A194. DOI: 10.1149/1.1407994. |
| [39] |
MYUNG S T, IZUMI K, KOMABA S, et al. Role of alumina coating on Li-Ni-Co-Mn-O particles as positive electrode material for lithium-ion batteries[J].
Journal of Materials Chemistry, 2005, 17(14): 3695–3704. DOI: 10.1021/cm050566s. |
| [40] |
DING Y, ZHANG P, JIANG Y, et al. Effect of rare earth elements doping on structure and electrochemical properties of LiNi1/3Co1/3Mn1/3O for lithium-ion battery[J].
Solid State Ionic, 2007, 178: 967–971. DOI: 10.1016/j.ssi.2007.04.012. |
| [41] |
LI N, AN R, SU Y F, et al. The role of yttrium content in improving electrochemical performance of layered lithium-rich cathode materials for Li-ion batteries[J].
Journal of Materials Chemistry A, 2013, 1: 9760–9767. DOI: 10.1039/c3ta11665d. |
| [42] |
SPEIGHT J G. Lange′s handbook of chemistry[M]. New York: Mc Graw-Hill: 2005.
|
| [43] |
DENG Z Q, MANTHIRAM A. Influence of cationic substitutions on the oxygen loss and reversible capacity of Lithium-rich layered oxide cathodes[J].
Journal of Physical Chemistry C, 2011, 115(14): 7097–7103. DOI: 10.1021/jp200375d. |
| [44] |
WU F, LI N, SU Y, et al. Can surface modification be more effective to enhance the electrochemical performance of lithium rich materials[J].
Journal of Materials Chemistry, 2012, 22(4): 1489–1497. |
 2019, Vol. 10
2019, Vol. 10


